Recovery of Alkali from Bayer Red Mud Using CaO and/or MgO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Analytical Methods
3. Results and Discussion
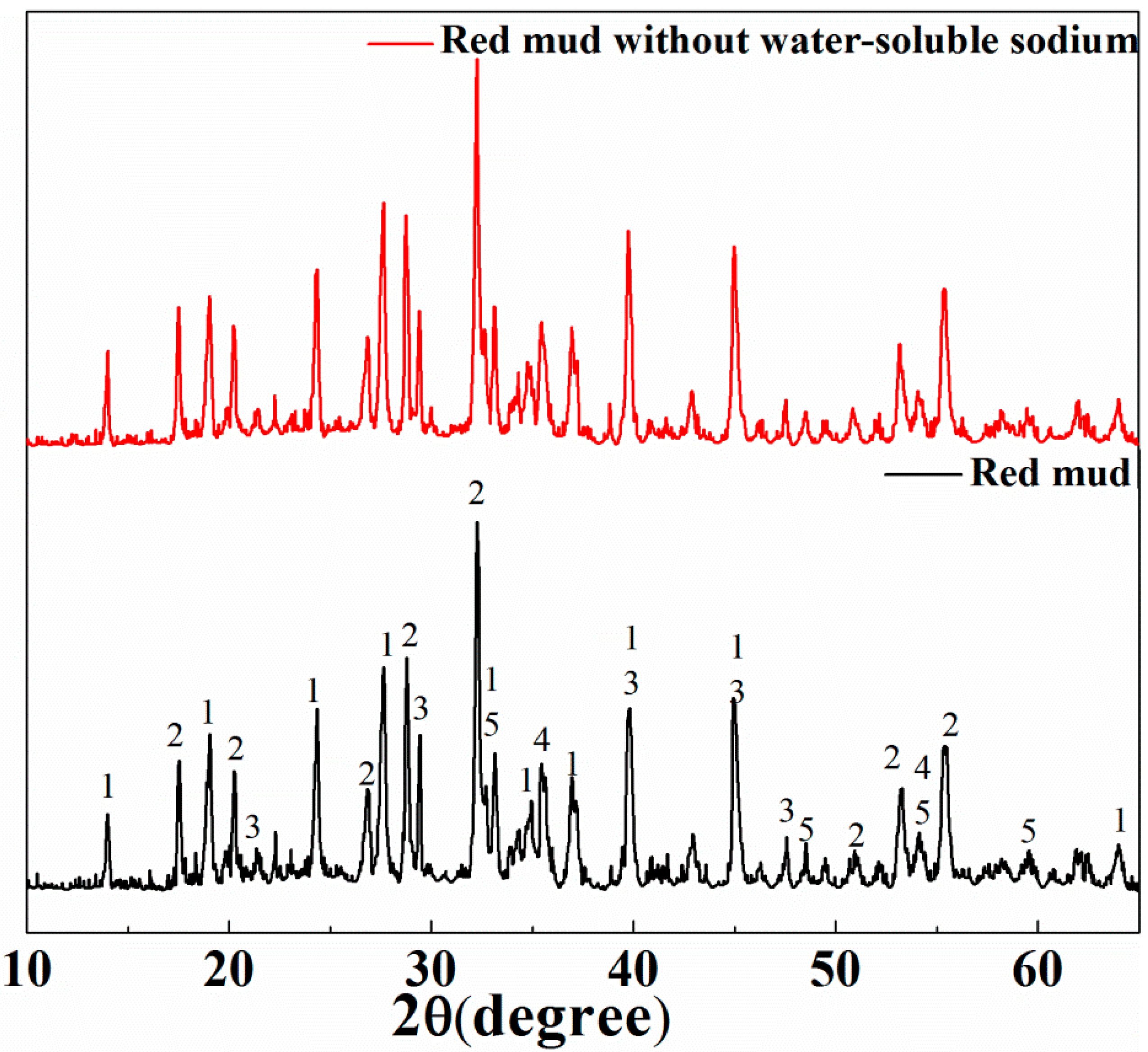
3.1. Characterization of Bayer Red Mud
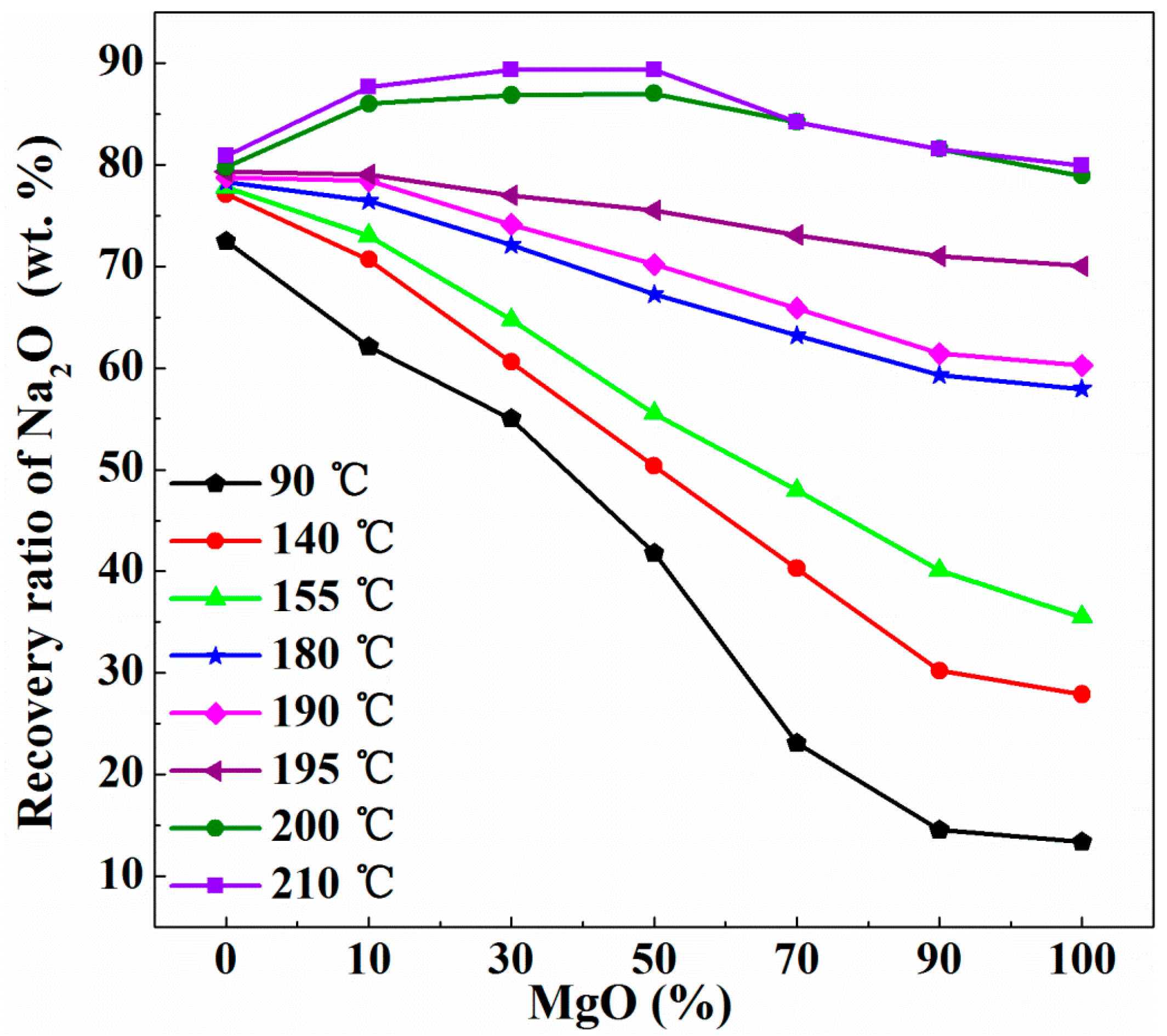
3.2. Recovery of Na2O Using CaO/MgO
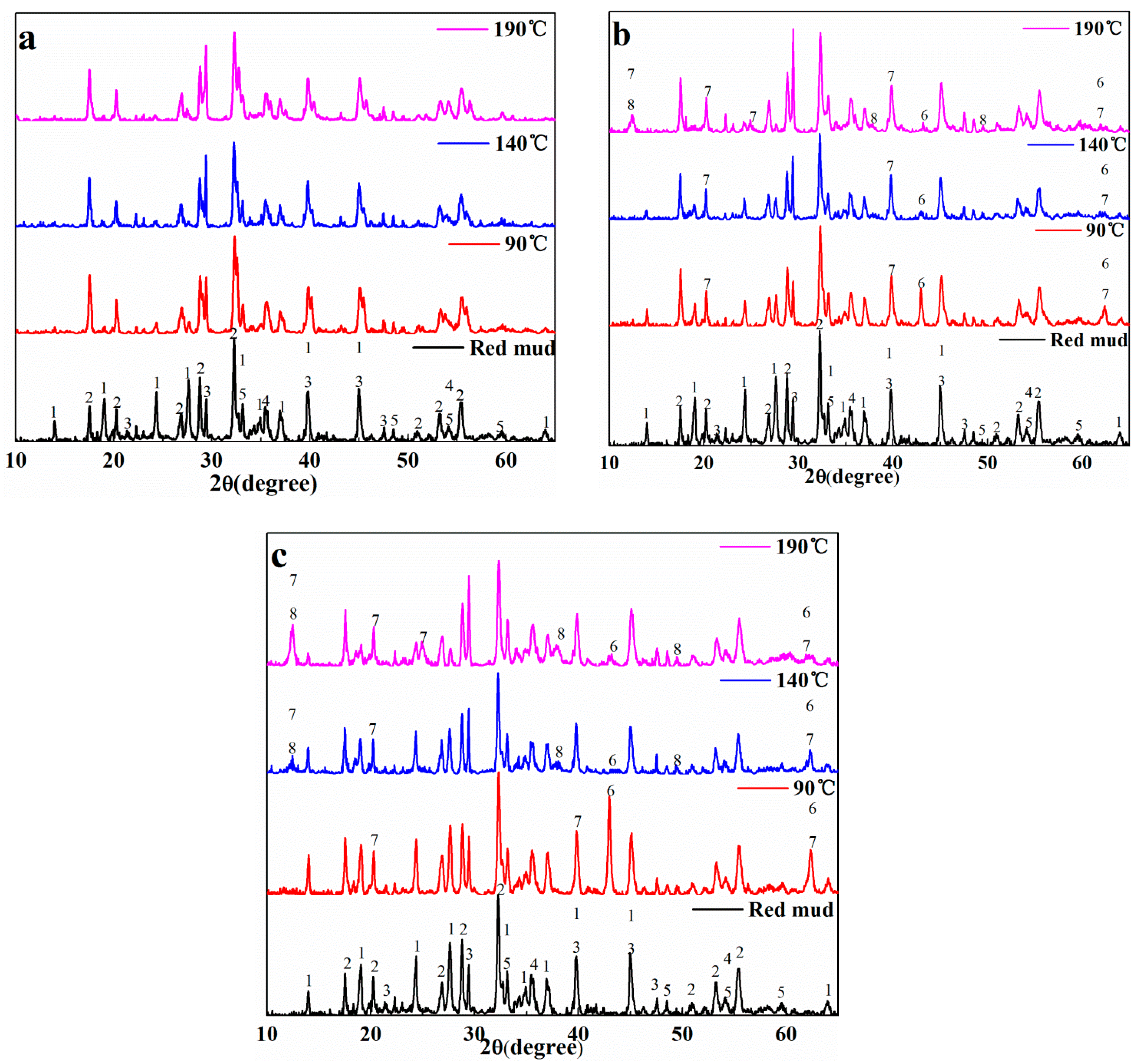
3.3. Phase Differences of CaO/MgO Substituting Na2O
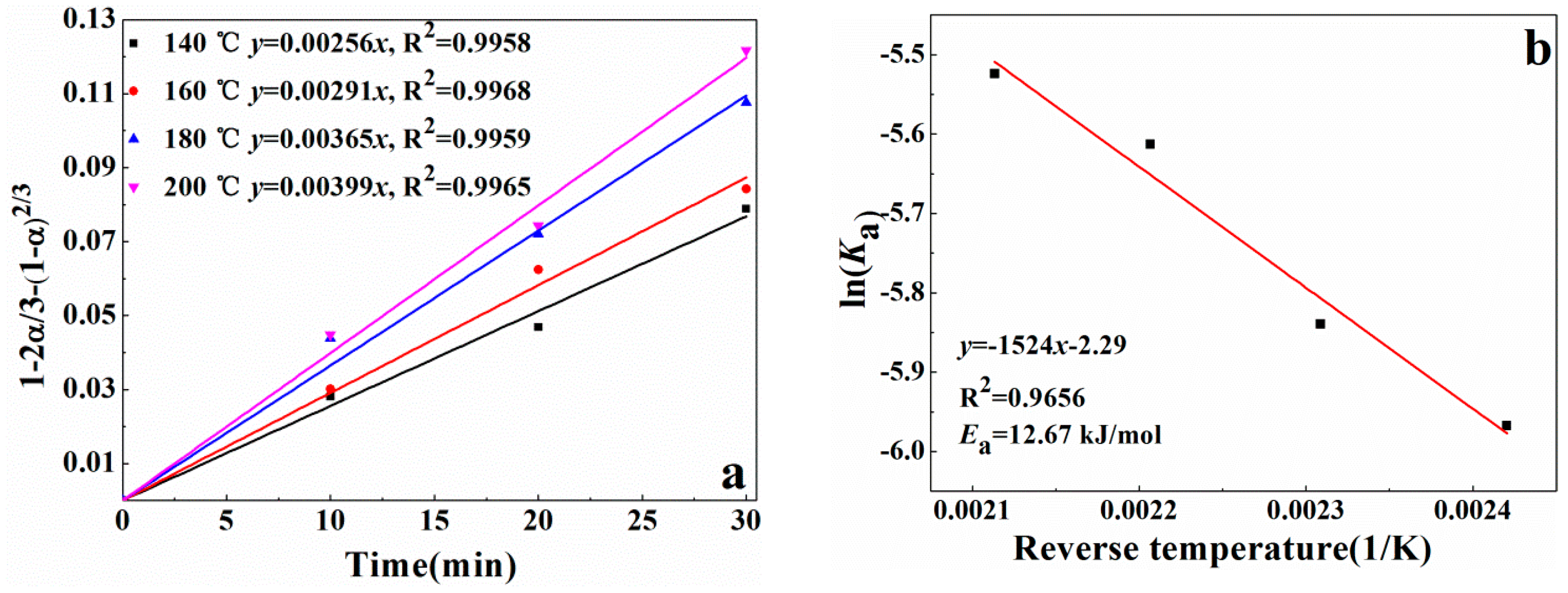
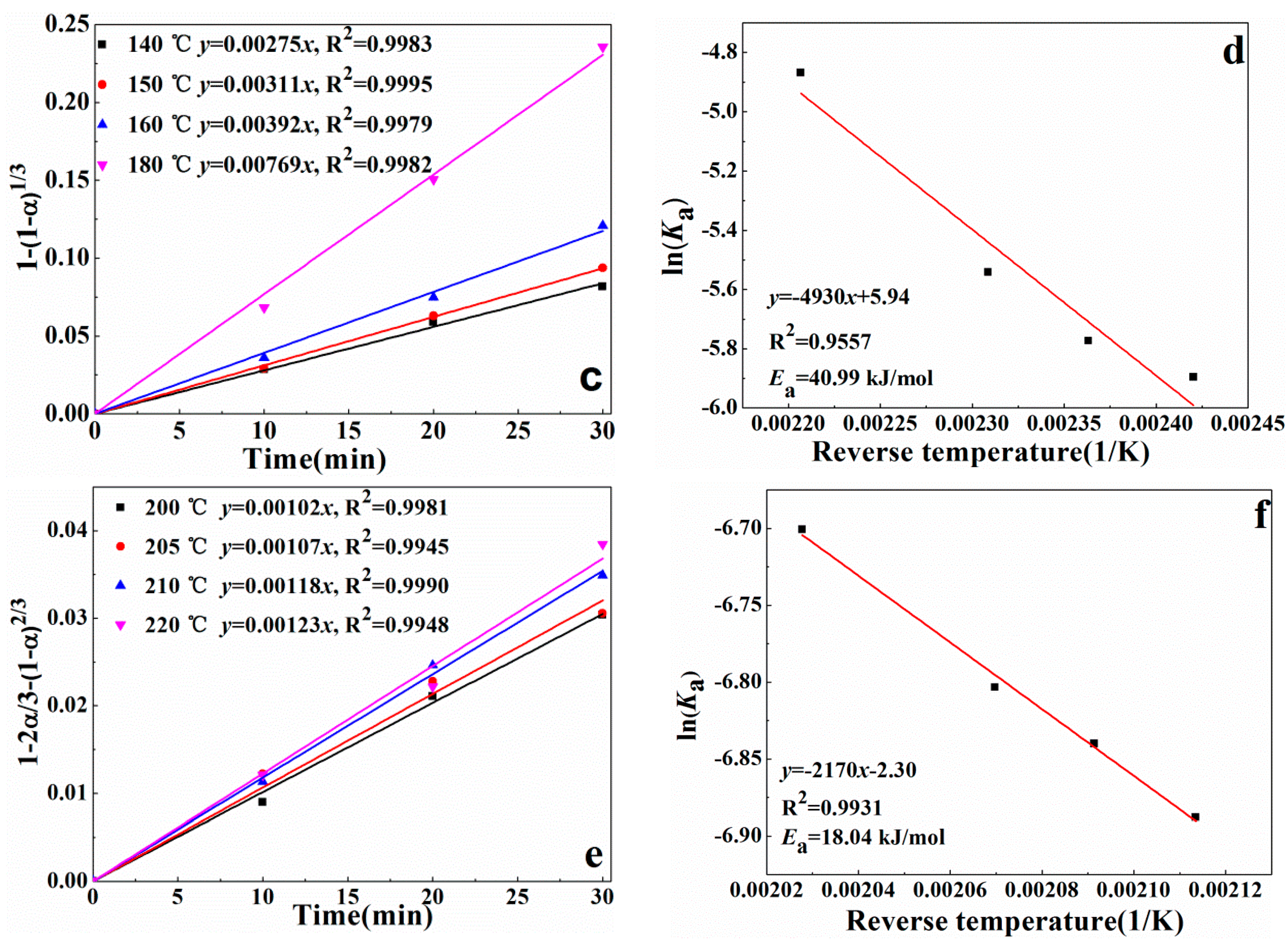
3.4. Reaction Kinetics of Alkali Recovery
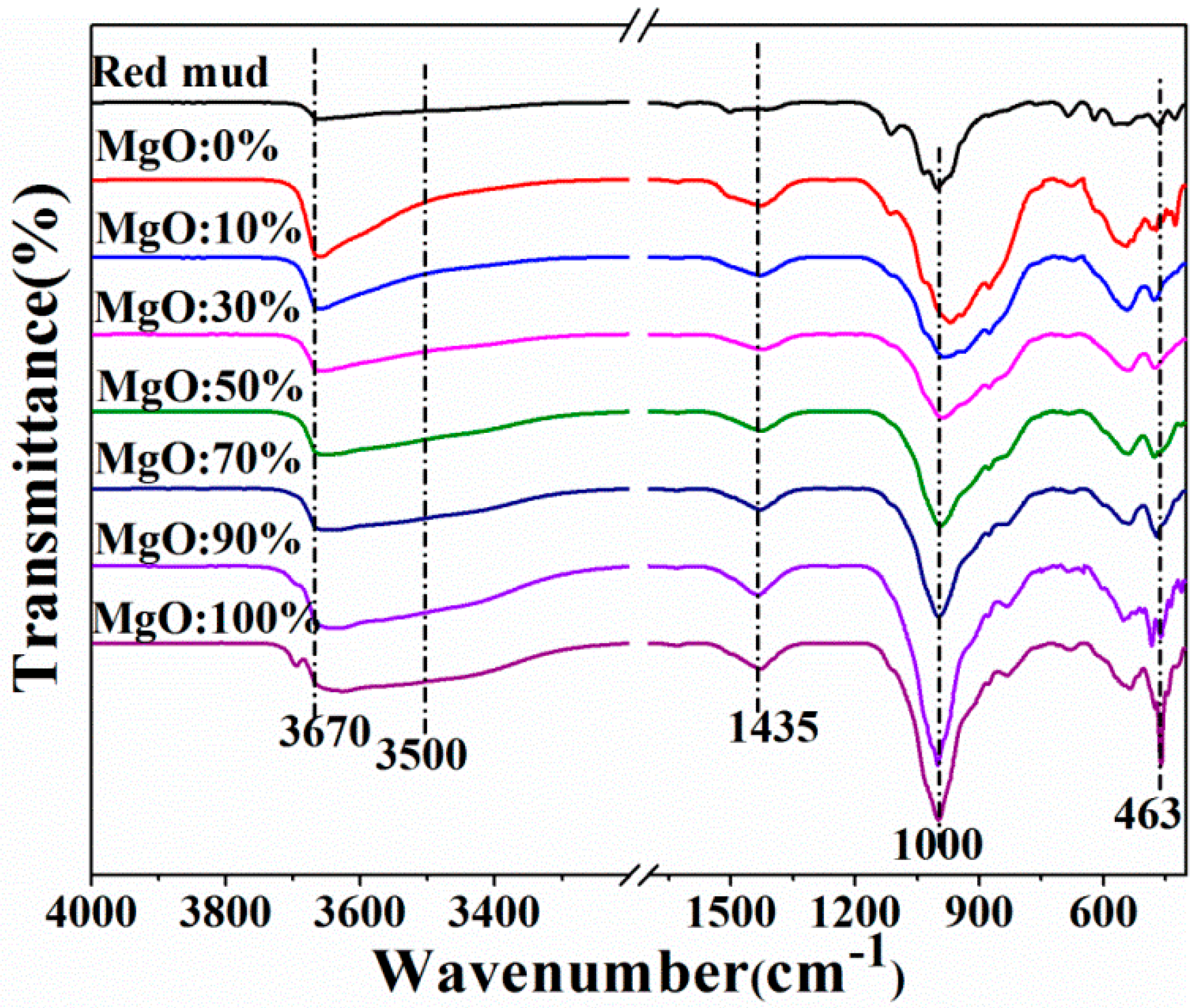
3.5. FTIR Spectra Analysis
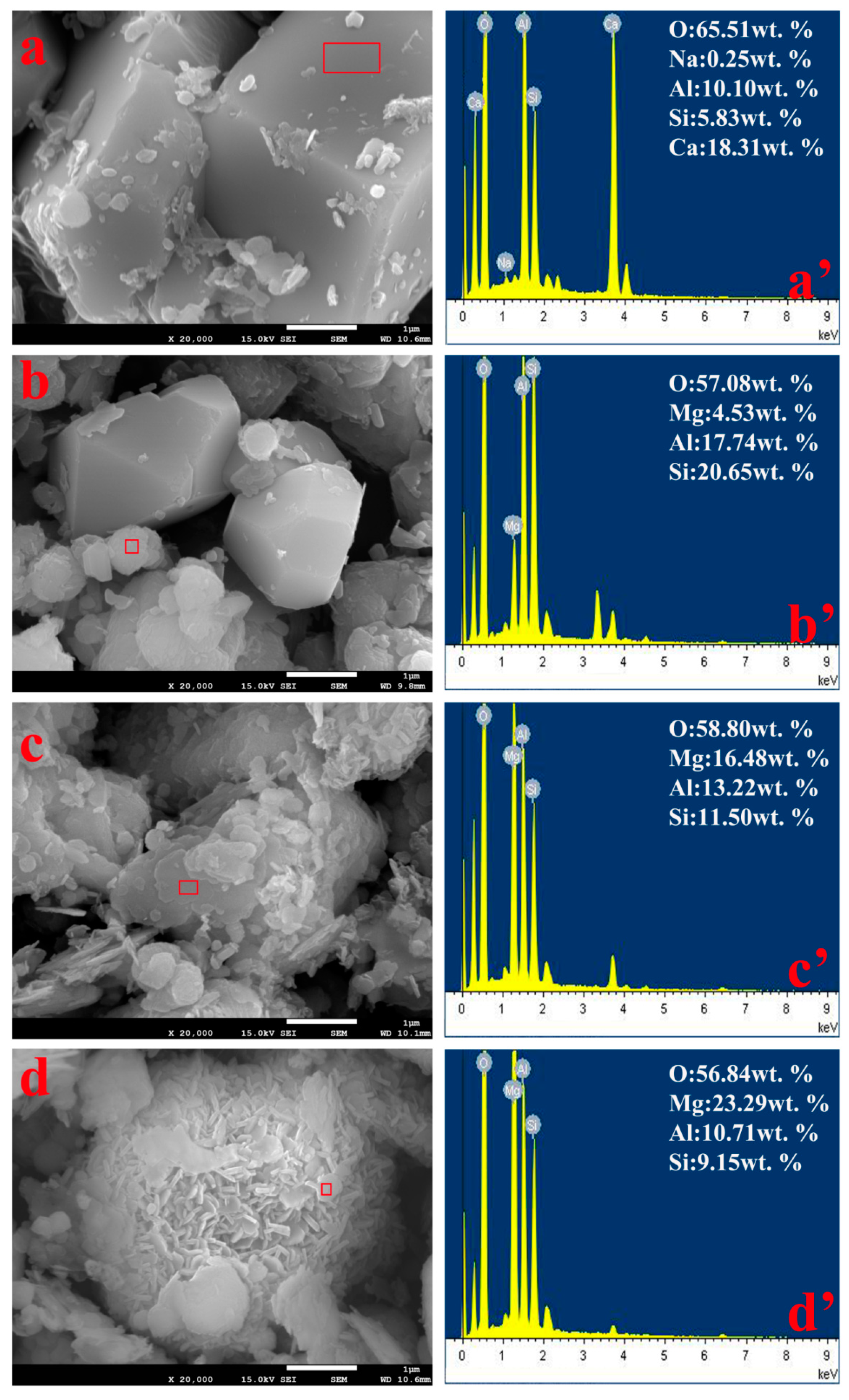
3.6. Solid Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Huo, Y.B.; Yuan, Y.; Shen, L.Y.; Xia, Y.Z.; Chai, Y.; Zhang, Q.; Wang, N.N. Regional statistics of alumina production in China from January to December 2017. China Alum. Mon. 2018, 230, 130–131. [Google Scholar]
- Roosen, J.; Van Roosendael, S.; Borra, C.R.; Van Gerven, T.; Mullens, S.; Binnemans, K. Recovery of scandium from leachates of Greek bauxite residue by adsorption on functionalized chitosan-silica hybrid materials. Green Chem. 2016, 18, 2005–2013. [Google Scholar] [CrossRef]
- Lehoux, A.P.; Lockwood, C.L.; Mayes, W.M.; Stewart, D.I.; Mortimer, R.J.G.; Gruiz, K.; Burke, I.T. Gypsum addition to soils contaminated by red mud: Implications for aluminium, arsenic, molybdenum and vanadium solubility. Environ. Geochem. Health 2013, 35, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. The processing of high silica bauxites-review of existing and potential processes. Hydrometallurgy 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Shoppert, A.A.; Loginova, I.V.; Rogozhnikov, D.A.; Karimov, K.A.; Chaikin, L.I. Increased as adsorption on maghemite-containing red mud prepared by the alkali fusion-leaching method. Minerals 2019, 9, 60. [Google Scholar] [CrossRef]
- Power, G.; Grafe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Liu, Z.B.; Li, H.X. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Enserink, M. Envrionment after red mud flood, scientists try to halt wave of fear and rumors. Science 2010, 330, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-scale consumption and zero-waste recycling method of red mud in steel making process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Brunori, C.; Cremisini, C.; Massanisso, P.; Pinto, V.; Torricelli, L. Reuse of a treated red mud bauxite waste: Studies on environmental compatibility. J. Hazard. Mater. 2005, 117, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Grafe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Hanahan, C.; McConchie, D.; Pohl, J.; Creelman, R.; Clark, M.; Stocksiek, C. Chemistry of seawater neutralization of bauxite refinery residues (red mud). Environ. Eng. Sci. 2004, 21, 125–138. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.L.; Agnihotri, A. Treatment of alumina refinery waste (red mud) through neutralization techniques: A review. Waste Manage. Res. 2017, 35, 563–580. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Wang, X.; Tian, W.; Shi, B.; Yao, M.; Zhang, Y. Bioleaching of major, rare earth, and radioactive elements from red mud by using indigenous chemoheterotrophic bacterium acetobacter sp. Minerals 2019, 9, 67. [Google Scholar] [CrossRef]
- Johnston, M.; Clark, M.W.; McMahon, P.; Ward, N. Alkalinity conversion of bauxite refinery residues by neutralization. J. Hazard. Mater. 2010, 182, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wasewar, K.L.; Lataye, D.H.; Mukhopadhyay, J.; Yoo, C.K. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef]
- Li, R.B.; Zhang, T.G.; Liu, Y.; Lv, G.Z.; Xie, L.Q. Calcification-carbonation method for red mud processing. J. Hazard. Mater. 2016, 316, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, S.L.; Wang, X.H.; Du, H.; Fang, Z.Z.; Zhang, Y. Reactive extraction of sodium hydroxide from alkali solutions for the separation of sodium and aluminum - part I. effect of extractant. Hydrometallurgy 2015, 154, 47–55. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, Y.F.; Zhang, Y. Extraction of alumina and sodium oxide from red mud by a mild hydro-chemical process. J. Hazard. Mater. 2009, 172, 1629–1634. [Google Scholar] [CrossRef]
- Luo, Z.T.; Xiao, Y.L.; Zhang, L.; Wang, X.; Zheng, Y.R.; Yang, J.J. Multi-stage cycle dealkalization and alkali recovery process of red mud slurry. J. Univ. Jinan Sci. Technol. 2013, 27, 369–372. [Google Scholar]
- Yang, J.J.; Li, J.W.; Xiao, Y.L.; Luo, Z.T.; Han, Y.F. Research on dealkalization of sintering process red mud by lime process at normal atmosphere and mechanism thereof. Inorg. Chem. Ind. 2012, 44, 40–42. [Google Scholar]
- Li, R.B.; Li, X.L.; Wang, D.X.; Liu, Y.; Zhang, T.A. Calcification reaction of red mud slurry with lime. Powder Technol. 2018, 333, 277–285. [Google Scholar] [CrossRef]
- Venancio, L.C.A.; Souza, J.A.S.; Macedo, E.N.; Botelho, F.A.; de Oliveira, A.M.; Fonseca, R.S. Bauxite residue amendment through the addition of Ca and or Mg followed by carbonation. In Light Metals; Ratvik, A.P., Ed.; Springer International Publishing Ag: Phoenix, AZ, USA, 2017; pp. 53–59. [Google Scholar]
- Zheng, X.F.; Hu, J.; Jiang, M.; Xue, Z.X. Study on optimization of dealkalization process on adding lime to red mud produced by low temperature Bayer process. Light Metals 2010, 4, 21–23. [Google Scholar]
- Amaral, L.F.; Oliveira, I.R.; Salomao, R.; Frollini, E.; Pandolfelli, V.C. Temperature and common-ion effect on magnesium oxide (MgO) hydration. Ceram. Int. 2010, 36, 1047–1054. [Google Scholar] [CrossRef]
- Maryska, M.; Blaha, J. Hydration kinetics of magnesium oxide—Part 3. Hydration rate of MgO in terms of temperature and time of its firing. Ceram. Silikaty 1997, 41, 121–123. [Google Scholar]
- Li, X.F.; Ye, Y.Z.; Xue, S.G.; Jiang, J.; Wu, C.; Kong, X.F.; Hartley, W.; Li, Y.W. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue. Trans. Nonferrous Met. Soc. China 2018, 28, 1248–1255. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle-shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Couperthwaite, S.J.; Johnstone, D.W.; Millar, G.J.; Frost, R.L. Neutralization of acid sulfate solutions using bauxite refinery residues and its derivatives. Ind. Eng. Chem. Res. 2013, 52, 1388–1395. [Google Scholar] [CrossRef]
- Qu, J.; Zhong, L.H.; Li, Z.; Chen, M.; Zhang, Q.W.; Liu, X.Z. Effect of anion addition on the syntheses of Ca-Al layered double hydroxide via a two-step mechanochemical process. Appl. Clay Sci. 2016, 124, 267–270. [Google Scholar] [CrossRef]
- Heraldy, E.; Nugrahaningtyas, K.D.; Sanjaya, F.B.; Darojat, A.A.; Handayani, D.S.; Hidayat, Y. In effect of reaction time and (Ca plus Mg)/Al molar ratios on crystallinity of Ca-Mg-Al layered double hydroxide. In Proceedings of the 10th Joint Conference on Chemistry, Solo, Indonesia, 8–9 September 2015. [Google Scholar]
- Del Arco, M.; Martin, C.; Martin, I.; Rives, V.; Trujillano, R. A FTIR spectroscopic study of surface acidity and basicity of mixed Mg, Al-oxides obtained by thermal decomposition of hydrotalcite. Spectrochim. Acta Part A Mol. Spectrosc. 1993, 49, 1575–1582. [Google Scholar] [CrossRef]
- Kuśtrowski, P.; Sułkowska, D.; Chmielarz, L.; Rafalska-Łasocha, A.; Dudek, B.; Dziembaj, R. Influence of thermal treatment conditions on the activity of hydrotalcite-derived Mg–Al oxides in the aldol condensation of acetone. Micropor. Mesopor. Mat. 2005, 78, 11–22. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Gaweł, A.; Bahranowski, K.; Olejniczak, Z.; et al. New insight into the preferred valency of interlayer anions in hydrotalcite-like compounds: The effect of Mg/Al ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]



| Chemicals | Al2O3 | SiO2 | CaO | Fe2O3 | Na2O | TiO2 | K2O | MgO | N/S |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 24.42 | 21.12 | 18.47 | 8.14 | 5.97 | 4.56 | 1.53 | 0.75 | 0.28 |
| MgO% | Temperature/℃ | MgO | Mg(OH)2 | (Mg4.5Al1.5)(Si2.5Al1.5)O10(OH)8 |
|---|---|---|---|---|
| 0 | 90 | x | x | x |
| 140 | x | x | x | |
| 190 | x | x | x | |
| 50 | 90 | √ | √ | x |
| 140 | √ | √ | x | |
| 190 | √ | √ | √ | |
| 100 | 90 | √ | √ | x |
| 140 | √ | √ | √ | |
| 190 | √ | √ | √ |
| MgO% | Ca10Mg2Al4(SiO4)5(Si2O7)2(OH)4 | Mg3Si2O5(OH)4 | (Mg4.5Al1.5)(Si2.5Al1.5)O10(OH)8 |
|---|---|---|---|
| 0 | x | x | x |
| 10 | √ | x | x |
| 30 | √ | √ | √ |
| 50 | √ | √ | √ |
| 70 | √ | √ | √ |
| 90 | √ | √ | √ |
| 100 | √ | √ | √ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Cao, S.; Chen, F.; Zhang, F.; Zhang, Y. Recovery of Alkali from Bayer Red Mud Using CaO and/or MgO. Minerals 2019, 9, 269. https://doi.org/10.3390/min9050269
Zhou B, Cao S, Chen F, Zhang F, Zhang Y. Recovery of Alkali from Bayer Red Mud Using CaO and/or MgO. Minerals. 2019; 9(5):269. https://doi.org/10.3390/min9050269
Chicago/Turabian StyleZhou, Bingxin, Shaotao Cao, Fangfang Chen, Fangfang Zhang, and Yi Zhang. 2019. "Recovery of Alkali from Bayer Red Mud Using CaO and/or MgO" Minerals 9, no. 5: 269. https://doi.org/10.3390/min9050269
APA StyleZhou, B., Cao, S., Chen, F., Zhang, F., & Zhang, Y. (2019). Recovery of Alkali from Bayer Red Mud Using CaO and/or MgO. Minerals, 9(5), 269. https://doi.org/10.3390/min9050269




