Interaction Mechanism of Ferric Ions with Celestite Surface and Implications for Flotation Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ferric Ion Released from Limonite
2.3. XPS Analysis
2.4. Adsorption Experiments
2.5. Flotation Experiments
2.6. Calculation Method
3. Results and Discussion
3.1. The Release of Ferric Ions from Limonite
3.2. Surface Properties of Celestite
3.3. Adsorption Mechanism of Ferric Ions on Celestite Surface
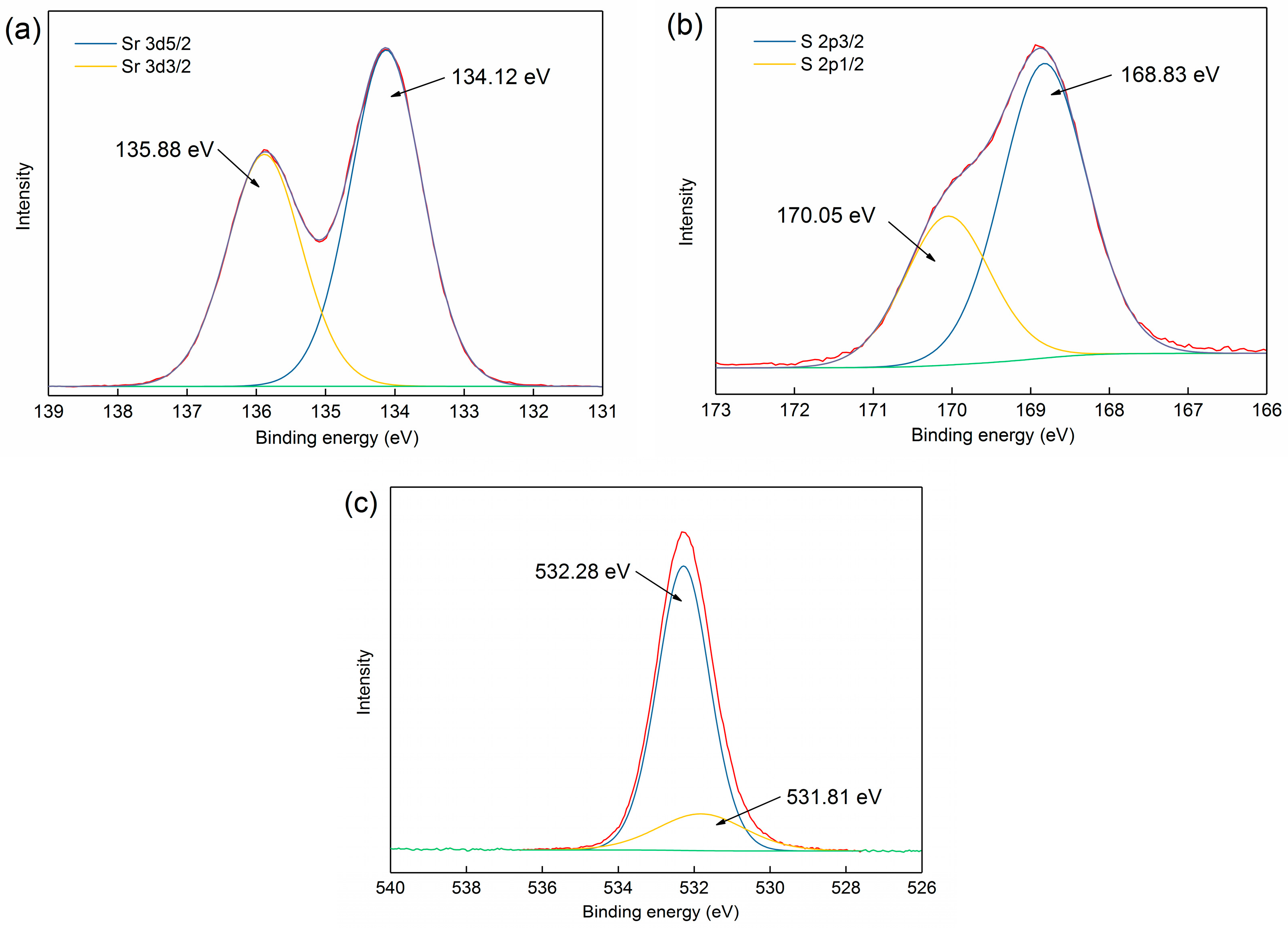
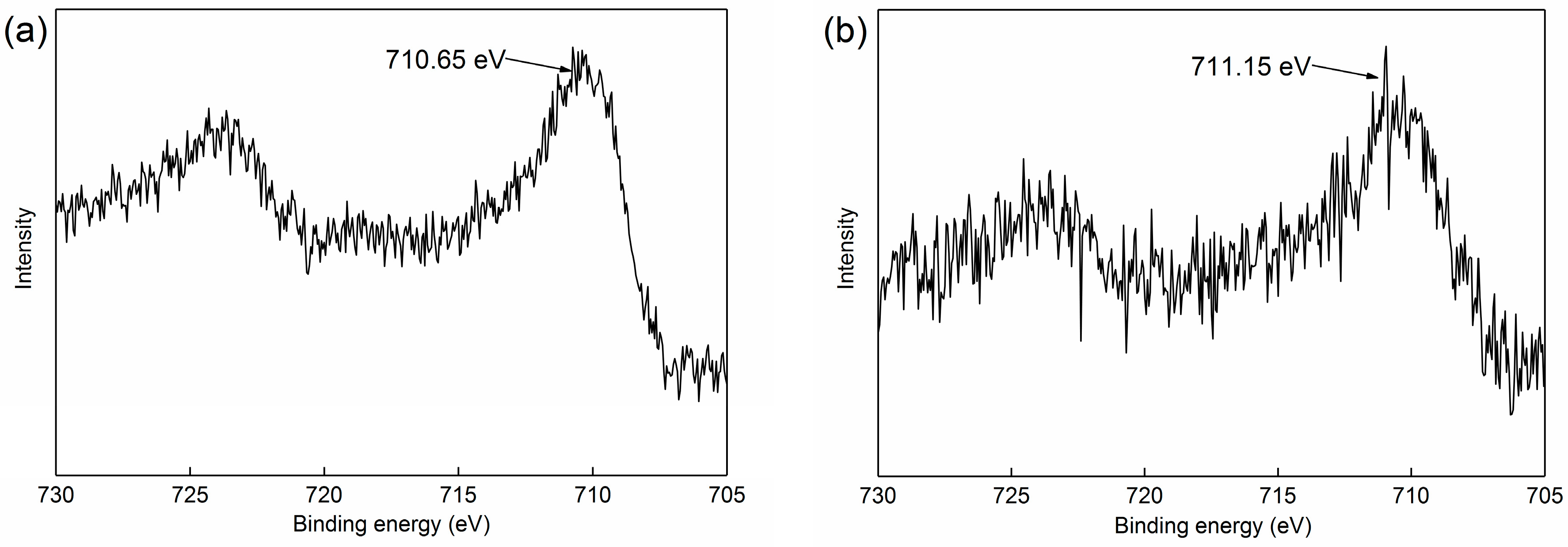
3.3.1. XPS Analysis
3.3.2. Adsorption Isotherm Investigation
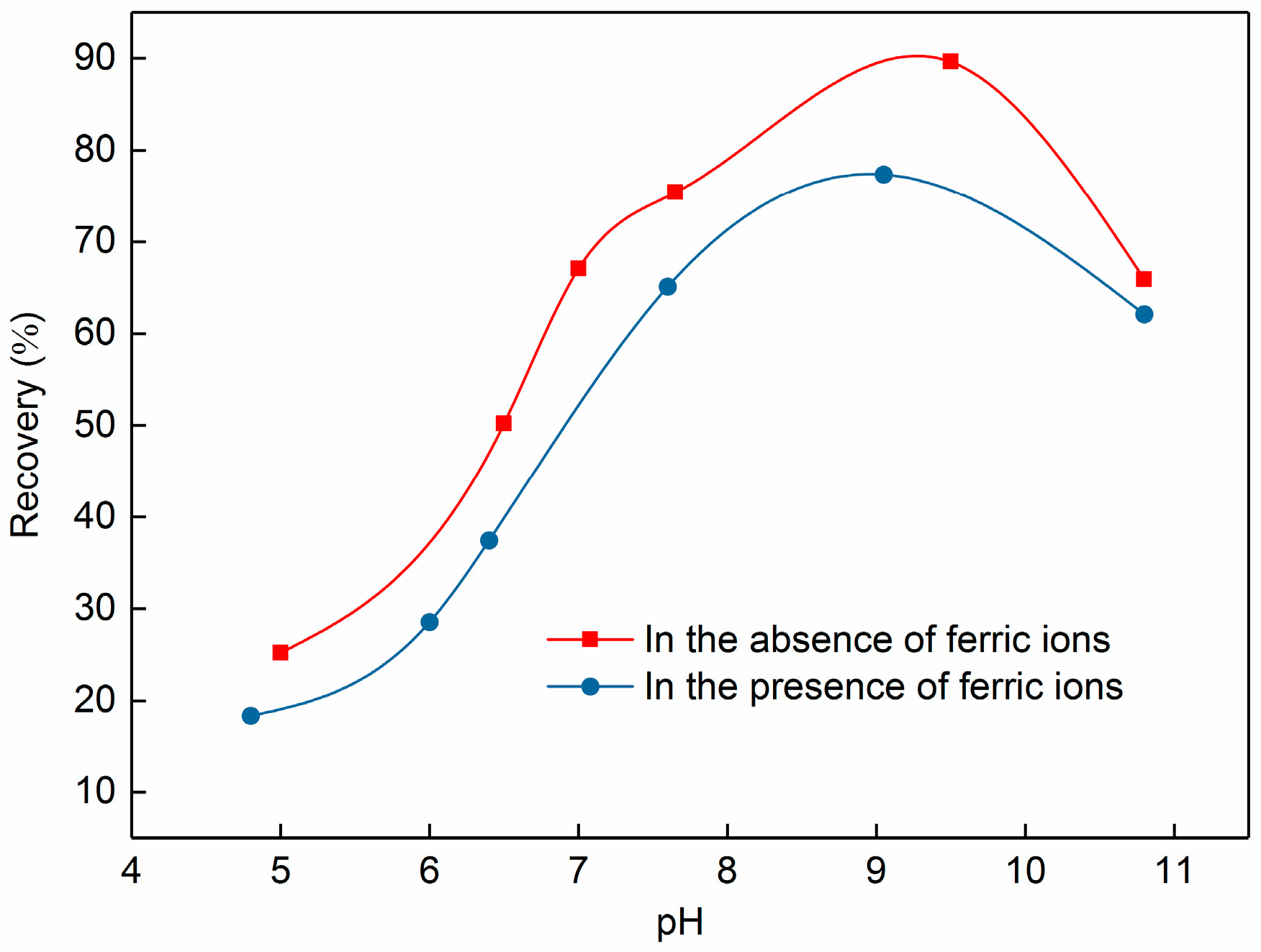
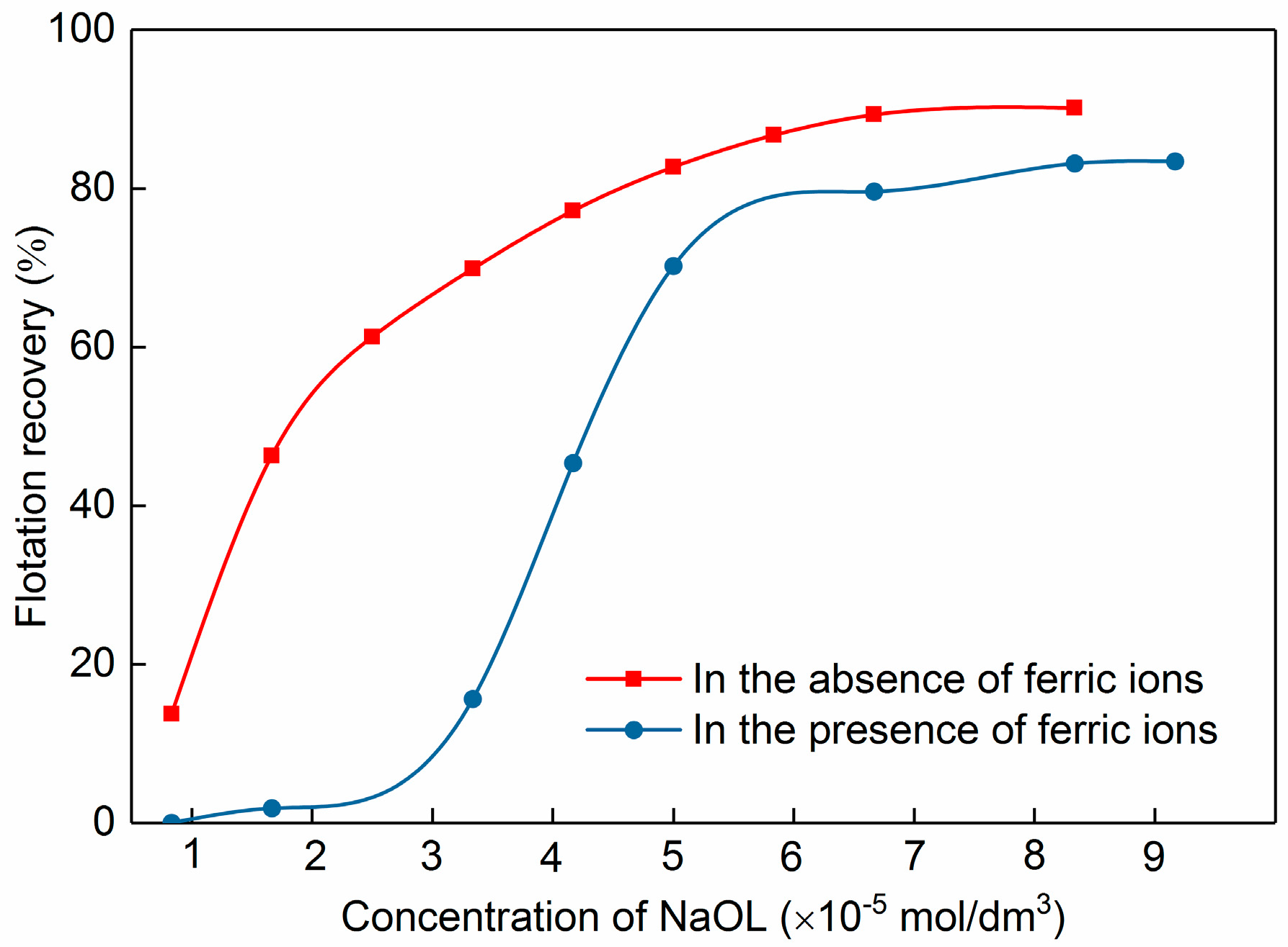
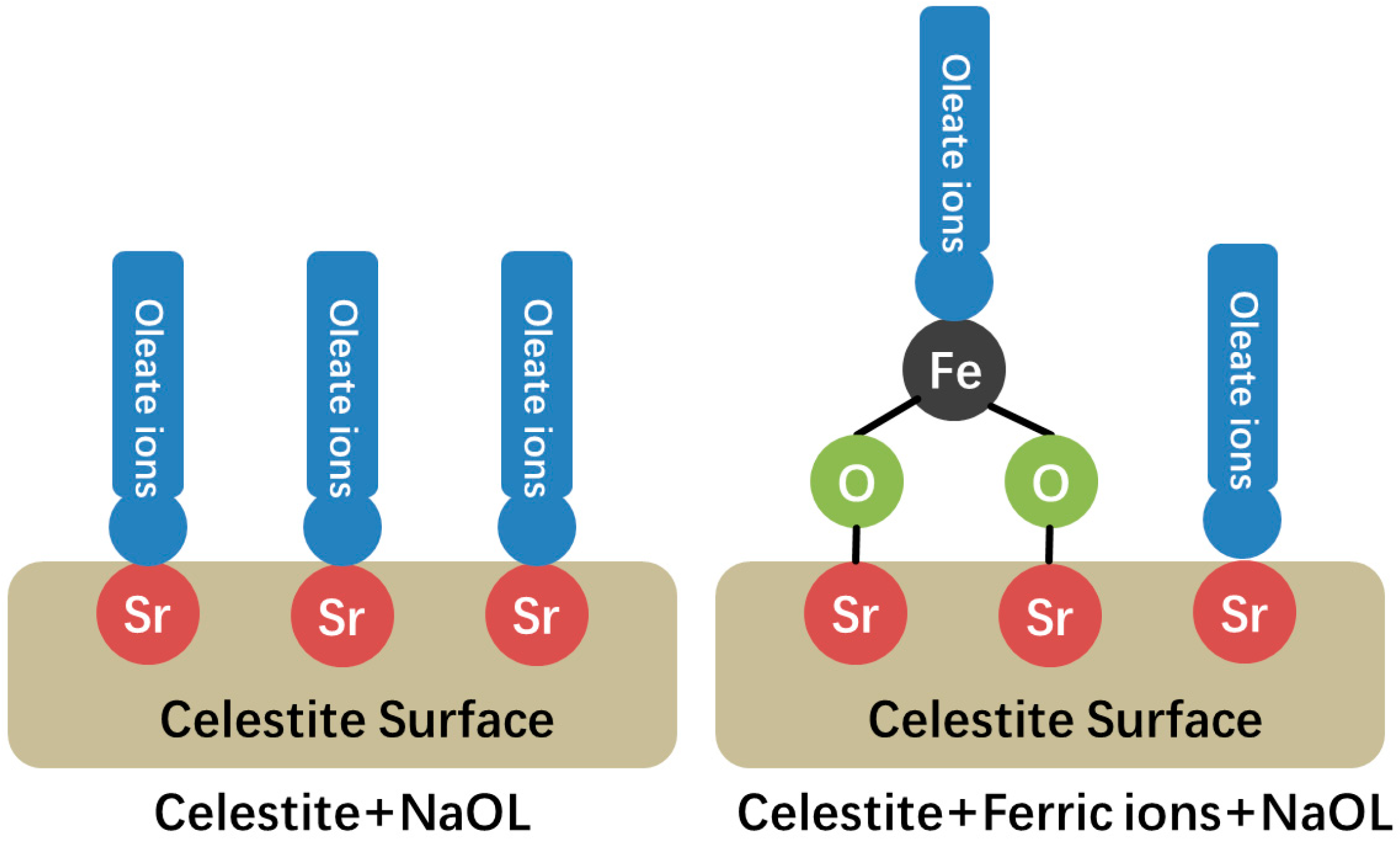
3.4. Implication Mechanism of Ferric Ions on Celestite Flotation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martínez-Luévanos, A.; Uribesalas, A.; López-Valdivieso, A. Mechanism of adsorption of sodium dodecylsulfonate on celestite and calcite. Miner. Eng. 1999, 12, 919–936. [Google Scholar] [CrossRef]
- Martinez, A.L.; Uribe, A.S. Interfacial properties of celestite and strontianite in aqueous solutions. Miner. Eng. 1995, 8, 1009–1022. [Google Scholar] [CrossRef]
- López-Valdivieso, A.; Robledo-Cabrera, A.; Uribe-Salas, A. Flotation of celestite with the anionic collector sodium dodecyl sulfate. Effect of carbonate ions. Int. J. Miner. Process. 2000, 60, 79–90. [Google Scholar] [CrossRef]
- Hernáinz Bermúdez De Castro, F.; Calero De Hoces, M. Flotation rate of celestite and calcite. Chem. Eng. Sci. 1996, 51, 119–125. [Google Scholar] [CrossRef]
- Hernáinz Bermúdez De Castro, F.; Gálvez Borrego, A. The influence of temperature during flotation of celestite and calcite with sodium oleate and quebracho. Int. J. Miner. Process. 1996, 46, 35–52. [Google Scholar] [CrossRef]
- Deng, J.S.; Wen, S.M.; Liu, J.; Xian, Y.J.; Wu, D.D.; Bai, S.J. New source of unavoidable ions in bornite Flotation Aqueous Solution: Fluid Inclusions. Ind. Eng. Chem. Res. 2013, 52, 4895–4901. [Google Scholar] [CrossRef]
- Deng, R.D.; Hu, Y.; Ku, J.G.; Ma, Y.Q.; Yang, Z.G. Ion migration law in flotation pulp and its influence on the separation of smithsonite and quartz. Sep. Sci. Technol. 2017, 53, 1–9. [Google Scholar]
- Shin-Nosuke, S.N.; Tanaka, T.; Yamamoto, K. Crystal structure control of the dissolution of rare earth elements in water-mineral interactions. Geochem. J. 2007, 40, 437. [Google Scholar] [CrossRef]
- Deng, J.S.; Wen, S.M.; Xian, Y.J.; Liu, J.; Bai, S.J. New discovery of unavoidable ions source in chalcopyrite flotation pulp: Fluid inclusions. Miner. Eng. 2013, 42, 22–28. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.M.; Wu, D.D.; Bai, S.J.; Liu, D. Determination of the concentrations of calcium and magnesium released from fluid inclusions of sphalerite and quartz. Miner. Eng. 2013, 45, 41–43. [Google Scholar] [CrossRef]
- Deng, R.D.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Yang, Z.G. Adsorption of Fe(III) on smithsonite surfaces and implications for flotation. Colloid. Surf. A. 2017, 533, 308–315. [Google Scholar] [CrossRef]
- Shi, H.L.; Zhu, W.L. Influence and corresponding mechanism of Fe3+ on spodumene flotation. Rare Met. Cem. Carbides 2015, 43, 5–9. (In Chinese) [Google Scholar]
- Jiang, C.L.; Wang, X.H.; Parekh, B.K.; Leonard, J.W. The surface and solution chemistry of pyrite flotation with xanthate in the presence of iron ions. Colloid. Surf. A 1998, 136, 51–62. [Google Scholar] [CrossRef]
- Cui, M.M. The Influence and Its Elimination of Unavoidable Ions in the Flotation Separation of Smithsonite and Quartz. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar] [CrossRef]
- Malati, M.A.; Estefan, S.F. Activation of quartz by alkaline earth cations in oleate flotation. J. Chem. Technol. Biot. 2010, 17, 209–212. [Google Scholar] [CrossRef]
- Mackenzie, J.M.W. Zeta potential of quartz in the presence of ferric iron. Trans. AIME 1966, 235, 82. [Google Scholar]
- Wang, D.Z.; Hu, Y.H. Flotation Solution Chemistry; Hunan Science and Technology Press: Changsha, China, 1988. (In Chinese) [Google Scholar]
- Zhu, Y.S.; Zhu, J.G. Chemical Principles of Flotation Reagents, Revised edition; Central South University of Technology Press: Changsha, China, 1996. (In Chinese) [Google Scholar]
- Li, F.D. Celestite-the primary strontium mineral. Chem. Min. Technol. 1984, 3, 39–40. (In Chinese) [Google Scholar]
- Tang, D.K.; Shen, J.P. Mineral processing technology of celestite. Chem. Min. Technol. 1988, 1, 24–26. (In Chinese) [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in lunsungen. J. Phys. Chem. 1915, 57, 387. [Google Scholar]
- Johnson, R.D.; Arnold, F.H. The Temkin Isotherm Describes Heterogeneous Protein Adsorption. Biochim. Biophys. Acta 1995, 1247, 293. [Google Scholar] [CrossRef]
- Cao, S.M.; Cao, Y.J.; Ma, Z.L.; Liao, Y.F. Metal Ion Release in Bastnaesite Flotation System and Implications for Flotation. Minerals 2018, 8, 203. [Google Scholar] [CrossRef]
- Welham, N.J.; Llewellyn, D.J. Mechanical enhancement of the dissolution of ilmenite. Miner. Eng. 1998, 11, 827–841. [Google Scholar] [CrossRef]
- Guy, P.; Trahar, W. The influence of grinding and flotation environments on the laboratory batch flotation of galena. Int. J. Miner. Process. 1984, 12, 15–38. [Google Scholar] [CrossRef]
- Jones, R.O.; Gunnarsson, O. The density functional formalism, its applications and prospects. Rev. Mod. Phys. 1989, 61, 689–746. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H. Mineral cleavage nature and surface energy: Anisotropic surface broken bonds consideration. T. Nonferr. Metal. Soc. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Wang, P. Systematic Mineralogy; Geological Publishing House: Beijing, China, 1982. (In Chinese) [Google Scholar]
- Sposito, G.S. Molecular models of iron adsorption on mineral surfaces. Rev. Mineral. Geochem. 1990, 23, 261–279. [Google Scholar]
- Parks, G.A. Surface Energy and Adsorption at Mineral/Water Interfaces: An Introduction. Rev. Miner. 1990, 23, 133–175. [Google Scholar]
- Cappellen, P.V.; Charlet, L.; Stumm, W.; Wersin, P. A surface complexation model of the carbonate mineral-aqueous solution interface. Geochim. Cosmochim. Acta 1993, 57, 3505–3518. [Google Scholar] [CrossRef]
- Oelhafen, P. Practical surface analysis by auger and X-ray photoelectron spectroscopy. J. Electron. Spectrosc. 1984, 34, 203. [Google Scholar] [CrossRef]
- Vasquez, R.P. X-ray photoelectron spectroscopy study of Sr and Ba compounds. J. Electron Spectrosc. Relat. Phenom. 1991, 56, 217–240. [Google Scholar] [CrossRef]
- Yu, Y.; Song, L.; Zhou, S.Z.; Sun, C.Y. Influence of multivalent metal ions on the flotation of calcium salt mineral. Ind. Miner. Proc. 2015, 9, 9–13. (In Chinese) [Google Scholar]
- Moulder, J.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1992; ISBN 0-9627026-2-5. [Google Scholar]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M.; Cao, Q.B. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Lu, S.S. Research of Crystal Chemistry on Flotation of Several Sulfate Minerals. Ph.D. Thesis, Northeastern University, Shenyang, China, 2008. [Google Scholar]
- Carter, M.C.; Kilduff, J.E.; Weber, W.J. Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 1995, 29, 1773. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X. Adsorption Science, 2nd ed.; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Jia, M.X. Surface Structure Study of Silicate Minerals and Its Adsorption Mechanism for Metal Ions. Ph.D. Thesis, Northeastern University, Shenyang, China, 2001. (In Chinese). [Google Scholar]
- Zhou, D.H.; Li, X.Y.; Xu, F.L.; Dong, Y.Y. Adsorption characteristics of heavy metal ions onto soil in mass-action model. J. Environ. Sci. 1996, 4, 425–430. (In Chinese) [Google Scholar]
- Wu, H.H.; Wu, D.Q.; Peng, J.L. Experimental study of the reaction between metal ions and quartz surface. Geochemistry 1998, 6, 521–531. (In Chinese) [Google Scholar]
- Ou, L.M.; Ye, J.S.; Zeng, W.W.; Wan, L. Influence and Mechanism of Ferric and Ferrous Ions on Flotation of Smithsonite and Quartz. Nonferr. Metals. 2012, 6, 79–82. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, G.F.; Zhu, Y.G.; Feng, Q.M.; Lu, Y.P.; Ou, L.M. Flotation mechanism of fine ilmenite by sodium oleate. Chin. J. Nonferr. Metal. 2009, 2, 372–377. (In Chinese) [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
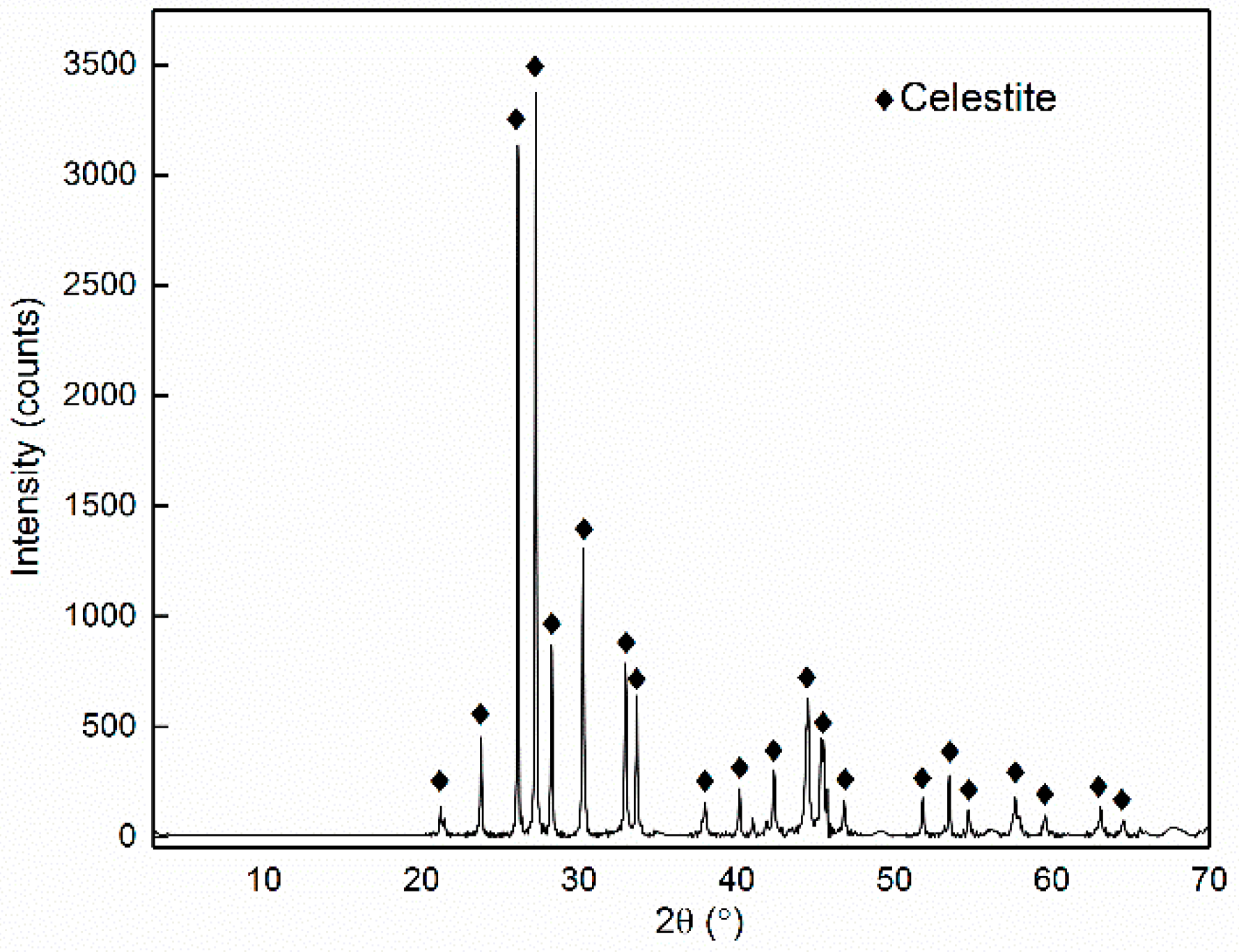
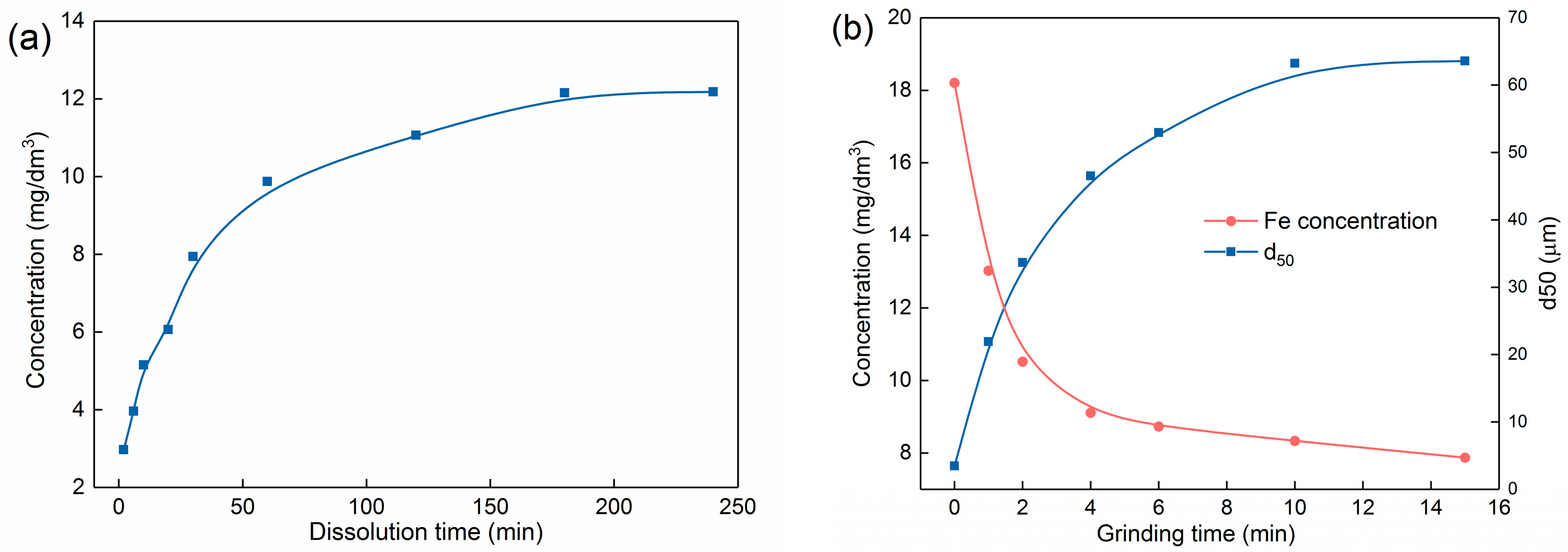
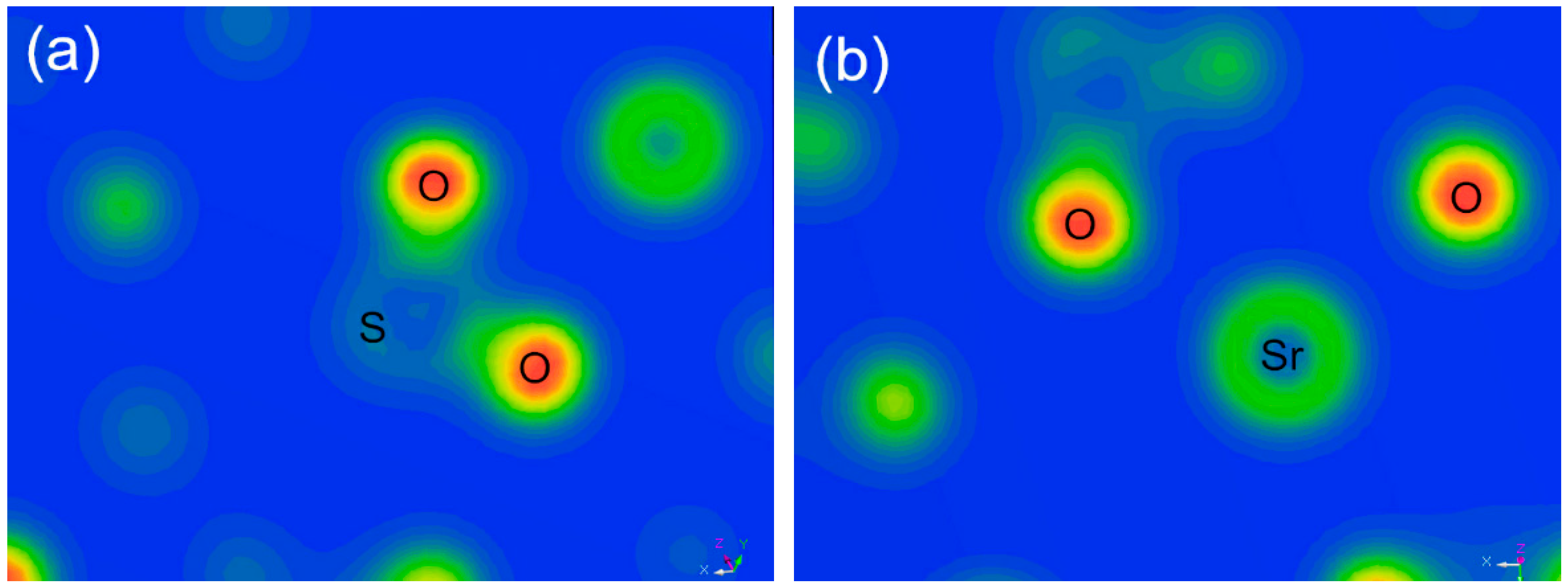
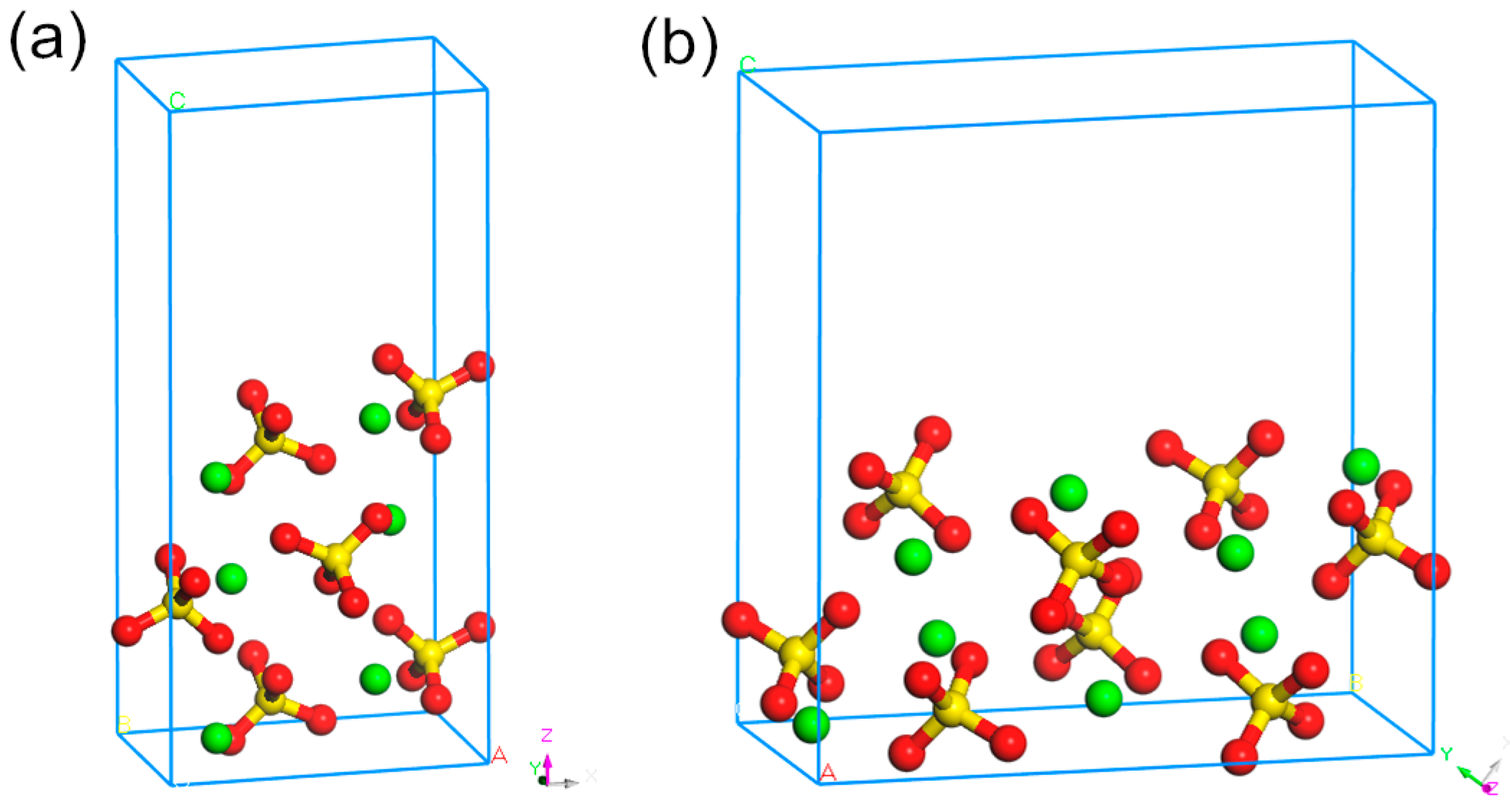

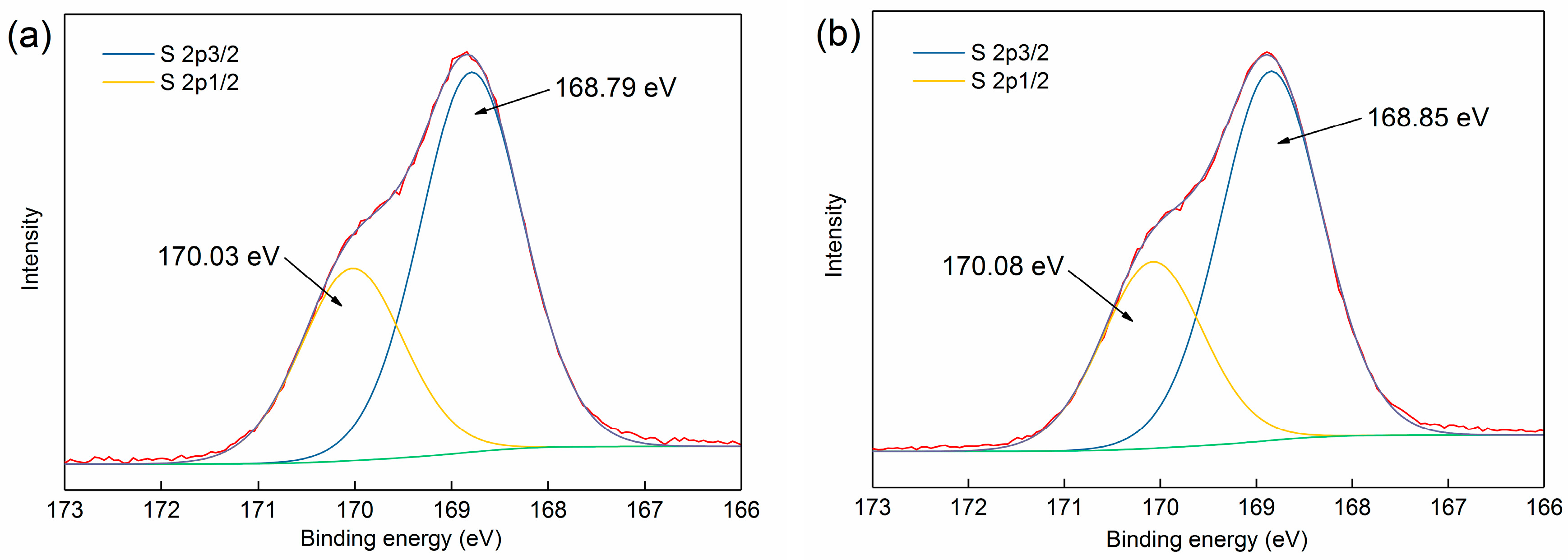
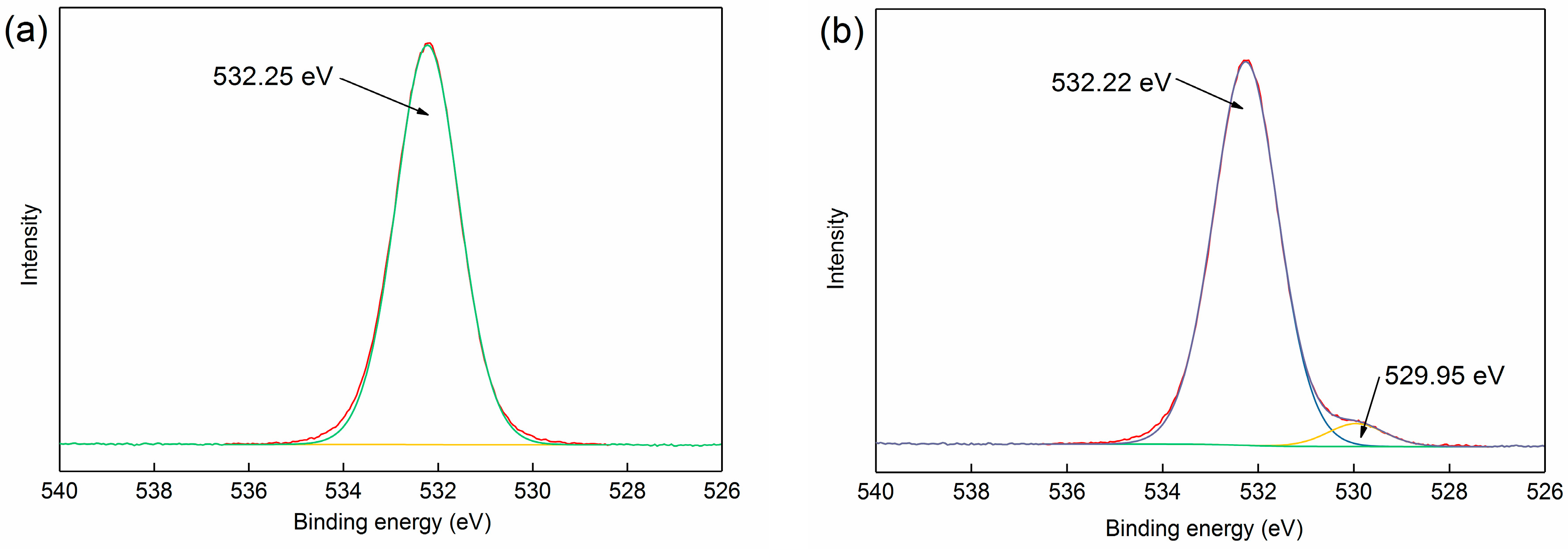
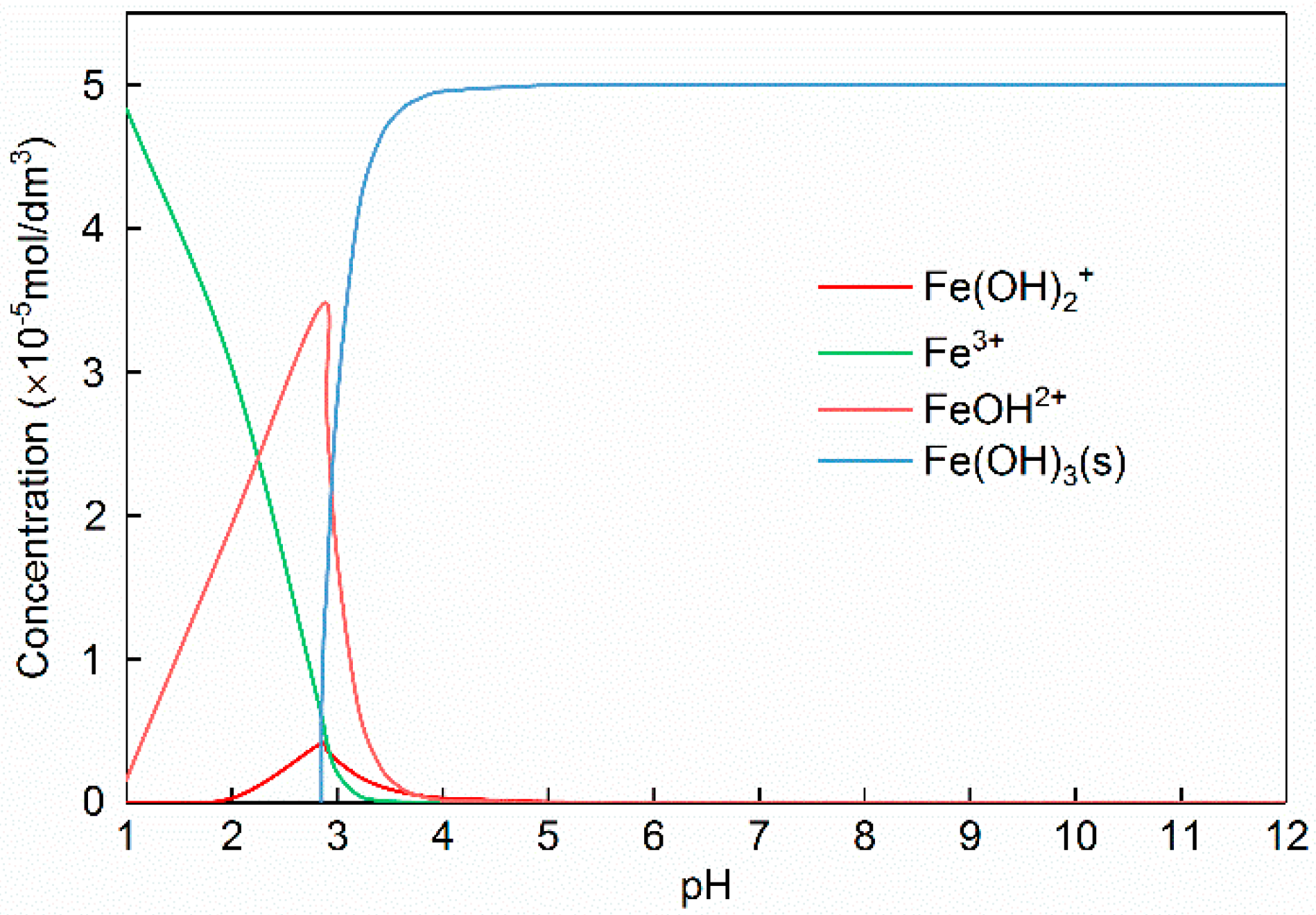
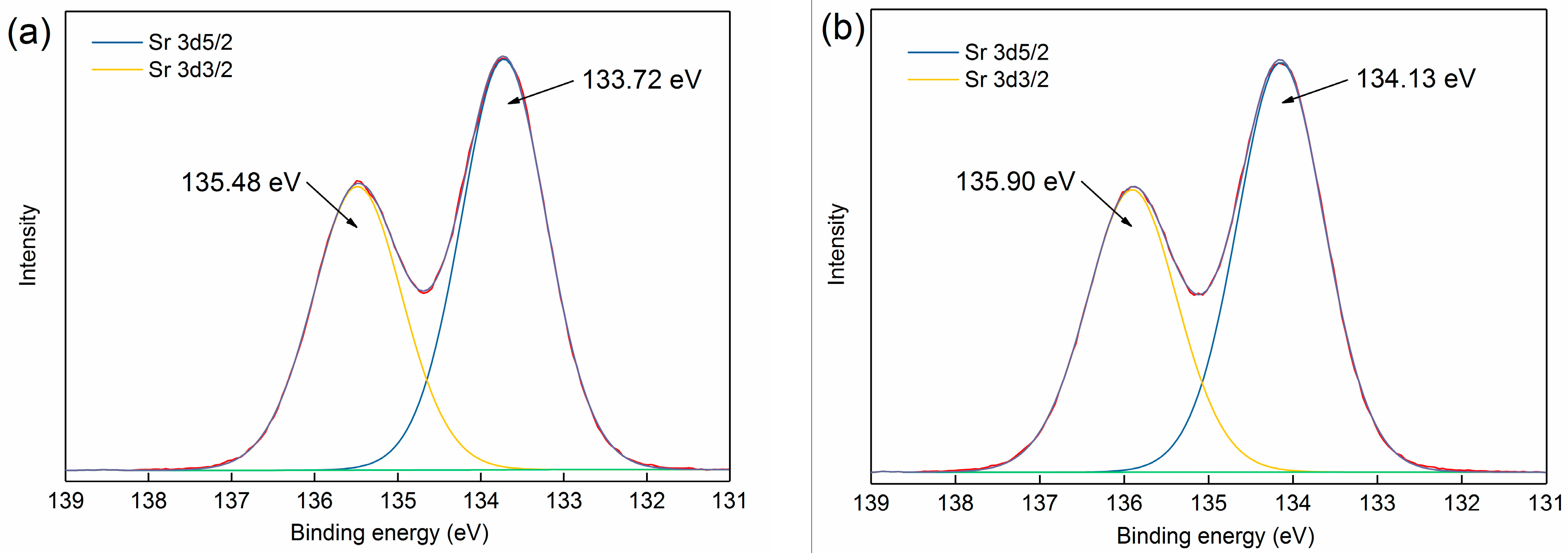


| Phase | Magnetite | Iron Oxide (Limonite) | Iron Sulphide | Iron Carbonate | Iron Silicate | Total Iron Content |
|---|---|---|---|---|---|---|
| Percentage | 0.37 | 33.37 | 0.21 | 0.68 | 0.42 | 35.05 |
| Distribution Rate | 1.06 | 95.20 | 0.60 | 1.94 | 1.20 | 100 |
| Bonds | Population | Length (Å) |
|---|---|---|
| S–O1 | 0.59 | 1.48 |
| S–O2 | 0.58 | 1.49 |
| S–O3 | 0.54 | 1.50 |
| Sr–O1 | 0.13 | 2.50 |
| Sr–O2 | 0.10 | 2.63 |
| Sr–O3 | 0.07 | 2.73 |
| Model | Langmuir | Freundlich | Temkin | |||
|---|---|---|---|---|---|---|
| Constants | Q0 | 0.16 | kF | 0.03 | KT | 1.44 |
| K1 | 0.29 | n | 1.83 | B | 0.10 | |
| R2 | 0.93 | R2 | 0.97 | R2 | 0.89 | |
| Atom | Atomic Concentration (%) | |
|---|---|---|
| Before Treatment | After Treatment | |
| Sr | 12.22 | 8.04 |
| S | 19.39 | 18.73 |
| O | 62.62 | 63.15 |
| C | 5.77 | 10.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Cao, Y.; Ma, Z.; Liao, Y.; Zhang, X. Interaction Mechanism of Ferric Ions with Celestite Surface and Implications for Flotation Recovery. Minerals 2019, 9, 405. https://doi.org/10.3390/min9070405
Cao S, Cao Y, Ma Z, Liao Y, Zhang X. Interaction Mechanism of Ferric Ions with Celestite Surface and Implications for Flotation Recovery. Minerals. 2019; 9(7):405. https://doi.org/10.3390/min9070405
Chicago/Turabian StyleCao, Shiming, Yijun Cao, Zilong Ma, Yinfei Liao, and Xiaolin Zhang. 2019. "Interaction Mechanism of Ferric Ions with Celestite Surface and Implications for Flotation Recovery" Minerals 9, no. 7: 405. https://doi.org/10.3390/min9070405
APA StyleCao, S., Cao, Y., Ma, Z., Liao, Y., & Zhang, X. (2019). Interaction Mechanism of Ferric Ions with Celestite Surface and Implications for Flotation Recovery. Minerals, 9(7), 405. https://doi.org/10.3390/min9070405







