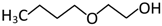
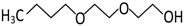
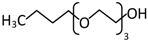
Abstract
This paper presents results of tests performed to determine the minimum concentration of a surfactant used at which the probability of occurrence of coalescence of air bubbles during flotation is low. Tests of the coalescence phenomenon were carried out using selected flotation frothing agents such as poly(ethylene glycol) butyl ethers, which are the ingredients of industrial flotation frothers known under the brand name of CORFLOT. Studies were carried out for three successive butyl ethers in the homologous series: ethylene glycol butyl ether (C4E1), diethylene glycol butyl ether (C4E2), triethylene glycol butyl ether (C4E3). Critical coalescence concentration (CCC) surfactant values were determined using the linear regression method. The investigation proved the existence of a clear relationship between the number of ethylene glycol groups in the ether molecule CnH2n+2(OC2O5)nOH and the value of the CCC. The results obtained show a correlation between the CCC values and the molecular weights (MW) of the tested frothers. This article shows the relationship between CCC value and hydrophilic–lipophilic balance (HLB)/MW for the family of polyglycol ethers. Results show the correlation between the critical coalescence concentration and the value of HLB index, being the measure of hydrophilic–lipophilic balance of surfactants (such as flotation frothers), expressed by the weight ratio of hydrophilic portion of the surfactant molecule and its molecular weight. Histograms of air bubbles created a distribution curve similar to the Gauss distribution pattern. The average diameter of air bubbles tends to decrease along with increasing concentration of the tested surfactants. Two characteristic zones that may be distinguished on the graphs show the relationship between Sauter mean diameter and frother concentration. The tests carried out demonstrate that the critical coalescence concentration may be used to characterize the flotation process, as, knowing the CCC values of the frothers used, we are able to control the consumption of foaming agents during mineral processing.
1. Introduction
Flotation is one of the enrichment processes most commonly used in mineral processing technology. One of the most important flotation-influencing factors is the selection of the reagents, particularly of the surfactants, which are the foaming agents called “frothers”. Surfactants are used to disperse gas, produce stable foam, and speed-up the flotation process. These reagents are adsorbed mainly at the interface of water and gas, and their sorption is accompanied by reduction of the surface tension of the aqueous solutions, which, in turn, causes a reduction of the sizes of mechanically produced air bubbles [1,2,3,4,5,6,7,8,9,10,11]. Stable foam keeps floated material at the water surface and allows it to be collected at the right time. The number and the size of air bubbles in a flotation cell have a direct impact on the performance of the flotation process [12]. For a given gas flow rate, a smaller size of air bubbles is more favorable for the flotation process [13]. Foam durability depends on the formation of a thin water film around the air bubbles of a structure which prevents water escape from the inter-bubble space [14,15,16]. It prevents bubble merging, called “coalescence” [2]. The surfactants, which are adsorbed at the bubble surface, cause the formation of thin rough films. Pure liquids do not foam, because without added frother, water leaks quickly and in an uncontrolled manner from thin layers between air bubbles, and the coalescence process (merging of air bubbles) occurs very quickly [17].
Parameters are used to evaluate frothers, and, among them, so called “critical coalescence concentration” (CCC) seems to be the most useful. This parameter was introduced in 2002 by Cho and Laskowski and co-authors [2,18,19,20]. Knowing the CCC value allows a reagent’s concentration to be selected so as to make the flotation process optimal. That is due to the fact that, usually, in laboratory conditions, flotation is carried out using concentrations close to the CCC. The influence of frothers on the size of bubbles and the flotation process has been analyzed by many authors [2,4,5,21,22]. Frothers reduce the size of bubbles, prevent their coalescence (CCC), and stabilize the foam. Surfactants used as flotation frothers are characterized by many parameters, such as coalescence concentration (CCC) [2,18,20,23]. Critical coalescence concentration is the minimum frother concentration that effectively prevents air bubbles from joining during flotation in a flotation chamber. It is generally observed that the higher the molar mass of a frother, the lower its CCC value [24]. Szyszka [25] and Kowalczuk [26] have shown that there is a strong correlation between CCC, molar mass, and hydrophilic–lipophilic balance. There are other indicators of the quality of flotation foams, including the dynamic foamability index (DFI) [27,28], rate of water transfer to froth at 25% gas content in the foam Jw,εg = 25% [29], and maximum mechanical entrainment of fine grains to foam εmax [30,31]. Preliminary analyses of those indicators suggest that, apart from the DFI, they are all similar [31]. It should also be mentioned that, according to Drzymała (unpublished data), the DFI becomes similar to other frother indicators if applied as 1/DFI. They found out that the increase of concentration of a given frother prevents bubble merging, i.e., coalescence. Further growth of frother concentration above the CCC value does not have any effect on the size of air bubbles.
The objective of this project was to determine the critical coalescence concentration (CCC) of selected poly(ethylene glycol) butyl ethers, which are the ingredients of industrial flotation frothers known under the brand name of CORFLOT. The critical coalescence concentration was determined by analysis of air bubble sizes for the tested compounds using a slightly modified graphical method developed by Cho and Laskowski [2,18]. In order to be able to determine the value of the coefficient more precisely, the linear regression method was used.
2. Materials and Methods
2.1. Materials
In the scope of this study, the solutions of the following frothers were tested: ethylene glycol butyl ether (C4E1), diethylene glycol butyl ether (C4E2), and triethylene glycol butyl ether (C4E3). The list of all parameters of the investigated surfactants and their structures are given in Table 1 and Table 2, respectively.

Table 1.
List of the tested frothers.

Table 2.
The structures of the investigated surfactants.
2.2. Methods
The lay-out of the measuring stand is shown in Figure 1 [32]. A peristaltic pump was used to deliver air with a constant flow of 5 cm3/min to generate air bubbles. The air was pressed through two capillaries (diameter 0.4 mm) rigidly fastened to a steel plate at a distance of 2 mm. The tests were carried out in an open flotation cell of volumetric capacity of 89 cm3. The process of air bubbles formation was filmed. A Sony α100 digital camera with a matrix of 10.8 M pixels and Industar-50-2 lens set on three intermediate rings ×10, ×5, ×3 was used to record test images. A minimum of 500 bubbles (typically 700 bubbles) was measured for each condition tested. Digital pictures were processed using IrfanView image editing software (version 4.40, IrfanView, Neustadt, Austria). Aqueous solutions of the investigated frothers were prepared using distilled water. All tests were carried out at room temperature of 21 ± 1 °C.
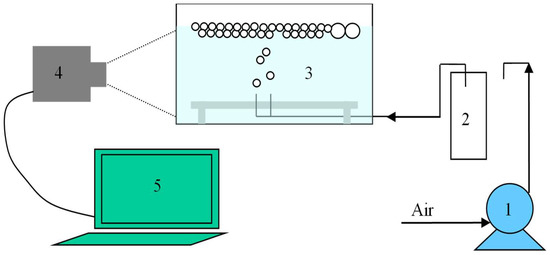
Figure 1.
Experimental set up for critical coalescence concentration (CCC) measurements. (1) peristaltic pump; (2) equalizing tank; (3) cell, (4) digital camera; (5) computer [32].
To pre-define initially the capability of a tested surfactant to flotation, the efficiency of multiparticle model flotation and the flotation of individual size fractions were determined in a flotation apparatus for laboratory use in the presence of only a foam-making agent at a CCC value pre-determined during the tests previously carried out.
The air bubble diameters were determined based on the received documentation using a free Meazure software (version 2.0.158, Softonic Corporate, Barcelona, Spain). The air bubble diameters obtained for the tested concentrations of the frothers were used to calculate the mean Sauter diameters (D32) of the air bubbles with an accuracy of 0.5 mm. The methodology of bubble size measurement was modified in the course of the research studies [26,31,32]. For instance the method of analyzing the air bubble images was modified to some extent. The conjugate and transverse diameter was measured and used in Equation (1) to determine the equivalent diameter of the elliptical air bubbles. The way of measuring the conjugate and transverse diameter is shown in Figure 2.
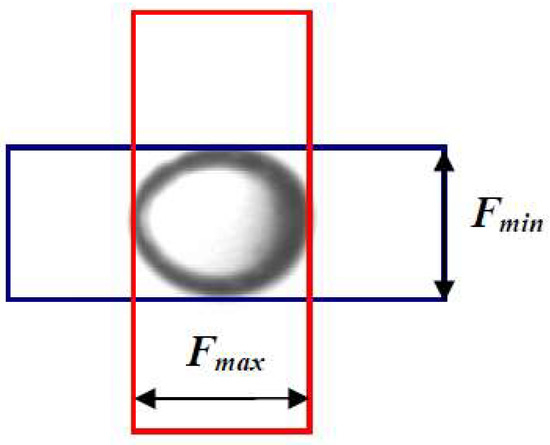
Figure 2.
Measurement of bubble diameter.
The graphs showing the relationship between the average values of air bubble diameters and the concentrations of tested solutions were plotted based on the measured values for the tested frothers. The CCC values were determined using the slightly modified graphical method described by Cho and Laskowski [2,18]. The critical coalescence concentration (CCC) was determined using the linear regression method—it is the point on the intersection of two regression lines obtained, projected down to the OX axis. Pearson’s correlation coefficient for the linear regression used to determine the CCC coefficient was in the range of 76%–89%. The average diameters of air bubbles were calculated from measured values using Equation (2):
which is called the Sauter mean diameter (D32) defined by Sauter [33].
3. Results and Discussion
The air bubble diameters obtained for the tested concentrations of the frothers were re-calculated into Sauter mean diameters and, subsequently, the graphs were plotted, which were used to determine the critical coalescence concentration (CCC) values (Figure 3).
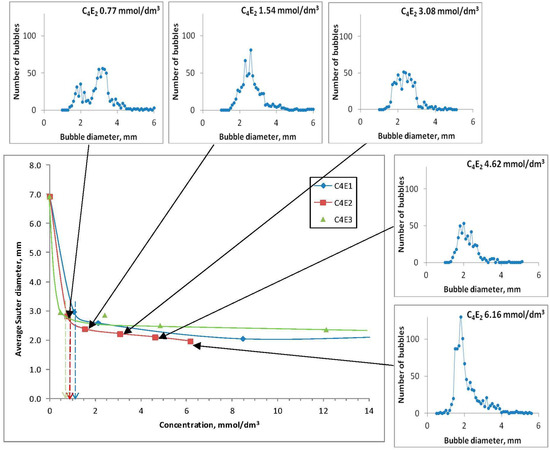
Figure 3.
Graphical determination of the CCC values for C4E1, C4E2, and C4E3.
It is to note that two ranges of frother concentrations are clearly distinguished on each graph representing the relationship between the Sauter mean diameter of the produced air bubbles and the tested frother concentrations. In the first range, the average air bubble size drops very quickly along with the increase of frother concentration. In the second range, the process is stabilized and air bubbles keep their sizes with increasing frother concentration [34,35]. Figure 3 does not show the last tested concentrations for C4E1 (16.92 mmol/dm3) and C4E3 (24.24 mmol/dm3) due to readability. The determined CCC values of all tested frothers are given in Table 3. Values presented in Table 3 characterize the relationship between the size of bubbles and the concentration of the studied surfactant. In the generated charts, two characteristic zones may be observed, showing the dependency between the Sauter mean diameter and frother concentration. The first zone is a vertical asymptote, typical for lower concentrations of the studied compounds, and the other zone is a horizontal asymptote typical for higher concentrations of the studied frothers. The vertical asymptote is prone to coalescence, which causes large-diameter air bubbles to form, up to the mean diameter of air bubbles in pure water (Figure 3). The horizontal asymptote, on the other hand, marks out the mean diameters of air bubbles that differ slightly. The increase in concentration of a frother above the CCC value has no impact on the size of air bubbles.

Table 3.
Critical coalescence concentration (CCC) values for the investigated frothers.
Figure 3 shows an example of air bubble distribution for C4E2 in the tested concentrations. This distribution pattern resembles the Gauss curve, which is a continuous distribution which approximates well the experimental distribution of the measurements, in which random uncertainties prevail. The Gauss distribution pattern is widely used in statistics because with increasing population of independent experiments all theoretical distribution patterns tend to converge on the normal distribution at a certain time. The Gauss curve is described by two parameters: expected value, which defines the highest value of the curve and standard deviation, which decides whether the curve is flatter or steeper [36]. The distribution of air bubbles for C4E1 at a concentration of 0.77 mmol/dm3 resembles the imposition of two Gauss distribution patterns with two peaks. Examples of air bubble images for individual frother concentrations are shown in Figure 4 and Figure 5.

Figure 4.
Examples of air bubble images for pure water.

Figure 5.
Examples of air bubble images for 4.62 mmol/dm3 C4E2.
The results obtained demonstrate that the sizes of air bubbles in tested polyglycol-based frothers tend to decrease along with the increasing number of “ethoxy” groups in the molecules of compounds in the homologous series (Figure 6). These results show a correlation between the CCC value and molecular weight of the tested frother and the CCC value versus the number of ethoxy-groups in the frother molecule. The CCC value drops with increasing number of ethoxy-groups in the molecule of polyglycol. This relationship is shown in Figure 7 and has already been described in the previous papers of the author [31]. The paper shows the relationship between critical coalescence concentration (CCC) value and hydrophilic–lipophilic balance (HLB)/molecular weight (MW) for the family of polyglycol ethers Figure 6 and Figure 7. Similar dependencies were observed by other researchers testing polyglycol frothers, also used as flotation frothers [26,37,38,39]. In Kowalczuk’s paper [26], the correlation between critical coalescence concentration and hydrophilic–lipophilic balance ratio/molecular weight was shown for surfactants used as flotation frothers. This correlation was based on experimental data published in the literature. The empirical equation obtained allows the critical coalescence concentration based only on the chemical structure of the frother to be accurately predicted. It has been found that the empirical equation based on one regulated parameter referred to by Kowalczuk as the frothing concentration constant may be used to predict the mean size of Sauter bubbles d32 for different flotation frothers. The CCC values of the frother belonging to the group of glycol ethers—tricosene ethyleneglycol 1-hexadecanoic ether (C16E23)—are also presented in Figure 6 and Figure 8. The CCC value of C16E23 was determined by the author in her previous studies on the foaming properties of surfactants [40]. In this paper the CCC value of C16E23 is quoted as the reference to the other test results to emphasize the impact of the number of ethoxy-groups in the molecule of the tested frother on its CCC value.
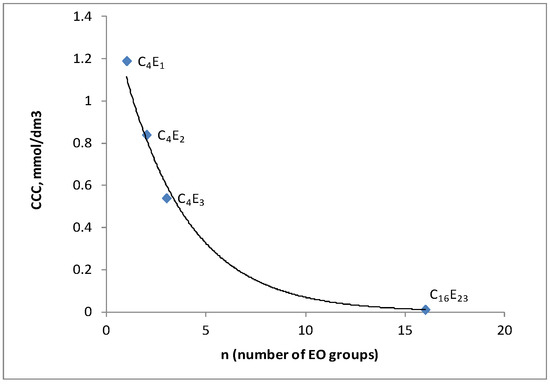
Figure 6.
Effect of a number of (EO) groups per molecule on CCC values for the polyglycol frothers.
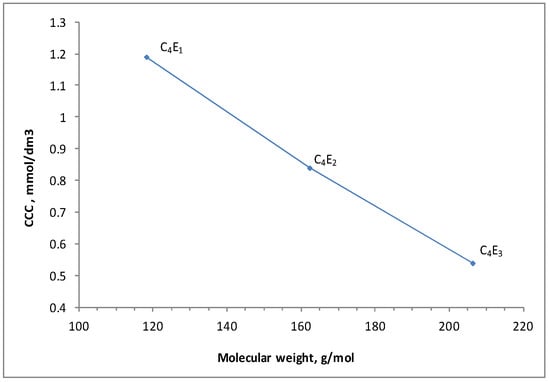
Figure 7.
Correlation between CCC and MW for C4E1, C4E2, and C4E3.
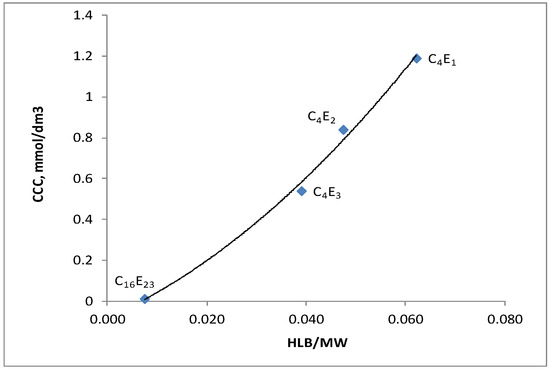
Figure 8.
Effect of HLB/MW on CCC values for the polyglycol frothers.
Figure 8 shows the relationship between (critical coalescence concentration) CCC value and hydrophilic–lipophilic balance (HLB)/molecular weight (MW) for the family of polyglycol ethers. This figure shows the correlation between the CCC and the value of the HLB index, being the measure of hydrophilic–lipophilic balance of surfactants (such as flotation frothers), expressed by the weight ratio of the hydrophilic portion of the surfactant molecule and its molecular weight. The relationship between the CCC, the HLB and the MW for various flotation frothers has been investigated and described in the literature by numerous authors [20,24].
Frothers which produce smaller air bubbles generate a more dynamically stable foam [21,27,28]. The higher dynamic foamability index (DFI) value indicates a more stable foam, in which air bubbles do not merge, because the CCC value of the frother is the concentration, at which bubble merging is fully prevented. The correlation between DFI and CCC was observed in the tests carried out on polyoxypropylene alkyl ethers [20,22,41,42].
The author of this paper plans to investigate the flotation properties of solutions containing blends of polyoxypropylene alkyl ethers of certain molar ratios as a function of their CCC values. These studies will be aimed at checking the effects of stronger frothers on weaker ones.
4. Conclusions
Determination of the critical coalescence concentration (CCC) of selected poly(ethylene glycol) butyl ethers which are the ingredients of industrial flotation frothers marketed under the brand name of CORFLOT allows to conclude as follows. The investigation proved the existence of a clear relationship between the number of ethylene glycol groups in the ether molecule CnH2n+2(OC2O5)nOH and the value of the critical coalescence concentration (CCC). The results obtained show a correlation between the CCC values and the molecular weights of the tested frothers.
Histograms of air bubbles created a distribution curve similar to the Gauss distribution pattern, and the values of the average diameters of air bubbles oscillated around the peaks of the graph, which confirms that the measurements were made correctly.
The average diameter of air bubbles tends to decrease along with increasing concentration of the tested surfactants. When the concentration of frother exceeds the CCC value, the average diameter of the air bubbles stabilizes and is equal to the original diameter of new bubbles. Air bubbles undergo coalescence below the CCC value, which causes growth of their size and foam thickness. Such foam is less stable, which leads subsequently to poorer concentrate quality.
Two characteristic zones may be distinguished on the graphs showing the relationship between Sauter mean diameter and frother concentration. The first zone forms a vertical asymptote, typical for lower concentrations of the tested compounds, and the second zone a horizontal asymptote typical for their higher concentrations. The vertical one is susceptible to the coalescence effect, which results in formation of air bubbles of large average diameters. The horizontal one describes constant average diameters of the air bubbles. A further increase of the foaming agent concentration above the CCC value does not have any significant effect on the size of air bubbles.
The carried out tests demonstrate that the critical coalescence concentration may be used to characterize the flotation process and may help to minimize the costs of mineral processing. Knowing the CCC values of the frothers used, we are able to control the consumption of the foaming agents.
Funding
This research was funded by Polish Statutory Research Grant 0401/0048/18 “New measuring, analytical, simulation and experimental research methods in mining and geology as well as in geodesy and cartography”.
Acknowledgments
The author also gratefully acknowledges the assistance of P. Skomorowski in the measurements.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Min. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Finch, J.A.; Gélinas, S.; Moyo, P. Frother-related research at McGill University. Miner. Eng. 2006, 19, 726–733. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuna, C. Role of frother in bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Grau, R.; Laskowski, J.S.; Heiskanen, K. Effect of frothers on bubble size. Int. J. Miner. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Bubble break-up and the role of frother and salt. Int. J. Miner. Process. 2009, 92, 153–161. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Effect of frother on initial bubble shape and velocity. Int. J. Miner. Process. 2010, 94, 115–120. [Google Scholar] [CrossRef]
- Kracht, W.; Rebolledo, H. Study of the local critical coalescence concentration (l-CCC) of alcohols and salts at bubble formation in two-phase systems. Miner. Eng. 2013, 50–51, 77–82. [Google Scholar] [CrossRef]
- Melo, F.; Laskowski, J.S. Fundamental properties of flotation frothers and their effect on flotation. Miner. Eng. 2006, 19, 766–773. [Google Scholar] [CrossRef]
- Nassif, M.; Finch, J.A.; Waters, K.E. Developing critical coalescence concentration curves for industrial process waters using dilution. Miner. Eng. 2013, 50–51, 64–68. [Google Scholar] [CrossRef]
- Finch, J.; Zhang, W. Frother function-structure relationship: Dependence of CCC95 on HLB and H-ratio. Miner. Eng. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Schwarz, S.; Alexander, D. Gas dispersion measurements in industrial cells. Miner. Eng. 2006, 19, 554–560. [Google Scholar] [CrossRef]
- Gorian, B.K.; Franzidis, J.P.; Manlaping, E.V. Studies on impeller type, impeller speed and air flow rate in an industrial flotation cell—Part 4 Effect of bubble surface area flux on flotation performance. Miner. Eng. 1997, 10, 367–379. [Google Scholar] [CrossRef]
- Harris, P.J. Chapter 13: Frothing phenomena and frothers. In Principles of Flotation; King, R.P., Ed.; Monograph Series 3; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1982. [Google Scholar]
- Kracht, W.; Finch, J.A. Using sound to study bubble coalescence. J. Colloid Interface Sci. 2009, 332, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–102. [Google Scholar] [CrossRef]
- Drzymala, J. Mineral. Processing, Foundations of Theory and Practice of Minerallurgy; Oficyna Wydawnicza Politechniki Wroclawskiej: Wroclaw, Poland, 2007. [Google Scholar]
- Cho, Y.S.; Laskowski, J.S. Bubble coalescence and its effect on bubble size and foam stability. Can. J. Chem. Eng. 2002, 80, 299–305. [Google Scholar] [CrossRef]
- Laskowski, J.S. Fundamental properties of flotation frothers. In Proceedings of the 22nd International Mineral Processing Congress, Cape Town, South Africa, 28 September–3 October 2003; Volume 2, pp. 788–797. [Google Scholar]
- Laskowski, J.S.; Tlhone, T.; Williams, P.; Ding, K. Fundamental properties of the polyoxypropylene alkyl ether flotation frothers. Int. J. Miner. Process. 2003, 72, 289–299. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Rao, R.; Finch, J.A. Characterizing Frothers through Critical Coalescence Concentration (CCC)95-Hydrophile-Lipophile Balance (HLB) Relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A. Effect of frothers on foamability, foam stability and bubble size. Coal Prep. 2007, 27, 107–125. [Google Scholar] [CrossRef]
- Laskowski, J.S. Testing flotation frothers. Physicochem. Probl. Miner. Process. 2004, 38, 13–22. [Google Scholar]
- Szyszka, D.; Glapiak, E.; Drzymała, J. Entrainment-flotation activity of quartz in the presence of selected frothers. Physicochem. Probl. Miner. Process. 2008, 42, 85–90. [Google Scholar]
- Szyszka, D. Krytyczne stężenie koalescencji potencjalnych spieniaczy do flotacji łupka miedzionośnego, In Lupek Miedzionosny II; Kowalczuk, P.B., Drzymala, J., Eds.; WGGG PWr: Wroclaw, Poland, 2016; pp. 222–227. (In Polish) [Google Scholar]
- Kowalczuk, P.B. Determination of Critical Coalescence Concentration and Bubble Size for Surfactants Used as Flotation Frothers. Ind. Eng. Chem. Res. 2013, 52, 11752–11757. [Google Scholar] [CrossRef]
- Małysa, K.; Czubak-Pawlikowska, J.; Pomianowski, A. Frothing properties of solutions and their influence on the floatability. In Proceedings of the 7th International Congress Surface Actives Substances, Moscow, Russia, 1978; Volume 3, pp. 513–520. [Google Scholar]
- Czarnecki, J.; Małysa, K.; Pomianowski, A. Dynamic frothability index. J. Colloid Interface Sci. 1982, 86, 570–572. [Google Scholar] [CrossRef]
- Moyo, P.; Gomez, C.O.; Finch, J.A. Characterizing frothers using water carrying rate. Can. Matallurgical Q. 2007, 46, 215–220. [Google Scholar] [CrossRef]
- Szyszka, D. Mechaniczna flotacja hydrofilnych ziarn kwarcu w obecności spieniacza. Min. Sci. 2007, 31, 81–88. (In Polish) [Google Scholar]
- Szyszka, D.; Drzymała, J.; Resiak, P.; Mielczarski, E.; Mielczarski, J. Entrainment of quartz in flotation tests with frothers. In Proceedings of the XXIV International Mineral Processing Congress, Beijing, China, 24–28 September 2008; pp. 1068–1073. [Google Scholar]
- Szyszka, D.; Drzymała, J.; Łuczyński, J.; Wilk, K.A.; Patkowski, J. Concentration of α-Terpineol and (2-Dodecanoyloxyethyl)trimethyl ammonium bromide required for prevention of air buble coalescence in aqueous solutions. Physicochem. Probl. Miner. Process. 2006, 40, 53–59. [Google Scholar]
- Szyszka, D. Critical coalescence concetration (CCC) as a parameter for evaluation of selected quaternary ammonium compounds. Min. Sci. 2013, 20, 101–113. [Google Scholar]
- Pacek, A.W.; Man, C.C.; Nienow, A.W. On the Sauter mean diameter and size distributions in turbulent liquid/liquid dispersions in a stirred vessel. Chem. Eng. Sci. 1998, 53, 2005–2011. [Google Scholar] [CrossRef]
- Grau, R.A. An Investigation of the Effect of Physical and Chemical Variables on Bubble Generation and Coalescence in Laboratory Scale Flotation; Helsinki University of Technology Doctoral Theses in Materials and Earth Sciences, 4; Laboratory of Mechanical Process Technology and Recycling: Espoo, Finland, 2006. [Google Scholar]
- Grau, R.; Heiskanen, K. Gas dispersion measurements in a flotation cell. Miner. Eng. 2003, 16, 1081–1089. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Quinn, J.J.; Sovechles, J.M.; Finch, J.A.; Waters, K.E. Critical coalescence concentration of inorganic salt solutions. Miner. Eng. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of Flotation Frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef]
- Sobczyk, M. Statistics Practical and Theoretical Aspects; Publisher UMTS: Lublin, Poland, 2006. [Google Scholar]
- Malysa, E.; Malysa, K.; Czarnecki, J. A Method of comparison of the frothing and collecting properties of frother. Colloids Surf. 1987, 23, 29–39. [Google Scholar] [CrossRef]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A.; Satish, P.; Pradip. Frother Characterization with Two-Phase Foam System. In Proceedings of the International Seminar on Mineral Processing Technology and Indo-Korean Workshop on Resource Recycling (MPT-2006), NML, Chennai, India, 8–10 March 2006. [Google Scholar]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).