Chemical Characteristics of Freshwater and Saltwater Natural and Cultured Pearls from Different Bivalves
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. LA-ICP-MS Results
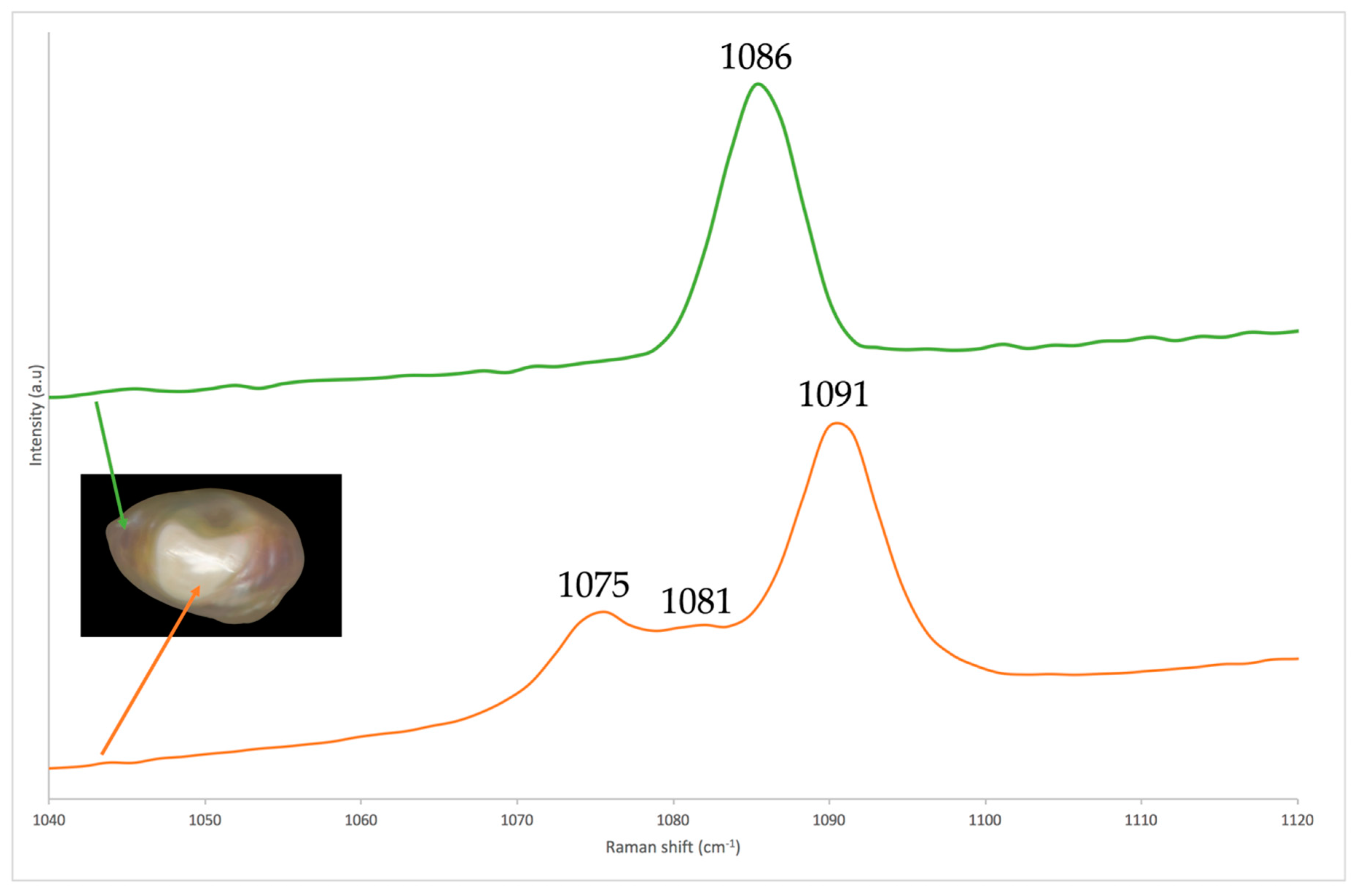
3.2. X-ray Luminescence and Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gauthier, J.P.; Karampelas, S. Pearls and corals: “Trendy biomineralizations”. Elements 2009, 5, 179–180. [Google Scholar] [CrossRef]
- Cartier, L.; Krzemnicki, M.S. New developments in cultured pearls production: Use of organic and baroque shell nuclei. Aust. Gemmol. 2013, 25, 6–13. [Google Scholar]
- Schiffmann, C.A. Pearl identification: Some laboratory experiments. J. Gemmol. 1971, 12, 284–296. [Google Scholar] [CrossRef]
- Lorenz, I.; Schmetzer, K. Possibilities and limitations in radiographic determination of pearls. J. Gemmol. 1986, 20, 114–123. [Google Scholar] [CrossRef]
- Hänni, H.A.; Kiefert, L.; Giese, P. X-ray luminescence, a valuable test in pearl identification. J. Gemmol. 2005, 29, 325–329. [Google Scholar] [CrossRef]
- Habermann, D.; Banerjee, A.; Meijer, J.; Stephan, A. Investigation of manganese in salt- and freshwater pearls. Nucl. Instrum. Methods 2001, 181, 739–743. [Google Scholar] [CrossRef]
- Gütmannsbauer, W.; Hänni, H.A. Structural and chemical investigations on shell and pearls of nacre forming salt- and freshwater bivalve mollusks. J. Gemmol. 1994, 24, 241–252. [Google Scholar] [CrossRef]
- Karampelas, S.; Kiefert, L. Gemstones and minerals. In Analytical Archaeometry: Selected Topics, 1st ed.; Edwards, H.G.M., Vandenabeele, P., Eds.; Royal Society of Chemistry: London, UK, 2012; pp. 291–317. ISBN 978-1-84973-162-1. [Google Scholar]
- Zhou, C.; Hodgins, G.; Lange, T.; Saruwatari, K.; Sturman, N.; Kiefert, L.; Schollenbruch, K. Saltwater pearls from the pre- to early Columbian era: A gemological and radiocarbon dating study. Gems Gemol. 2017, 53, 286–295. [Google Scholar] [CrossRef]
- Goebel, M.; Dirlam, D.M. Polynesian black pearls. Gems Gemol. 1989, 25, 130–148. [Google Scholar] [CrossRef]
- Elen, S. Spectral reflectance and fluorescence characteristics of natural-color and heat-treated “Golden” south sea cultured pearls. Gems Gemol. 2001, 37, 114–123. [Google Scholar] [CrossRef]
- Jacob, D.E.; Wehrmeister, U.; Hager, T.; Hofmeister, W. Identifying Japanese freshwater cultured pearls from Lake Kasumigaura. J. Gemmol. 2006, 22, 539–541. [Google Scholar]
- Scarratt, K.; Bracher, P.; Bracher, M.; Atawi, A.; Safar, A.; Saeseaw, S.; Homkrajae, A.; Sturman, N. Natural pearls from Australian Pinctada maxima. Gems Gemol. 2012, 48, 236–261. [Google Scholar] [CrossRef]
- Hänni, H.A.; Cartier, L.E. Tracing cultured pearls from farm to consumer: A review of potential methods and solutions. J. Gemmol. 2013, 33, 185–191. [Google Scholar] [CrossRef]
- Sturman, N.; Homkrajae, A.; Manustrong, A.; Somsaard, N. Observations on pearls reportedly from the Pinnidae family (pen pearls). Gems Gemol. 2014, 50, 202–215. [Google Scholar] [CrossRef]
- Homkrajae, A.; Sun, Z.; Blodgett, T.; Zhou, C. Provenance discrimination of freshwater pearls by laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) and linear discriminant analysis (LDA). Gems Gemol. 2019, 55, 47–60. [Google Scholar]
- Perkins, W.T.; Fuge, R.; Pearce, N.J.G. Quantitative analysis of trace elements in carbonates using laser ablation inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectr. 1991, 6, 445–449. [Google Scholar] [CrossRef]
- Sinclair, D.J.; Kinsley, L.P.J.; McCulloch, M.T. High resolution analysis of trace elements in corals by laser-ablation ICP-MS. Geochim. Cosmochim. Acta 1998, 62, 1889–1901. [Google Scholar] [CrossRef]
- Gillikin, D.P. Geochemistry of Marine Bivalve Shells: The Potential for Paleoenvironmental Reconstruction. Ph.D. Thesis, Vrije University, Brussel, Belgium, 2005; 265p. [Google Scholar]
- Jacob, D.E.; Soldati, A.L.; Wirth, R.; Huth, J.; Wehrmeister, U.; Hofmeister, W. Nanostructure, composition and mechanisms of bivalve shell growth. Geochim. Cosmochim. Acta 2009, 72, 5401–5415. [Google Scholar] [CrossRef]
- Mertz-Kraus, R.; Brachert, T.C.; Jochum, K.P.; Reuter, M.; Stoll, B. LA-ICP-MS analyses on coral growth increments reveal heavy winter rain in the eastern Mediterranean at 9 Ma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 273, 25–40. [Google Scholar] [CrossRef]
- Phung, A.T.; Baeyens, W.; Leermakers, M.; Goderis, S.; Vanhaecke, F.; Gao, Y. Reproducibility of laser ablation–inductively coupled plasma–mass spectrometry (LA–ICP–MS) measurements in mussel shells and comparison with micro-drill sampling and solution ICP–MS. Talanta 2013, 396, 42–50. [Google Scholar] [CrossRef]
- Strasser, C.A.; Mullineaux, L.S.; Walther, B.D. Growth rate and age effects on Mya Arenaria shell chemistry: Implications for biogeochemical studies. J. Exp. Mar. Biol. Ecol. 2008, 355, 153–163. [Google Scholar] [CrossRef]
- Jochum, K.P.; Scholz, D.; Stoll, B.; Weis, U.; Wilson, S.A.; Yang, Q.; Schwalb, A.; Borner, N.; Jacob, D.E.; Andreae, M.O. Accurate trace element analysis of speleothems and biogenic calcium carbonates by LA-ICP-MS. Chem. Geol. 2012, 318–319, 31–44. [Google Scholar] [CrossRef]
- Gordon, C.M.; Carr, R.A.; Larson, R.E. The influence of environmental factors on the sodium and manganese content of barnacle shells. Limnol. Oceanogr. 1970, 15, 461–466. [Google Scholar] [CrossRef]
- Soldati, A.L.; Jacob, D.E.; Glatzel, P.; Swarbrick, J.C.; Geck, J. Element substitution by living organisms: The case of manganese in mollusc shell aragonite. Sci. Rep. 2016, 6, 22514. [Google Scholar] [CrossRef] [PubMed]
- Geeza, T.J.; Gillikin, D.P.; Goodwin, D.H.; Evans, S.D.; Watters, T.; Warner, N.R. Controls on magnesium, manganese, strontium, and barium concentrations recorded in freshwater mussel shells from Ohio. Chem. Geol. 2019. [Google Scholar] [CrossRef]
- Carroll, M.; Romanek, C.S. Shell layer variation in trace element concentration for the freshwater bivalve Elliptio complanta. Geo-Mar. Lett. 2008, 28, 369–381. [Google Scholar] [CrossRef]
- Kelemen, Z.; Gillikin, D.P.; Bouillon, S. Relationship between river water chemistry and shell chemistry of two tropical African freshwater bivalve species. Chem. Geol. 2019. [Google Scholar] [CrossRef]
- Poulain, C.; Gillikin, D.P.; Thebault, J.; Munaron, J.M.; Bohn, M.; Robert, R.; Paulet, Y.M.; Lorrain, A. An evaluation of Mg/Ca, Sr/Ca, and Ba/Ga ratios as environmental proxies bivalve shells. Chem. Geol. 2015, 396, 42–50. [Google Scholar] [CrossRef]
- Wanamaker, D.; Gillikin, D.P. Strontium, magnesium and barium incorporation in aragonitic shells of junevile Arctica islandica: Insights from temperature controlled experiments. Chem. Geol. 2019. [Google Scholar] [CrossRef]
- Stecher, H.A.; Krantz, D.E.; Lord, C.J.; Luther, G.W.; Bock, K.W. Profiles of strontium and barium in Mercenaria mercenaria and Spisula solidisima shells. Geochim. Cosmochim. Acta 1996, 60, 3445–3456. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Tipton, I.H.; Nason, A.P. Trace metals in man: Strontium and barium. J. Chronic Dis. 1972, 25, 491–517. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Dehairs, F.; Lorrain, A.; Steenmans, D.; Baeyens, W.; Andre, L. Barium uptake into the shells of the common mussel (Mytilus edulis) and the potentials for estuarine paleo-chemistry reconstruction. Geochim. Cosmochim. Acta 2006, 70, 395–407. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Lorrain, A.; Paulet, Y.M. Synchronous barium peaks in high-resolution profiles of calcite and aragonite marine bivalve shells. Geo-Mar. Lett. 2008, 28, 351–358. [Google Scholar] [CrossRef]
- Zhao, L.; Schone, B.R.; Mertz-Kraus, R.; Yang, F. Controls on strontium and barium incorporation into freshwater bivalve shells (Corbicula fluminea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 465, 386–394. [Google Scholar] [CrossRef]
- Zhao, L.; Schone, B.R.; Mertz-Kraus, R. Sodium provides unique insights into transgenerational effects of ocean acidification on bivalve shell formation. Sci. Total Environ. 2017, 577, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Herath, D.; Jacob, D.E.; Jones, H.; Fallon, S.J. Potential of shells of three species of Eastern Australian freshwater mussels (Bivalvia: Hyriidae) as environmental proxy archives. Mar. Freshw. Res. 2018, 70, 255–269. [Google Scholar] [CrossRef]
- Foster, L.C.; Finch, A.A.; Allison, N.; Andersson, C.; Clarke, L.J. Mg in aragonitic bivalve shells: Seasonal variations and mode of incorporation in Arctica islandica. Chem. Geol. 2008, 254, 113–119. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Dehairs, F.; Baeyens, W.; Navez, J.; Lorrain, A.; André, L. Inter- and intra-annual variations of Pb/Ca ratios in clam shells (Mercenaria mercenaria): A record of anthropogenic lead pollution. Mar. Pollut. Bull. 2005, 50, 1530–1540. [Google Scholar] [CrossRef]
- Collins, D. Thousands of Pearls Found in Shipwreck. Available online: https://www.cbsnews.com/news/thousands-of-pearls-found-in-shipwreck/ (accessed on 20 March 2019).
- Sommer, S.E. Cathodoluminescence of carbonates 1. Characterization of cathodoluminescence from carbonate solid solutions. Chem. Geol. 1972, 9, 257–273. [Google Scholar] [CrossRef]
- Götte, T.; Richter, D.K. Quantitative aspects of Mn activated cathodoluminescence of natural and synthetic aragonite. Sedimentology 2009, 56, 483–492. [Google Scholar] [CrossRef]
- Hainschwang, T.; Karampelas, S.; Fritsch, E.; Notari, F. Luminescence spectroscopy and microscopy applied to study gem materials: A case study of C centre containing diamonds. Mineral. Petrol. 2013, 107, 393–413. [Google Scholar] [CrossRef]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Häger, T.; Hofmeister, W. Vaterite in freshwater cultured pearls from China and Japan. J. Gemmol. 2007, 31, 269–276. [Google Scholar] [CrossRef]
- Qiao, L.; Feng, Q.L.; Li, Z. Special vaterite in freshwater lackluster pearls. Cryst. Growth Des. 2006, 7, 275–279. [Google Scholar] [CrossRef]
- Ma, H.; Su, A.; Zhang, B.; Li, R.K.; Zhou, L.; Wang, B. Vaterite or aragonite observed in the prismatic layer of freshwater-cultured pearls from South China. Prog. Nat. Sci. 2009, 19, 817–820. [Google Scholar] [CrossRef]

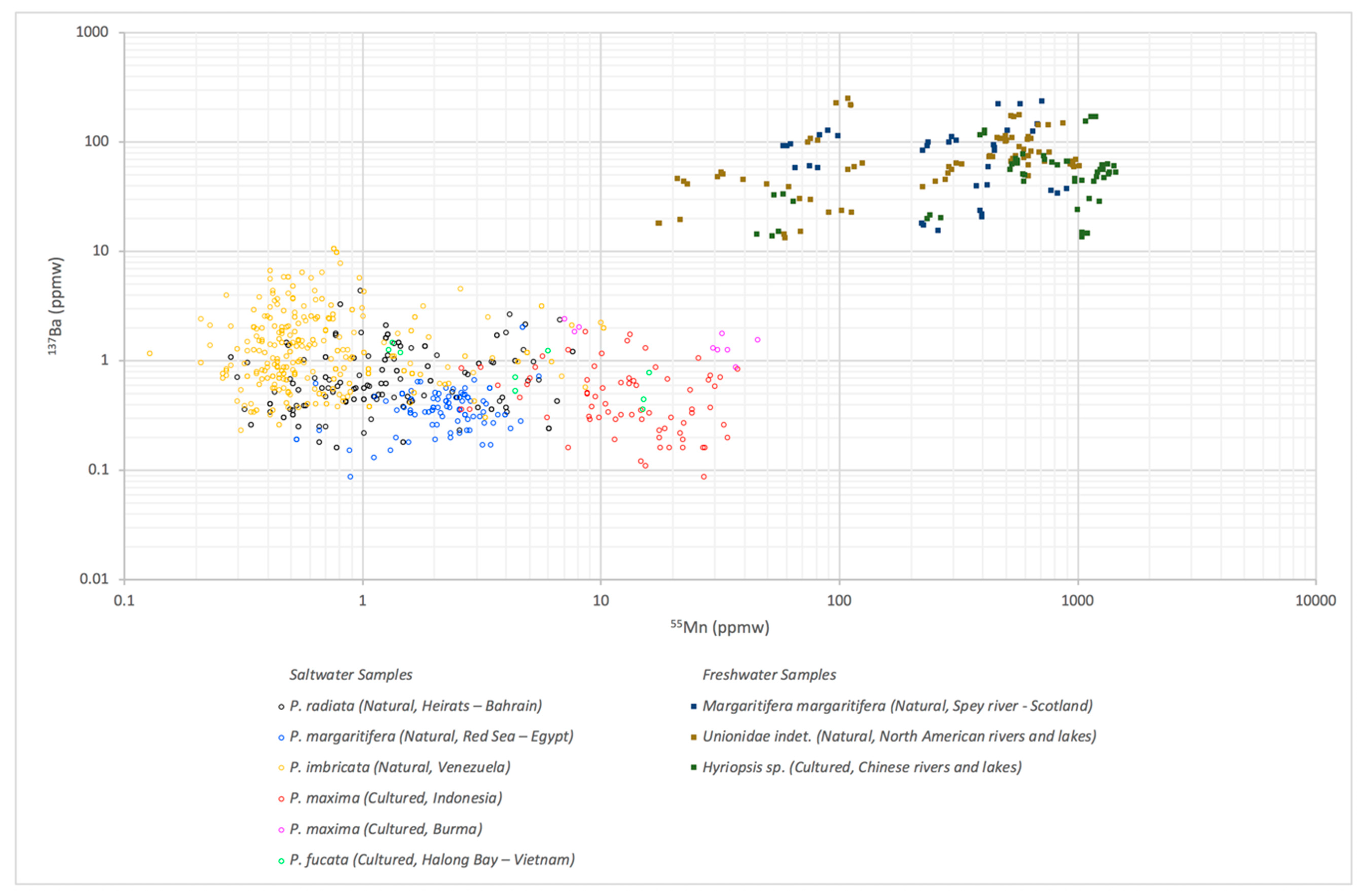
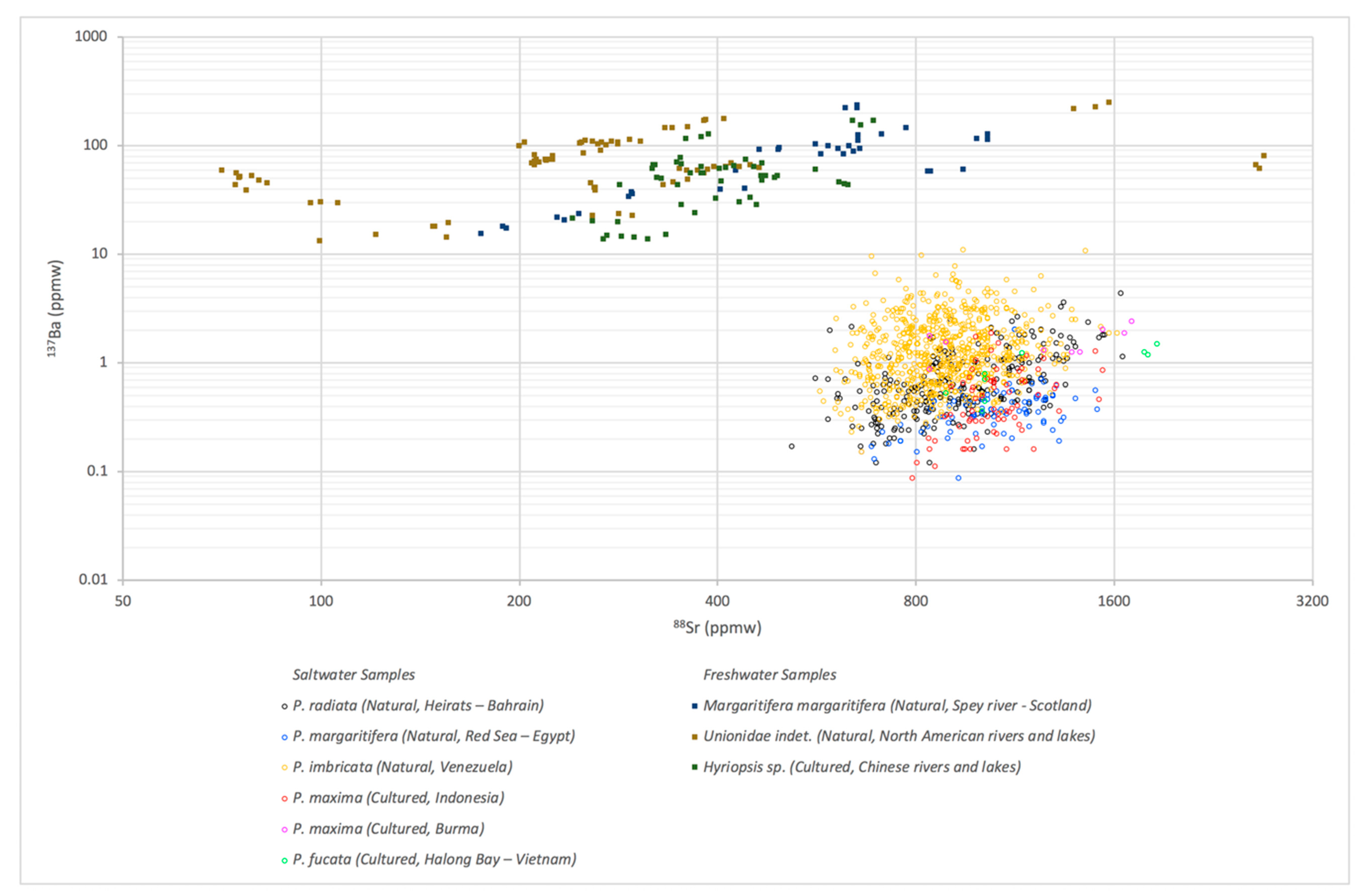
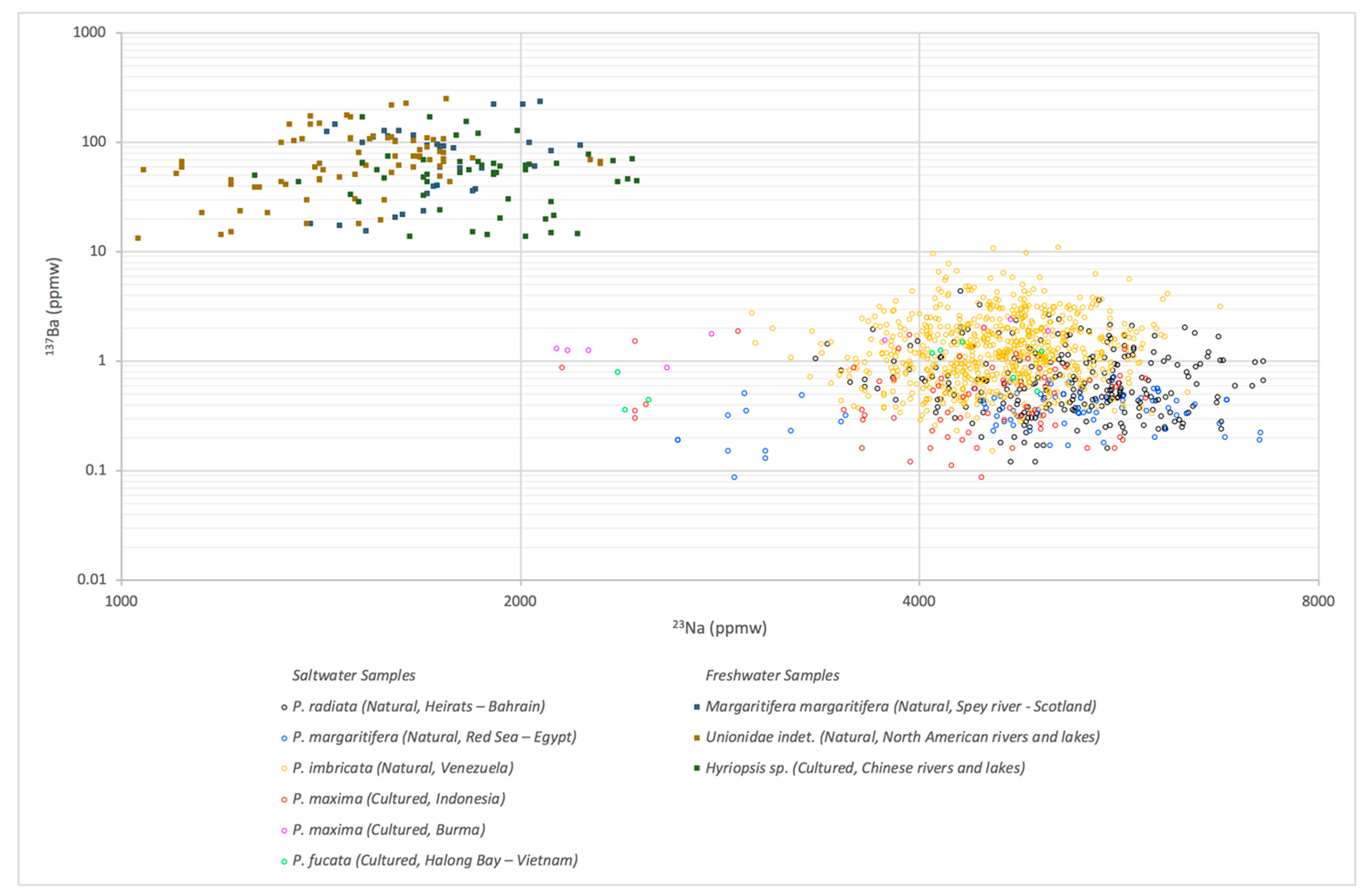
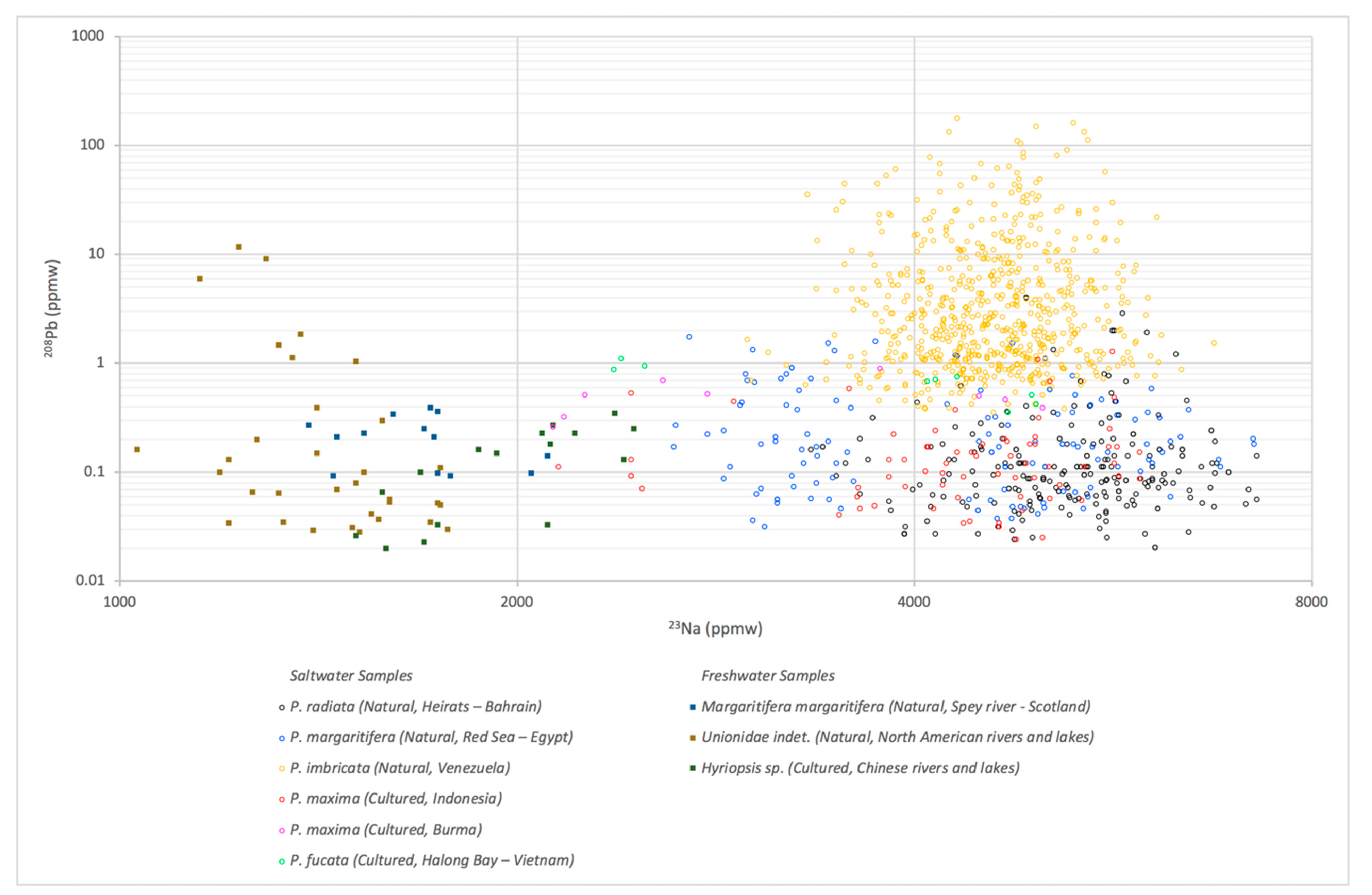
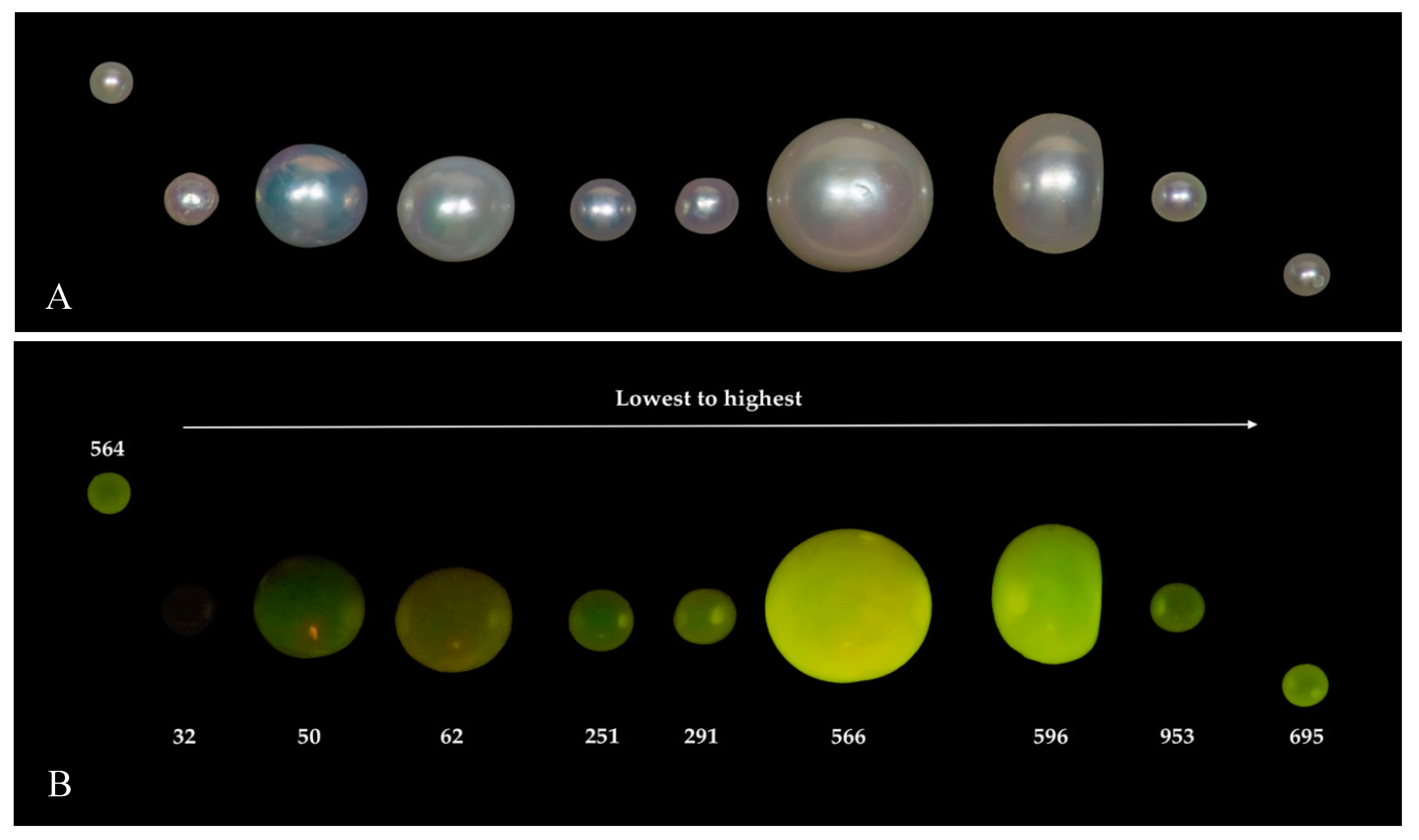
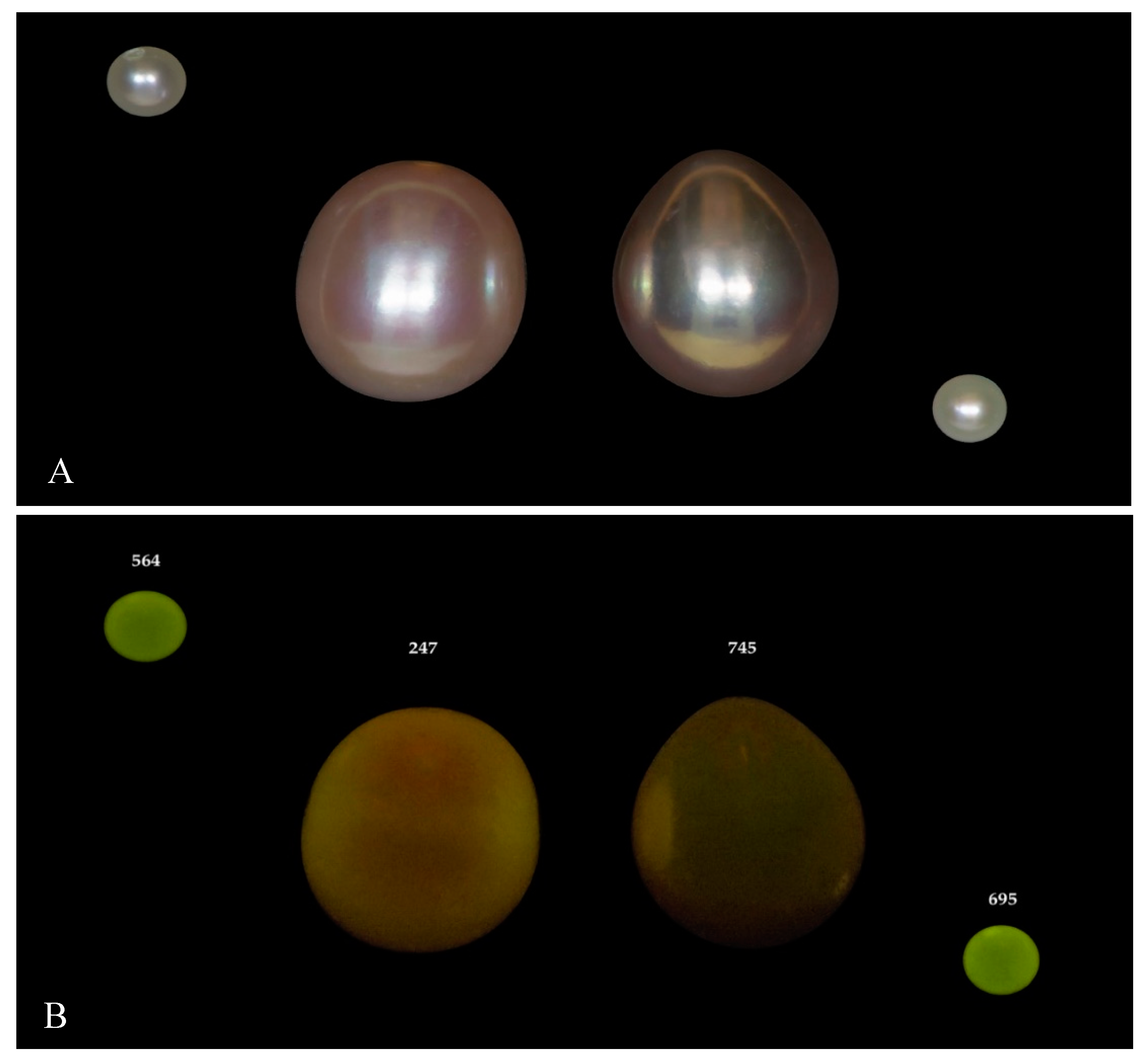
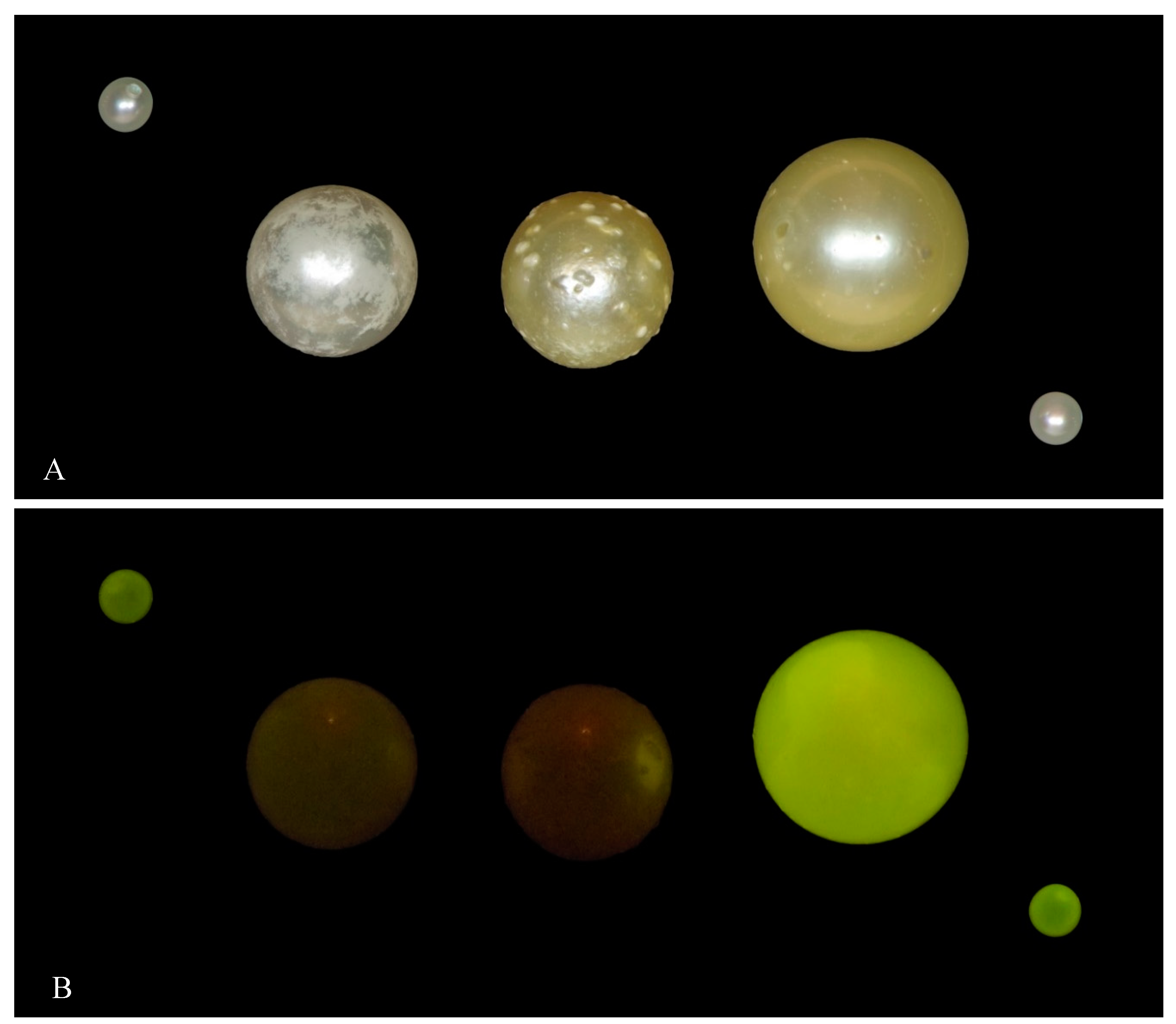
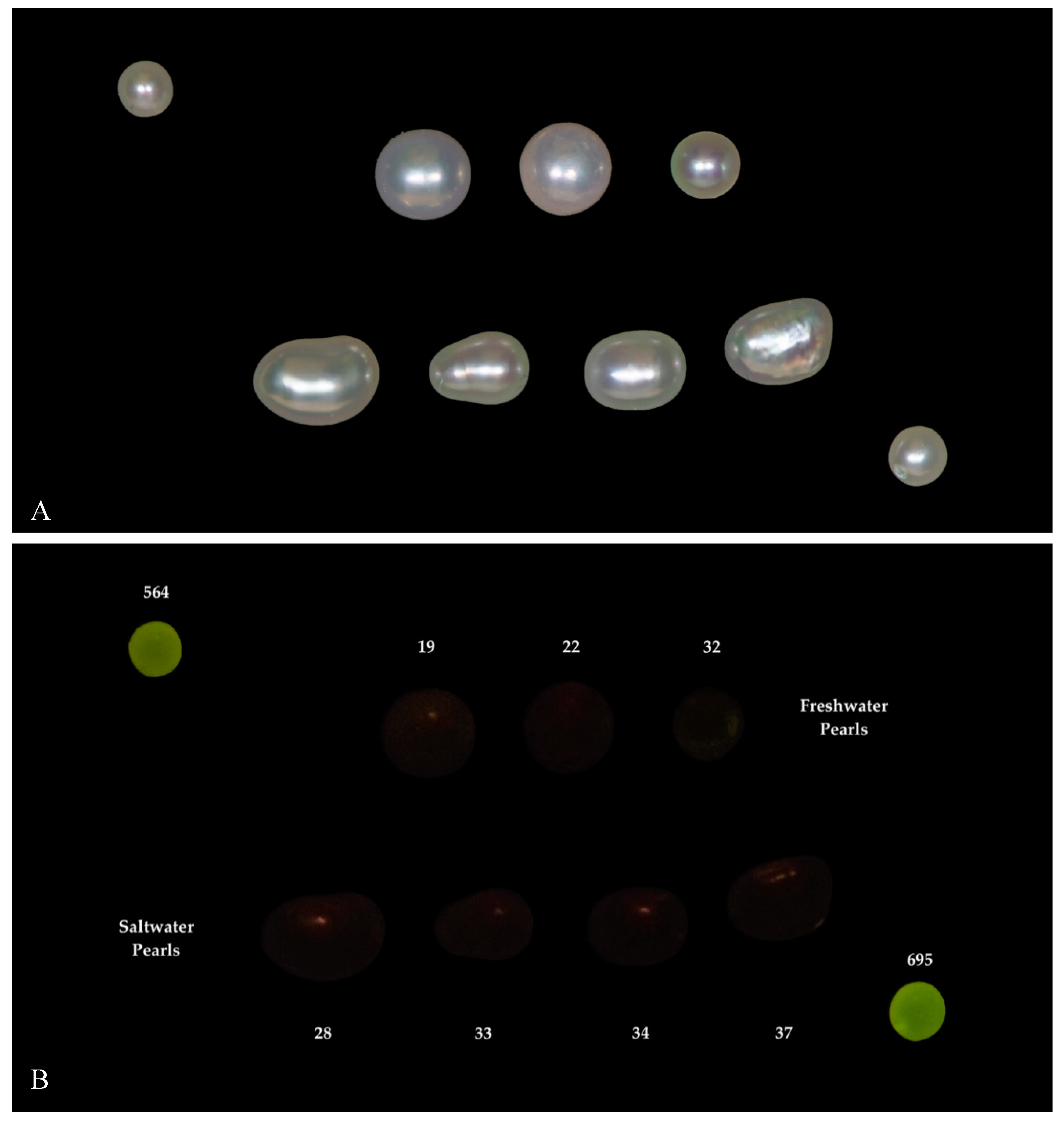
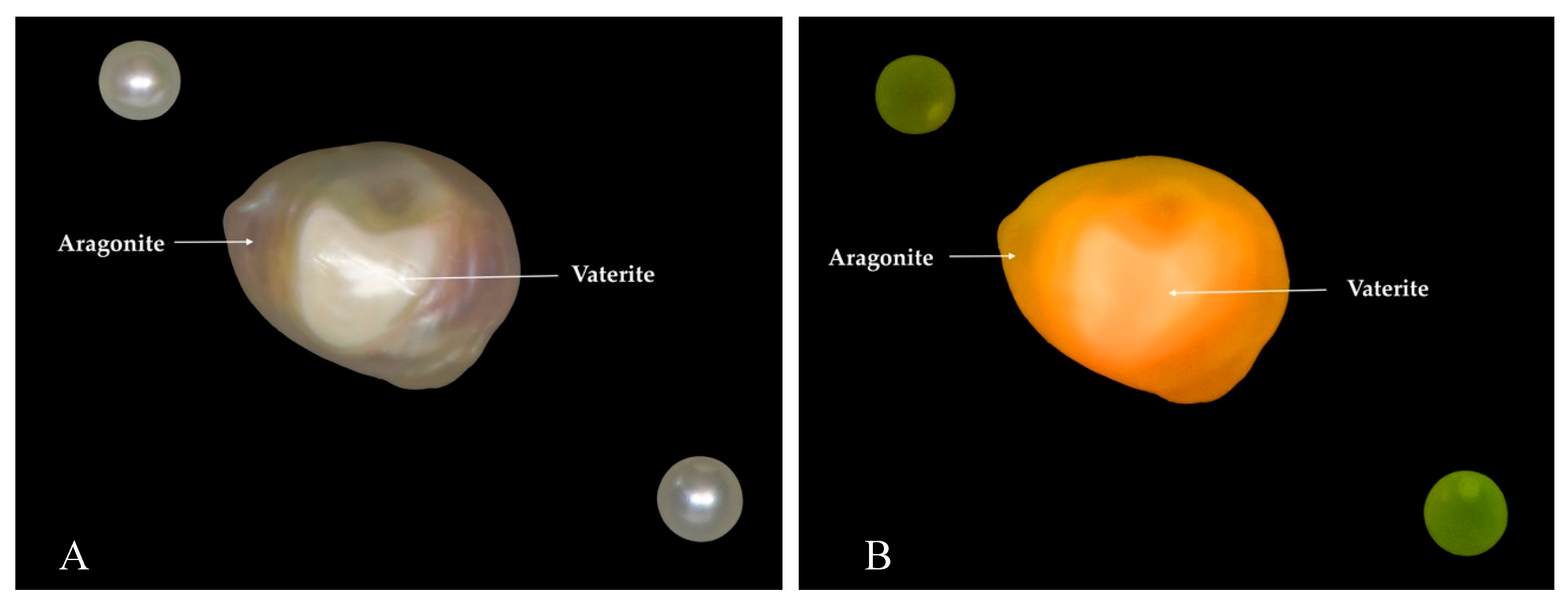
| Environment | Bivalve | Area | No. of Samples | |
|---|---|---|---|---|
| Saltwater | P. radiata * | Arabian Gulf (Heirats, Bahrain) | 248 **** | 1058 |
| P. margaritifera * | Red Sea (Hurghada, Egypt) | 80 (****) | ||
| P. imbricata * | Venezuela | 671 **** | ||
| P. maxima **/*** | Indonesia | 53 (****) | ||
| P. maxima *** | Burma | 3 | ||
| P. fucata *** | Vietnam (Halong Bay) | 3 | ||
| Freshwater | Margaritifera margaritifera * | Scotland (Spey river) | 12 | 55 |
| Unionidae indet. * | North American rivers | 26 | ||
| Hyriopsis sp. *** | Chinese rivers and lakes | 17 | ||
| Limits | 23Na (ppmw) | 24Mg (ppmw) | 55Mn (ppmw) | 88Sr (ppmw) | 137Ba (ppmw) | 208Pb (ppmw) |
|---|---|---|---|---|---|---|
| LOD | 1.62–70.84 | 0.02–0.57 | 0.09–0.65 | 0.01–0.05 | 0.01–0.39 | 0.01–0.02 |
| LOQ | 5.35–233.77 | 0.66–1.88 | 0.21–1.18 | 0.03-0.16 | 0.17–1.18 | 0.02–0.07 |
| Samples | Element | Min–Max (ppmw) | Average (SD) (ppmw) | Median (ppmw) |
|---|---|---|---|---|
| Saltwater | 23Na | 2130–7270 | 4711.27 (816.6) | 4720 |
| 24Mg | 29.5–950 | 241.29 (111.13) | 240 | |
| 55Mn | BQL–45.7 | 1.84 (5.01) | BQL | |
| 88Sr | 518–1860 | 941.2 (197.31) | 912 | |
| 137Ba | BQL–11 | 1.18 (1.17) | 0.84 | |
| 208Pb | BQL–177 | 5.18 (14.88) | 0.95 | |
| Freshwater | 23Na | 1030–2450 | 1672.81 (303.89) | 1680 |
| 24Mg | 5.58–81.3 | 30.87 (16.45) | 28.3 | |
| 55Mn | 17.4–1440 | 504.95 (386.34) | 473 | |
| 88Sr | 70.6–2700 | 431.84 (389.41) | 350 | |
| 137Ba | 13.2–249 | 72.76 (48.25) | 61.6 | |
| 208Pb | BQL–11.6 | 0.24 (1.24) | BQL |
| Samples | Element | Min–Max (ppmw) | Average (SD) (ppmw) | Median (ppmw) |
|---|---|---|---|---|
| P. radiata (Heirats, Bahrain) Natural | 23Na | 3340–7270 | 5249.52 (800.85) | 5250 |
| 24Mg | 29.5–477 | 147.45 (88.18) | 131 | |
| 55Mn | BQL–7.62 | 0.79 (1.38) | BQL | |
| 88Sr | 518–1650 | 943.9 (219.42) | 902 | |
| 137Ba | BQL–4.32 | 0.78 (0.6) | 0.58 | |
| 208Pb | BQL–3.91 | 0.17 (0.4) | 0.08 | |
| P. margaritifera (Red Sea, Egypt) Natural | 23Na | 2630–7240 | 4442.05 (1204.07) | 4595 |
| 24Mg | 67.6–675 | 362.72 (109.6) | 365 | |
| 55Mn | BQL–5.5 | 2.24 (1) | 2.31 | |
| 88Sr | 663–1560 | 1052.57 (180.98) | 1030 | |
| 137Ba | BQL–2.02 | 0.26 (0.26) | 0.28 | |
| 208Pb | BQL–1.74 | 0.3 (0.36) | 0.17 | |
| P. imbricata (Venezuela) Natural | 23Na | 3200–6240 | 4621.64 (564.29) | 4600 |
| 24Mg | 108–531 | 264.34 (72.01) | 258 | |
| 55Mn | BQL–10.3 | 0.33 (0.95) | BQL | |
| 88Sr | 572–1620 | 898.07 (164.56) | 880 | |
| 137Ba | BQL–11 | 1.56 (1.32) | 1.18 | |
| 208Pb | 0.35–177 | 8.58 (18.68) | 2.42 | |
| P. maxima (Indonesia) Cultured | 23Na | 2150–5930 | 4430.28 (860.33) | 4460 |
| 24Mg | 71.1–279 | 125.76 (55.38) | 110 | |
| 55Mn | 2.6–37.4 | 15.37 (8.65) | 13.5 | |
| 88Sr | 791–1540 | 1061.54 (151.92) | 1050 | |
| 137Ba | 0.09–1.85 | 0.53 (0.38) | 0.38 | |
| 208Pb | BQL–1.27 | 0.17 (0.22) | 0.11 | |
| P. maxima (Burma) Cultured | 23Na | 2130–5000 | 3317.78 (1170.44) | 2790 |
| 24Mg | 107–172 | 139.67 (23.58) | 139 | |
| 55Mn | 7.04–45.7 | 25.82 (14.42) | 30.90 | |
| 88Sr | 840–1700 | 1279.78 (364.37) | 1380 | |
| 137Ba | 0.86–2.39 | 1.58 (0.47) | 1.55 | |
| 208Pb | 0.2–1.16 | 0.43 (0.32) | 0.3 | |
| P. fucata (Halong Bay, Vietnam) Cultured | 23Na | 2370–4950 | 3821.11 (1092.03) | 4150 |
| 24Mg | 237–950 | 476.11 (298.01) | 306 | |
| 55Mn | 1.29–15.9 | 7.2 (6.31) | 4.38 | |
| 88Sr | 888–1860 | 1284.22 (403.18) | 1020 | |
| 137Ba | 0.36–1.47 | 0.88 (0.41) | 0.78 | |
| 208Pb | 0.35–1.08 | 0.7 (0.24) | 0.70 | |
| Margaritifera margaritifera (Spey river, Scotland) Natural | 23Na | 1390–2220 | 1733.61 (202.26) | 1725 |
| 24Mg | 11.1–60.6 | 31.37 (11.79) | 29.15 | |
| 55Mn | 58–896 | 355.25 (234.92) | 343.5 | |
| 88Sr | 175–1030 | 559.94 (244.16) | 599 | |
| 137Ba | 15.30–233 | 85.96 (56.2) | 90.45 | |
| 208Pb | BQL–0.39 | 0.08 (0.12) | BQL | |
| Unionidae indet. (North American rivers and lakes) Natural | 23Na | 1030–2300 | 1519.48 (BQL) | 1510 |
| 24Mg | 9.21–81.3 | 34.06 (16.96) | 33.45 | |
| 55Mn | 17.40–1020 | 382.07 (298.5) | 427.5 | |
| 88Sr | 70.6–2700 | 389.12 (527.14) | 260.5 | |
| 137Ba | 13.2–249 | 77.04 (48.49) | 65.9 | |
| 208Pb | BQL–11.6 | 0.44 (1.79) | BQL | |
| Hyriopsis sp. (Chinese rivers and lakes) Cultured | 23Na | 1260–2450 | 1880.39 (BQL) | 1870 |
| 24Mg | 5.58–76.9 | 25.65 (17.51) | 19.20 | |
| 55Mn | 45–1440 | 798.54 (431.41) | 898 | |
| 88Sr | 241–690 | 406.75 (111.39) | 378 | |
| 137Ba | 13.6–169 | 56.88 (37.33) | 52 | |
| 208Pb | BQL–0.35 | 0.04 (0.09) | BQL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampelas, S.; Mohamed, F.; Abdulla, H.; Almahmood, F.; Flamarzi, L.; Sangsawong, S.; Alalawi, A. Chemical Characteristics of Freshwater and Saltwater Natural and Cultured Pearls from Different Bivalves. Minerals 2019, 9, 357. https://doi.org/10.3390/min9060357
Karampelas S, Mohamed F, Abdulla H, Almahmood F, Flamarzi L, Sangsawong S, Alalawi A. Chemical Characteristics of Freshwater and Saltwater Natural and Cultured Pearls from Different Bivalves. Minerals. 2019; 9(6):357. https://doi.org/10.3390/min9060357
Chicago/Turabian StyleKarampelas, Stefanos, Fatima Mohamed, Hasan Abdulla, Fatema Almahmood, Latifa Flamarzi, Supharart Sangsawong, and Abeer Alalawi. 2019. "Chemical Characteristics of Freshwater and Saltwater Natural and Cultured Pearls from Different Bivalves" Minerals 9, no. 6: 357. https://doi.org/10.3390/min9060357
APA StyleKarampelas, S., Mohamed, F., Abdulla, H., Almahmood, F., Flamarzi, L., Sangsawong, S., & Alalawi, A. (2019). Chemical Characteristics of Freshwater and Saltwater Natural and Cultured Pearls from Different Bivalves. Minerals, 9(6), 357. https://doi.org/10.3390/min9060357





