Abstract
Detailed characterization and recovery of apatite from a sedimentary phosphate ore slime of a gravity plant of Yichang, China, were investigated. The phosphate ore slime consisted mainly of apatite, quartz, dolomite, and clay. To start with, the ore slime was characterized in sufficient detail to reveal that it is extremely fine in distribution with a d50 of 65 μm. Other different characterizations, such as apatite grain size distribution, apatite liberation, particle density distribution, and theoretical grade recovery, were also analyzed by mineral liberation analyser (MLA). Based on the results, a new gravity-flotation process, which was comprised of spiral gravity and reverse flotation, was proposed to process the slime. In the new process, the apatite was firstly recovered by two-stage spiral gravity, avoiding direct flotation. The gravity concentrate was the feed for the reverse flotation. A phosphate concentrate of 30.51% P2O5 with a P2O5 recovery of 89.00% can be produced from the slime analyzing 24.25% P2O5. Compared to the conventional direct-reverse flotation process, it was found that the reagent cost of the new gravity-flotation process was lower a quarter than that of the direct-reverse flotation flowsheet.
1. Introduction
Phosphate deposits are abundant in China, mainly distributed in Yunnan, Sichuan, Hubei, Guizhou, and Hunan, etc. As one of five important phosphate ore producing area in China, Hubei province has approximate 1.8 billion tons available reserves of phosphate ore, of which about 52% distribute in Yichang region. Over 88% of the phosphate ore resources in Yichang are medium- or low-grade phosphate ore, the average grade of P2O5 is 22.2%, and most ores are characterized as siliceous-calcareous phosphate ore [1]. To meet the requirement of fertilizer industry, phosphate ore should be enriched to near 30% P2O5 [2]. Thus, the separation of apatite from impurity minerals, such as dolomite, clay, and quartz, is the critical step in phosphate ore processing [3,4].
Many separation techniques of Yichang siliceous-calcareous phosphate ore, such as direct-reverse flotation (or reverse-direct flotation) and gravity-flotation process have been researched and developed by various researchers [5,6,7]. Although the flotation process is now drawing more attention and has been improved with the use of more efficient collectors and modifier, the high reagent consumption of the direct flotation stage remains problematic. Currently, the gravity-flotation process has been successfully commercialized. In the gravity stage, heavy medium separation techniques are used for discarding coarse dolomite [8,9]. The typical heavy medium separation equipment used in Yichang phosphate ore is heavy medium cyclone. The gravity concentrate is then subjected to double reverse flotation for further discarding of carbonate and silicate minerals. However, in the silicate minerals flotation step, cationic collectors are often used, which can cause problems such as sensitivity to slimes, sticky froths, and bad fluidity [6,10,11]. In addition, gravity separation has lower size limit, which is generally accepted to be 0.5 mm for heavy medium cyclone [12]. Thus, in the gravity-flotation process for Yichang phosphate ore, the fine fraction (–0.5 mm), which account for about 14% of the feed, are pre-separated by screening [13]. Compared to the feed, the slime has a slightly lower P2O5 grade and MgO content, and a slightly higher SiO2 content.
In order to recover apatite from Yichang slimes, a host of explorations have been carried out by some scholars. The direct-reverse flotation (or reverse-direct flotation) has been proven to be effective [14,15,16,17]. Although it is possible to obtain qualified phosphate concentrate, the problem of high reagent consumption of direct flotation is still the main reason for restricting its application.
Prior studies have demonstrated that pre-recovery of apatite by spiral separation could significantly reduce the flotation reagent consumption in direct flotation [18,19]. The results of the prior study showed that the fine spirals were capable of recovering of apatite from ultra-fine phosphate ore. This study focuses on the investigation of a new process for recovery of apatite from the gravity slime thereby avoiding the high reagent consumption. Based on the characterization and separation studies, a new combined process is proposed. The feasibility of the proposed process was also evaluated by comparison against the direct-reverse flotation process.
2. Materials and Methods
2.1. Materials
Phosphate ore slime was obtained from Yichang, Hubei Province, China. The chemical composition of the ore was analyzed by ICP-AES (inductively-coupled plasma atomic emission spectrometry) performed on an IRIS Advantage ER/S instrument (Thermo Elemental, Waltham, MA, USA). The results are shown in Table 1. The ore contained 24.25% P2O5, 2.64% MgO, and 25.17% SiO2.

Table 1.
Chemical composition of slime sample.
The mineral composition of the sample was obtained by the comprehensive analysis of the chemical composition and MLA (mineral liberation analysis). The results are shown in Table 2. The raw ore contains 57.5% apatite, 12.5% quartz, 14.8% dolomite and 9.2% clay.

Table 2.
Mineral composition of the slime.
Characterization of the slime, such as particle size distribution and apatite grain size distribution, apatite liberation, particle density distribution, and theoretical grade recovery, were analyzed by using a mineral liberation analyser (MLA).
The particle size distribution/apatite grain size distribution results (Figure 1) illustrate that the slime has a d50 of 65 μm and the contained apatite has a d50 of 58 μm.
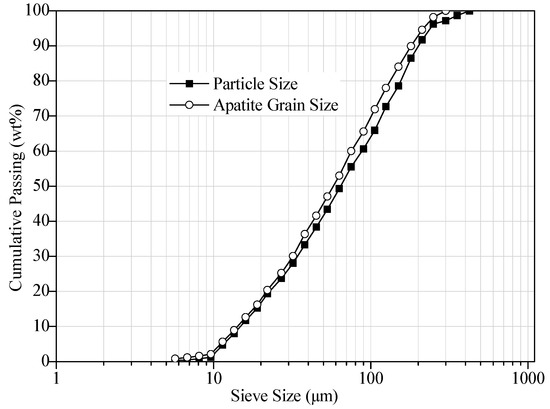
Figure 1.
Particle size distribution and apatite grain size distribution of received sample.
The mineral liberation of apatite by particle composition presented in Figure 2 clearly shows that more than 50% of the apatite particles are completely liberated and nearly 80% of the apatite particles (above 90% liberation class) could be separated as phosphate concentrate.
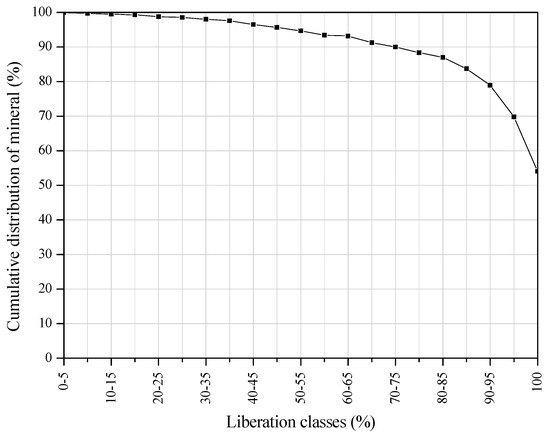
Figure 2.
Mineral liberation of apatite by particle composition.
The particle density distribution results (Figure 3) show that about 45% of the particles have density distributed in a range of 3.1–3.2 g/cm3. It was concluded from the mineral composition and liberation study results that these particles are mainly liberated apatite particles. The density of gangue minerals is mainly distributed in the range of <3.1 g/cm3, among which 2.5–2.7 g/cm3 is the greatest portion. The characterization study results show that although the particle size of the slime is ultra-fine, the liberation of apatite is good, and the density of most gangue mineral particles is lower than apatite. Therefore, the phosphate ore slime has a certain gravity separability. The theoretical grade-recovery curve for apatite was drawn. Theoretical grade-recovery curves are defined by the maximal expected recovery of apatite at a given grade. These curves are related to the particle size and determined from the liberation characteristics. It should be noted that theoretical grade-recovery curves are defined for the value minerals (e.g., apatite) and not based on a final product to be recovered. Furthermore, it is important to advise that the theoretical grade-recovery curve provided by the MLA is generated from 2D liberation measurements and, therefore, overestimate the true liberation by a certain amount [20]. Nevertheless, the theoretical grade-recovery curve for apatite (Figure 4) gives a reason to expect the best results for apatite recovery from the phosphate ore slime.
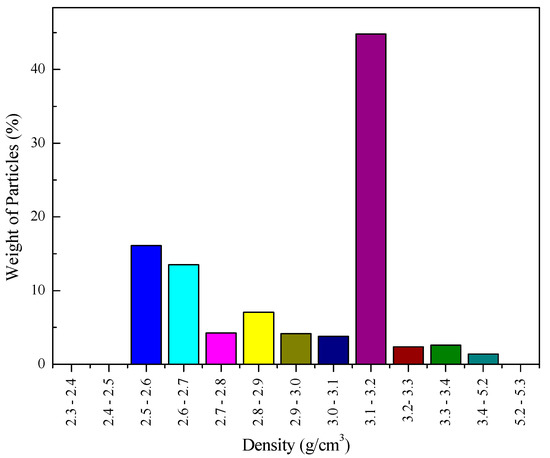
Figure 3.
Particle density distribution.
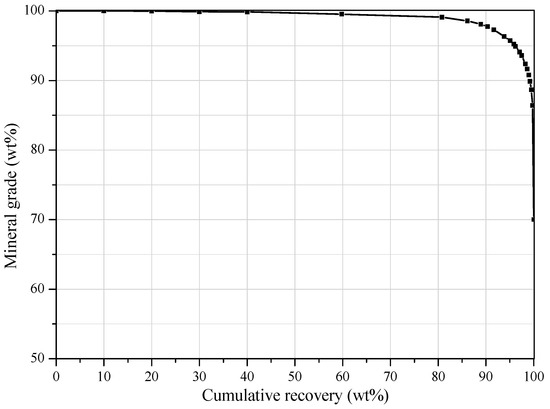
Figure 4.
Theoretical grade recovery curve for apatite mineral grains.
The sample of phosphate ore slime was further subjected to a wet screening separation. Each of the size fractions produced was wet assayed. The results tabulated in Table 3 show that phosphorous values are well distributed in all size fractions except the last one, i.e., −38 μm. It is also observed that magnesia tends to concentrate in the fraction −38 μm. The composite sample was separately analyzed to have 24.25% P2O5 and 2.68% MgO. Thus, a mere classification at around 25 μm would yield a product of 26.60% P2O5, 2.58% MgO.

Table 3.
Particle size and chemical composition of the slime.
2.2. Methods
Phosphate ore slime separation tests were carried out with the direct-reverse flotation process and gravity-flotation process. The separation process where spiral gravity and reverse flotation were employed was termed as the new process (Figure 5a) and that were direct-reverse flotation was employed was termed as the conventional process (Figure 5b).
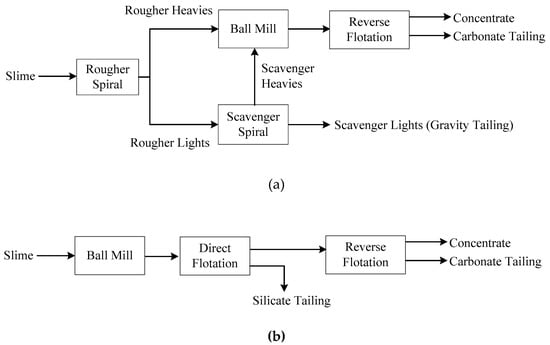
Figure 5.
Flowsheet of the new gravity-flotation process (a) and the conventional direct-reverse flotation process (b).
The original sample of phosphate ore slime was split into two fractions; one of the fractions was subjected to separation tests of the new process. As shown in the Figure 5a, the products of gravity separation included spiral concentrate and tailing. The spiral concentrate was wet ground to −74 μm accounting for 70.6% in a laboratory ball mill (model HLXMQ-Φ240 × 90) (Wuhan Exploring Machinery Factory, Wuhan, China) at 50 wt % solids. The ground product was the feed for reverse flotation. The other fraction was subjected to separation tests of the conventional process. The grind fineness of the feed for direct flotation was −74 μm accounting for 73.1%
2.2.1. Gravity Separation Tests
The gravity tests were comprised of rougher and scavenger. The physical structure parameters of the spiral used in the gravity are tabulated in Table 4. The setup diagram of the gravity separation test is shown in Figure 6. According to the particle size and chemical composition results of the raw sample, the +74 μm content of the slime accounts for 44.75% with a P2O5 grade of 27.92%. Thus, rougher spiral separation was firstly carried out to recover of the coarse fractions from the slime. Then spiral scavenger separation was conducted for recovery of fine apatite from the rougher tailing. In the rougher stage, approximate 40 L pulp was prepared, with a solid content of 20 wt %. The rougher separation was conducted under the condition of 348 L/h feed rate and 66 L/h wash water. In the scavenger stage, about 20 L pup at a solid content of 15 wt % was prepared. The scavenger separation was conducted under the condition of 210 L/h feed rate and no wash water. The products of gravity separation tests included rougher heavies, rougher lights, scavenger heavies, and scavenger lights.

Table 4.
Physical structure parameters of the spiral.
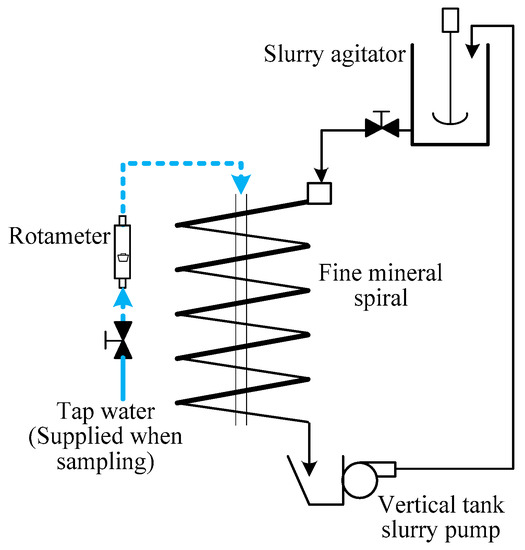
Figure 6.
Spiral setup diagram.
2.2.2. Flotation Separation Tests
Flotation tests were carried out with a 0.75/1.0 L XFD-IV single cell flotation machine (Jilin Provincial Machine Factory of Ore Exploration, Changchun, China). Tests were conducted at an impeller speed of 1750 rpm and pulp temperature of 25 °C. A 1.0 L flotation cell was prepared for the direct flotation tests, and a 0.75 L flotation cell for the reverse flotation. Commercial product CXY-P (a fatty acid mixture) from Chuxiang Phosphorus Technology Ltd. (Wuhan, China) was used as collector, which was selective for apatite at pH 10 and dolomite at pH 4.5. For each direct flotation test, the pulp containing 330 g of feed ore was transferred into the flotation cell and stirred for 1 min. Na2CO3 (Xilong Chemical Co., Ltd., Wuhan, China) was added into the pulp to achieve a pH at 10 with 2.0 min of conditioning time. Then water glass (Xiangxing Co. Ltd., Loudi, China) was added, and conditioned for another 2.0 min. Next, the collector CXY-P was added and the slurry was stirred for 2.0 min. All conditioning processes were conducted in the absence of airflow. For each reverse flotation test, the feed was the concentrate from either the gravity or the direct flotation. H2SO4 (Pingmei Group Co. Ltd., Pingdingshan, China) was firstly added to achieve a pH at 4.5 with 0.5 min conditioning time. CXY-P was added and conditioned for 2.0 min before flotation. Batch experiments were conducted to find the optimal dosages of reagents by single factor test method, and using the parallel test to increase the stability. The optimal flotation conditions are shown in Figure 7.
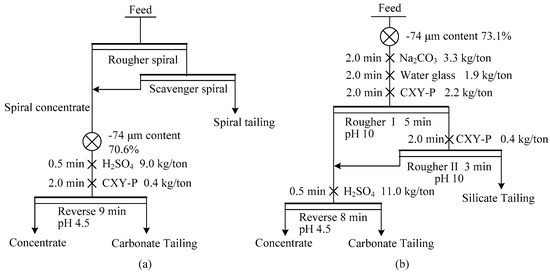
Figure 7.
The flotation flowsheet for the new gravity-flotation process (a) and the conventional direct-reverse flotation process (b).
3. Results and Discussion
3.1. Gravity-Flotation Flowsheet
3.1.1. Gravity Separation Tests results
The results of gravity separation tests are given in Table 5. It is indicated from this table that rougher heavies can be produced at a yield of 47.88% and a P2O5 grade of 28.08%. After having gone through two stages of spiral separation, a concentrate of 27.25% P2O5 at 91.37% recovery was produced from the feed of 24.25% P2O5. It is also observed that the effect of removing MgO by gravity separation is weak. The weak removal effect may be the result of small density differences between apatite and dolomite. Figure 8 shows the size distribution of particles in the products of gravity separation. The size distribution in the heavies and the lights show that the coarser particles are passed to the heavies. It can also be seen from this figure that the spiral concentrate is quite coarse having a d50 of 90 μm that is highly desirable.

Table 5.
Gravity separation results.
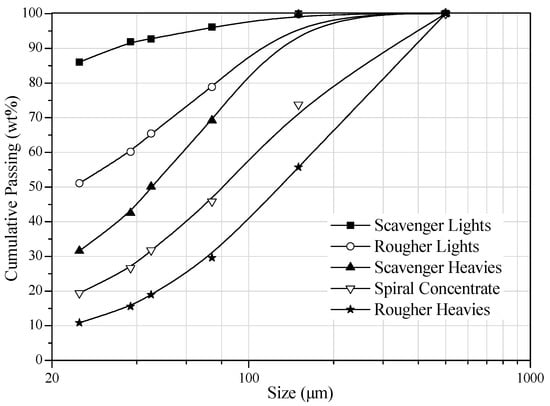
Figure 8.
Particle size distribution in the products of rougher and scavenger.
The partition curves for the phosphorus carriers obtained in the rougher and scavenger spirals are shown in Figure 9. The rougher spiral exhibit a recovery of fine phosphate ore particles (−74 μm) below 50%. In the scavenger spiral separation, the partition factor for the phosphorus mineral carriers increases up to particle size of 45 μm and then reaches a plateau. The recovery of phosphorus carriers in the size range of 45–115 μm is practically 100%, but drops for coarser size ranges.
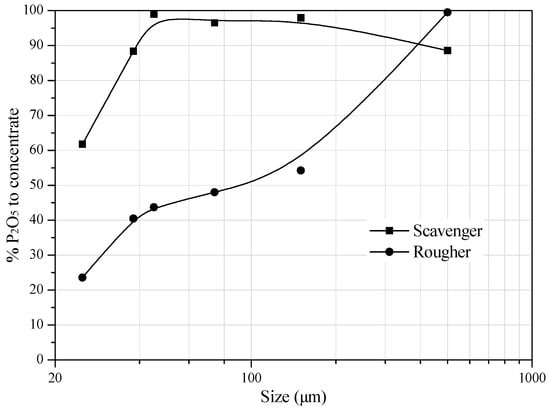
Figure 9.
Partition curves of phosphorus carries in the rougher and scavenger spirals.
3.1.2. Reverse Flotation Tests Results
The reverse flotation tests were carried out for discarding dolomite from the spiral concentrate and the results were tabulated in Table 6. It is shown that a concentrate, which has index of yield 87.00%, P2O5 grade 30.51%, and recovery 97.12%, can be obtained by reverse flotation.

Table 6.
Flotation tests results of the gravity-flotation process.
3.2. Direct-Reverse Flowsheet
Flotation tests were carried out with the conventional flowsheet and the results are shown in Table 7. A concentrate of 32.76% P2O5 was produced at a P2O5 recovery of 89.65%.

Table 7.
Flotation tests results of the direct-reverse flotation process.
3.3. Comparative Analysis of Flowsheets
The summary of the results for the flowsheet is tabulated in Table 8. In the conventional direct-reverse flotation flowsheet, the product quality is enriched to higher than 32% P2O5, but the reagent consumption is high, which may not be accepted in the economics view. It is recognized as one of the main reasons for high reagent consumption that more than 77% of the feed need to be floated in direct flotation stage. In the case of the new gravity-flotation flowsheet, the direct flotation was replaced by gravity separation, which is comprised of spiral rougher and scavenger. The spiral concentrate was then treated in a similar reverse flotation operation as in the conventional direct-reverse flotation flowsheet. Treated through the new gravity-flotation flowsheet, the concentrate was upgraded to 30.51% P2O5, having MgO content of 0.59%, with 89.00% P2O5 recovery. It is seen that with the incorporation of spiral separation and reverse flotation, the yield of concentrate increased which resulted in lower P2O5 grade. The reagent dosages of the flowsheet are shown in Table 9. Compared to the conventional flowsheet, the reagent dosages of the new flowsheet decreased significantly. Based the average price of each reagent in the year 2016, the reagents costs decreased from 56.7 CNY to 14.0 CNY for processing 1 t of concentrate.

Table 8.
Summary of the results in both flowsheets.

Table 9.
Optimum reagent dosages of two flowsheets.
4. Conclusions
- (1)
- The generation of phosphate ore slime was about 14% from the heavy medium cyclone gravity process in the Yichang region. The slime contained 24.30% P2O5, 2.64% MgO, and 25.17% SiO2. Although the particle size of the slime was fine in distribution with d50 of 65 μm, nearly 80% of the apatite particles were liberated (above 90% liberation class), and the density of gangue minerals were mostly distributed at 2.5–2.7 g/cm3, which was lower than apatite mineral density of 3.1–3.2 g/cm3. It was concluded that the phosphate ore slime has a certain gravity separability.
- (2)
- Two different flowsheets were studied to recover the apatite. The first flowsheet comprised of the gravity concentration and reverse flotation, which produced of the concentrate assaying of 30.51% P2O5 and 0.59% MgO. Moreover, the second flowsheet comprised of the direct flotation and reverse flotation, which produced a concentrate assaying of 32.86% P2O5 and 0.59% MgO. Although in the second flowsheet, the product quality was enriched to higher than 32% P2O5, the reagent consumption was high, which may not be adopted from the economical of view. Compared to the direct-reverse flotation flowsheet, the reagent cost decreased from 56.7 CNY to 14.0 CNY for producing 1 t concentrate when using the gravity-flotation flowsheet, which is an optimal processing methods.
Acknowledgments
This work was financially supported by the Science Technology Support Programs of Hubei Province, China (No. 2014BCB029), the Natural Science Foundation of Hubei Province, China (2016CFB197), and the National Natural Science Foundation of China (No. 51604197).
Author Contributions
Xin Liu and Yimin Zhang conceived and designed the experiments; Kun Sun performed the experiments; Xin Liu and Kun Sun analyzed the data; Tao Liu and Zhenlei Cai contributed reagents/materials/analysis tools; and Xin Liu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, N.F. Analysis and proposal about rational utilization of middle-low grade phosphate rock in Hubei province. Ind. Miner. Process. 2005, 11, 1–4. (In Chinese) [Google Scholar]
- Zafar, Z.I.; Anwar, M.M.; Pritchard, D.W. A new route for the beneficiation of low grade calcareous phosphate rocks. Fertil. Res. 1996, 44, 133–142. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Gan, S.P.; Zeng, X.B.; Yu, Y.F. Double reverse flotation process of collophanite and regulating froth action. Trans. Nonferr. Met. Soc. China 2008, 18, 449–453. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M. Physical and thermal treatment of phosphate ores-An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Liu, Q.; Chi, R. Reactive oily bubble technology for flotation of apatite, dolomite and quartz. Int. J. Miner. Process. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.Y.; Guo, X.L.; Guo, W.B. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Lin, J. Application of double reverse flotation process in commercial production of difficult separated collophane in Yichang. Ind. Miner. Process. 2013, 8, 30–31. (In Chinese) [Google Scholar]
- Le, H.; Shao, T. Promoting of heavy medium cyclone and new technology application. Coal Sci. Technol. 2008, 5, 5–9. [Google Scholar]
- Tan, M.; Wei, M. Progress in beneficiation of phosphorite ores. Min. Metall. 2010, 4, 1–6. (In Chinese) [Google Scholar]
- Boulos, T.R.; Yehia, A.; Ibrahim, S.S.; Yassin, K.E. A modification in the flotation process of a calcareous–siliceous phosphorite that might improve the process economics. Miner. Eng. 2014, 69, 97–101. [Google Scholar] [CrossRef]
- Al-Thyabat, S. Empirical evaluation of the role of sodium silicate on the separation of silica from Jordanian siliceous phosphate. Sep. Purif. Technol. 2009, 67, 289–294. [Google Scholar] [CrossRef]
- Chen, J.; Chu, K.W.; Zou, R.P.; Yu, A.B.; Vince, A.; Barnett, G.D.; Barnett, P.J. Systematic study of the effect of particle density distribution on the flow and performance of a dense medium cyclone. Powder Technol. 2016, 314, 510–523. [Google Scholar] [CrossRef]
- Wei, X.S.; Huang, Q.S.; Li, Y.X. Study on dense media separation and its’ application in Huaguoshu phosphorite deposit. Geol. Chem. Miner. 2010, 3, 186–188. (In Chinese) [Google Scholar]
- Luo, H.H.; Liu, L.K.; Zhu, D.P.; Qu, D.J.; Miao, H.J.; Hu, X.C.; Hu, Z.; Zhang, Z.L. Recovery of fine-grain and low-grade collophane from gravity tailings of Yichang phosphate by flotation. Multipurp. Util. Miner. Resour. 2016, 3, 67–70. (In Chinese) [Google Scholar]
- Luo, H.H.; Shu, C.; Tong, Y.L.; Qu, D.J.; Miao, H.J.; Hu, X.C.; Hu, Z.; Zhang, Z.L. Recovery of Yichang phosphate by heavy fluid-separation and flotation combined process. Ind. Miner. Process. 2016, 7, 6–8. (In Chinese) [Google Scholar]
- Zhang, L.Y.; Hong, W.; Qiu, Y.S.; Song, Y.H. Experimental study on flotation of fine low grade collophane. Non-Met. Mines 2012, 2, 21–23. (In Chinese) [Google Scholar]
- Wu, C.B.; Duan, X.X. Studies on processing phosphorus ores in China. Yunnan Metall. 2000, 163, 19–22. (In Chinese) [Google Scholar]
- Liu, X.; Zhang, Y.M.; Liu, T.; Cai, Z.L.; Chen, T.J.; Sun, K. Beneficiation of a sedimentary phosphate ore by a combination of spiral gravity and direct-reverse flotation. Minerals 2016, 6, 38. [Google Scholar] [CrossRef]
- Sun, K.; Liu, T.; Zhang, Y.M.; Liu, X.; Wang, B.; Xu, C.B. Application and mechanism of anionic collector sodium dodecyl sulfate (SDS) in phosphate beneficiation. Minerals 2017, 7, 29. [Google Scholar] [CrossRef]
- Sandmann, D.; Gutzmer, J. Use of mineral liberation analysis (MLA) in the characterization of lithium-bearing micas. Miner. Mater. Charact. Eng. 2013, 1, 285–292. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).