Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction–Magnetic Separation and Acid Leaching Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
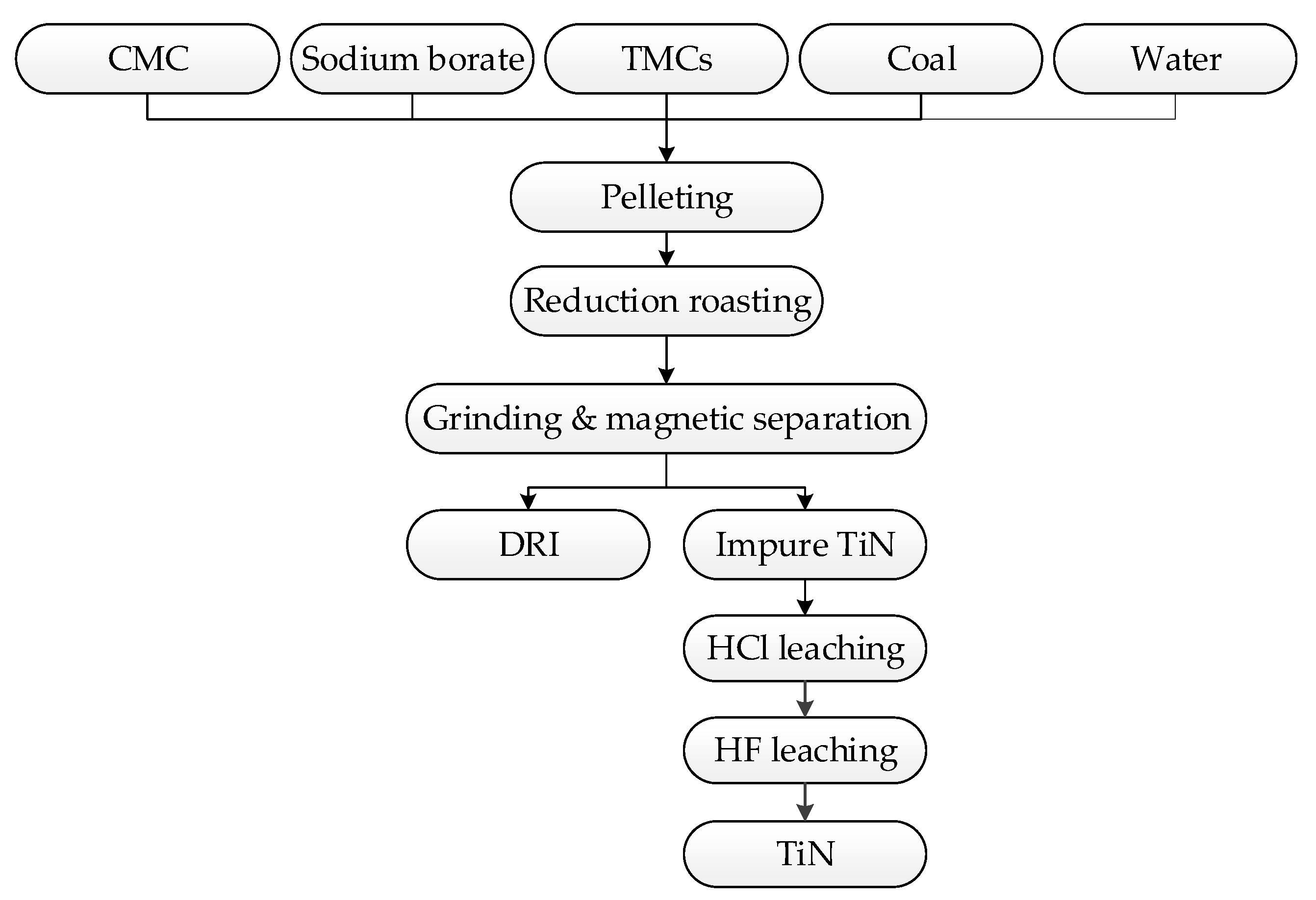
2.2. Methods
2.3. Characterization
3. Results and Discussions
3.1. Titanomagnetite Concentrate Composition
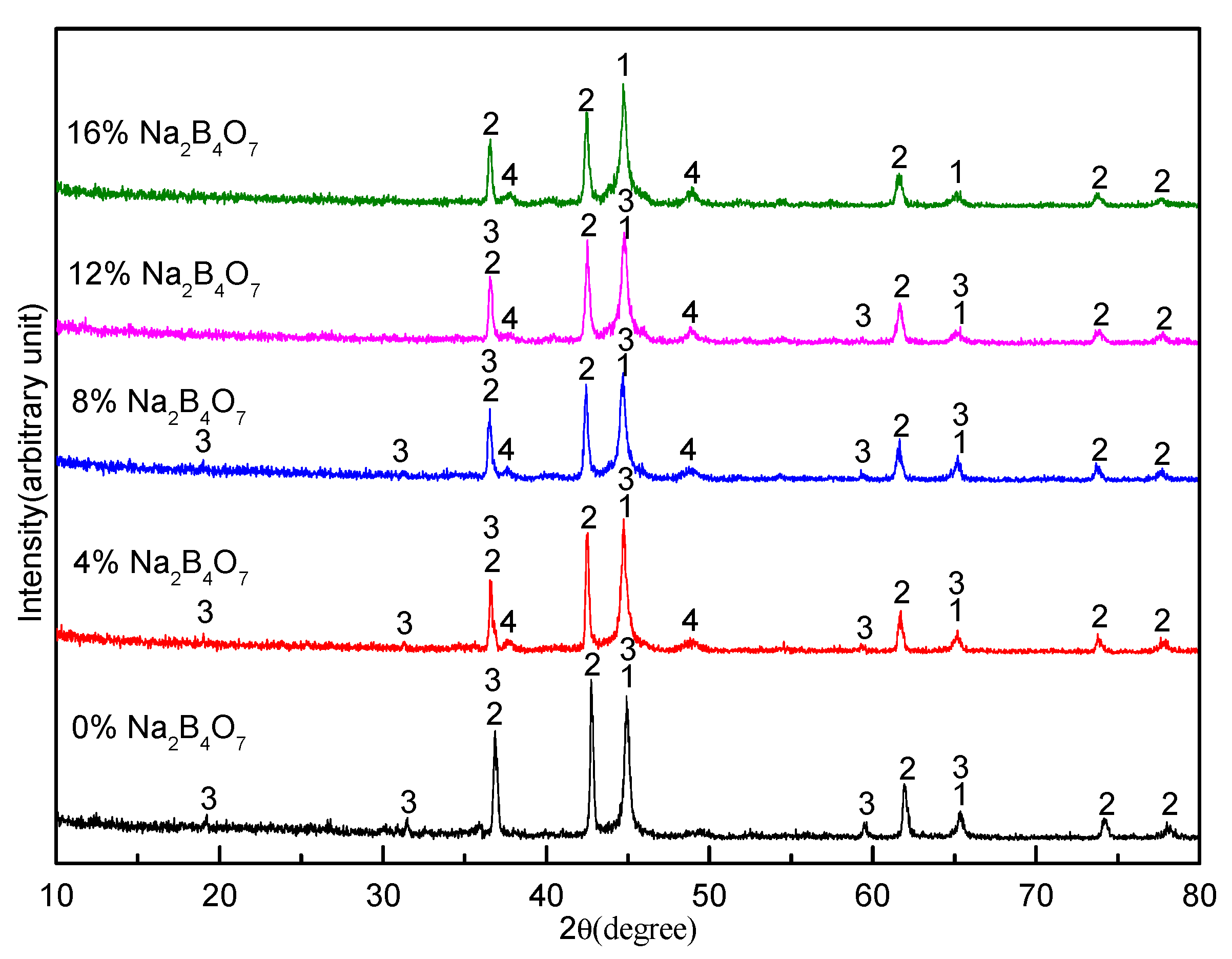
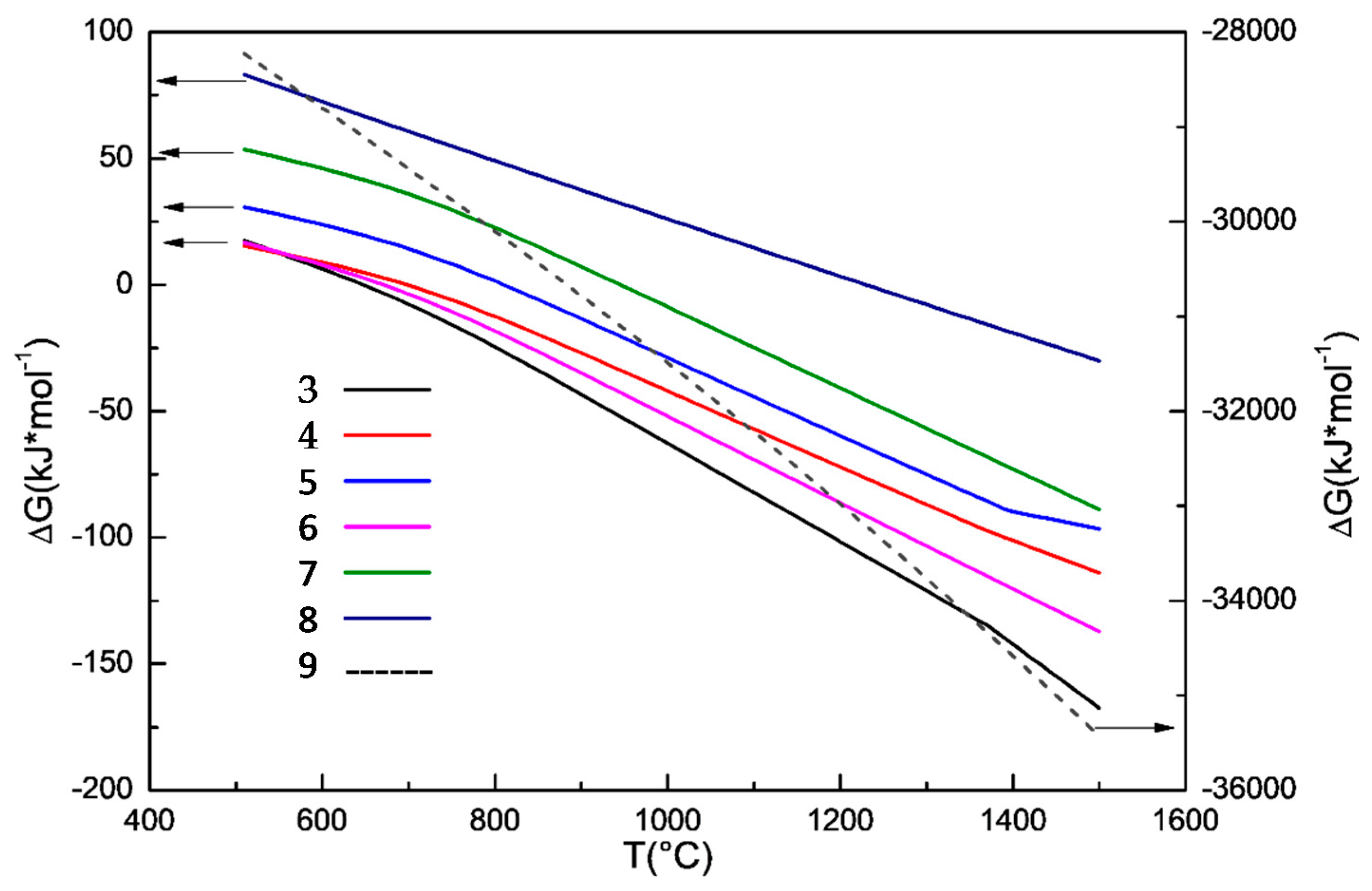
3.2. Effect of Sodium Borate on the Formation of MgAl2O4
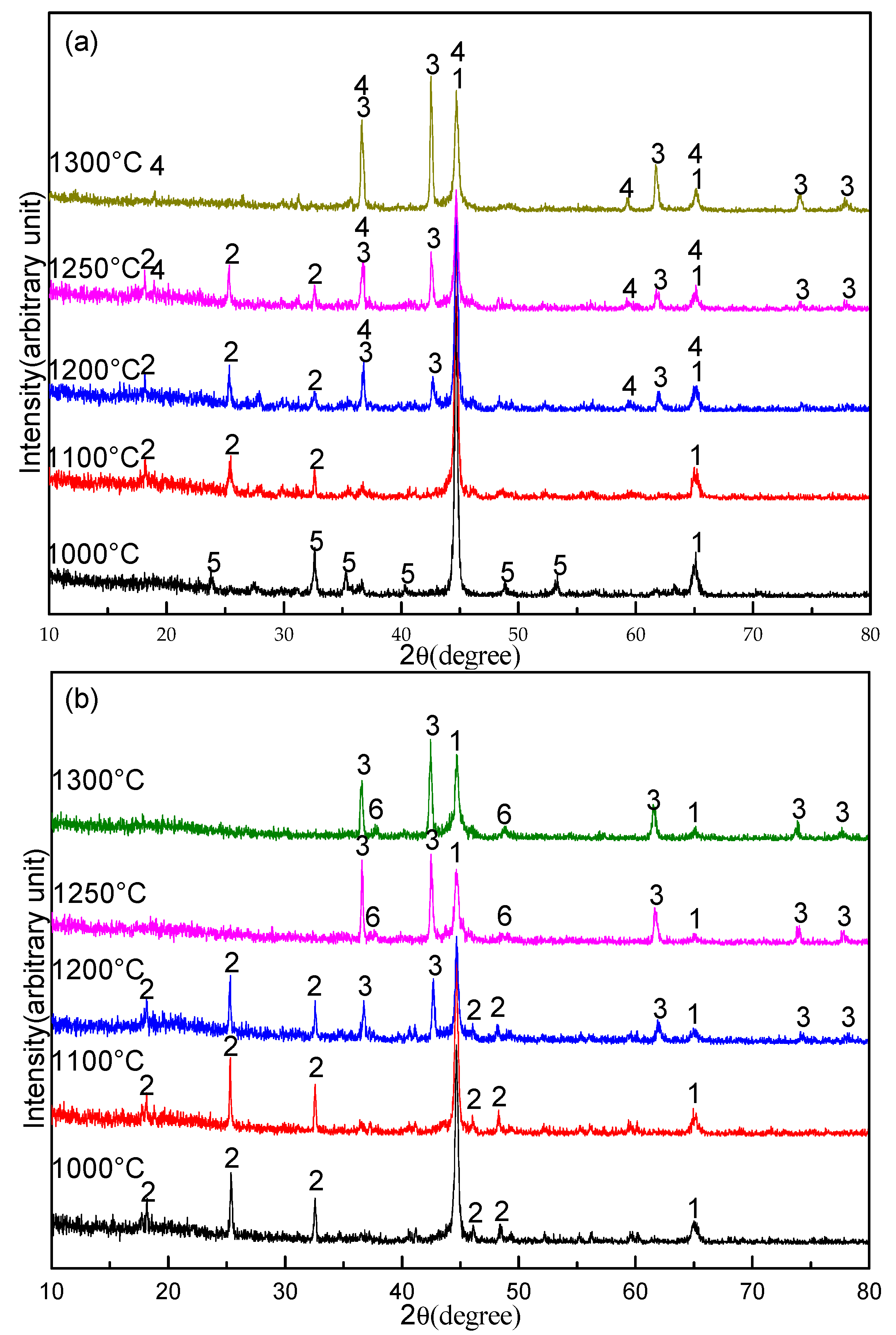
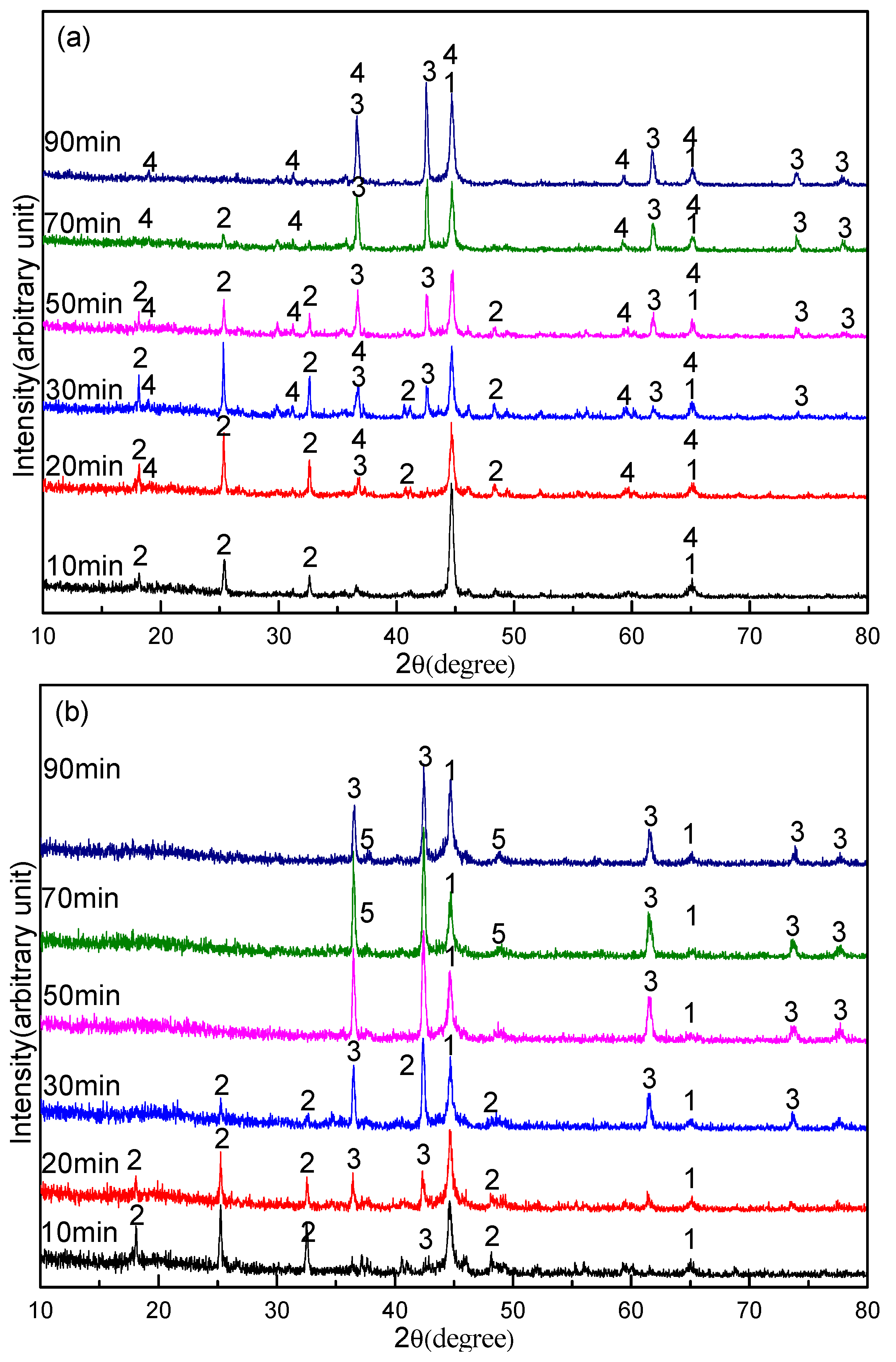
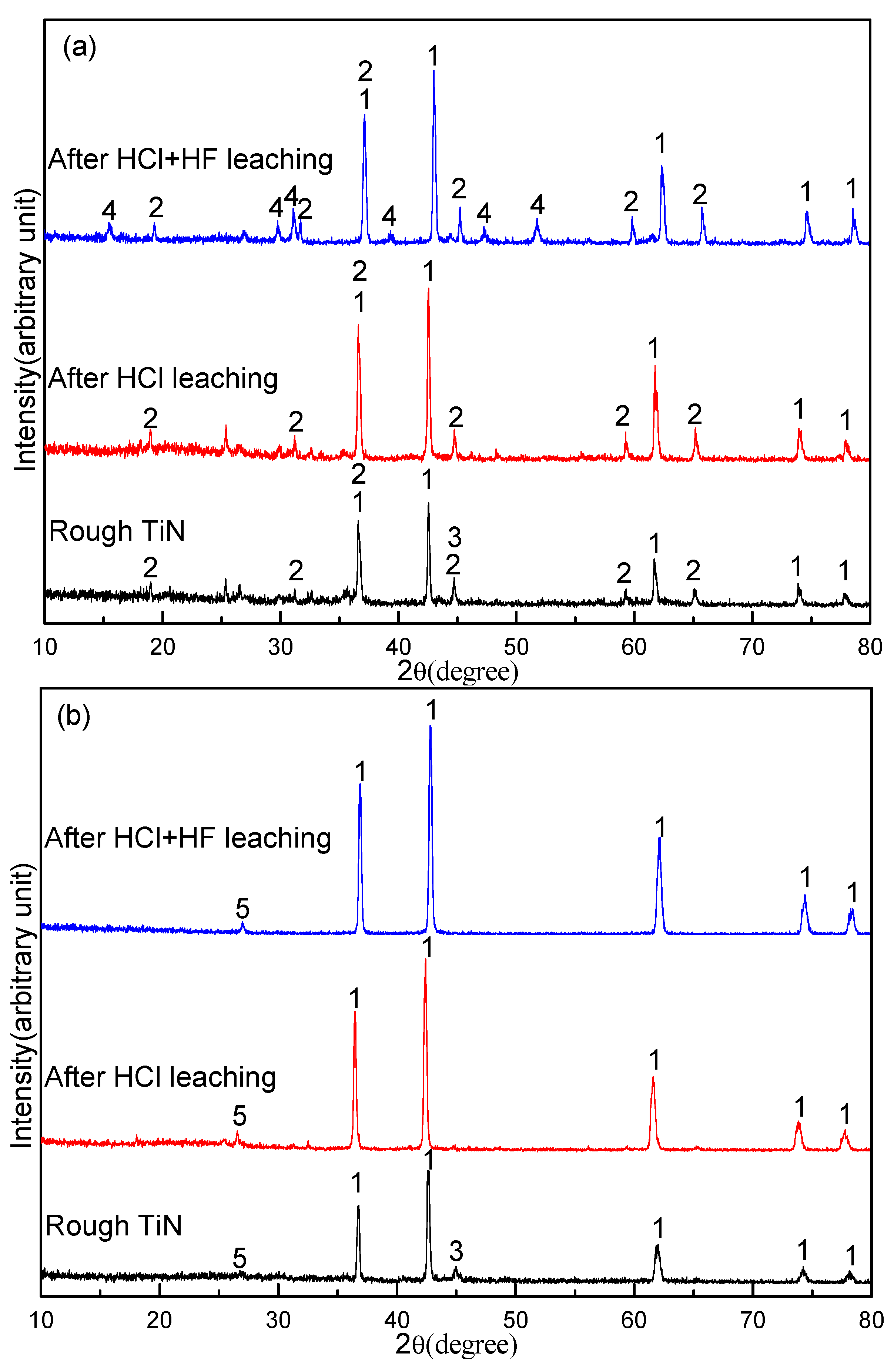
3.3. Effect of Sodium Borate on the Formation of TiN
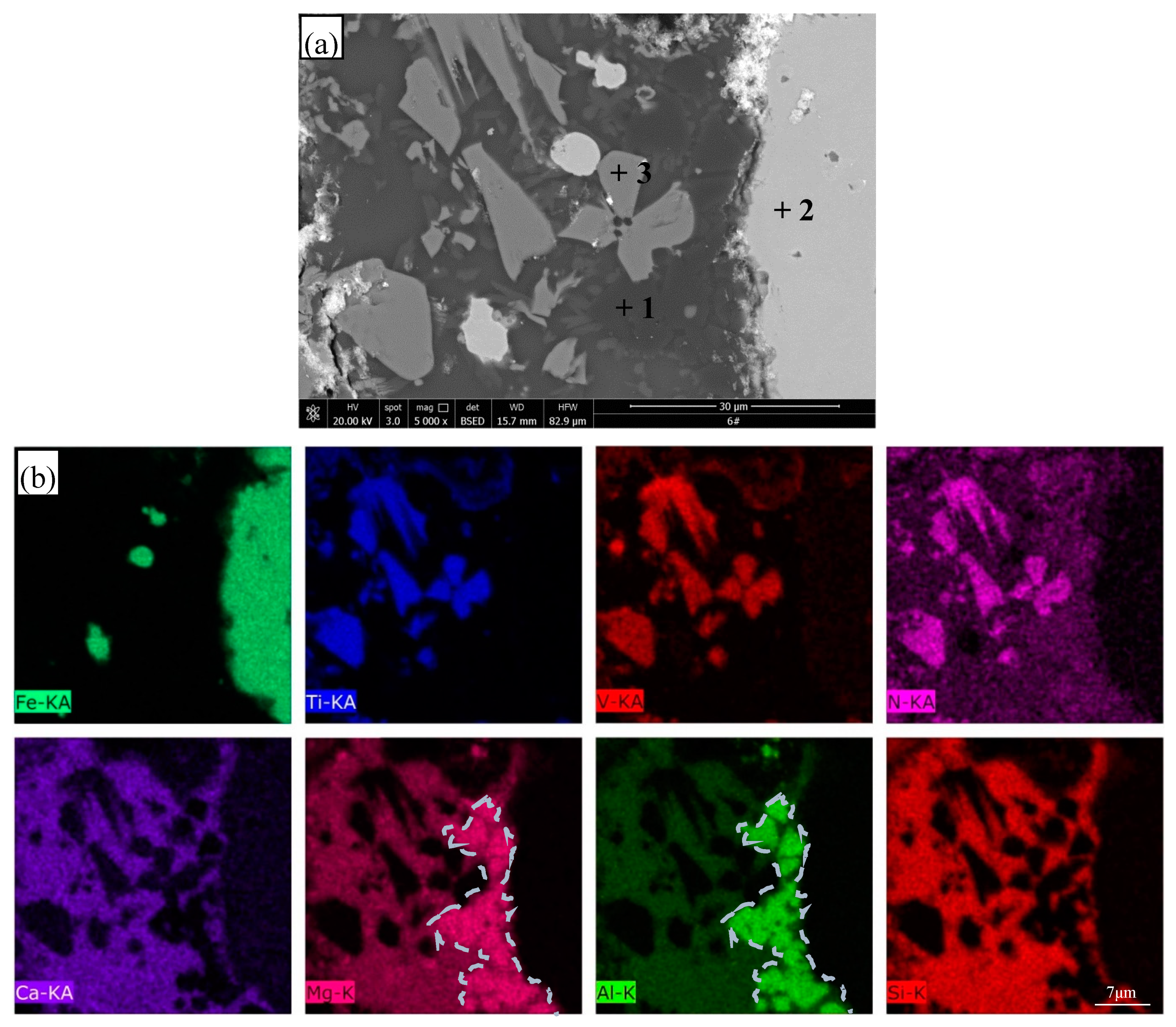
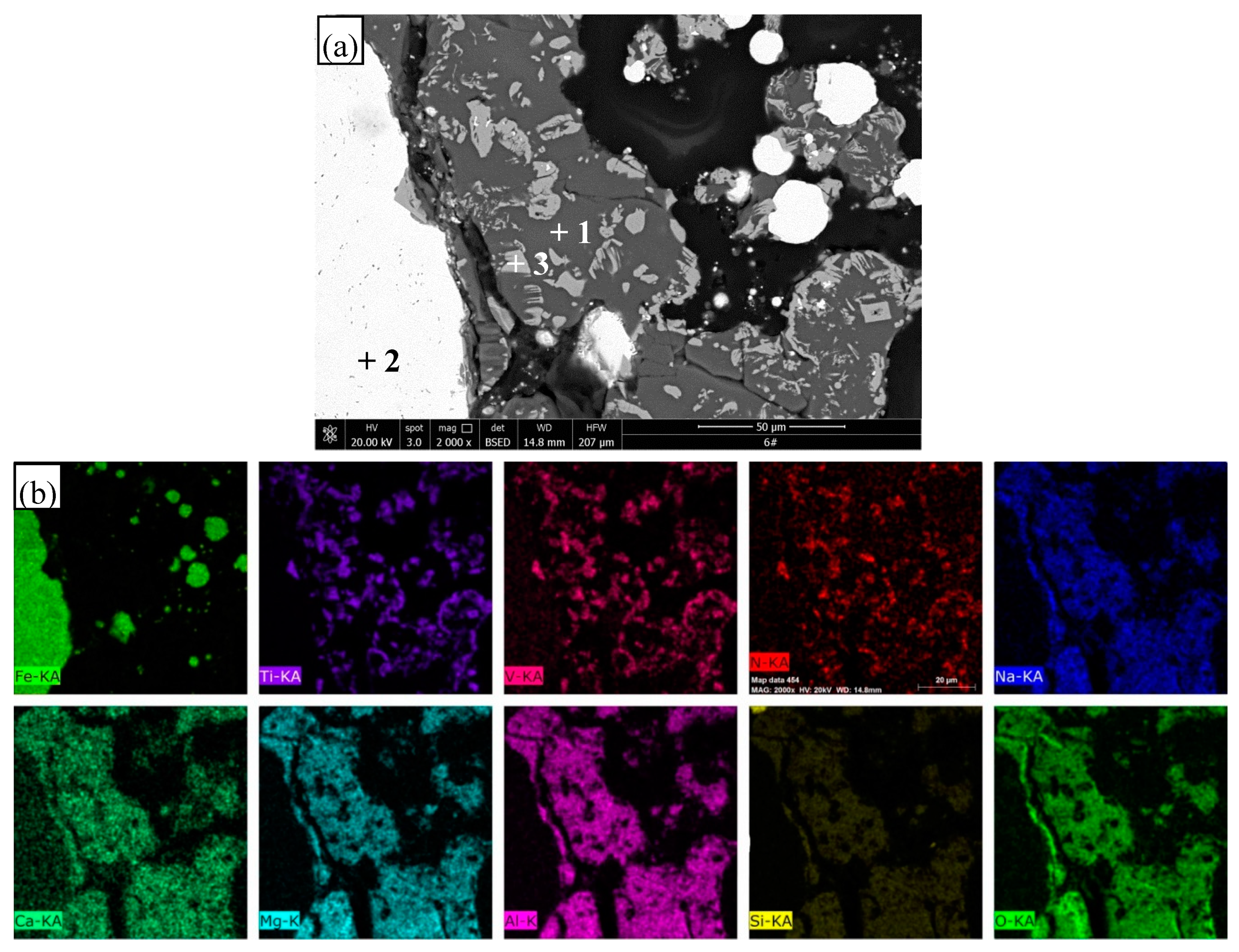
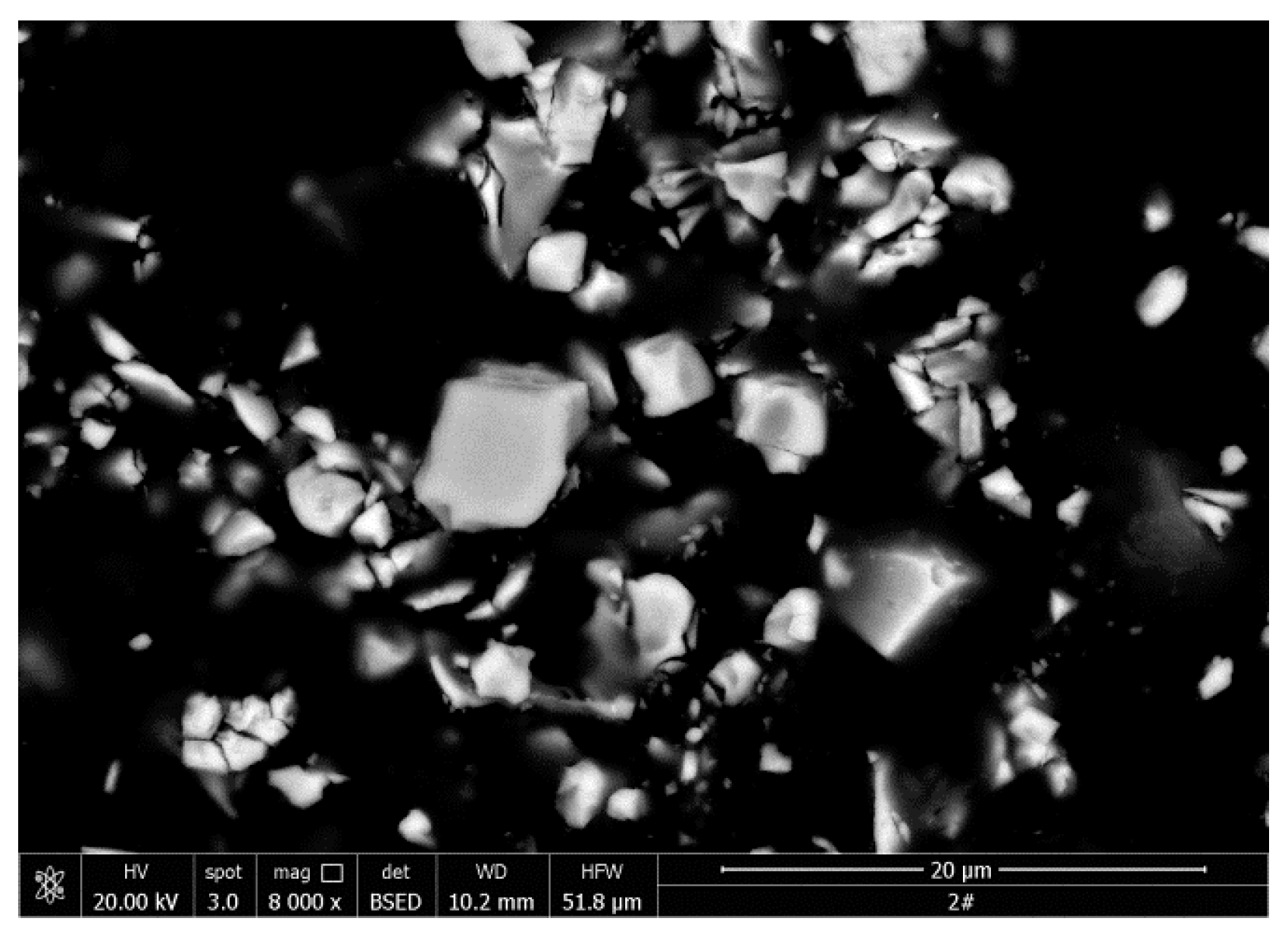
3.4. SEM Observation and EDS Analysis
3.5. Effect of Sodium Borate on the Separation of Metallic Iron and TiN
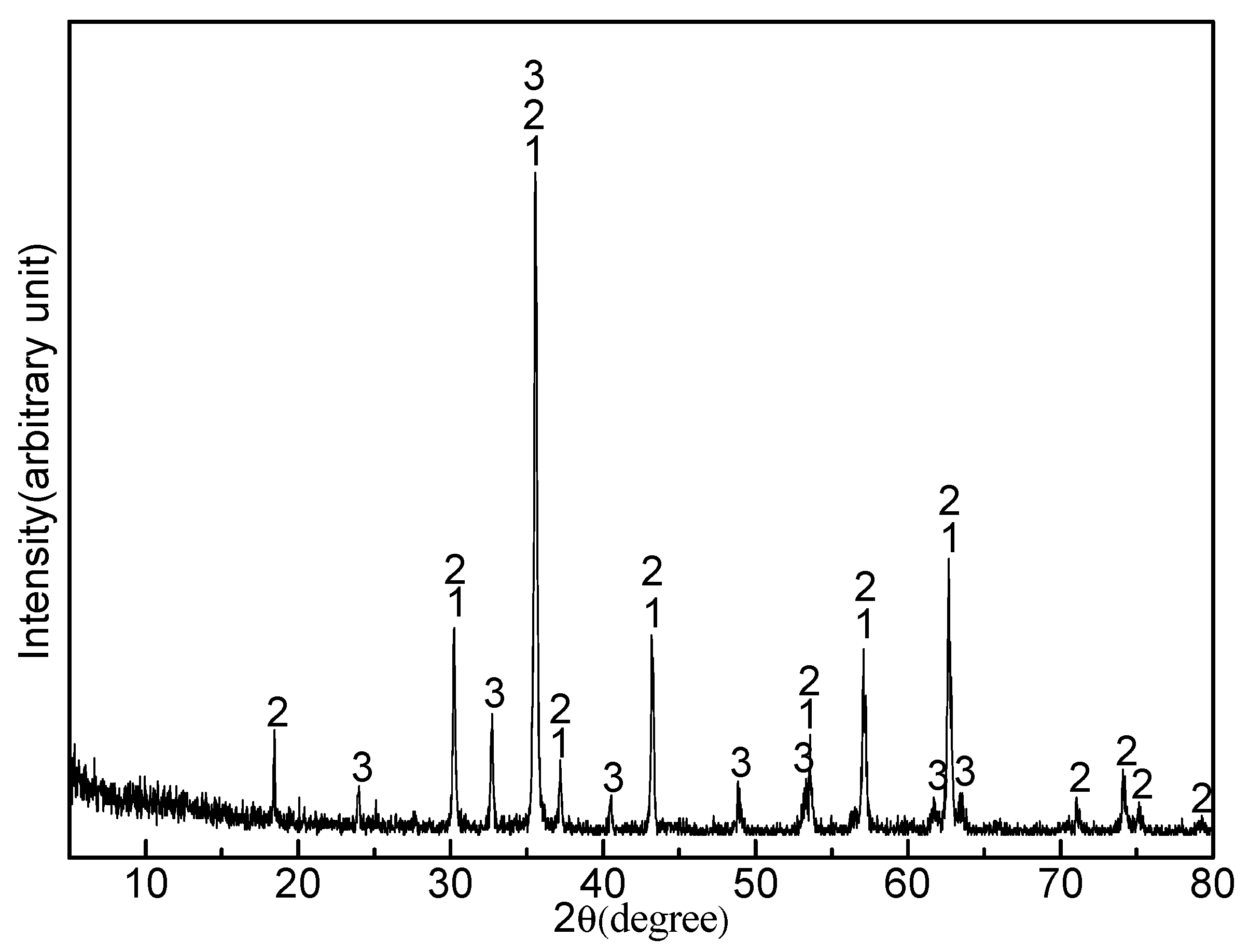
3.6. Effect of Sodium Borate on the Purification of TiN
4. Conclusions
- (1)
- During the carbothermal reduction of TMCs, the addition of sodium borate not only inhibits the formation of MgAl2O4, but also promotes the reduction and nitridation of TMCs.
- (2)
- The promotion mechanism of sodium borate on the formation of TiN can be summarized as follows: sodium borate first promotes the formation of Fe and M3O5 by catalyzing coal gasification and then promotes the reduction and nitridation of titanium oxide to TiN by accelerating the carburization of iron.
- (3)
- Sodium borate reacts with MgO, Al2O3, and other components to form complex compounds of Na2O–MgO–Al2O3–CaO–SiO2–FeO–TiO2 during the carbothermal reduction of TMCs.
- (4)
- Sodium borate slightly affects the separation of metallic Fe and TiN. Adding 16% sodium borate resulted in DRI containing 94.3% Fe, 0.6% Ti, and 0.1% V after magnetic separation. The recoveries of Fe, Ti, and V in this DRI were 91.2%, 5.1%, and 17.3%, respectively.
- (5)
- After HCl + HF leaching, a TiN product containing 74.1% Ti and 2.8% V was obtained with a Ti recovery of 94.6% and V recovery of 58.3%. In contrast the resulting TiN product contained a considerable amount of MgAl2O4 and CaMg2Al2F2 without the addition of sodium borate.
Author Contributions
Funding
Conflicts of Interest
References
- Pierson, H.O. Handbook of Refractory Carbides & Nitrides: Properties, Characteristics, Processing and Apps; Noyes Publications: Saddle River, NY, USA, 1996; p. 193. [Google Scholar]
- Li, Y.; Zeng, M.Q.; Liu, J.W.; Lu, Z.C. Evolution of metal nitriding and hydriding reactions during ammonia plasma-assisted ball milling. Ceram. Int. 2018, 44, 18329–18336. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Yang, L.; Yan, Y.; Qian, Y. Reduction-Nitridation Synthesis of Titanium Nitride Nanocrystals. J. Am. Ceram. Soc. 2003, 86, 206–208. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Xu, C.; Zhang, Q.; Wang, D.; Gong, K. Synthesis of TiN from FeTiO3 by microwave-assisted carbothermic reduction–nitridation. J. Alloy. Compd. 2014, 583, 121–127. [Google Scholar] [CrossRef]
- Zakorzhevskii, V.V.; Kovalev, I.D.; Barinov, Y.N. Self-propagating high-temperature synthesis of titanium nitride with the participation of ammonium chloride. Inorg. Mater. 2017, 53, 278–286. [Google Scholar] [CrossRef]
- Krishna, M.; Kumar, S.M. Synthesis of nanocrystalline titanium nitride by microwave plasma technique. SN Appl. Sci. 2019, 1, 833. [Google Scholar] [CrossRef]
- Tang, W.; Yang, S.; Yang, H.; Xue, X. Effect of Co2O3 on Oxidation Induration and Reduction Swelling of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas. Metals 2019, 9, 16. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, F.; Guo, Y.; Jiang, T.; Travyanov, A.Y.; Qiu, G. Kinetics of Hydrochloric Acid Leaching of Titanium from Titanium-Bearing Electric Furnace Slag. JOM 2016, 68, 1476–1484. [Google Scholar] [CrossRef]
- Song-tao, Y.; Mi, Z.; Tao, J.; Xiang-xin, X. Isothermal reduction kinetics and mineral phase of chromium-bearing vanadium–titanium sinter reduced with CO gas at 873–1273 K. Int. J. Min. Met. Mater. 2018, 25, 145–152. [Google Scholar]
- Shahbazi, H.; Shokrollahi, H.; Alhaji, A. Optimizing the gel-casting parameters in synthesis of MgAl2O4 spinel. J. Alloy. Compd. 2017, 712, 732–741. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, D.; Wang, W.; Liu, Y.; Zhao, H.; Qi, T. A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite. Int. J. Min. Met. Mater. 2018, 25, 131–144. [Google Scholar] [CrossRef]
- Sui, Y.; Guo, Y.; Jiang, T.; Xie, X.; Wang, S.; Zheng, F. Gas-based reduction of vanadium titano-magnetite concentrate: Behavior and mechanisms. Int. J. Min. Met. Mater. 2017, 24, 10–17. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, K.; Yue, H. Theoretical Analyses and Experimental Investigations of Selective Carbothermal Reactions of Vanadium-Bearing Titanomagnetite Concentrates for Preparation of Iron-Based Wear-Resistant Material. JOM 2016, 68, 2525–2532. [Google Scholar] [CrossRef]
- Wu, E.; Zhu, R.; Yang, S.; Li, H.; Li, J.; Hou, J. Preparation of Fe-Ti(C,N) Composites from Titanomagnetite Concentrate by Carbothermal Reduction in Air Atmosphere. Iron Steel Vanadium Titan. 2016, 37, 46–50. (In Chinese) [Google Scholar]
- Yu, W.; Wen, X.; Chen, J.; Kuang, J.; Tang, Q.; Tian, Y.; Fu, J.; Huang, W.; Qiu, T. Preparation of Direct Reduced Iron and Titanium Nitride from Panzhihua Titanomagnetite Concentrate Through Carbothermic Reduction-Magnetic Separation. Minerals 2017, 7, 220. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Bao, S.; Yuan, Y.; Jian, X.; Li, R. Selective vanadium extraction from vanadium bearing ferro-phosphorus via roasting and pressure hydrogen reduction. Sep. Purif. Technol. 2019, 220, 293–299. [Google Scholar] [CrossRef]
- Wu, D.; Liu, W.; Han, J.; Jiao, F.; Xu, J.; Gu, K.; Qin, W. Direct preparation of sodium stannate from lead refining dross after NaOH roasting-water leaching. Sep. Purif. Technol. 2019, 227, 115683. [Google Scholar] [CrossRef]
- Song, B.; Lv, X.; Miao, H.H.; Han, K.; Zhang, K.; Huang, R. Effect of Na2B4O7 Addition on Carbothermic Reduction of Ilmenite Concentrate. ISIJ Int. 2016, 56, 2140–2146. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, F.; Jiang, T.; Chen, F.; Wang, S.; Qiu, G. Effects of Borax on the Reduction of Pre-oxidized Panzhihua Ilmenite. JOM 2018, 70, 23–28. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Cui, Q.; Xu, C.; Kou, J. Effect of Coal Type on the Reduction and Magnetic Separation of a High-phosphorus Oolitic Hematite Ore. ISIJ Int. 2015, 55, 536–543. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Y.; Lei, M.; Chen, S.; Qiu, T.; Tang, Q. Preparation of micro-electrolysis material from flotation waste of copper slag and its application for degradation of organic contaminants in water. J. Hazard. Mater. 2019, 361, 221–227. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, W.T.; Wang, C.Y.; Prevec, S.A.; Liu, P.P.; Howarth, G.H. Two stages of immiscible liquid separation in the formation of Panzhihua-type Fe-Ti-V oxide deposits, SW China. Geosci. Front. 2013, 4, 481–502. [Google Scholar] [CrossRef]
- Chen, J. Re-Exploring on Catalyzing Mechanism of Carbonate in Pack Carburizing. Heat Treat. Met. 2000, 20, 37–41. [Google Scholar]
- Coetsee, T.; Pistorius, C. Preliminary Observations on Phase Relations in the “V2O3–FeO” and V2O3–TiO2 Systems from 1400 °C to 1600 °C in Reducing Atmospheres. J. Am. Ceram. Soc. 2000, 83, 1485–1488. [Google Scholar] [CrossRef]
- Rezan, S.A.; Zhang, G.; Ostrovski, O. Carbothermal Reduction and Nitridation of Ilmenite Concentrates. ISIJ Int. 2012, 52, 363–368. [Google Scholar] [CrossRef]
- Gou, H.; Zhang, G.; Yuan, X.; Chou, K. Formation of Titanium Carbonitride via Carbothermic Reduction of Ilmenite Concentrate in Nitrogen Atmosphere. ISIJ Int. 2016, 56, 744–751. [Google Scholar] [CrossRef]
- He, X.; Ye, J.W.; Liu, Y.; Chen, B.Q.; Jiang, Z.T.; Zou, H.W.; Deng, L.; Tu, M.J. Phase transition and microstructure evolution during the carbothermal preparation of Ti(C,N) powders in an open system. Adv. Powder Technol. 2010, 21, 448–451. [Google Scholar] [CrossRef]
- Sun, H.; Dong, X.; She, X.; Xue, Q.; Wang, J. Solid State Reduction of Titanomagnetite Concentrate by Graphite. ISIJ Int. 2013, 53, 564–569. [Google Scholar] [CrossRef]
- Welham, N.J.; Willis, P.E. Formation of TiN/TiC-Fe composites from ilmenite (FeTiO3) concentrate. Metall. Mater. Trans. B 1998, 29, 1077–1083. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.; Bai, C.; Lun, Z.; Qiu, G. Reduction Behavior of Panzhihua Titanomagnetite Concentrates with Coal. Metall. Mater. Trans. B 2013, 44, 252–260. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E. Fact-Web Suite of Interactive Programs. Available online: www.factsage.com (accessed on 25 October 2019).
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Gu, L.; Zhou, X.; Wang, H.; Liu, Z.; Han, P.; Yao, J.; Wang, L.; Cui, G. TiN/VN composites with core/shell structure for supercapacitors. Mater. Res. Bull. 2011, 46, 835–839. [Google Scholar] [CrossRef]
- Kiesler, D.; Bastuck, T.; Theissmann, R.; Kruis, F.E. Plasma synthesis of titanium nitride, carbide and carbonitride nanoparticles by means of reactive anodic arc evaporation from solid titanium. J. Nanopart. Res. 2015, 17, 152. [Google Scholar] [CrossRef]
| Fe | TiO2 | V2O5 | Al2O3 | MgO | SiO2 | CaO | S |
|---|---|---|---|---|---|---|---|
| 56.7 | 10.5 | 0.6 | 2.6 | 3.0 | 3.5 | 0.4 | 0.6 |
| Point | C | N | O | Mg | Al | Si | Ca | Ti | V | Fe | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.9 | – | 48.0 | 14.3 | 28.2 | 2.5 | 0.9 | 2.7 | 0.1 | 0.5 | Spinel |
| 2 | 5.0 | – | – | – | – | 0.5 | – | – | – | 94.5 | Iron |
| 3 | 2.0 | 19.3 | – | – | – | – | 0.3 | 74.8 | 3.1 | 0.7 | TiN |
| Point | C | N | O | Mg | Al | Si | Ca | Ti | Na | V | Fe | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.9 | – | 55.9 | 8.9 | 11.5 | 9.1 | 3.8 | 1.1 | 4.8 | – | 4.9 | Slag |
| 2 | 11.2 | – | – | – | – | 0.4 | – | – | – | – | 88.4 | Iron |
| 3 | 7.9 | 21.8 | – | – | – | – | – | 66.9 | – | 3.5 | – | TiN |
| Additive | Yield * | Element Content in DRI | Recovery | |||||
|---|---|---|---|---|---|---|---|---|
| DRI | Impure TiN | Fe | Ti | V | Fe | Ti | V | |
| No additive | 53.6 | 30.9 | 95.3 | 0.5 | 0.1 | 90.1 | 3.9 | 15.5 |
| 16% sodium borate | 54.9 | 32.0 | 94.3 | 0.6 | 0.1 | 91.2 | 5.1 | 17.3 |
| Component | Ti | V | Fe | Al | Mg | Si | Ca | Na | Total |
|---|---|---|---|---|---|---|---|---|---|
| Impure TiN | 19.5 | 0.7 | 8.5 | 5.1 | 4.6 | 5.2 | 2.4 | 2.6 | 48.6 |
| TiN Product | 74.1 | 2.8 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 77.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Wen, X.; Chen, J.; Tang, Q.; Dong, W.; Zhong, J. Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction–Magnetic Separation and Acid Leaching Process. Minerals 2019, 9, 675. https://doi.org/10.3390/min9110675
Yu W, Wen X, Chen J, Tang Q, Dong W, Zhong J. Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction–Magnetic Separation and Acid Leaching Process. Minerals. 2019; 9(11):675. https://doi.org/10.3390/min9110675
Chicago/Turabian StyleYu, Wen, Xiaojin Wen, Jiangan Chen, Qiongyao Tang, Wen Dong, and Jingen Zhong. 2019. "Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction–Magnetic Separation and Acid Leaching Process" Minerals 9, no. 11: 675. https://doi.org/10.3390/min9110675
APA StyleYu, W., Wen, X., Chen, J., Tang, Q., Dong, W., & Zhong, J. (2019). Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction–Magnetic Separation and Acid Leaching Process. Minerals, 9(11), 675. https://doi.org/10.3390/min9110675





