The Depression and Adsorption Mechanism of Polyglutamic Acid on Chalcopyrite and Pyrrhotite Flotation Systems
Abstract
:1. Introduction
2. Methodology
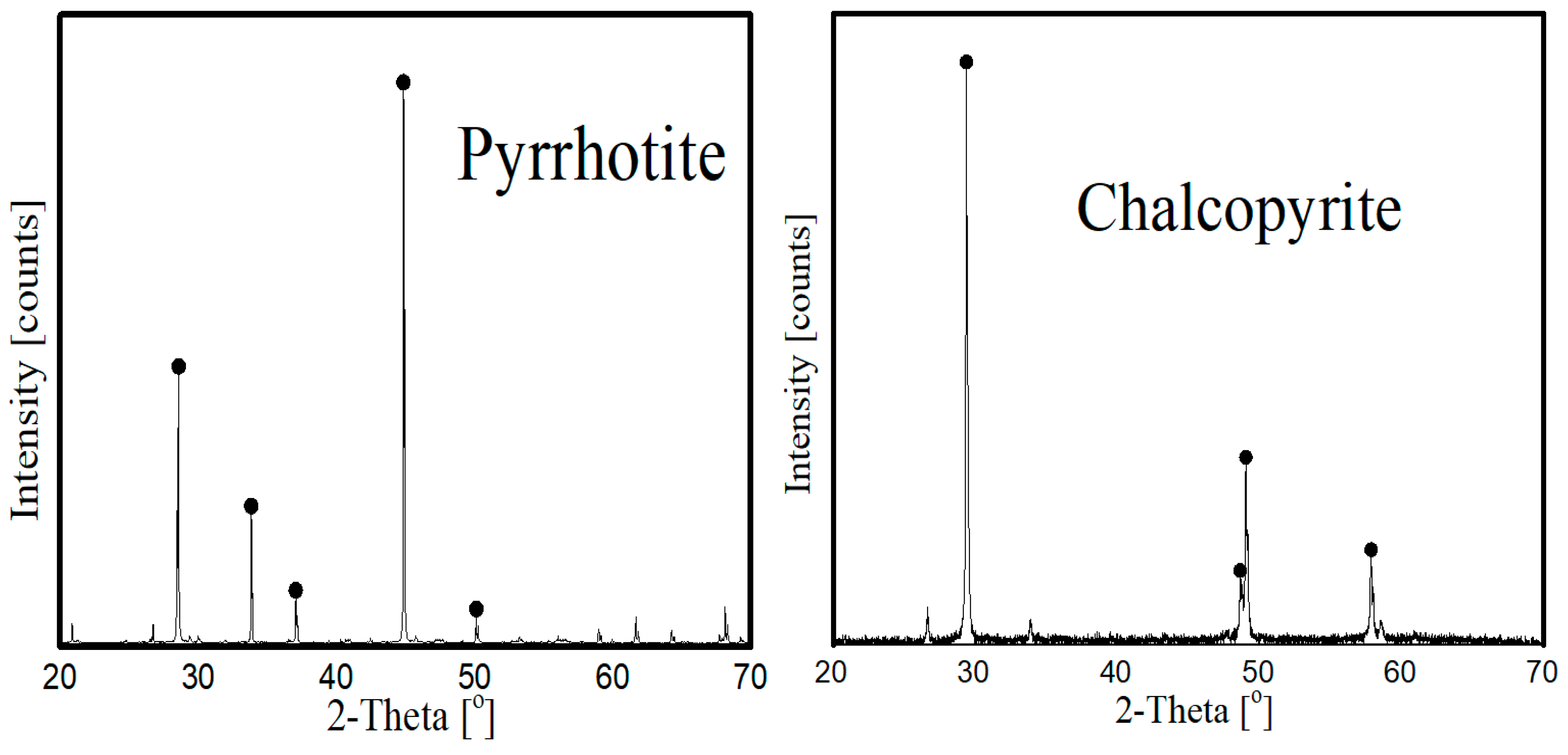
2.1. Samples and Reagents
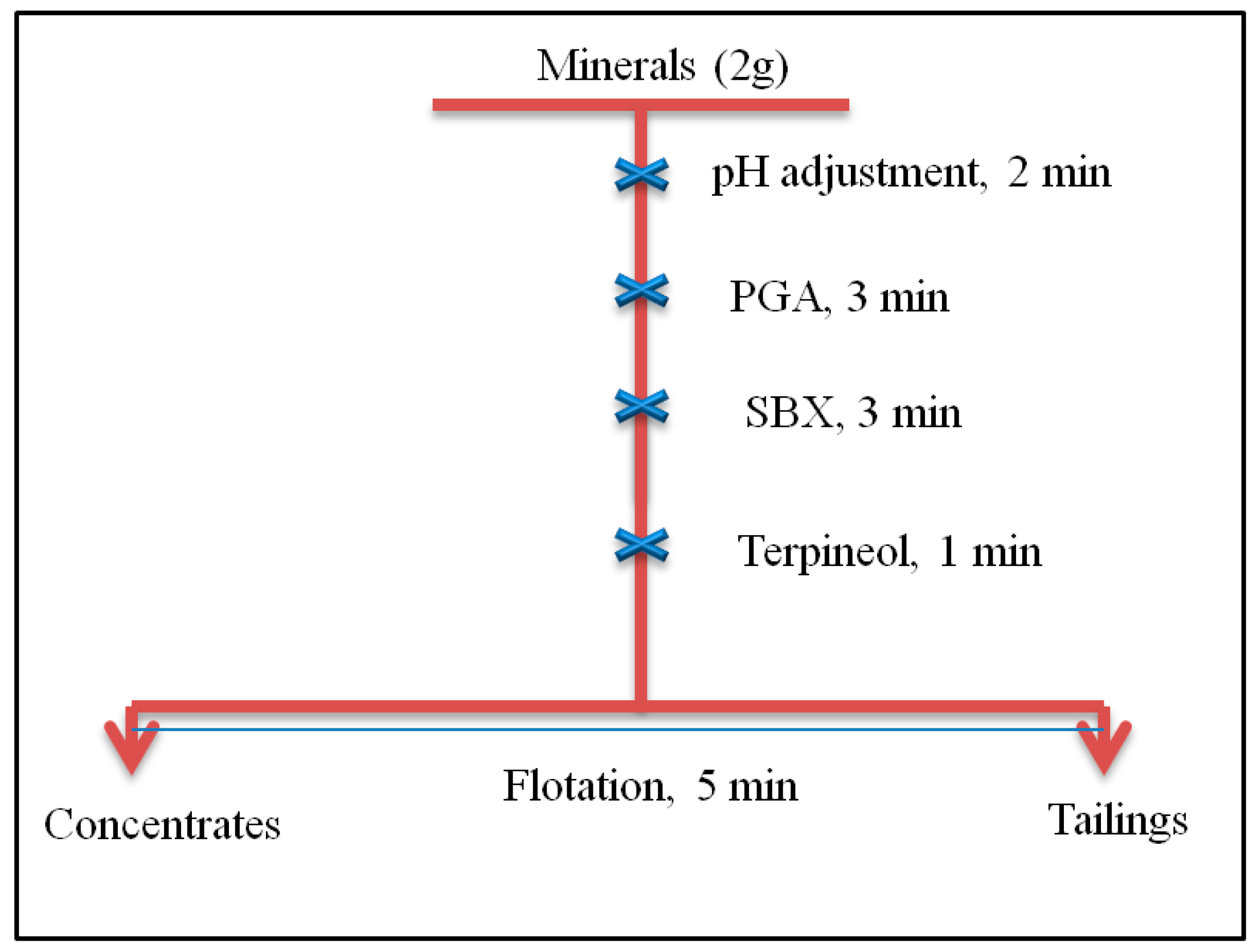
2.2. Procedure of Flotation Experiments
2.3. Zeta Potential of Minerals
2.4. Reagent Adsorption Measurements
2.5. Infrared Spectroscopy Analysis
2.6. X-ray Photoelectron Spectroscopy Analysis
3. Results and Discussion
3.1. Single Mineral Flotation
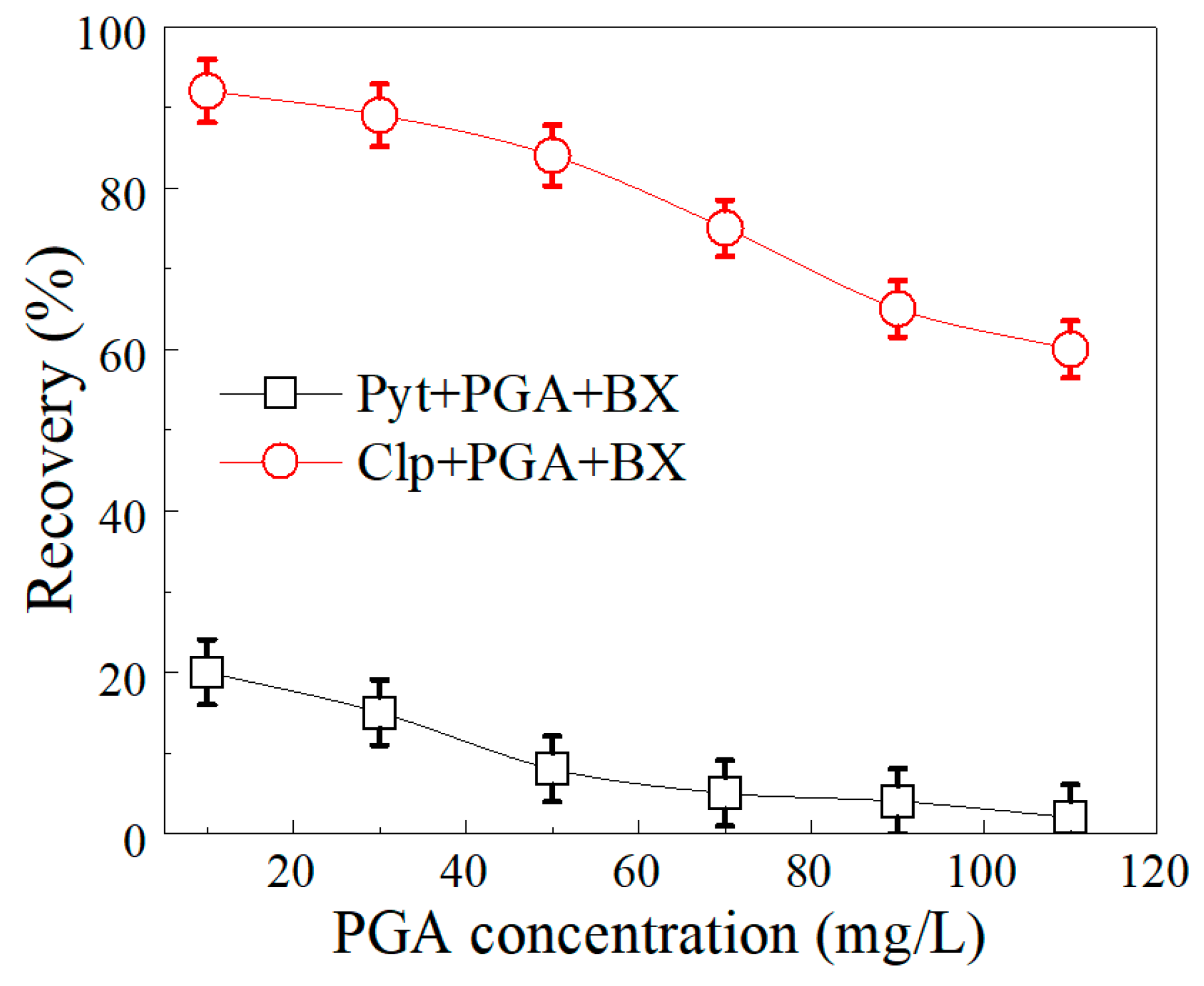
3.2. Selective Flotation
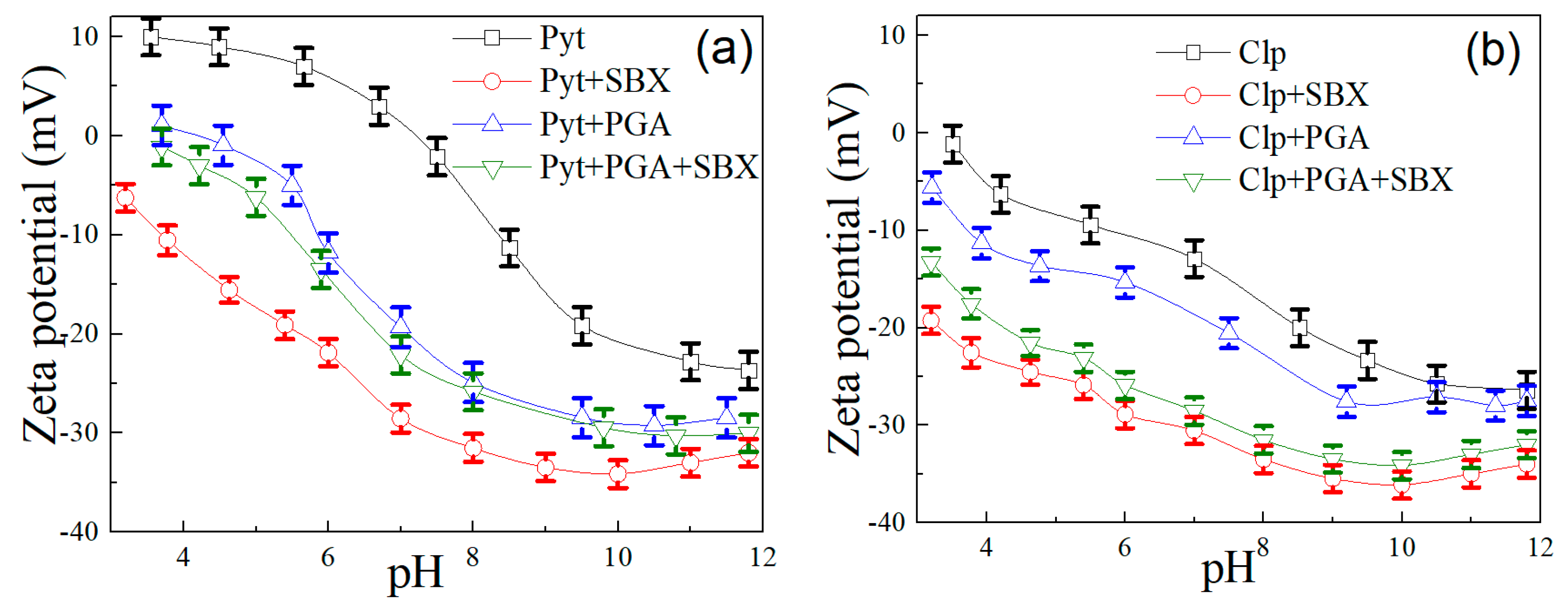
3.3. Zeta Potential
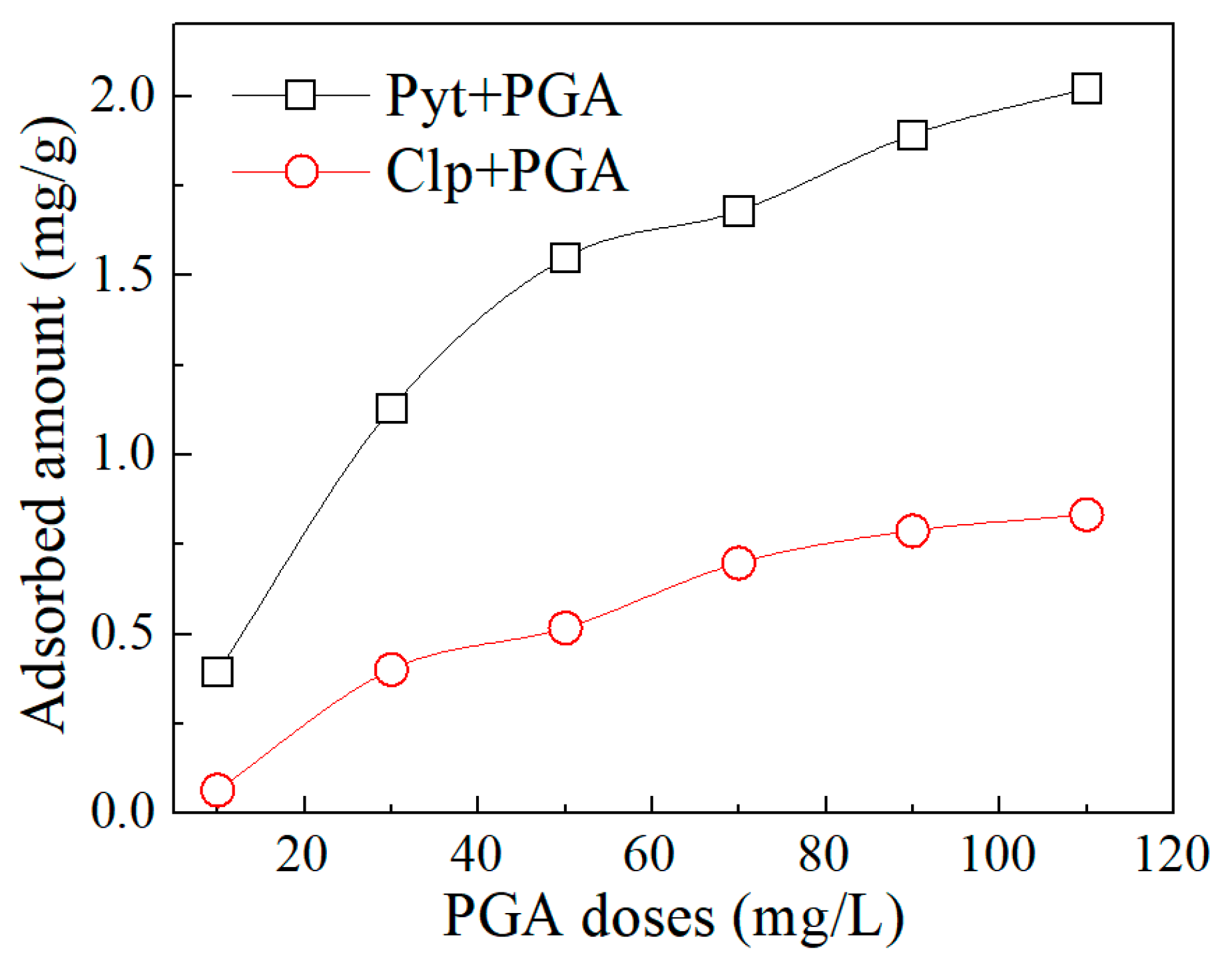
3.4. Reagent Adsorption
3.5. Infrared Spectroscopy
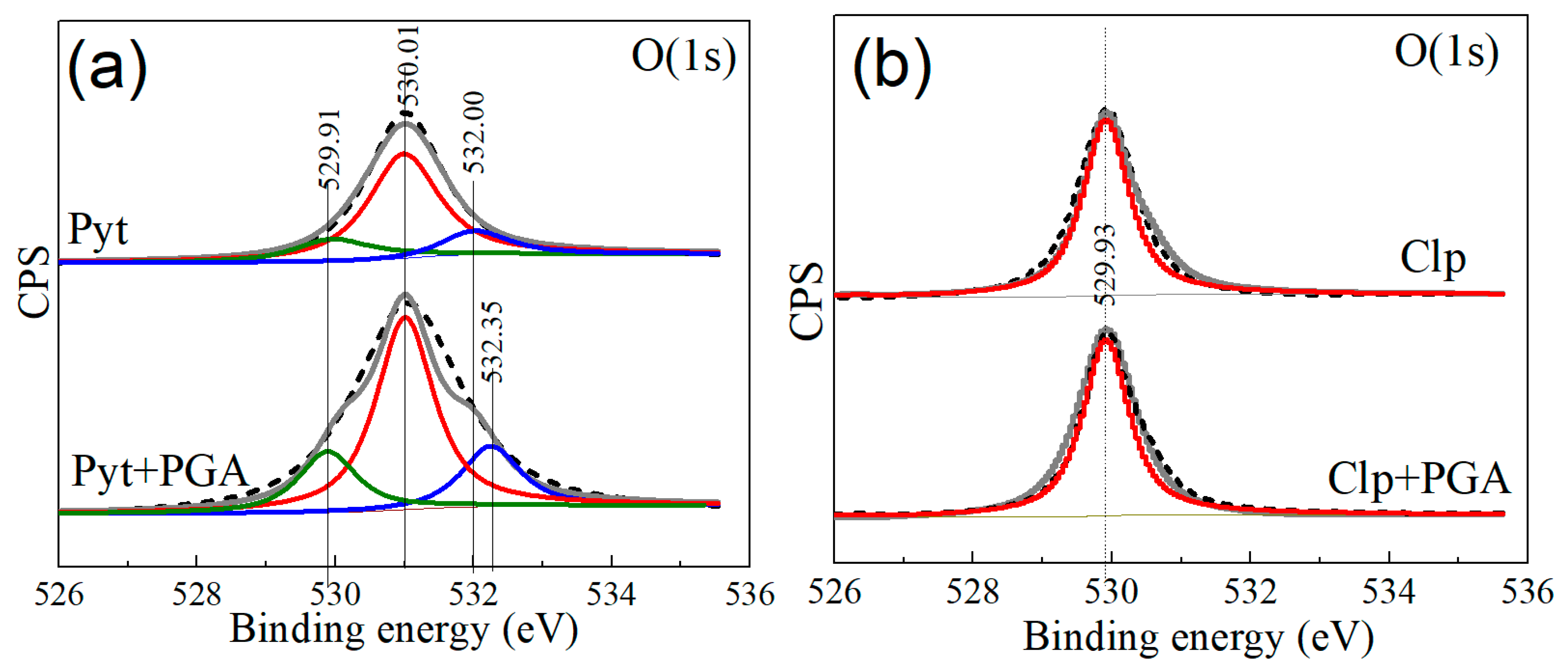
3.6. X-ray Photoelectron Spectroscopy
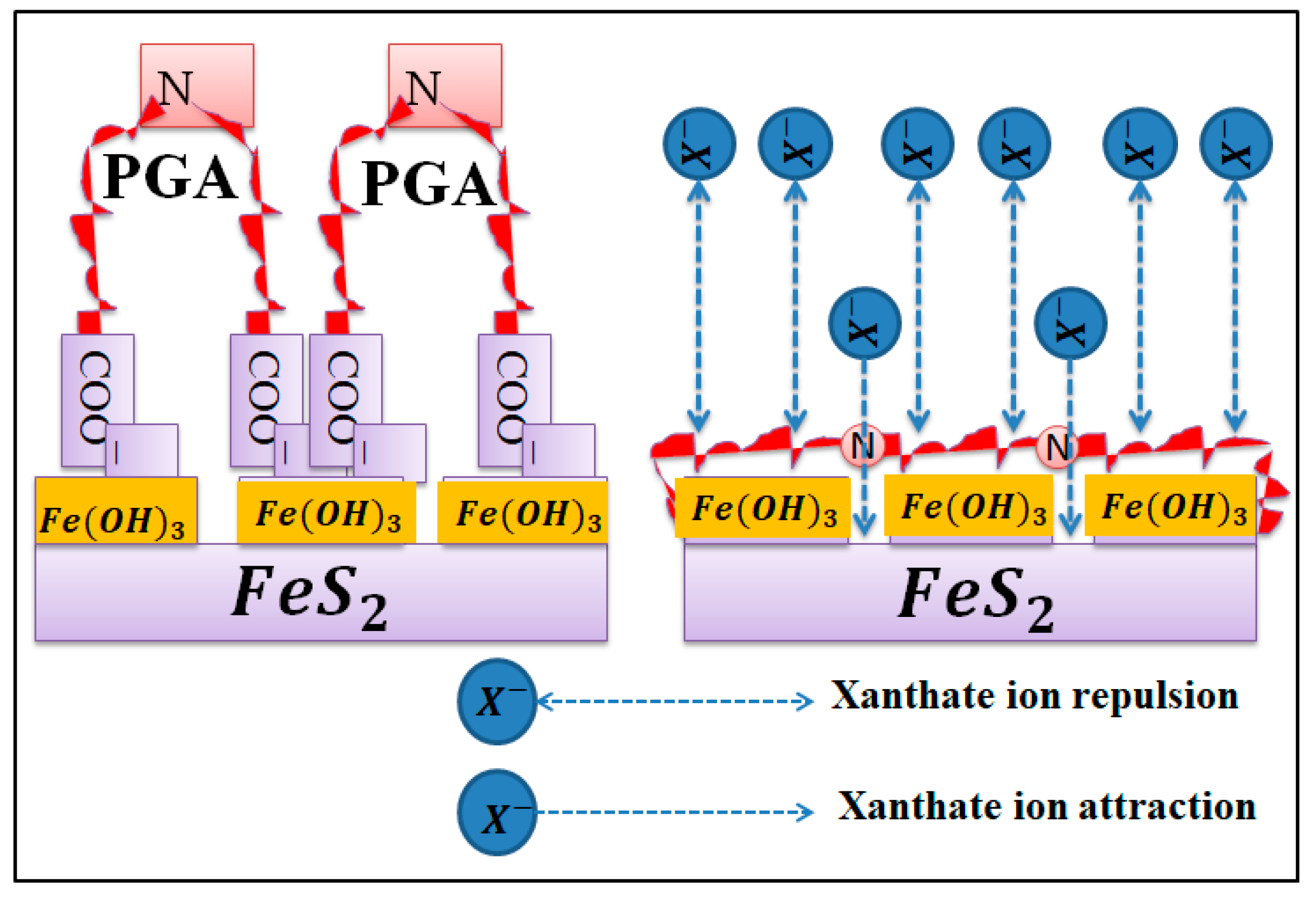
3.7. Adsorption Model and Depression Mechanism of PGA on Pyrrhotite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agar, G.E. Flotation of chalcopyrite, pentlandite, pyrrhotite ores. Int. J. Miner. Process. 1991, 33, 1–19. [Google Scholar] [CrossRef]
- Cheng, X.; Iwasaki, I. Effect of chalcopyrite and pyrrhotite interaction on flotation separation. Min. Met. Explor. 1992, 9, 73–79. [Google Scholar] [CrossRef]
- Allison, S.; O’Connor, C. An investigation into the flotation behaviour of pyrrhotite. Int. J. Miner. Process. 2011, 98, 202–207. [Google Scholar] [CrossRef]
- Chimbganda, T.; Becker, M.; Broadhurst, J.; Harrison, S.; Franzidis, J.P.; Broadhurst, J. A comparison of pyrrhotite rejection and passivation in two nickel ores. Miner. Eng. 2013, 46, 38–44. [Google Scholar] [CrossRef]
- Moslemi, H.; Shamsi, P.; Habashi, F. Pyrite and pyrrhotite open circuit potentials study: Effects on flotation. Miner. Eng. 2011, 24, 1038–1045. [Google Scholar] [CrossRef]
- Bozkurt, V.; Xu, Z.; Finch, J. Pentlandite/pyrrhotite interaction and xanthate adsorption. Int. J. Miner. Process. 1998, 52, 203–214. [Google Scholar] [CrossRef]
- Becker, M.; De Villiers, J.; Bradshaw, D. The flotation of magnetic and non-magnetic pyrrhotite from selected nickel ore deposits. Miner. Eng. 2010, 23, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Xu, M. Method for improving selectivity and recovery in the flotation of nickel sulphide ores that contain pyrrhotite by exploiting the synergy of multiple depressants. U.S. Patent 20140305848A1, 12 July 2016. [Google Scholar]
- Khoso, S.A.; Abro, M.I.; Agheem, M.H. Mineralogical Study of Zard Koh and Kulli Koh Iron Ore Deposits of Pakistan. Mehran Univ. Res. J. Eng. Technol. 2017, 36, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Kelebek, S.; Wells, P.; Fekete, S. Differential flotation of chalcopyrite, pentlandite and pyrrhotite in Ni-Cu sulphide ores. Can. Metall. Q. 1996, 35, 329–336. [Google Scholar]
- Multani, R.S.; Williams, H.; Johnson, B.; Li, R.; Waters, K.E. The effect of superstructure on the zeta potential, xanthate adsorption, and flotation response of pyrrhotite. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 551, 108–116. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Wu, B.Z.; Chen, J.H. Electronic structure and flotation behavior of monoclinic and hexagonal pyrrhotite. J. Cent. South Univ. 2015, 22, 466–471. [Google Scholar] [CrossRef]
- Qi, C.; Liu, J.; Malainey, J.; Kormos, L.J.; Coffin, J.; Deredin, C.; Liu, Q.; Fragomeni, D. The role of Cu ion activation and surface oxidation for polymorphic pyrrhotite flotation performance in Strathcona Mill. Miner. Eng. 2019, 134, 87–96. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Ralston, J.; Smart, R.S. The competitive adsorption of cyanide and ethyl xanthate on pyrite and pyrrhotite surfaces. Int. J. Miner. Process. 1993, 38, 205–233. [Google Scholar] [CrossRef]
- Da Silva, G.; Waters, K. The effects of microwave irradiation on the floatability of chalcopyrite, pentlandite and pyrrhotite. Adv. Powder Technol. 2018, 29, 3049–3061. [Google Scholar] [CrossRef]
- Tukel, C.; Kelebek, S. Modulation of xanthate action by sulphite ions in pyrrhotite deactivation/depression. Int. J. Miner. Process. 2010, 95, 47–52. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Mao, G. Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine. Minerals 2018, 8, 472. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, S.; Han, Y.; Li, Y. The effects of various activators on flotation performance of lime–depressed pyrrhotite. Can. Metall. Q. 2018, 58, 1–8. [Google Scholar]
- Miller, J.; Li, J.; Davidtz, J.; Vos, F.; Vos, C. A review of pyrrhotite flotation chemistry in the processing of PGM ores. Miner. Eng. 2005, 18, 855–865. [Google Scholar] [CrossRef]
- Arvidson, B.; Klemetti, M.; Knuutinen, T.; Kuusisto, M.; Man, Y.; Hughes-Narborough, C. Flotation of pyrrhotite to produce magnetite concentrates with a sulphur level below 0.05% w/w. Miner. Eng. 2013, 50, 4–12. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, D.; Chen, J.; Li, Y.; Chen, Y.; Li, W. The interaction of cyanide with pyrite, marcasite and pyrrhotite. Miner. Eng. 2016, 95, 131–137. [Google Scholar] [CrossRef]
- October, L.; Corin, K.; Schreithofer, N.; Manono, M.; Wiese, J. Water quality effects on bubble-particle attachment of pyrrhotite. Miner. Eng. 2019, 131, 230–236. [Google Scholar] [CrossRef]
- Multani, R.S.; Waters, K.E. Flotation recovery–by–size comparison of pyrrhotite superstructures with and without depressants. Miner. Eng. 2019, 130, 92–100. [Google Scholar] [CrossRef]
- Sun, W.; Liu, R.-Q.; Cao, X.-F.; Hu, Y.-H. Flotation separation of marmatite from pyrrhotite using DMPS as depressant. Trans. Nonferrous Met. Soc. China 2006, 16, 671–675. [Google Scholar] [CrossRef]
- Chen, X.; Guohua, G.; Lijuan, L.; Zhixiang, C. Effect of food–grade guar gum on flotation separation of chalcopyrite and monoclinic pyrrhotite in low–alkali systems. Physicochem. Probl. Miner. Process. 2019, 55, 437–447. [Google Scholar]
- Yoon, R.H.; Basilio, C.; Marticorena, M.; Kerr, A.; Stratton-Crawley, R. A study of the pyrrhotite depression mechanism by diethylenetriamine. Miner. Eng. 1995, 8, 807–816. [Google Scholar] [CrossRef]
- Kelebek, S.; Tukel, C. The effect of sodium metabisulfite and triethylenetetramine system on pentlandite–pyrrhotite separation. Int. J. Miner. Process. 1999, 57, 135–152. [Google Scholar] [CrossRef]
- Gu, G.; Zhao, K.; Qiu, G.; Hu, Y.; Sun, X. Effects of Leptospirillum ferriphilum and Acidithiobacillus caldus on surface properties of pyrrhotite. Hydrometally 2009, 100, 72–75. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems–A Literature review. Miner. Eng. 2016, 96, 143–156. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of copper-activated pyrite in flotation by biopolymers with different compositions. Miner. Eng. 2016, 96, 113–122. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Lyu, F.; Liu, R.; Sun, W. Selective separation of chalcopyrite from pyrite with a novel non-hazardous biodegradable depressant. J. Clean. Prod. 2019, 232, 888–897. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Liu, R.; Tian, M.; Sun, W.; Gao, Y.; Han, H.; Gao, Z. Selective depression of pyrite with a novel functionally modified biopolymer in a Cu–Fe flotation system. Miner. Eng. 2019, 135, 55–63. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.H. Froth Flotation: A Century of Innovation; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2007. [Google Scholar]
- Valdivieso, A.L.; Cervantes, T.C.; Song, S.; Cabrera, A.R.; Laskowski, J. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector. Miner. Eng. 2004, 17, 1001–1006. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Iron hydroxide complexes and their influence on the interaction between ethyl xanthate and pyrite. J. Colloid Interface Sci. 1992, 151, 225–235. [Google Scholar] [CrossRef]
- Neuberger, A. Dissociation constants and structures of glutamic acid and its esters. Biochem. J. 1936, 30, 2085–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, M.S.; Koetzle, T.F.; Hamilton, W.C. Precision neutron diffraction structure determination of protein and nucleic acid components. VIII: The crystal and molecular structure of the β–form of the amino acid l–glutamic acid. J. Cryst. Mol. Struct. 1972, 2, 225–233. [Google Scholar] [CrossRef]
- Clarke, P.; Fornasiero, D.; Ralston, J.; Smart, R. A study of the removal of oxidation products from sulfide mineral surfaces. Miner. Eng. 1995, 8, 1347–1357. [Google Scholar] [CrossRef]
- Shrimali, K.; Atluri, V.; Wang, X.; Miller, J.D. Adsorption of corn starch molecules at hydrophobic mineral surfaces. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 546, 194–202. [Google Scholar] [CrossRef]
- Liu, Q.; Laskowski, J. The interactions between dextrin and metal hydroxides in aqueous solutions. J. Colloid Interface Sci. 1989, 130, 101–111. [Google Scholar] [CrossRef]
- Khosla, N.K.; Bhagat, R.P.; Gandhi, K.S.; Biswas, A.K. Calorimetric and other interaction studies on mineral—starch adsorption systems. Colloids Surf. 1984, 8, 321–336. [Google Scholar] [CrossRef]
- López–Valdivieso, A.; Sánchez–López, A.A.; Padilla–Ortega, E.; Robledo–Cabrera, A.; Galvez, E.; Cisternas, L. Pyrite depression by dextrin in flotation with xanthates. Adsorption and floatability studies. Physicochem. Probl. Miner. Process 2018, 54, 1159–1171. [Google Scholar]
- Lopez Valdivieso, A.; Sánchez López, A.; Song, S.; García Martínez, H.; Licón Almada, S. Dextrin as a regulator for the selective flotation of chalcopyrite, galena and pyrite. Can. Metall. Q. 2007, 46, 301–309. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Varnek, V.; Asanov, I.; Tomashevich, Y.; Okotrub, A.; Livshits, A.; Selyutin, G.; Pashkov, G. Reactivity of pyrrhotite (Fe9S10) surfaces: Spectroscopic studies. Phys. Chem. Chem. Phys. 2000, 2, 4393–4398. [Google Scholar] [CrossRef]
- Moreira, G.F.; Peçanha, E.R.; Monte, M.B.M.; Leal Filho, L.S.; Stavale, F. XPS study on the mechanism of starch–hematite surface chemical complexation. Miner. Eng. 2017, 110, 96–103. [Google Scholar] [CrossRef]
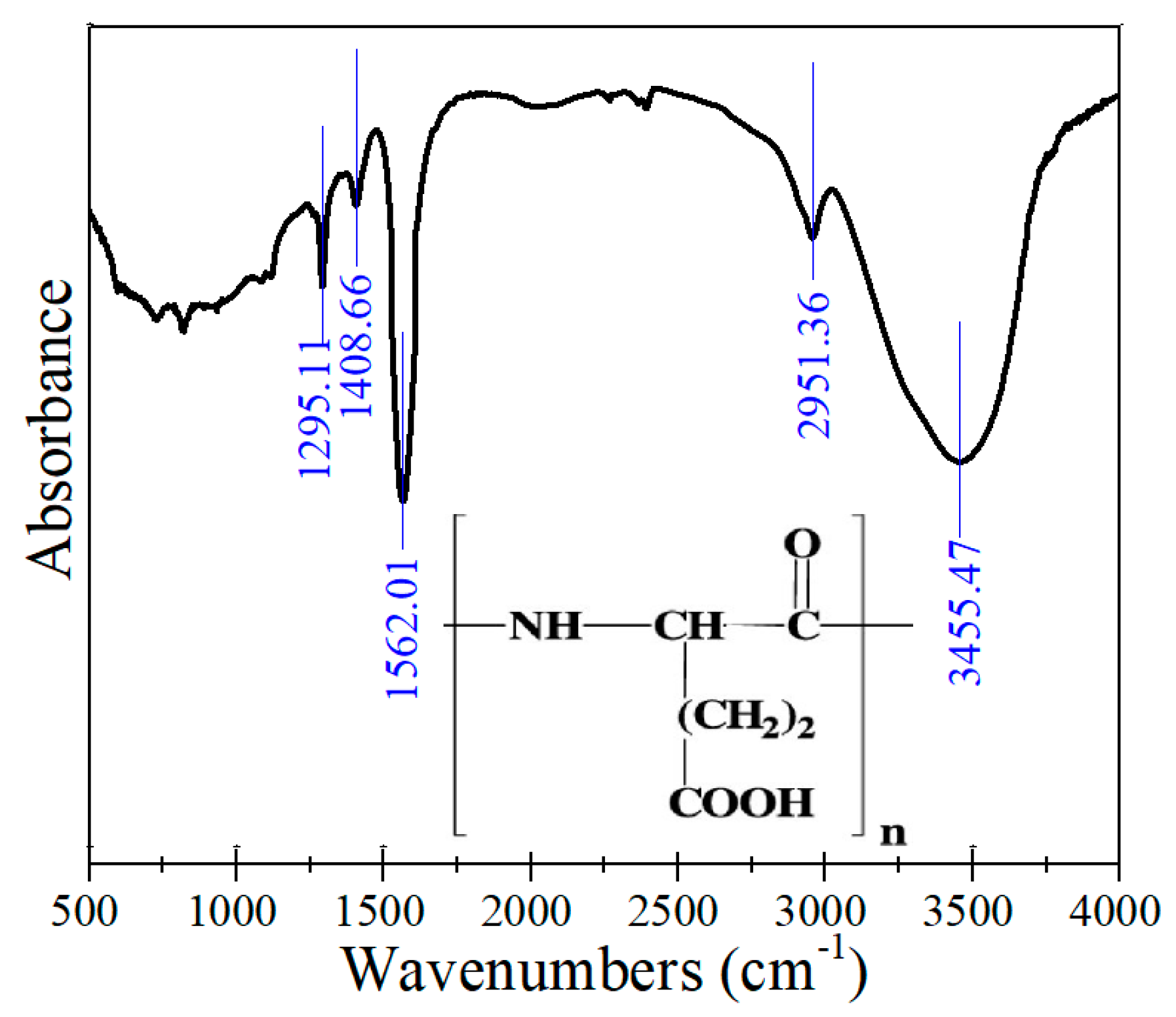

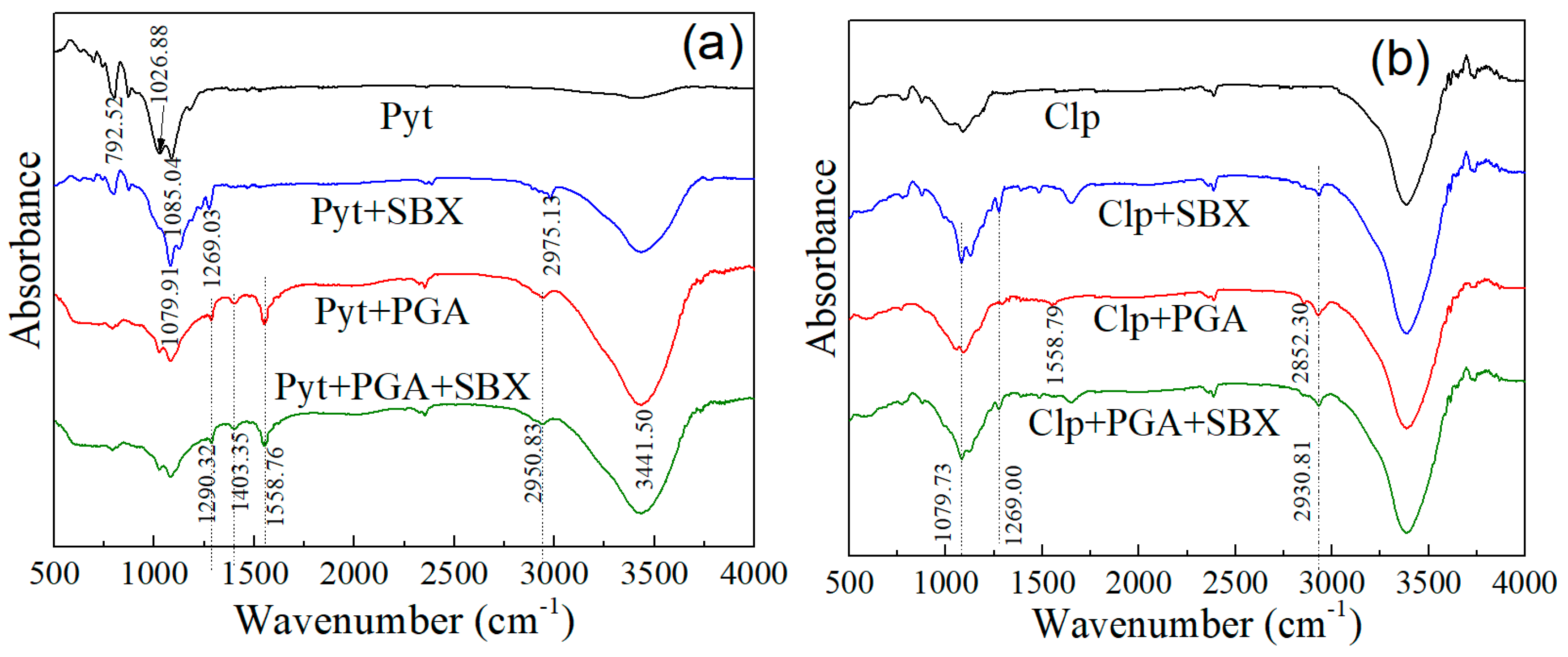

| Wavenumber (cm−1) | Assignment |
|---|---|
| 1295.11 | C–N stretching |
| 1408.66 | CO2− symmetric stretching |
| 1562.01 | CO2− asymmetric stretching |
| 2951.36 | C–H stretching |
| 3455.47 | N–H stretching |
| Products | Yield | Recovery (%) | Grade (%) | ||
|---|---|---|---|---|---|
| (wt. %) | CuFeS2 | Fe1−xS | CuFeS2 | Fe1−xS | |
| Concentrate | 53.00 ± 0.68 | 85.73 ± 0.37 | 20.20 ± 0.33 | 80.97 ± 0.40 | 19.03 ± 0.40 |
| Tailing | 47.00 ± 0.68 | 14.27 ± 0.37 | 79.80 ± 0.33 | 15.20 ± 0.37 | 84.80 ± 0.37 |
| Flotation feed | 100 | 100 | 100 | 50 | 50 |
| Sample | Elemental Concentration (%) | ||||
|---|---|---|---|---|---|
| Cu | Fe | S | O | C | |
| Pyrrhotite | - | 32.93 | 41.96 | 21.89 | 3.21 |
| Pyrrhotite with PGA | - | 19.31 | 27.01 | 34.11 | 19.51 |
| Chalcopyrite | 27.03 | 16.31 | 31.45 | 21.63 | 3.51 |
| Chalcopyrite with PGA | 23.39 | 14.93 | 29.38 | 23.37 | 8.89 |
| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu(2p) | Fe(2p) | S(2p) | O(1s) | C(1s) | Cu(2p) | Fe(2p) | S(2p) | O(1s) | C(1s) | |
| Pyrrhotite | - | 707.45 | 161.47 | 530.33 | 284.33 | - | - | - | - | - |
| Pyrrhotite with PGA | - | 708.01 | 162.00 | 531.03 | 284.93 | - | 0.56 | 0.53 | 0.70 | 0.60 |
| Chalcopyrite | 931.81 | 707.51 | 161.49 | 529.91 | 284.79 | - | - | - | - | |
| Chalcopyrite with PGA | 931.89 | 707.56 | 161.52 | 529.98 | 284.84 | 0.08 | 0.05 | 0.03 | 0.07 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoso, S.A.; Gao, Z.; Meng, X.; Hu, Y.; Sun, W. The Depression and Adsorption Mechanism of Polyglutamic Acid on Chalcopyrite and Pyrrhotite Flotation Systems. Minerals 2019, 9, 510. https://doi.org/10.3390/min9090510
Khoso SA, Gao Z, Meng X, Hu Y, Sun W. The Depression and Adsorption Mechanism of Polyglutamic Acid on Chalcopyrite and Pyrrhotite Flotation Systems. Minerals. 2019; 9(9):510. https://doi.org/10.3390/min9090510
Chicago/Turabian StyleKhoso, Sultan Ahmed, Zhiyong Gao, Xiangsong Meng, Yuehua Hu, and Wei Sun. 2019. "The Depression and Adsorption Mechanism of Polyglutamic Acid on Chalcopyrite and Pyrrhotite Flotation Systems" Minerals 9, no. 9: 510. https://doi.org/10.3390/min9090510
APA StyleKhoso, S. A., Gao, Z., Meng, X., Hu, Y., & Sun, W. (2019). The Depression and Adsorption Mechanism of Polyglutamic Acid on Chalcopyrite and Pyrrhotite Flotation Systems. Minerals, 9(9), 510. https://doi.org/10.3390/min9090510