Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
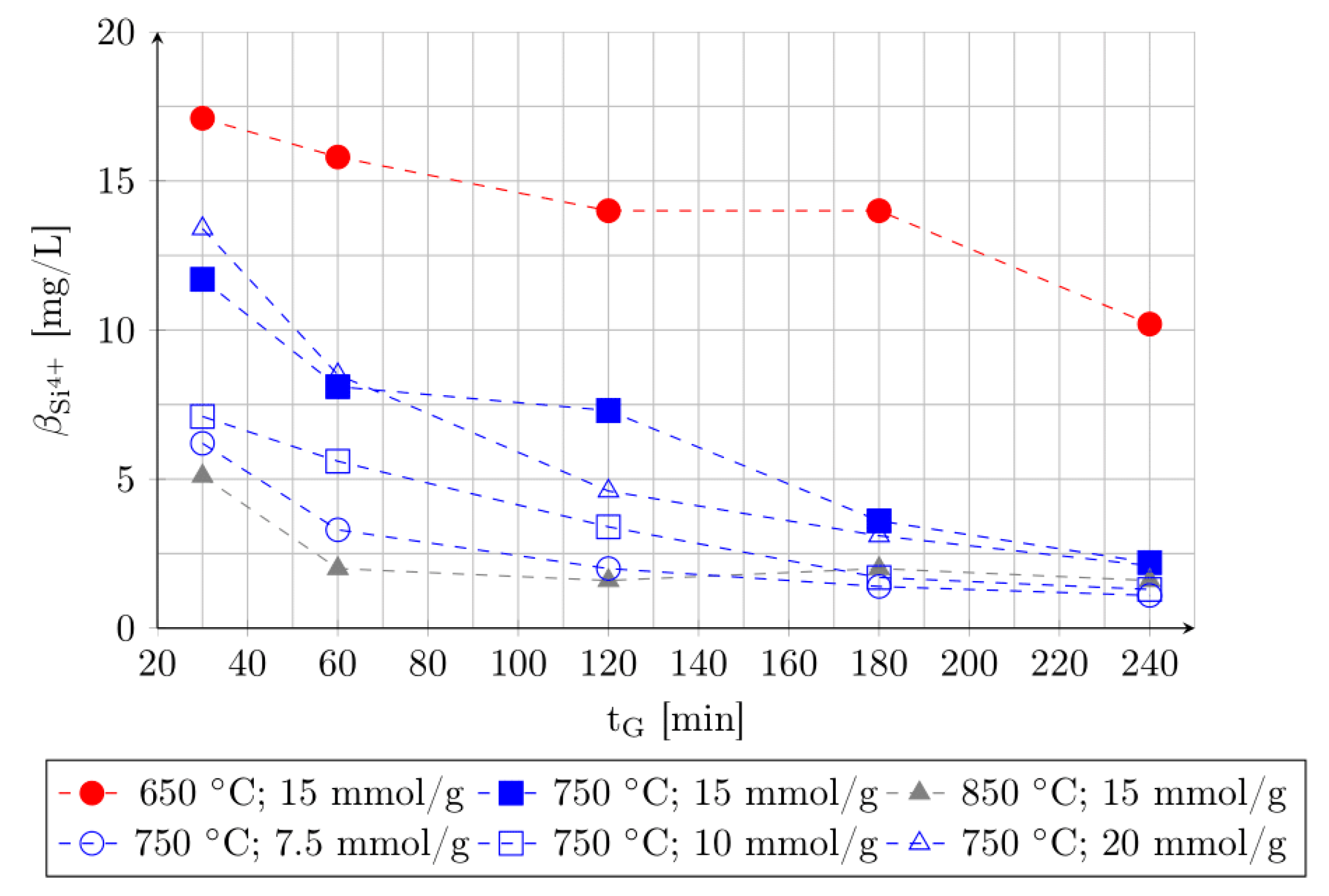
3.1. Effect of Roasting Time, Roasting Temperature and Acid Addition
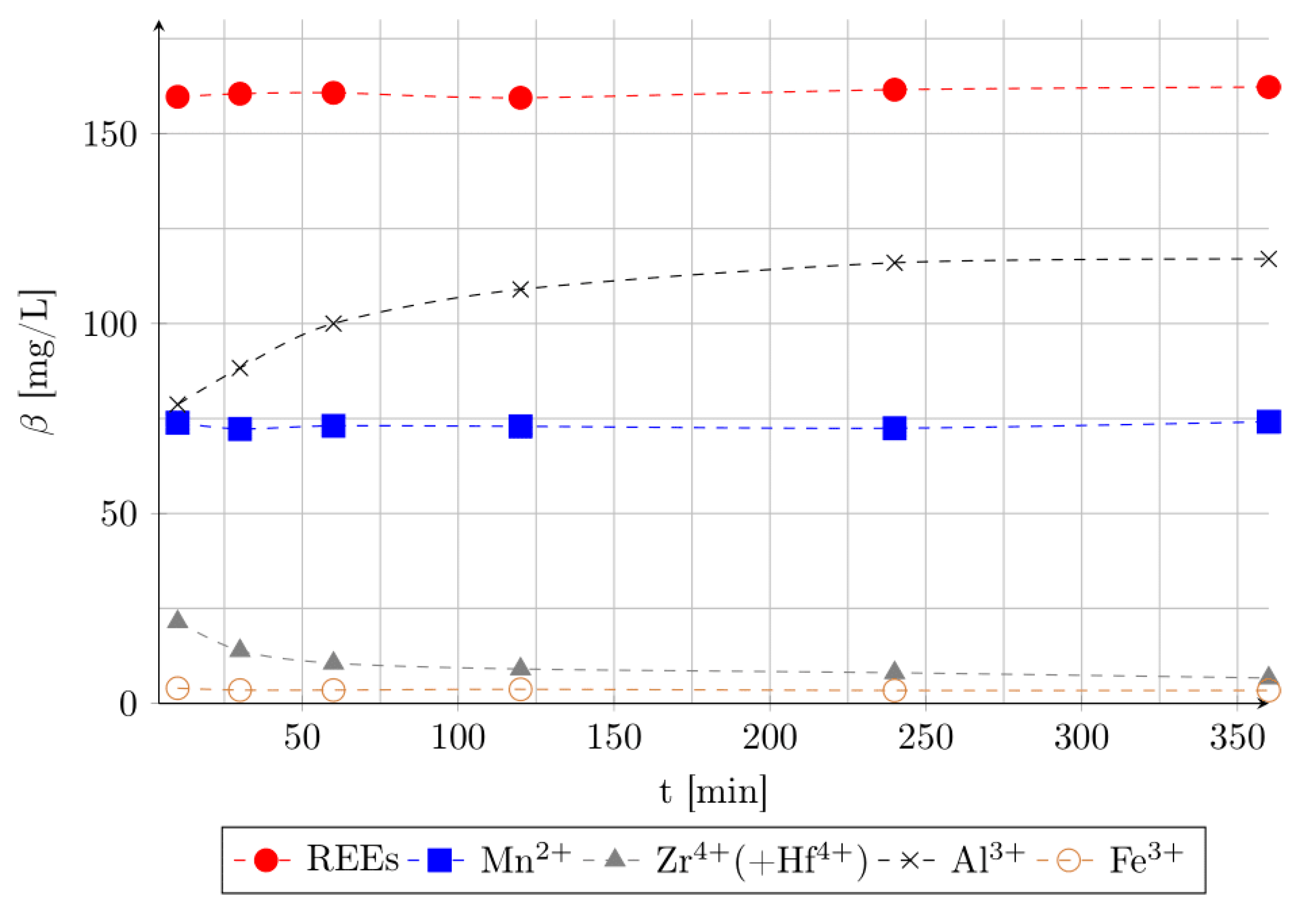
3.2. Effect of Leaching Time and pH Value of Leaching Medium
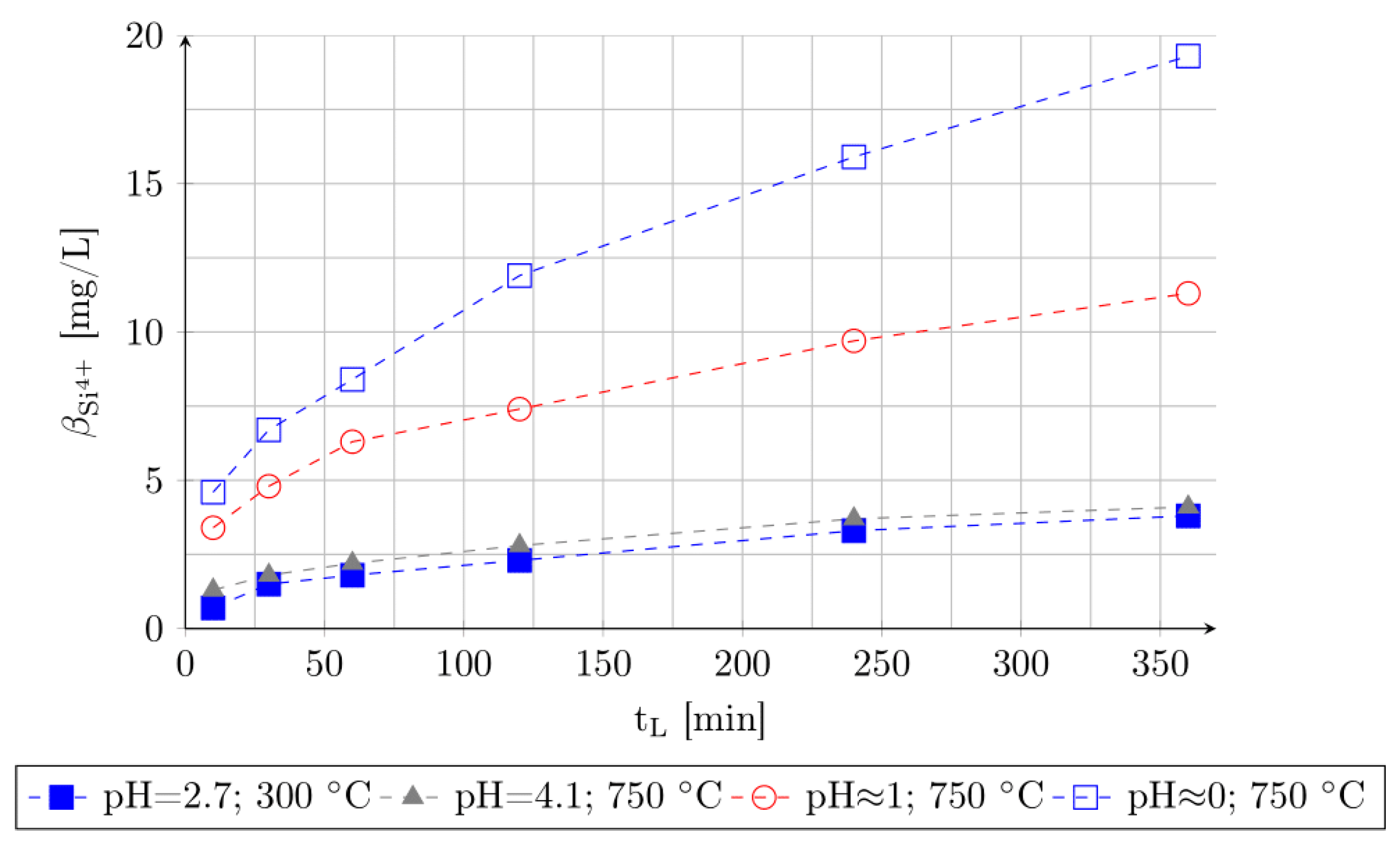
3.3. Stability of Silica
3.4. Effect of Pulp Density during Leaching
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; Publisher: Boca Raton, FL, USA, 2015. [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Qi, D. Extraction of Rare Earths from RE Concentrates. In Hydrometallurgy of Rare Earths, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–185. [Google Scholar]
- Yang, X.J.; Lin, A.; Li, X.-L.; Wu, Y.; Zhou, W.; Chen, Z. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Hoshino, M.; Sanematsu, K.; Watanabe, Y. REE Mineralogy and Resources. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.C., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 129–291. [Google Scholar]
- Krasikov, S.A.; Upolovnikova, A.G.; Sitnikova, O.A.; Ponomarenko, A.A.; Agafonov, S.N.; Zhidovinova, S.V.; Maiorov, D.V. Phase formation during the carbothermic reduction of eudialyte concentrate. Russ. Metall. (Met.) 2013, 2013, 482–485. [Google Scholar] [CrossRef]
- Lipin, B.R.; Boynton, W.V. Geochemistry and Mineralogy of Rare Earth Elements. In Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1989; Volume 21. [Google Scholar]
- Balinski, A.; Atanasova, P.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Recovery of REEs, Zr(+Hf), Mn and Nb by H2SO4 leaching of eudialyte concentrate. Hydrometallurgy 2019, 186, 176–186. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from eudialyte concentrate by suppressing silica gel formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Voßenkaul, D.; Birich, A.; Müller, N.; Stoltz, N.; Friedrich, B. Hydrometallurgical Processing of Eudialyte Bearing Concentrates to Recover Rare Earth Elements Via Low-Temperature Dry Digestion to Prevent the Silica Gel Formation. J. Sustain. Metall. 2017, 3, 79–89. [Google Scholar] [CrossRef]
- Davidson, T. Amended and Restated Prefeasibility Study-NI 43-101-Technical report for the Norra Kärr Rare Earth Element Deposit; 0465-RPT-014 Rev 1; GBM: Gränna, Sweden, 2015. [Google Scholar]
- Litvinova, T.E. Development of Physico-Chemical Bases of Hydrometallurgical Processing of Rare-Metal Eudialyte Ores. Ph.D. Thesis, Saint Petersburg Mining University, Saint Petersburg, Russia, 1998. [Google Scholar]
- Ma, Y.; Stopic, S.; Friedrich, B. Hydrometallurgical Treatment of an Eudialyte Concentrate for Preparation of Rare Earth Carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Motov, D.L.; Leshtaeva, T.G. Khimicheskaya Tekhnologiya Redkometall’nogo syr’ya (Chemical Technology of Rare-Earth Raw Materials); Nauka: Moscow, Russia, 1966; pp. 16–26. [Google Scholar]
- Zubryckyj, N.; Evans, D.J.I.; Mackiw, V.N. Promotion Agents in the Sulphation of Oxidized Nickel and Cobalt Bearing Ores. U.S. Patent 3,367,740A, 6 February 1968. [Google Scholar]
- Naumann, D.; Goode, J.R. Rare Earth Ore Processing Methods by Acid Mixing, Sulphating and Decomposing. Patent WO2017,1009,33A1, 22 June 2017. [Google Scholar]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB Magnets Using Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- King, M.; Moats, M.; Davenport, W. Sulfuric Acid Manufacture, 2nd ed.; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Stern, K.H.; Weise, E.L. High Temperature Properties and Decomposition of Inorganic Salts. Part 1. Sulfates; U.S. Department of Commerce National Bureau of Standards: Washington, WA, USA, 1966. [Google Scholar]
- Monhemius, A.J. Precipitation diagrams for metal hydroxides, sulfides, arsenates and phosphates. Trans. Inst. Min. Metall. 1977, 86, C202–C206. [Google Scholar]
- Terry, B. The acid decomposition of silicate minerals part II. Hydrometallurgical applications. Hydrometallurgy 1983, 10, 151–171. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Forrester, K.; Leijd, M.; Oczlon, M.; Holmström, H.; Saxon, M. Beneficiation of rare earth element enriched eudialyte from the Norra Kärr peralkaline intrusion with wet high intensity magnetic separation. In Proceedings of the Conference of Metallurgists, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Qi, D. Extractants Used in Solvent Extraction-Separation of Rare Earths: Extraction Mechanism, Properties, and Features. In Hydrometallurgy of Rare Earths, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–389. [Google Scholar]
- Zhang, J.; Zhao, B.; Schreiner, B. Separation Hydrometallurgy of Rare Earth Elements, 1st ed.; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
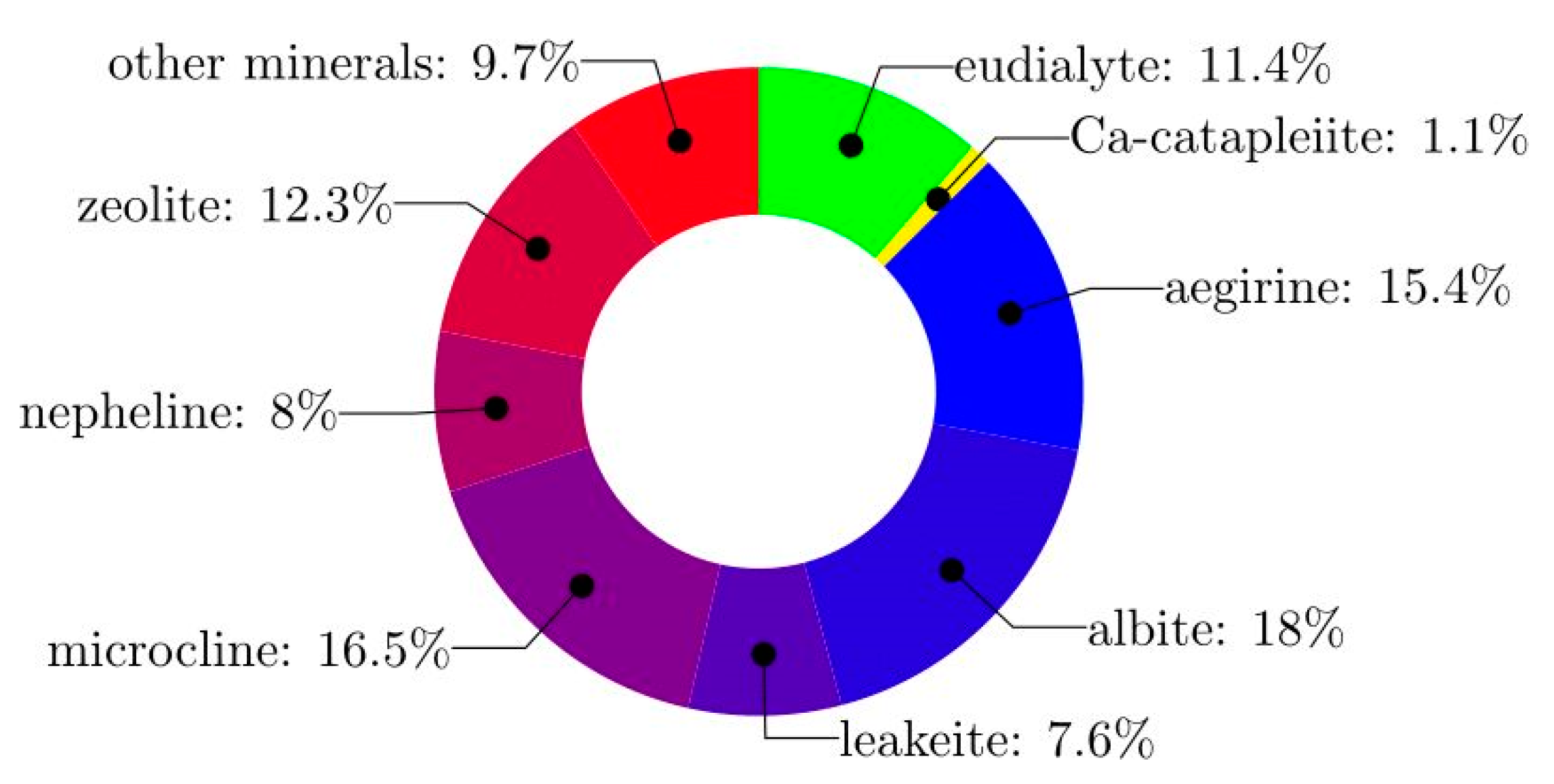
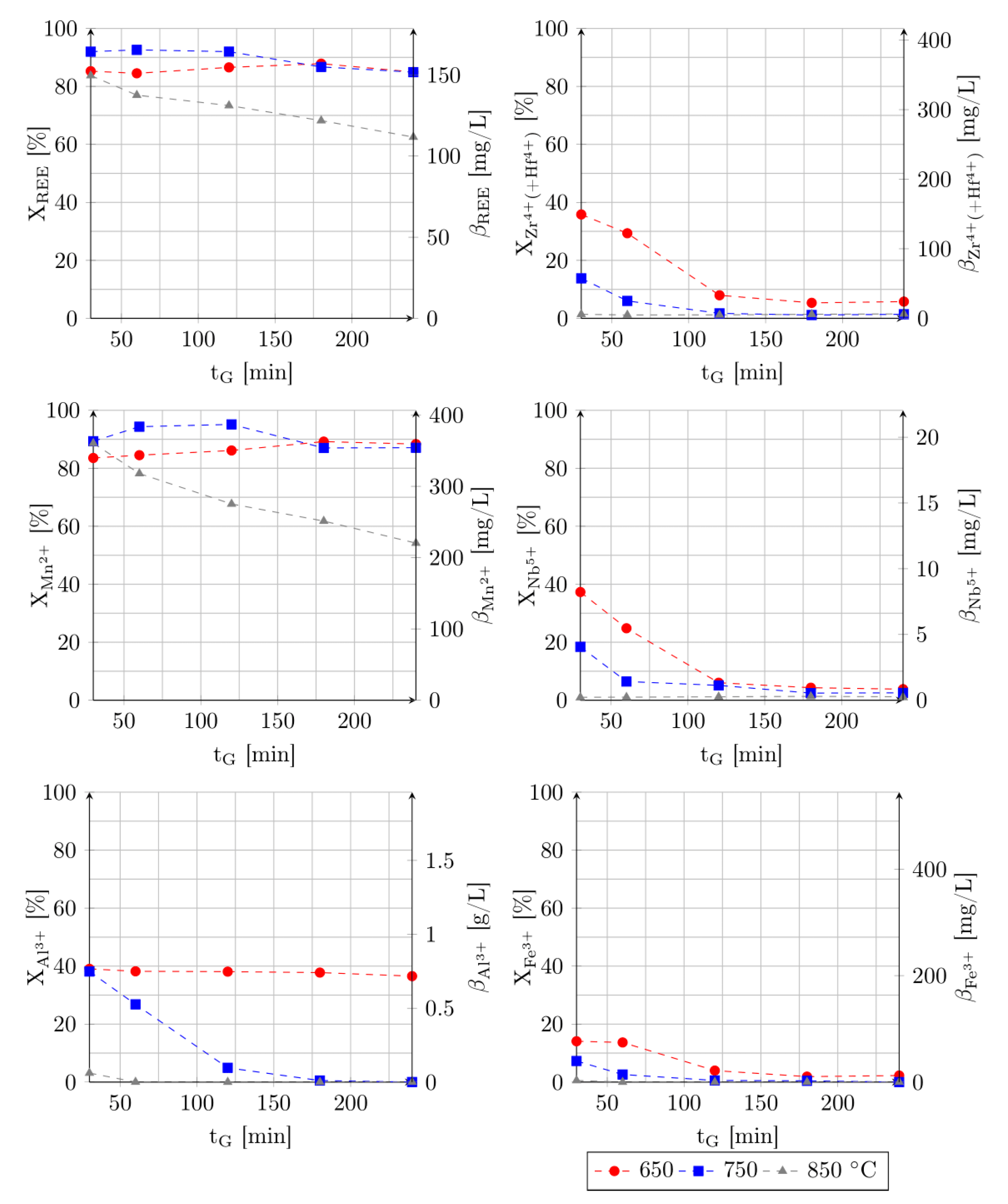
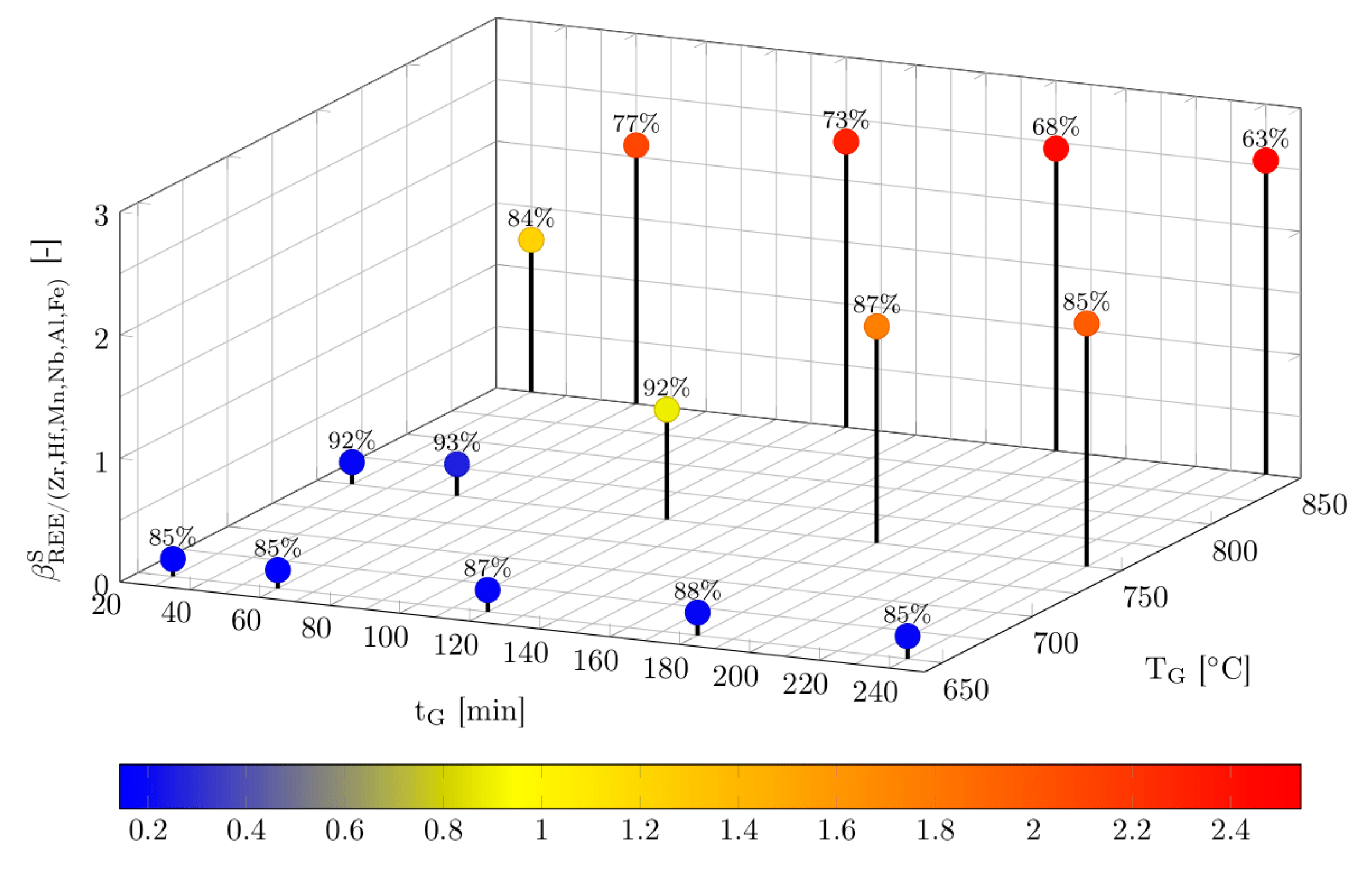


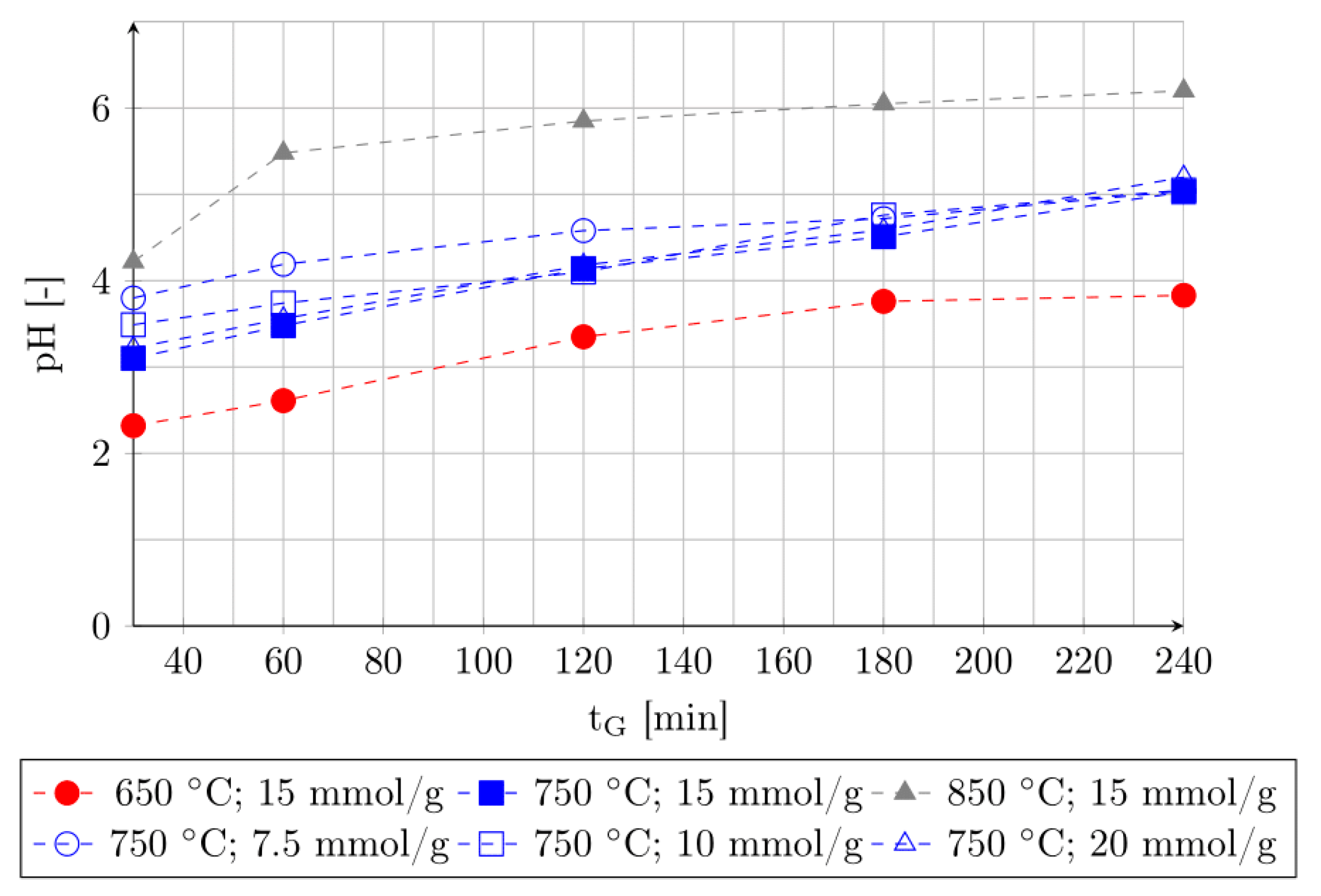


| Valuable Components | Content (mg/kg) | Other | Content (mg/kg) |
|---|---|---|---|
| Sc | 49 | Na | 71,600 |
| Y | 1908 | K | 60,700 |
| La–Eu (LREE) | 3669 | Mg | 2500 |
| Gd–Lu (HREE) | 1151 | Al | 78,500 |
| Si | 197,000 | ||
| Mn | 3210 | Ca | 10,900 |
| Zr | 16,270 | Fe | 21,800 |
| Hf | 400 | Th | 20 |
| Nb | 882 | U | 21 |
| 25 | 50 | 100 | 200 | 300 | |
|---|---|---|---|---|---|
| (mg/L) (X (%)) | 164 (92) | 322 (90) | 624 (88) | 1043 (73) | 1229 (57) |
| (mg/L) (X (%)) | 91 (91) | 178 (89) | 345 (87) | 508 (64) | 469 (39) |
| (mg/L) (X (%)) | 74 (93) | 144 (91) | 279 (89) | 535 (85) | 760 (80) |
| (mg/L) (X (%)) | 76 (95) | 149 (93) | 290 (91) | 584 (91) | 891 (92) |
| (mg/L) | 7 (2) | 8 (1) | 25 (2) | 110 (3) | 252 (5) |
| (mg/L) | 1 (5) | 1 (3) | 4 (5) | 18 (10) | 43 (17) |
| (mg/L) | 97 | 148 | 505 | 1030 | 1580 |
| (mg/L) | 3 | 7 | 18 | 61 | 149 |
| 0.89 | 0.78 | 0.74 | 0.58 | 0.42 | |
| (mg/L) (X (%)) | 240 (88) | 470 (87) | 558 (52) | 506 (23) | 523 (16) |
| (mg/L) | 820 | 1670 | 3280 | 6420 | 9410 |
| (mg/L) | 4 | 8 | 14 | 22 | 31 |
| pH value | 4.1 | 3.9 | 3.8 | 3.7 | 3.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balinski, A.; Atanasova, P.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates. Minerals 2019, 9, 522. https://doi.org/10.3390/min9090522
Balinski A, Atanasova P, Wiche O, Kelly N, Reuter MA, Scharf C. Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates. Minerals. 2019; 9(9):522. https://doi.org/10.3390/min9090522
Chicago/Turabian StyleBalinski, Adam, Petya Atanasova, Oliver Wiche, Norman Kelly, Markus Andreas Reuter, and Christiane Scharf. 2019. "Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates" Minerals 9, no. 9: 522. https://doi.org/10.3390/min9090522
APA StyleBalinski, A., Atanasova, P., Wiche, O., Kelly, N., Reuter, M. A., & Scharf, C. (2019). Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates. Minerals, 9(9), 522. https://doi.org/10.3390/min9090522






