Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Extraction of Cu(II) and Fe(III)
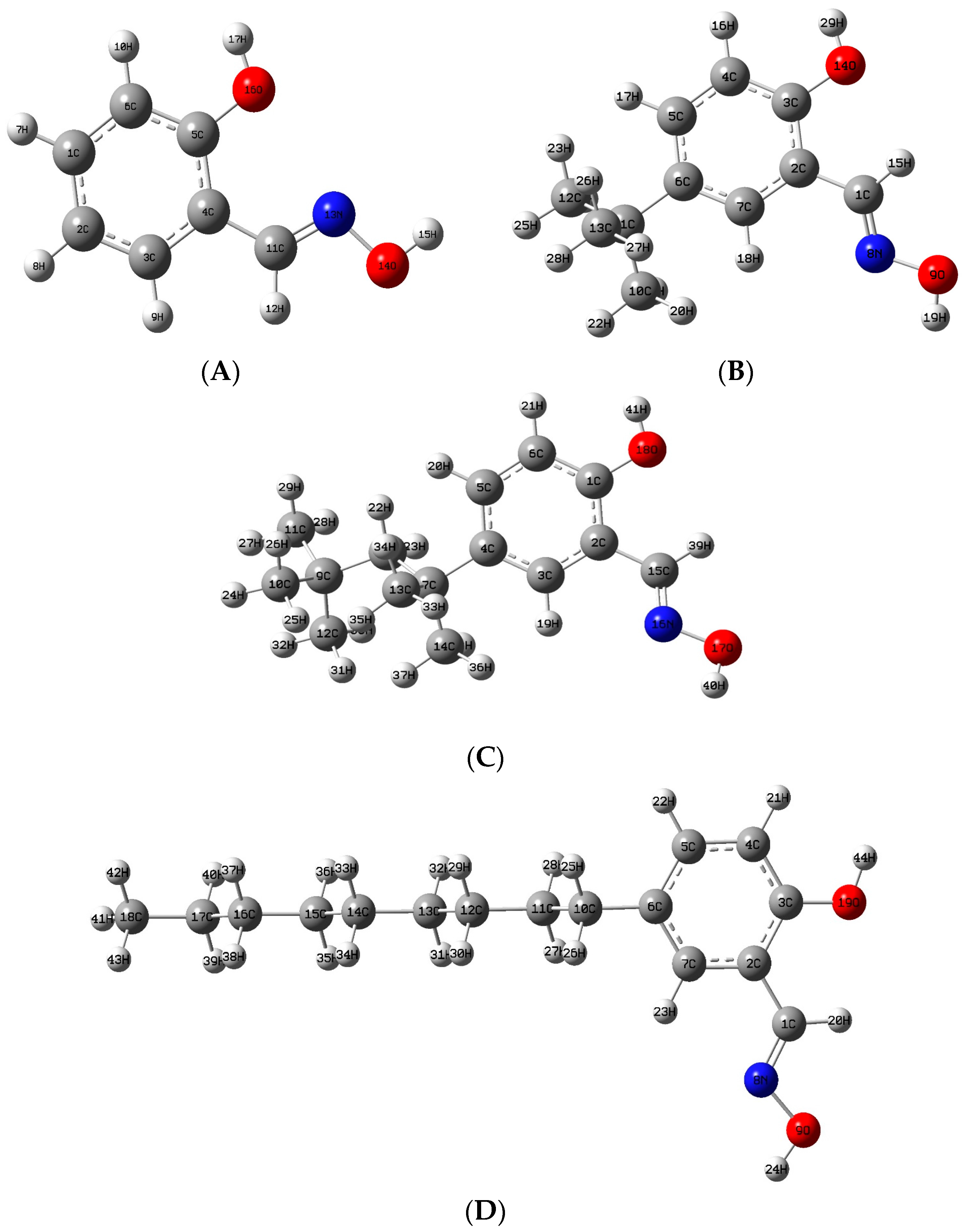
2.2.2. The Structure Properties of Reagents
3. Results and Discussion
3.1. The Structures of the Extractants
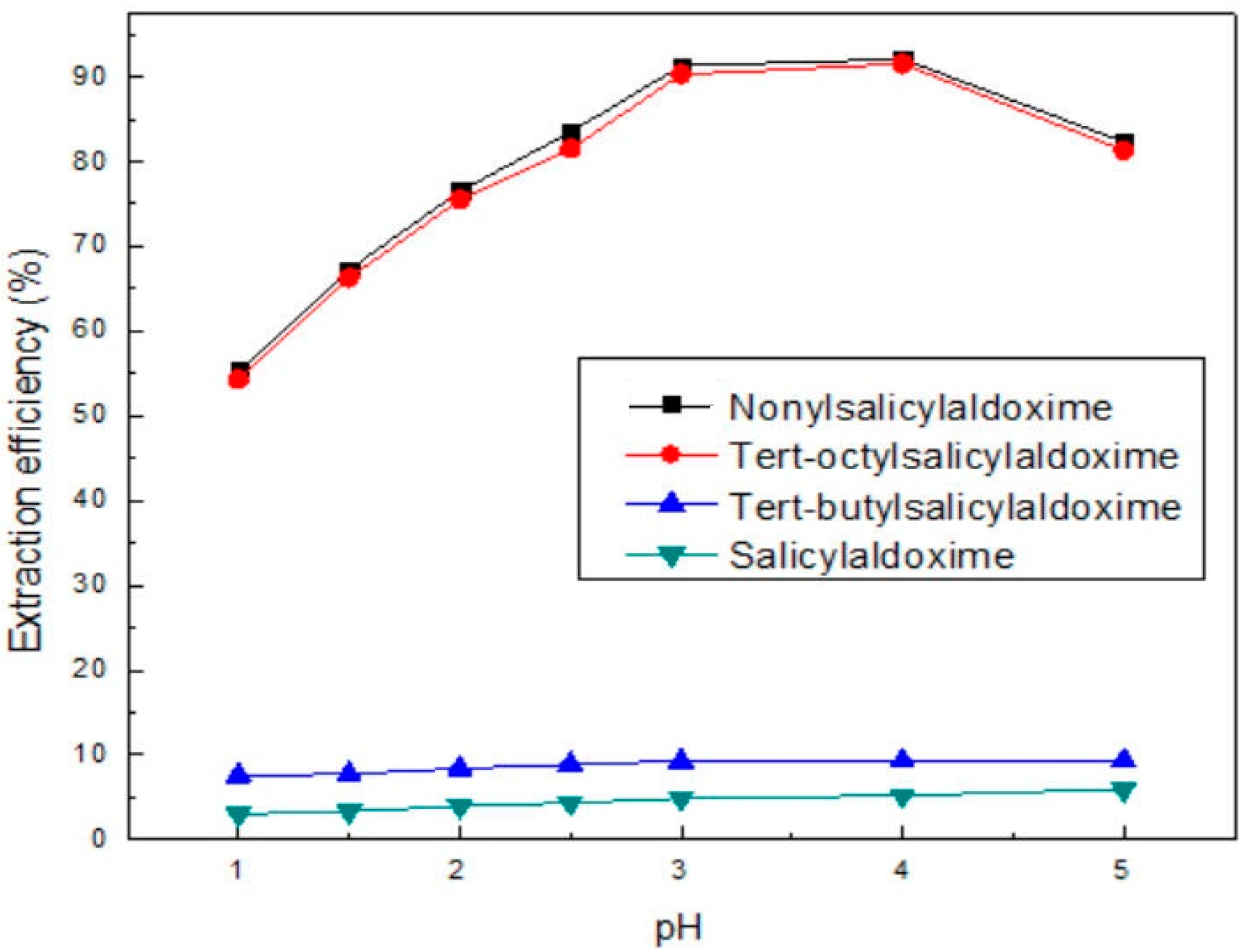
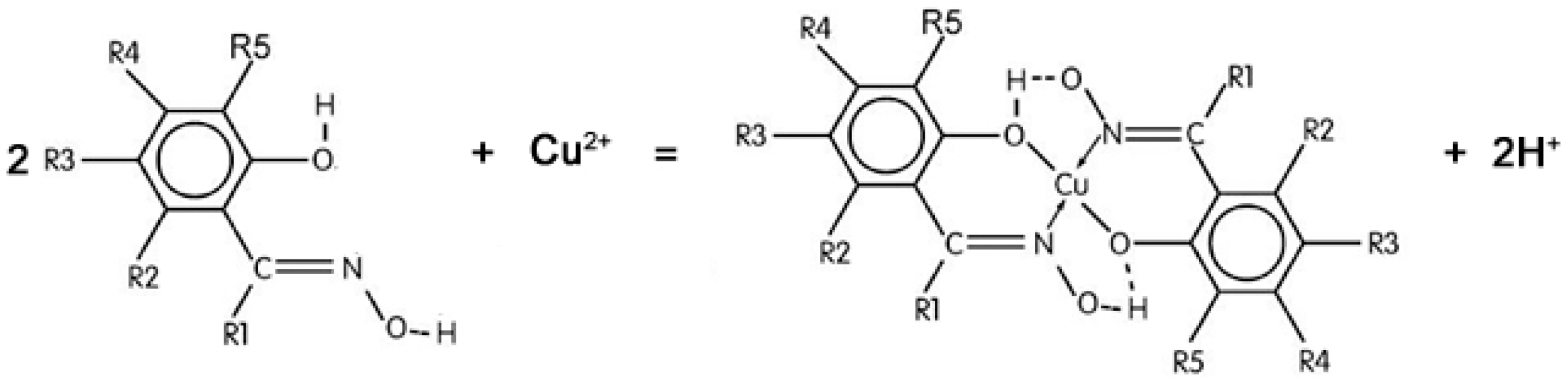
3.2. The Extraction of Cu(II)
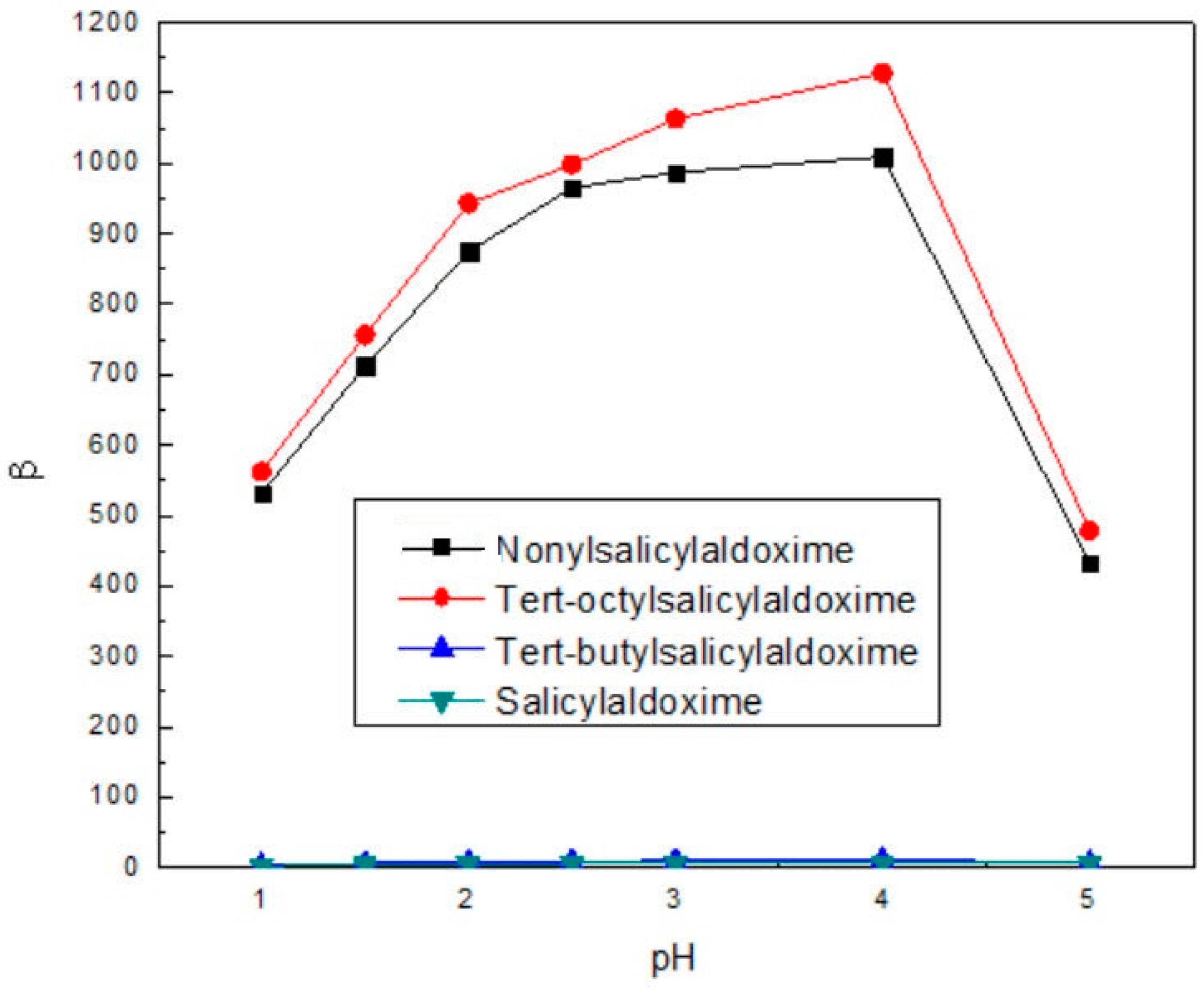
3.3. Separation of Cu(II) and Fe(III)
3.4. Hydrophobicity (Log P) and Molecular Sizes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naderi, H.; Abdollahy, M.; Mostoufi, N.; Koleini, M.J.; Shojaosadati, S.A.; Manafi, Z. Kinetics of chemical leaching of chalcopyrite from low grade copper ore: Behavior of different size fractions. Int. J. Miner. Metall. Mater. 2011, 18, 638–645. [Google Scholar] [CrossRef]
- Ramanathan, T.; Ting, Y.P. Selective copper bioleaching by pure and mixed cultures of alkaliphilic bacteria isolated from a fly ash landfill site. Water Air Soil Pollut. 2015, 226, 374–387. [Google Scholar] [CrossRef]
- Watling, H.R.; Collinson, D.M.; Li, J.; Mutch, L.A.; Perrot, F.A.; Rea, S.M.; Reith, F.; Watkin, E.L.J. Bioleaching of a low-grade copper ore, linking leach chemistry and microbiology. Miner. Eng. 2014, 56, 35–44. [Google Scholar] [CrossRef]
- Naderi, H.; Abdollahy, M.; Mostoufi, N. Kinetics of chemical leaching of chalcocite from low-grade copper ore: size-distribution behavior. J. Min. Environ. 2015, 6, 109–118. [Google Scholar]
- Rotuska, K.; Chmielewski, T. Growing role of solvent extraction in copper ores processing. Physicochem. Probl. Miner. Process. 2008, 42, 29–36. [Google Scholar]
- Jain, V.; Pradip; Rai, B. Density functional theory computations for design of salicylaldoxime derivatives as selective reagents in solvent extraction of copper. Trans. Indian Inst. Met. 2016, 69, 135–141. [Google Scholar] [CrossRef]
- Pouramini, Z.; Moradi, A. Characterization of 5-nonylsalicylaldoxime production and the effects of modifiers on its extracting/stripping properties. Res. Chem. Intermed. 2012, 38, 2401–2409. [Google Scholar] [CrossRef]
- Gameiro, M.L.; Machado, R.M.; Ismael, M.R.; Reis, M.T.; Carvalho, J.M. Copper extraction from ammoniacal medium in a pulsed sieve-plate column with LIX 84-I. J. Hazard. Mater. 2010, 183, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Sengupta, R.; Subrahmanyam, N. Copper extraction into emulsion liquid membranes using LIX 984N-C®. Hydrometallurgy 2006, 81, 67–73. [Google Scholar] [CrossRef]
- Tyman, J.H.; Iddenten, S.J. The synthesis of oxime reagents from natural and semi-synthetic phenolic lipid and alkanoic acid resources for the solvent recovery of copper(II). J. Chem. Technol. Biotechnol. 2005, 80, 1319–1328. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrera-Urbina, R.; McGlashan, D.W. Studies on the applicability of chelating agents as universal collectors for copper minerals. Int. J. Miner. Process. 2000, 58, 15–33. [Google Scholar] [CrossRef]
- Reddy, B.R.; Priya, N.D.; Park, K.H. Separation and recovery of cadmium(II), cobalt(II) and nickel(II) from sulphate leach liquors of spent Ni–Cd batteries using phosphorus based extractants. Sep. Purif. Technol. 2005, 45, 161–166. [Google Scholar] [CrossRef]
- Kul, M.; Çetinkaya, Ü. Recovery of copper by LIX 984N-C from electroplating rinse bath solution. Hydrometallurgy 2009, 98, 86–91. [Google Scholar] [CrossRef]
- Reddy, B.R.; Park, K.H.; Mohapatra, D. Process development for the separation and recovery of copper from sulphate leach liquors of synthetic Cu–Ni–Co–Fe matte using LIX 84 and LIX 973N. Hydrometallurgy 2007, 87, 51–57. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D. Recovery of copper cyanide from waste cyanide solution by LIX 7950. Miner. Eng. 2009, 22, 190–195. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Agarwal, S.; Machado, R.M.; Gameiro, M.L.F.; Santos, S.M.C.; Reis, M.T.A.; Ismael, M.R.C.; Correia, M.J.N.; Carvalho, J.M.R. Extraction of copper from acidic leach solution with Acorga M5640 using a pulsed sieve plate column. Hydrometallurgy 2010, 104, 66–75. [Google Scholar] [CrossRef]
- Li, L.; Liao, C.; Tang, Y.; Yang, L. A Tertiary Butyl Salicylaldoxime and Its Synthesis Method. Patent ZL201410444168.1, 3 September 2014. [Google Scholar]
- Huang, Z.; Zhong, H.; Wang, S.; Xia, L.; Zhao, G.; Liu, G. Gemini trisiloxane surfactant: Synthesis and flotation of aluminosilicate minerals. Miner. Eng. 2014, 56, 145–154. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Landowska, L.; Konefał-Góral, J. Improvement of copper FAAS determination conditions via preconcentration procedure with the use of salicylaldoxime complex trapped in polymer matrix. Talanta 2012, 96, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Effect of pH, concentration and temperature on copper and zinc hydroxide formation/precipitation in solution. In Proceedings of the Chemeca 2011—Engineering a Better World, Sydney, Australia, 18–21 September 2011; pp. 2100–2110. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, Q.; Hu, H.; Niu, C.; Zhu, Q. Solvent extraction of copper from ammoniacal chloride solutions by sterically hindered β-diketone extractants. Sep. Purif. Technol. 2011, 80, 52–58. [Google Scholar] [CrossRef]
- Cierpiszewski, R.; Hebrant, M.; Szymanowski, J.; Tondre, C. Copper(II) complexation kinetics with hydroxyoximes in CTAB micelles. Effect of extractant hydrophobicity and additives. J. Chem. Soc. Faraday Trans. 1996, 92, 249–255. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. Influence of the steric hindrance, ligand hydrophobicity, and DN of solvents on structure and extraction of Cu(II) complexes of 1-alkyl-2-ethylimidazoles. Sep. Sci. Technol. 2008, 43, 794–814. [Google Scholar] [CrossRef]
| Items | Log P | Molecular Sizes (nm3) |
|---|---|---|
| Salicylaldoxime | 1.78 | 100.15 |
| tert-Butylsalicylaldoxime | 3.48 | 170.50 |
| tert-Octylsalicylaldoxime | 5.12 | 241.27 |
| Nonylsalicylaldoxime | 5.61 | 254.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, Y.; An, W.; Bao, S. Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II). Minerals 2017, 7, 61. https://doi.org/10.3390/min7040061
Li L, Wang Y, An W, Bao S. Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II). Minerals. 2017; 7(4):61. https://doi.org/10.3390/min7040061
Chicago/Turabian StyleLi, Liqing, Yi Wang, Wenjuan An, and Shenxu Bao. 2017. "Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II)" Minerals 7, no. 4: 61. https://doi.org/10.3390/min7040061
APA StyleLi, L., Wang, Y., An, W., & Bao, S. (2017). Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II). Minerals, 7(4), 61. https://doi.org/10.3390/min7040061






