Gem-Quality Green Cr-Bearing Andradite (var. Demantoid) from Dobšiná, Slovakia
Abstract
1. Introduction
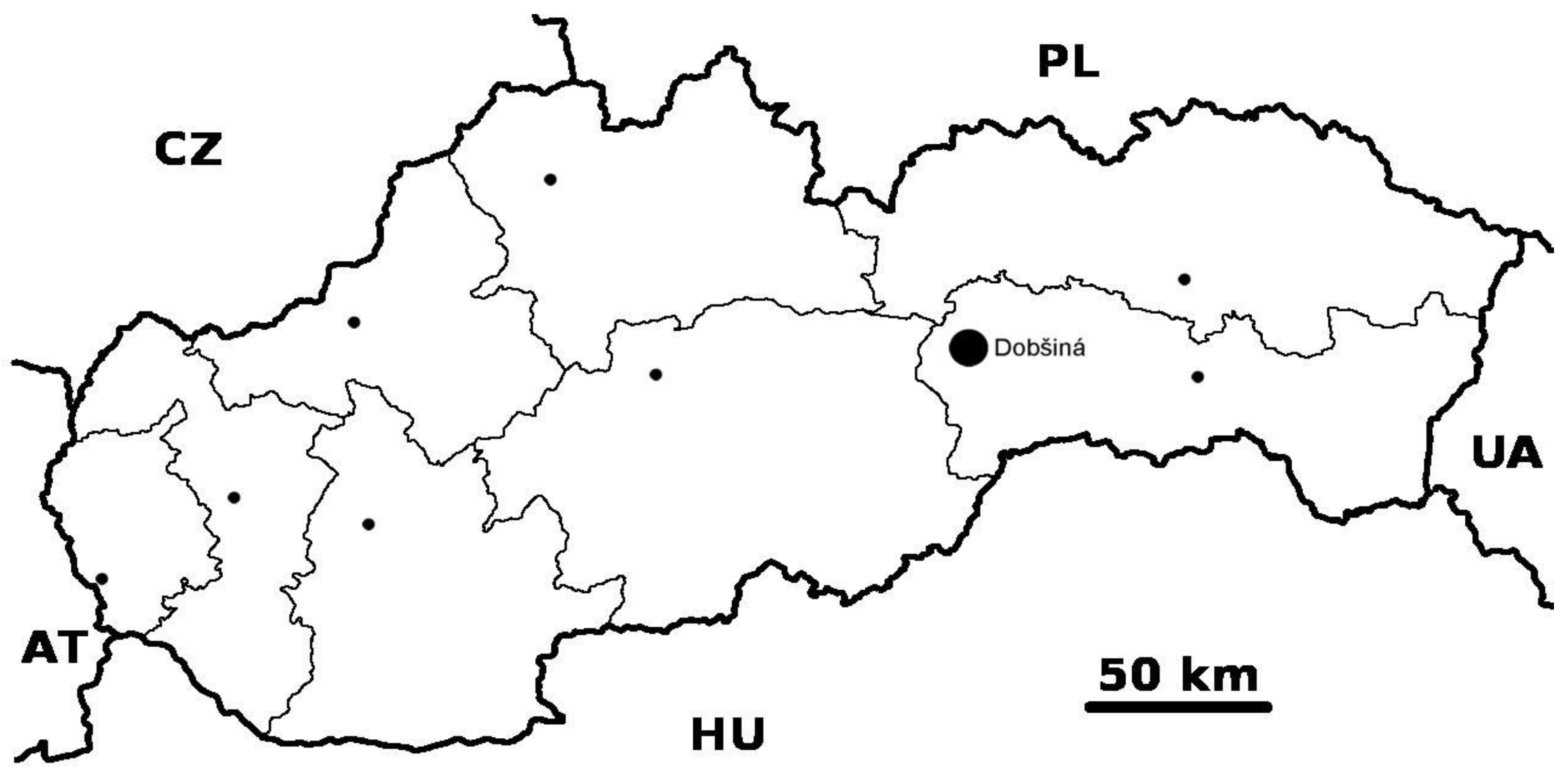
Geological Setting
2. Materials and Methods
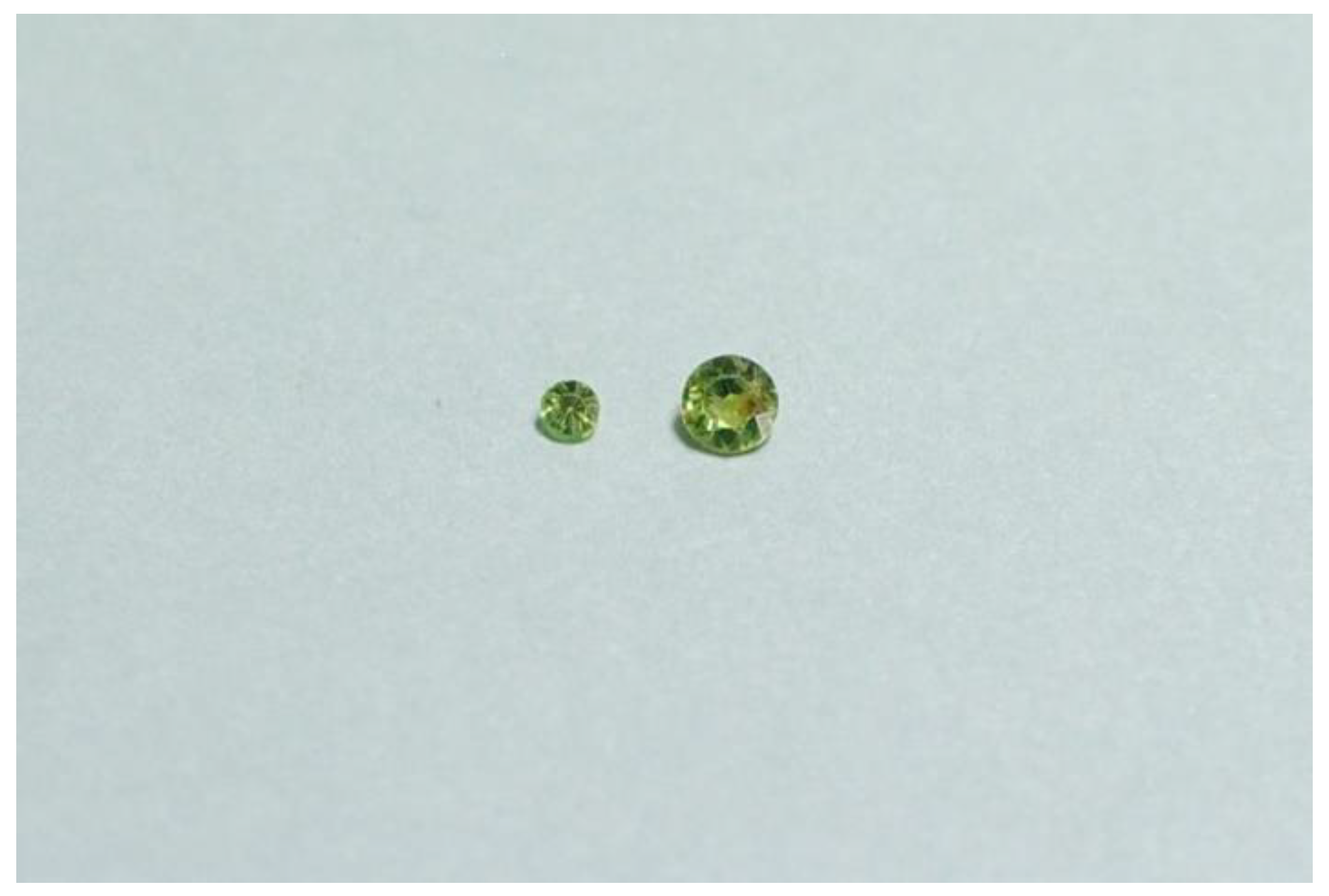
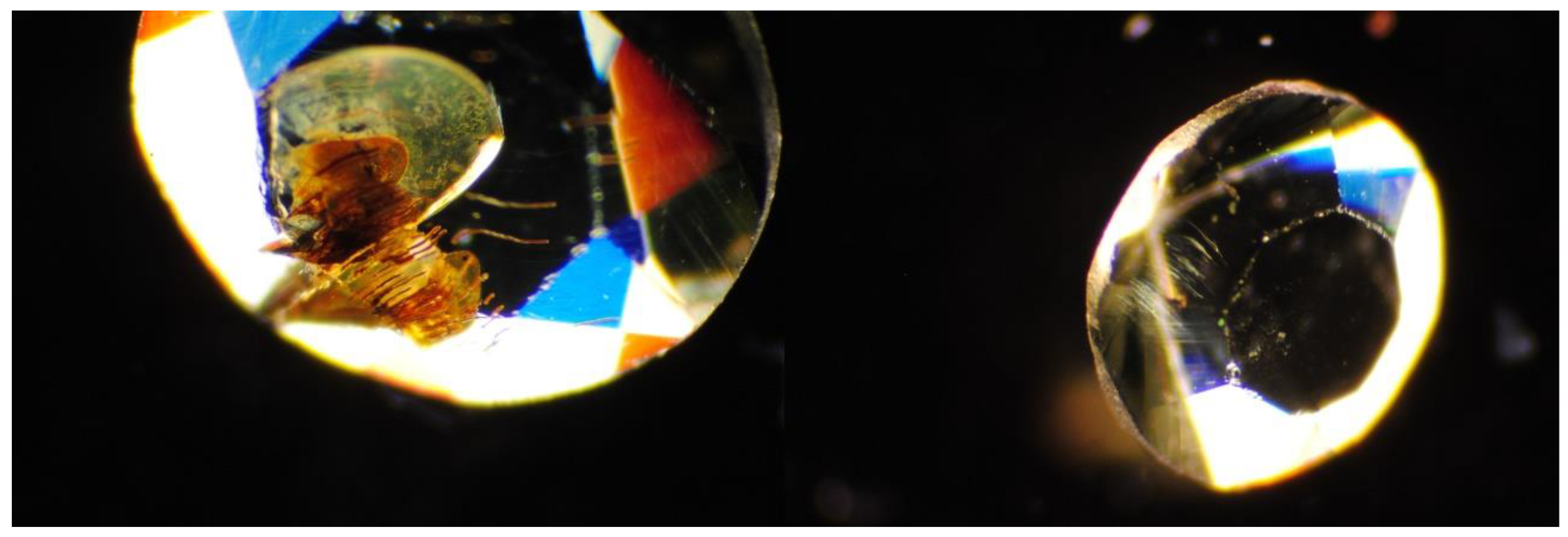
2.1. Sample Description
2.2. Analytical Methods
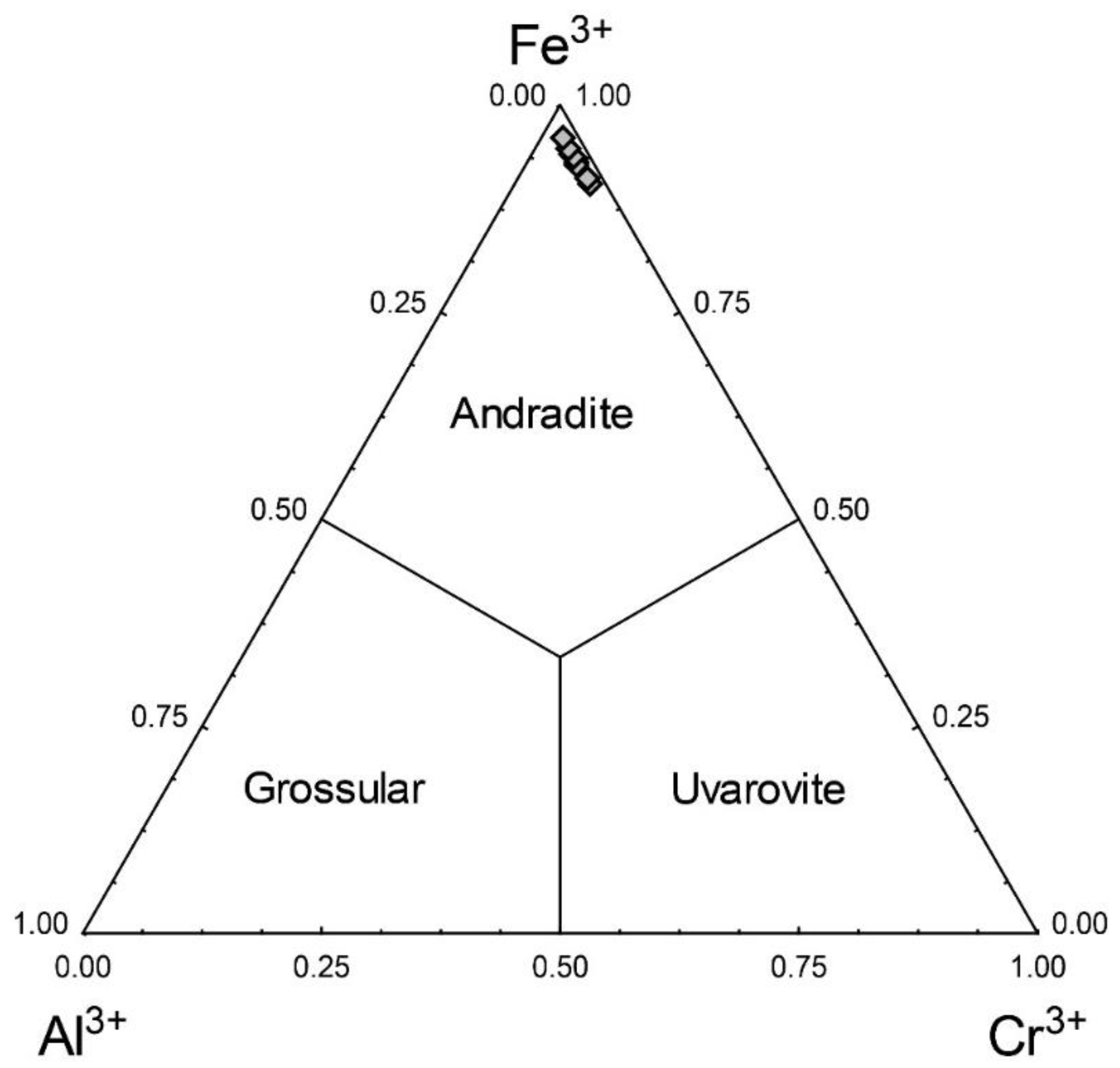
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaličiak, M.; Ďud’a, R.; Burda, P.; Kaličiaková, E. Structural geological characteristics of the Dubník opal deposits. Zborník Vychodoslovenského Múzea v Košiciach 1976, 17, 7–22. [Google Scholar]
- Rondeau, B.; Fritsch, E.; Guiraud, M.; Renac, C. Opals from Slovakia (“Hungarian” opals): A re-assessment of the conditions of formation. Eur. J. Mineral. 2004, 16, 789–799. [Google Scholar] [CrossRef]
- Gaillou, E.; Fritsch, E.; Aguilar-Reyes, B.; Rondeau, B.; Post, J.; Barreau, A.; Ostroumov, M. Common gem opal: An investigation of micro- to nano-structure. Am. Mineral. 2008, 93, 1865–1873. [Google Scholar] [CrossRef]
- Gaillou, E.; Delaunay, A.; Rondeau, B.; Bouhnik-le-Coz, M.; Fritsch, E.; Cornen, G.; Monnier, C. The geochemistry of gem opals as evidence of their origin. Ore Geol. Rev. 2008, 34, 113–126. [Google Scholar] [CrossRef]
- Kiefert, L.; Karampelas, S. Use of the Raman spectrometer in gemmological laboratories: Review. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Caucia, F.; Marinoni, L.; Leone, A.; Adamo, I. Investigation on the gemological, physical and compositional properties of some opals from Slovakia (“Hungarian” opals). Periodico di Mineralogia 2013, 82, 251–261. [Google Scholar]
- Caucia, F.; Ghisoli, C.; Marinoni, L.; Bordoni, V. Opal, a beautiful gem between myth and reality. Neues Jahrbuch fur Mineralogie Abhandlungen 2013, 190, 1–9. [Google Scholar] [CrossRef]
- Ďuďa, R. Gemmological and genetic classification of precious and trim stones of Slovakia. Mineral. Slovaca 1987, 19, 353–362. (In Slovakian) [Google Scholar]
- Fridrichová, J.; Bačík, P.; Illášová, Ľ.; Kozáková, P.; Škoda, R.; Pulišová, Z.; Fiala, A. Raman and optical spectroscopic investigation of gem-quality smoky quartz crystals. Vib. Spectrosc. 2016, 85, 71–78. [Google Scholar] [CrossRef]
- Fridrichová, J.; Bačík, P.; Malíčková, I.; Illášová, Ľ.; Pulišová, Z. Optical-spectroscopic study of gem sphalerite from Banská Štiavnica and Hodruša-Hámre. Esemestník 2017, 6, 13–15. (In Slovakian) [Google Scholar]
- Uher, P.; Sabol, M.; Konečný, P.; Gregáňová, M.; Táborský, Z.; Puškelová, Ľ. Sapphire from Hajnáčka Cérová Highland, southern Slovakia. Slovak Geol. Mag. 1999, 5, 273–280. [Google Scholar]
- Uher, P.; Sabol, M.; Konečný, P.; Gregáňová, M.; Táborský, Z.; Puškelová, Ľ. Zafír vo výplni vrchnopliocénneho maaru pri Hajnáčke. Miner. Slovaca 2001, 33, 307–308. [Google Scholar]
- Uher, P.; Giuliani, G.; Szakáll, S.; Fallick, A.; Strunga, V.; Vaculovič, T.; Ozdín, D.; Gregáňová, M. Sapphires related to alkali basalts from the Cerová Highlands, Western Carpathians (southern Slovakia): Composition and origin. Geol. Carpath. 2012, 63, 71–82. [Google Scholar] [CrossRef]
- Quareni, S.; De Pieri, R. La struttura dell’andradite. Atti. e Memorie dell’. Accademia Patavina di Science Lettere ed Arti 1966, 78, 151–170. [Google Scholar]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mineral. 1971, 56, 791–825. [Google Scholar]
- Hazen, R.M.; Finger, L.W. High-pressure crystal chemistry of andradite and pyrope: Revised procedure for high-pressure diffraction experiments. Am. Mineral. 1989, 74, 352–359. [Google Scholar]
- Lager, G.A.; Armbruster, T.; Rotella, F.J.; Rossman, G.R. OH substitution in garnets: X-ray and neutron diffraction, infrared, and geometric-modeling studies. Am. Mineral. 1989, 74, 840–851. [Google Scholar]
- Armbruster, T.; Geiger, C.A. Andradite crystal chemistry, dynamic X-site disorder and structural strain in silicate garnets. Eur. J. Mineral. 1993, 5, 59–71. [Google Scholar] [CrossRef]
- Armbruster, T.; Birrer, J.; Libowitzky, E.; Beran, A. Crystal chemistry of Ti-bearing andradites. Eur. J. Mineral. 1998, 10, 907–921. [Google Scholar] [CrossRef]
- Pavese, A.; Diella, V.; Pischedda, V.; Merli, M.; Bocchio, R.; Mezouar, M. Pressure-temperature equation of state of andradite and grossular, by high-pressure and -temperature powder diffraction. Phys. Chem. Miner. 2001, 28, 242–248. [Google Scholar] [CrossRef]
- Bocchio, R.; Adamo, I.; Diella, V. Trace-element profiles in gem-quality green andradite from classic localities. Can. Mineral. 2010, 48, 1205–1216. [Google Scholar] [CrossRef]
- Adamo, I.; Gatta, G.D.; Rotiroti, N.; Diella, V.; Pavese, A. Green andradite stones: Gemmological and mineralogical characterisation. Eur. J. Mineral. 2011, 23, 91–100. [Google Scholar] [CrossRef]
- Balčiūnaitė, I.; Kleišmantas, A.; Norkus, E. Chemical composition of rare garnets, their colours and gemmological characteristics. Chemija 2015, 26, 18–24. [Google Scholar]
- O’Donoghue, M. Gems, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2006; p. 936. [Google Scholar]
- Adamo, I.; Bocchio, R.; Diella, V.; Caucia, F.; Schmetzer, K. Demantoid from Balochistan, Pakistan: Gemmological and Mineralogical Characterization. J. Gemmol. 2015, 34, 428–433. [Google Scholar] [CrossRef]
- Phillips, W.R.; Talantsev, A.S. Russian demantoid, czar of the garnet family. Gems Gemol. 1996, 32, 100–111. [Google Scholar] [CrossRef]
- Krzemnicki, M.S. Diopside needles as inclusions in demantoid garnet from Russia: A Raman microspectrometric study. Gems Gemol. 1999, 35, 192–195. [Google Scholar] [CrossRef]
- Laurs, B.M. A new find of demantoid at a historic side in Kladovka, Russia. Gems Gemol. 2003, 39, 54–55. [Google Scholar]
- Mayerson, W.M. Lab Notes: Large demantoid of exceptional color. Gems Gemol. 2006, 42, 261–262. [Google Scholar]
- Adamo, I.; Bocchio, R.; Diella, V.; Pavese, A.; Vignola, P.; Prosperi, L.; Palanza, V. Demantoid from Val Malenco, Italy: Review and update. Gems Gemol. 2009, 45, 280–287. [Google Scholar] [CrossRef]
- Douman, M.; Dirlam, D. Update on demantoid and cat´s-eye from Iran. Gems Gemol. 2004, 40, 67–68. [Google Scholar]
- Du Toi, G.; Mayerson, W.; van Der Bogert, C.; Douman, M.; Befi, R.; Koivula, J.I.; Kiefert, L. Demantoid from Iran. Gems Gemol. 2006, 42, 131. [Google Scholar]
- Karampelas, S.; Gaillou, E.; Fritsch, E.; Douman, M. Les grenats andradites-démantoïde d’Iran: Zonage de couleur et inclusions. Rev. Gemmol. 2007, 160, 14–20. [Google Scholar]
- Fritz, E.A.; Laurs, B.M. Gem News International: Andradite from Balochistan, Pakistan. Gems Gemol. 2007, 43, 373. [Google Scholar]
- Milisenda, C.C.; Henn, U.; Henn, J. Demantoide aus Pakistan. Gemmol. Z. Dtsch. Gemmol. Ges. 2001, 50, 51–56. [Google Scholar]
- Quinn, E.P.; Laurs, B.M. Demantoid from northern Pakistan. Gems Gemol. 2005, 41, 176–177. [Google Scholar]
- Palke, A.C.; Pardieu, V. Gem News International: Demantoid from Baluchistan Province in Pakistan. Gems Gemol. 2014, 50, 302–303. [Google Scholar]
- Lind, T.; Henn, U.; Bank, H. New occurrence of demantoid in Namibia. Aust. Gemmol. 1998, 20, 75–79. [Google Scholar]
- Cairncross, B.; Bahmann, U. The Erongo Mountains, Namibia. Mineral. Rec. 2006, 37, 361–470. [Google Scholar]
- Koller, F.; Niedermayar, G.; Pintér, Z.; Syabó, C. The demantoid garnets of the Green Dragon Mine (Tubussi, Erongo region, Namibia). Acta Mineral.-Petrogr. 2012, 7, 72. [Google Scholar]
- Kaiser, H.; Enzersdorf, M. Demantoid aus der Green Dragon Mine, Erongo Region, Namibia. Gemmo News 2012, 32, 5. [Google Scholar]
- Danet, F. Gem News International: New discovery of demantoid from Ambanja, Madagascar. Gems Gemol. 2009, 45, 218–219. [Google Scholar]
- Rondeau, B.; Mocquet, B.; Lulzac, Y.; Fritsch, E. Les nouveaux grenats démantoïde d’Ambanja, Province d’Antsiranana, Madagascar. Le Règne Mineral 2009, 90, 41–45. [Google Scholar]
- Rondeau, B.; Fritsch, E.; Mocquet, B.; Lulyac, Y. Ambanja (Madagascar)—New source of gem demantoid garnet. InColor 2009, 11, 16–20. [Google Scholar]
- Praszkier, T.; Gajowniczek, J. Demantoide aus Antetezambato auf Madagascar. Miner. Welt 2010, 21, 32–41. [Google Scholar]
- Pezzotta, F. Andradite from Antetezambato, North Madagascar. Mineral. Rec. 2010, 41, 209–229. [Google Scholar]
- Pezzotta, F.; Adamo, I.; Diella, V. Demantoid and topazolite from Antetezambato, northern Madagascar: Review and new data. Gems Gemol. 2011, 47, 2–14. [Google Scholar] [CrossRef]
- Wilson, B.S. Coloured gemstones from Canada. Rocks Miner. 2009, 85, 24–43. [Google Scholar] [CrossRef]
- Ostrooumov, M. Mexican demantoid from new deposits. Gems Gemol. 2015, 51, 450–452. [Google Scholar]
- Guobin, L.; Xu, K.; Lin, Z. On the Genesis of Demantoid from Xinjiang, China. Chin. J. Geochem. 1986, 5, 381–390. [Google Scholar] [CrossRef]
- Mock, R.; Sýkora, M.; Aubrecht, R.; Ožvoldová, L.; Kronome, B.; Reichwalder, P.; Jablonský, J. Petrology and stratigraphy of the Meliaticum near the Meliata and Jaklovce Villages, Slovakia. Slovak Geol. Mag. 1998, 4, 223–260. [Google Scholar]
- Kozur, H. The Evolution of the Meliata-Hallstatt ocean and its significance for the early evolution of the Eastern Alps and Western Carpathians. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 87, 109–135. [Google Scholar] [CrossRef]
- Stampfli, G.M. The Intra-Alpine terrain: A Paleotethyan remnant in the Alpine Variscides. Eclogae Geol. Helv. 1996, 89, 13–42. [Google Scholar]
- Ivan, P. Relics of the Meliata Ocean crust: Geodynamic implications of mineralogical, petrological and geochemical proxies. Geol. Carpath. 2002, 53, 245–256. [Google Scholar]
- Faryad, S.W.; Spišiak, J.; Horváth, P.; Hovorka, D.; Dianiška, I.; Józsa, S. Petrological and geochemical features of the Meliata mafic rocks from the sutured Triassic Oceanic Basin, Western Carpathians. Ofioliti 2005, 30, 27–35. [Google Scholar]
- Kozur, H.; Mock, R. New paleogeographic and tectonic interpretations in the Slovakian Carpathians and their implications for correlations with the Eastern Alps and other parts of the Western Tethys, Part II: Inner Western Carpathians. Miner. Slovaca 1997, 29, 164–209. [Google Scholar]
- Kozur, H.; Mock, R. Erster Nachweis von Jura der Meliata-Einheit der südlichen Westkarpaten. Geologisch-Paläontologische Mitteilungen Innsbruck 1985, 13, 223–238. [Google Scholar]
- Putiš, M.; Radvanec, M.; Hain, M.; Koller, F.; Koppa, M.; Snárska, B. 3-D analysis of perovskite in serpentinite (Dobšiná quarry) by X-ray micro-tomography. In Proceedings of the Petros Symposium; Ondrejka, M., Šarinová, K., Eds.; Univerzita Komenského: Bratislava, Slovakia, 2011; pp. 33–37. [Google Scholar]
- Dallmeyer, R.D.; Neubauer, F.; Handler, R.; Fritz, H.; Müller, W.; Pana, D.; Putiš, M. Tectonothermal evolution of the internal Alps and Carpathians: 40Ar/39Ar mineral and whole rock data. Eclogae Geol. Helv. 1996, 89, 203–277. [Google Scholar]
- Putiš, M.; Yang, Y.-H.; Koppa, M.; Dyda, M.; Šmál, P. U/Pb LA-ICP-MS age of metamorphic-metasomatic perovskite from serpentinized harzburgite in the Meliata Unit at Dobšiná, Slovakia: Time constraint of fluid-rock interaction in an accretionary wedge. Acta Geol. Slovaca 2015, 7, 63–71. [Google Scholar]
- Faryad, S.W.; Henjes-Kunst, F. Petrological and K-Ar and 40Ar/39Ar age constraints for the tectonothermal evolution of the high-pressure Meliata unit, Western Carpathians (Slovakia). Tectonophysics 1997, 280, 141–156. [Google Scholar] [CrossRef]
- Plašienka, D. Cretaceous tectonochronology of the central western Carpathians, Slovakia. Geol. Carpath. 1997, 48, 99–111. [Google Scholar]
- Putiš, M.; Frank, W.; Plašienka, D.; Siman, P.; Sulák, M.; Biroň, A. Progradation of the Alpidic Central Western Carpathians orogenic wedge related to two subductions: Constrained by 40Ar/39Ar ages of white micas. Geodin. Acta 2009, 22, 31–56. [Google Scholar] [CrossRef]
- Dal Piaz, G.; Martin, S.; Villa, I.M.; Gosso, G.; Marschalko, R. Late Jurassic blueschist facies pebbles from the Western Carpathians orogenic wedge and paleostructural implications for Western Tethys evolution. Tectonics 1995, 14, 874–885. [Google Scholar] [CrossRef]
- Putiš, M.; Gawlick, H.J.; Frisch, W.; Sulák, M. Cretaceous transformation from passive to active continental margin in the Western Carpathians as indicated by the sedimentary record in the Infratatric Unit. Int. J. Earth Sci. 2008, 97, 799–819. [Google Scholar] [CrossRef]
- Putiš, M.; Danišík, M.; Ružička, P.; Schmiedt, I. Constraining exhumation pathway in an accretionary wedge by (U-Th)/He thermochronology—Case study on Meliatic nappes in the Western Carpathians. J. Geodyn. 2014, 81, 80–90. [Google Scholar] [CrossRef]
- Fediuková, E.; Hovorka, D.; Greguš, J. Compositional zoning of andradite from serpentinite at Dobšiná (West Carpathias). Věst. Ústr. Úst. Geol. 1976, 51, 339–345. [Google Scholar]
- Hovorka, D.; Jaroš, J.; Kratochvíl, M.; Mock, R. The Mesozoic ophiolites of the Western Carpathians. Krystalinikum 1984, 17, 143–157. [Google Scholar]
- Hovorka, D.; Ivan, P.; Jaroš, J.; Kratochvíl, M.; Reichwalder, P.; Rojkovič, I.; Spišiak, J.; Turanová, L. Ultramafic rocks of the Western Carpathians, Czechoslovakia; Geological Institute of Dionýz Štúr Publishers: Bratislava, Czechoslovakia, 1985; p. 258. [Google Scholar]
- Putiš, M.; Koppa, M.; Snárska, B.; Koller, F.; Uher, P. The blueschist-associated perovskite-andradite-bearing serpentinized harzburgite from Dobšiná (the Meliata Unit), Slovakia. J. Geosci. 2012, 57, 221–240. [Google Scholar] [CrossRef]
- Putiš, M.; Yang, Y.-H.; Vaculovič, T.; Koppa, M.; Li, X.-H.; Uher, P. Perovskite, reaction product of a harzburgite with Jurassic-Cretaceous accretionary wedge fluids (Western Carpathians, Slovakia): Evidence from the whole-rock and mineral trace element data. Geol. Carpath. 2016, 67, 133–146. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Chopelas, A. Vibrational spectroscopy of end-member silicate garnets. Phys. Chem. Miner. 1991, 17, 503–526. [Google Scholar] [CrossRef]
- Uher, P.; Kováčik, M.; Kubiš, M.; Shtukenberg, A.; Ozdín, D. Metamorphic vanadian-chromian silicate mineralization in carbon-rich amphibole schists from the Malé Karpaty Mountains, Western Carpathians, Slovakia. Am. Mineral. 2008, 93, 63–73. [Google Scholar] [CrossRef]
- Bačík, P.; Uher, P.; Kozakova, P.; Števko, M.; Ozdín, D.; Vaculovič, T. Vanadian and chromian garnet- and epidote-supergroup minerals in metamorphosed Paleozoic black shales from Cierna Lehota, Strazovske vrchy Mountains, Slovakia: Crystal chemistry and evolution. Mineral. Mag. 2018, 82, 889–911. [Google Scholar] [CrossRef]
- Peters, T. A water-bearing andradite from the Totalp serpentine (Davos, Switzerland). Am. Mineral. 1965, 50, 1482–1486. [Google Scholar]
- Passaglia, E.; Rinaldi, R. Katoite, a new member of the Ca3Al2(SiO4)3-Ca3Al2(OH)12 series and a new nomenclature for the hydrogrossular group of minerals. Bulletin de la Société Française de Minéralogie et de Cristallographie 1984, 107, 605–618. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V. Garnets of the hydrogrossular-‘‘hydroandradite”-‘‘hydroschorlomite” series. Spec. Pap. Mineral. Soc. Pol. 2003, 22, 54–57. [Google Scholar]
- Galuskin, E. Minerals of the Vesuvianite Group from the Achtarandite Rocks (Wiluy River, Yakutia); University of Silesia Publishing House: Katowice, Poland, 2005; p. 192. [Google Scholar]
- Moore, R.K.; White, W.B. Electronic spectra of transition metal ions in silicate garnets. Can. Mineral. 1972, 11, 791–811. [Google Scholar]
- Manning, P.G. Optical absorption spectra of Fe3+ in octahedral and tetrahedral sites in natural garnets. Can. Mineral. 1972, 11, 826–839. [Google Scholar]
- Amthauer, G. Crystal chemistry and colour of chromium bearing garnets. Neues Jahrbuch für Mineralogie Abhandlungen 1976, 126, 158–186. [Google Scholar]
- Stockton, C.M.; Manson, D.V. Gem andradite garnets. Gems Gemol. 1983, 19, 202–208. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions II. J. Phys. Soc. Jpn. 1954, 9, 766–779. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions I. J. Phys. Soc. Jpn. 1954, 5, 753–766. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions III. J. Phys. Soc. Jpn. 1956, 11, 864–877. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| P2O5 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.00 | 0.02 | 0.02 |
| SiO2 | 35.21 | 35.41 | 35.38 | 35.33 | 35.47 | 35.43 | 35.74 | 35.72 |
| TiO2 | 0.05 | 0.05 | 0.04 | 0.03 | 0.00 | 0.03 | 0.08 | 0.04 |
| Al2O3 | 0.33 | 0.34 | 0.31 | 0.32 | 0.40 | 0.34 | 0.37 | 0.32 |
| V2O3 | 0.09 | 0.07 | 0.07 | 0.08 | 0.07 | 0.09 | 0.06 | 0.05 |
| Cr2O3 | 1.24 | 1.50 | 2.31 | 2.14 | 1.54 | 1.34 | 0.97 | 0.63 |
| Fe2O3 | 29.15 | 29.15 | 27.69 | 28.34 | 28.64 | 28.82 | 29.16 | 29.65 |
| MgO | 0.20 | 0.23 | 0.20 | 0.17 | 0.34 | 0.23 | 0.20 | 0.21 |
| CaO | 33.88 | 33.80 | 33.74 | 33.72 | 33.85 | 33.85 | 33.74 | 33.65 |
| F | 0.08 | 0.11 | 0.09 | 0.11 | 0.09 | 0.08 | 0.09 | 0.10 |
| O=F | −0.04 | −0.05 | −0.04 | −0.06 | −0.04 | −0.04 | −0.05 | −0.05 |
| Total | 100.19 | 100.63 | 99.80 | 100.20 | 100.40 | 100.16 | 100.38 | 100.34 |
| P5+ | 0.002 | 0.001 | 0.001 | 0.001 | 0.003 | 0.000 | 0.001 | 0.001 |
| Si4+ | 2.975 | 2.977 | 2.993 | 2.981 | 2.984 | 2.990 | 3.003 | 3.004 |
| F− | 0.021 | 0.028 | 0.023 | 0.030 | 0.023 | 0.022 | 0.024 | 0.026 |
| Σ | 2.997 | 3.006 | 3.018 | 3.012 | 3.010 | 3.012 | 3.029 | 3.031 |
| Ti4+ | 0.003 | 0.003 | 0.003 | 0.002 | 0.000 | 0.002 | 0.005 | 0.003 |
| Al3+ | 0.033 | 0.034 | 0.031 | 0.032 | 0.040 | 0.034 | 0.037 | 0.032 |
| V3+ | 0.006 | 0.005 | 0.005 | 0.006 | 0.005 | 0.006 | 0.004 | 0.003 |
| Cr3+ | 0.083 | 0.100 | 0.155 | 0.143 | 0.102 | 0.089 | 0.064 | 0.042 |
| Fe3+ | 1.845 | 1.837 | 1.757 | 1.793 | 1.806 | 1.823 | 1.840 | 1.873 |
| Σ | 1.969 | 1.979 | 1.950 | 1.975 | 1.953 | 1.954 | 1.950 | 1.952 |
| Mg2+ | 0.025 | 0.029 | 0.026 | 0.022 | 0.043 | 0.028 | 0.025 | 0.026 |
| Ca2+ | 3.067 | 3.044 | 3.059 | 3.049 | 3.052 | 3.060 | 3.037 | 3.032 |
| Σ | 3.092 | 3.073 | 3.084 | 3.071 | 3.095 | 3.089 | 3.063 | 3.058 |
| K | Sc | Ti | V51 | Cr53 | Mn | Ge72 | As75 | Y89 | Mo95 | Gd157 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 392 | 16 | 103 | 305 | 3814 | 103 | 4 | 11 | 2 | 0 | 0 |
| Max | 787 | 40 | 389 | 572 | 13331 | 224 | 13 | 22 | 5 | 1 | 2 |
| Average | 523 | 25 | 244 | 444 | 9199 | 143 | 9 | 16 | 3 | 1 | 1 |
| LOD | 48.7 | 2.1 | 11.4 | 0.7 | 9.2 | 7.8 | 3.8 | 9.1 | 0.1 | 0.1 | 0.1 |
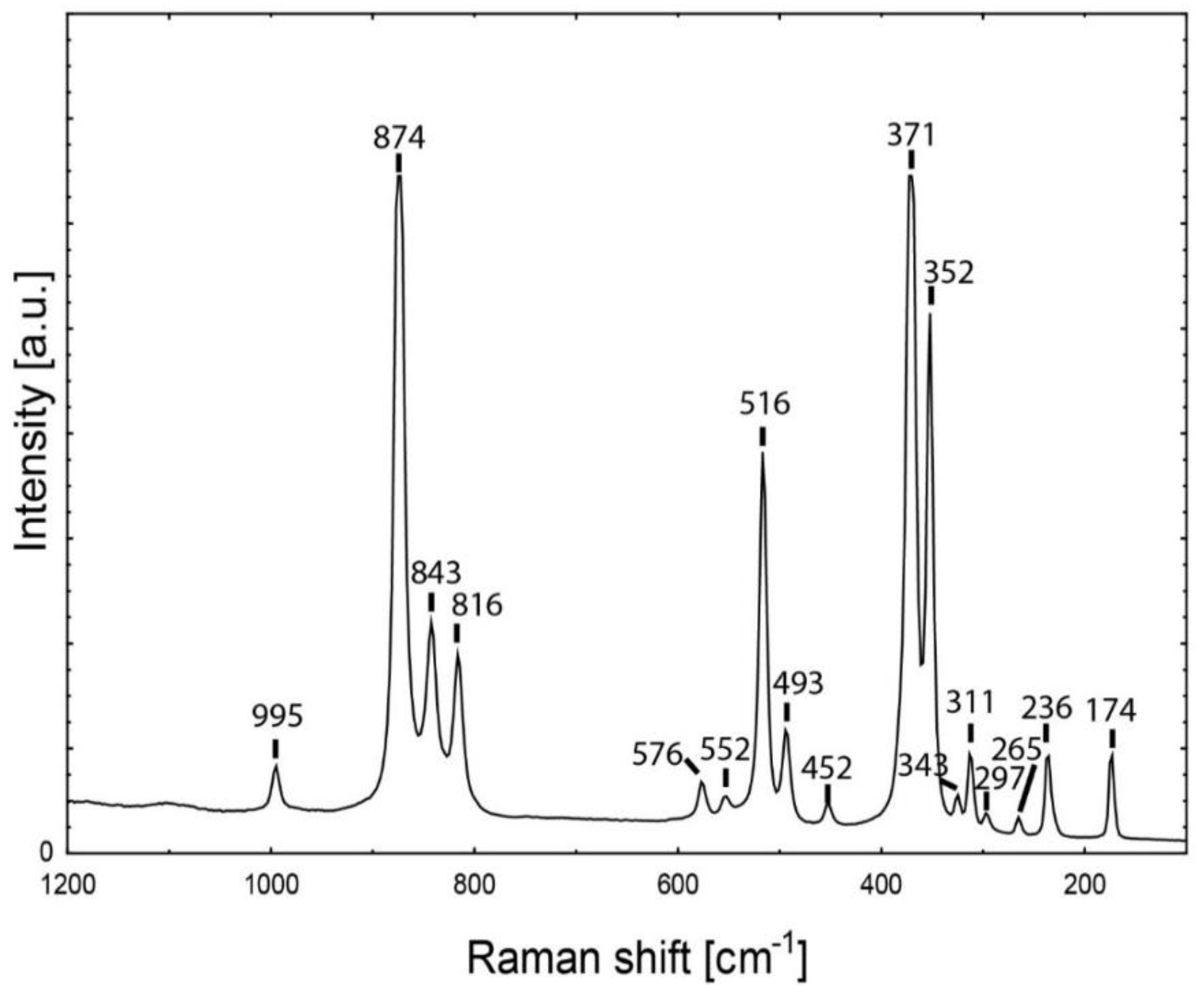
| Symmetry | Assignment | Demantoid Dobšiná | Andradite [72] |
|---|---|---|---|
| T2g + T1u | T(M) | 174 | 173 |
| T2g + T1u | T(M) | 236 | 235 |
| T2g | T(SiO4)mix | 265 | 264 |
| Eg | T(SiO4) | 297 | 296 |
| T2g + T1u | T(SiO4) | 311 | 311 |
| ? | ? | 343 | - |
| Eg | ν2 | 352 | 352 |
| A1g | R(SiO4) | 371 | 370 |
| T2g + T1u | ν2 | 452 | 452 |
| Eg | ν2 | 493 | 494 |
| A1g | ν2 | 516 | 516 |
| T2g + T1u | ν4 | 552 | 553 |
| Eg | ν4 | 576 | 576 |
| T2g + T1u | ν3 | 816 | 816 |
| T2g + T1u | ν3 | 843 | 842 |
| Eg | ν3 | 874 | 874 |
| T2g + T1u | ν3 | 995 | 995 |
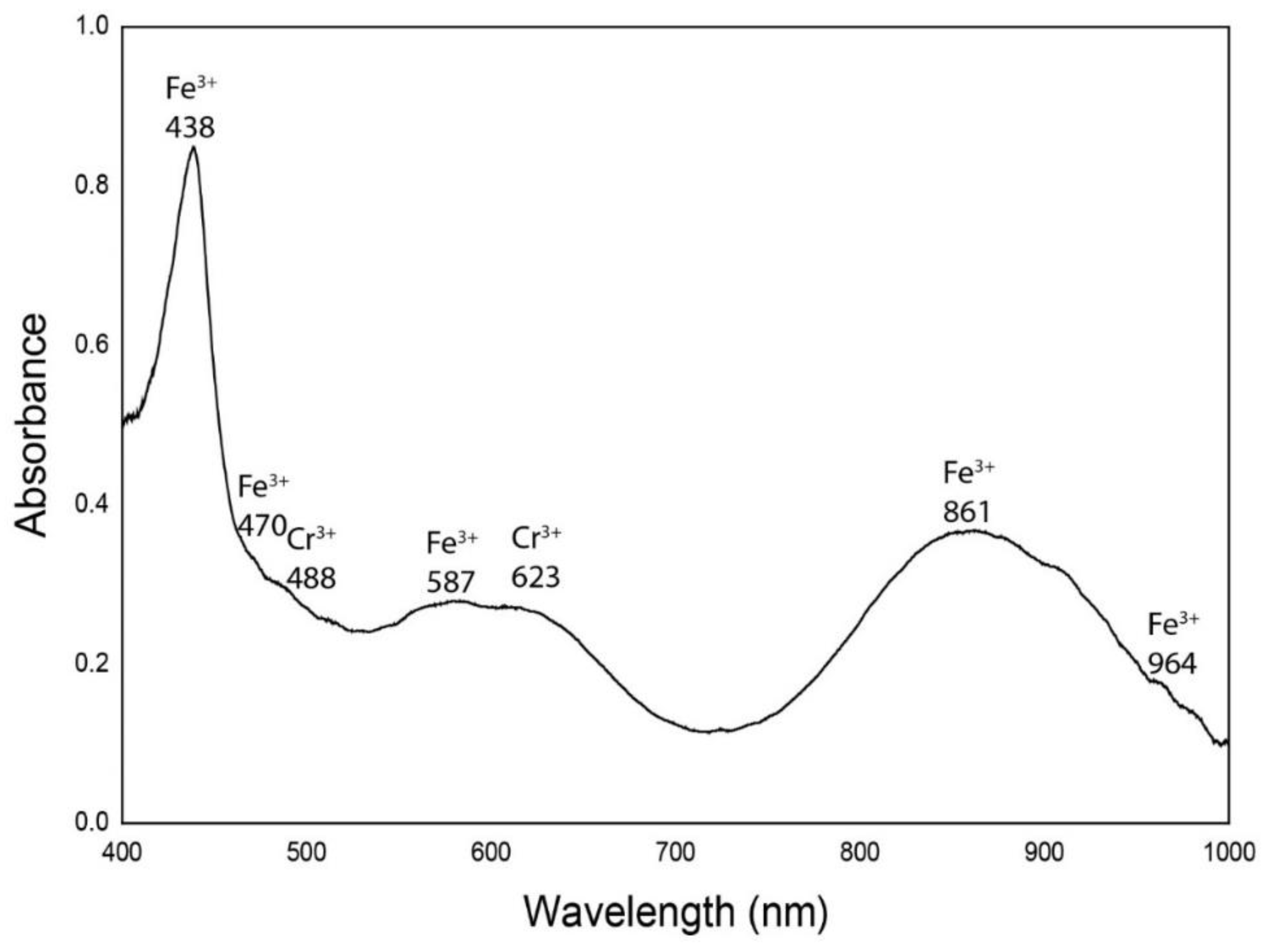
| Chromophores | Demantoid Dobšiná |
|---|---|
| Fe3+ | 438 |
| Fe3+ | 470 |
| Cr3+ | 488 |
| Fe3+ | 587 |
| Cr3+ | 623 |
| Fe3+ | 861 |
| Fe3+ | 964 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štubňa, J.; Bačík, P.; Fridrichová, J.; Hanus, R.; Illášová, Ľ.; Milovská, S.; Škoda, R.; Vaculovič, T.; Čerňanský, S. Gem-Quality Green Cr-Bearing Andradite (var. Demantoid) from Dobšiná, Slovakia. Minerals 2019, 9, 164. https://doi.org/10.3390/min9030164
Štubňa J, Bačík P, Fridrichová J, Hanus R, Illášová Ľ, Milovská S, Škoda R, Vaculovič T, Čerňanský S. Gem-Quality Green Cr-Bearing Andradite (var. Demantoid) from Dobšiná, Slovakia. Minerals. 2019; 9(3):164. https://doi.org/10.3390/min9030164
Chicago/Turabian StyleŠtubňa, Ján, Peter Bačík, Jana Fridrichová, Radek Hanus, Ľudmila Illášová, Stanislava Milovská, Radek Škoda, Tomáš Vaculovič, and Slavomír Čerňanský. 2019. "Gem-Quality Green Cr-Bearing Andradite (var. Demantoid) from Dobšiná, Slovakia" Minerals 9, no. 3: 164. https://doi.org/10.3390/min9030164
APA StyleŠtubňa, J., Bačík, P., Fridrichová, J., Hanus, R., Illášová, Ľ., Milovská, S., Škoda, R., Vaculovič, T., & Čerňanský, S. (2019). Gem-Quality Green Cr-Bearing Andradite (var. Demantoid) from Dobšiná, Slovakia. Minerals, 9(3), 164. https://doi.org/10.3390/min9030164





