Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ochre Composition
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.3.1. Powder X-Ray Diffraction (PXRD)
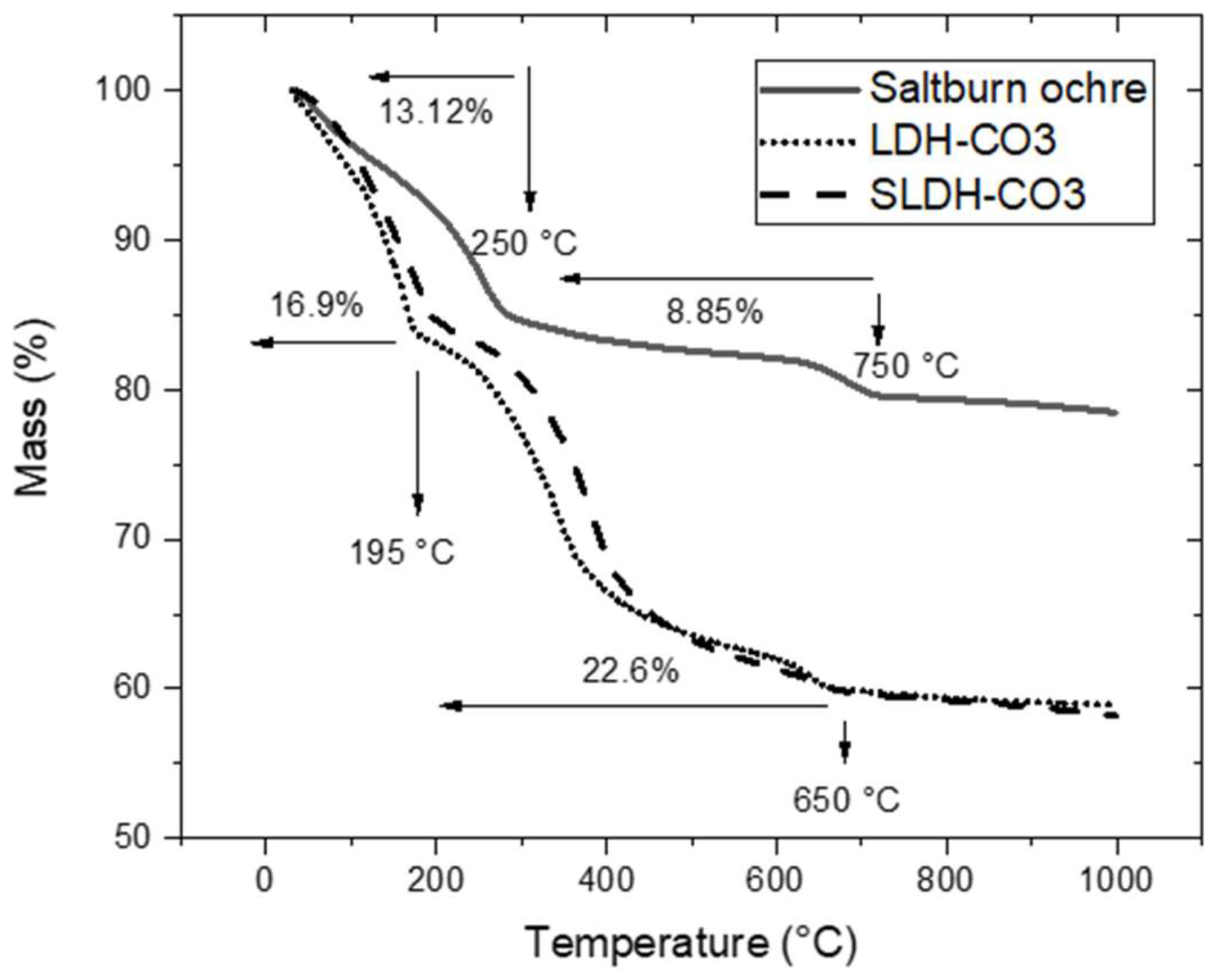
2.3.2. Thermogravimetric Analysis (TGA)
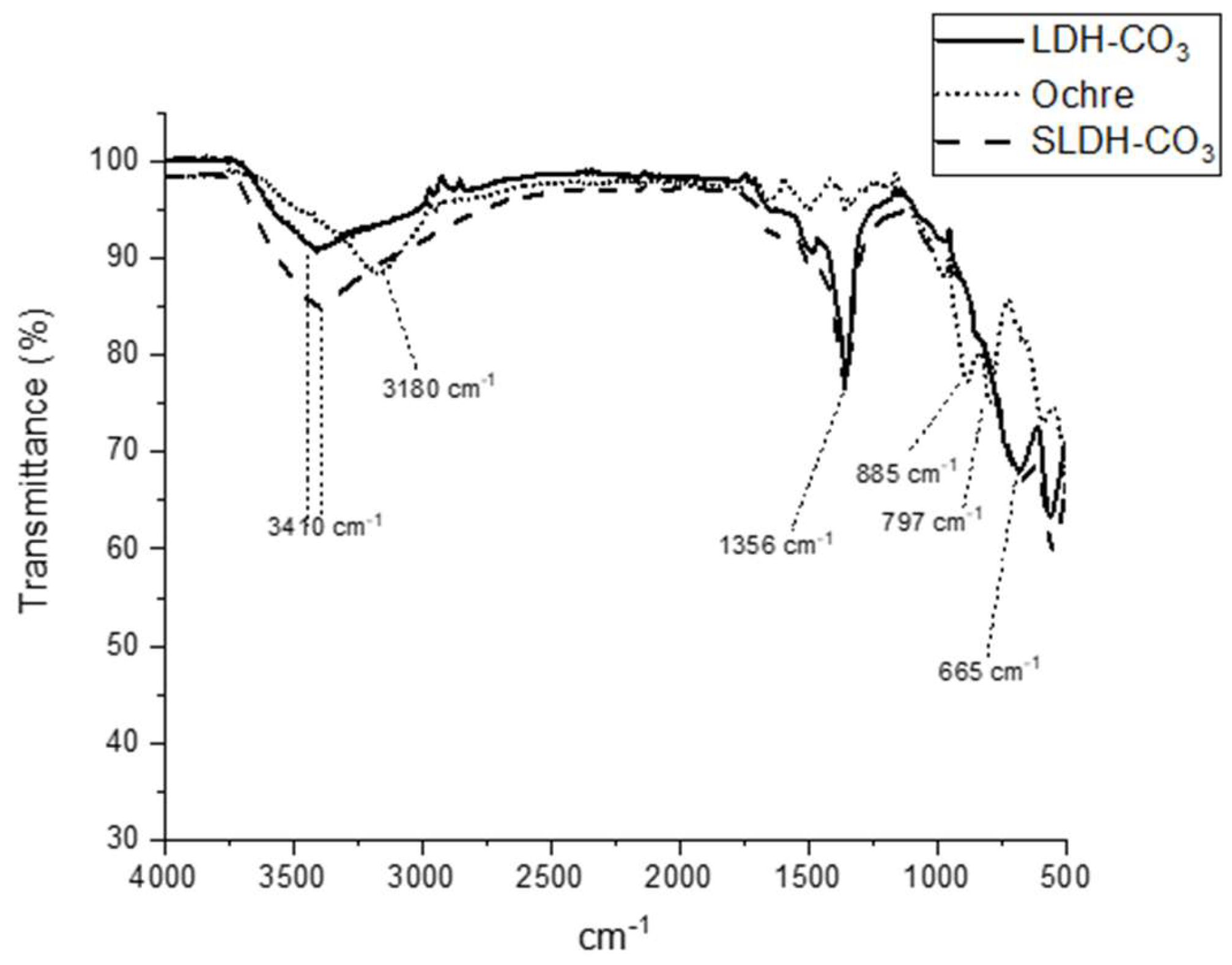
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.4. Inductively-Coupled Plasma–Optical Emission Spectrometry (ICP-OES)
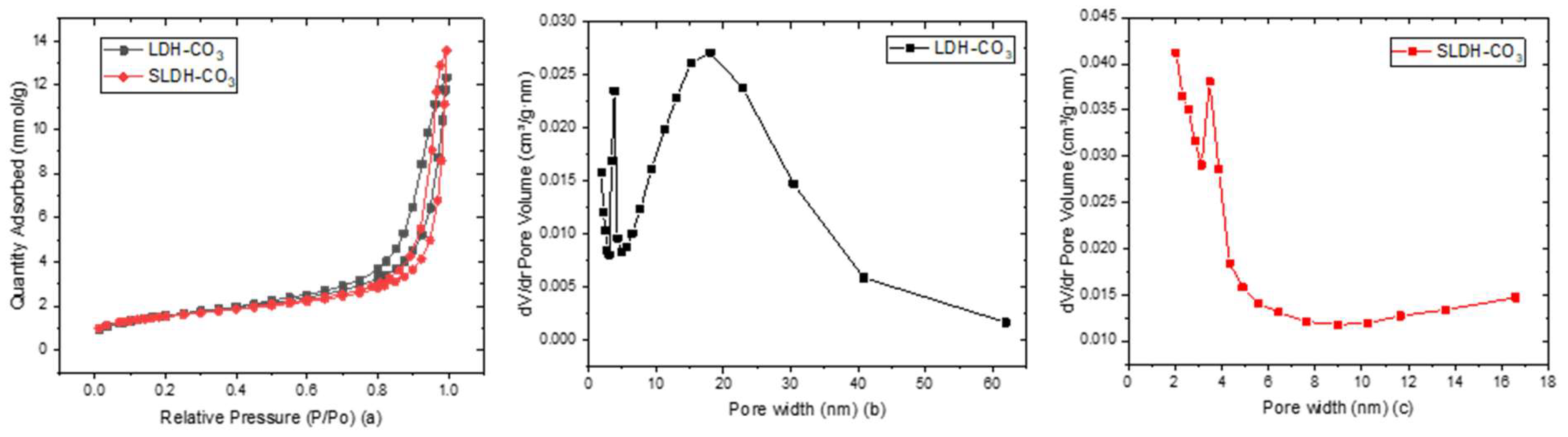
2.3.5. Brunauer-Emmett-Teller (BET) N2 Adsorption and Desorption Measurements
2.4. Ketonic Decarboxylation Reaction Procedure
Analysis of Crude Reaction Product
3. Results
3.1. Saltburn Ochre and As-Synthesized LDH-CO3 (Ochre-Derived and Synthetic) Characterization
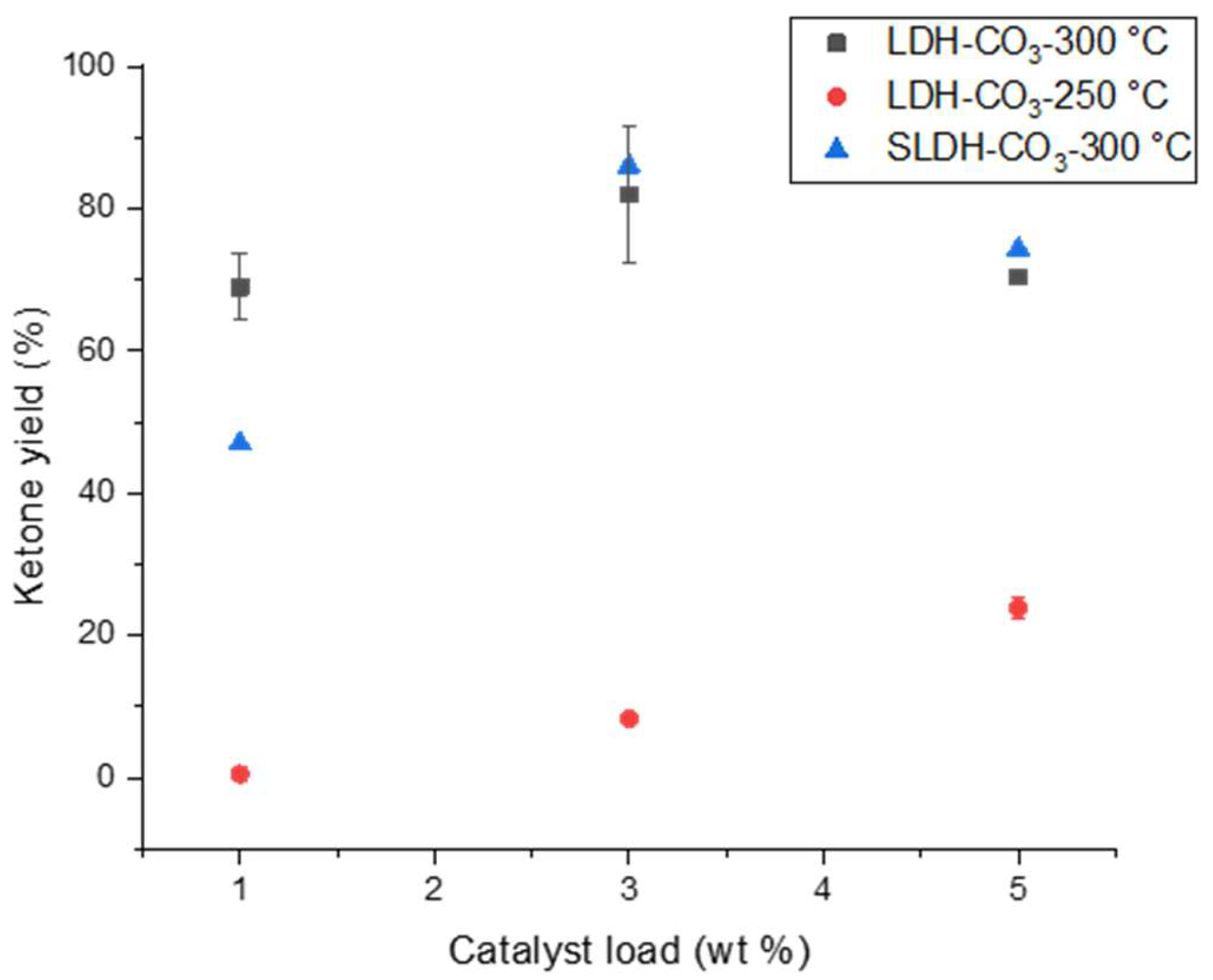
3.2. LDH-CO3 as a Catalyst for the Ketonic Decarboxylation of Lauric Acid
4. Discussion
4.1. Characterisation of Ochre to LDH Conversion
4.2. Ketonic Decarboxylation of Lauric Acid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Noraini, M.Y.; Ong, H.C.; Badrul, M.J.; Chong, W.T. A review on potential enzymatic reaction for biofuel production from algae. Renew. Sustain. Energy Rev. 2014, 39, 24–34. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Janampelli, S.; Darbha, S. Selective and reusable Pt-WOx/Al2O3 catalyst for deoxygenation of fatty acids and their esters to diesel-range hydrocarbons. Catal. Today 2017, 309, 219–226. [Google Scholar] [CrossRef]
- Na, J.-G.; Han, J.K.; Oh, Y.-K.; Park, J.-H.; Jung, T.S.; Han, S.S.; Yoon, H.C.; Chung, S.H.; Kim, J.-N.; Ko, C.H. Decarboxylation of microalgal oil without hydrogen into hydrocarbon for the production of transportation fuel. Catal. Today 2012, 185, 313–317. [Google Scholar] [CrossRef]
- Na, J.-G.; Yi, B.E.; Kim, J.N.; Yi, K.B.; Park, S.-Y.; Park, J.-H.; Kim, J.-N.; Ko, C.H. Hydrocarbon production from decarboxylation of fatty acid without hydrogen. Catal. Today 2010, 156, 44–48. [Google Scholar] [CrossRef]
- Snell, R.W.; Shanks, B.H. Insights into the Ceria-Catalyzed Ketonization Reaction for Biofuels Applications. ACS Catal. 2013, 3, 783–789. [Google Scholar] [CrossRef]
- Simakova, I.L.; Murzin, D.Y. Transformation of bio-derived acids into fuel-like alkanes via ketonic decarboxylation and hydrodeoxygenation: Design of multifunctional catalyst, kinetic and mechanistic aspects. J. Energy Chem. 2016, 25, 208–224. [Google Scholar] [CrossRef]
- Corma, A.; Renz, M.; Schaverien, C. Coupling Fatty Acids by Ketonic Decarboxylation Using Solid Catalysts for the Direct Production of Diesel, Lubricants, and Chemicals. ChemSusChem 2008, 1, 739–741. [Google Scholar] [CrossRef]
- Smith, B.; Li, L.; Perera-Solis, D.; Gildea, L.; Zholobenko, V.; Dyer, P.; Greenwell, H. Ketone Formation via Decarboxylation Reactions of Fatty Acids Using Solid Hydroxide/Oxide Catalysts. Inorganics 2018, 6, 121. [Google Scholar] [CrossRef]
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion. ACS Catal. 2013, 3, 2456–2473. [Google Scholar] [CrossRef]
- Pulido, A.; Oliver-Tomas, B.; Renz, M.; Boronat, M.; Corma, A. Ketonic Decarboxylation Reaction Mechanism: A Combined Experimental and DFT Study. ChemSusChem 2013, 6, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Dobbie, K.E.; Heal, K.V.; Aumônier, J.; Smith, K.A.; Johnston, A.; Younger, P.L. Evaluation of iron ochre from mine drainage treatment for removal of phosphorus from wastewater. Chemosphere 2009, 75, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Heal, K.V.; Smith, K.; Younger, P.; McHaffie, H.; Batty, L. Removing phosphorus from sewage effluent and agricultural runoff using recovered ochre. In Phosphorus in Environmental Technology: Principles and Applications; Valsami-Jones, E., Ed.; IWA Publishing: London, UK, 2004; pp. 321–335. [Google Scholar]
- Sapsford, D.; Santonastaso, M.; Thorn, P.; Kershaw, S. Conversion of coal mine drainage ochre to water treatment reagent: Production, characterisation and application for P and Zn removal. J. Environ. Manag. 2015, 160, 7–15. [Google Scholar] [CrossRef]
- Littler, J.; Geroni, J.N.; Sapsford, D.J.; Coulton, R.; Griffiths, A.J. Mechanisms of phosphorus removal by cement-bound ochre pellets. Chemosphere 2013, 90, 1533–1538. [Google Scholar] [CrossRef]
- Satterley, C. (The Coal Authority, Mansfield, United Kingdom). Personal communication, 2019.
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Bumajdad, A.; Ali, S.; Mathew, A. Characterization of iron hydroxide/oxide nanoparticles prepared in microemulsions stabilized with cationic/non-ionic surfactant mixtures. J. Colloid Interface Sci. 2011, 355, 282–292. [Google Scholar] [CrossRef]
- Song, S.; Jia, F.; Peng, C. Study on decomposition of goethite/siderite in thermal modification through XRD, SEM and TGA measurements. Surf. Rev. Lett. 2014, 21, 1450019. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Rui, X.; Chen, J.; Sim, D.; Shi, W.; Hng, H.H.; Lim, T.M.; Yan, Q. Synthesis of hexagonal-symmetry α-iron oxyhydroxide crystals using reduced graphene oxide as a surfactant and their Li storage properties. CrystEngComm 2012, 14, 147–153. [Google Scholar] [CrossRef]
- Shekoohi, K.; Hosseini, F.S.; Haghighi, A.H.; Sahrayian, A. Synthesis of some Mg/Co-Al type nano hydrotalcites and characterization. MethodsX 2017, 4, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Borges, M.E.; Garín, M.; Hernández, A. Biodiesel Production from Waste Oil Using Mg−Al Layered Double Hydroxide Catalysts. Energy Fuels 2009, 23, 2952–2958. [Google Scholar] [CrossRef]
- del Arco, M.; Malet, P.; Trujillano, R.; Rives, V. Synthesis and Characterization of Hydrotalcites Containing Ni(II) and Fe(III) and Their Calcination Products. Chem. Mater. 1999, 11, 624–633. [Google Scholar] [CrossRef]
- Novillo, C.; Guaya, D.; Allen-Perkins Avendaño, A.; Armijos, C.; Cortina, J.L.; Cota, I. Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 2014, 138, 72–79. [Google Scholar] [CrossRef]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Brian, G.; Erwan, A.; Christian, R.; Cedric, C. Tuning and Investigating the Structure of MII-FeIII Layered Double Hydroxides (MII = NiII, CoII and MgII) in Relation to their Composition: From Synthesis to Anionic Exchange Properties. Curr. Inorg. Chem. 2015, 5, 169–183. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J. Colloid Interface Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, L.; Fang, Y.; Zhang, X.; Lyu, M.; Wang, S. The Adsorption of Dextranase onto Mg/Fe-Layered Double Hydroxide: Insight into the Immobilization. Nanomaterials 2018, 8, 173. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gasser, M.S. Adsorption study of anionic reactive dye from aqueous solution to Mg–Fe–CO3 layered double hydroxide (LDH). Appl. Surf. Sci. 2012, 259, 650–656. [Google Scholar] [CrossRef]
- Jia, X.; Li, D.; Evans, D.G.; Lin, Y. Optimization of the wash process and wash water recycling in the preparation of MgZnAl–CO3 layered double hydroxides. Particuology 2010, 8, 231–233. [Google Scholar] [CrossRef]
- Morterra, C.; Chiorlno, A.; Borello, E. An IR spectroscopic characterization of α-FeOOH (goethite). Mater. Chem. Phys. 1984, 10, 119–138. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Shiva Prasad, K.; Krishna Chaitanya, V.; Babu, E.V.S.S.K.; Sreedhar, B.; Ramana Murthy, S. In situ FTIR study on the dehydration of natural goethite. J. Asian Earth Sci. 2006, 27, 503–511. [Google Scholar] [CrossRef]
- Xiao, W.; Jones, A.M.; Collins, R.N.; Bligh, M.W.; Waite, T.D. Use of fourier transform infrared spectroscopy to examine the Fe(II)-Catalyzed transformation of ferrihydrite. Talanta 2017, 175, 30–37. [Google Scholar] [CrossRef]
- Richards, R. Surface and Nanomolecular Catalysis; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Das, J.; Sairam Patra, B.; Baliarsingh, N.; Parida, K.M. Calcined Mg–Fe–CO3 LDH as an adsorbent for the removal of selenite. J. Colloid Interface Sci. 2007, 316, 216–223. [Google Scholar] [CrossRef]
- Cocheci, L.; Barvinschi, P.; Pode, R.; Popovici, E.; Seftel, E. Structural characterization of some Mg/Zn-Al type hydrotalcites prepared for chromate sorption from wastewater. Chem. Bull. Politeh. Univ. 2010, 55, 17. [Google Scholar]
- Yang, K.; Yan, L.-G.; Yang, Y.-M.; Yu, S.-J.; Shan, R.-R.; Yu, H.-Q.; Zhu, B.-C.; Du, B. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Yu, J.; Shu, Z. Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J. Hazard. Mater. 2011, 192, 1114–1121. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Mesoporous materials: Versatile supports in heterogeneous catalysis for liquid phase catalytic transformations. RSC Adv. 2015, 5, 24363–24391. [Google Scholar] [CrossRef]
- Grégoire, B.; Ruby, C.; Carteret, C. Hydrolysis of mixed Ni2+–Fe3+ and Mg2+–Fe3+ solutions and mechanism of formation of layered double hydroxides. Dalton Trans. 2013, 42, 15687–15698. [Google Scholar] [CrossRef]
- Masuda, T.; Kondo, Y.; Miwa, M.; Shimotori, T.; Mukai, S.R.; Hashimoto, K.; Takano, M.; Kawasaki, S.; Yoshida, S. Recovery of useful hydrocarbons from oil palm waste using ZrO2 supporting FeOOH catalyst. Chem. Eng. Sci. 2001, 56, 897–904. [Google Scholar] [CrossRef]
- Masuda, T.; Miwa, Y.; Hashimoto, K.; Ikeda, Y. Recovery of oil from waste poly(ethylene terephthalate) without producing any sublimate materials. Polym. Degrad. Stab. 1998, 61, 217–224. [Google Scholar] [CrossRef]
- Oliver-Tomas, B.; Gonell, F.; Pulido, A.; Renz, M.; Boronat, M. Effect of the Cα substitution on the ketonic decarboxylation of carboxylic acids over m-ZrO2: The role of entropy. Catal. Sci. Technol. 2016, 6, 5561–5566. [Google Scholar] [CrossRef]
- Ignatchenko, A.V. Density Functional Theory Study of Carboxylic Acids Adsorption and Enolization on Monoclinic Zirconia Surfaces. J. Phys. Chem. C 2011, 115, 16012–16018. [Google Scholar] [CrossRef]
- Takagi, K.; Shichi, T.; Usami, H.; Sawaki, Y. Controlled photocycloaddition of unsaturated carboxylates intercalated in hydrotalcite clay interlayers. J. Am. Chem. Soc. 1993, 115, 4339–4344. [Google Scholar] [CrossRef]
- Deng, L.; Fu, Y.; Guo, Q.-X. Upgraded Acidic Components of Bio-oil through Catalytic Ketonic Condensation. Energy Fuels 2009, 23, 564–568. [Google Scholar] [CrossRef]
- Maher, K.D.; Kirkwood, K.M.; Gray, M.R.; Bressler, D.C. Pyrolytic Decarboxylation and Cracking of Stearic Acid. Ind. Eng. Chem. Res. 2008, 47, 5328–5336. [Google Scholar] [CrossRef]
- Torok, B.; Dransfield, T. Green Chemistry: An Inclusive Approach; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]

| Element | Concentration (ppm) in 5 g of Saltburn Ochre | SD * | Concentration (ppm) in 15 mg of LDH-CO3 | SD * | Concentration (ppm) in 15 mg of SLDH-CO3 | SD * |
|---|---|---|---|---|---|---|
| Mg | 32.4 | 1.4 | 270 | 37.8 | 190 | 30.5 |
| Fe | 6091 | 17.6 | 210 | 15.2 | 210 | 20.8 |
| Ca | 499.1 | 1.5 | 15 | 2.1 | 0.52 | 0.01 |
| Si | 12 | 0.12 | 8 | 2.8 | 6.6 | 2.8 |
| Ba | 0.4 | 0.1 | 0.94 | 0.5 | 0.94 | 0.5 |
| Tb | 1 | 0.012 | 0.77 | 2.3 | 0.31 | 2.3 |
| K | 0.09 | 0.1 | 0.72 | 0.6 | 0.56 | 0.6 |
| Mn | 6.3 | 0.1 | 0.62 | 0.1 | 1.1 | 0.1 |
| Na | 2 | 0.1 | 0.57 | 0.1 | 4.1 | 0.1 |
| Sr | 10.8 | 0.1 | 0.55 | 0.04 | - | - |
| Parameter of the Material | LDH-CO3 | SLDH-CO3 |
|---|---|---|
| Specific surface area (m2∙g−1) | 125.91 | 20.92 |
| Pore volume (cm3/g) | 0.39 | 0.13 |
| Pore width (nm) | 14.90 | 2.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera-Solis, D.D.; Pimlott, M.; Fidment, E.; Whiting, A.; Greenwell, H.C. Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation. Minerals 2019, 9, 681. https://doi.org/10.3390/min9110681
Perera-Solis DD, Pimlott M, Fidment E, Whiting A, Greenwell HC. Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation. Minerals. 2019; 9(11):681. https://doi.org/10.3390/min9110681
Chicago/Turabian StylePerera-Solis, Diego D., Matilda Pimlott, Ella Fidment, Andrew Whiting, and Hugh Christopher Greenwell. 2019. "Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation" Minerals 9, no. 11: 681. https://doi.org/10.3390/min9110681
APA StylePerera-Solis, D. D., Pimlott, M., Fidment, E., Whiting, A., & Greenwell, H. C. (2019). Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation. Minerals, 9(11), 681. https://doi.org/10.3390/min9110681






