Adsorption of N2, NO2 and CO2 on Epistilbite Natural Zeolite from Jalisco, Mexico after Acid Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. CO2 and NO2 Adsorption
3. Results and Discussion
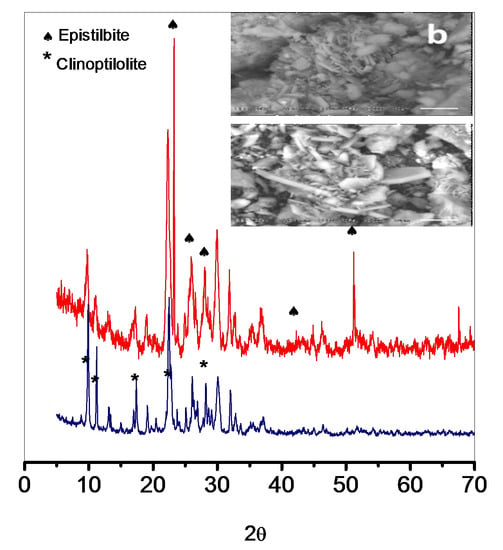
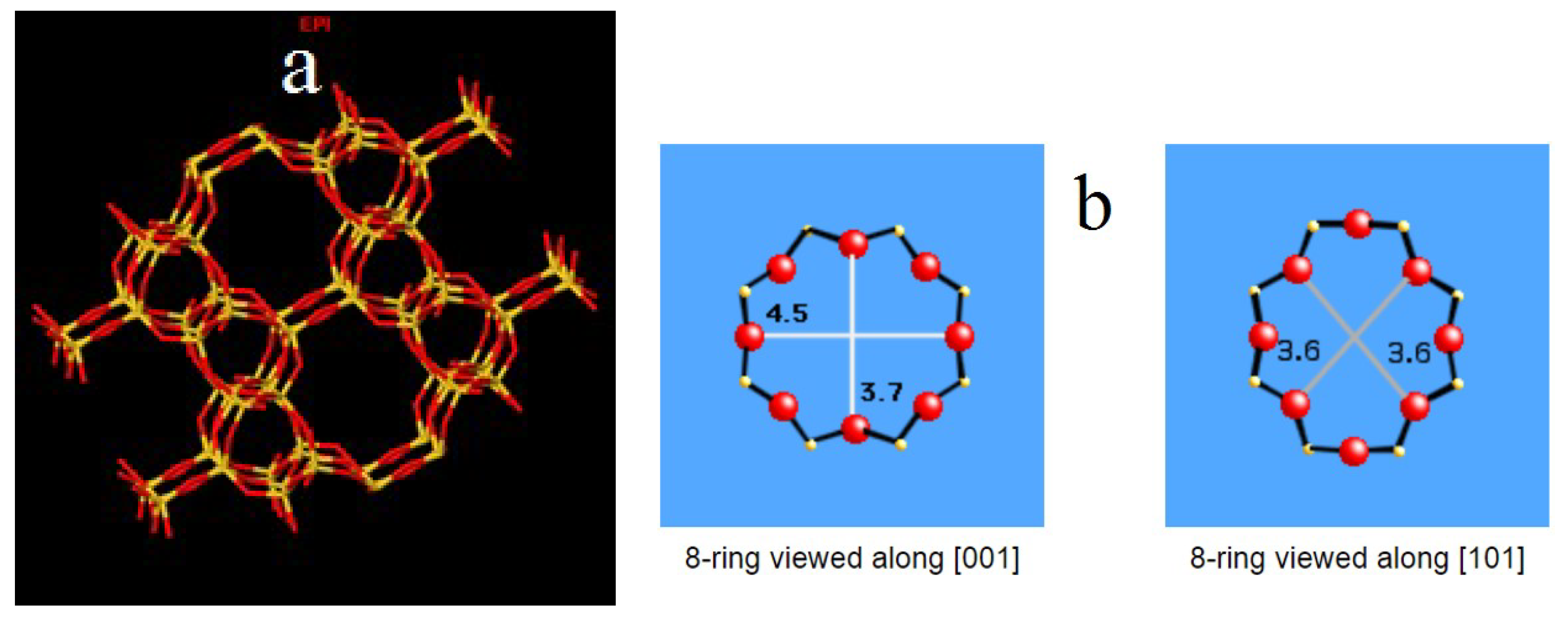
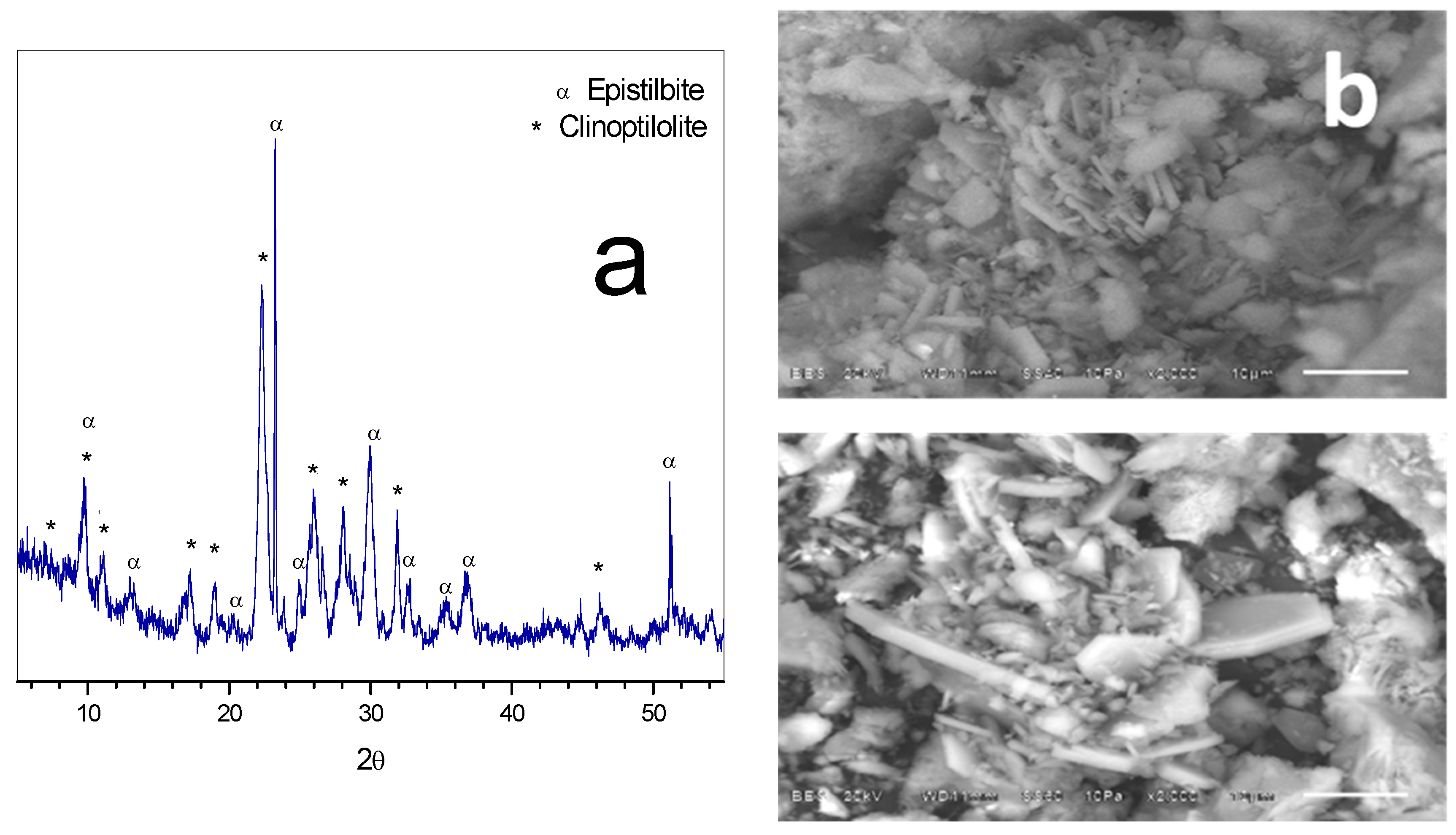
3.1. Morphology, Chemical Composition and X-ray Diffraction
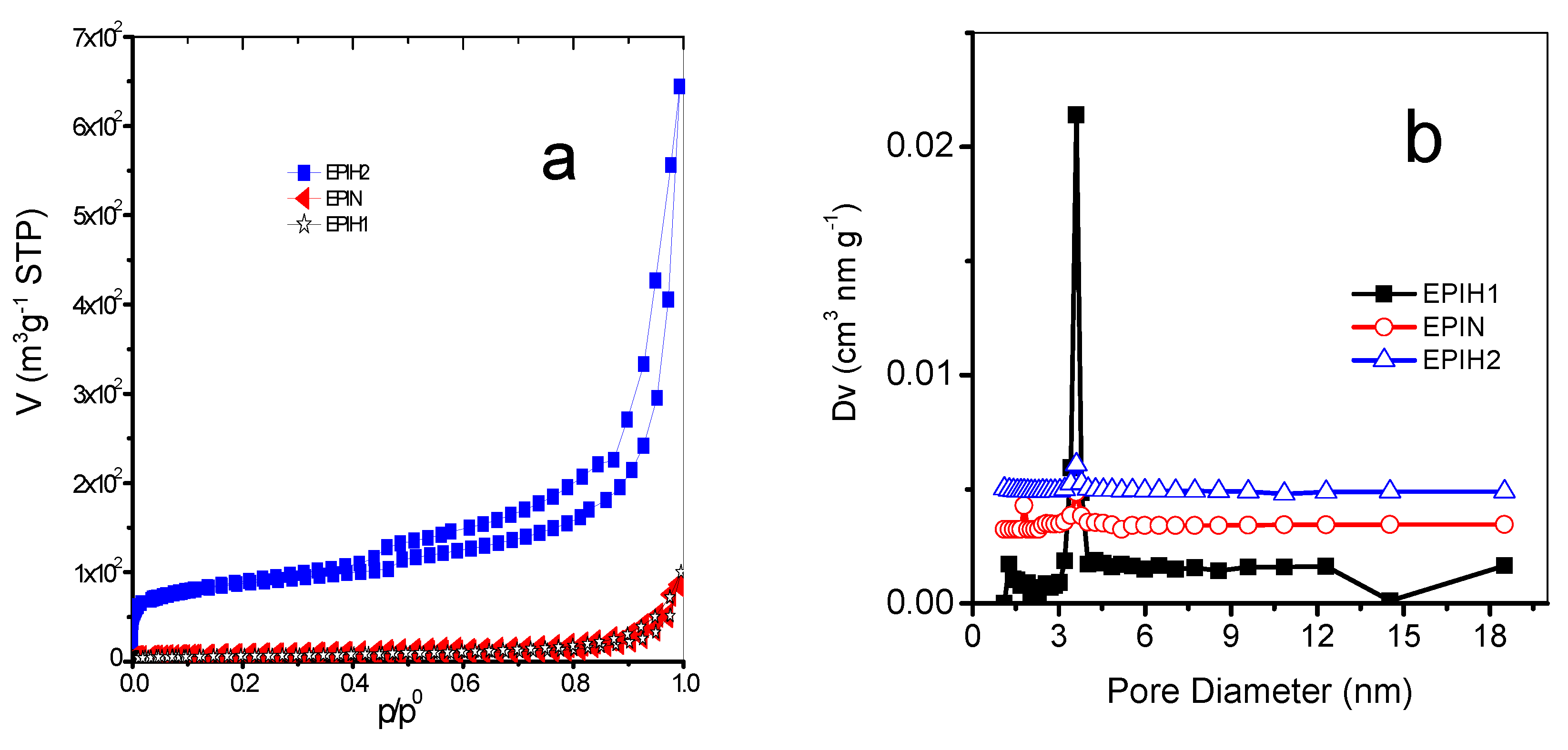
3.2. N2 Adsorption
3.3. Adsorption of CO2 and NO2
3.4. Isosteric Heat of Adsorption and Standard Adsorption Energies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 13 May 2017).
- International Zeolite Association (IZA). Available online: http://www.iza-online.org/ (accessed on 14 May 2017).
- Index of Natural Zeolites Datasheets. Available online: http://www.iza-online.org/natural/IZA-NZ_Datasheets.htm (accessed on 13 May 2017).
- Tiwari, A.; Titinchi, S. Advanced Catalytic Materials; Schivener Publishing: Wiley, NJ, USA, 2015; p. 472. [Google Scholar]
- Yang, S.; Lach-hab, M.; Vaismal, L.; Blaisten-Barojas, E.; Li, X.; Karen, V. Framework-Type determination for zeolite structures in the inorganic crystal structure database. J. Phys. Chem. Ref. Data 2010, 39, 1–44. [Google Scholar] [CrossRef]
- Perego, C.; Carati, A. Zeolites and zeolite-like materials in industrial catalysis. In Zeolites: From Model Materials to Industrial Catalysts; Cejka, J., Perez-Pariente, J., Roth, W.J., Eds.; Transworld Research Network: Kerala, India, 2008; pp. 357–389. [Google Scholar]
- Moliner, M.; Martinez, C.; Corma, A. Synthesis strategies for preparing useful small pore zeolites and zeotypes for gas separations and catalysis. Chem. Mater. 2014, 26, 246–258. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: New York, NY, USA, 2014. [Google Scholar]
- Foster, M.D.; Rivin, I.; Treacy, M.M.J.; Delgado, F.O. A geometric solution to the Largest-Free-Sphere problem in zeolite frameworks. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Cheung, O.; Hedin, N. Zeolites and related sorbents with narrow pores for CO2 separation from flue gas. RSC Adv. 2014, 4, 14480–14494. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; Wiley-Interscience: New York, NY, USA, 1974; pp. 644–652. [Google Scholar]
- Yaluris, G.; Zhao, X.; Ziebarth, M.S. Reducing Nitrogen Oxide Emissions Involves Incorporating a Nitrogen Oxide Reduction Zeolite Component with a Catalytically Cracking Catalyst Inventory That Is Being Circulated Throughout a Fluid Catalytic Cracking Unit. Patent No. US7641787-B2, 5 January 2010. [Google Scholar]
- Tumsek, F.; Inel, O. Evaluation of the thermodynamic parameters for the adsorption of some n-alkanes on a type zeolite crystals by inverse gas chromatography. Chem. Eng. J. 2003, 94, 57–66. [Google Scholar] [CrossRef]
- Khabashesku, V.; Mazyar, O.; Chakraborty, S.; Agrawal, G.; Hain, T. Functionalized Silicate Nanoparticle Composition, Removing and Exfoliating Asphaltenes with Same. Patent No. US9012377-B2, 31 December 2012. [Google Scholar]
- Field, B.F. Apparatus for Cleaning a Surface Comprises A Mobile Body; First Liquid Line Configured to Receive a Liquid; a Container Coupled to the First Liquid Line; a Second Liquid Line Coupled to the Container; and Dispenser. Patent No. US2010276301-A1, 4 November 2010. [Google Scholar]
- Cruciani, G.; Martucci, A.; Meneghini, C. Dehydration dynamics of epistilbite by in situ time resolved synchrotron powder diffraction. Eur. J. Miner. 2003, 15, 257–266. [Google Scholar] [CrossRef]
- Hernández, M.A.; Portillo, R.; Salgado, M.A.; Rojas, F.; Petranovskii, V.; Salas, R.; Pérez, G. Comparación de la capacidad de adsorción de CO2 en clinoptilolitas naturales y tratadas químicamente. Superf. Vacio 2010, 23, 67–72. [Google Scholar]
- Choudary, V.R.; Mantri, K. Adsorption of aromatic hydrocarbons on highly siliceous MCM-41. Langmuir 2000, 16, 7031–7037. [Google Scholar] [CrossRef]
- Treacy, M.; Higgins, J. Collection of Simulated XRD Powder Patterns for Zeolites; Elsevier: New York, NY, USA, 2001; pp. 49–50. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Atci, E.; Erucar, I.; Keskin, S. Adsorption and transport of CH4, CO2, H2 mixtures in a bio-MOF material from molecular simulations. J. Phys. Chem. C 2011, 115, 6833–6840. [Google Scholar] [CrossRef]
- Canet, X.; Nokerman, J.; Frere, M. Determination of the Henry constant for zeolite-VOC systems using massic and chromatographic adsorption data. Adsorption 2005, 11, 213–216. [Google Scholar] [CrossRef]
- Hernández, M.A.; Asomoza, M.; Rojas, F.; Solís, S.; Salgado, M.A.; Portillo, R.; Jimenez, D. VOCs physisorption on micro-mesoporous solids: Application for dichloroethylene, trichloroethylene, and tetrachloroethylene on SiO2 and Ag/SiO2. J. Environ. Chem. Eng. 2013, 967–974. [Google Scholar] [CrossRef]
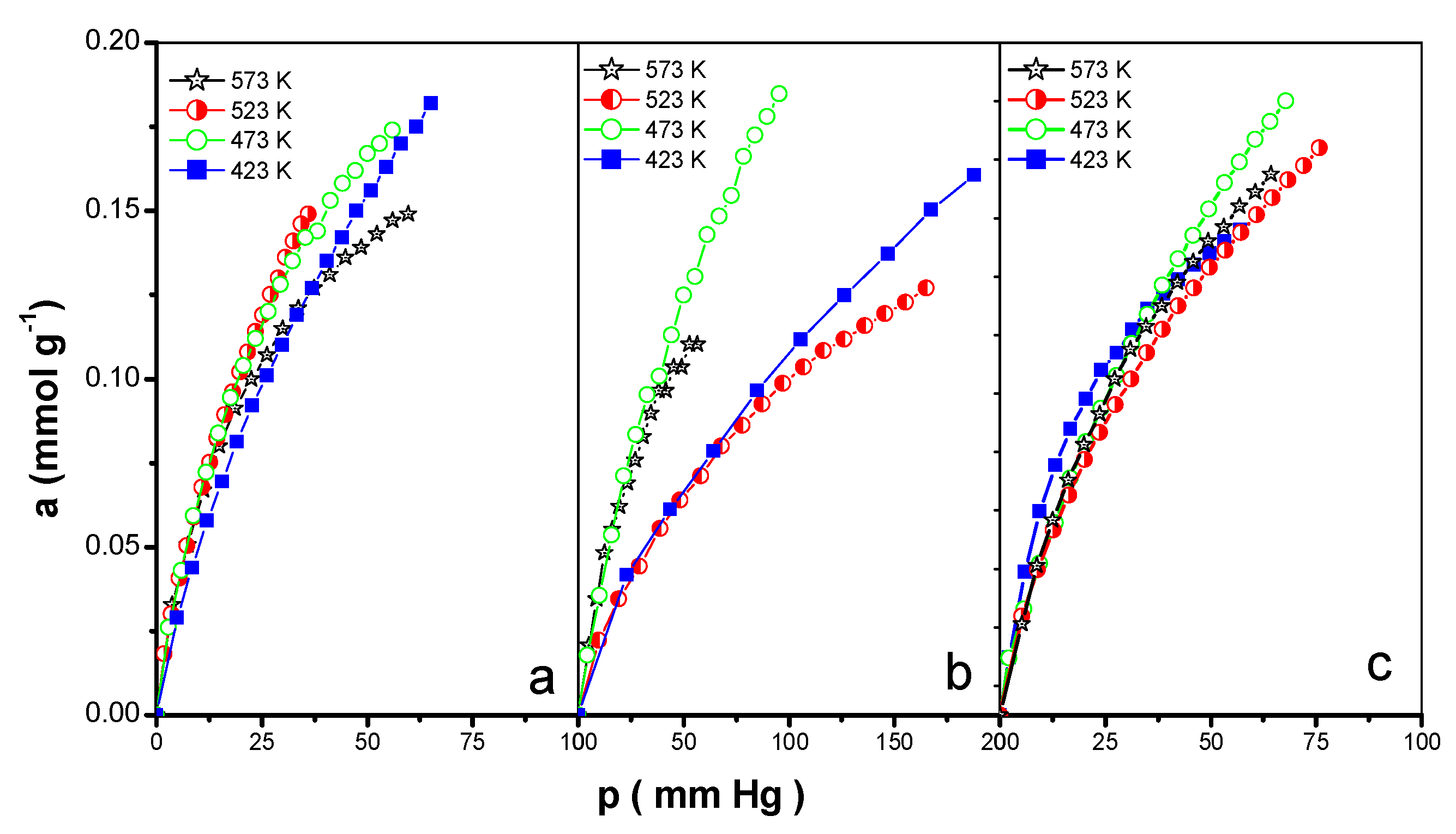
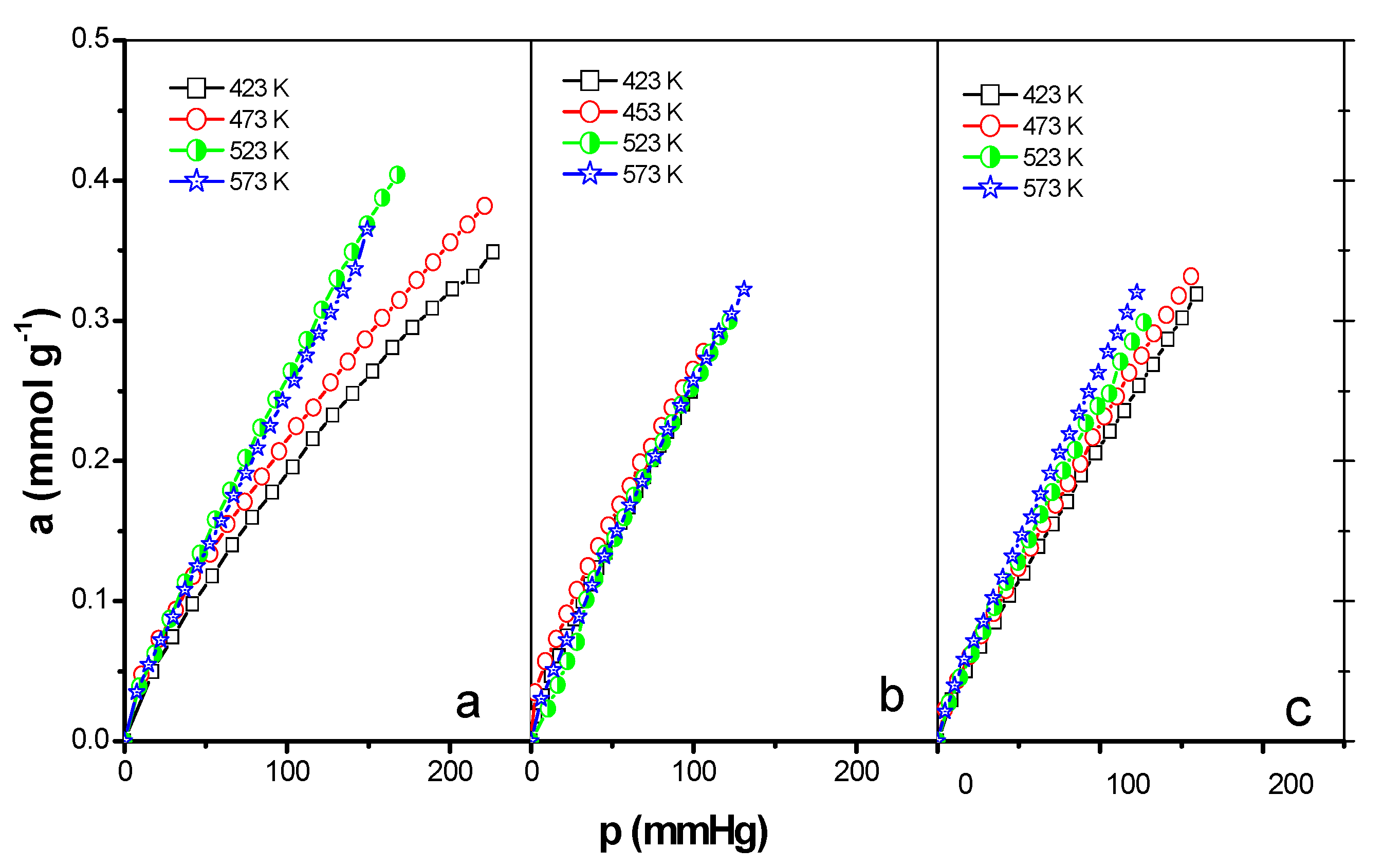
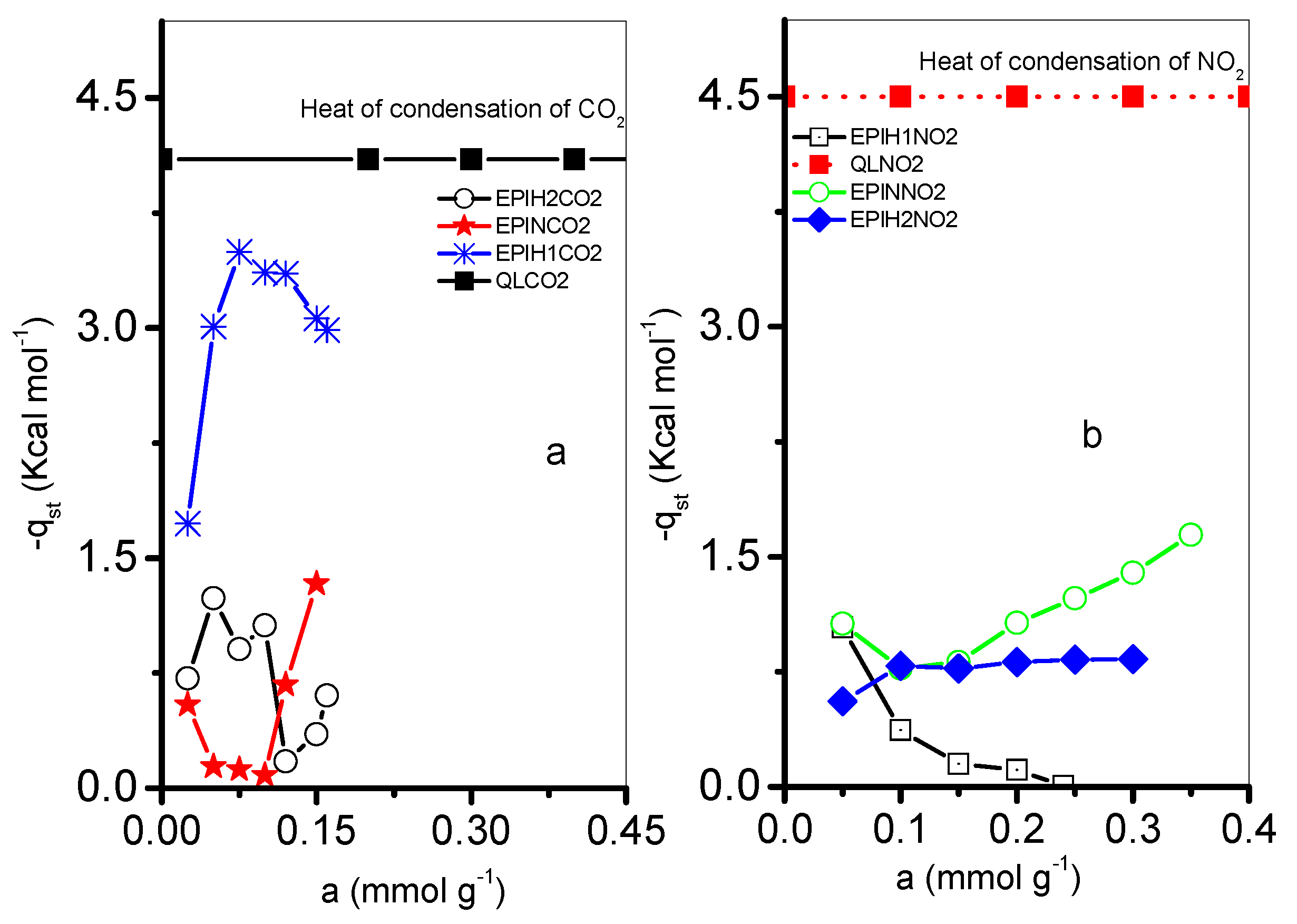
| Element | EPIN | EPIH1 | EPIH2 |
|---|---|---|---|
| Si | 66.374 | 68.361 | 69.103 |
| Al | 1.837 | 1.246 | 1.263 |
| Fe | 21.786 | 19.907 | 21.473 |
| Ca | 1.816 | 2.745 | 2.098 |
| Sr | 2.973 | 0.426 | 0.7045 |
| K | 1.765 | 1.545 | 1.855 |
| Rb | 1.248 | 1.222 | 1.647 |
| Y | Nd | 1.222 | Nd |
| Ni | Nd | 0.596 | Nd |
| Cu | 0.292 | 0.426 | 1.020 |
| Zn | Nd | 0.427 | 0.785 |
| Ti | 0.345 | Nd | Nd |
| V | Nd | 0.796 | Nd |
| As | Nd | Nd | Nd |
| Zr | 1.558 | 2.580 | Nd |
| Cl | 0.001 | 0.001 | 0.007 |
| Total % | 99.995 | 99.955 | 99.955 |
| Sample | ASL (m2/g) | ASB (m2/g) | CB | V∑ (cm3/g) | W0t (cm3/g) | Dp (nm) | δr (g/cm3) | ε (%) |
|---|---|---|---|---|---|---|---|---|
| EPIN | 45.48 | 62.69 | 111 | 0.0511 | 0.00184 | 30.6 | 1.39 | 0.0625 |
| EPIH1 | 42.5 | 20.90 | 203 | 0.05631 | 0.00184 | 38.2 | 1.91 | 0.0368 |
| EPIH2 | 415 | 306 | −32 | 0.452 | 0.0424 | 58.8 | 1.74 | 0.0726 |
| Sample | T (K) | Kf × 102 (mmol·g−1·mmHg−1) | n | RF | am (mmol·g−1) | KH × 103 (mmol·g−1·mmHg−1) | RL |
|---|---|---|---|---|---|---|---|
| EPIN | 423 | 1.8 | 0.539 | 0.993 | 0.183 | 8.9 | 0.996 |
| 473 | 1.4 | 0.635 | 0.996 | 0.229 | 9.6 | 0.997 | |
| 523 | 1.0 | 0.705 | 0.999 | 0.281 | 6.3 | 0.998 | |
| 573 | 1.2 | 0.701 | 0.999 | 0.191 | 10.6 | 0.995 | |
| 423 | 1.2 | 0.673 | 0.984 | 0.299 | 6.6 | 0.998 | |
| EPIH1 | 473 | 0.9 | 0.750 | 0.999 | 0.459 | 5.9 | 0.999 |
| 523 | 0.8 | 0.654 | 0.995 | 0.329 | 3.0 | 0.992 | |
| 573 | 0.8 | 0.621 | 0.999 | 0.224 | 3.6 | 0.992 | |
| 423 | 1.6 | 0.611 | 0.991 | 0.238 | 9.9 | 0.999 | |
| EPIH2 | 473 | 1.2 | 0.643 | 0.996 | 0.255 | 7.1 | 0.996 |
| 523 | 1.1 | 0.681 | 0.999 | 0.311 | 6.7 | 0.999 | |
| 573 | 1.1 | 0.708 | 0.998 | 0.218 | 9.2 | 0.987 |
| Sample | T (K) | Kf × 102 (mmol·g−1·mmHg−1) | n | RF | am (mmol·g−1) | KH × 103 (mmol·g−1·mmHg−1) | RL |
|---|---|---|---|---|---|---|---|
| EPIN | 423 | 1.8 | 0.761 | 0.993 | 0.465 | 3.4 | 0.993 |
| 473 | 1.4 | 0.699 | 0.996 | 0.305 | 5.0 | 0.984 | |
| 523 | 1.0 | 0.830 | 0.999 | 0.453 | 3.9 | 0.998 | |
| 573 | 1.2 | 0.799 | 0.999 | 0.419 | 3.9 | 0.993 | |
| 423 | 1.2 | 0.761 | 0.999 | 0.239 | 6.2 | 0.982 | |
| EPIH1 | 473 | 0.9 | 0.699 | 0.998 | 0.195 | 1.48 | 0.983 |
| 523 | 0.8 | 0.830 | 0.998 | 0.843 | 2.2 | 0.994 | |
| 573 | 0.8 | 0.799 | 0.995 | 0.386 | 4.8 | 0.987 | |
| 423 | 1.6 | 0.831 | 0.998 | 0.563 | 3.0 | 0.996 | |
| EPIH2 | 473 | 1.2 | 0.782 | 0.995 | 0.414 | 4.6 | 0.984 |
| 523 | 1.1 | 0.844 | 0.998 | 0.589 | 4.2 | 0.991 | |
| 573 | 1.1 | 0.841 | 0.998 | 0.414 | 4.7 | 0.987 |
| T (K) | Kp | α1 |
|---|---|---|
| EPIN | ||
| 423 | 2.18 | 2.93 |
| 473 | 2.27 | 2.05 |
| 523 | 1.80 | 1.48 |
| 573 | 2.17 | 2.26 |
| EPIH1 | ||
| 423 | 1.98 | 1.06 |
| 473 | 1.52 | 2.48 |
| 523 | 1.49 | 1.36 |
| 573 | 2.00 | 1.34 |
| EPIH2 | ||
| 423 | 2.27 | 2.89 |
| 473 | 1.96 | 1.41 |
| 523 | 1.83 | 1.71 |
| 573 | 2.06 | 2.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Espinosa, M.A.; Quiroz-Estrada, K.; Petranovskii, V.; Rojas, F.; Portillo, R.; Salgado, M.A.; Marcelo, M.; Rubio, E.; Felipe, C. Adsorption of N2, NO2 and CO2 on Epistilbite Natural Zeolite from Jalisco, Mexico after Acid Treatment. Minerals 2018, 8, 196. https://doi.org/10.3390/min8050196
Hernández-Espinosa MA, Quiroz-Estrada K, Petranovskii V, Rojas F, Portillo R, Salgado MA, Marcelo M, Rubio E, Felipe C. Adsorption of N2, NO2 and CO2 on Epistilbite Natural Zeolite from Jalisco, Mexico after Acid Treatment. Minerals. 2018; 8(5):196. https://doi.org/10.3390/min8050196
Chicago/Turabian StyleHernández-Espinosa, Miguel Angel, Karla Quiroz-Estrada, Vitalii Petranovskii, Fernando Rojas, Roberto Portillo, Martha Alicia Salgado, Miguel Marcelo, Efraín Rubio, and Carlos Felipe. 2018. "Adsorption of N2, NO2 and CO2 on Epistilbite Natural Zeolite from Jalisco, Mexico after Acid Treatment" Minerals 8, no. 5: 196. https://doi.org/10.3390/min8050196
APA StyleHernández-Espinosa, M. A., Quiroz-Estrada, K., Petranovskii, V., Rojas, F., Portillo, R., Salgado, M. A., Marcelo, M., Rubio, E., & Felipe, C. (2018). Adsorption of N2, NO2 and CO2 on Epistilbite Natural Zeolite from Jalisco, Mexico after Acid Treatment. Minerals, 8(5), 196. https://doi.org/10.3390/min8050196