Basic Characteristics of Hemimorphite and Its Transformation Mechanism with Na2CO3
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. SEM-EDS Analysis
2.3. XPS Analysis
2.4. Computational Methods
3. Results and Discussion
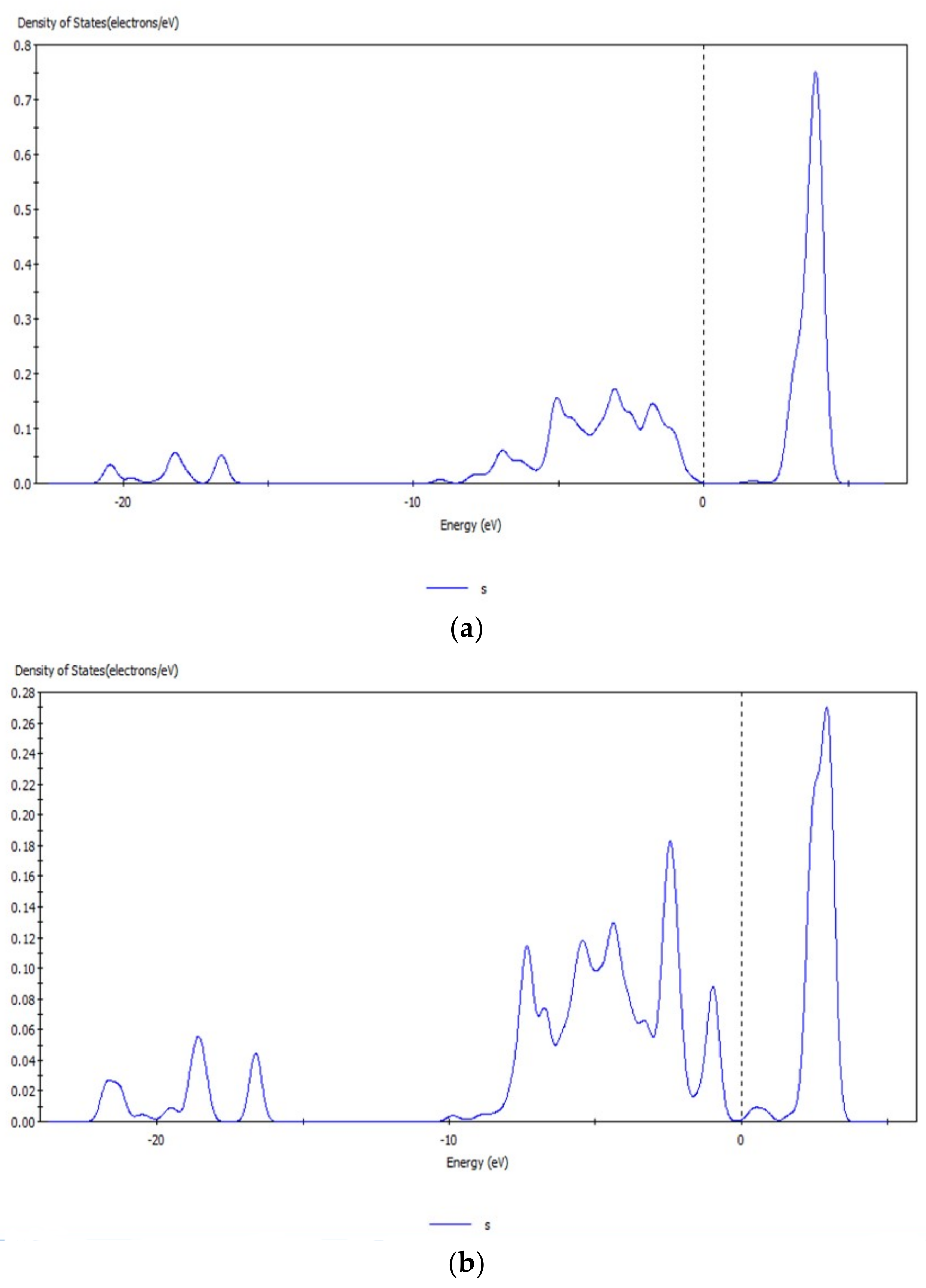
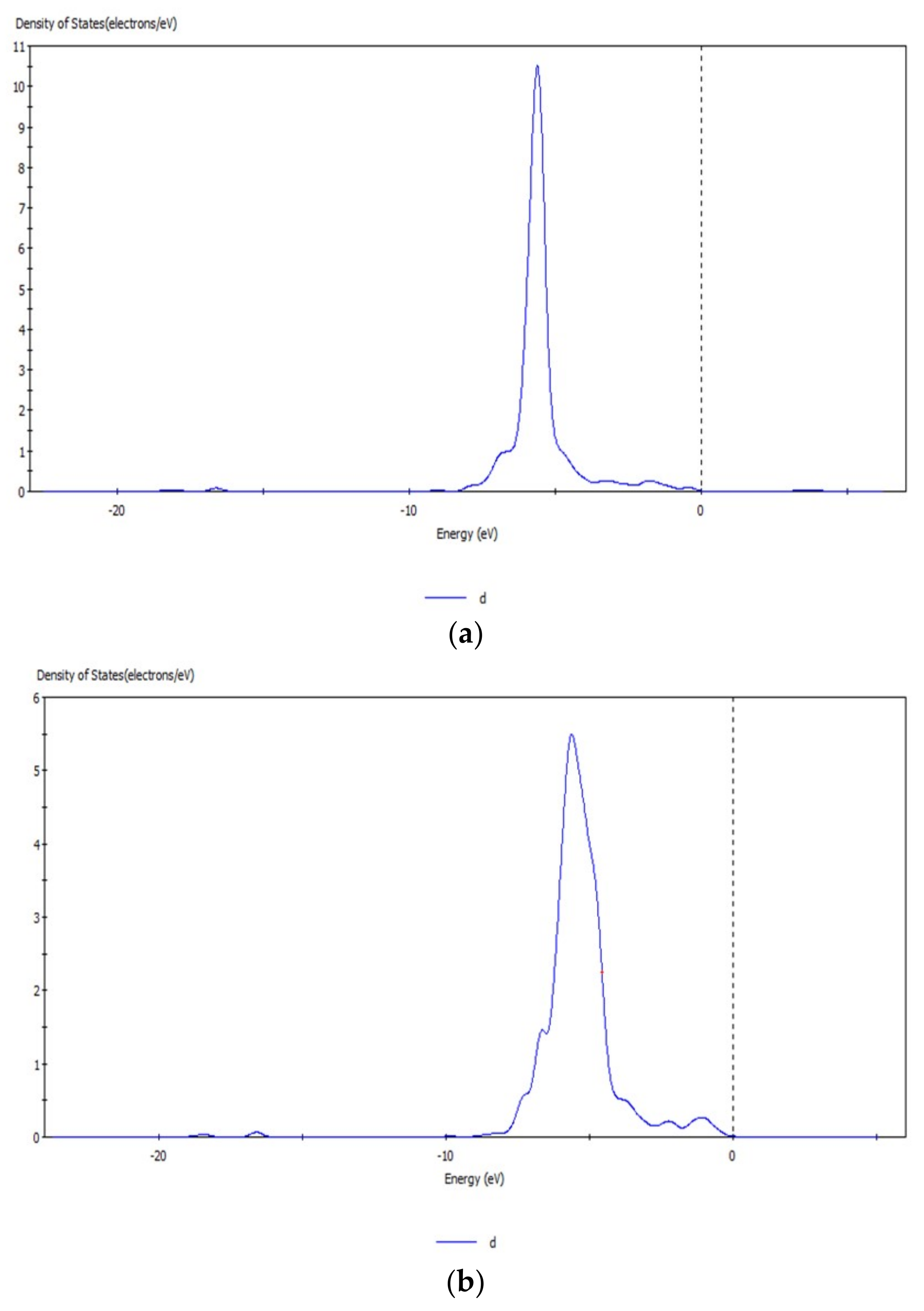
3.1. Analysis of Energy Band Structure and Density of States of Hemimorphite
3.2. Mulliken Population Analysis
3.3. Charge Density Difference of Hemimorphite
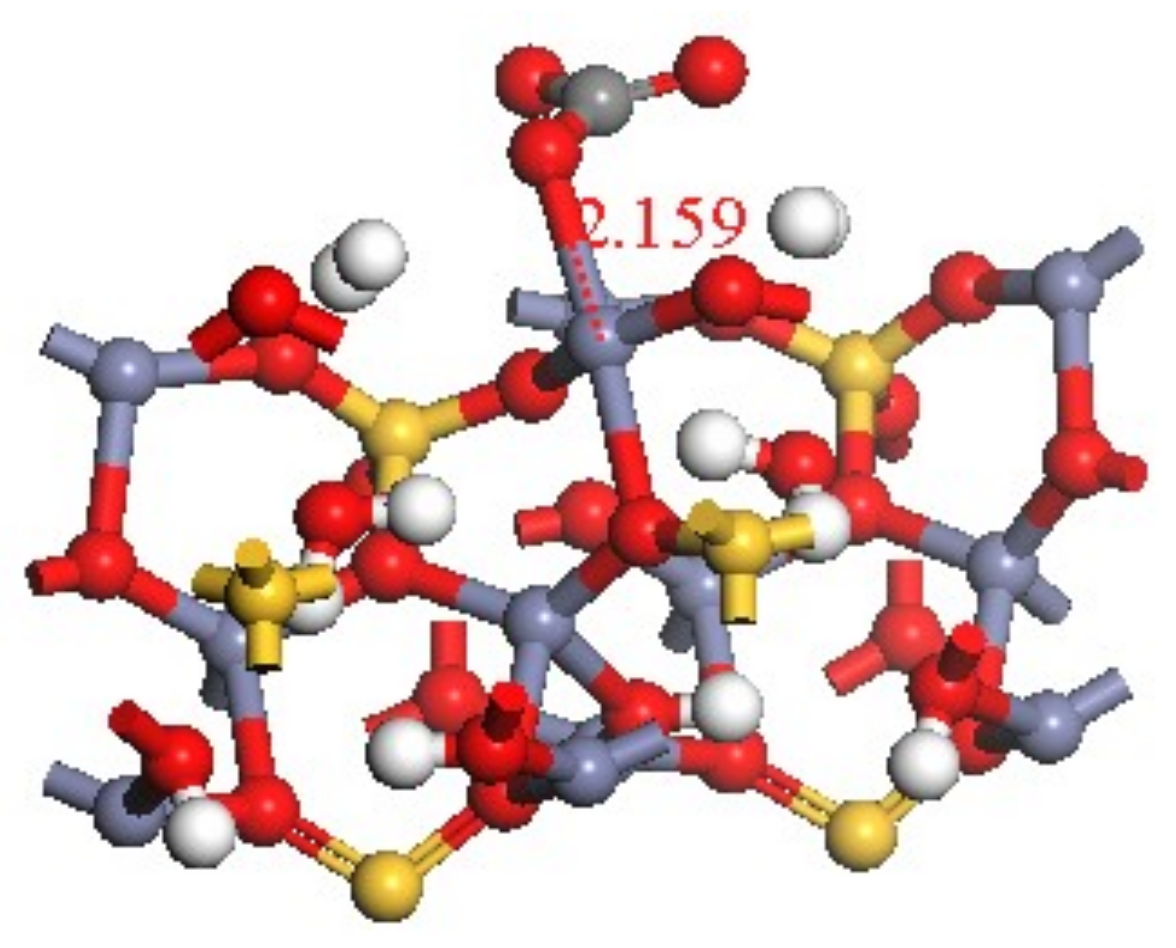
3.4. Transformation Mechanism of CO32− on Hemimorphite
3.5. XPS Measurements for the Transformation of CO32− on Hemimorphite
3.6. Analysis of Density of States of Hemimorphite after Interacting with CO32−
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yan, L.G.; Wang, A.J.; Chen, Q.S.; Li, J.W. Dynamic material flow analysis of zinc resources in China. Resour. Conserv. Recycl. 2013, 75, 23–31. [Google Scholar] [CrossRef]
- Zhang, X.F.; Hu, Y.H.; Sun, W.; Xu, L.H. The Effect of Polystyrene on the Carrier Flotation of Fine Smithsonite. Minerals 2017, 7, 52. [Google Scholar] [CrossRef]
- Dai, S.F.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dong, Y.H. Mineral Processing Research on a Complicated Refractory Zinc Oxide Ore. Hunan Nonferrous Met. 2015, 31, 23–26. (In Chinese) [Google Scholar]
- Zhou, Q.; Yang, Y.Z.; Liang, Y.Q. Study on Mineral Processing Technology for Refractory Zinc Oxide Ore. Min. Metall. Eng. 2014, 34, 54–57. (In Chinese) [Google Scholar]
- Pereira, C.A.; Peres, A.E.C. Reagents in calamine zinc ores flotation. Miner. Eng. 2005, 18, 275–277. [Google Scholar] [CrossRef]
- Zhou, W. Study on Activation Behavior of α-Nitroso-β-Naphthol in Flotation of Hemimorphite. Master’s Thesis, Hunan University, Changsha, China, 2010; pp. 35–40. (In Chinese). [Google Scholar]
- Chen, Y.; Chen, J.H.; Wu, B.Z.; Mu, X.; Wei, Z.W. Effect of synergism between hydroxy radical and sulfhydryl radical on the amine flotation of hemimorphite. Met. Mine 2009, 39, 65–68. (In Chinese) [Google Scholar]
- Jia, K.; Feng, Q.M.; Zhang, G.F.; Shi, Q.; Chang, Z.Y. Understanding the roles of Na2S and Pb(II)in the flotation of hemimorphite. Miner. Eng. 2017, 111, 167–173. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.M.; Zhang, G.F.; Ma, W.K.; Meng, Q.Y.; Chen, Y.F. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 89, 163–167. [Google Scholar] [CrossRef]
- Yuan, T.C.; Cao, Q.Y.; Li, J. Effects of mechanical activation on physicochemical properties and alkaline leaching of hemimorphite. Hydrometallurgy 2010, 104, 136–141. [Google Scholar]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Bakakin, V.V. Structural evolution of hemimorphite at high pressure up to 4.2 Gpa. Phys. Chem. Miner. 2011, 38, 679–684. [Google Scholar] [CrossRef]
- Validžić, I.L.; Mitrić, M.; Jokić, B.M.; Čomor, M.I. Structural and optical characterization of hemimorphite with flower-like morphology synthesized by a novel low-temperature method. Mater. Lett. 2012, 85, 138–141. [Google Scholar] [CrossRef]
- Mao, M.; Li, Z.; Pan, Y. Phase transitions and proton ordering in hemimorphite: New insights from single-crystal EPR experiments and DFT calculations. Phys. Chem. Miner. 2012, 40, 133–143. [Google Scholar] [CrossRef]
- Topsakal, M.; Cahangirov, S.; Bekaroglu, E.; Ciraci, S. A first-principles study of zinc oxide honeycomb structures. Phys. Rev. B 2009, 80, 235119. [Google Scholar] [CrossRef]
- Guo, L.Q.; Wu, H.N.; Liu, J.H.; Ma, H.; Song, K.Y.; Li, D.Y. Density Functional theory study on energy band and density of states of ZnO. J. Synth. Cryst. 2009, 2, 440–444. (In Chinese) [Google Scholar]
- Iokibe, K.; Sakamoto, T.; Tachikawa, H.; Azumi, K. Density functional theory study of zinc clusters. J. Surf. Finish. Soc. Jpn. 2009, 60, 592–597. [Google Scholar] [CrossRef]
- Mohamad, A.A.; Hassan, M.S.; Yaakob, M.K.; Taib, M.F.M.; Badrudin, F.W.; Hassan, O.H.; Yahya, M.Z.A. First-principles calculation on electronic properties of zinc oxide by zinc–air system. J. King Saud Univ. Eng. Sci. 2017, 29, 278–283. [Google Scholar] [CrossRef]
- Wu, D.D. Research on Ammonium Salton Strengthen Suifide Flotation Theory for Smithsonite. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2015; pp. 60–69. (In Chinese). [Google Scholar]
- Wu, L.; Hou, T.J.; Wang, Y.; Zhao, Y.F.; Guo, Z.Y.; Li, Y.Y.; Lee, S.T. First-principles study of doping effect on the phase transition of zinc oxide with transition metal doped. J. Alloys Compd. 2012, 541, 250–255. [Google Scholar] [CrossRef]
- Shaik, S. A personal story on a renaissance in valence bond theory: A theory coming of age! Comput. Theor. Chem. 2017, 2, 1–30. [Google Scholar] [CrossRef]
- Andrés, R.B.M.; Florentino, L.U.; Mauricio, T.; Humberto, T. Effect of impurities on the electronic and magnetic properties of zinc oxide nanostructures. Chem. Phys. Lett. 2010, 492, 82–88. [Google Scholar]
- Magnasco, V. Chapter 15: Valence bond theory and the chemical bond. In Elementary Methods of Molecular Quantum Mechanics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 599–680. [Google Scholar]
- Hu, Z.Q. Molecular orbital theory studies on bond energy I. theoretic equation. Acta Chim. Sin. 1998, 56, 353–358. (In Chinese) [Google Scholar]
- Ikeda, A.; Nakao, Y.; Sato, H.; Sakaki, S. A resonance theory consistent with Mulliken-population concept. Chem. Phys. Lett. 2011, 505, 148–153. [Google Scholar] [CrossRef]
- Ogorodnikova, N.A. On invariance of the Mulliken substituent-induced charge changes in quantum-chemical calculations of different levels. J. Mol. Struct. THEOCHEM 2009, 894, 41–49. [Google Scholar] [CrossRef]
- Magnasco, V. Chapter 10: Valence bond theory and the chemical bond. In Elementary Methods of Molecular Quantum Mechanics; Elsevier: Amsterdam, The Netherlands, 2007; pp. 473–575. [Google Scholar]
- Pipek, J. Unique positive definite extension of Mulliken’s charge populations of non-orthogonal atomic basis functions. J. Mol. Struct. THEOCHEM 2000, 501–502, 395–401. [Google Scholar] [CrossRef]
- Kaupp, M.; Danovich, D.; Shaik, S. Chemistry is about energy and its changes: A critique of bond-length/bond-strength correlations. Coord. Chem. Rev. 2017, 344, 355–362. [Google Scholar] [CrossRef]
- Gunnarsson, O.; Jones, R.O. Self-interaction corrections in the density functional formalism. Solid State Commun. 1981, 37, 249–252. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H. Mineral cleavage nature and surface energy: Anisotropic surface broken bonds consideration. Trans. Nonferrous Met. Soc. China 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Ren, S.P.; Tong, X.; Zhou, Y.C. Study and application of desilication process in mineral processing. Min. Metall. 2013, 22, 32–36. (In Chinese) [Google Scholar]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Zhang, X.L.; Jing, M.; Rao, F.; Wu, L.Q.; Li, K.K.; Cao, S.M. A review of forming process and flotation mechanism of hemimorphite. Chin. J. Process Eng. 2017, 17, 903–910. (In Chinese) [Google Scholar]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M.; Cao, Q.B. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Deng, J.S.; Zhao, W.J. Combined DFT and XPS investigation of enhanced adsorption of sulfide species onto cerussite by surface modification with chloride. Appl. Surf. Sci. 2017, 425, 8–15. [Google Scholar] [CrossRef]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M. Surface modification of malachite with ethanediamine and its effect on sulfidization flotation. Appl. Surf. Sci. 2018, 436, 823–831. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sadowski, T.; Ramprasad, R. Density functional theory study of ZnX (X = O, S, Se, Te), under uniaxial strain. Phys. Rev. B 2010, 81, 144120. [Google Scholar] [CrossRef]
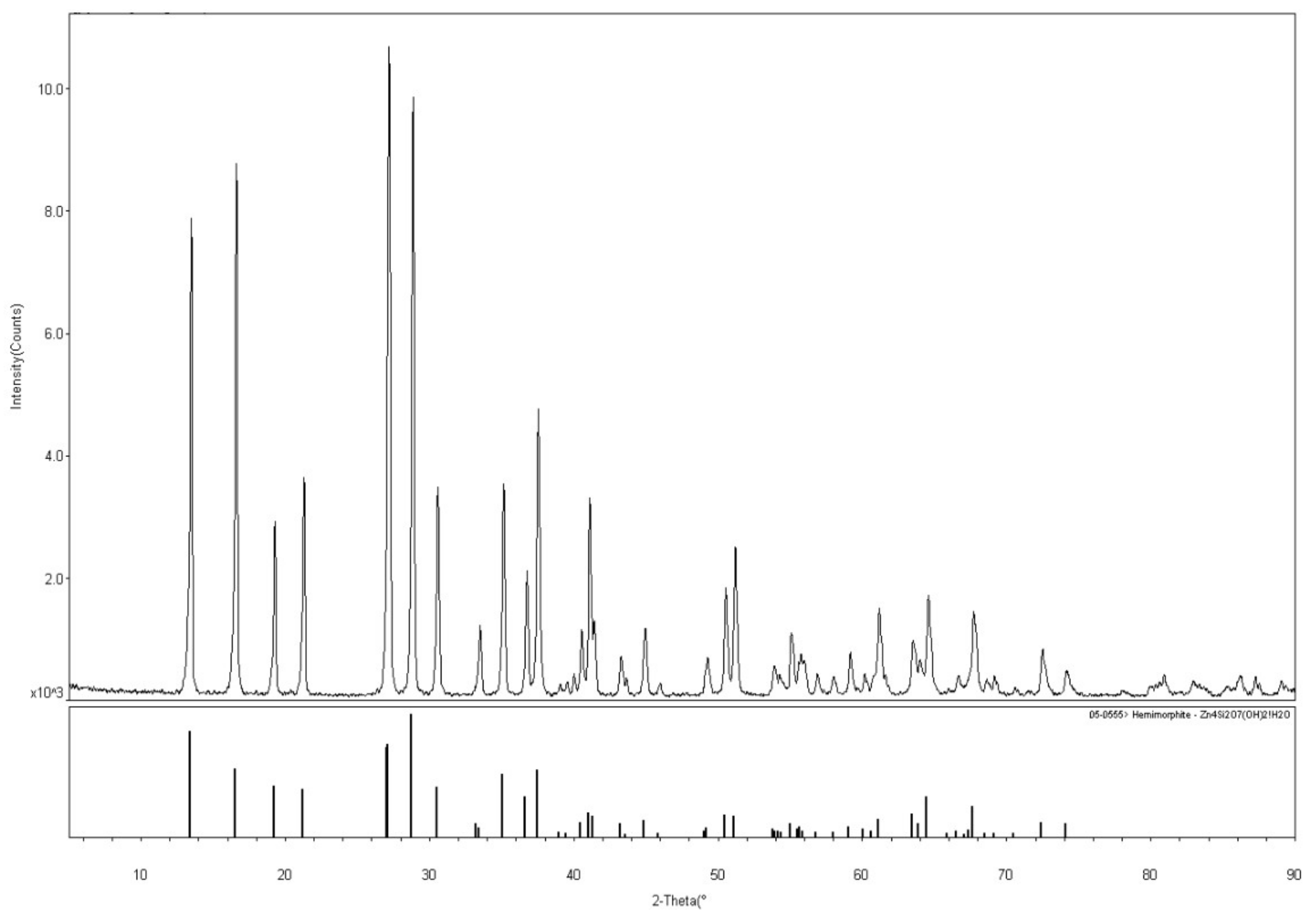
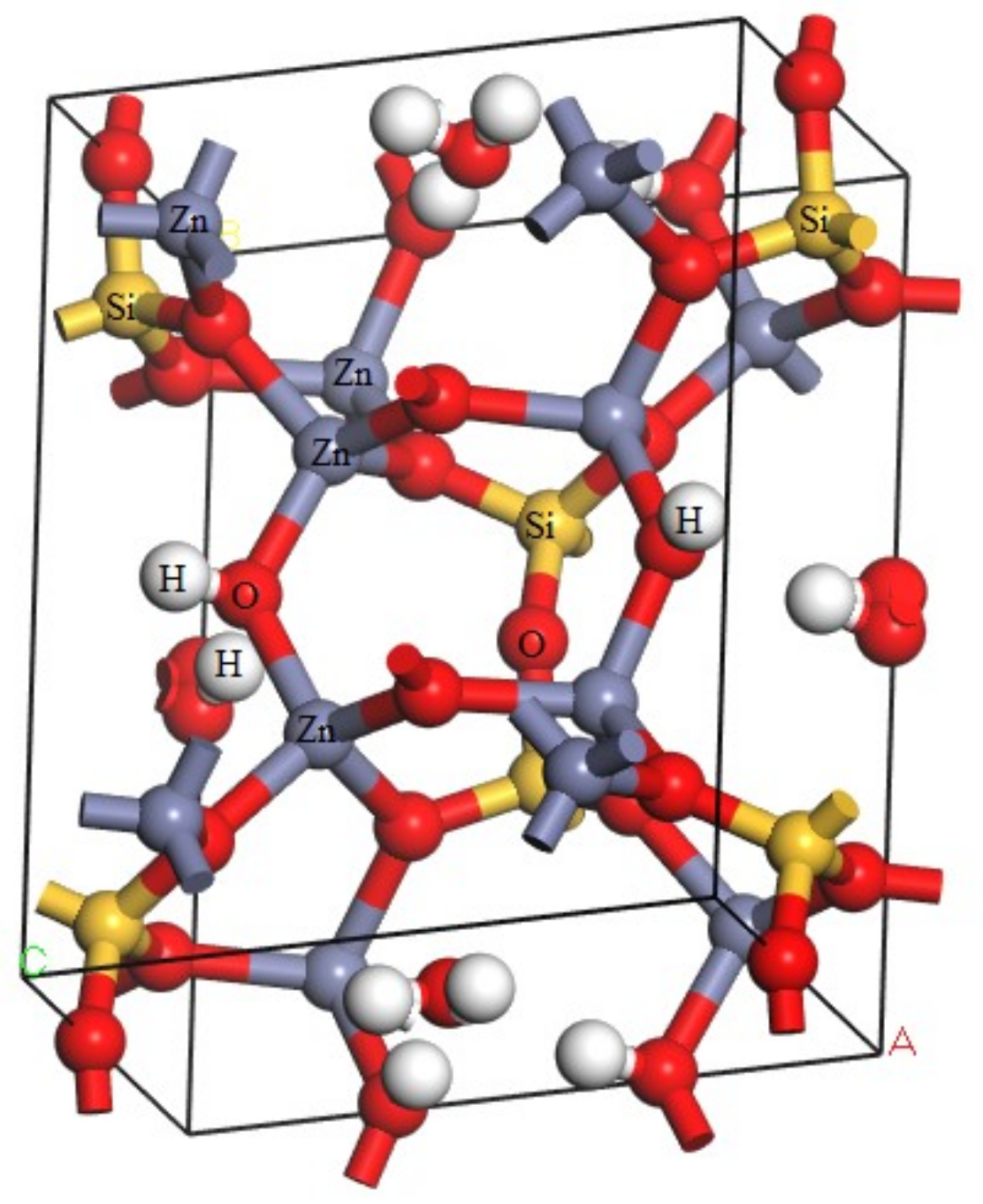
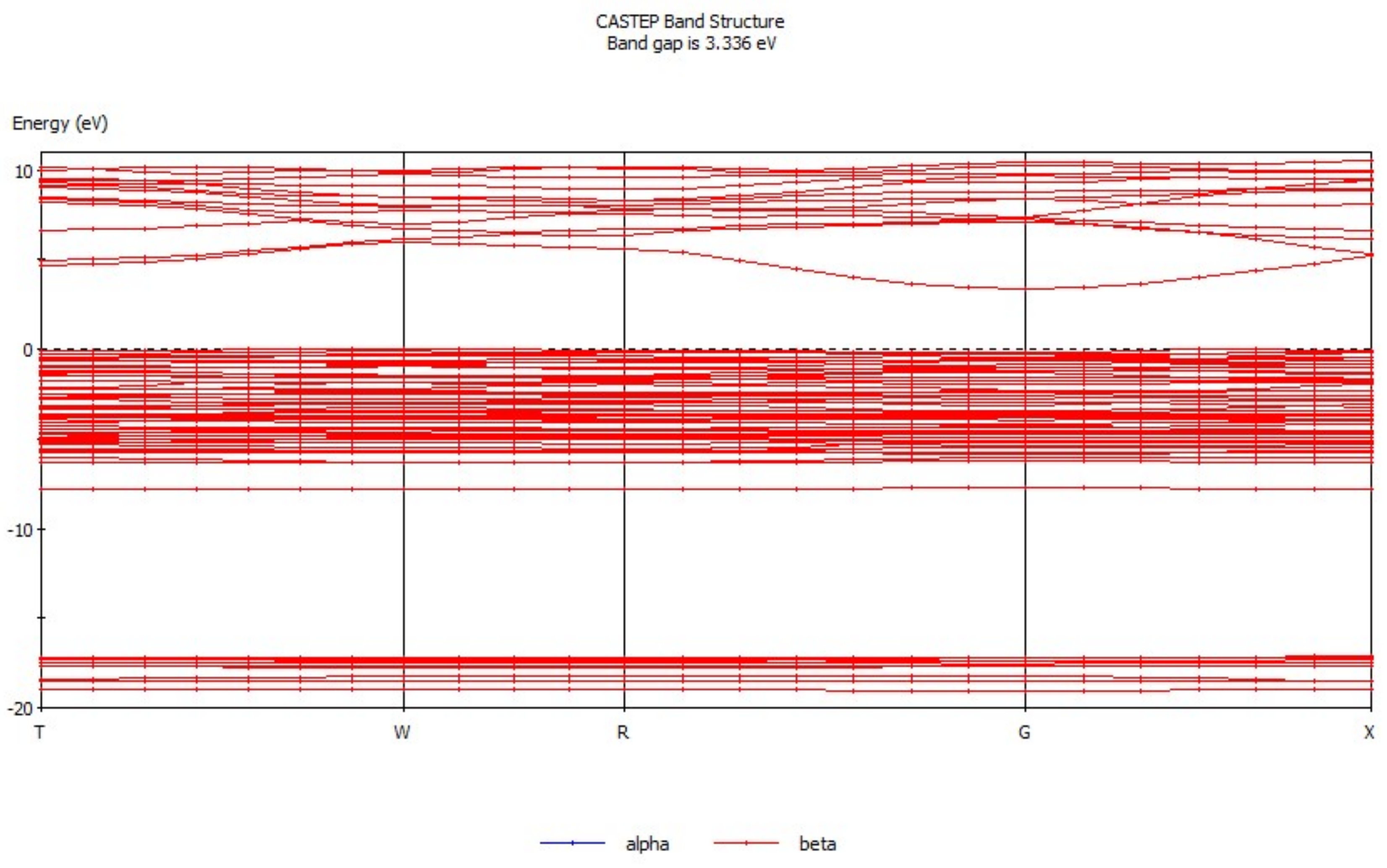
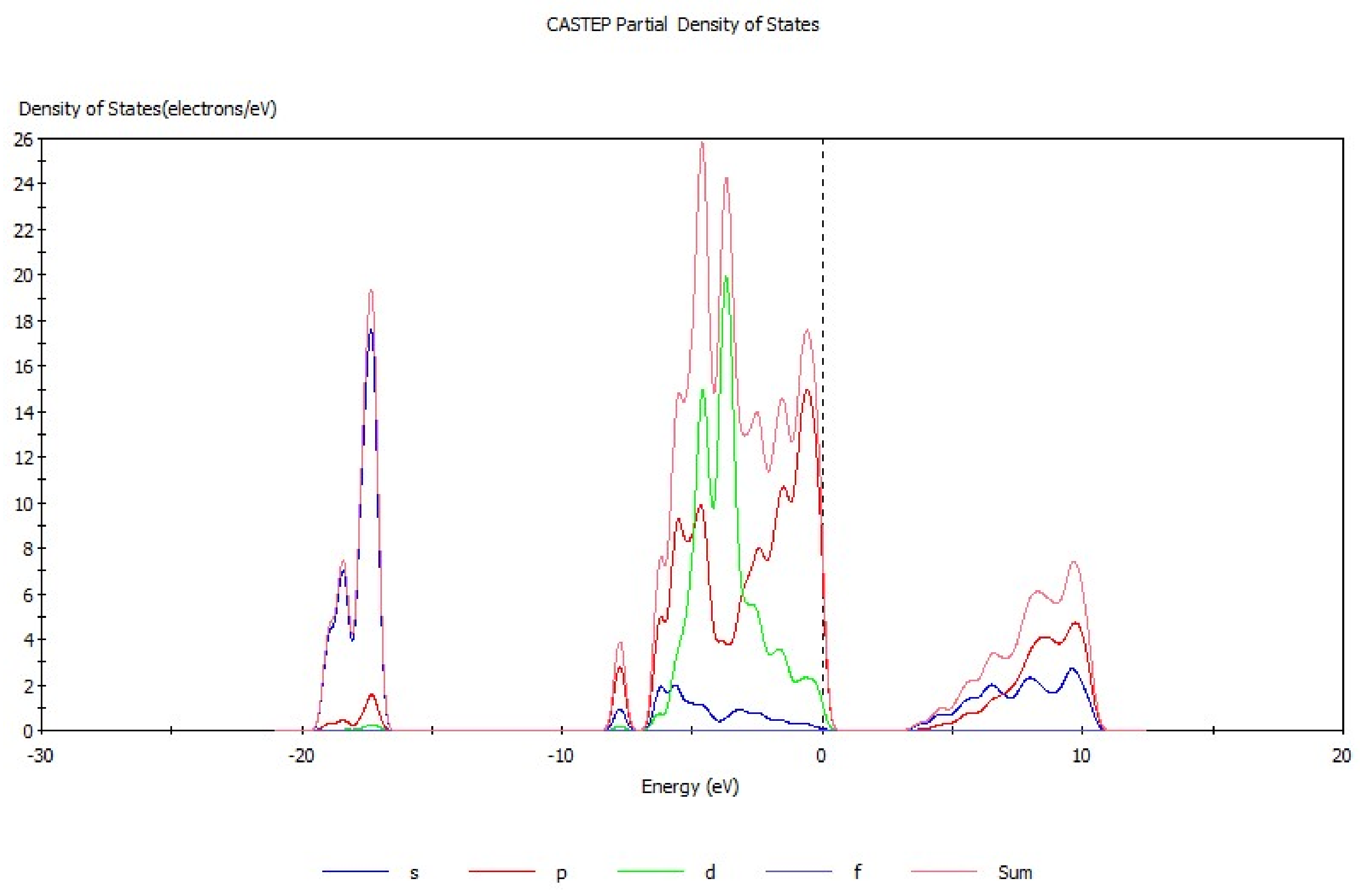
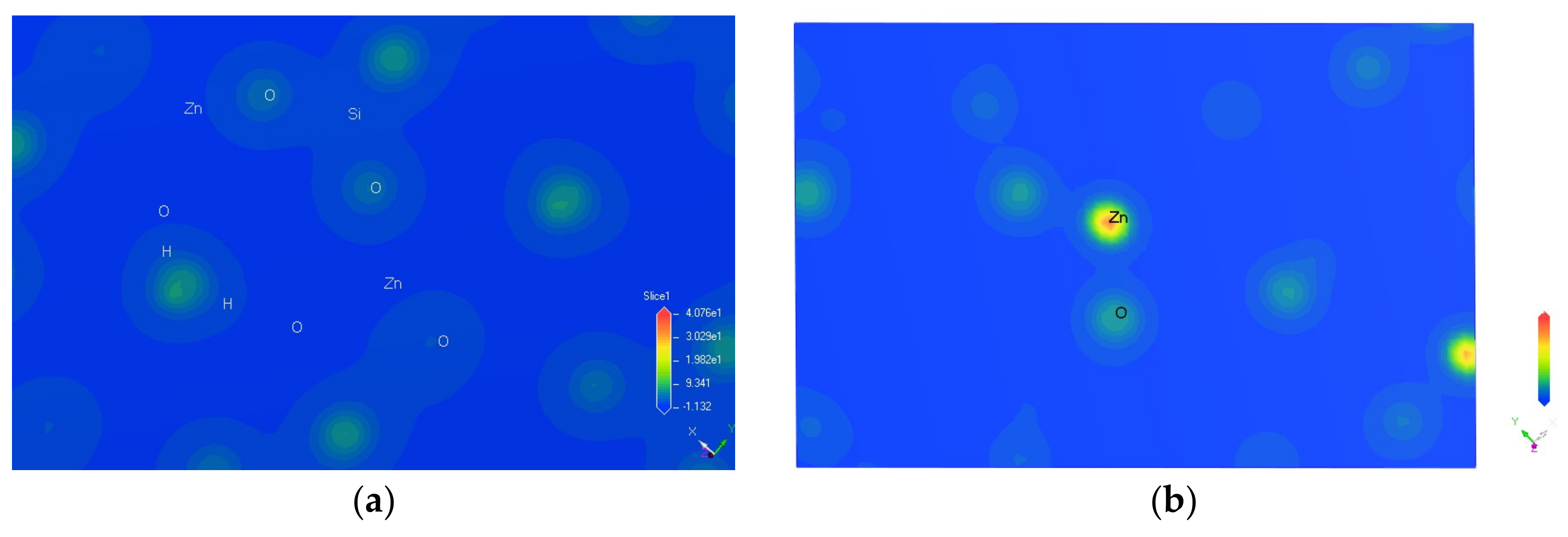
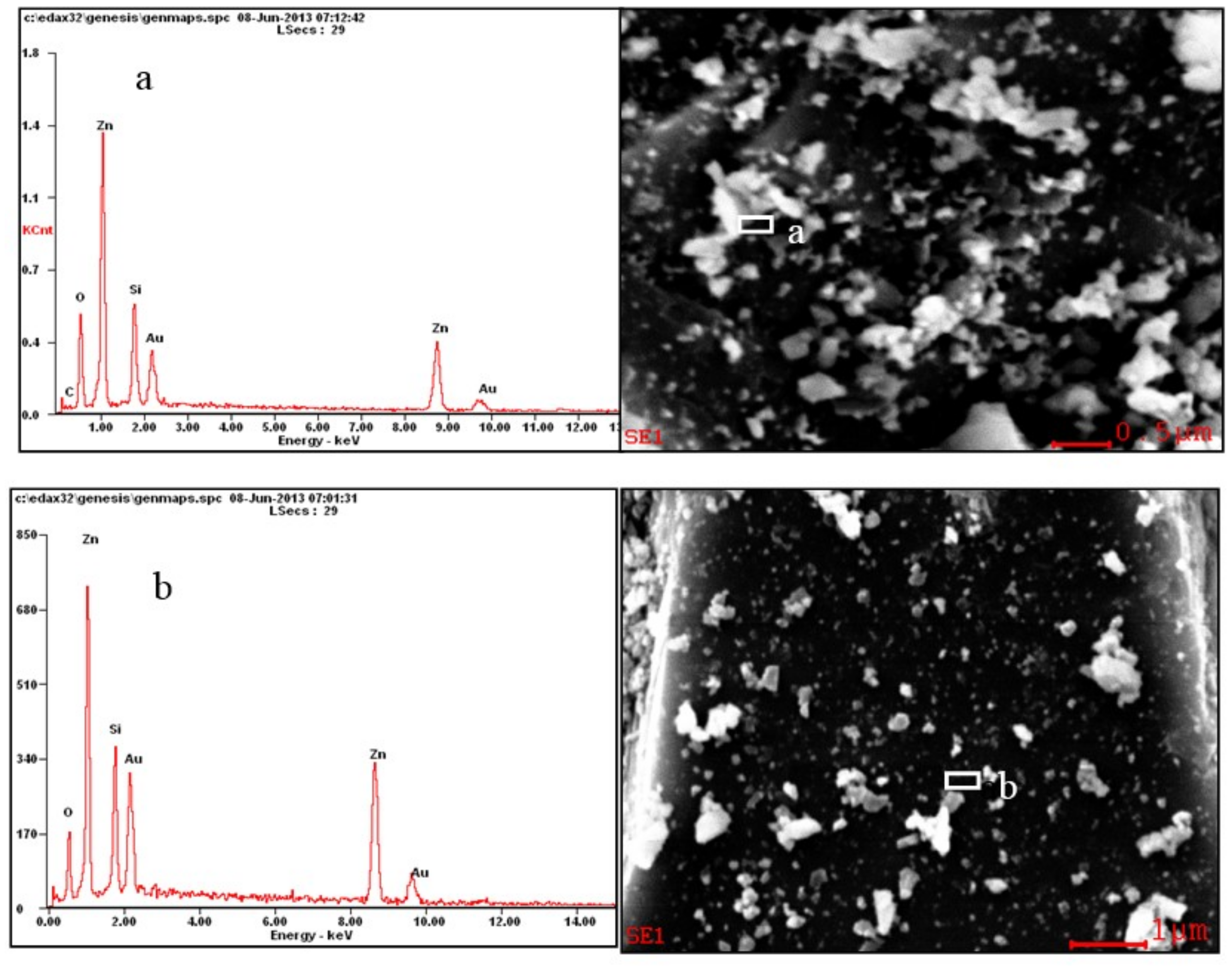
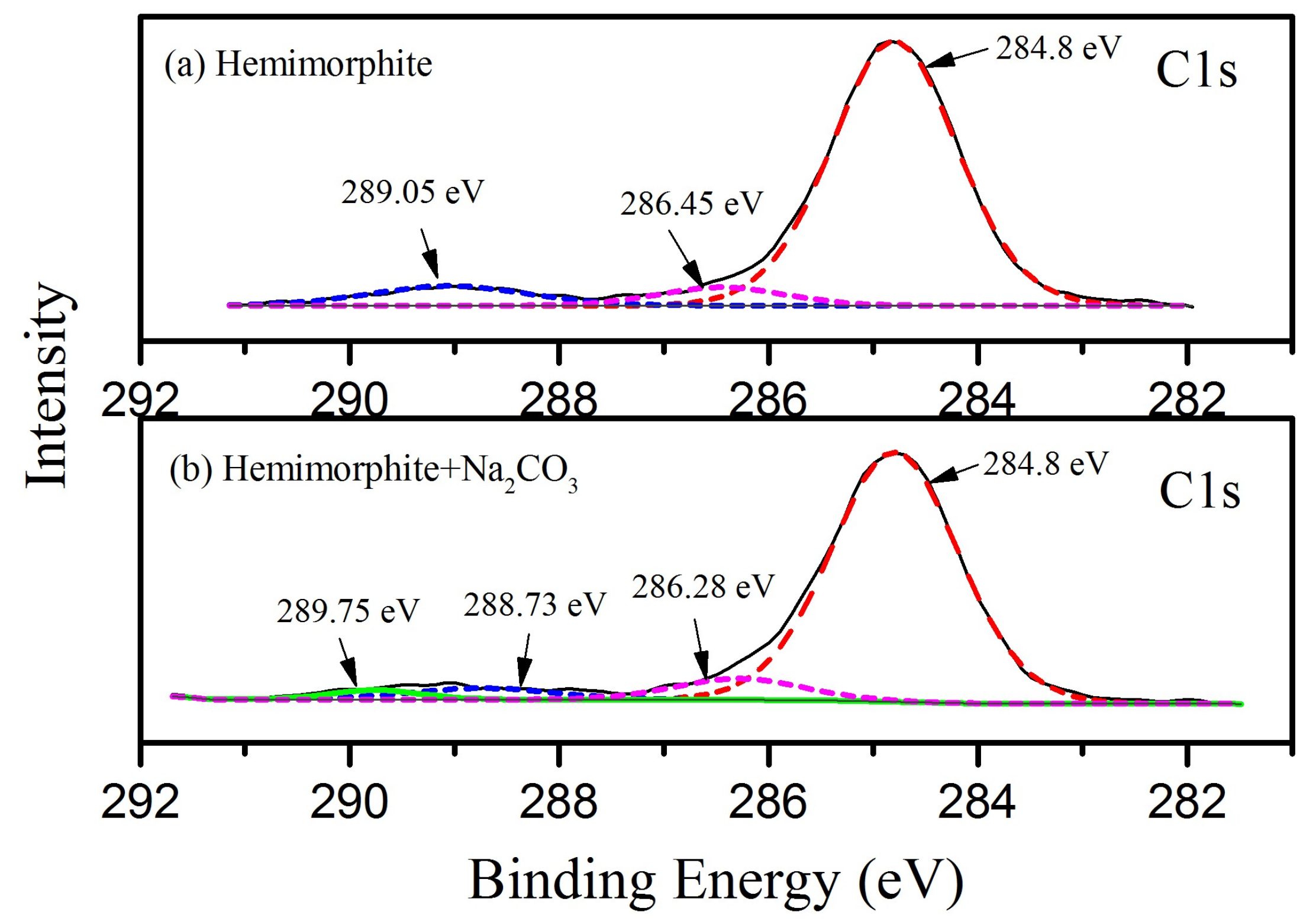
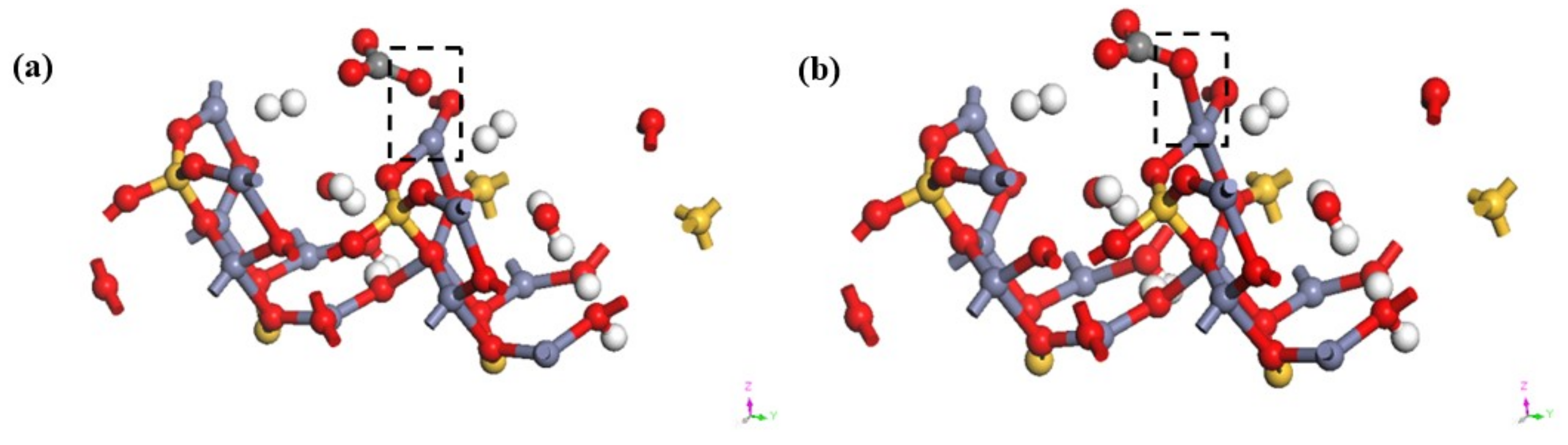
| Bond | Population | Spin | Length (Å) | Bond | Population | Spin | Length (Å) |
|---|---|---|---|---|---|---|---|
| O 004–Si 001 | 0.62 | 0.00 | 1.60910 | O 005–Zn 001 | 0.40 | 0.00 | 1.94416 |
| O 003–Si 002 | 0.62 | 0.00 | 1.60910 | O 006–Zn 002 | 0.40 | 0.00 | 1.94416 |
| O 001–Si 001 | 0.62 | 0.00 | 1.60910 | O 005–Zn 004 | 0.40 | 0.00 | 1.94416 |
| O 002–Si 002 | 0.62 | 0.00 | 1.60910 | O 006–Zn 003 | 0.40 | 0.00 | 1.94416 |
| O 009–Si 002 | 0.56 | 0.00 | 1.62123 | O 003–Zn 004 | 0.33 | 0.00 | 1.96457 |
| O 009–Si 001 | 0.56 | 0.00 | 1.62123 | O 002–Zn 001 | 0.33 | 0.00 | 1.96457 |
| O 006–Si 002 | 0.59 | 0.00 | 1.62158 | O 001–Zn 002 | 0.33 | 0.00 | 1.96457 |
| O 005–Si 001 | 0.59 | 0.00 | 1.62158 | O 004–Zn 003 | 0.33 | 0.00 | 1.96457 |
| O 001–Zn 001 | 0.36 | 0.00 | 1.93171 | O 008–Zn 004 | 0.35 | 0.00 | 1.97100 |
| O 004–Zn 004 | 0.36 | 0.00 | 1.93171 | O 007–Zn 003 | 0.35 | 0.00 | 1.97100 |
| O 003–Zn 003 | 0.36 | 0.00 | 1.93171 | O 007–Zn 001 | 0.35 | 0.00 | 1.97100 |
| O 002–Zn 002 | 0.36 | 0.00 | 1.93171 | O 008–Zn 002 | 0.35 | 0.00 | 1.97100 |
| Elment | wt (%) | At (%) | ||
|---|---|---|---|---|
| a (Before Adsorption) | b (After Adsorption) | a (Before Adsorption) | b (After Adsorption) | |
| CK | 00.60 | 03.61 | 02.02 | 14.11 |
| OK | 19.06 | 08.10 | 47.87 | 23.74 |
| SiK | 11.53 | 04.93 | 16.50 | 08.24 |
| ZnK | 47.63 | 70.99 | 29.29 | 50.96 |
| AuL | 21.18 | 12.37 | 04.32 | 02.95 |
| Bond | Population | Spin | Length (Å) |
|---|---|---|---|
| O 024–Zn 006 | 0.22 | 0.00 | 2.11216 |
| O 005–Zn 006 | 0.18 | 0.00 | 2.12877 |
| O 029–Zn 011 | 0.21 | 0.00 | 2.15893 |
| O 016–Zn 009 | 0.11 | 0.00 | 2.16948 |
| O 014–Zn 007 | 0.14 | 0.00 | 2.17600 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, X.; Liu, D.; Cao, S.; Song, K.; Jing, M.; Li, K.; Wu, L.; Liu, R. Basic Characteristics of Hemimorphite and Its Transformation Mechanism with Na2CO3. Minerals 2018, 8, 143. https://doi.org/10.3390/min8040143
Wang Q, Zhang X, Liu D, Cao S, Song K, Jing M, Li K, Wu L, Liu R. Basic Characteristics of Hemimorphite and Its Transformation Mechanism with Na2CO3. Minerals. 2018; 8(4):143. https://doi.org/10.3390/min8040143
Chicago/Turabian StyleWang, Qihong, Xiaolin Zhang, Dianwen Liu, Shiming Cao, Kaiwei Song, Man Jing, Kangkang Li, Luqing Wu, and Ruizeng Liu. 2018. "Basic Characteristics of Hemimorphite and Its Transformation Mechanism with Na2CO3" Minerals 8, no. 4: 143. https://doi.org/10.3390/min8040143
APA StyleWang, Q., Zhang, X., Liu, D., Cao, S., Song, K., Jing, M., Li, K., Wu, L., & Liu, R. (2018). Basic Characteristics of Hemimorphite and Its Transformation Mechanism with Na2CO3. Minerals, 8(4), 143. https://doi.org/10.3390/min8040143






