Mineral Composition, Pore Structure, and Mechanical Characteristics of Pyroxene Granite Exposed to Heat Treatments
Abstract
1. Introduction
2. Experimental Material and Methodology
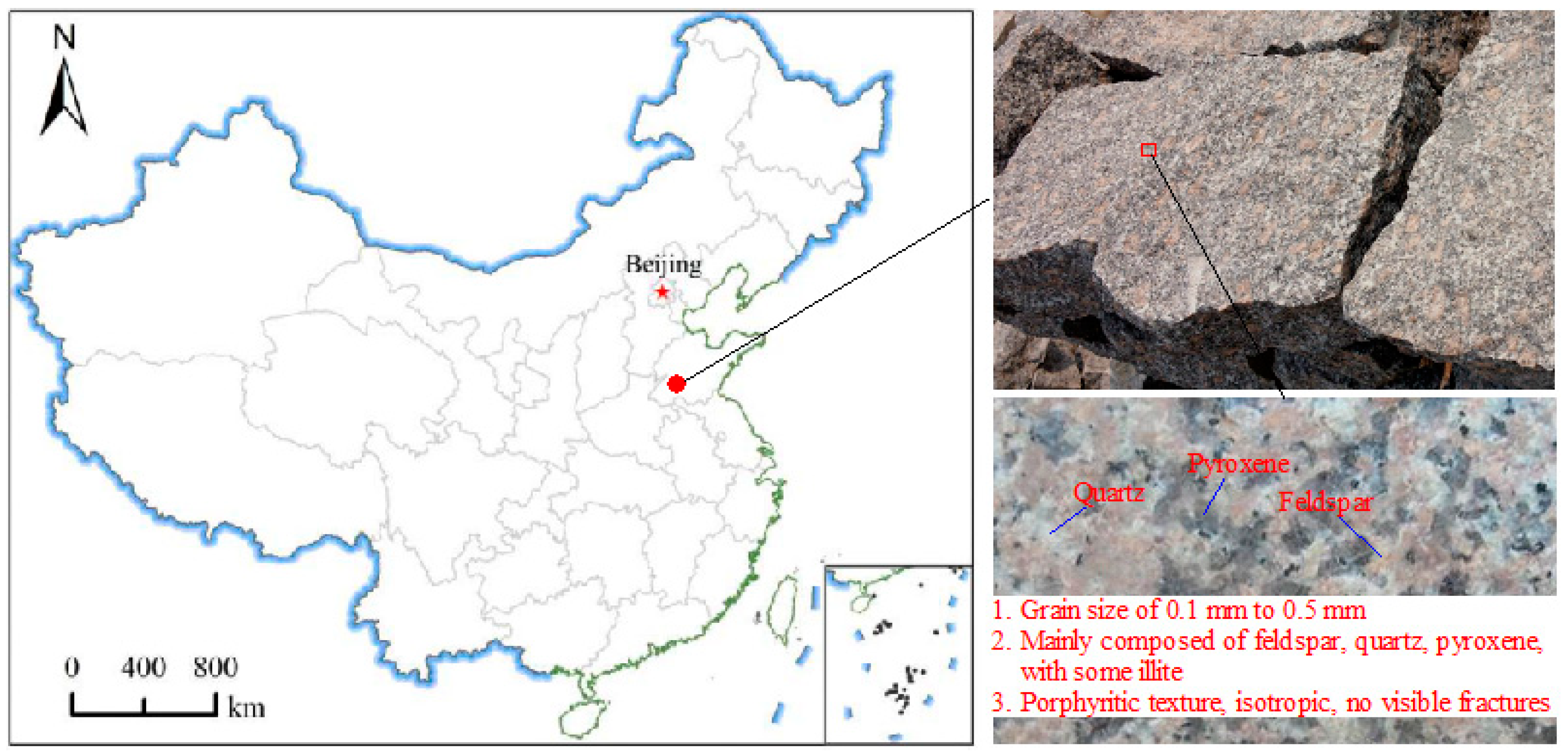
2.1. Sample Description
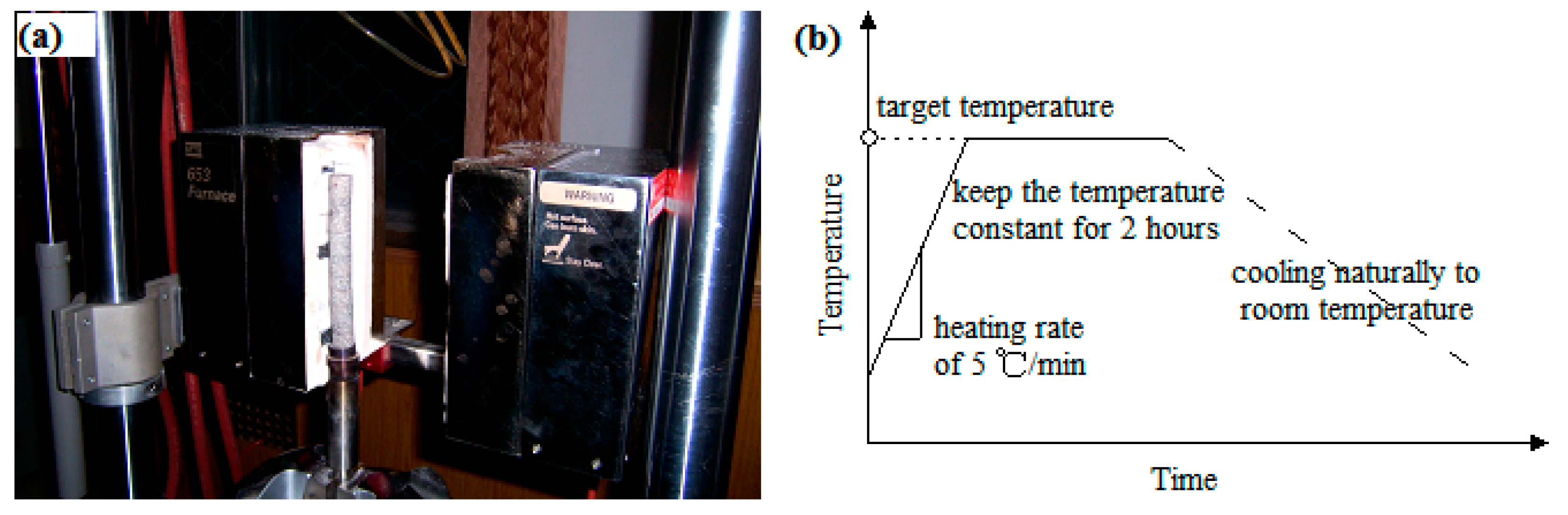
2.2. Thermal Treatment
2.3. Experimental Procedure and Instruments
3. Results
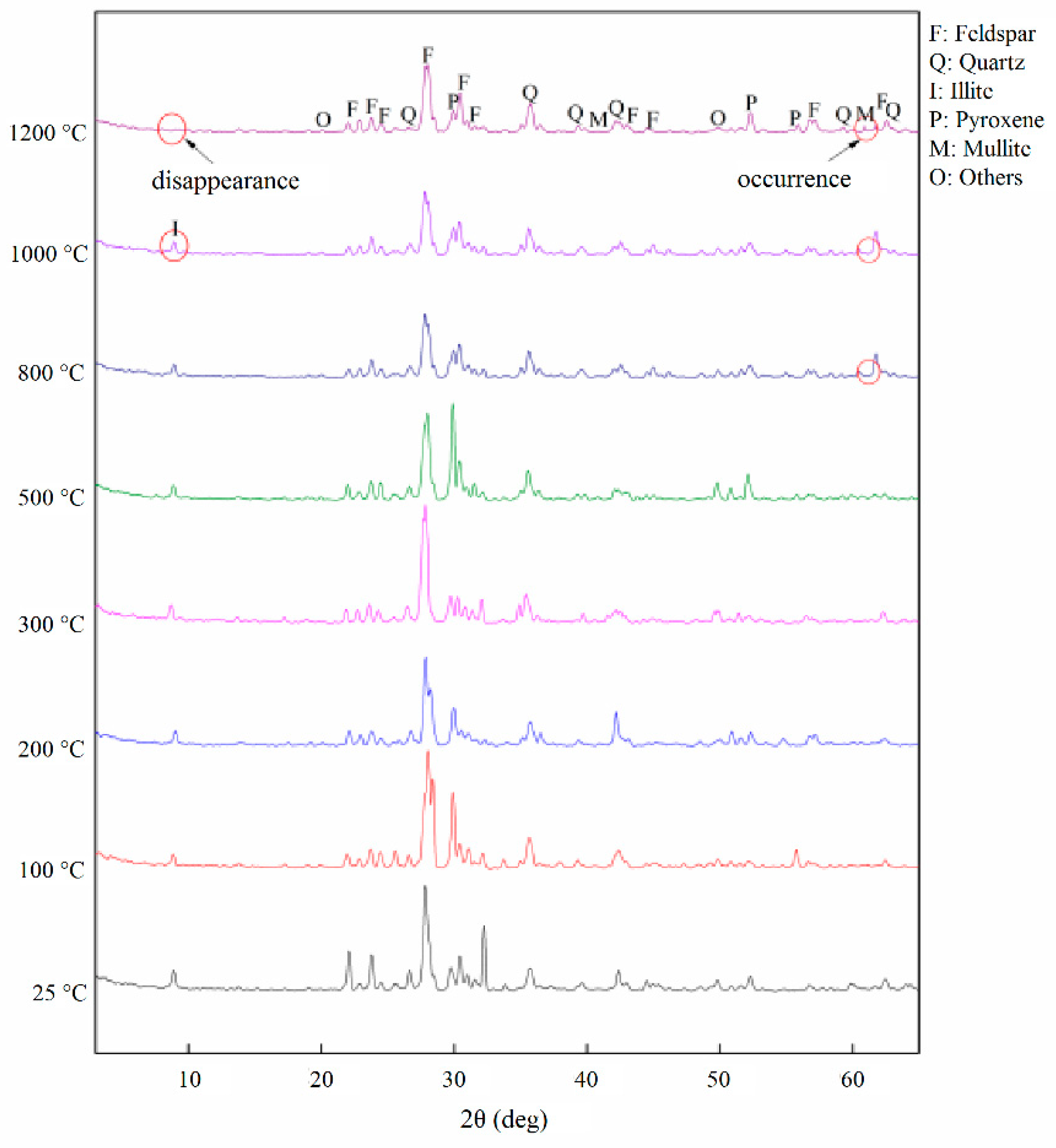
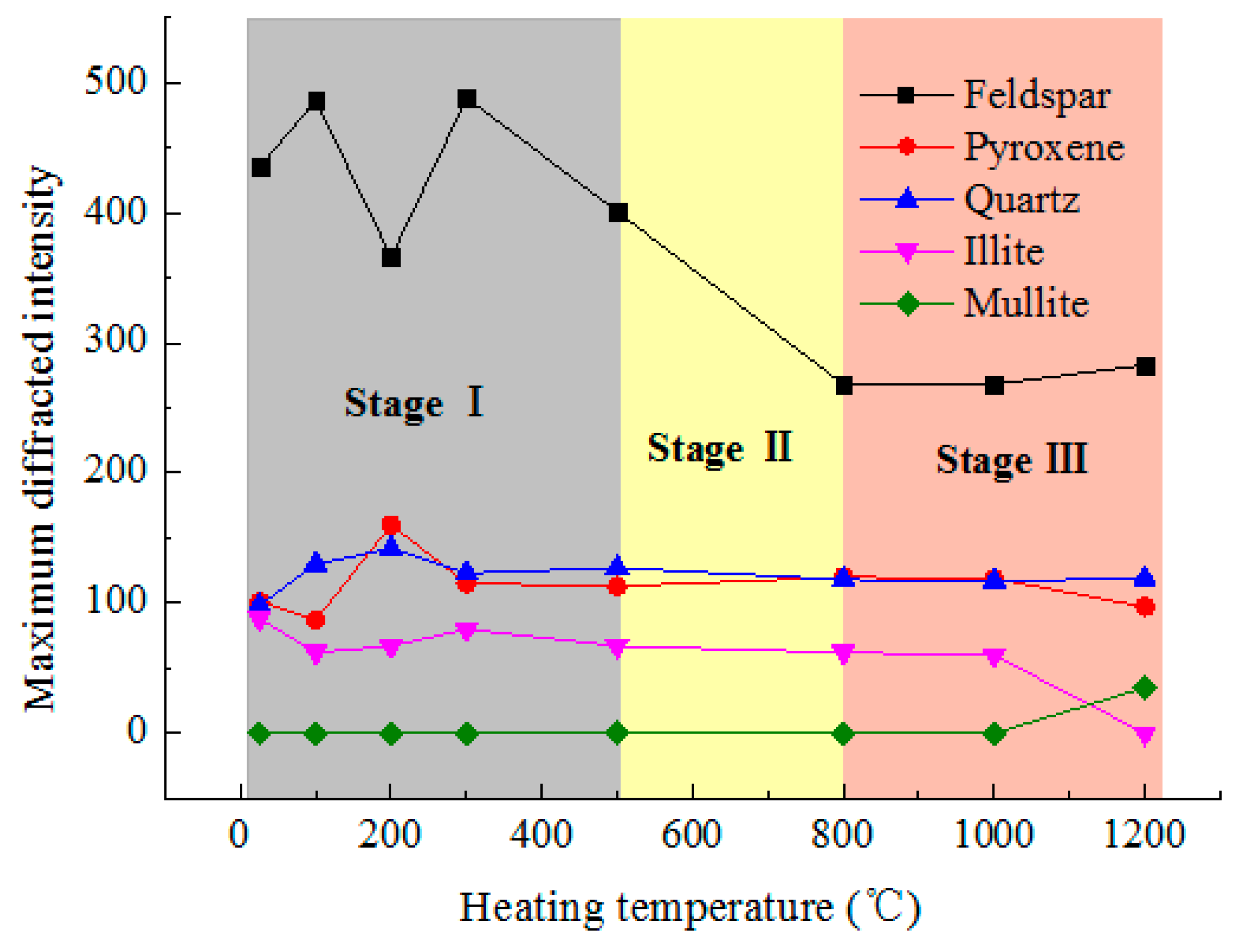
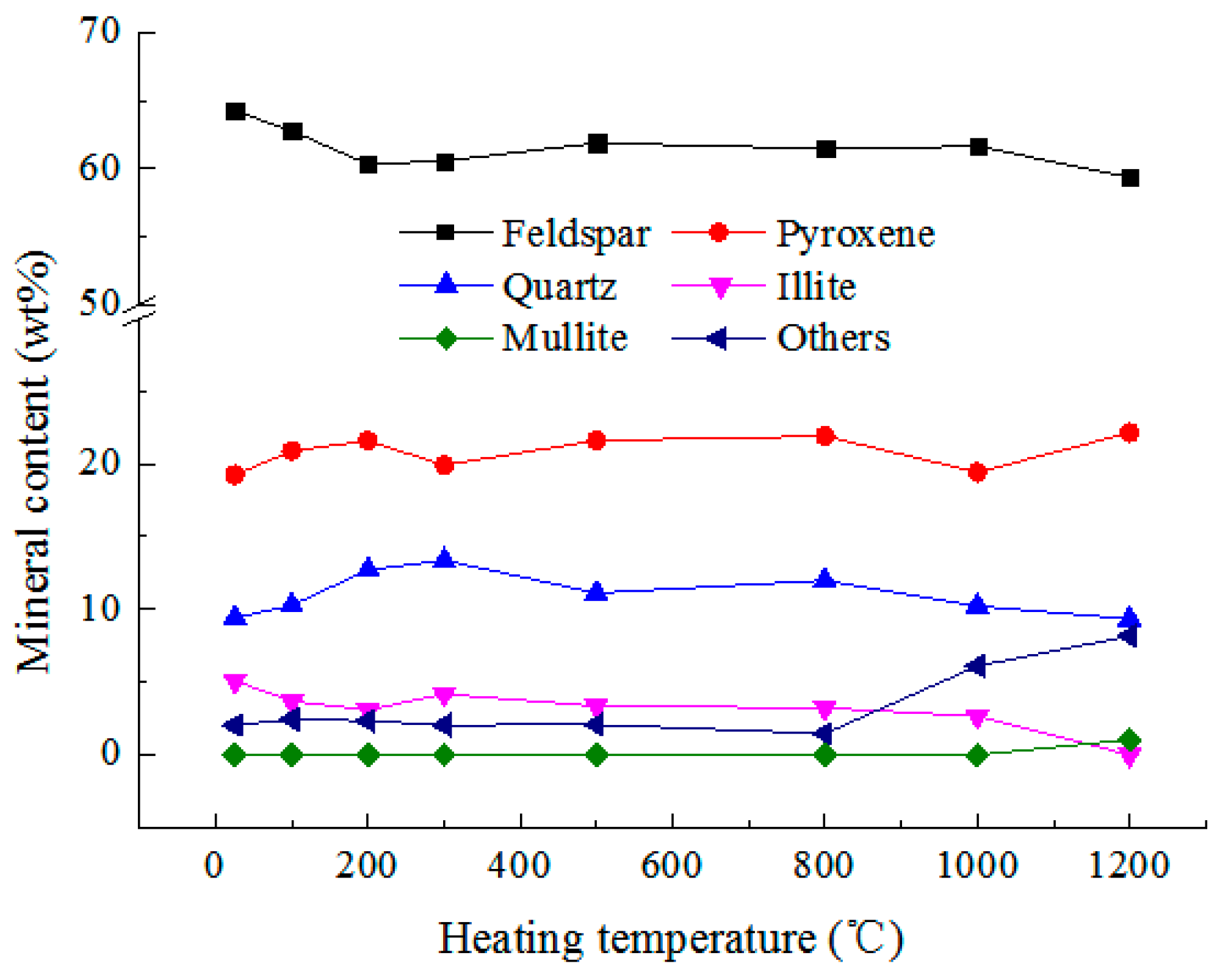
3.1. Mineral Composition
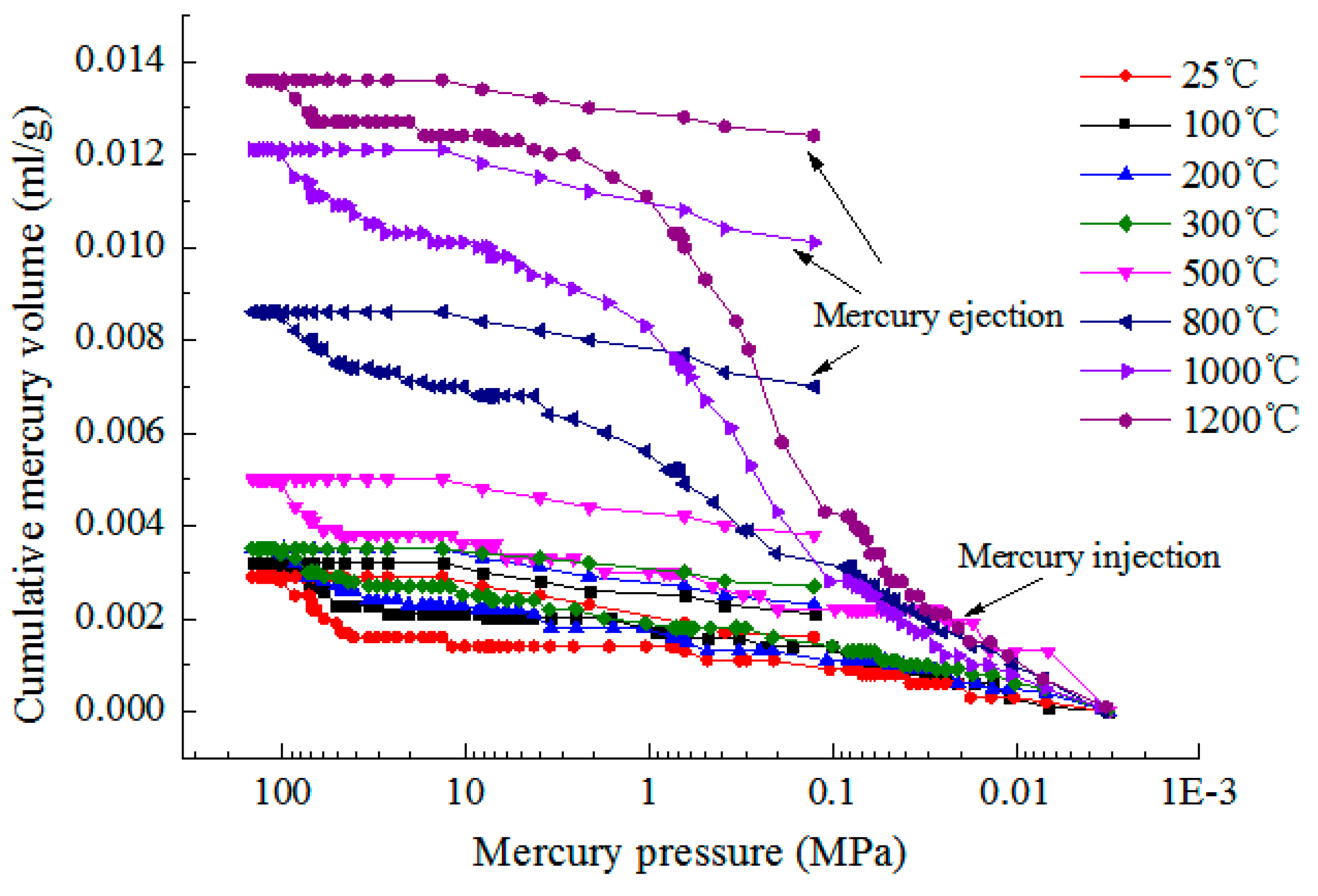
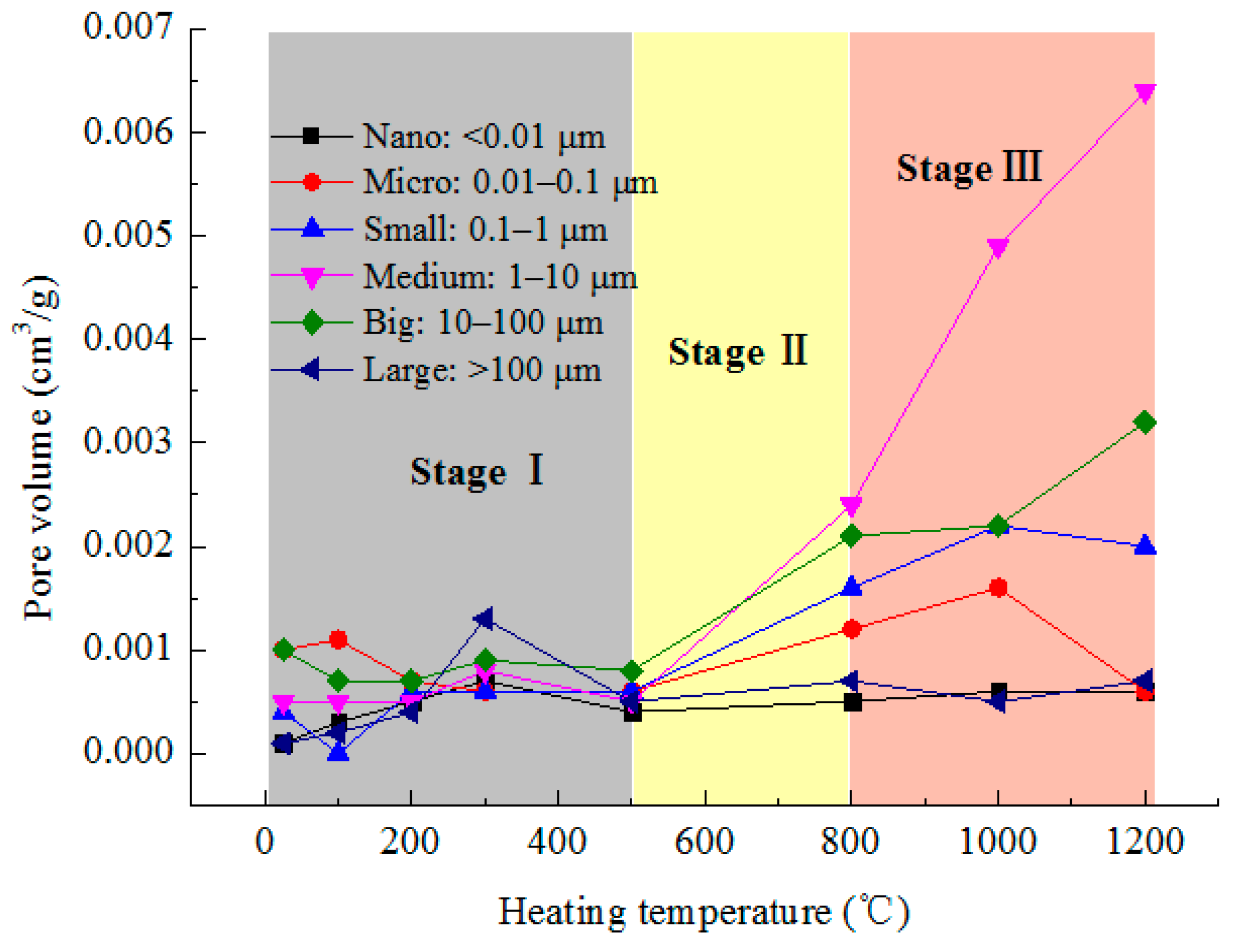
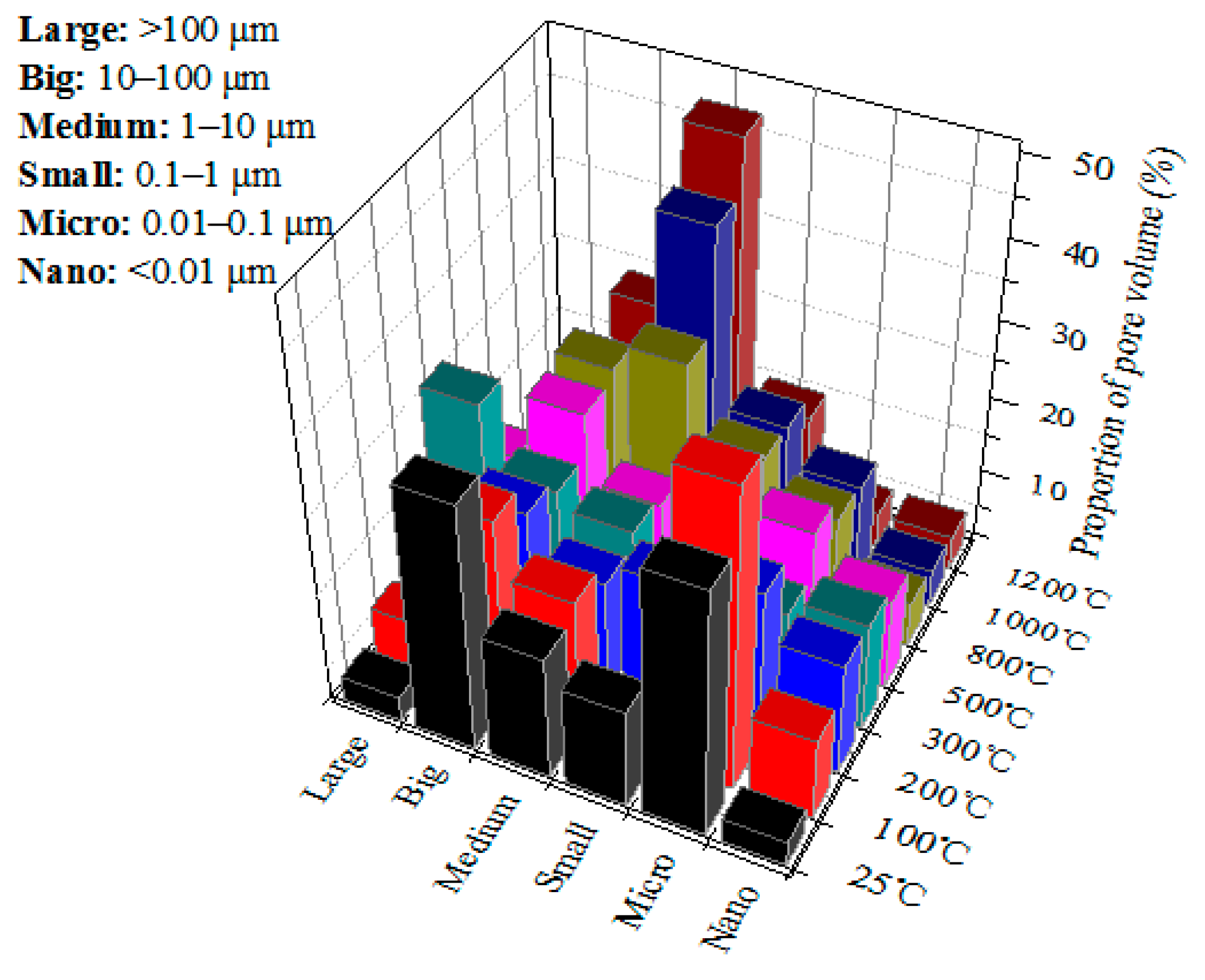
3.2. Pore Structure
3.2.1. Pore Distribution of Granite Samples at Different Heating Temperatures
3.2.2. Thermal Effect on the Porosity of Granite Samples
3.3. Mechanical Characteristics
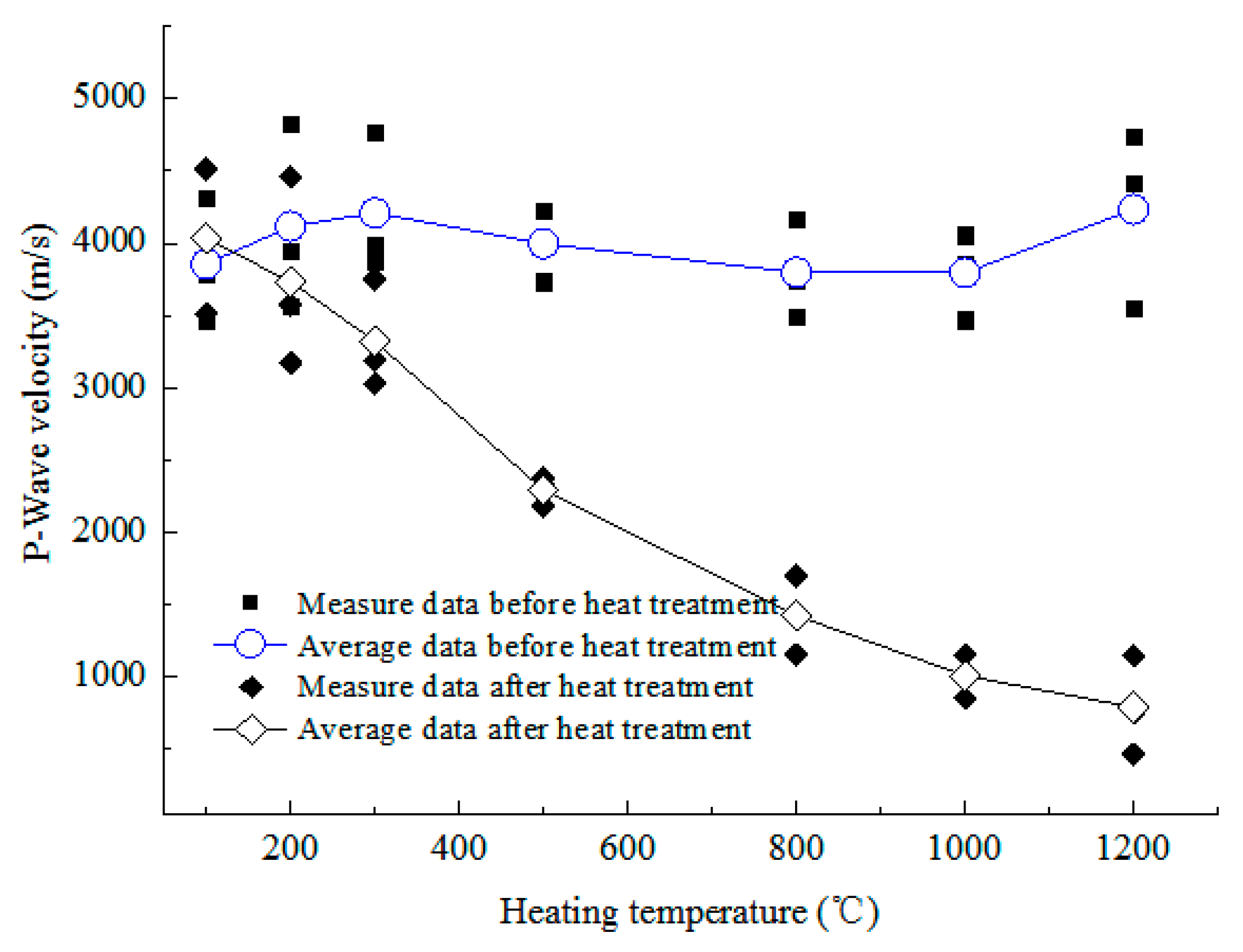
3.3.1. P-Wave Velocity
3.3.2. UCS and Peak Strain
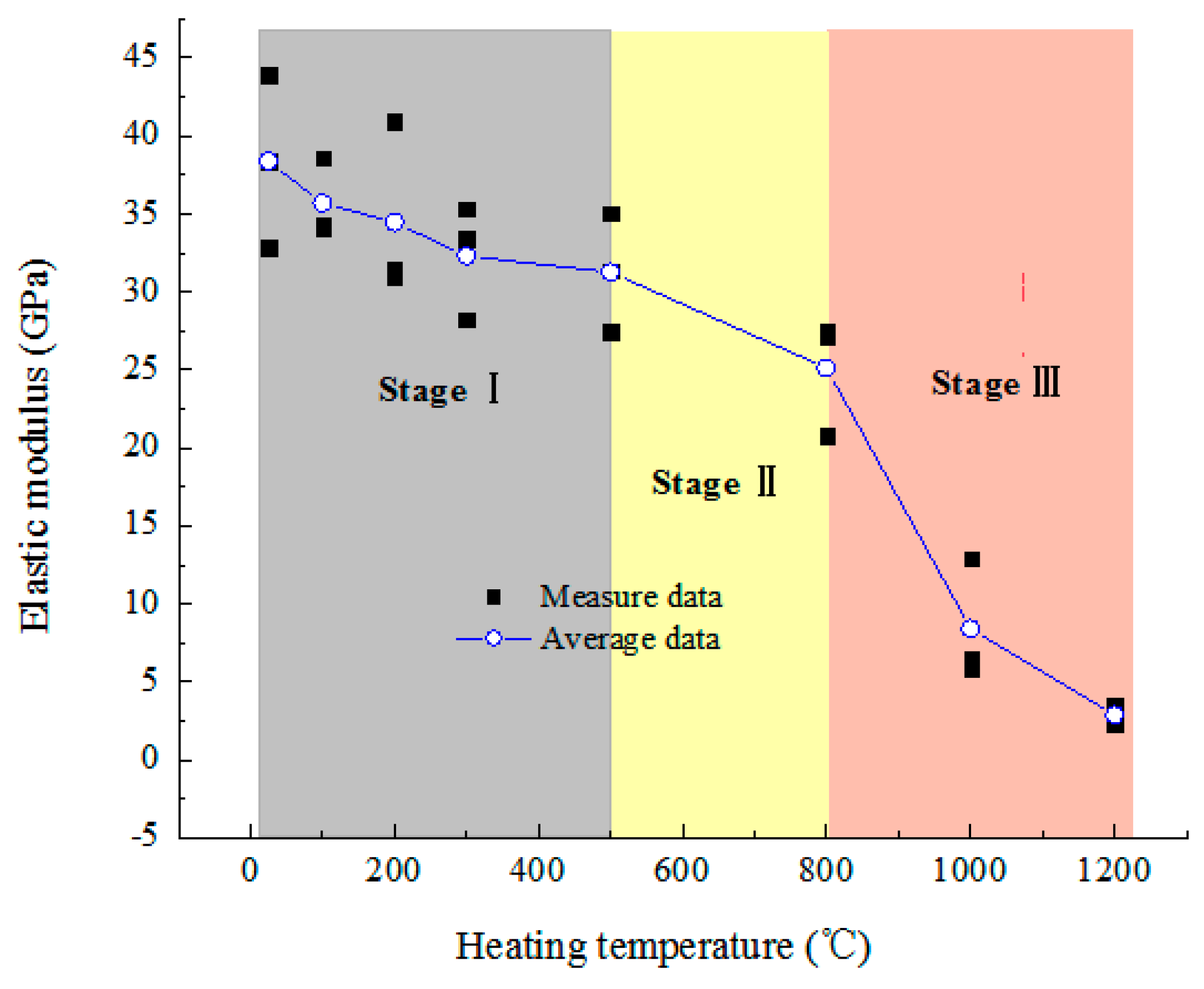
3.3.3. Elastic Modulus
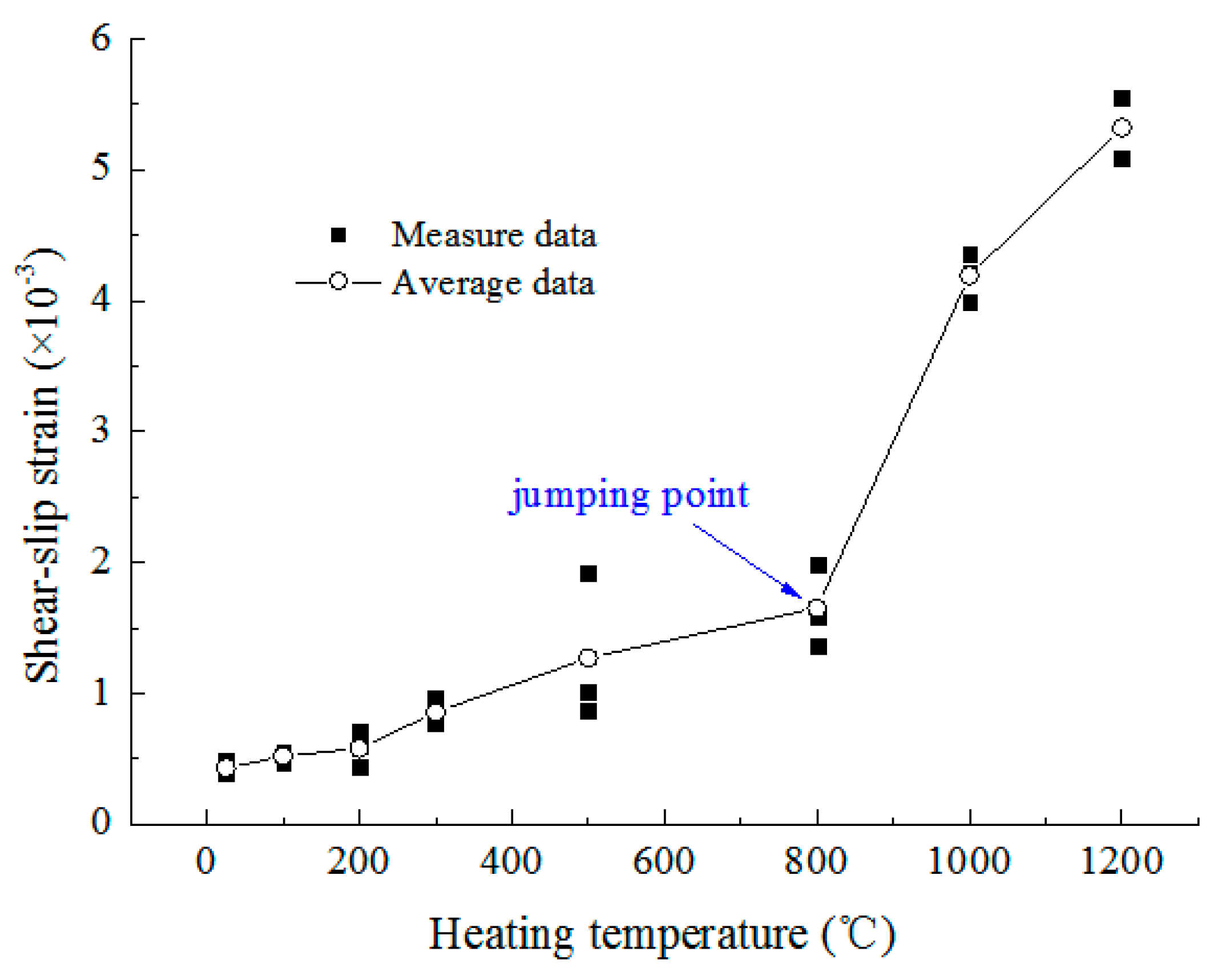
3.3.4. Shear-Slip Strain
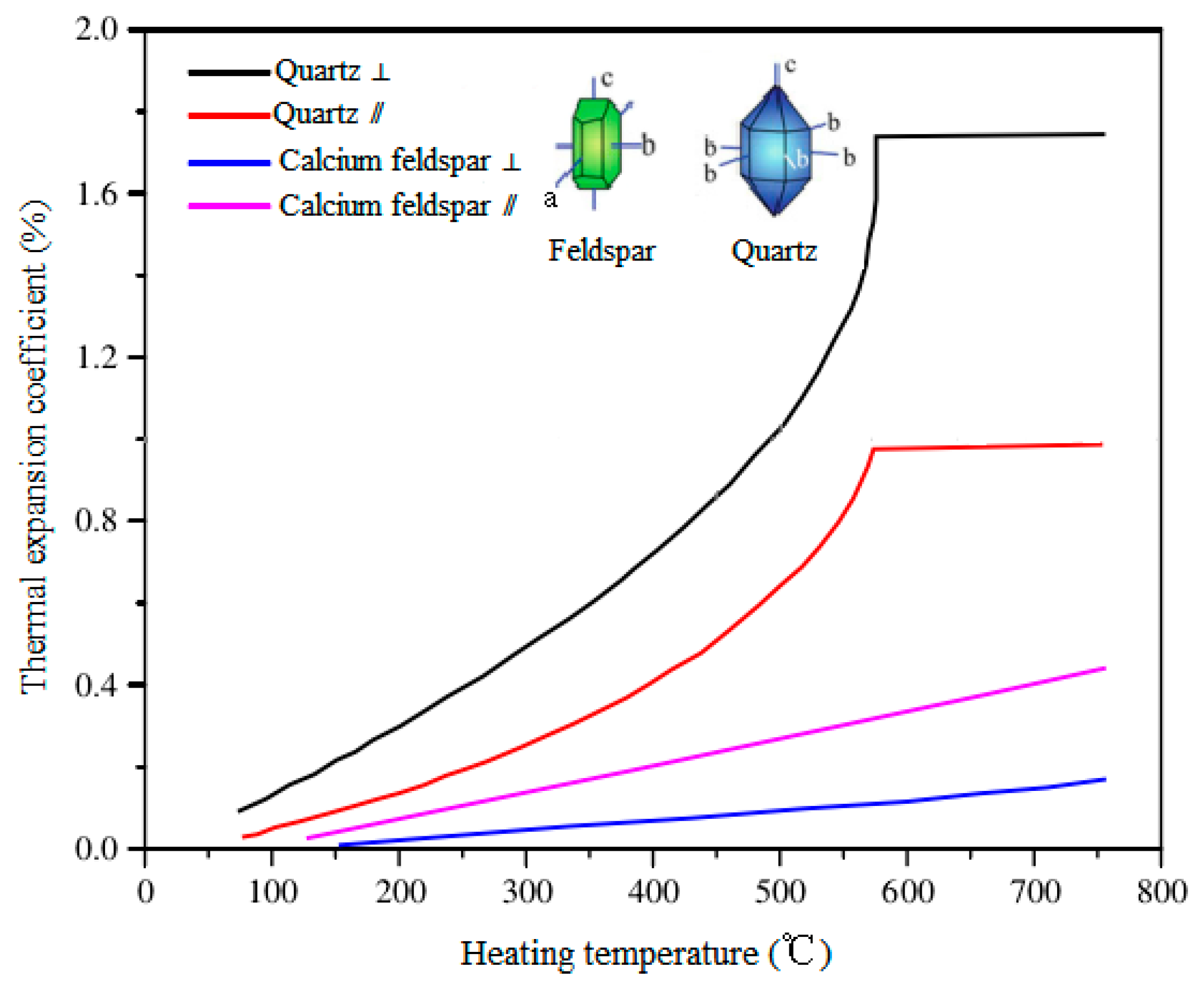
4. Discussion
5. Conclusions
- (1)
- Mineral composition. The main components of the examined pyroxene granite are feldspar, quartz, pyroxene, and some illite. Below 500 °C, the MDIs of the minerals are comparatively stable. In 500–800 °C, the diffraction angles become wider, but the chemical composition does not change. Above 800 °C, illite and quartz react chemically to produce mullite and the crystal state of the minerals deteriorate dramatically.
- (2)
- Pore structure. Considering the significance of pore size for gas and liquid adsorption and seepage, the pores are divided into six categories (nanopore, micropore, small pore, medium pore, big pore, large pore), and the effects of the heating temperature on them were obtained. Below 500 °C, the six pore volumes change little. In 500–1200 °C, the volume of medium and big pores increases greatly. The newly created pores caused by high-heat treatment are mainly medium ones. The rock porosity increases exponentially with heating temperature.
- (3)
- Mechanical characteristics. As the temperature increases, the P-wave velocity increases slightly at 100 °C, then decreases linearly, the UCS and elastic modulus both decrease consistently, and the peak strain and shear-slip strain increase. Below 500 °C, they all change slightly, but above 800 °C, the UCS and elastic modulus decrease dramatically and the increasing rate of shear-slip strain is obviously accelerated, indicating the deformation and failure mode of rock changes from brittle to plastic.
- (4)
- The high temperature heat treatment changes the mineralogical characteristics of granite, including dehydration, phase transformation, and anisotropic and uncoordinated thermal expansion of minerals. The variation of minerals further changes the pore structure and mechanical properties of the granite rock.
Author Contributions
Funding
Conflicts of Interest
References
- Lei, Z.H.; Zhang, Y.J.; Yu, Z.W.; Hu, Z.J.; Li, L.Z.; Zhang, S.Q.; Fu, L.; Zhou, L.; Xie, Y.Y. Exploratory research into the enhanced geothermal system power generation project: The Qiabuqia geothermal field, Northwest China. Renew. Energy 2019, 139, 52–70. [Google Scholar] [CrossRef]
- Hossain, M.E.; Mousavizadegan, S.H.; Islam, M.R. The effects of thermal alterations on formation permeability and porosity. Pet. Sci. Technol. 2008, 26, 1282–1302. [Google Scholar] [CrossRef]
- Cao, Z.D.; Lin, B.Q.; Liu, T. The impact of depositional environment and tectonic evolution on coalbed methane occurrence in West Henan, China. Int. J. Min. Sci. Technol. 2019, 29, 297–305. [Google Scholar] [CrossRef]
- Kamble, A.D.; Saxena, V.K.; Chavan, P.D.; Mendhe, V.A. Co-gasification of coal and biomass an emerging clean energy technology: Status and prospects of development in Indian context. Int. J. Min. Sci. Technol. 2019, 29, 171–186. [Google Scholar] [CrossRef]
- Prentice, D.P.; Walkley, B.; Bernal, S.A.; Bankhead, M.; Hayes, M.; Provis, J.L. Thermodynamic modelling of BFS-PC cements under temperature conditions relevant to the geological disposal of nuclear wastes. Cem. Concr. Res. 2019, 119, 21–35. [Google Scholar] [CrossRef]
- Chaki, S.; Takarli, M.; Agbodjan, W.P. Influence of thermal damage on physical properties of a granite rock: Porosity, permeability and ultrasonic wave evolutions. Constr. Build. Mater. 2008, 22, 1456–1461. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhang, Y.L.; Gao, F.; Yang, G.S.; Lai, X.P. Influence of temperature on the microstructure deterioration of sandstone. Energies 2018, 11, 1753. [Google Scholar] [CrossRef]
- Sirdesai, N.N.; Mahanta, B.; Ranjith, P.G.; Singh, T.N. Effects of thermal treatment on physico-morphological properties of Indian fine-grained sandstone. Bull. Eng. Geol. Environ. 2019, 78, 883–897. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, S.R.; Ni, J.; Azzam, R.; Fernandez-Steeger, T.M. An experimental study of the mechanical properties of granite after high temperature exposure based on mineral characteristics. Eng. Geol. 2017, 220, 234–242. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Sun, Q.; Zhang, Y.L.; Xue, L.; Kong, F.F. Porosity and wave velocity evolution of granite after high-temperature treatment: A review. Environ. Earth Sci. 2018, 77, 350. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Keshavarz, V.; Kazemzadeh, E.; Hosseini, S.A.; Rajabi-Kochi, M.; Salimidelshad, Y.; Naderi, H.; Bakhtiari, H.A. Experimental and mathematical model evaluation of asphaltene fractionation based on adsorption in porous media: Part 1. Calcite reservoir rock. J. Pet. Sci. Eng. 2019, 177, 24–40. [Google Scholar] [CrossRef]
- Kammlein, M.; Stollhofen, H. Pore-fluid-dependent controls of matrix and bulk thermal conductivity of mineralogically heterogeneous sandstones. Geotherm. Energy 2019, 7, 13. [Google Scholar] [CrossRef]
- Shang, X.J.; Wang, J.G.; Zhang, Z.Z. Iterative analytical solutions for nonlinear two-phase flow with gas solubility in shale gas reservoirs. Geofluids 2019. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Peng, W.H.; Shang, X.J.; Wang, K.; Li, H.; Ma, W.M. Temperature field distribution of coal seam in heat injection. Therm. Sci. 2017, 21, S39–S45. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zheng, K.Y.; Li, Z.W.; Li, Z.H.; Si, L.L.; Hou, S.S.; Duan, Y.J. Experimental study on pore-fracture evolution law in the thermal damage process of coal. Int. J. Rock Mech. Min. Sci. 2019, 116, 13–24. [Google Scholar] [CrossRef]
- Liu, P.; Cui, S.G.; Li, Z.H.; Xu, X.F.; Guo, C. Influence of surrounding rock temperature on mechanical property and pore structure of concrete for shotcrete use in a hot-dry environment of high-temperature geothermal tunnel. Constr. Build. Mater. 2019, 207, 329–337. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Sun, Q.; Zhu, S.Y.; Wang, B. Experimental study on mechanical and porous characteristics of limestone affected by high temperature. Appl. Therm. Eng. 2017, 110, 356–362. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.Q.; Su, T.M.; Zhu, S.Y. Variation of wave velocity and porosity of sandstone after high temperature heating. Acta Geophys. 2016, 64, 633–648. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, Q.; He, H.; Cao, L.W.; Zhang, W.Q.; Wang, B. Pore characteristics and mechanical properties of sandstone under the influence of temperature. Appl. Therm. Eng. 2017, 113, 537–543. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, Q.; Cao, L.W.; Geng, J.S. Pore, mechanics and acoustic emission characteristics of limestone under the influence of temperature. Appl. Therm. Eng. 2017, 123, 1237–1244. [Google Scholar] [CrossRef]
- Meng, T.; Liu, R.C.; Meng, X.X.; Zhang, D.H.; Hu, Y.Q. Evolution of the permeability and pore structure of transversely isotropic calcareous sediments subjected to triaxial pressure and high temperature. Eng. Geol. 2019, 253, 27–35. [Google Scholar] [CrossRef]
- Glatz, G.; Castanier, L.M.; Kovscek, A.R. Visualization and quantification of thermally induced porosity alteration of immature source rock using X-ray computed tomography. Energy Fuels 2016, 30, 8141–8149. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.S.; Pan, Z.J.; Niu, X.L.; Yu, Y.; Meng, S.Z. Pore structure and its fractal dimensions of transitional shale: A cross-section from east margin of the Ordos Basin, China. Fuel 2019, 241, 417–431. [Google Scholar] [CrossRef]
- Hazra, B.; Wood, D.A.; Kumar, S.; Saha, S.; Dutta, S.; Kumari, P.; Singh, A.K. Fractal disposition, porosity characterization and relationships to thermal maturity for the Lower Permian Raniganj basin shales, India. J. Nat. Gas Sci. Eng. 2018, 59, 452–465. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhai, C.; Liu, S.M.; Qin, L.; Dong, R.W. Investigation of temperature effects from LCO2 with different cycle parameters on the coal pore variation based on infrared thermal imagery and low-field nuclear magnetic resonance. Fuel 2018, 215, 528–540. [Google Scholar] [CrossRef]
- Schmitt, M.; Poffo, C.M.; de Lima, J.C.; Fernandes, C.P.; dos Santos, V.S.S. Application of photoacoustic spectroscopy to characterize thermal diffusivity and porosity of caprocks. Eng. Geol. 2017, 220, 183–195. [Google Scholar] [CrossRef]
- Ugur, I.; Sengun, N.; Demirdag, S.; Altindag, R. Analysis of the alterations in porosity features of some natural stones due to thermal effect. Ultrasonics 2014, 54, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Haffen, S.; Geraud, Y.; Rosener, M.; Diraison, M. Thermal conductivity and porosity maps for different materials: A combined case study of granite and sandstone. Geothermics 2017, 66, 143–150. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gao, F.; Gao, Y.N.; Xu, X.L.; Hou, P.; Teng, T.; Shang, X.J. Fractal structure and model of pore size distribution of granite under high temperatures. Chin. J. Rock Mech. Eng. 2016, 35, 2426–2438. [Google Scholar]
- Peng, J.; Yang, S.Q. Comparison of mechanical behavior and acoustic emission characteristics of three thermally-damaged rocks. Energies 2018, 11, 2350. [Google Scholar] [CrossRef]
- Rong, G.; Peng, J.; Yao, M.D.; Jiang, Q.H.; Wong, L.N.Y. Effects of specimen size and thermal-damage on physical and mechanical behavior of a fine-grained marble. Eng. Geol. 2018, 232, 46–55. [Google Scholar] [CrossRef]
- Gautam, P.K.; Verma, A.K.; Jha, M.K.; Sharma, P.; Singh, T.N. Effect of high temperature on physical and mechanical properties of Jalore granite. J. Appl. Geophys. 2018, 159, 460–474. [Google Scholar] [CrossRef]
- Kumari, W.G.P.; Beaumont, D.M.; Ranjith, P.G.; Perera, M.S.A.; Isaka, B.L.A.; Khandelwal, M. An experimental study on tensile characteristics of granite rocks exposed to different high-temperature treatments. Geomech. Geophys. Geo-Energy Geo-Resour. 2019, 5, 47–64. [Google Scholar] [CrossRef]
- Zuo, J.P.; Li, Y.L.; Zhang, X.Y.; Zhao, Z.H.; Wang, T.Z. The effects of thermal treatments on the subcritical crack growth of Pingdingshan sandstone at elevated high temperatures. Rock Mech. Rock Eng. 2018, 51, 3439–3454. [Google Scholar] [CrossRef]
- Wang, Z.L.; Shi, H.; Wang, J.G. Mechanical behavior and damage constitutive model of granite under coupling of temperature and dynamic loading. Rock Mech. Rock Eng. 2018, 51, 3045–3059. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gao, F.; Xu, X.L. Experimental study of temperature effect of mechanical properties of granite. Rock Soil Mech. 2011, 32, 2346–2352. [Google Scholar]
- Zhang, Z.Z.; Gao, F.; Gao, Y.N.; Xu, X.L. Experimental study of brittle stress drop coefficient of granite endured high temperature. J. Exp. Mech. 2010, 25, 589–597. [Google Scholar]
- Zhang, W.Q.; Sun, Q.; Zhu, Y.M.; Guo, W.H. Experimental study on response characteristics of micro-macroscopic performance of red sandstone after high-temperature treatment. J. Therm. Anal. Calorim. 2019, 136, 1935–1945. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Q.; Deng, W.N.; Zhang, W.Q.; Lu, C. Temperature effect on microstructure and P-wave propagation in Linyi sandstone. Appl. Therm. Eng. 2017, 115, 913–922. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Xu, X.L.; Sun, Q.P.; Dong, Y. Effect of thermal treatment on fractals in acoustic emission of rock material. Adv. Mater. Sci. Eng. 2016, 2016, 6309856. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhang, Z.Z. Acoustic emission and damage characteristics of granite subjected to high temperature. Adv. Mater. Sci. Eng. 2018, 2018, 8149870. [Google Scholar] [CrossRef]
- Wang, Z.L.; He, A.L.; Shi, G.Y.; Mei, G.X. Temperature effect on AE energy characteristics and damage mechanical behaviors of granite. Int. J. Geomech. 2018, 18, 04017163. [Google Scholar] [CrossRef]
- Yang, S.Q.; Ranjith, P.G.; Jing, H.W.; Tian, W.L.; Ju, Y. An experimental investigation on thermal damage and failure mechanical behavior of granite after exposure to different high temperature treatments. Geothermics 2017, 65, 180–197. [Google Scholar] [CrossRef]
- Gautam, P.K.; Jha, M.K.; Verma, A.K.; Singh, T.N. Evolution of absorption energy per unit thickness of damaged sandstone. J. Therm. Anal. Calorim. 2019, 136, 2305–2318. [Google Scholar] [CrossRef]
- Zhang, R.R.; Jing, L.W.; Ma, Q.Y. Experimental study on thermal damage and energy evolution of sandstone after high temperature treatment. Shock Vib. 2018, 2018, 3845353. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gao, F.; Liu, Z.J. Research on rockburst proneness and its microcosmic mechanism of granite considering temperature effect. Chin. J. Rock Mech. Eng. 2010, 29, 1591–1602. [Google Scholar]
- Ersoy, H.; Kolayli, H.; Karahan, M.; Karahan, H.H.; Sunnetci, M.O. Effect of thermal damage on mineralogical and strength properties of basic volcanic rocks exposed to high temperatures. Bull. Eng. Geol. Environ. 2019, 78, 1515–1525. [Google Scholar] [CrossRef]
- Laita, E.; Bauluz, B.; Yuste, A. High-temperature mineral phases generated in natural clinkers by spontaneous combustion of coal. Minerals 2019, 9, 213. [Google Scholar] [CrossRef]
- Liu, X.F.; Yuan, S.Y.; Sieffert, Y.; Fityus, S.; Buzzi, O. Changes in mineralogy, microstructure, compressive strength and intrinsic permeability of two sedimentary rocks subjected to high-temperature heating. Rock Mech. Rock Eng. 2016, 49, 2985–2998. [Google Scholar] [CrossRef]
- Yang, S.Q.; Xu, P.; Li, Y.B.; Huang, Y.H. Experimental investigation on triaxial mechanical and permeability behavior of sandstone after exposure to different high temperature treatments. Geothermics 2017, 69, 93–109. [Google Scholar] [CrossRef]
- Nowak, J. The influence of thermal transformation of coal mining wastes on their petrographic and mineralogical composition. Gospod. Surowcami Miner. Miner. Resour. Manag. 2014, 30, 143–159. [Google Scholar] [CrossRef]
- Mahanta, B.; Singh, T.N.; Ranjith, P.G. Influence of thermal treatment on mode I fracture toughness of certain Indian rocks. Eng. Geol. 2016, 210, 103–114. [Google Scholar] [CrossRef]
- Seipold, U.; Schilling, F.R. Heat transport in serpentinites. Tectonophysics 2003, 370, 147–162. [Google Scholar] [CrossRef]
- Maeshima, T.; Noma, H.; Sakiyama, M.; Mitsuda, T. Natural 1.1 and 1.4 nm tobermorites from Fuka, Okayama, Japan: Chemical analysis, cell dimensions, Si-29 NMR and thermal behavior. Cem. Concr. Res. 2003, 33, 1515–1523. [Google Scholar] [CrossRef]
- Plevova, E.; Kozusnikova, A.; Vaculikova, L.; Martynkova, G.S. Thermal behavior of selected Czech marble samples. J. Therm. Anal. Calorim. 2010, 101, 657–664. [Google Scholar] [CrossRef]
- Teixeira, A.M.D.; Garrido, F.; Medeiros, M.E.; Sampaio, J.A. Effect of thermal treatments on the potassium and sodium availability in phonolite rock powder. Int. J. Miner. Process. 2015, 145, 57–65. [Google Scholar] [CrossRef]
- Saidi, R.; Tlili, A.; Jamoussi, F. The effect of heating on mineral composition and grain size distribution of flux calcined porcelanites from the Gafsa-Metlaoui basin, southwestern Tunisia. J. Afr. Earth Sci. 2016, 124, 189–198. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, Q.; Geng, J.S. Microstructural characterization of limestone exposed to heat with XRD, SEM and TG-DSC. Mater. Charact. 2017, 134, 285–295. [Google Scholar] [CrossRef]
- Liu, L.; Chu, G.; Li, Y.G.; Yang, X.Y.; Santosh, M.; Hu, Q. Mesozoic high- and low-SiO2 adakites and a-type granites in the lower Yangtze River belt, eastern China: Implications for petrogenesis and metallogeny. Minerals 2018, 8, 328. [Google Scholar] [CrossRef]
- Shu, B.; Zhu, R.J.; Tan, J.Q.; Zhang, S.H.; Liang, M. Evolution of permeability in a single granite fracture at high temperature. Fuel 2019, 242, 12–22. [Google Scholar] [CrossRef]
- Wang, G.X.; Wang, Z.T.; Feng, B.; Rudolph, V.; Jiao, J.L. Semi-industrial tests on enhanced underground coal gasification at Zhong-Liang-Shan coal mine. Asia-Pac. J. Chem. Eng. 2009, 4, 771–779. [Google Scholar] [CrossRef]
- Xin, L.; Wang, Z.T.; Huang, W.G.; Kang, G.J.; Lu, X.F.; Zhang, P.; Wang, J.H. Temperature field distribution of burnt surrounding rock in UCG stope. Int. J. Min. Sci. Technol. 2014, 24, 573–580. [Google Scholar] [CrossRef]
- Shao, S.S.; Ranjith, P.G.; Wasantha, P.L.P.; Chen, B.K. Experimental and numerical studies on the mechanical behaviour of Australian Strathbogie granite at high temperatures: An application to geothermal energy. Geothermics 2015, 54, 96–108. [Google Scholar] [CrossRef]
- Ding, Q.L.; Ju, F.; Mao, X.B.; Ma, D.; Yu, B.Y.; Song, S.B. Experimental investigation of the mechanical behavior in unloading conditions of sandstone after high-temperature treatment. Rock Mech. Rock Eng. 2016, 49, 2641–2653. [Google Scholar] [CrossRef]
- Sang, L.K.; Ma, C.Q. Petrology, 2nd ed.; Geological Publishing House: Beijing, China, 2012. [Google Scholar]
- RaigonPichardo, M.; GarciaRamos, G.; SanchezSoto, P.J. Characterization of a waste washing solid product of mining granitic tin-bearing sands and its application as ceramic raw material. Resour. Conserv. Recycl. 1996, 17, 109–124. [Google Scholar] [CrossRef]
- Oikonomopoulos, I.K.; Perraki, M.; Tougiannidis, N.; Perraki, T.; Kasper, H.U.; Gurk, M. Clays from Neogene Achlada lignite deposits in Florina basin (Western Macedonia, N. Greece): A prospective resource for the ceramics industry. Appl. Clay Sci. 2015, 103, 1–9. [Google Scholar] [CrossRef]
- Yokoyama, T.; Takeuchi, S. Porosimetry of vesicular volcanic products by a water-expulsion method and the relationship of pore characteristics to permeability. J. Geophys. Res. Solid Earth 2009. [Google Scholar] [CrossRef]
- Guo, X.B.; Huang, Z.L.; Zhao, L.B.; Han, W.; Ding, C.; Sun, X.W.; Yan, R.T.; Zhang, T.H.; Yang, X.J.; Wang, R.M. Pore structure and multi-fractal analysis of tight sandstone using MIP, NMR and NMRC methods: A case study from the Kuqa depression, China. J. Pet. Sci. Eng. 2019, 178, 544–558. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, J.; Wang, L.; Qi, L.L. Influence of pore size distribution of different metamorphic grade of coal on adsorption constant. J. China Coal Soc. 2013, 38, 294–300. [Google Scholar]
- Wu, E.J.; Han, B.P.; Wang, G.L.; Peng, Z.C.; Liu, G.J. Pore structure test of Jurassic red-bed in yanzhou mining area, shandong province, and its affecting factors. Geol. J. China Univ. 2005, 11, 442–452. [Google Scholar]
- Brady, B.H.G.; Brown, E.T. Rock Mechanics for Underground Mining; Springer: Berlin, Germany, 2006. [Google Scholar]
- Zhang, Z.Z.; Gao, F. Energy Evolution Mechanism During Rock Deformation and Failure; China University of Mining and Technology Press: Xuzhou, China, 2014. [Google Scholar]
- Han, L.J.; He, Y.N.; Zhang, H.Q. Study on complete stress-strain experiment and destruction characteristics of rock under lower confining pressure. In Progresses in Fracture and Strength of Materials and Structures; Zhou, Y., Tu, S.T., Xie, X., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007; Volume 353, pp. 70–73. [Google Scholar]
- Yang, D.F.; Zhang, D.Y.; Niu, S.J.; Dang, Y.H.; Feng, W.L.; Ge, S.S. Experiment and study on mechanical property of sandstone post-peak under the cyclic loading and unloading. Geotech. Geol. Eng. 2018, 36, 1609–1620. [Google Scholar] [CrossRef]
- Yang, S.Q.; Jing, H.W.; Wang, S.Y. Experimental investigation on the strength, deformability, failure behavior and acoustic emission locations of red sandstone under triaxial compression. Rock Mech. Rock Eng. 2012, 45, 583–606. [Google Scholar] [CrossRef]
- Becattini, V.; Motmans, T.; Zappone, A.; Madonna, C.; Haselbacher, A.; Steinfeld, A. Experimental investigation of the thermal and mechanical stability of rocks for high-temperature thermal-energy storage. Appl. Energy 2017, 203, 373–389. [Google Scholar] [CrossRef]
- Luque, A.; Ruiz-Agudo, E.; Cultrone, G.; Sebastian, E.; Siegesmund, S. Direct observation of microcrack development in marble caused by thermal weathering. Environ. Earth Sci. 2011, 62, 1375–1386. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Wan, Z.J.; Feng, Z.J.; Xu, Z.H.; Liang, W.G. Evolution of mechanical properties of granite at high temperature and high pressure. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 3, 199–210. [Google Scholar] [CrossRef]
- David, C.; Menendez, B.; Darot, M. Influence of stress-induced and thermal cracking on physical properties and microstructure of La Peyratte granite. Int. J. Rock Mech. Min. Sci. 1999, 36, 433–448. [Google Scholar] [CrossRef]
- Dwivedi, R.D.; Goel, R.K.; Prasad, V.V.R.; Sinha, A. Thermo-mechanical properties of Indian and other granites. Int. J. Rock Mech. Min. Sci. 2008, 45, 303–315. [Google Scholar] [CrossRef]
- Isaka, B.L.A.; Ranjith, P.G.; Rathnaweera, T.D.; Perera, M.S.A.; De Silva, V.R.S. Quantification of thermally-induced microcracks in granite using X-ray CT imaging and analysis. Geothermics 2019, 81, 152–167. [Google Scholar] [CrossRef]
- Hajpal, M.; Torok, A. Mineralogical and colour changes of quartz sandstones by heat. Environ. Geol. 2004, 46, 311–322. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Z.Z.; Xue, L.; Zhu, S.Y. Physico-mechanical properties variation of rock with phase transformation under high temperature. Chin. J. Rock Mech. Eng. 2013, 32, 935–942. [Google Scholar]
- Winkler, E.M. Stone in Architecture: Properties, Durability, 3rd ed.; Spring: Berlin, Germany, 1994. [Google Scholar]
- Ohno, I. Temperature variation of elastic properties of alpha-quartz up to the alpha-beta transition. J. Phys. Earth 1995, 43, 157–169. [Google Scholar] [CrossRef]
- Hartlieb, P.; Toifl, M.; Kuchar, F.; Meisels, R.; Antretter, T. Thermo-physical properties of selected hard rocks and their relation to microwave-assisted comminution. Miner. Eng. 2016, 91, 34–41. [Google Scholar] [CrossRef]
- Chen, S.W.; Yang, C.H.; Wang, G.B. Evolution of thermal damage and permeability of Beishan granite. Appl. Therm. Eng. 2017, 110, 1533–1542. [Google Scholar] [CrossRef]
- Zhao, Z.K.; Sun, Q.Z.; Zhang, P.Q.; Jing, H.O.; Sun, Y.M. Effect of calcinatin on phase transformation and expansibility of quartz sands during heating. Foundry 2006, 55, 961–964. [Google Scholar]
- Huang, B.L. Mineral Differential Thermal Analysis and Identification Manual; Science Press: Beijing, China, 1987. [Google Scholar]
- Vagnon, F.; Colombero, C.; Colombo, F.; Comina, C.; Ferrero, A.M.; Mandrone, G.; Vinciguerra, S.C. Effects of thermal treatment on physical and mechanical properties of Valdieri Marble-NW Italy. Int. J. Rock Mech. Min. Sci. 2019, 116, 75–86. [Google Scholar] [CrossRef]
- Yavuz, H.; Demirdag, S.; Caran, S. Thermal effect on the physical properties of carbonate rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 94–103. [Google Scholar] [CrossRef]
- Zhang, Z.Z. Fractal dimension of fracture surface in rock material after high temperature. Adv. Mater. Sci. Eng. 2015, 2015, 468370. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhang, Z.Z. Fractal characteristics of rock fracture surface under triaxial compression after high temperature. Adv. Mater. Sci. Eng. 2016, 2016, 2181438. [Google Scholar] [CrossRef]
- Rong, G.; Yao, M.D.; Peng, J.; Sha, S.; Tan, J. Influence of initial thermal cracking on physical and mechanical behaviour of a coarse marble: Insights from uniaxial compression tests with acoustic emission monitoring. Geophys. J. Int. 2018, 214, 1886–1900. [Google Scholar]
- Dang, J.X.; Zhou, Y.S.; Rybacki, E.; He, C.R.; Dresen, G. An experimental study on the brittle-plastic transition during deformation of granite. J. Asian Earth Sci. 2017, 139, 30–39. [Google Scholar] [CrossRef]
- Masotta, M.; Laumonier, M.; McCammon, C. Transport of melt and volatiles in magmas inferred from kinetic experiments on the partial melting of granitic rocks. Lithos 2018, 318, 434–447. [Google Scholar] [CrossRef]
- Zuo, J.P.; Wang, J.T.; Sun, Y.J.; Chen, Y.; Jiang, G.H.; Li, Y.H. Effects of thermal treatment on fracture characteristics of granite from Beishan, a possible high-level radioactive waste disposal site in China. Eng. Fract. Mech. 2017, 182, 425–437. [Google Scholar] [CrossRef]
- Xi, D.Y. Physical characteristics of mineral phase transition in the granite. Acta Mineral. Sin. 1994, 14, 223–227. [Google Scholar]
- Fan, L.F.; Gao, J.W.; Wu, Z.J.; Yang, S.Q.; Ma, G.W. An investigation of thermal effects on micro-properties of granite by X-ray CT technique. Appl. Therm. Eng. 2018, 140, 505–519. [Google Scholar] [CrossRef]




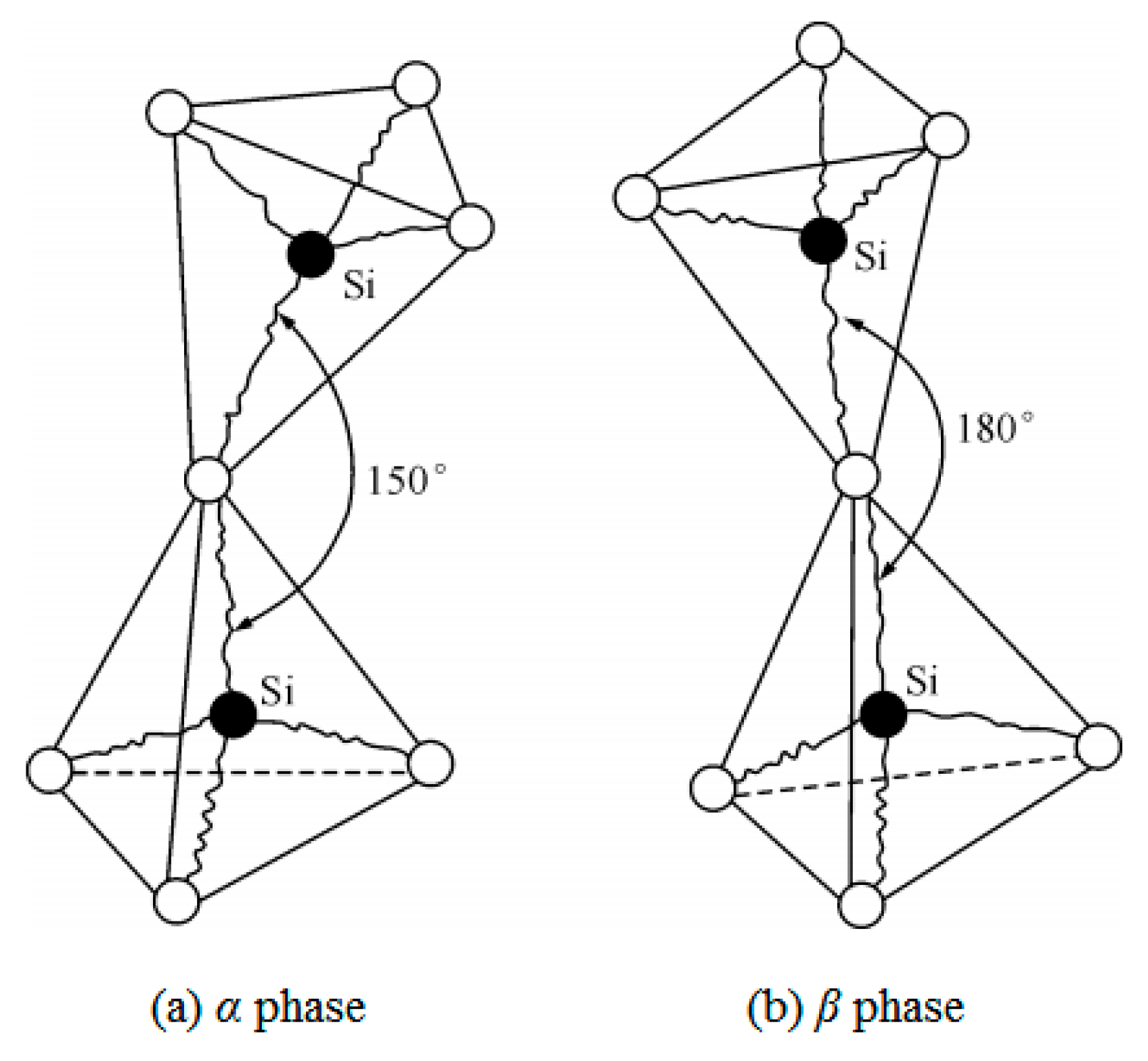


| Temperature (°C) | 25 | 100 | 200 | 300 | 500 | 800 | 1000 | 1200 |
|---|---|---|---|---|---|---|---|---|
| Feldspar (cps) | 435 | 486 | 366 | 488 | 401 | 268 | 268 | 283 |
| Pyroxene (cps) | 101 | 87 | 160 | 115 | 113 | 120 | 119 | 97 |
| Quartz (cps) | 98 | 130 | 142 | 123 | 127 | 118 | 117 | 119 |
| Illite (cps) | 88 | 62 | 67 | 80 | 67 | 62 | 60 | 0 |
| Mullite (cps) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35 |
| Temperature (°C) | 25 | 100 | 200 | 300 | 500 | 800 | 1000 | 1200 | |
|---|---|---|---|---|---|---|---|---|---|
| Feldspar (%) | 64.25 | 62.76 | 60.33 | 60.56 | 61.88 | 61.46 | 61.63 | 59.35 | |
| Pyroxene (%) | 19.23 | 20.87 | 21.56 | 19.89 | 21.59 | 21.88 | 19.4 | 22.12 | |
| Quartz (%) | 9.4 | 10.26 | 12.69 | 13.35 | 11.11 | 11.98 | 10.22 | 9.32 | |
| Illite (%) | 5.1 | 3.67 | 3.09 | 4.16 | 3.35 | 3.23 | 2.65 | 0 | |
| Mullite (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.05 | |
| Others (%) | 2.02 | 2.44 | 2.33 | 2.04 | 2.07 | 1.45 | 6.1 | 8.16 | |
| Temperature (°C) | Properties | Pore Radius (μm) | |||||
|---|---|---|---|---|---|---|---|
| <0.01 (Nano) | 0.01–0.1 (Micro) | 0.1–1 (Small) | 1–10 (Medium) | 10–100 (Big) | >100 (Large) | ||
| 25 | Volume (cm3/g) | 0.0001 | 0.001 | 0.0004 | 0.0005 | 0.001 | 0.0001 |
| Proportion (%) | 3.23 | 32.26 | 12.90 | 16.13 | 32.26 | 3.23 | |
| 100 | Volume (cm3/g) | 0.0003 | 0.0011 | 0 | 0.0005 | 0.0007 | 0.0002 |
| Proportion (%) | 10.71 | 39.29 | 0 | 17.86 | 25.00 | 7.14 | |
| 200 | Volume (cm3/g) | 0.0005 | 0.0007 | 0.0006 | 0.0005 | 0.0007 | 0.0004 |
| Proportion (%) | 14.71 | 20.59 | 17.65 | 14.71 | 20.59 | 11.76 | |
| 300 | Volume (cm3/g) | 0.0007 | 0.0006 | 0.0006 | 0.0008 | 0.0009 | 0.0013 |
| Proportion (%) | 14.29 | 12.24 | 12.24 | 16.33 | 18.37 | 26.53 | |
| 500 | Volume (cm3/g) | 0.0004 | 0.0006 | 0.0006 | 0.0005 | 0.0008 | 0.0005 |
| Proportion (%) | 11.76 | 17.65 | 17.65 | 14.71 | 23.53 | 14.71 | |
| 800 | Volume (cm3/g) | 0.0005 | 0.0012 | 0.0016 | 0.0024 | 0.0021 | 0.0007 |
| Proportion (%) | 5.88 | 14.12 | 18.82 | 28.24 | 24.71 | 8.24 | |
| 1000 | Volume (cm3/g) | 0.0006 | 0.0016 | 0.0022 | 0.0049 | 0.0022 | 0.0005 |
| Proportion (%) | 5.00 | 13.33 | 18.33 | 40.83 | 18.33 | 4.17 | |
| 1200 | Volume (cm3/g) | 0.0006 | 0.0006 | 0.0020 | 0.0064 | 0.0032 | 0.0007 |
| Proportion (%) | 4.44 | 4.44 | 14.81 | 47.41 | 23.70 | 5.19 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Zhang, Z.; Xu, X.; Liu, T.; Xing, Y. Mineral Composition, Pore Structure, and Mechanical Characteristics of Pyroxene Granite Exposed to Heat Treatments. Minerals 2019, 9, 553. https://doi.org/10.3390/min9090553
Shang X, Zhang Z, Xu X, Liu T, Xing Y. Mineral Composition, Pore Structure, and Mechanical Characteristics of Pyroxene Granite Exposed to Heat Treatments. Minerals. 2019; 9(9):553. https://doi.org/10.3390/min9090553
Chicago/Turabian StyleShang, Xiaoji, Zhizhen Zhang, Xiaoli Xu, Tingting Liu, and Yan Xing. 2019. "Mineral Composition, Pore Structure, and Mechanical Characteristics of Pyroxene Granite Exposed to Heat Treatments" Minerals 9, no. 9: 553. https://doi.org/10.3390/min9090553
APA StyleShang, X., Zhang, Z., Xu, X., Liu, T., & Xing, Y. (2019). Mineral Composition, Pore Structure, and Mechanical Characteristics of Pyroxene Granite Exposed to Heat Treatments. Minerals, 9(9), 553. https://doi.org/10.3390/min9090553







