Adsorption Properties of Sepiolite in Relation to Uranium and Lanthanide Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorption Determination
2.3. Methods
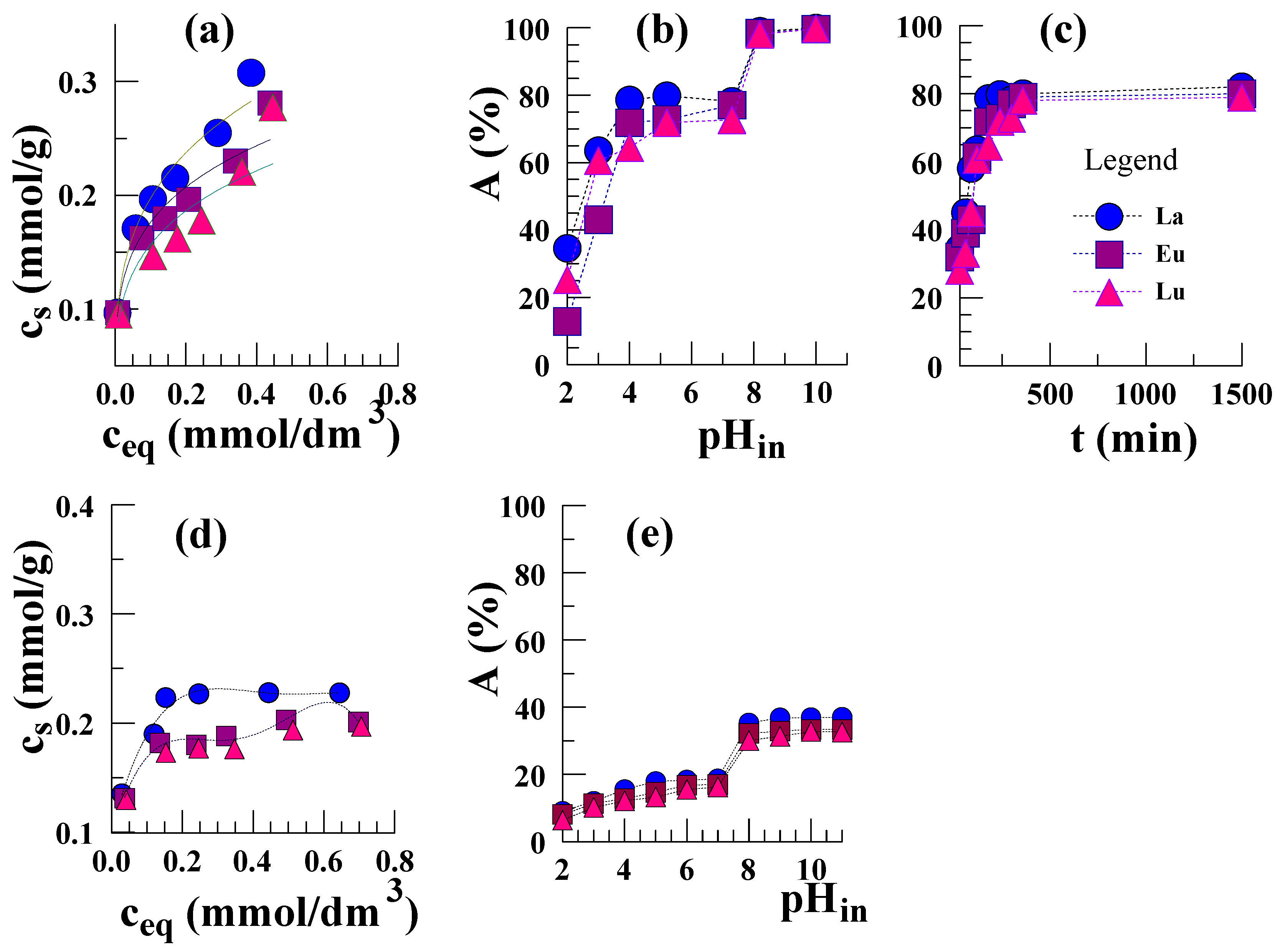
3. Results and Discussion
3.1. Surface Propetries of the Sepiolite
3.2. Scanning Electron Microscopy Analysis
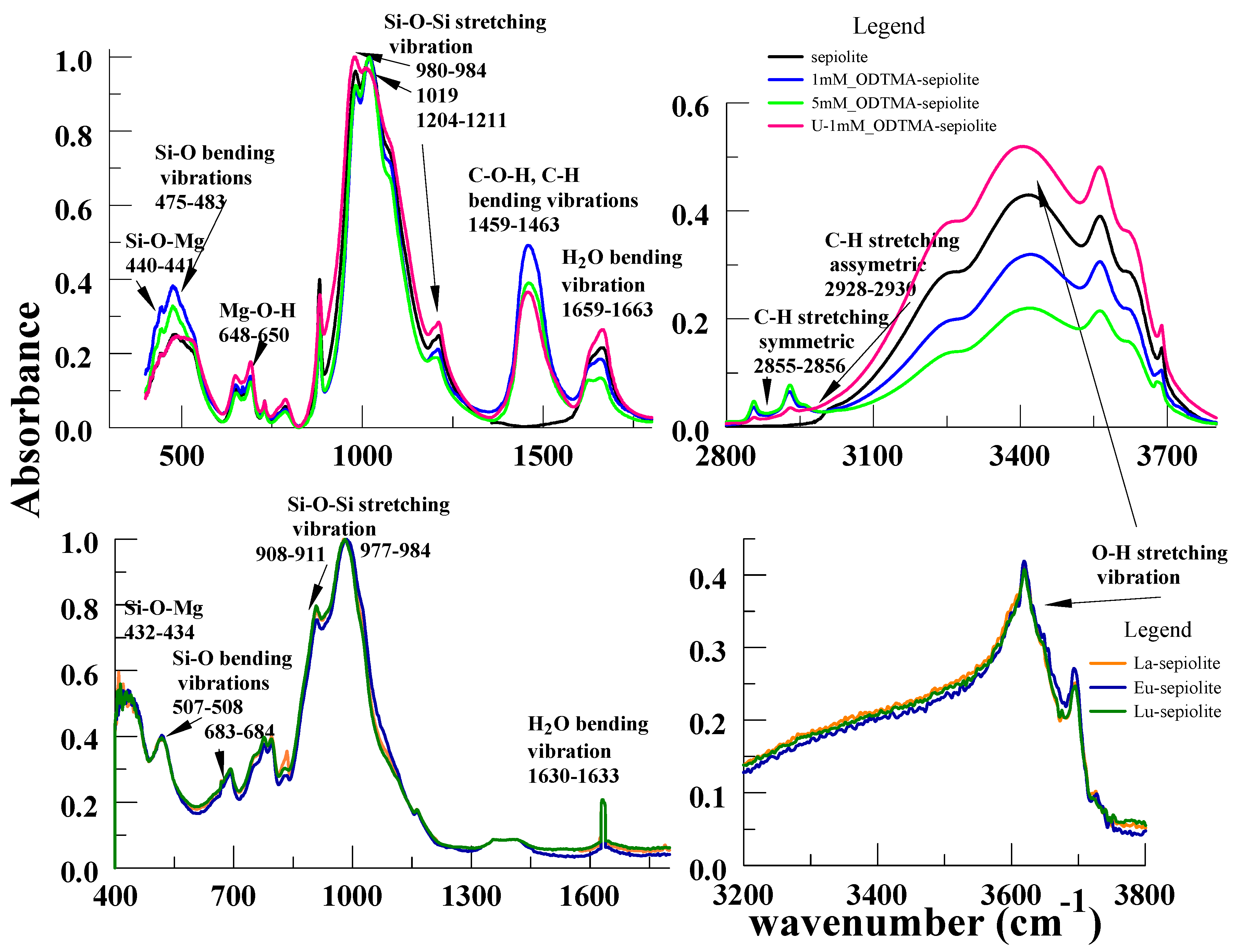
3.3. Fourier-Transform Infrared Spectroscopy and Fluorescence Spectra of Sepiolite
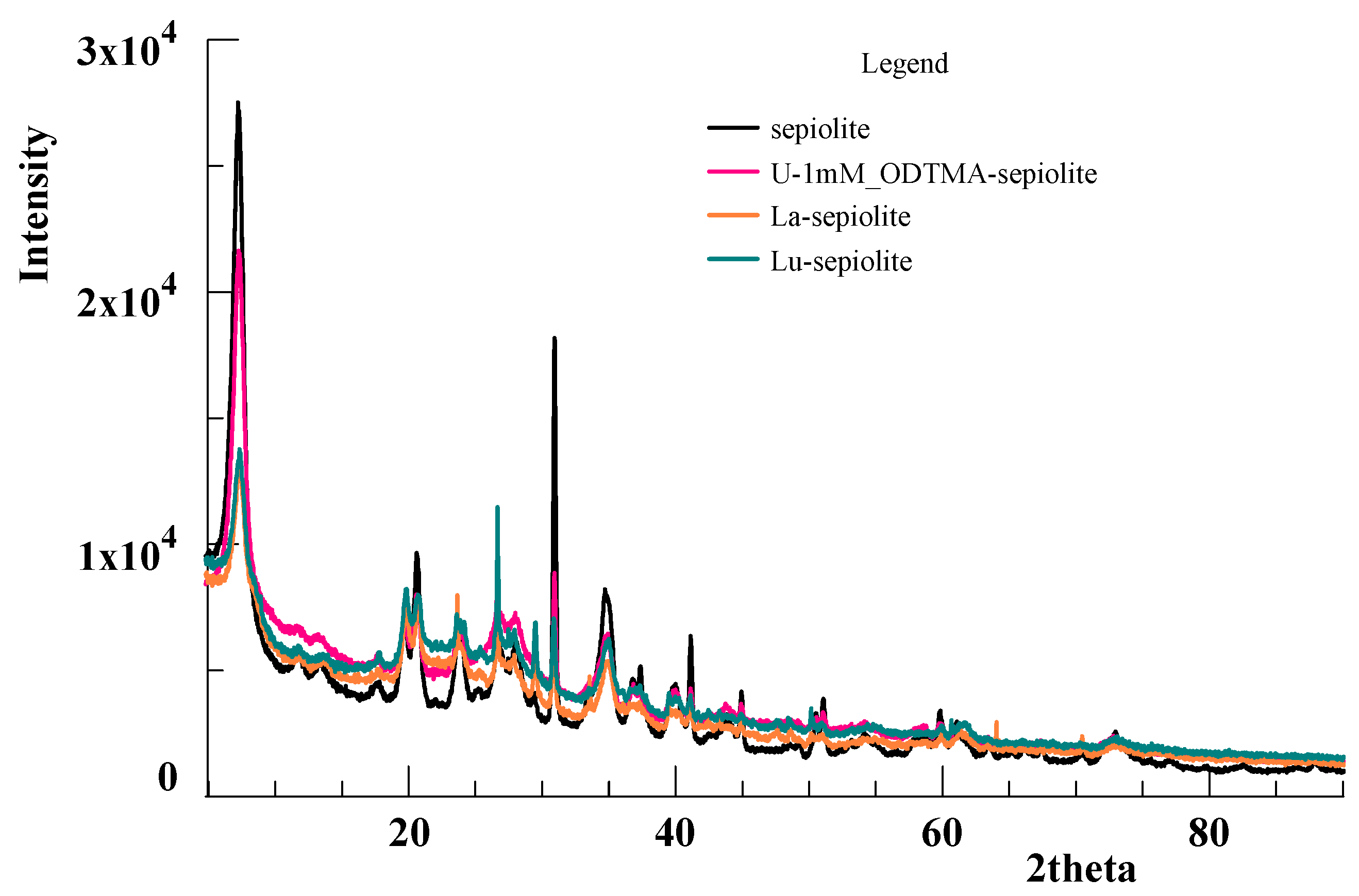
3.4. X-ray Photoelectron Spectroscopy Analysis
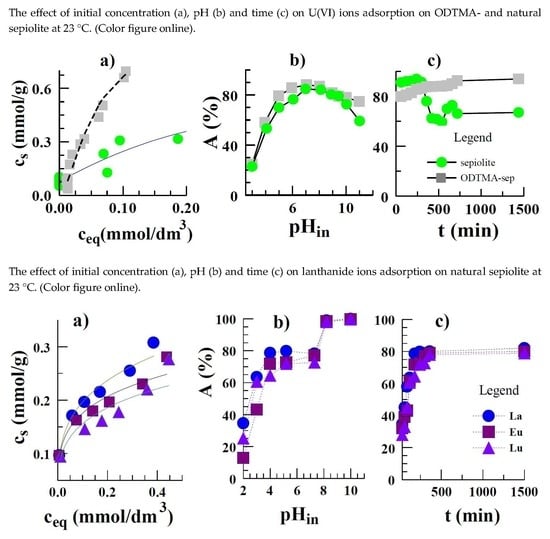
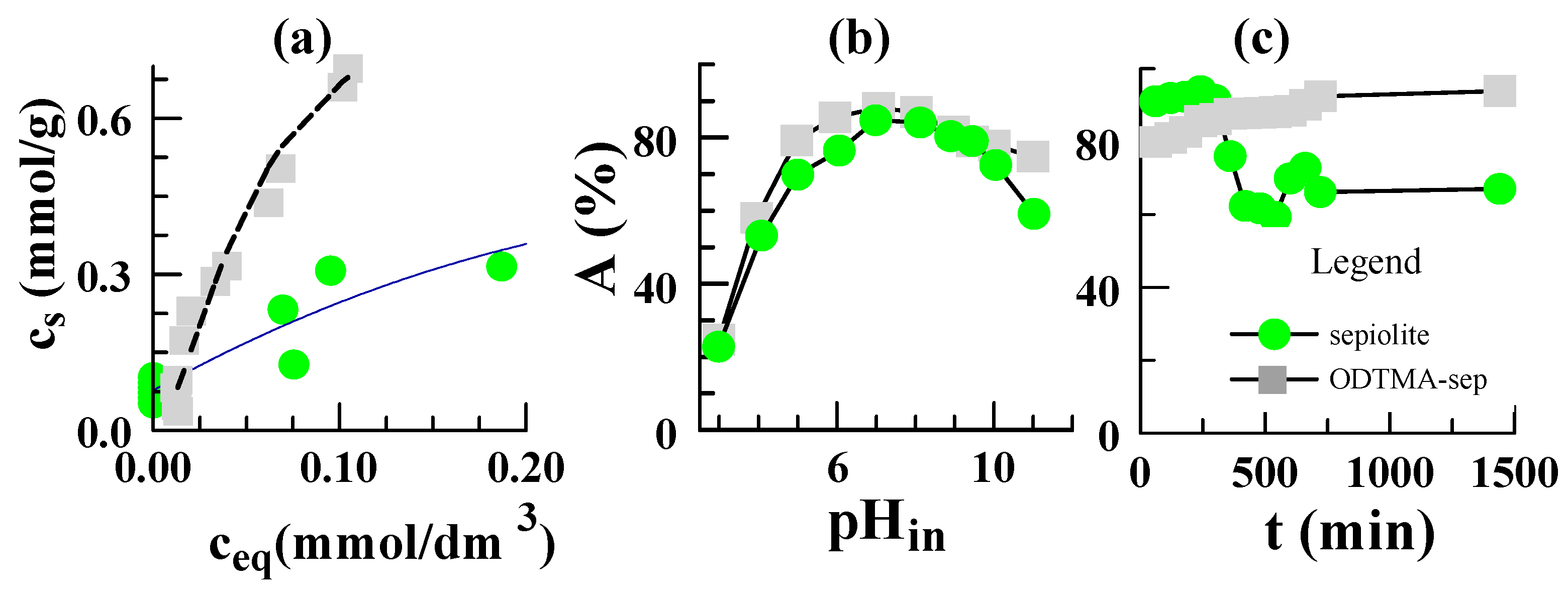
3.5. Equilibrium Data of Sorption on Sepiolite
3.6. Proposed Mechanism of Adsorption
3.7. Comparison of ODTMA-Sepiolite with Other Adsorbents
4. Conclusions
Funding
Conflicts of Interest
References
- Qafoku, N.P.; Icenhower, J.P. Interactions of aqueous U(VI) with soil minerals in slightly alkaline natural systems. Rev. Environ. Sci. Biotechnol. 2008, 7, 355–361. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C.; Zhou, X.; Yang, J.; Zhang, X.; Wang, J. Removal of uranium (VI) from aqueous solution by adsorption of hematite. J. Environ. Radioact. 2009, 100, 162–168. [Google Scholar]
- Zakutevskii, O.I.; Psareva, T.S.; Strelko, V.V.; Kartel, N.T. Sorption of U(VI) from Aqueous Solutions with Carbon Sorbents. Radiochemistry 2007, 49, 67–73. [Google Scholar] [CrossRef]
- Yusan, S.; Erenturk, S.A. Adsorption equilibrium and kinetics of U(VI) on beta type of akaganeite. Desalination 2010, 263, 233–239. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, C.; Wang, J.; Liu, Q.; Li, R.; Yang, P.; Zhang, M. Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: Kinetic and thermodynamic investigation. Chem. Eng. J. 2012, 198, 412–420. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Li, J.; Wang, X. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2015, 349, 129–133. [Google Scholar] [CrossRef]
- Arnold, T.; Zorn, T.; Zanker, H.; Bernhard, G.; Nitsche, H. Sorption behavior of U(VI) on phyllite: Experiments and modeling. J. Contam. Hydrol. 2001, 47, 219–225. [Google Scholar] [CrossRef]
- Filipi, R.; Nesmerak, K.; Rucki, M.; Roth, Z.; Hanzlikova, I.; Tichy, M. Acute toxicity of rare earth elements and their compounds. Chem. Listy 2007, 101, 793–798. [Google Scholar]
- Gladysz-Plaska, A.; Majdan, M.; Ferenc, W.; Sarzynski, J. Comparison of covalency in the lanthanide chloride and nitrate complexes based on the adsorption data on zeolite Y. J. Mol. Struct. 2011, 3, 469–474. [Google Scholar] [CrossRef]
- Majdan, M.; Pikus, S.; Gladysz-Plaska, A.; Fuks, L.; Zieba, E. Adsorption of light lanthanides on the zeolite A Surface. Colloids Surf. A 2002, 209, 27–35. [Google Scholar] [CrossRef]
- Donat, R. The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J. Chem. Thermodyn. 2009, 41, 829–834. [Google Scholar] [CrossRef]
- Özdemir, O.; Cınar, M.; Sabah, E.; Arslan, F.; Celik, M.S. Adsorption of anionic surfactants onto sepiolite. J. Hazard. Mater. 2007, 147, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Structure of organo-clays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2001, 277, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming montmorillonite surfaces using a cationic surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef]
- He, H.; Ding, Z.; Zhu, J.; Yuan, P.; Xi, Y.; Yang, D.Y.; Frost, R.L. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005, 53, 287–293. [Google Scholar] [CrossRef]
- He, H.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Xi, Y.; Kloprogge, T.J. Changes in the morphology of organoclays with HDTMA+ surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef]
- Cody, C.A.; Kemnetz, S. Process for the Removal of Heavy Metals from Aqueous Systems Using Organoclays. European Patent EPO765842, 1999. [Google Scholar]
- Dolatyari, L.; Yaftian, M.R.; Rostamnia, S. Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J. Environ. Manag. 2016, 169, 8–15. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Grabias, E.; Majdan, M. Simultaneous adsorption of uranium(VI) and phosphate on red clay. Prog. Nucl. Energy 2018, 104, 150–159. [Google Scholar] [CrossRef]
- Majdan, M.; Pikus, S.; Gajowiak, A.; Sternik, D.; Zieba, E. Uranium sorption on bentonite modified by octadecyltrimethylammonium bromide. J. Hazard. Mater. 2010, 184, 662–667. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, J.H.; Jin, J.M.; Lee, S.H. Adsorption characteristics of volatile organic compounds onto lyocell-based activated carbon fibers. Carbon Lett. 2019, 39, 1–10. [Google Scholar]
- Liu, X.; Wang, L.; Zheng, Z.; Kang, M.; Li, C.; Liu, C. Molecular dynamics simulation of the diffusion of uranium species in clay pores. J. Hazard. Mater. 2013, 245, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, J.; Zheng, S.; Hashisho, Z. Adsorption of volatile organic compounds onto natural porous materials. J. Hazard. Mater. 2019, 364, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Buekens, A.; Zyaykina, N.N. Adsorbents and Adsorption Processes for Pollution Control, Pollution Control Technologies—Vol. II. In Adsorbents and Adsorption Processes for Pollution Control; Encyclopedia of Life Support Systems (EOLSS): Brussel, Belgium, 2013; pp. 1–10. [Google Scholar]
- Huang, M.-C.; Chou, C.-H.; Teng, H. Pore-Size Effects on Activated-Carbon Capacities for Volatile Organic Compound Adsorption. Mater. Interfaces Electrochem. Phenom. 2002, 48, 1804–1810. [Google Scholar] [CrossRef]
- Artner, C.; Kronister, S.; Czakler, M.; Schubert, U. Ion-Size-Dependent Formation of Mixed Titanium/Lanthanide Oxo Clusters. Eur. J. Inorg. Chem. 2014, 32, 5596–5602. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Qin, X. Preparation and characterization of mercapto functionalized sepiolite and their application for sorption of lead and cadmium. Chem. Eng. J. 2011, 174, 436–444. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, C.; Tu, H.; Yang, J.; Li, F.; Li, D.; Liao, X.; Yang, Y.; Tang, J.; Liu, N. U(VI) adsorption onto cetyltrimethylammonium bromide modified bentonite in the presence of U(VI)-CO3 complexes. Appl. Clay Sci. 2017, 135, 64–74. [Google Scholar] [CrossRef]
- Křepelovǎ, A. Influence of Humic Acid on the Sorption of Uranium(VI) and Americium(III), Disseration; University of Technology: Dresden, Germany, 2007. [Google Scholar]
- Baumann, N.; Brendler, V.; Arnold, T.; Geipel, G.; Bernhard, G. Uranyl sorption onto gibbsite studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS). J. Colloid Interface Sci. 2005, 290, 318–322. [Google Scholar] [CrossRef]
- Trepte, P. Sorption von Radionukliden an Tongestein: Spektroskopische Referenzdaten. Master’s Thesis, University of Applied Science, Dresden, Germany, 2006. [Google Scholar]
- Wang, G.; Wang, X.; Chai, X.; Liu, J.; Deng, N. Adsorption of uranium (VI) from aqueous solution on calcined and acid-activated kaolin. Appl. Clay Sci. 2010, 47, 448–455. [Google Scholar] [CrossRef]
- Gajowiak, A.; Gładysz-Płaska, A.; Sternik, D.; Pikus, S.; Sabah, E.; Majdan, M. Sorption of uranyl ions on organosepiolite. Chem. Eng. J. 2013, 219, 459–468. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kovaluk, I.; Buszewski, B. The separation of uranium ions by natural and modified diatomite from aqueous solution. J. Hazard. Mater. 2010, 181, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 2009, 34, 451–459. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Cao, X.; Wang, Y.; Jiang, X.; Li, M.; Hua, M.; Zhang, Z. Removal of uranium(VI) from aqueous solutions by CMK-3 and its polymer composite. Appl. Surf. Sci. 2013, 285, 258–264. [Google Scholar] [CrossRef]
- Han, R.P.; Zou, W.H.; Wang, Y.; Zhu, L. Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: Discussion of adsorption isotherms and pH effect. J. Environ. Radioact. 2007, 93, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhen, J.; Zhou, L. Adsorption and photocatalytic reduction of U(VI) in aqueous TiO2 suspensions enhanced with sodium formate. J. Radioanal. Nucl. Chem. 2015, 304, 579–585. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, Z.B.; Liu, Y.H. Adsorption of uranium from aqueous solution using HDTMA+-pillared bentonite: Isotherm, kinetic and thermodynamic aspects. J. Radioanal. Nucl. Chem. 2012, 293, 231–239. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Majdan, M.; Tarasiuk, B.; Sternik, D.; Grabias, E. The use of halloysite functionalized with isothiouronium salts as an organic/inorganic hybrid adsorbent for uranium(VI) ions removal. J. Hazard. Mater. 2018, 354, 133–144. [Google Scholar] [CrossRef]
- Youssef, W.M. Uranium Adsorption from Aqueous Solution Using Sodium Bentonite Activated Clay. J. Chem. Eng. Process Technol. 2017, 8, 157–170. [Google Scholar]
- Turanov, A.; Karandashev, V.; Sukhinina, N.; Masalov, V.; Zhokhov, A.; Emelchenko, G. A novel sorbent for lanthanide adsorption based on tetraoctyldiglycolamide, modified carbon inverse opals. RSC Adv. 2015, 5, 529–535. [Google Scholar] [CrossRef]
- Li, W.; Lin, P.; Dai, S.; Sun, X.; Shen, Y. Preparation of a mesocellular siliceous foam supported lanthanide-sensitive polymer for the selective adsorption of lanthanides. Dalton Trans. 2018, 47, 4840–4849. [Google Scholar] [CrossRef]
- Kusrini, E.; Kinastiti, D.D.; Wilson, L.D.; Usman, A.; Rahman, A. Adsorption of lanthanide ions from an aqueous solution in multicomponent systems using activated carbon from banana peels (Musa paradisiaca L.). Int. J. Technol. 2018, 6, 1132–1139. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Zabłocka-Malicka, M.; Pasiecznik, I.; Pohl, P.; Rutkowski, P. Adsorption of La3+ and Dy3+ ions on biohydroxyapatite obtained from pork bones gasified with steam. Environ. Prot. Eng. 2018, 44, 29–40. [Google Scholar]
- Andres, Y.; MacCordick, H.J.; Hubert, J.-C. Adsorption of several actinide (Th, U) and lanthanide (La, Eu, Yb) ions by Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 1993, 39, 413–417. [Google Scholar] [CrossRef]
- Alakhras, F. Kinetic Studies on the Removal of Some Lanthanide Ions from Aqueous Solutions Using Amidoxime-Hydroxamic Acid Polymer. J. Anal. Methods Chem. 2018, 2018, 4058503. [Google Scholar] [CrossRef]
- Belgacem, A.; Rebiai, R.; Hadoun, H.; Khemaissia, S.; Belmedani, M. The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ. Sci. Pollut. Res. 2014, 21, 684–689. [Google Scholar] [CrossRef]
- Durán-Blanco, J.M.; López-Muñoz, B.E.; Olguín, M.T. Influence of pH on U(VI) Adsorption using a Thermally-Treated Mg-Al Hydrotalcite and a Natural Zeolite in a Batch System. Separ. Sci. Technol. 2013, 48, 797–809. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 42.19 | 0.87 | 0.48 | 0.006 | 22.90 | 10.30 | <0.01 | 0.19 | 0.05 | 0.02 |
| Sample | BET Total Surface Area (m2/g) | Micropore Area (m2/g) | Micropore Volume (cm3/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (Å) |
|---|---|---|---|---|---|
| Sepiolite | 342 | 157 | 0.069 | 0.399 | 45 |
| 1mM-ODTMA-sepiolite | 212 | 26 | 0.011 | 0.380 | 86 |
| 5mM-ODTMA-sepiolite | 177 | 18 | 0.007 | 0.364 | 95 |
| U-1mM-ODTMA-sepiolite | 162 | 10 | 0.007 | 0.323 | 98 |
| La-sepiolite | 248 | 89 | 0.025 | 0.383 | 55 |
| Eu-sepiolite | 253 | 91 | 0.027 | 0.384 | 58 |
| Lu-sepiolite | 257 | 92 | 0.027 | 0.385 | 59 |
| Band Assignment | Wavenumber, cm−1 | ||||||
|---|---|---|---|---|---|---|---|
| Sepiolite | 1 mM_ODTMA-Sepiolite | 5 mM_ODTMA-Sepiolite | U-1·mM_ODTMA-Sepiolite | La-Sepiolite | Eu-Sepiolite | Lu-Sepiolite | |
| Si–O–Mg bending | 440 | 441 | 441 | 440 | 432 | 433 | 434 |
| Si–O–Si bending | 479 | 475 | 475 | 483 | 507 | 507 | 508 |
| Mg–O–H bending | 648 | 648 | 650 | 648 | - | - | - |
| Si–O bending | 690 | 690 | 690 | 690 | 683 | 684 | 683 |
| Si–O–Si stretching | 980 1019 1211 | 983 1019 1209 | 984 1019 1204 | 980 1019 1211 | 910 984 | 908 980 | 911 977 |
| C–H bending | - | 1463 | 1463 | 1459 | - | - | - |
| H2O bending | 1663 | 1659 | 1659 | 1663 | 1630 | 1631 | 1633 |
| C–H stretching symmetric | - | 2855 | 2855 | 2856 | - | - | - |
| C–H stretching asymmetric | - | 2928 | 2929 | 2930 | - | - | - |
| O–H stretching | 3416 3562 | 3420 3562 | 3420 3562 | 3400 3562 | 3616 | 3619 | 3619 |
| Region | C1s | O1s | Mg1s | Si2p | F1s | U4f/Ln3d | |
|---|---|---|---|---|---|---|---|
| Peak position | sepiolite | 284.7 | 532.7 | 1304.7 | 103.2 | 686.2 | - |
| 1mM_ODTMA-sepiolite | 284.7 | 532.7 | 1304.7 | 103.2 | 686.7 | - | |
| U-1mM_ODTMA-sepiolite | 284.7 | 531.2 | 1303.8 | 102.7 | 685.2 | 382.2 | |
| La-sepiolite | 285.6 | 532.1 | 1312.3 | 102.8 | 686.1 | 835.6 | |
| Eu-sepiolite | 285.7 | 532.2 | 1312.5 | 102.8 | 686.1 | 1136 | |
| Lu-sepiolite | 285.9 | 532.4 | 1313.2 | 102.6 | 685.4 | 1345 | |
| Intensity (CPS) | sepiolite | 25,026.5 | 417,224 | 250,349 | 54,183.9 | 14,863.2 | - |
| 1mM_ODTMA-sepiolite | 31,044.6 | 412,383 | 242,361 | 55,103.5 | 13,149.4 | - | |
| U-1mM_ODTMA-sepiolite | 23,433.7 | 374,534 | 209,122 | 47,450.8 | 13,278.8 | 94,042.8 | |
| La-sepiolite | 21,345.3 | 321,689 | 190,234 | 43,555 | 11,023 | 72,346 | |
| Eu-sepiolite | 22,454 | 313,222 | 198,645 | 44,356 | 12,008 | 68,955 | |
| Lu-sepiolite | 22,635 | 317,890 | 197,653 | 43,864 | 12,024 | 69,034 | |
| % At Conc | sepiolite | 9.6 | 54.9 | 8.6 | 25.6 | 1.3 | - |
| 1mM_ODTMA-sepiolite | 11.8 | 53.3 | 8.2 | 25.6 | 1.1 | - | |
| U-1mM_ODTMA-sepiolite | 10.1 | 54.9 | 8 | 24.9 | 1.3 | 0.8 | |
| La-sepiolite | 8.9 | 54.6 | 6.8 | 24.3 | 1.1 | 0.5 | |
| Eu-sepiolite | 8.3 | 56.2 | 6.6 | 24.8 | 1.1 | 0.49 | |
| Lu-sepiolite | 8.8 | 55.7 | 6.8 | 24.9 | 1.1 | 0.5 | |
| % Mass Conc | sepiolite | 6 | 45.1 | 10.8 | 36.9 | 1.3 | - |
| 1mM_ODTMA-sepiolite | 7.3 | 44.1 | 10.3 | 37.1 | 1.1 | ||
| U-1mM_ODTMA-sepiolite | 5.7 | 41.5 | 9.2 | 33.1 | 1.2 | 9.3 | |
| La-sepiolite | 5.2 | 40.8 | 8.2 | 32.2 | 1.1 | 6.6 | |
| Eu-sepiolite | 5.3 | 41.8 | 8.0 | 32.4 | 1.1 | 6.4 | |
| Lu-sepiolite | 5.5 | 41.0 | 8.2 | 33.1 | 1.1 | 6.6 | |
| Model | Parameters | U(VI) + Sepiolite | La(III) + Sepiolite | Eu(III) + Sepiolite | Lu(III) + Sepiolite | U + ODTMA-Sepiolite |
|---|---|---|---|---|---|---|
| Langmuir-Freundlich | KL-F (dm3/mol) N a (mmol/g) R2 | 64610.1 0.81 0.6 0.981 | 4567 0.45 0.66 0.974 | 3488 0.48 0.6 0.977 | 4550 0.46 0.6 0.978 | 132,375.3 0.68 1.2 0.973 |
| Langmuir | KL (dm3/mol) amax (mmol/g) R2 | 6642.2 0.55 0.967 | 478.8 0.42 0.965 | 568.5 0.44 0.966 | 599.6 0.43 0.966 | 7621.1 0.92 0.966 |
| Dubinin-Radushke-vich | qm (mol/g) KD-R (mol2/kJ2) E (kJ/mol) R2 | 0.000247 3.51 × 10−9 14.5 0.948 | 0.00033 2.11 × 10−9 11.2 0.944 | 0.0003 2.31 × 10−9 11.4 0.956 | 0.0003 2.23 × 10−9 11.5 0.943 | 0.000938 4.35 × 10−9 13.3 0.952 |
| Pseudo-first –order kinetics | k1 (1/min) qe calculated (mol/g) R2 | 0.0016 1.39 × 10−5 0.756 | 0.002 3.01·10−5 0.823 | 0.0018 2.99 × 10−5 0.818 | 0.002 2.49 × 10−5 0.822 | 0.00235 1.62 × 10−5 0.752 |
| Pseudo-second-order kinetics | k2 (g/mol·min) qe calculated (mol/g) R2 | 0.000313 6.01 × 10−4 0.999 | 342 4.6 × 10−4 0.998 | 333.25 4.4 × 10−4 0.993 | 367.5 4.2 × 10−4 0.996 | 30581 1.1 × 10−3 0.999 |
| Intraparti-cle diffusion | k3 (mg/g·min0.5) R2 | 4.55 0.758 | 3.45 0.736 | 3.23 0.722 | 3.33 0.721 | 3.3 0.663 |
| Sorbent | Sorption Capacity, qmax, mg/g | References | |||
|---|---|---|---|---|---|
| La(III) | Eu(III) | Lu(III) | U(VI) | ||
| TiO2 | 44 | [38] | |||
| HDTMA+-pillared bentonite Na-bentonite | 106.465 | [39] | |||
| Organo-halloysite | 157 | [40] | |||
| HDTMA-sepiolite | 212 | [33] | |||
| Sodium bentonite activated clay | 11.8 | [41] | |||
| Carbon modified with tetraoctyldiglycolamide (TODGA) | 18 | [42] | |||
| Montmorillonite-carbon composite | 20.8 | [6] | |||
| Mesocellular siliceous foam polymer | 136 | 138 | 147 | [43] | |
| Activated Carbon from Banana Peels (Musa Paradisiaca L.) | 67 | - | - | - | [44] |
| Biohydroxyapatite | 17 | [45] | |||
| Biomass Mycobacterium smegmatis | 55 | 49 | 114 | [46] | |
| Amidoxime-Hydroxamic Acid Polymer | 69 | [47] | |||
| Activated carbon | 158 | [48] | |||
| Zeolite-MnO2 | 180 | [37] | |||
| Kaolin | 4.5 | [32] | |||
| Calcined Mg-Al hydrotalcite | 42.4 | [49] | |||
| Sepiolite | 91.6 | 91.4 | 104.9 | 142.8 | This work |
| ODTMA-sepiolite | 285.6 | This work | |||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gładysz-Płaska, A. Adsorption Properties of Sepiolite in Relation to Uranium and Lanthanide Ions. Minerals 2019, 9, 686. https://doi.org/10.3390/min9110686
Gładysz-Płaska A. Adsorption Properties of Sepiolite in Relation to Uranium and Lanthanide Ions. Minerals. 2019; 9(11):686. https://doi.org/10.3390/min9110686
Chicago/Turabian StyleGładysz-Płaska, Agnieszka. 2019. "Adsorption Properties of Sepiolite in Relation to Uranium and Lanthanide Ions" Minerals 9, no. 11: 686. https://doi.org/10.3390/min9110686
APA StyleGładysz-Płaska, A. (2019). Adsorption Properties of Sepiolite in Relation to Uranium and Lanthanide Ions. Minerals, 9(11), 686. https://doi.org/10.3390/min9110686