PGE–(REE–Ti)-Rich Micrometer-Sized Inclusions, Mineral Associations, Compositional Variations, and a Potential Lode Source of Platinum-Group Minerals in the Sisim Placer Zone, Eastern Sayans, Russia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Placer and Lode Occurrences of Chromian Spinel
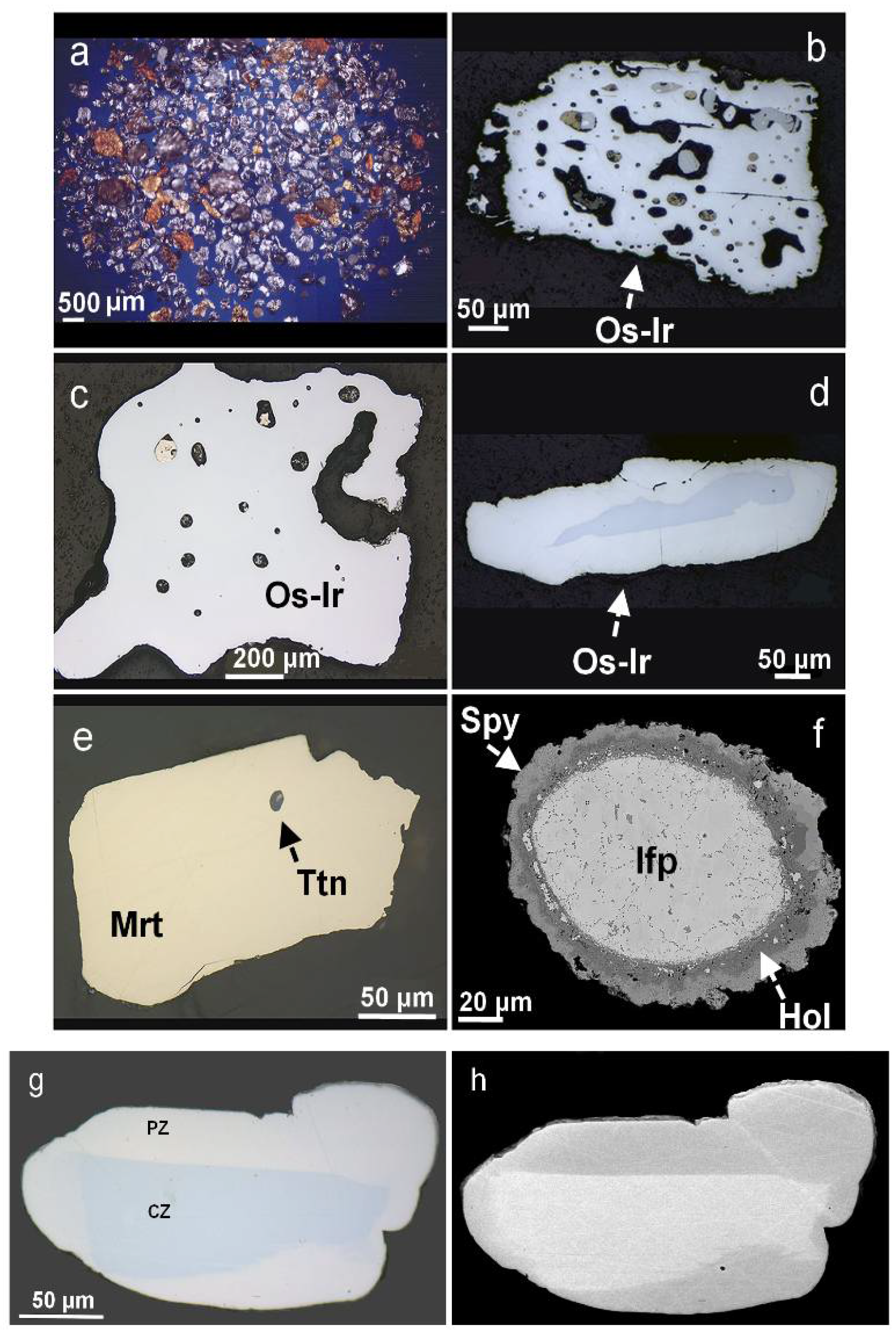
3.2. Placer Grains of Os–Ir–(Ru) and Pt–Fe Alloys
3.3. Inclusions of Hydrous Silicates and High-Ti Micrometer-Sized Mixtures
3.4. Inclusions of REE- and Ti-Rich Minerals Coexisting with PGM
3.5. Variations and Element Correlations in the Laurite–Erlichmanite Series at Sisim
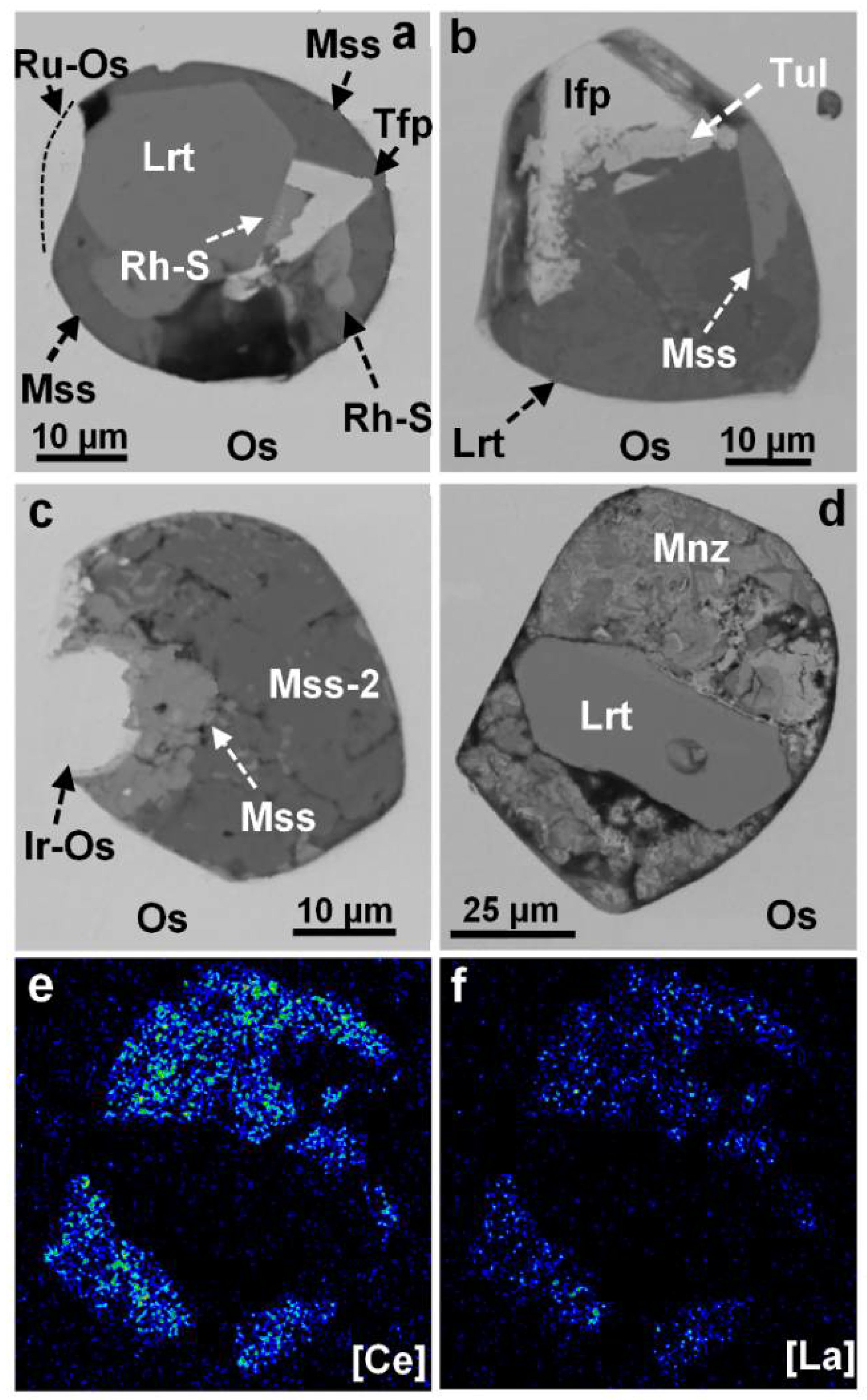
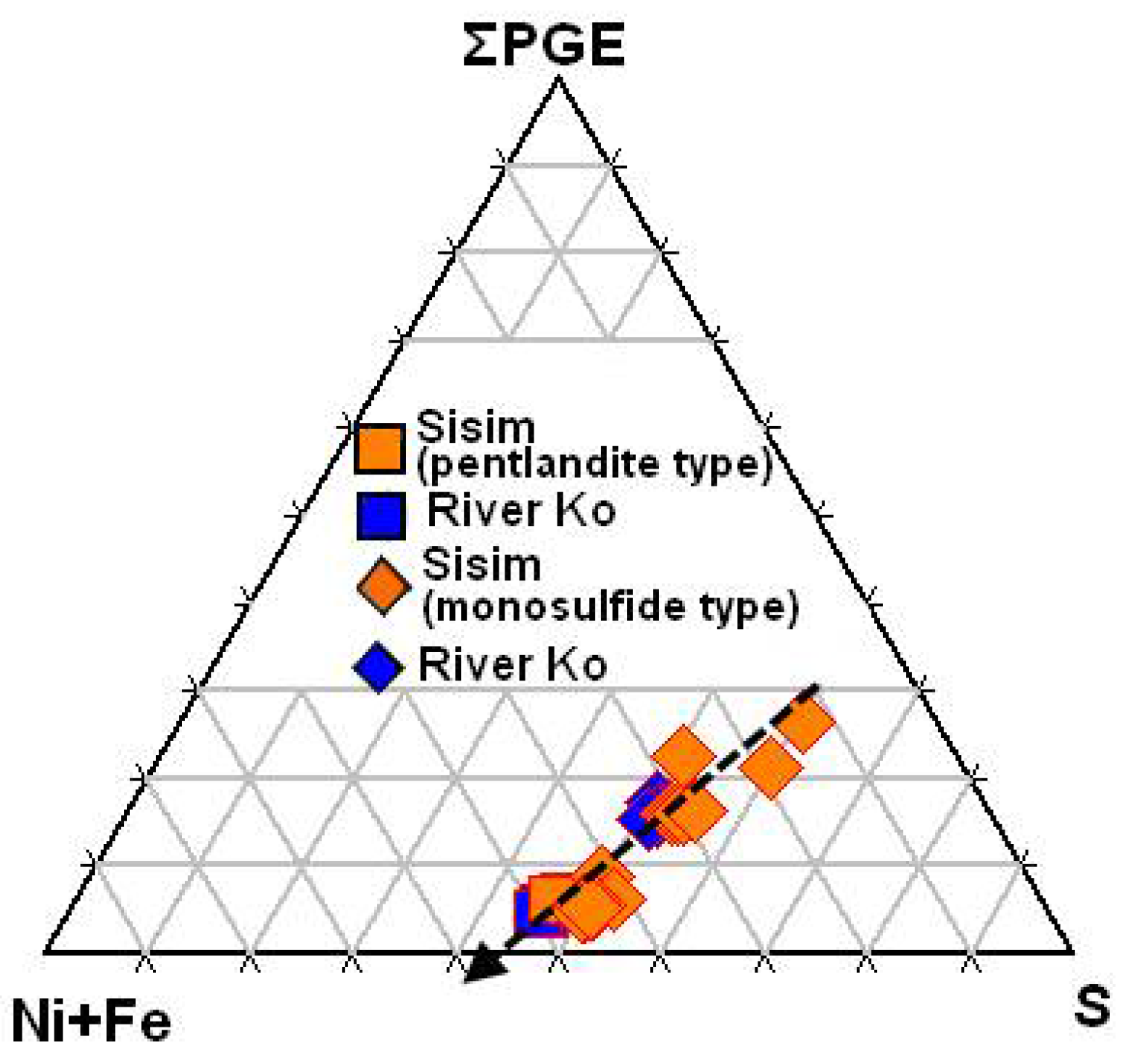
3.6. Monosulfide and Pentlandite-Type Inclusions in Grains of PGE Alloys
3.7. Other Unusual Phases in Micrometric Inclusions at Sisim
4. Discussion
4.1. Crystallization History of Associations of PGM at Sisim
4.2. Contrasting Behavior of Ir and Mechanisms of Element Substitutions
4.3. Potential Provenance of PGM in the Sisim Placer Zone
5. Conclusions
- (1)
- We attribute the PGM-bearing placer deposits in the Sisim watershed to the Lysanskiy ultramafic–mafic layered complex, Eastern Sayans. The PGE mineralization is strongly dominated by Os–Ir alloy minerals poor in Ru and is thus distinct from deposits in an ophiolite setting.
- (2)
- The Os–Ir–(Ru) alloy minerals and associated Pt–Fe alloys were likely derived from chromitite units of the complex, whose unusual degree of Mg enrichment suggest a picritic parental melt, unusual, however, for its level of titanium. The completeness of the magnesiochromite–chromite series in the placer grains suggests that large volumes of the source rocks were completely eroded.
- (3)
- The limitation of the Os–Ir–(Ru) alloys at Sisim to the Ru-poor portion of the Os–Ir–Ru system by the line Ru/Ir = 1 implies a close geochemical relationship of Ir and Ru, manifested by the scheme Ir + Ru → 2Os. A drop in temperature, leading to a decrease in Os, is recorded in zoned grains. This zonation indicates the existence of a simple and effective mechanism of fractionation of Os from Ru and Ir in natural systems.
- (4)
- In contrast, we document a strong positive covariation of Ir and Os along with a negative Ir–Ru correlation in the laurite–erlichmanite series, likely promoted by locally high levels of fS2. This relationship points to the scheme [Os2+ + 2Ir3+ + □] → 4Ru2+. Alternatively, the IrS2 component [Ir2+(S2)2–] is involved; if so, the incorporation of essential, though limited amounts of Ir is governed by the scheme Os2+ + Ir2+ → 2Ru2+.
- (5)
- The inferred sequence of crystallization of PGE alloys at Sisim is as follows: (1) grains (Os-rich) of Os–Ir–(Ru) alloy; the core (Os-rich) of the zoned grains of Os–Ir–(Ru) alloy → (2) grains (Ir-rich) and periphery zones enriched relatively in Ir–Ru of the zoned grains of Os–Ir–(Ru) alloy → (3) isoferroplatinum or ferroan platinum (rich in Ir → poor in Ir) → (4) various Pt–(Pd)–Fe–Cu–Ni alloys, all likely formed under subsolidus conditions → (5) various S–As-rich phases deposited to form inclusions (or a late rim) as a result of buildup in levels of fS2 and fAs2 in the micro-environments.
- (6)
- Inclusions of the PGE-bearing phases of monosulfide and pentlandite types, hosted by grains of PGE alloys, follow a linear trend of crystallization, which reflects a decrease in temperature. The decrease in ΣPGE and overall S was accompanied by an increase in Ni and Fe. The observed S-excess in the ΣPGE-rich sulfide phases likely compensates the excess in positive charges owing to the Rh3+ (+Ir3+)-for-(Ni + Fe)2+ substitution.
- (7)
- A unique association of laurite with micrometer-sized particles of monazite-(Ce) is documented in a composite inclusion. The juxtaposition reflects an increase in levels of incompatible elements (S, P, and the REE) in a residual microvolume of aqueous fluid. This could be another expression of the unusual character of the parental magma of the Lysanskiy complex.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vysotskiy, N.K. Platinum and areas of its mining. In The Natural Producing Forces of Russia. Part 5—A Review of Platinum Deposits Outside The Urals; The USSR Academy of Sciences: Leningrad, Russia, 1933; p. 240. (In Russian) [Google Scholar]
- Krivenko, A.P.; Tolstykh, N.D.; Nesterenko, G.V.; Lazareva, E.V. Types of mineral assemblages of platinum metals in auriferous placers of the Altai-Sayan region. Russian Geol. Geophys. 1994, 35, 58–65. [Google Scholar]
- Tolstykh, N.D.; Krivenko, A.P. On the composition of sulfides containing the platinum-group elements. Zap. Vseross Miner. Obshch 1994, 123, 41–49. (In Russian) [Google Scholar]
- Barkov, A.Y.; Tolstykh, N.D.; Shvedov, G.I.; Martin, R.F. Ophiolite-related associations of platinum-group minerals at Rudnaya, western Sayans, and Miass, southern Urals, Russia. Mineral. Mag. 2018, in press. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E.F. Petrogenesis of the ophiolitic giant chromite deposits of Kempirsai, Kazakhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 38, 1419–1458. [Google Scholar] [CrossRef]
- Harris, D.C.; Cabri, L.J. Nomenclature of platinum-group-element alloys: Review and revision. Can. Mineral. 1991, 29, 231–237. [Google Scholar]
- Barkov, A.Y.; Fleet, M.E.; Nixon, G.T.; Levson, V.M. Platinum-group minerals from five placer deposits in British Columbia, Canada. Can. Mineral. 2005, 43, 1687–1710. [Google Scholar] [CrossRef]
- Karimova, O.; Zoloratev, A.; Evstigneeva, T.L.; Johanson, B. Mertieite-II, Pd8Sb2.5As0.5, crystal structure refinement and formula revision. Mineral. Mag. 2018. [Google Scholar] [CrossRef]
- Begizov, V.D.; Zavyalov, E.N. Ferhodsite (Fe,Rh,Ir,Ni,Cu,Co,Pt)9-xS8—A new mineral from the Nizhniy Tagil ultramafic complex. New Data Miner. 2016, 51, 8–11. (In Russian) [Google Scholar]
- Barkov, A.Y.; Halkoaho, T.A.A.; Laajoki, K.V.O.; Alapieti, T.T.; Peura, R.A. Ruthenian pyrite and nickeloan malanite from the Imandra layered complex, northwestern Russia. Can. Mineral. 1997, 35, 887–897. [Google Scholar]
- Roberts, P.M. Introduction to Brazing Technology; CRC Press: Boca Raton, FL, USA, 2016; p. 340. [Google Scholar] [CrossRef]
- Andrews, D.R.A.; Brenan, J.M. Phase-equilibrium constraints on the magmatic origin of laurite and Os–Ir alloy. Can. Mineral. 2002, 40, 1705–1716. [Google Scholar] [CrossRef]
- Bockrath, C.; Ballhaus, C.; Holzheid, A. Stabilities of laurite RuS2 and monosulfide liquid solution at magmatic temperature. Chem. Geol. 2004, 208, 265–271. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Fleet, M.E. An unusual association of hydrothermal platinum-group minerals from the Imandra layered complex, Kola Peninsula, northwestern Russia. Can. Mineral. 2004, 42, 455–467. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Nikiforov, A.A.; Tolstykh, N.D.; Shvedov, G.I.; Korolyuk, V.N. Compounds of Ru–Se–S, alloys of Os–Ir, framboidal Ru nanophases and laurite–clinochlore intergrowths in the Pados-Tundra complex, Kola Peninsula, Russia. Eur. J. Mineral. 2017, 29, 613–622. [Google Scholar] [CrossRef]
- Cabri, L.J. (Ed.) The Geology, Geochemistry, Mineralogy, Mineral Beneficiation of the Platinum-Group Elements; Canadian Institute of Mining, Metallurgy and Petroleum: Westmount, QC, Canada, 2002; Volume 54, p. 852. [Google Scholar]
- Junge, M.; Oberthür, T.; Melcher, F. Cryptic variation of chromite chemistry, platinum group element and platinum group mineral distribution in the UG-2 chromitite: An example from the Karee mine, western Bushveld complex, South Africa. Econ. Geol. 2014, 109, 795–810. [Google Scholar] [CrossRef]
- Junge, M.; Wirth, R.; Oberthür, T.; Melcher, F.; Schreiber, A. Mineralogical siting of platinum-group elements in pentlandite from the Bushveld Complex, South Africa. Miner. Depos. 2014, 50, 41–54. [Google Scholar] [CrossRef]
- Makovicky, E.; Karup-Møller, S. The Pd–Ni–Fe–S phase system at 550 and 400 °C. Can. Mineral. 2016, 54, 377–400. [Google Scholar] [CrossRef]
- Makovicky, E.; Makovicky, M.; Rose-Hansen, J. The system Fe–Rh–S at 900° and 500 °C. Can. Mineral. 2002, 40, 519–526. [Google Scholar] [CrossRef]
- Makovicky, E.; Karup-Møller, S. The phase system Fe–Ir–S at 1100, 1000 and 800 °C. Mineral. Mag. 1999, 63, 379–385. [Google Scholar] [CrossRef]
- Majzlan, J.; Makovicky, M.; Makovicky, E.; Rose-Hansen, J. The system Fe–Pt–S at 1100 °C. Can. Mineral. 2002, 40, 509–517. [Google Scholar] [CrossRef]
- Karup-Møller, S.; Makovicky, E. The system Fe–Os–S at 1180°, 1100° and 900 °C. Can. Mineral. 2002, 40, 499–507. [Google Scholar] [CrossRef]
- Cabri, L.J.; Harris, D.C.; Weiser, T.W. Mineralogy and distribution of platinum-group mineral (PGM) placer deposits of the world. Explor. Min. Geol. 1996, 5, 73–167. [Google Scholar]
- Barkov, A.Y.; Fleet, M.E.; Martin, R.F.; Alapieti, T.T. Zoned sulfides and sulfarsenides of the platinum-group elements from the Penikat layered complex, Finland. Can. Mineral. 2004, 42, 515–537. [Google Scholar] [CrossRef]
- Stockman, H.W.; Hlava, P. Platinum-group minerals in Alpine chromitites from Southwestern Oregon. Econ. Geol. 1984, 79, 491–508. [Google Scholar] [CrossRef]
- Glazunov, O.M. The Geochemistry and Petrology of the Gabbro-Pyroxenite Formation of the Eastern Sayans; Nauka: Novosibirsk, Russia, 1975; p. 188. (In Russian) [Google Scholar]
- Hulbert, L.J.; von Gruenewaldt, G. Textural and compositional features of chromite in the Lower and Critical zones of the Bushveld complex south of Potgietersrus. Econ. Geol. 1985, 80, 872–895. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Korolyuk, V.N.; Martin, R.F. The maximum extent of Mg-enrichment in olivine of layered intrusions: Evidence from Monchepluton (Fo96) and Pados-Tundra (Fo93), Kola Peninsula, Russia. Eur. J. Mineral. 2018. under review. [Google Scholar]
- Hickmann, K.; John, V.; Oertel, A.; Koempe, K.; Haase, M. Investigation of the early stages of growth of monazite-type lanthanide phosphate nanoparticles. J. Phys. Chem. C 2009, 113, 4763–4767. [Google Scholar] [CrossRef]



| # | TiO2 | Al2O3 | Cr2O3 | FeO(t) | FeO(calc.) | Fe2O3(calc.) | MnO | MgO | NiO | Total | |
| 1 | 0.52 | 13.83 | 46.45 | 36.72 | 31.8 | 5.47 | 0.34 | 1.64 | 0.04 | 100.21 | |
| 2 | 1.33 | 13.54 | 43.31 | 37.59 | 30.37 | 8.03 | 0.27 | 2.92 | 0.18 | 100.1 | |
| 3 | 1.05 | 16.24 | 48.09 | 18.97 | 12.71 | 6.96 | 0.17 | 14.63 | 0.24 | 100.22 | |
| 4 | 1.01 | 15.04 | 45.6 | 29.53 | 23.21 | 7.02 | 0.32 | 7.55 | 0.14 | 100.05 | |
| 5 | 0.38 | 13.57 | 42.48 | 40.34 | 32.57 | 8.64 | 0.68 | 0.51 | 0.06 | 99.03 | |
| 6 | 0.9 | 16.12 | 48.64 | 15.88 | 9.33 | 7.28 | 0.09 | 16.59 | 0.26 | 99.33 | |
| 7 | 1.16 | 14.68 | 45.02 | 27.42 | 18.13 | 10.32 | 0.2 | 11.12 | 0.21 | 100.95 | |
| 8 | 0.89 | 16.46 | 48.82 | 16.78 | 11.01 | 6.41 | 0.12 | 15.59 | 0.24 | 99.64 | |
| 9 | 1.34 | 15.07 | 45.61 | 23.1 | 15.62 | 8.31 | 0.16 | 12.55 | 0.18 | 99.1 | |
| 10 | 1.15 | 14.54 | 46.13 | 27.58 | 20.28 | 8.11 | 0.22 | 9.6 | 0.16 | 100.31 | |
| Atomic Proportions (O = 4) | |||||||||||
| # | Cr | Al | Fe3+ | Ti | Mg | Fe2+ | Mn | Ni | Mg# | Cr# | Fe3+# |
| 1 | 1.27 | 0.56 | 0.14 | 0.01 | 0.08 | 0.92 | 0.01 | 0.001 | 8 | 69 | 7 |
| 2 | 1.18 | 0.55 | 0.21 | 0.03 | 0.15 | 0.87 | 0.01 | 0.005 | 15 | 68 | 11 |
| 3 | 1.19 | 0.6 | 0.16 | 0.02 | 0.68 | 0.33 | <0.01 | 0.006 | 67 | 67 | 8 |
| 4 | 1.19 | 0.59 | 0.17 | 0.03 | 0.37 | 0.64 | 0.01 | 0.004 | 36 | 67 | 9 |
| 5 | 1.19 | 0.56 | 0.23 | 0.01 | 0.03 | 0.96 | 0.02 | 0.002 | 3 | 68 | 12 |
| 6 | 1.2 | 0.59 | 0.17 | 0.02 | 0.77 | 0.24 | <0.01 | 0.006 | 76 | 67 | 9 |
| 7 | 1.14 | 0.55 | 0.25 | 0.03 | 0.53 | 0.49 | 0.01 | 0.005 | 52 | 67 | 13 |
| 8 | 1.2 | 0.6 | 0.15 | 0.02 | 0.72 | 0.29 | <0.01 | 0.006 | 71 | 67 | 8 |
| 9 | 1.16 | 0.57 | 0.2 | 0.03 | 0.6 | 0.42 | <0.01 | 0.005 | 59 | 67 | 10 |
| 10 | 1.19 | 0.56 | 0.2 | 0.03 | 0.47 | 0.55 | 0.01 | 0.004 | 46 | 68 | 10 |
| # | Ru | Os | Ir | Rh | Pt | Fe | Ni | Total | ||
| 1 | Os-dominant | Matrix | 6.72 | 55.45 | 37 | 0.06 | 0.23 | 0.13 | 0.03 | 99.62 |
| 2 | 11.58 | 45.6 | 38.48 | 1.13 | 2.86 | 0.16 | 0.02 | 99.83 | ||
| 3 | 5.82 | 74.44 | 20 | 0.05 | 0.04 | 0.03 | bdl | 100.38 | ||
| 4 | 5.42 | 65.97 | 27.4 | 0.24 | 0.43 | 0.03 | 0.05 | 99.54 | ||
| 5 | 11.94 | 42.79 | 41.28 | 1.1 | 1.61 | 0.26 | 0.05 | 99.03 | ||
| 6 | Inclusion | 11.76 | 53.15 | 34.38 | 0.24 | 0.39 | 0.11 | 0.09 | 100.12 | |
| 7 | 0.25 | 77.34 | 21.83 | bdl | 0.09 | 0.06 | 0.03 | 99.6 | ||
| 8 | 0.89 | 88.05 | 10.5 | bdl | bdl | 0.06 | bdl | 99.5 | ||
| 9 | 3.08 | 71.01 | 25.65 | bdl | bdl | 0.06 | 0.05 | 99.85 | ||
| 10 | 4.54 | 54.2 | 39.53 | 0.26 | 1.58 | 0.18 | bdl | 100.3 | ||
| 11 | Ir-dominant | Matrix | 6.36 | 14.58 | 75.29 | 0.52 | 1.81 | 0.58 | 0.09 | 99.23 |
| 12 | 3.55 | 12.63 | 82.53 | 0.14 | 0.79 | 0.8 | 0.09 | 100.53 | ||
| 13 | 1.11 | 27.12 | 70.78 | bdl | bdl | 0.35 | 0.06 | 99.41 | ||
| 14 | 5 | 7.21 | 84.83 | 0.15 | 0.68 | 1.07 | 0.15 | 99.08 | ||
| 15 | Inclusion | 13.17 | 37.08 | 48.38 | bdl | 0.55 | 0.23 | 0.04 | 99.45 | |
| Atomic Proportions (per a Total of 100 at. %) | ||||||||||
| # | Ru | Os | Ir | Rh | Pt | Fe | Ni | |||
| 1 | 12 | 52.5 | 34.7 | 0.1 | 0.21 | 0.42 | 0.1 | |||
| 2 | 19.6 | 41.1 | 34.3 | 1.88 | 2.51 | 0.49 | 0.06 | |||
| 3 | 10.4 | 70.6 | 18.8 | 0.09 | 0.03 | 0.1 | 0 | |||
| 4 | 9.8 | 63.2 | 26 | 0.42 | 0.4 | 0.11 | 0.14 | |||
| 5 | 20.3 | 38.6 | 36.9 | 1.84 | 1.42 | 0.81 | 0.14 | |||
| 6 | 20 | 48 | 30.7 | 0.39 | 0.35 | 0.34 | 0.26 | |||
| 7 | 0.5 | 77.5 | 21.6 | 0 | 0.08 | 0.2 | 0.11 | |||
| 8 | 1.7 | 87.8 | 10.4 | 0 | 0 | 0.19 | 0.01 | |||
| 9 | 5.7 | 69.2 | 24.7 | 0 | 0 | 0.21 | 0.15 | |||
| 10 | 8.2 | 51.9 | 37.4 | 0.47 | 1.47 | 0.59 | 0.01 | |||
| 11 | 11.3 | 13.7 | 70.3 | 0.91 | 1.66 | 1.85 | 0.28 | |||
| 12 | 6.4 | 12 | 77.8 | 0.25 | 0.73 | 2.59 | 0.28 | |||
| 13 | 2.1 | 27 | 69.6 | 0 | 0 | 1.18 | 0.18 | |||
| 14 | 8.9 | 6.8 | 79.5 | 0.26 | 0.63 | 3.45 | 0.44 | |||
| 15 | 22.3 | 33.3 | 43.1 | 0 | 0.48 | 0.72 | 0.1 | |||
| # | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Cu | Total | |
| 1 | Matrix | 0.41 | 0.71 | 4.96 | bdl | 84.62 | bdl | 7.15 | 0.03 | 0.42 | 98.3 |
| 2 | 1.12 | 0.3 | 5.44 | 5.6 | 79.63 | bdl | 6.8 | 0.15 | 1.07 | 100.1 | |
| 3 | 1.2 | 0.31 | 5.81 | 4.56 | 80.01 | bdl | 7.38 | 0.09 | 0.65 | 100 | |
| 4 | 0.9 | 2.19 | 4.55 | 4.38 | 80.02 | bdl | 5.65 | 0.01 | 1.93 | 99.6 | |
| 5 | 0.79 | 2.67 | 5.24 | 4.55 | 79.28 | bdl | 5.82 | 0.01 | 1.75 | 100.1 | |
| 6 | 2.52 | 2.35 | 6.82 | 1.03 | 79.82 | bdl | 6 | 0.12 | 0.81 | 99.5 | |
| 7 | Inclusion | bdl | 0.15 | 9.72 | 0.27 | 77.55 | 0.25 | 8.72 | 0.62 | 0.11 | 97.4 |
| 8 | bdl | 0.06 | 8.33 | 0.28 | 78.98 | 0.3 | 9 | 0.7 | 0.2 | 97.9 | |
| 9 | bdl | 0.16 | 10.84 | 0.22 | 76.4 | 0.18 | 8.69 | 0.59 | 0.11 | 97.2 | |
| 10 | 0.15 | 0.08 | 9.57 | 0.38 | 78.92 | bdl | 8.7 | 0.68 | 0.22 | 98.7 | |
| 11 | 0.15 | 0.31 | 8.82 | 8.47 | 69.51 | bdl | 9.19 | 0.85 | 0.4 | 97.7 | |
| 12 | bdl | bdl | 8.9 | 4.2 | 71.6 | bdl | 12.7 | 3.7 | 0.9 | 102 | |
| 13 | bdl | bdl | 20.9 | 3.5 | 64.4 | bdl | 10.2 | 2.6 | bdl | 101.6 | |
| 14 | bdl | bdl | 10.6 | 7.9 | 66.9 | bdl | 10.5 | 2.6 | bdl | 98.5 | |
| 15 | bdl | bdl | 4.8 | 2.9 | 69.8 | 1.2 | 13.9 | 5.6 | 1.5 | 99.7 | |
| 16 | bdl | bdl | 10.8 | 6.7 | 73.7 | bdl | 10.1 | 1.9 | bdl | 103.2 | |
| 17 | bdl | bdl | bdl | bdl | 71.7 | 2.5 | 12.8 | 4.4 | 6.1 | 97.5 | |
| 18 | bdl | bdl | bdl | bdl | 80.2 | 6.9 | 10.8 | 1.6 | 0.9 | 100.4 | |
| 19 | bdl | bdl | bdl | bdl | 92.9 | bdl | 8.8 | 0.7 | bdl | 102.4 | |
| 20 | bdl | bdl | bdl | bdl | 92 | bdl | 8.6 | 0.7 | bdl | 101.3 | |
| 21 | bdl | bdl | bdl | bdl | 66.7 | 12.8 | 11.3 | 0.7 | 11.3 | 102.8 | |
| Atomic Proportions (per a Total of 100 at. %) | |||||||||||
| # | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Cu | ΣPGE/(Fe + Ni + Cu) | |
| 1 | 0.7 | 0.6 | 4.3 | 0 | 72 | 0 | 21.2 | 0.1 | 1.1 | 3.46 | |
| 2 | 1.7 | 0.2 | 4.4 | 8.4 | 63.3 | 0 | 18.9 | 0.4 | 2.6 | 3.57 | |
| 3 | 1.8 | 0.3 | 4.7 | 6.9 | 63.9 | 0 | 20.6 | 0.2 | 1.6 | 3.46 | |
| 4 | 1.4 | 1.8 | 3.8 | 6.8 | 65.3 | 0 | 16.1 | 0 | 4.8 | 3.77 | |
| 5 | 1.2 | 2.2 | 4.3 | 7 | 64.3 | 0 | 16.5 | 0 | 4.4 | 3.79 | |
| 6 | 4.1 | 2 | 5.8 | 1.6 | 66.6 | 0 | 17.5 | 0.3 | 2.1 | 4.03 | |
| 7 | 0 | 0.1 | 8.1 | 0.4 | 63.9 | 0.4 | 25.1 | 1.7 | 0.3 | 2.69 | |
| 8 | 0 | 0.1 | 6.9 | 0.4 | 64.2 | 0.4 | 25.6 | 1.9 | 0.5 | 2.58 | |
| 9 | 0 | 0.1 | 9.1 | 0.3 | 63.2 | 0.3 | 25.1 | 1.6 | 0.3 | 2.7 | |
| 10 | 0.2 | 0.1 | 7.9 | 0.6 | 64.1 | 0 | 24.7 | 1.8 | 0.6 | 2.69 | |
| 11 | 0.2 | 0.2 | 6.8 | 12.2 | 53 | 0 | 24.5 | 2.1 | 0.9 | 2.63 | |
| 12 | 0 | 0 | 6.1 | 5.4 | 48.4 | 0 | 30 | 8.3 | 1.9 | 1.49 | |
| 13 | 0 | 0 | 15.5 | 4.9 | 47.2 | 0 | 26.1 | 6.3 | 0 | 2.08 | |
| 14 | 0 | 0 | 7.8 | 10.9 | 48.5 | 0 | 26.6 | 6.3 | 0 | 2.04 | |
| 15 | 0 | 0 | 3.2 | 3.6 | 45.3 | 1.4 | 31.5 | 12.1 | 3 | 1.15 | |
| 16 | 0 | 0 | 7.9 | 9.1 | 53 | 0 | 25.4 | 4.5 | 0 | 2.34 | |
| 17 | 0 | 0 | 0 | 0 | 46.5 | 3 | 29 | 9.5 | 12.1 | 0.98 | |
| 18 | 0 | 0 | 0 | 0 | 57.8 | 9.1 | 27.2 | 3.8 | 2 | 2.03 | |
| 19 | 0 | 0 | 0 | 0 | 73.8 | 0 | 24.4 | 1.8 | 0 | 2.81 | |
| 20 | 0 | 0 | 0 | 0 | 74 | 0 | 24.2 | 1.9 | 0 | 2.84 | |
| 21 | 0 | 0 | 0 | 0 | 40 | 14.1 | 23.7 | 1.4 | 20.8 | 1.18 | |
| # | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Total | ||
| 1 | 45.92 | 1.2 | 10.28 | 0.16 | 6.59 | 0.14 | 18.15 | 10.56 | 2.03 | 0.15 | 95.18 | ||
| 2 | 45.83 | 0.84 | 8.27 | 0.18 | 7.95 | 0.2 | 19.04 | 8.38 | 1.53 | 0.11 | 92.33 | ||
| 3 | 44.31 | 1.63 | 10.26 | 0.27 | 11.78 | 0.17 | 14.97 | 10.2 | 0.61 | 0.22 | 94.42 | ||
| 4 | 43.61 | 1.62 | 9.91 | 0.2 | 12.05 | 0.22 | 14.7 | 10.49 | 0.7 | 0.21 | 93.71 | ||
| 5 | 47.04 | 1 | 10.34 | 0.17 | 10.63 | 0.19 | 14.31 | 8.16 | 1.04 | 0.02 | 92.9 | ||
| 6 | 48.63 | 1.02 | 8.2 | 0.21 | 11.32 | 0.34 | 13.48 | 11.93 | 0.73 | 0.84 | 96.7 | ||
| 7 | 48.37 | 1.5 | 13.32 | 0.28 | 9.9 | 0.18 | 10.28 | 9.02 | 2.17 | 1.06 | 96.08 | ||
| 8 | 44.39 | 1.75 | 10.1 | 0.9 | 12.06 | 0.16 | 13.2 | 5.68 | 3.64 | 0.25 | 92.13 | ||
| 9 | 48.67 | 1.72 | 13.6 | 0.12 | 6.78 | 0.18 | 7.81 | 13.81 | 2.38 | 0.38 | 95.45 | ||
| 10 | 51.11 | 1.3 | 14.4 | 0.13 | 6.65 | 0.17 | 8.45 | 12.18 | 2.4 | 0.38 | 97.17 | ||
| Atomic Proportions (O = 23) | |||||||||||||
| # | Si | [4]Al | Al | Fe3+ | Ti | Cr | Fe2+ | Mn | Mg | Ca | Na | K | Mg# |
| 1 | 6.68 | 1.32 | 0.44 | 0 | 0.13 | 0.02 | 0.8 | 0.02 | 3.94 | 1.65 | 0.57 | 0.03 | 82.8 |
| 2 | 6.84 | 1.16 | 0.29 | 0.2 | 0.09 | 0.02 | 0.8 | 0.03 | 4.24 | 1.34 | 0.44 | 0.02 | 83.6 |
| 3 | 6.58 | 1.42 | 0.37 | 0.44 | 0.18 | 0.03 | 1.02 | 0.02 | 3.31 | 1.62 | 0.18 | 0.04 | 76.1 |
| 4 | 6.54 | 1.46 | 0.29 | 0.55 | 0.18 | 0.02 | 0.97 | 0.03 | 3.29 | 1.68 | 0.2 | 0.04 | 76.7 |
| 5 | 7.02 | 0.98 | 0.83 | 0 | 0.11 | 0.02 | 1.33 | 0.02 | 3.18 | 1.3 | 0.3 | <0.01 | 70.2 |
| 6 | 7.1 | 0.9 | 0.52 | 0 | 0.11 | 0.02 | 1.38 | 0.04 | 2.94 | 1.87 | 0.21 | 0.16 | 67.4 |
| 7 | 6.99 | 1.01 | 1.26 | 0 | 0.16 | 0.03 | 1.2 | 0.02 | 2.22 | 1.4 | 0.61 | 0.2 | 64.5 |
| 8 | 6.81 | 1.19 | 0.63 | 0 | 0.2 | 0.11 | 1.55 | 0.02 | 3.02 | 0.93 | 1.08 | 0.05 | 65.8 |
| 9 | 7.04 | 0.96 | 1.36 | 0 | 0.19 | 0.01 | 0.82 | 0.02 | 1.69 | 2.14 | 0.67 | 0.07 | 66.8 |
| 10 | 7.18 | 0.82 | 1.57 | 0 | 0.14 | 0.01 | 0.78 | 0.02 | 1.77 | 1.83 | 0.65 | 0.07 | 68.9 |
| # | P2O5 | SiO2 | TiO2 | Al2O3 | Cr2O3 | Ce2O3 | La2O3 | Sm2O3 | Nd2O3 | Pr2O3 | FeO | CaO | Na2O | Total |
| 1 | 28.52 | bdl | bdl | bdl | bdl | 24.91 | 12.42 | bdl | 8.38 | 2.22 | bdl | 2.83 | bdl | 79.3 |
| 2 | 30.93 | bdl | bdl | bdl | bdl | 24 | 14.84 | 1.09 | 9.91 | 2.71 | 1.22 | 3.13 | bdl | 87.8 |
| 3 | 0 | 29.77 | 37.22 | 0.46 | 0.12 | bdl | bdl | bdl | bdl | bdl | 0.65 | 26.81 | 0.06 | 95.1 |
| Atomic Proportions | ||||||||||||||
| # | P | Si | Ti | Al | Cr | Ce | La | Sm | Nd | Pr | Fe | Ca | Na | Σ(REE + Ca) |
| 1 | 1.08 | 0 | 0 | 0 | 0 | 0.41 | 0.2 | 0 | 0.13 | 0.04 | 0 | 0.14 | 0 | 0.92 |
| 2 | 1.06 | 0 | 0 | 0 | 0 | 0.36 | 0.22 | 0.02 | 0.14 | 0.04 | 0.04 | 0.14 | <0.01 | 0.94 |
| 3 | 0 | 1.02 | 0.96 | 0.02 | <0.01 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0.99 | 0 | 1.01 |
| # | Mineral | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Co | Cu | S | As | Sb | Total |
| 1 | Mertieite-II | bdl | bdl | bdl | bdl | bdl | 72 | bdl | bdl | bdl | bdl | bdl | 3.48 | 23.5 | 99 |
| 2 | bdl | bdl | bdl | bdl | bdl | 71.86 | bdl | bdl | bdl | bdl | bdl | 3.5 | 23.28 | 98.6 | |
| 3 | Hollingworthite | 0.95 | 0.29 | 5.89 | 31.14 | 13.2 | bdl | 0.85 | 0.03 | bdl | bdl | 9.82 | 34.58 | bdl | 96.8 |
| 4 | 3.03 | 0.73 | 18.24 | 17.5 | 15.95 | bdl | 0.1 | 0.06 | bdl | bdl | 10.78 | 31.3 | bdl | 97.7 | |
| 5 | Cherepanovite | 3.51 | bdl | 2.1 | 47.11 | 5 | bdl | 0.13 | 0.02 | bdl | bdl | 3.13 | 39.62 | bdl | 100.6 |
| 6 | 2.73 | bdl | 2.6 | 45.52 | 6.27 | bdl | 0.06 | 0.02 | bdl | bdl | 3.43 | 39.41 | bdl | 100 | |
| 7 | Laurite (Fe–Ni-rich) | 31.5 | bdl | 4.9 | 6.9 | bdl | bdl | 9.1 | 8.9 | bdl | 2.7 | 36.4 | bdl | bdl | 100.4 |
| 8 | Laurite (As-rich) | 46.8 | 3.7 | 3 | bdl | bdl | bdl | 1.1 | 0.6 | 1.8 | bdl | 22.1 | 23.4 | bdl | 102.5 |
| 9 | Kashinite | bdl | bdl | 77.8 | 0.7 | bdl | bdl | bdl | bdl | bdl | bdl | 22.5 | bdl | bdl | 101 |
| 10 | Kashinite (Cu-rich) | bdl | bdl | 64.5 | bdl | bdl | bdl | 3.7 | bdl | bdl | 6.4 | 24.6 | bdl | bdl | 99.2 |
| Atomic Proportions | |||||||||||||||
| # | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Co | Cu | S | As | Sb | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 8.12 | 0 | 0 | 0 | 0 | 0 | 0.56 | 2.32 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 8.13 | 0 | 0 | 0 | 0 | 0 | 0.56 | 2.3 | ||
| 3 | 0.02 | <0.01 | 0.08 | 0.76 | 0.17 | 0 | 0.04 | <0.01 | 0 | 0 | 0.77 | 1.16 | 0 | ||
| 4 | 0.08 | 0.01 | 0.25 | 0.45 | 0.22 | 0 | <0.01 | <0.01 | 0 | 0 | 0.89 | 1.1 | 0 | ||
| 5 | 0.06 | 0 | 0.02 | 0.79 | 0.04 | 0 | <0.01 | <0.01 | 0 | 0 | 0.17 | 0.91 | 0 | ||
| 6 | 0.05 | 0 | 0.02 | 0.77 | 0.06 | 0 | <0.01 | <0.01 | 0 | 0 | 0.19 | 0.92 | 0 | ||
| 7 | 0.49 | 0 | 0.04 | 0.11 | 0 | 0 | 0.26 | 0.24 | 0 | 0.07 | 1.89 | 0 | 0 | ||
| 8 | 0.89 | 0.04 | 0.03 | 0 | 0 | 0 | 0 | 0.02 | 0.06 | 0 | 1.33 | 0.6 | 0 | ||
| 9 | 0 | 0 | 1.82 | 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 3.15 | 0 | 0 | ||
| 10 | 0 | 0 | 1.32 | 0 | 0 | 0 | 0.26 | 0 | 0 | 0.4 | 3.02 | 0 | 0 | ||
| # | Ru | Os | Ir | Rh | Pt | Fe | Ni | S | As | Total |
| 1 | 14.91 | 45.58 | 10.79 | bdl | bdl | bdl | 0.02 | 28.49 | bdl | 99.79 |
| 2 | 49.16 | 6.67 | 5.79 | 0.82 | bdl | 0.01 | 0.01 | 36.01 | bdl | 98.47 |
| 3 | 1.7 | 57.75 | 14.09 | bdl | bdl | 0.24 | 0.2 | 26.02 | 0.17 | 100.17 |
| 4 | 34.24 | 22.2 | 8.71 | 0.66 | bdl | bdl | bdl | 32.94 | 0.08 | 98.83 |
| 5 | 46.17 | 9.55 | 6.8 | 0.34 | bdl | bdl | 0.04 | 35.31 | 0.08 | 98.29 |
| 6 | 28.8 | 27.8 | 9.8 | bdl | bdl | bdl | bdl | 34.2 | bdl | 100.6 |
| 7 | 35.6 | 20.6 | 8 | bdl | bdl | bdl | bdl | 35.3 | bdl | 99.5 |
| 8 | 57.3 | 1.9 | 2 | bdl | bdl | bdl | bdl | 37.9 | bdl | 99.1 |
| 9 | 4.44 | 34.39 | 31.63 | 0.33 | 0.09 | 0.28 | bdl | 26.43 | bdl | 97.6 |
| 10 | 3.46 | 44.46 | 26.6 | bdl | bdl | bdl | bdl | 24.58 | 0.86 | 99.96 |
| Atomic Proportions (per a Total of 3 a.p.f.u.) | ||||||||||
| # | Ru | Os | Ir | Rh | Pt | Fe | Ni | S | As | |
| 1 | 0.33 | 0.54 | 0.13 | 0 | 0 | 0 | 0.001 | 2 | 0 | |
| 2 | 0.87 | 0.06 | 0.05 | 0.01 | 0 | 0 | 0 | 2 | 0 | |
| 3 | 0.04 | 0.75 | 0.18 | 0 | 0 | 0.01 | 0.008 | 2 | 0.006 | |
| 4 | 0.66 | 0.23 | 0.09 | 0.01 | 0 | 0 | 0 | 2.01 | 0.002 | |
| 5 | 0.83 | 0.09 | 0.06 | 0.01 | 0 | 0 | 0.001 | 2 | 0.002 | |
| 6 | 0.55 | 0.28 | 0.1 | 0 | 0 | 0 | 0 | 2.07 | 0 | |
| 7 | 0.66 | 0.2 | 0.08 | 0 | 0 | 0 | 0 | 2.06 | 0 | |
| 8 | 0.96 | 0.02 | 0.02 | 0 | 0 | 0 | 0 | 2 | 0 | |
| 9 | 0.11 | 0.44 | 0.4 | 0.01 | <0.01 | 0.01 | 2.02 | 0 | ||
| 10 | 0.09 | 0.59 | 0.35 | 0 | 0 | 0 | 0 | 1.94 | 0.03 | |
| # | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Co | Cu | S | Total |
| 1 | bdl | bdl | 0.95 | 12.69 | 0.25 | 0.02 | 23.49 | 29.26 | 0.26 | 0.41 | 30.65 | 98 |
| 2 | bdl | bdl | 0.57 | 12.25 | 0.13 | 0.15 | 24.23 | 30.11 | 0.2 | 0.23 | 30.02 | 97.9 |
| 3 | 6.98 | bdl | 0.88 | 10.34 | bdl | bdl | 20.54 | 25.03 | bdl | 0.81 | 31.14 | 95.7 |
| 4 | bdl | bdl | bdl | 11.5 | bdl | bdl | 18.7 | 33.1 | bdl | bdl | 32.1 | 95.4 |
| 5 | bdl | bdl | bdl | 11.7 | bdl | bdl | 19.3 | 34 | bdl | bdl | 33.8 | 98.8 |
| 6 | bdl | bdl | bdl | 11.3 | bdl | bdl | 19.5 | 33.7 | bdl | bdl | 34 | 98.5 |
| 7 | bdl | bdl | bdl | 10.5 | bdl | bdl | 24.7 | 31 | bdl | bdl | 34.6 | 100.8 |
| 8 | bdl | bdl | 3.1 | 10.2 | bdl | 1 | 22.3 | 25.6 | bdl | bdl | 34.4 | 96.6 |
| 9 | bdl | bdl | bdl | 10.8 | bdl | bdl | 23 | 32.1 | bdl | bdl | 33.9 | 99.8 |
| 10 | bdl | bdl | bdl | 12.1 | bdl | bdl | 18.9 | 35.3 | bdl | bdl | 34.9 | 101.2 |
| 11 | 31.5 | bdl | 4.9 | 6.9 | bdl | bdl | 9.1 | 8.9 | bdl | 2.7 | 36.4 | 100.4 |
| 12 | bdl | bdl | bdl | 11.4 | bdl | bdl | 24 | 28.7 | bdl | bdl | 33.5 | 97.6 |
| 13 | bdl | bdl | 2.7 | 29.6 | bdl | bdl | 11.6 | 20.1 | bdl | 1.9 | 31.9 | 97.8 |
| 14 | bdl | bdl | 2.6 | 29.8 | bdl | bdl | 10.9 | 20.4 | bdl | 2.3 | 32.5 | 98.5 |
| 15 | bdl | bdl | 27.4 | 10.2 | 4.5 | bdl | 9.6 | 13.7 | bdl | 5.9 | 28.8 | 100.1 |
| 16 | 1.5 | 13.4 | 26 | 7.6 | 5.5 | bdl | 7.3 | 10.1 | bdl | 5.2 | 23.5 | 100.1 |
| 17 | 2.7 | bdl | 27.1 | 11.8 | bdl | bdl | 10.9 | 11.5 | bdl | 6.3 | 30.5 | 100.8 |
| 18 | bdl | bdl | 64.5 | bdl | bdl | bdl | 3.7 | bdl | bdl | 6.4 | 24.6 | 99.2 |
| Atomic Proportions (per a Total of 100 at. %) | ||||||||||||
| # | Ru | Os | Ir | Rh | Pt | Pd | Fe | Ni | Co | Cu | S | Me/S |
| 1 | 0 | 0 | 0.2 | 6.1 | 0.06 | 0.01 | 20.9 | 24.7 | 0.2 | 0.3 | 47.4 | 1.11 |
| 2 | 0 | 0 | 0.1 | 5.9 | 0.03 | 0.07 | 21.5 | 25.5 | 0.2 | 0.2 | 46.5 | 1.15 |
| 3 | 3.5 | 0 | 0.2 | 5.1 | 0 | 0 | 18.8 | 21.8 | 0 | 0.7 | 49.8 | 1.01 |
| 4 | 0 | 0 | 0 | 5.6 | 0 | 0 | 16.6 | 28 | 0 | 0 | 49.8 | 1.01 |
| 5 | 0 | 0 | 0 | 5.4 | 0 | 0 | 16.5 | 27.7 | 0 | 0 | 50.4 | 0.99 |
| 6 | 0 | 0 | 0 | 5.2 | 0 | 0 | 16.7 | 27.4 | 0 | 0 | 50.7 | 0.97 |
| 7 | 0 | 0 | 0 | 4.7 | 0 | 0 | 20.6 | 24.5 | 0 | 0 | 50.2 | 0.99 |
| 8 | 0 | 0 | 0.8 | 4.9 | 0 | 0.46 | 19.6 | 21.5 | 0 | 0 | 52.8 | 0.89 |
| 9 | 0 | 0 | 0 | 4.9 | 0 | 0 | 19.4 | 25.8 | 0 | 0 | 49.9 | 1.01 |
| 10 | 0 | 0 | 0 | 5.5 | 0 | 0 | 15.8 | 28 | 0 | 0 | 50.7 | 0.97 |
| 11 | 16.4 | 0 | 1.3 | 3.5 | 0 | 0 | 8.6 | 8 | 0 | 2.2 | 59.9 | 0.67 |
| 12 | 0 | 0 | 0 | 5.3 | 0 | 0 | 20.7 | 23.6 | 0 | 0 | 50.4 | 0.99 |
| 13 | 0 | 0 | 0.7 | 15.3 | 0 | 0 | 11.1 | 18.2 | 0 | 1.6 | 53 | 0.89 |
| 14 | 0 | 0 | 0.7 | 15.3 | 0 | 0 | 10.3 | 18.3 | 0 | 1.9 | 53.5 | 0.87 |
| 15 | 0 | 0 | 8.6 | 6 | 1.39 | 0 | 10.3 | 14 | 0 | 5.6 | 54.1 | 0.85 |
| 16 | 1 | 4.9 | 9.4 | 5.1 | 1.96 | 0 | 9.1 | 11.9 | 0 | 5.7 | 50.9 | 0.96 |
| 17 | 1.5 | 0 | 8.2 | 6.7 | 0 | 0 | 11.3 | 11.4 | 0 | 5.8 | 55.2 | 0.81 |
| 18 | 0 | 0 | 26.4 | 0 | 0 | 0 | 5.2 | 0 | 0 | 7.9 | 60.4 | 0.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkov, A.Y.; Shvedov, G.I.; Martin, R.F. PGE–(REE–Ti)-Rich Micrometer-Sized Inclusions, Mineral Associations, Compositional Variations, and a Potential Lode Source of Platinum-Group Minerals in the Sisim Placer Zone, Eastern Sayans, Russia. Minerals 2018, 8, 181. https://doi.org/10.3390/min8050181
Barkov AY, Shvedov GI, Martin RF. PGE–(REE–Ti)-Rich Micrometer-Sized Inclusions, Mineral Associations, Compositional Variations, and a Potential Lode Source of Platinum-Group Minerals in the Sisim Placer Zone, Eastern Sayans, Russia. Minerals. 2018; 8(5):181. https://doi.org/10.3390/min8050181
Chicago/Turabian StyleBarkov, Andrei Y., Gennadiy I. Shvedov, and Robert F. Martin. 2018. "PGE–(REE–Ti)-Rich Micrometer-Sized Inclusions, Mineral Associations, Compositional Variations, and a Potential Lode Source of Platinum-Group Minerals in the Sisim Placer Zone, Eastern Sayans, Russia" Minerals 8, no. 5: 181. https://doi.org/10.3390/min8050181
APA StyleBarkov, A. Y., Shvedov, G. I., & Martin, R. F. (2018). PGE–(REE–Ti)-Rich Micrometer-Sized Inclusions, Mineral Associations, Compositional Variations, and a Potential Lode Source of Platinum-Group Minerals in the Sisim Placer Zone, Eastern Sayans, Russia. Minerals, 8(5), 181. https://doi.org/10.3390/min8050181






