1. Introduction
The name rathite was given by Baumhauer [
1], in honor of Gerhard von Rath, Professor of Mineralogy, Bonn, Germany, to a new mineral of the sartorite–dufrénoysite series from the Lengenbach deposit (Binntal, Canton Wallis, Switzerland) with the formula 12PbS·5As
2S
3·Sb
2S
3 (=Pb
12As
10Sb
2S
30). One century later, Berlepsch et al. [
2], on the basis of new single-crystal structure determination of chemically well-characterized rathite and summarizing past achievements in unraveling name, chemistry, structure, and classification made by Solly [
3], Giuşcă [
4], Berry [
5], Le Bihan [
6], Nowacki et al. [
7], Marumo and Nowacki [
8], Makovicky [
9], Laroussi et al. [
10], Pring [
11], and Berlepsch et al. [
12], presented the last chapter of the complex story of this rare mineral. They concluded that rathite is a member of sartorite homologous series with space group symmetry
P2
1/
c and reported the unit-cell parameters
a = 8.496(1),
b = 7.969(1),
c = 25.122(3) Å, β = 100.704(2)° for a sample with the composition Ag
2.01Tl
1.37Pb
9.25(As
19.09Sb
0.83)
Σ19.92S
40.00. Berlepsch et al. [
2] also pointed out that the substitution involving Ag is essential for the formation of rathite and that it distinguishes rathite from another N = 4; 4 member of the sartorite homologous series,
viz. dufrénoysite Pb
16As
16S
40 [space group
P2
1,
a = 8.47(4),
b = 25.74(4),
c = 7.90(1) Å, β = 90.35(12)°]. Furthermore, these authors suggested that the Tl and Sb substitutions in rathite are to be considered optional.
No subsequent studies of rathite were conducted prior to our present work, although Makovicky and Topa [
13] recently present a set of analytical formulae for the calculation of the homolog order N
calc and the substitution degree for Tl and Ag in the sartorite homologous series (Tl
subst and Ag
subst), from quantitative electron microprobe analyses. In the meantime, barikaite (Topa et al. [
14]), philrothite (Bindi et al. [
15]), and carducciite (Biagioni et al. [
16]) were described as homeotypes of rathite, and polloneite (Topa et al. [
17]) as a threefold superstructure of dufrénoysite (
Table 1).
As part of an indepth and long-term study of the sulphosalt mineralogy of the Lengenbach deposit by the first author since 2013, the present paper deals with the crystal chemistry of rathite and aims to provide an understanding of the observed substitutional schemes on the basis of numerous electron microprobe datasets and a larger number of single-crystal structure refinements of samples from the Lengenbach and Reckibach occurrences.
2. Examined Material, Appearance, and Optical Properties
Euhedral crystals and massive sulphosalt accumulations (patches) of the sartorite homologs from the world-famous Lengenbach deposit (e.g., Graeser et al. [
20] and references therein) and the Reckibach sulphosalt occurrence (Cannon et al. [
21]) were obtained from the collection of the Natural History Museum, Vienna, and the Natural History Museum, Basel, as well as from private collections of Horst Geuer (Germany), Frank Keutsch (USA), the second author (Austria), Thomas Raber (Germany), Phillippe Roth (Switzerland), and Ralph Cannon (Germany). These samples were collected or acquired in the time range between 1816 and 2017.
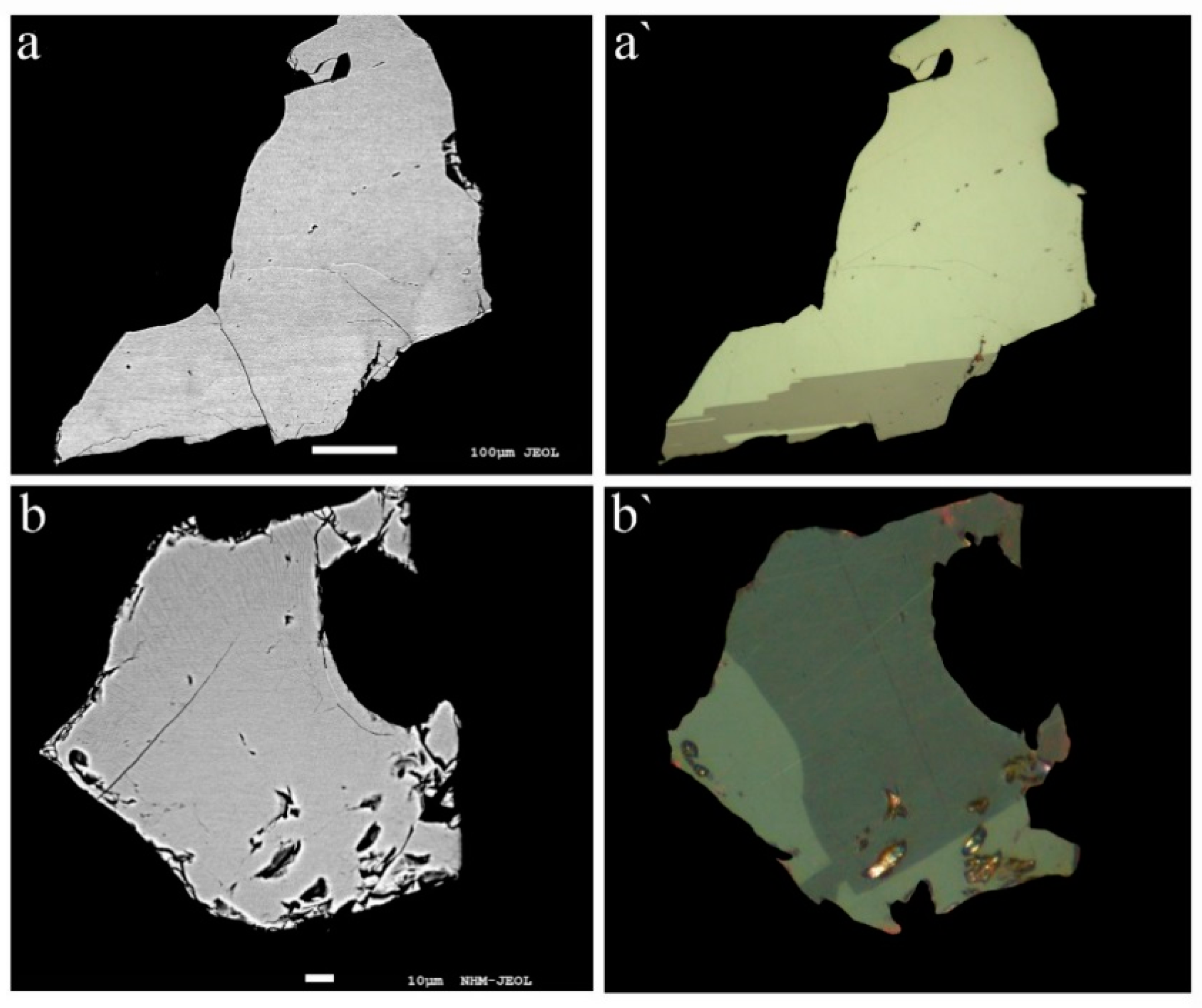
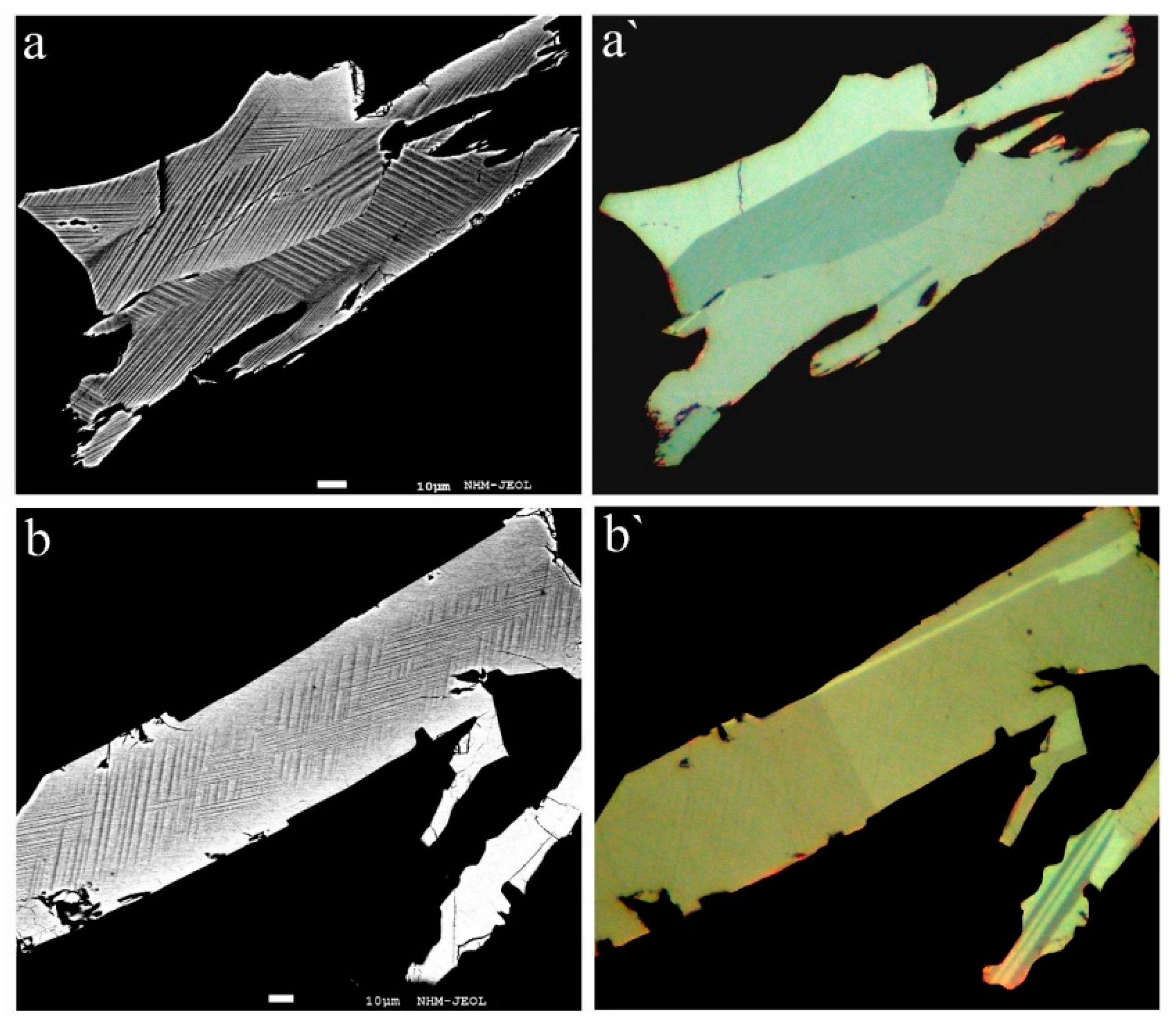
Our studies show that rathite is commonly intimately intergrown with sartorite, baumhauerite, liveingite, and dufrénoysite, but also occurs as larger, single-phase homogenous grains. Macroscopically, the mineral is always blackish (independent of the Tl content), with metallic to dull luster. In reflected light, the color of rathite is greyish-white with strong anisotropy. No twin lamellae were observed in the structurally studied fragments, but twinning was partly observed in other fragments and may be present in other samples. Representative back-scattered electron and optical images of rathite grains analysed by EPMA are shown in
Figure 1,
Figure 2 and
Figure 3: homogeneous rathite grains (aggregates) with low and high Tl content are shown in
Figure 1, of Tl-rich rathite with exsolved lamellae of a Tl-richer rathite-type phase “SR” (for details see below) in
Figure 2 and of Sb-bearing rathites from Reckibach and Lengenbach in
Figure 3.
3. Chemical Data
Up to twenty separate sulphosalt grains, depending on quantity of available material in a given sample, were mechanically extracted from the dolomite-hosted sulphosalt aggregates or crystals and subsequently embedded in an epoxy-mount, polished, studied in reflected light, examined by qualitative back-scattered electron (BSE) images and energy dispersive spectrometry (EDS) analyses) and quantitative wave-length dispersive spectrometry (WDS) electron microprobe analyses. This procedure was separately applied to a large number of samples.
Chemical analyses of rathite and accompanying sulphosalt minerals from Lengenbach and Reckibach deposits, Binntal, Canton Wallis, Switzerland, were carried out using a JEOL “Hyperprobe” JXA 8530F field-emission gun electron microprobe (FE-EPMA) in the Central Research Laboratories at the Natural History Museum, Vienna, employing JEOL and Probe for EPM analysis software (WDS mode, 25 kV, 20 nA, 2 μm beam diameter, count time 10 s on peak and 5 s on background positions). The following emission lines and standards were used: As-Lα, Tl-Lα and S-Kα (lorándite, TlAsS2), Pb-Mα (galena), Ag-Lα (Ag metal), and Sb-Lα (stibnite). Other elements, such as Hg, Cu, and Fe, were sought but not detected.
The formula calculations for the sartorite homologue order N
calc, described by Makovicky and Topa [
13], are designed to identify the minerals of the family from the results of electron microprobe investigations, and are used here to select all point analyses representative for rathite and rathite-related phases with N = 4. More than 2700 point analyses, of more than 800 samples containing rathite, with calculated homologue number N
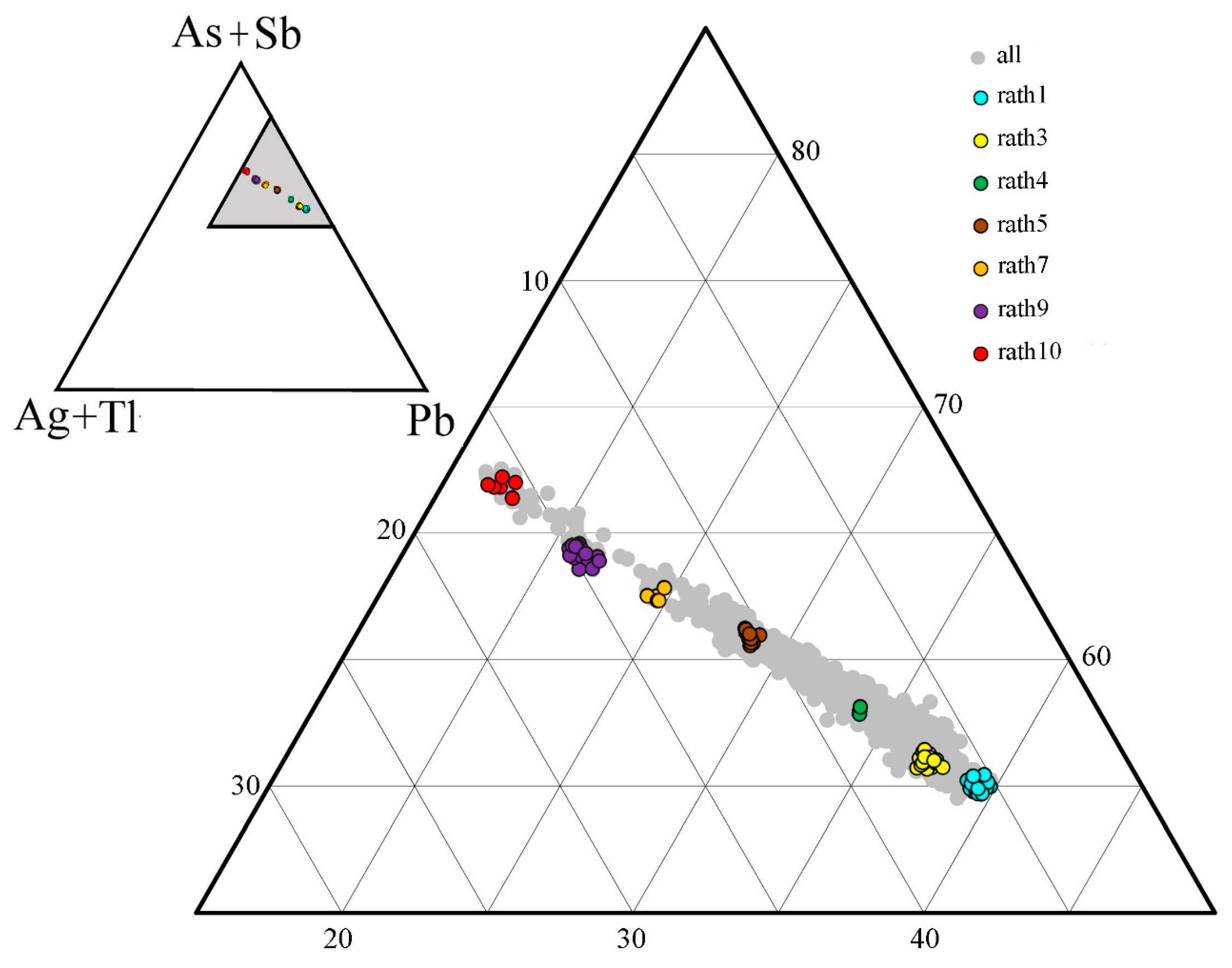
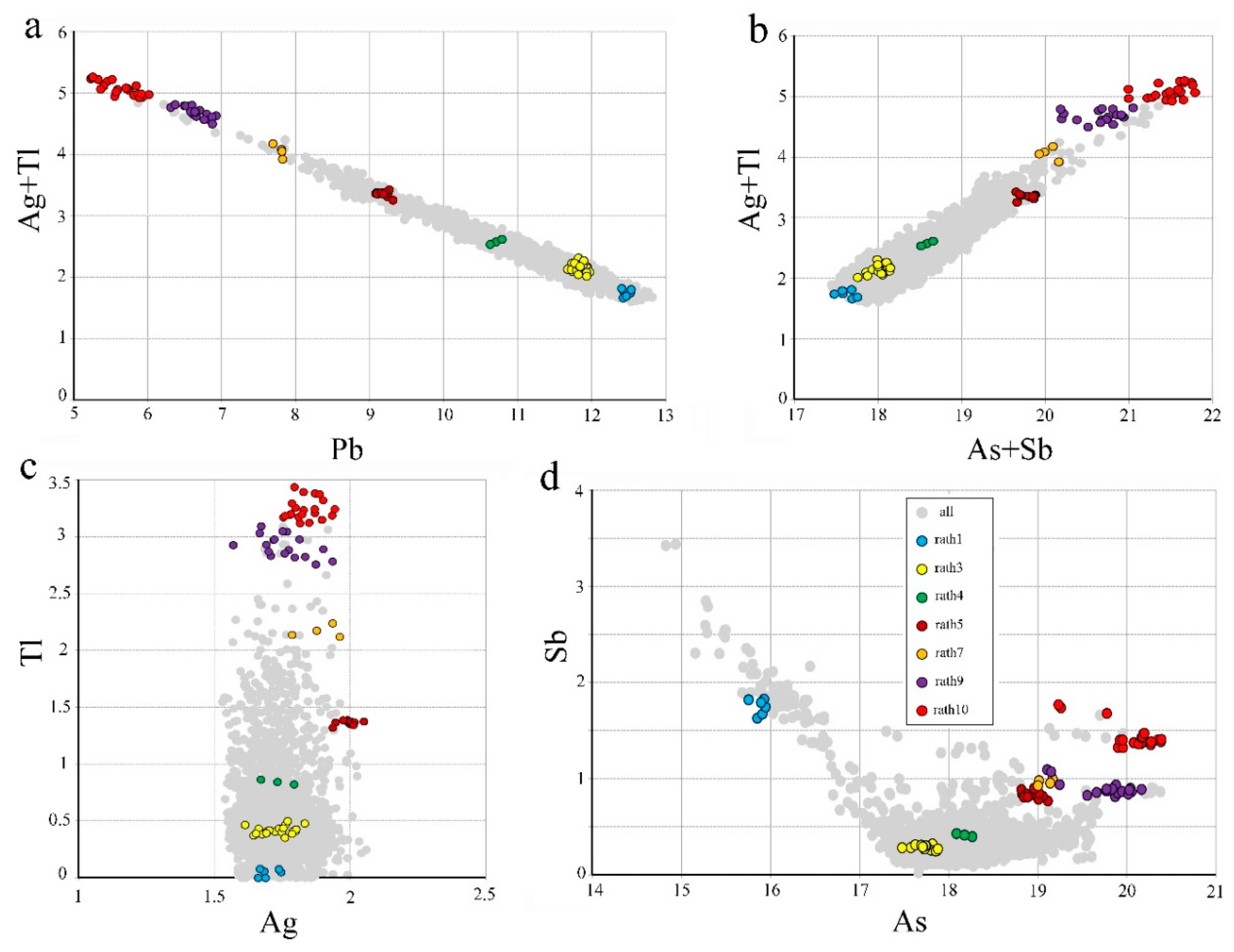
calc ranging between 3.9 and 4.1, and with a good charge balance (calculated from at % values) ranging between –2.5 and +2.5, were selected and represent the best analytical data base of rathite compositions at present. The entire content of the database is plotted in the (As + Sb) − (Ag + Tl) − Pb ternary diagram (
Figure 4) as a grey background and gives a first hint of the chemical variations.
The representative mean chemical compositions of rathite with different degree of (Ag
+ + As
3+)-for-2Pb
2+, (Tl
+ + As
3+)-for-2Pb
2+ and Sb-for-As substitutions (some used for subsequent crystal-structure studies), are presented in
Table 2 and
Table 3, whereas individual points of the first group of samples (used for the structural studies) are plotted in different colours in the ternary diagram of
Figure 4. The range of Ag, Tl, and Sb variations found are considerably larger than those reported by Berlepsch et al. [
2]. The degree of Ag and Tl substitutions (Ag
subst and Tl
subst) are calculated with formulae given by Makovicky and Topa [
13]. The Ag
subst value varies between 43.4 and 47.9, whereas the Tl
subst value shows a large variation between 0.5 and 41.9, with strong implications for the crystal chemistry and range of compositional variability of rathite.
The empirical formulae of the chemically most different rathite compositions (based on 72 atoms per formula unit (
apfu), 32Me + 40S) are presented in the footnote of
Table 2 and
Table 3 and demonstrate a limited Ag substitution combined with large Tl and Sb substitutions ranging between Ag
1.70Tl
0.04Pb
12.47(As
15.88Sb
1.75)
Σ17.63S
40.17 (Tl-poorest sample) to Ag
1.76Tl
2.91Pb
6.67(As
19.77Sb
0.90)
Σ20.66S
39.99 (Tl-richest sample) and Ag
1.82Tl
0.80Pb
10.79(As
14.88Sb
3.43)
Σ18.31S
40.28 (Sb-richest sample).
The binary plots a, b, c, d in
Figure 5, based on
apfu, depict the correlations of (Ag + Tl) vs. Pb, (Ag + Tl) vs. (As + Sb), Tl vs. Ag, and Sb vs. As, respectively, and allowed to determine the range of variations and a revised general formula of rathite: Ag
xTl
yPb
16−2(x+y)As
16+x+y−zSb
zS
40 (with 1.6 <
x < 2, 0 <
y < 3, 0 <
z < 3.5). An Ag-free natural rathite does not exist, in agreement with studies of the system PbS-As
2S
3 between 250 and 750 °C (Kutolglu [
22]), although synthetic “rathite” (insufficiently characterized) was observed in an earlier, hydrothermal study of this system (Rösch and Hellner [
23]). The conclusion of Berlepsch et al. [
2] that the presence of Ag in the crystal structure of rathite is essential for this mineral, agrees with the Kutolglu study.
In Lengenbach, the Sb content of rathite, sartorite, baumhauerite, liveingite, and dufrénoysite generally ranges between 0 wt% and ~2 wt% Sb. An unusually high Sb content in rathite (5 wt%–7 wt%) is found normally in samples coming from the Reckibach deposit, except for a single sample from Lengenbach (
Table 3). A conspicuous Sb content in rathite was pointed out by Baumhauer [
1].
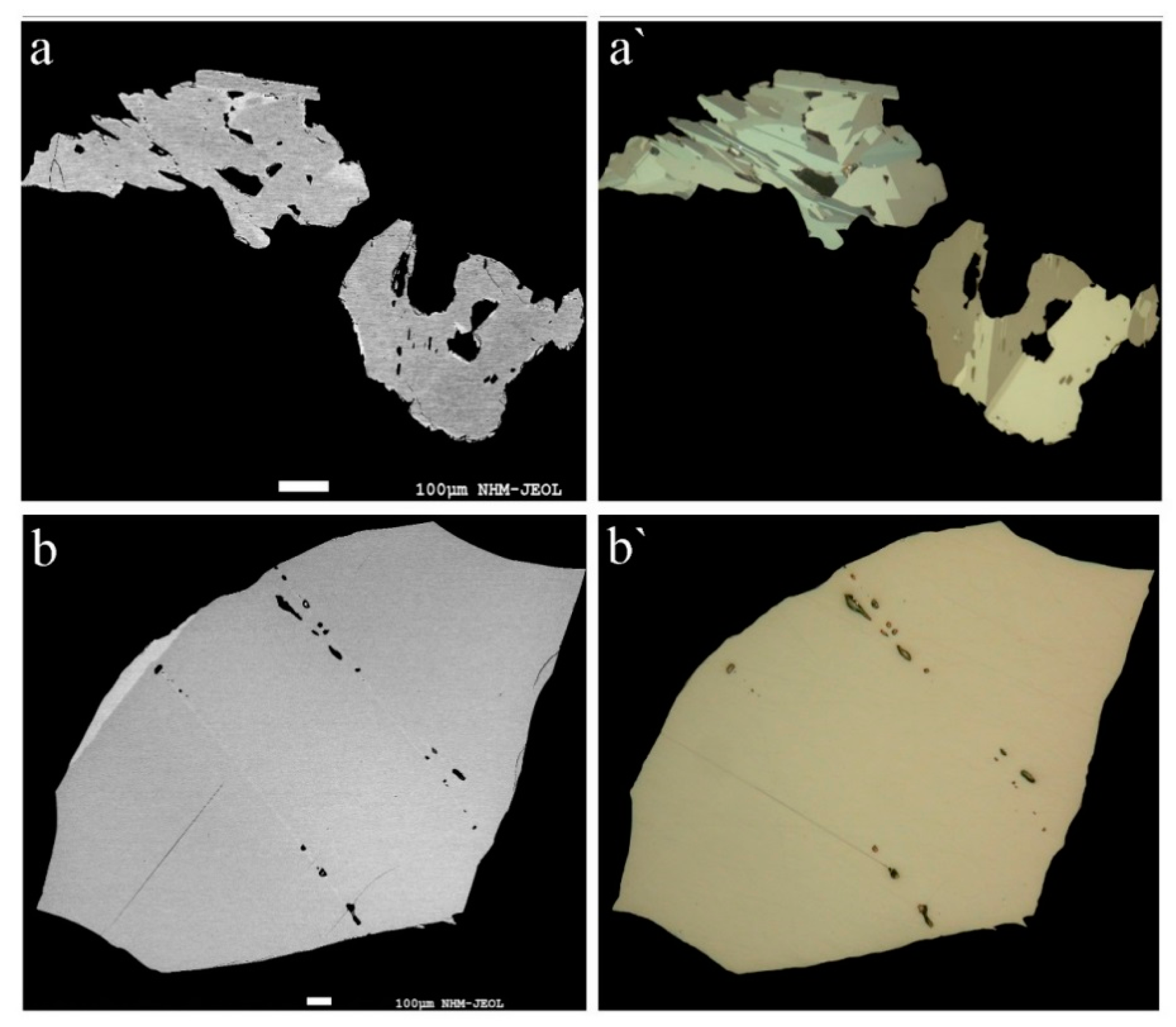
EPMA studies revealed a specific limit to the Tl content in rathite. The back-scattered images in
Figure 2 show two aggregates of mixed exsolution lamellae of the Tl-richest rathite (bright grey phase, rath9 in
Table 2; 11.78 wt% Tl) and a possibly new, still Tl-richer species, still under investigation, here designated “SR” (dark-grey phase, rath10 in
Table 2; 13.25 wt% Tl). The empirical formula of phase “SR” is Ag
1.84Tl
3.24Pb
5.62(As
20.05Sb
1.44)
Σ21.49S
39.81, with N
calc = 3.9, which clearly indicates its membership of the rathite group. Note the small gap between the chemical compositions of rath9 and phase “SR” (rath10), in the ternary plot of
Figure 4 and binary plots of
Figure 5, where all data are considered. The meaning and importance of that are discussed later, in connection with the results of crystal structures and crystal chemistry of the studied rathite samples.
4. Crystallography
Single-crystal studies were performed on a total of eight rathite fragments, mechanically extracted after quantitative electron microprobe analysis, from samples containing the groups mentioned in
Table 2. All fragments were found to show good diffracting quality; no twinning or other anomalies were noted. We were so far unable to obtain structural information for phase “SR” due to the thinness of its exsolution lamellae, but the search for suitable fragments is still ongoing.
Single-crystal X-ray diffraction data were collected for all samples except rath9 on a Bruker SMART APEX CCD-three-circle-diffractometer at the University of Salzburg. Intensity data were collected with graphite-monochromatised Mo
Kα X-radiation (50 kV, 30 mA) using SMART software (Bruker AXS [
24]). The crystal-to-detector distance was set to 60 mm for all rathite crystals. The detector was positioned at –28° 2θ using an ω-scan mode strategy at four different ϕ positions (0°, 90°, 180°, and 270°). In total, 630 frames with Δω = 0.3° were acquired for each run, with a 40 s/frame time of acquisition. Intensity data were integrated using SAINT software (Bruker AXS [
25]). The semiempirical absorption correction based on pseudo ω scans was done with XPREP software (Bruker AXS [
26]). Sample rath9 (and three other samples not reported here) were measured at the University of Vienna using a Nonius KappaCCD single-crystal diffractometer (graphite-monochromatised Mo
Kα X-radiation, 50 kV, 30 mA; crystal-to-detector distance 30 mm, 1126 frames, 330 s/frame) that was equipped with a 300 μm diameter capillary-optics collimator to provide increased resolution. The data were processed with the Nonius program suite DENZO-SMN and corrected for Lorentz, polarization, background, and by multiscan absorption correction (Otwinowski and Minor [
27]), absorption effects.
The centrosymmetric space group
P2
1/
n proposed for all fragments by XPREP was chosen in accordance with systematic extinctions and intensity statistics and was afterward transformed in
P2
1/
c standard settings. Rathite structures with different Tl content were solved by direct methods (SHELXS-97; Sheldrick [
28]), which revealed most of the atom positions. The crystal-structure model and atom-site labeling scheme of Berlepsch et al. [
2] were used as starting points for subsequent refinements. Occupancies of split sites were assigned based on geometric features and chemical considerations based on EPMA data. Between about 20 and 50 “bad” reflections (from a total of about 2300 to 6000 reflections) were omitted in order to “sharpen” the obtained models and to improve the distinguishing of the split sites; in the case of rath9 four of the omitted reflections were blocked by the beam stop. We checked that this omission did not seriously affect our models. Taking in account that Pb and Tl cannot be distinguished in X-ray diffraction experiments, no attempt to localize Tl in the structure refinement was made. Based on refinements for low-Sb samples (rath4 and rath3), the distinction between Ag and Sb was possible and found to be in accordance with the Berlepsch et al. [
2] model.
Out of the eight studied crystals, we selected five (samples rath1, rath3, rath4, rath7, and rath9) that best represent a series of increasing Tl contents. Details on data collection and results of the structure refinement of these samples are given in
Table 4, unit-cell dimensions are compared in
Table 5, fractional atomic coordinates and anisotropic atomic displacement parameters of rath1 (Tl-poorest) and rath9 (Tl-richest) are compiled in
Table 6 and
Table 7, and a comparison of corresponding site occupancies is given in
Table 8, while
Table 9 and
Table 10 give selected bond lengths for rath1 and rath9.
The full set of data is accessible via the CIF files freely available online as
Supplementary Material linked to this article. Supplementary tables of fractional atomic coordinates, anisotropic atomic displacement parameters, bond lengths, and polyhedron characteristics for samples rath3, rath4, rath5 (partly recalculated from [
2]), and rath7 are available online (
Tables S1–S3, respectively).
The single-crystal diffraction data of all studied rathites indicated, independent of the individual Tl contents, a monoclinic cell, with space group
P2
1/
c and
a~8.5,
b~8,
c~25 Å, β~100.6°. The small but conspicuous changes in unit-cell parameters are shown in
Table 4. A clear increase of
a,
b, and
V, and a slight decrease of
c with increasing Tl content are masked somewhat by a random variation of the Sb content.
The asymmetric unit in the crystal structure of rathite contains eight cation sites and ten sulphur sites. The rathite structure model of Berlepsch et al. [
2] consists of two fully occupied Pb sites, three fully occupied As sites, and three mixed split positions Me3 (As
0.735,Sb
0.265), Me5 (As
0.526,Ag
0.474), and Me6 (Pb
0.739,As
0.261). Our refinement results, compared with those of Berlepsch et al. [
2] are presented in
Table 6.
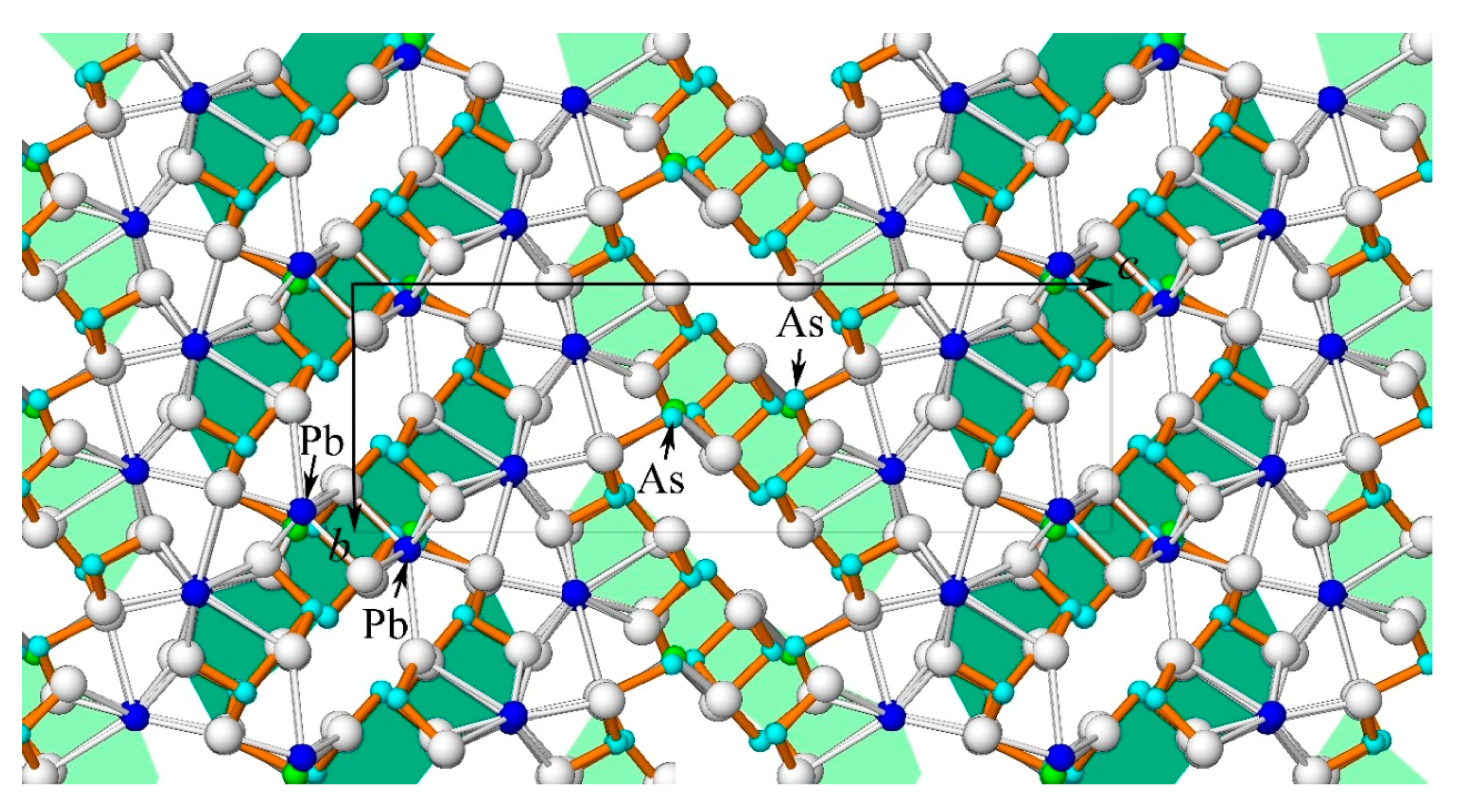
Figure 6 depicts the crystal structure of rath1 (a) and rath9 (b), projected along the
a = 8.4 Å axis. The 18 independent sites form a layer (L
1 or L
2 in
Figure 6a) four coordination polyhedra long and two polyhedra thick, based on an SnS-like archetype, built by six (mainly) As-centered coordination polyhedra (CN = 5–7) with a crankshaft arrangement of short As–S bonds, and terminating with two Pb-centred tricapped trigonal coordination prisms (CN = 9) at one end. Two such identical layers, symmetry related by inversion centers in rathite, form a tightly bonded double layer (ribbon), and the adjacent parallel double layers are separated by the lone-electron pair micelle. Along the
a and
b axes, the infinite rows of ribbons form a (001) slab, related by the 2
1 screw axis and the
c glide plane to the next one. The dark-green ribbon of rath1 is Pb-rich due to the 0.961 Pb occupancy of the Me6 site and the light-green ribbon of rath9 is As-rich due to the 0.576 As occupancy of the Me6 site.
4.1. Crystal Chemistry
4.1.1. Pb Sites
Thallium is supposed to enter into the structure of rathite (as in the sartorite family) on the Pb sites and investigations of those sites reveal the Tl role.
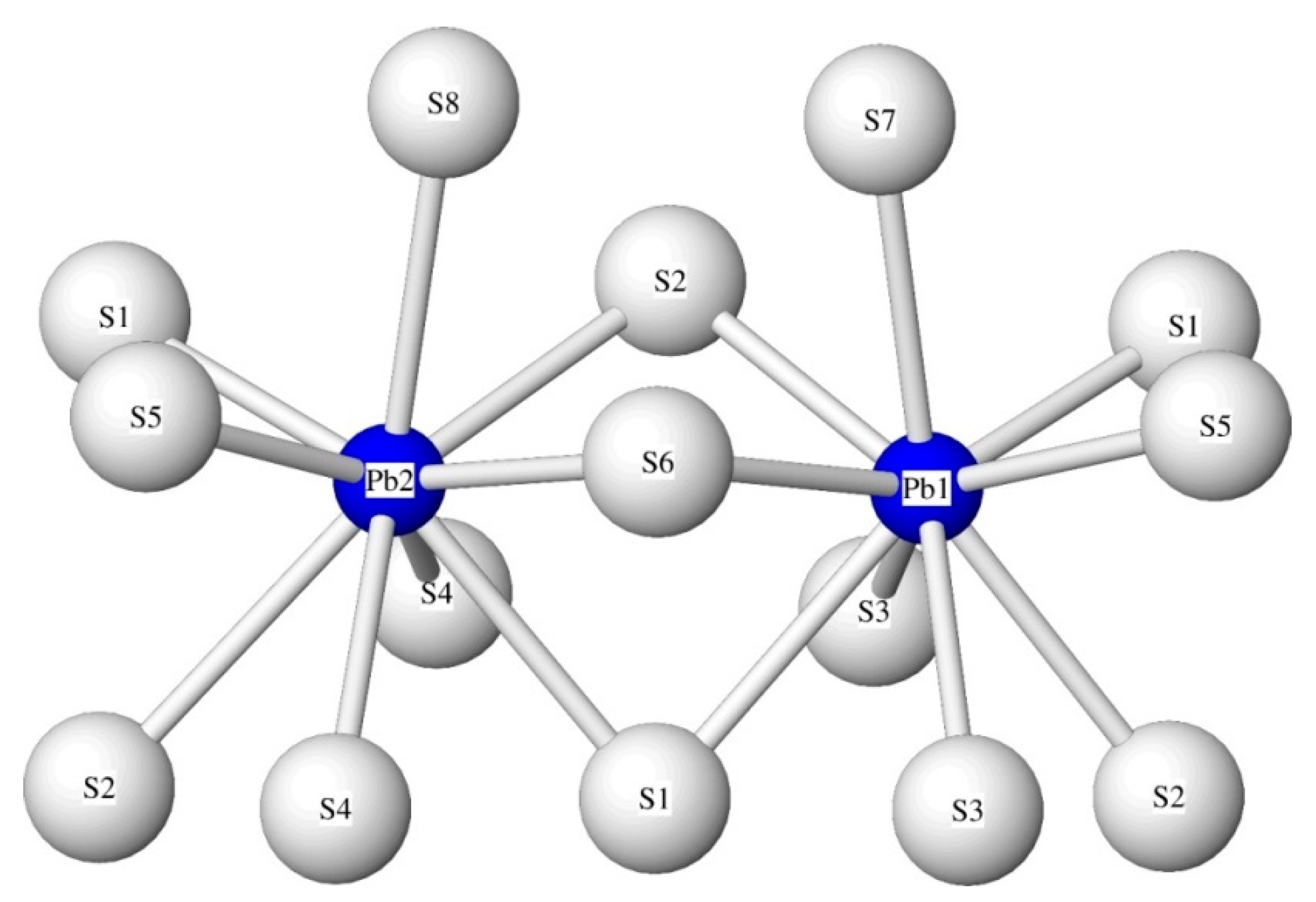
Figure 7 shows the coordination polyhedra of
[9]Pb1 and
[9]Pb2 sharing the S1, S2, and S6 sulphur ligands. The S5, S6, S7, and S8 sulphur ligands are close to the Me3, Me5, and Me6 sites, and the Pb–S bond lengths are slightly affected by the different substitution at these Me sites.
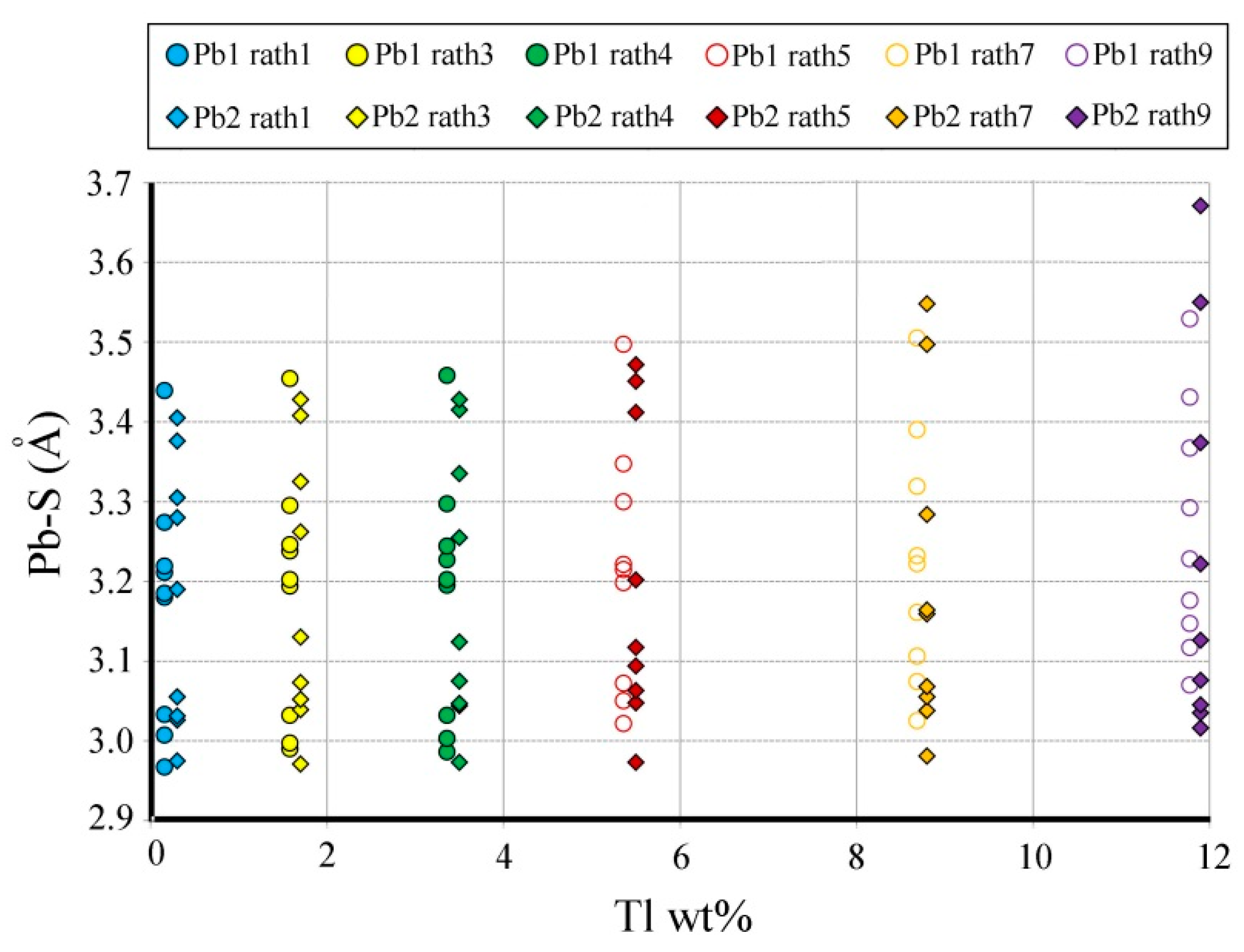
The incorporation of Tl on the two Pb sites, Pb1 and Pb2, is expressed by an increase of the (Pb,Tl)–S bond lengths that is positively correlated with the Tl content (
Figure 8,
Table 9 and
Table 10). For the samples with high Tl content (above 5 wt%), the first two short (Pb,Tl)–S bond lengths for Pb1 and Pb2 are better separated in comparison with the corresponding bond lengths in the low-Tl samples, thus indicating that Pb1 incorporates more Tl than Pb2.
As shown in
Table 11,
Table 12 and
Table 13, this incorporation is also reflected by the polyhedron characteristics, as defined in IVTON (Balić Žunić and Vicković [
29]; Makovicky and Balić Žunić [
30]): radius r
s in Å of a circumscribed sphere least-squares fitted to the coordination polyhedron, the volume distortion υ, the ’volume-based’ eccentricity ECC
V, the volume in Å
3 of the circumscribed sphere, the volume in Å
3 of coordination polyhedron, the average bond distance and the standard deviation of the bond distance all increase with increasing Tl content, while the bond-valence sum notably decreases.
4.1.2. As, (As/Ag), (As,Sb), and (Pb/As) Sites
The As sites behave differently in rathite. The As–S bond lengths (
Table 9 and
Table 10) of the As1, As2, and A4 sites comprise regular three short (2.2–2.37 Å) and two long (2.9–3.4 Å) distances, typical for pure As sites (CN = 5) and indicate that As1, As2, and As4 sites are unaffected by the observed substitutions.
The Ag substitution in rathite occurs on the Me5 site that is split into an Me5b site (As) and a Me5a site (Ag), with a fairly constant ratio of roughly 1:1, also reflected by the fairly constant Ag contents in all chemically analyzed samples (3.1 wt%–4.1 wt%, mean 3.6 wt%). This substitution feature is explained by the fixed location of the Ag atom within the ribbon, as is also observed for other sartorite-group members, such as argentobaumhauerite [
31] and argentoliveingite [
32], in both of which, however, the Ag site is fully occupied.
The Me6 site is split and shared between Pb (Me6a) and As (Me6b). With increasing Tl content, and therefore increased [Tl
+ + As
3+] ↔ 2Pb
2+ substitution, the occupancies change from Pb0.961(3) and As0.039(3) (Tl-poorest sample rath1) to Pb0.424(4) and As0.576(4) (Tl-richest sample rath9). The Me6 site is located in the central part of the ribbon (
Figure 6,
Figure 9, and
Figure 10).
Sb substitution is seemingly only affecting the Me3 site. In the Sb-richest, structurally studied sample (rath1), the occupancy of this (then split) site is Me3a = As0.532(9) and Me3b = Sb0.468(9). The left side of the binary plot of Sb vs. As in
Figure 5d depicts the linear decrease of As with increasing Sb due to direct Sb-for-As substitution, whereas the right side expresses the increase of As due to the Ag and Tl substitutions.
Since our EPMA data revealed even Sb-richer samples (with up to 7.71 wt% Sb), for which currently no crystal-structure refinements are available, these might theoretically represent a new mineral species with Sb dominant on the Me3 site (according to the current IMA rules on the definition of new mineral species). However, we cannot exclude that minor Sb might also be present on the Me5b (As) subsite of the split Ag/As Me5 site.
4.1.3. Crankshaft Chains
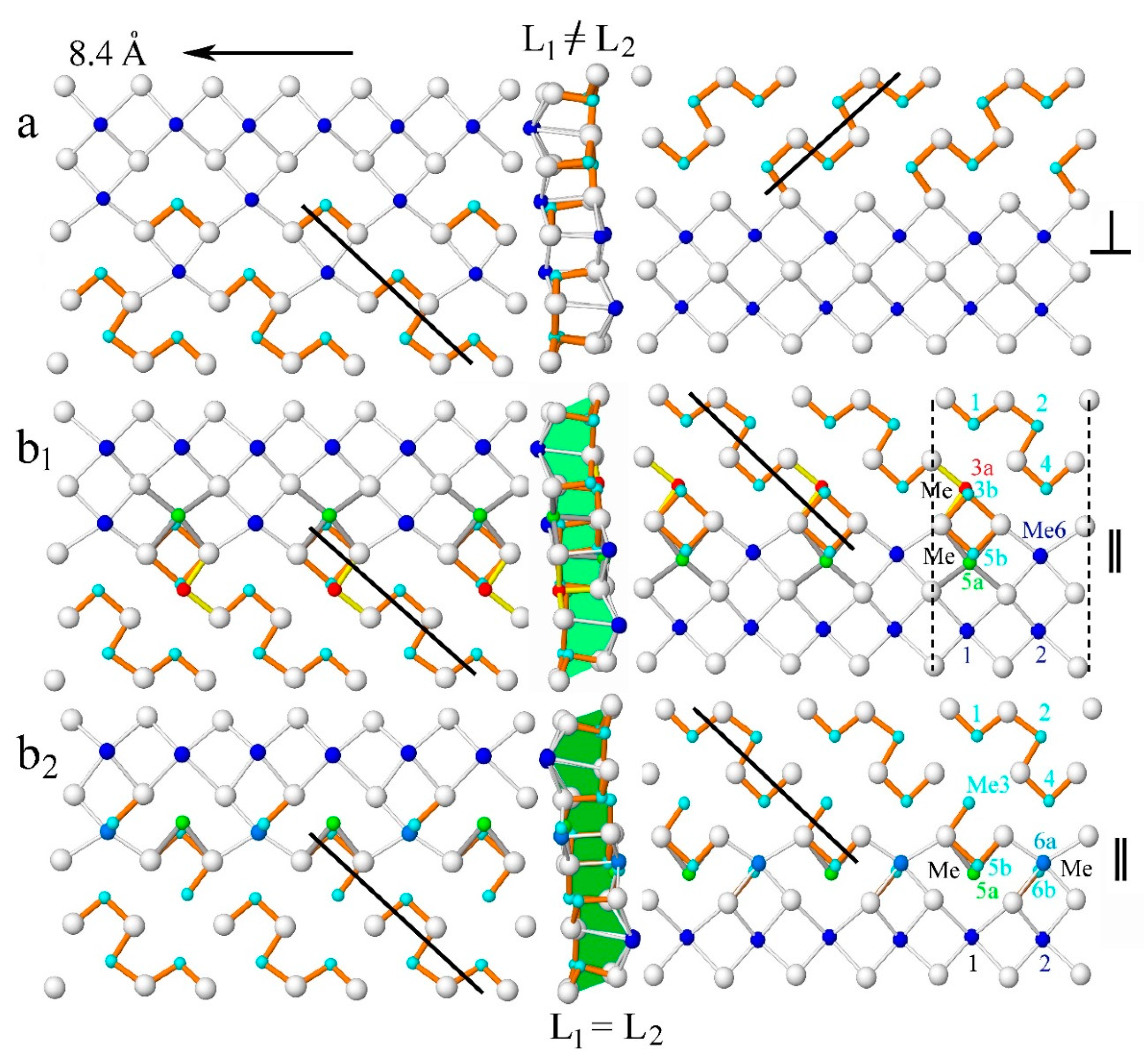
Another way to visualize the differences between the structures of rath1 and rath9 is the analysis of the crankshaft chains (alternating short–long bond distribution present in the highly covalent structures), as an unbroken or fragmented chain of short Me–S bonds. The crankshaft chains of the tightly bonded double SnS-like layer ribbon in rath1 and rath2 (
Figure 9b
1,b
2) and their chemistries are compared with the one of dufrénoysite (
Figure 9a).
To achieve that, three unit-cell broad ribbons along the 8.4 Å axis, oriented with the Pb-dominated layer for dufrénoysite on the left side, are sliced in two, 90° clockwise-rotated layers (L
1 and L
2), and placed on the left and right sides of each ribbon (
Figure 9). The short As–S bonds are shown in orange color, while the Sb–S short bonds are indicated in yellow color. For each ribbon, the length and direction of the crankshaft chains are indicated. The L
2 SnS-layer of dufrénoysite shows an unbroken four-polyhedra crankshaft chain of As. All other ribbons additionally possess one- or two-polyhedra fragmented crankshaft chains beside the unbroken three-polyhedra chain.
One can distinguish parallel and almost perpendicular directions of the chain directions between the left and right sides of the layers in each ribbon (thick black lines in
Figure 9). In the centrosymmetric structures of rathite (exemplified by rath1 and rath9) individual SnS-like layers (L
1 and L
2) of the tightly bonded double layer are symmetry related by inversion centers and their direction is parallel to each other, a feature not seen in the acentric structure of dufrénoysite, where the direction are perpendicular to each other.
5. Phase “SR”
The phase “SR” (rath10) forms thin exsolution lamellae (~2 micron thick, dark in BSE images) with the Tl-richest rathite rath9 (bright in BSE images), in two separate grains coming from different samples, with almost the same chemistry (
Figure 2a,b and
Table 1). The presence of two kinds of lamellae implies two distinct phases with different chemistries and structures. The exsolved intergrowths between the phase “SR” and rath9 may have formed during cooling of a previously stable, homogeneous higher-temperature Tl-rich phase.
The chemical composition of “SR” differs from that of rath9, but due to the thinness of the lamellae (measured with fully focused beam) an accurate determination of its composition and its range is difficult to achieve. For rath9 a homogenous grain was used for chemistry and structure determination (
Figure 1b,
Table 2). The calculated homolog order N
calc is 3.9 for “SR”, indicating a close relationship with rathite and the existence of a periodicity based on a ..44.. sequence (as in rathite). The degree of Ag and Sb substitution in “SR” is equivalent to that in rathite, but the degree of Tl substitution is 16% higher than in rath9.
A hypothetical crystal structure for phase “SR” is presented in
Figure 10. It could have the same metric as rathite, but should have a lower symmetry (monoclinic
P2
1 or triclinic
P-1) in order to produce two asymmetric ribbons with different chemistry. One ribbon (light green in
Figure 10) is As-rich (Me6 site fully occupied by As) similar to the (As,Ag)-rich ribbon from the argentobaumhauerite structure (Topa and Makovicky [
31]). The other ribbon (dark green) may be Pb-rich (Me6 site fully occupied by Pb, as in rath1) similar to one of the (Pb,Ag)-rich ribbons in the argentoliveingite structure (Topa et al. [
32]). Both ribbons in phase “SR” are only similar to, but not identical with the mentioned ones, because of the partial presence of Ag at the site Me5. The suggested periodicity sequence ..44.. is based on two chemically and structurally different ribbons, whereas in rathite there is only one ribbon.
A structure determination of phase “SR” would necessitate careful electron-diffraction studies and/or simultaneous refinement of two phases in the same fragment.