Microbial Diversity and Community Assembly across Environmental Gradients in Acid Mine Drainage
Abstract
:1. Introduction
2. Microbial Diversity and Community Dynamics in AMD Ecosystems
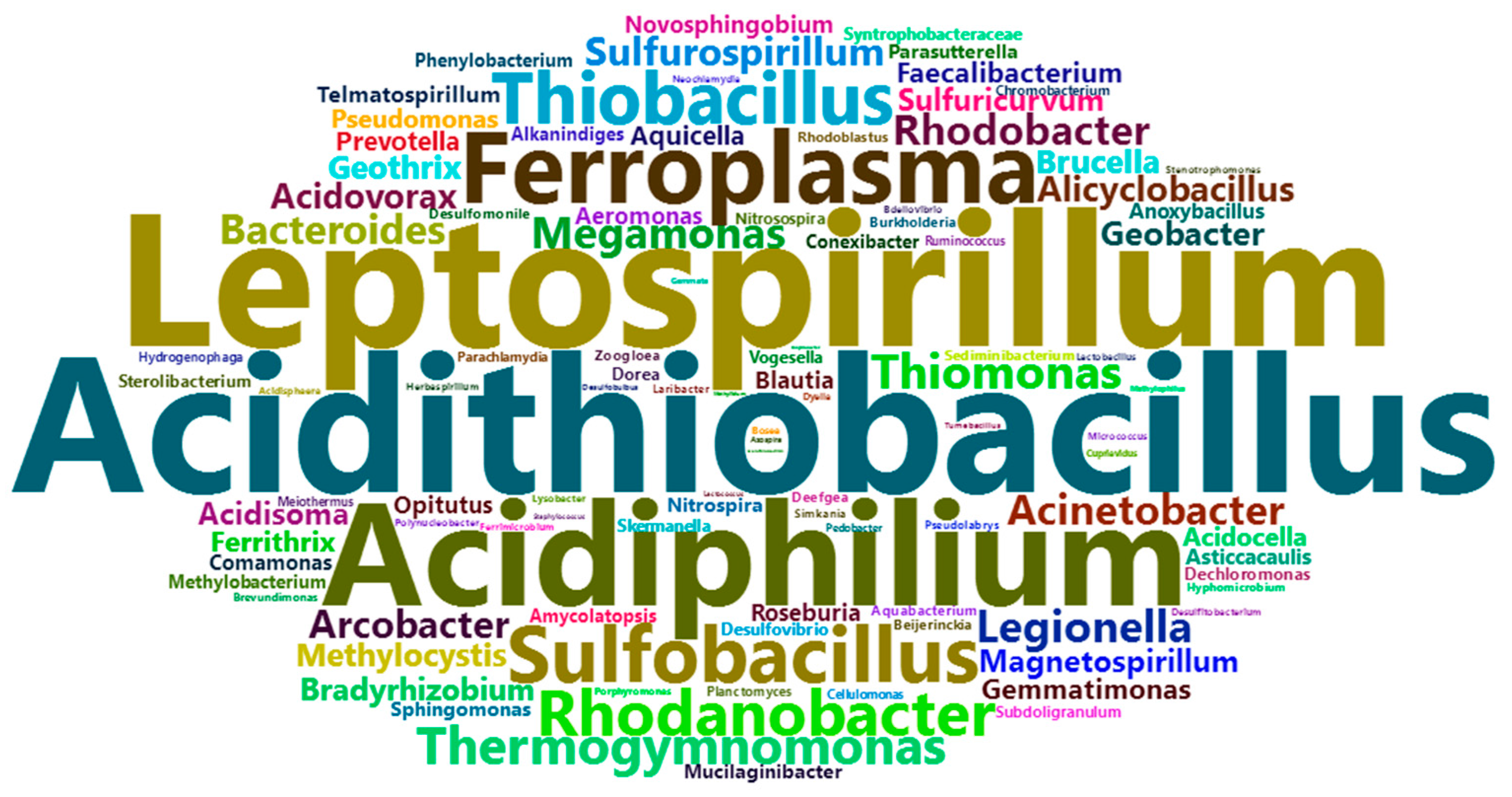
2.1. AMD Generation and Associated Microorganisms
2.2. Microbial Community Function and Dynamics During AMD Generation
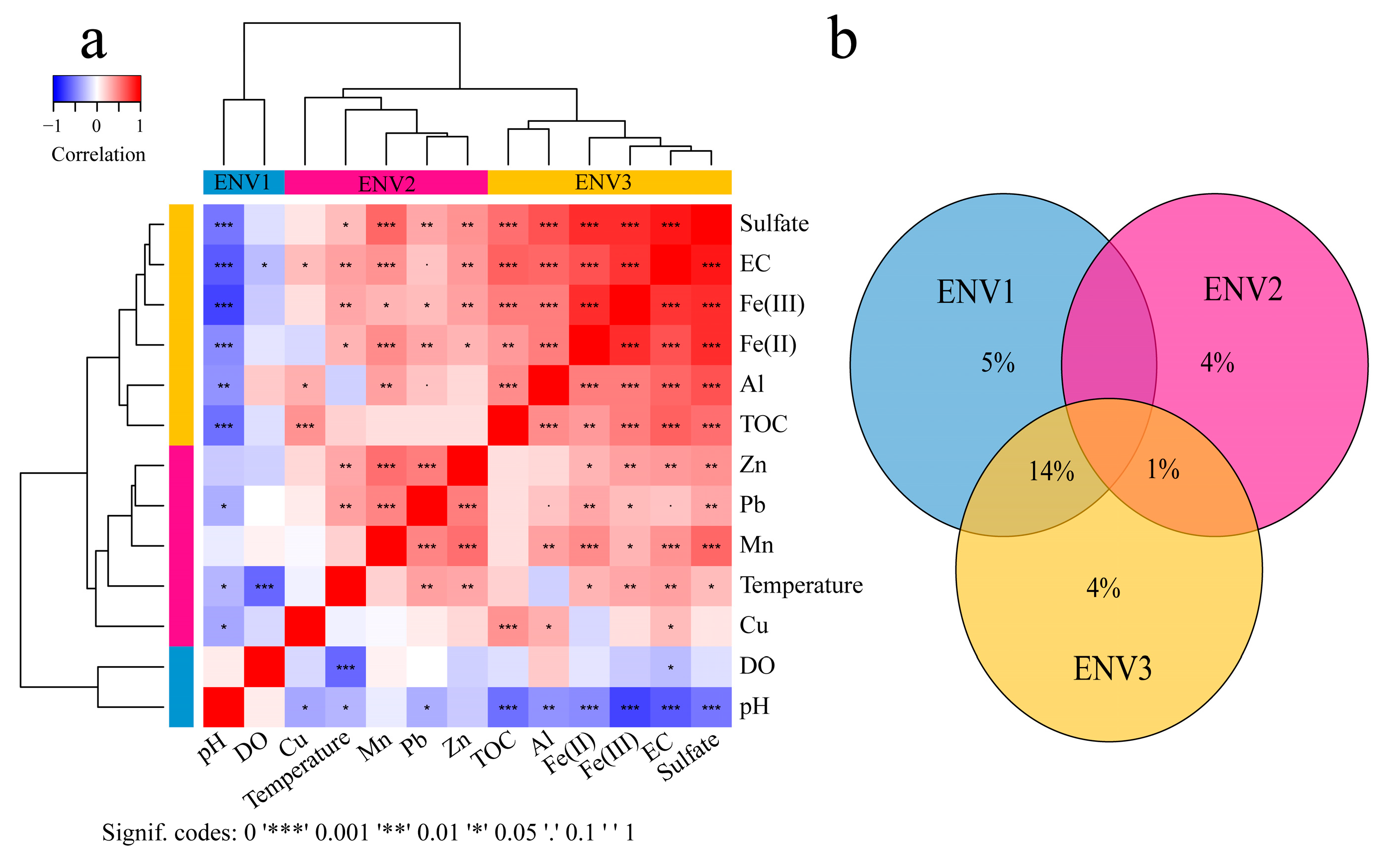
3. Microbial Community Assembly across Environmental Gradients in AMD Ecosystems
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T.L. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.N.; Kuang, J.L.; Shu, W.S. Microbial ecology and evolution in the acid mine drainage model system. Trends Microbiol. 2016, 24, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. The microbiology of acidic mine waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef]
- Johnson, D.B.; Rolfe, S.; Hallberg, K.B.; Iversen, E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned norwegian copper mine. Environ. Microbiol. 2001, 3, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.B.; Johnson, D.B. Biodiversity of acidophilic prokaryotes. Adv. Appl. Microbiol. 2001, 49, 37–84. [Google Scholar] [PubMed]
- Edwards, K.J.; Schrenk, M.O.; Hamers, R.; Banfield, J.F. Microbial oxidation of pyrite; experiments using microorganisms from an extreme acidic environment. Am. Mineral. 1998, 83, 1444–1453. [Google Scholar] [CrossRef]
- Edwards, K.J.; Gihring, T.M.; Banfield, J.F. Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl. Environ. Microbiol. 1999, 65, 3627–3632. [Google Scholar] [PubMed]
- Hallberg, K.B. New perspectives in acid mine drainage microbiology. Hydrometallurgy 2010, 104, 448–453. [Google Scholar] [CrossRef]
- Hug, L.A.; Baker, B.J.; Anantharaman, K.; Brown, C.T.; Probst, A.J.; Castelle, C.J.; Butterfield, C.N.; Hernsdorf, A.W.; Amano, Y.; Ise, K. A new view of the tree of life. Nat. Microbiol. 2016, 1, 16048. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Bohannan, B.J.M.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Volant, A.; Bruneel, O.; Desoeuvre, A.; Héry, M.; Casiot, C.; Bru, N.; Delpoux, S.; Fahy, A.; Javerliat, F.; Bouchez, O. Diversity and spatiotemporal dynamics of bacterial communities: Physicochemical and other drivers along an acid mine drainage. FEMS Microbiol. Ecol. 2014, 90, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.A.; Vlaar, S.; Nguyen, N.; Leppard, G.G.; Finan, T.M. Effects of bioavailable heavy metal species, arsenic, and acid drainage from mine tailings on a microbial community sampled along a pollution gradient in a freshwater ecosystem. Geomicrobiol. J. 2015, 32, 724–750. [Google Scholar] [CrossRef]
- Tan, G.L.; Shu, W.S.; Zhou, W.H.; Li, X.L.; Lan, C.Y.; Huang, L.N. Seasonal and spatial variations in microbial community structure and diversity in the acid stream draining across an ongoing surface mining site. FEMS Microbiol. Ecol. 2009, 70, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-P.; You, S.-J.; Pai, T.-Y.; Chen, K.-W. Effect of cadmium on composition and diversity of bacterial communities in activated sludges. Int. Biodeterior. Biodegrad. 2005, 55, 285–291. [Google Scholar] [CrossRef]
- Li, J.T.; Duan, H.N.; Li, S.P.; Kuang, J.L.; Zeng, Y.; Shu, W.S. Cadmium pollution triggers a positive biodiversity-productivity relationship: Evidence from a laboratory microcosm experiment. J. Appl. Ecol. 2010, 47, 890–898. [Google Scholar] [CrossRef]
- Miller, S.R.; Strong, A.L.; Jones, K.L.; Ungerer, M.C. Bar-coded pyrosequencing reveals shared bacterial community properties along the temperature gradients of two alkaline hot springs in yellowstone national park. Appl. Environ. Microbiol. 2009, 75, 4565–4572. [Google Scholar] [CrossRef] [PubMed]
- Zengler, K.; Palsson, B.O. A road map for the development of community systems (cosy) biology. Nat. Rev. Microbiol. 2012, 10, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Denef, V.J.; Mueller, R.S.; Banfield, J.F. Amd biofilms: Using model communities to study microbial evolution and ecological complexity in nature. ISME J. 2010, 4, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Huang, L.N.; Méndezgarcía, C.; Kuang, J.L.; Hua, Z.S.; Liu, J.; Shu, W.S. Microbial communities, processes and functions in acid mine drainage ecosystems. Curr. Opin. Biotechnol. 2016, 38, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dagmar Kock, A.S. Quantitative microbial community analysis of three different sulfidic mine tailing dumps generating acid mine drainage. Appl. Environ. Microbiol. 2008, 74, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.L.; Huang, L.N.; Chen, L.X.; Hua, Z.S.; Li, S.J.; Hu, M.; Li, J.T.; Shu, W.S. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013, 7, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hua, Z.S.; Chen, L.X.; Kuang, J.L.; Li, S.J.; Shu, W.S.; Huang, L.N. Correlating microbial diversity patterns with geochemistry in an extreme and heterogeneous environment of mine tailings. Appl. Environ. Microbiol. 2014, 80, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.L.; Shu, W.S.; Hallberg, K.B.; Li, F.; Lan, C.Y.; Huang, L.N. Cultivation-dependent and cultivation-independent characterization of the microbial community in acid mine drainage associated with acidic Pb/Zn mine tailings at Lechang, Guangdong, China. FEMS Microbiol. Ecol. 2007, 59, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Goltsman, D.S.A.; Comolli, L.R.; Thomas, B.C.; Banfield, J.F. Community transcriptomics reveals unexpected high microbial diversity in acidophilic biofilm communities. ISME J. 2015, 9, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.S.; Han, Y.J.; Chen, L.X.; Liu, J.; Hu, M.; Li, S.J.; Kuang, J.L.; Chain, P.S.; Huang, L.N.; Shu, W.S. Ecological roles of dominant and rare prokaryotes in acid mine drainage revealed by metagenomics and metatranscriptomics. ISME J. 2015, 9, 1280–1294. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Huang, L.; He, Z.; Chen, L.; Hua, Z.; Jia, P.; Li, S.; Liu, J.; Li, J.; Zhou, J. Predicting taxonomic and functional structure of microbial communities in acid mine drainage. ISME J. 2016, 10, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, K.; Kutschke, S.; Matys, S.; Kostudis, S.; Hopfe, S.; Raff, J. Novel biotechnological approaches for the recovery of metals from primary and secondary resources. Minerals 2016, 6, 54. [Google Scholar] [CrossRef]
- Johnson, D. Recent developments in microbiological approaches for securing mine wastes and for recovering metals from mine waters. Minerals 2014, 4, 279–292. [Google Scholar] [CrossRef]
- Molson, J.; Fala, O.; Aubertin, M.; Bussière, B. Numerical simulations of pyrite oxidation and acid mine drainage in unsaturated waste rock piles. J. Contam. Hydrol. 2005, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [PubMed]
- Marchand, E.A.; Silverstein, J. Influence of heterotrophic microbial growth on biological oxidation of pyrite. Environ. Sci. Technol. 2002, 36, 5483–5490. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, V.P.; Zhang, Y.L. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Méndez-García, C.; Peláez, A.I.; Mesa, V.; Sánchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar] [PubMed]
- Zirnstein, I.; Arnold, T.; Krawczykbärsch, E.; Jenk, U.; Bernhard, G.; Röske, I. Eukaryotic life in biofilms formed in a uranium mine. Microbiologyopen 2012, 1, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Falagan, C.; Johnson, D.B. Acidithiobacillus ferriphilus sp. nov.: A facultatively anaerobic iron- and sulfur-metabolising extreme acidophile. Int. J. Syst. Evol. Microbiol. 2015, 66, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.W.; Lo, I.; Baker, B.J.; Allen, E.E.; Hugenholtz, P.; Banfield, J.F. Genome-directed isolation of the key nitrogen fixer leptospirillum ferrodiazotrophum sp. nov. from an acidophilic microbial community. Appl. Environ. Microbiol. 2005, 71, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Bond, P.L.; Gihring, T.M.; Banfield, J.F. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 2000, 287, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.V. Environmental, biogeographic, and biochemical patterns of archaea of the family ferroplasmaceae. Appl. Environ. Microbiol. 2011, 77, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Craig, B.A.; Andrew, H.; Bowman, J.P.; Bond, P.L. Characterization of ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 2004, 70, 2079–2088. [Google Scholar]
- Welch, D.B.M.; Huse, S.M. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar]
- Hallberg, K.B.; Coupland, K.; Kimura, S.; Johnson, D.B. Macroscopic streamer growths in acidic, metal-rich mine waters in north wales consist of novel and remarkably simple bacterial communities. Appl. Environ. Microbiol. 2006, 72, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.L.; Shu, W.S.; Hallberg, K.B.; Li, F.; Lan, C.Y.; Zhou, W.H.; Huang, L.N. Culturable and molecular phylogenetic diversity of microorganisms in an open-dumped, extremely acidic Pb/Zn mine tailings. Extremophiles 2008, 12, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Küsel, K. Microbial cycling of iron and sulfur in acidic coal mining lake sediments. Water Air Soil Pollut. 2003, 3, 67–90. [Google Scholar] [CrossRef]
- Chen, Y.T.; Li, J.T.; Chen, L.X.; Hua, Z.S.; Huang, L.N.; Liu, J.; Xu, B.B.; Liao, B.; Shu, W.S. Biogeochemical processes governing natural pyrite oxidation and release of acid metalliferous drainage. Environ. Sci. Technol. 2014, 48, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Neilson, J.W.; Maier, R.M. Characterization of a bacterial community in an abandoned semiarid lead-zinc mine tailing site. Appl. Environ. Microbiol. 2008, 74, 3899–3907. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.N.; Zhou, W.H.; Hallberg, K.B.; Wan, C.Y.; Li, J.; Shu, W.S. Spatial and temporal analysis of the microbial community in the tailings of a Pb-Zn mine generating acidic drainage. Appl. Environ. Microbiol. 2011, 77, 5540–5544. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Li, J.T.; Chen, Y.T.; Huang, L.N.; Hua, Z.S.; Hu, M.; Shu, W.S. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ. Microbiol. 2013, 15, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Liljeqvist, M.; Ossandon, F.J.; Carolina, G.; Rajan, S.; Stell, A.; Valdes, J.; Holmes, D.S.; Dopson, M. Metagenomic analysis reveals adaptations to a cold-adapted lifestyle in a low-temperature acid mine drainage stream. FEMS Microbiol. Ecol. 2015, 91, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Hu, M.; Huang, L.N.; Hua, Z.S.; Kuang, J.L.; Li, S.J.; Shu, W.S. Comparative metagenomic and metatranscriptomic analyses of microbial communities in acid mine drainage. ISME J. 2015, 9, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Korehi, H.; Blöthe, M.; Schippers, A. Microbial diversity at the moderate acidic stage in three different sulfidic mine tailings dumps generating acid mine drainage. Res. Microbiol. 2014, 165, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L. Experimental analysis of intermediate disturbance and initial floristic composition: Decoupling cause and effect. Ecology 1995, 76, 486–492. [Google Scholar] [CrossRef]
- Howard, L.F.; Lee, T.D. Temporal patterns of vascular plant diversity in southeastern new hampshire forests. For. Ecol. Manag. 2003, 185, 5–20. [Google Scholar] [CrossRef]
- Jones, D.S.; Schaperdoth, I.; Macalady, J.L. Biogeography of sulfur-oxidizing acidithiobacillus populations in extremely acidic cave biofilms. ISME J. 2016, 10, 2879–2891. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Garcíamoyano, A.; Gonzáleztoril, E.; Aguilera, Á.; Amils, R. Comparative microbial ecology study of the sediments and the water column of the Rio Tinto, an extreme acidic environment. FEMS Microbiol. Ecol. 2012, 81, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gonzáleztoril, E.; Aguilera, Á.; Souzaegipsy, V.; Pamo, E.L.; España, J.S.; Amils, R. Geomicrobiology of La Zarza-Perrunal acid mine effluent (Iberian Pyritic Belt, Spain). Appl. Environ. Microbiol. 2011, 77, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.I.; Sun, Q.Y. Archaeal and bacterial communities in acid mine drainage from metal-rich abandoned tailing ponds, Tongling, China. Trans. Nonferr. Met. Soc. China 2014, 24, 3332–3342. [Google Scholar] [CrossRef]
- Li, S.P.; Li, J.T.; Kuang, J.L.; Duan, H.N.; Zeng, Y.; Shu, W.S. Effects of species richness on cadmium removal efficiencies of algal microcosms. J. Appl. Ecol. 2012, 49, 261–267. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Comolli, L.R.; Banfield, J.F. Inter-species interconnections in acid mine drainage microbial communities. Front. Microbiol. 2014, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- González-Toril, E.; Aguilera, A.; Rodriguez, N.; Fernández-Remolar, D.; Gómez, F.; Diaz, E.; García-Moyano, A.; Sanz, J.L.; Amils, R. Microbial ecology of Río Tinto, a natural extreme acidic environment of biohydrometallurgical interest. Hydrometallurgy 2010, 104, 329–333. [Google Scholar] [CrossRef]
- Larsen, P.E.; Field, D.; Gilbert, J.A. Predicting bacterial community assemblages using an artificial neural network approach. Nat. Methods 2012, 1260, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Bohannan, B.J.; Whitaker, R.J. Microbial biogeography: From taxonomy to traits. Science 2008, 320, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Mcgill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Response to kearney and porter: Both functional and community ecologists need to do more for each other. Trends Ecol. Evol. 2006, 21, 482–483. [Google Scholar] [CrossRef]
- Raes, J.; Letunic, I.; Yamada, T.; Jensen, L.J.; Bork, P. Toward molecular trait-based ecology through integration of biogeochemical, geographical and metagenomic data. Mol. Syst. Biol. 2011, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Nancucheo, I.; Johnson, D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. 2011, 5, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Lutz, M.A.; Dawson, S.C.; Bond, P.L.; Banfield, J.F. Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl. Environ. Microbiol. 2004, 70, 6264–6271. [Google Scholar] [CrossRef] [PubMed]
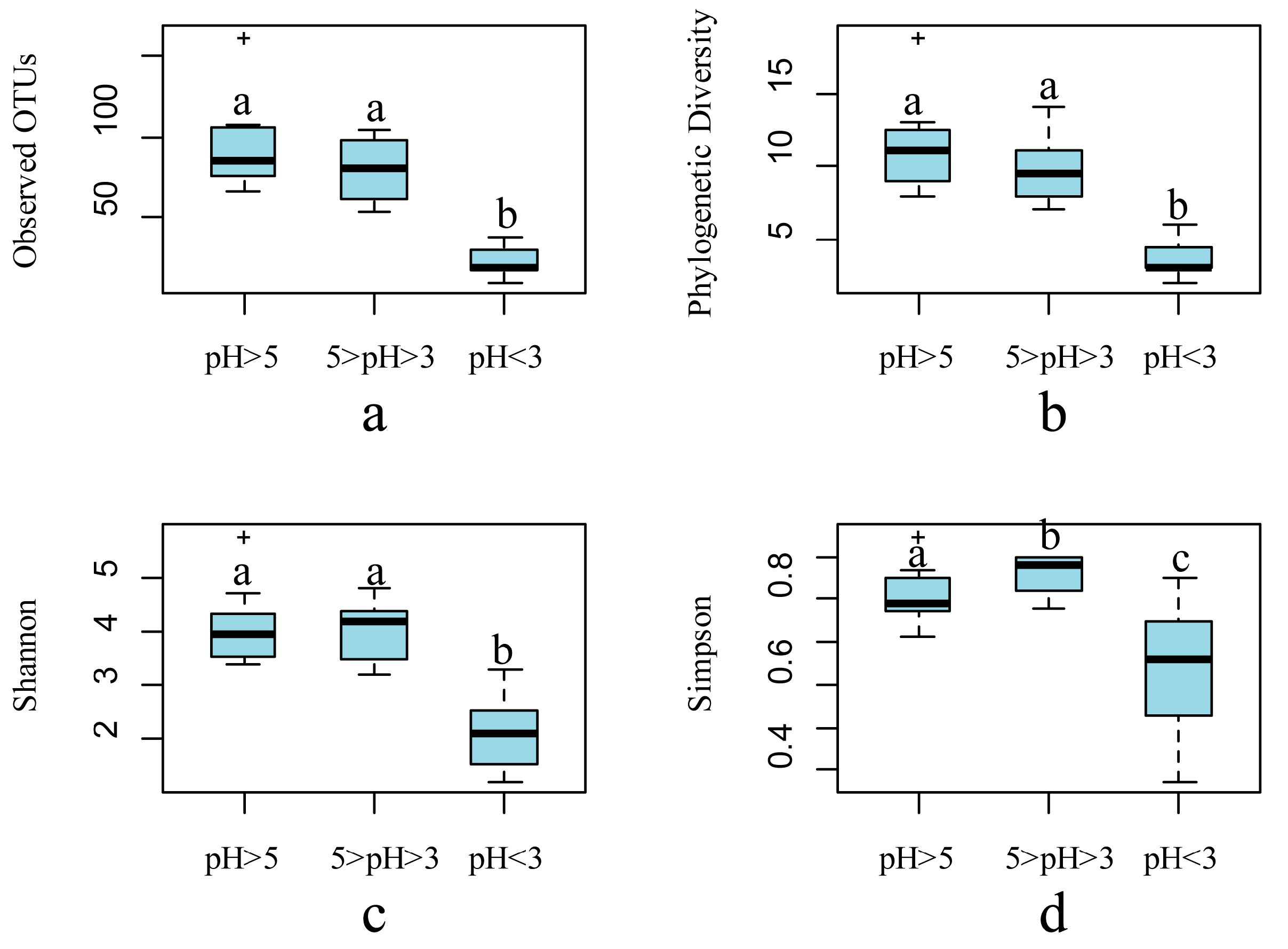

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.; Kuang, J.; Luo, Z.; Shu, W. Microbial Diversity and Community Assembly across Environmental Gradients in Acid Mine Drainage. Minerals 2017, 7, 106. https://doi.org/10.3390/min7060106
Teng W, Kuang J, Luo Z, Shu W. Microbial Diversity and Community Assembly across Environmental Gradients in Acid Mine Drainage. Minerals. 2017; 7(6):106. https://doi.org/10.3390/min7060106
Chicago/Turabian StyleTeng, Wenkai, Jialiang Kuang, Zhenhao Luo, and Wensheng Shu. 2017. "Microbial Diversity and Community Assembly across Environmental Gradients in Acid Mine Drainage" Minerals 7, no. 6: 106. https://doi.org/10.3390/min7060106
APA StyleTeng, W., Kuang, J., Luo, Z., & Shu, W. (2017). Microbial Diversity and Community Assembly across Environmental Gradients in Acid Mine Drainage. Minerals, 7(6), 106. https://doi.org/10.3390/min7060106



