Interlayer Structures and Dynamics of Arsenate and Arsenite Intercalated Layered Double Hydroxides: A First Principles Study
Abstract
:1. Introduction
2. Methods
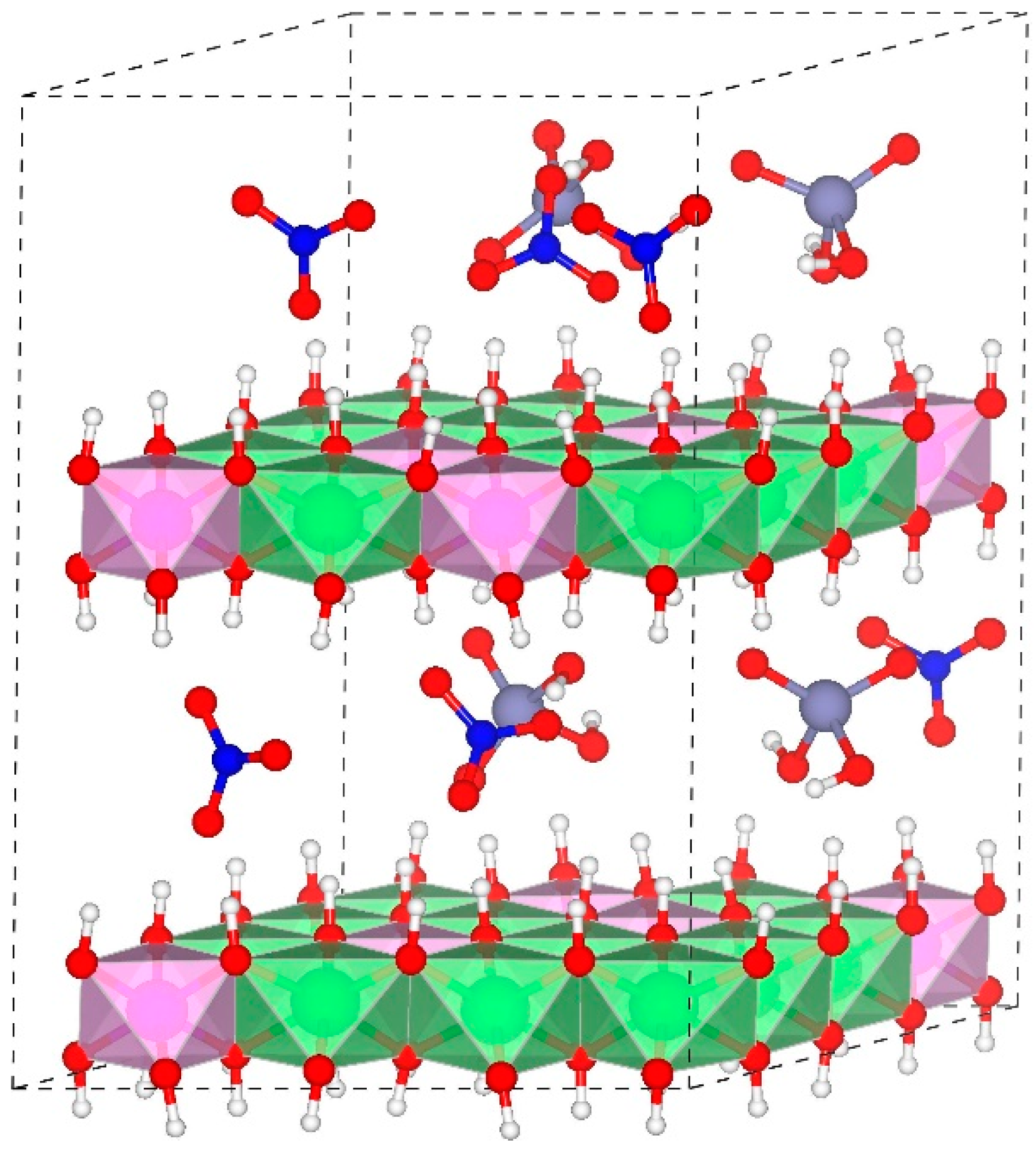
2.1. Models
2.2. Simulation Details
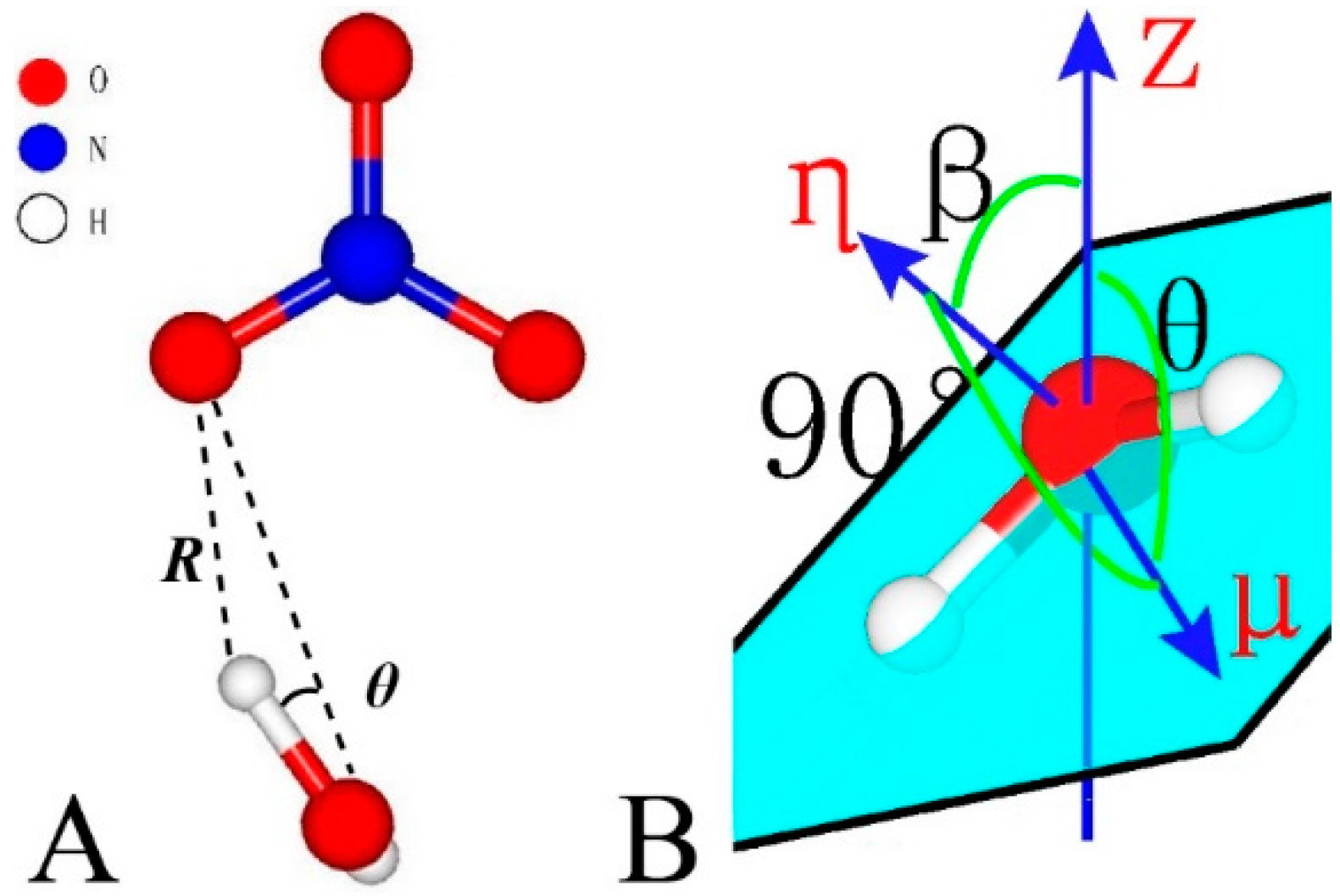
2.3. Simulation Analyses
3. Results and Discussion
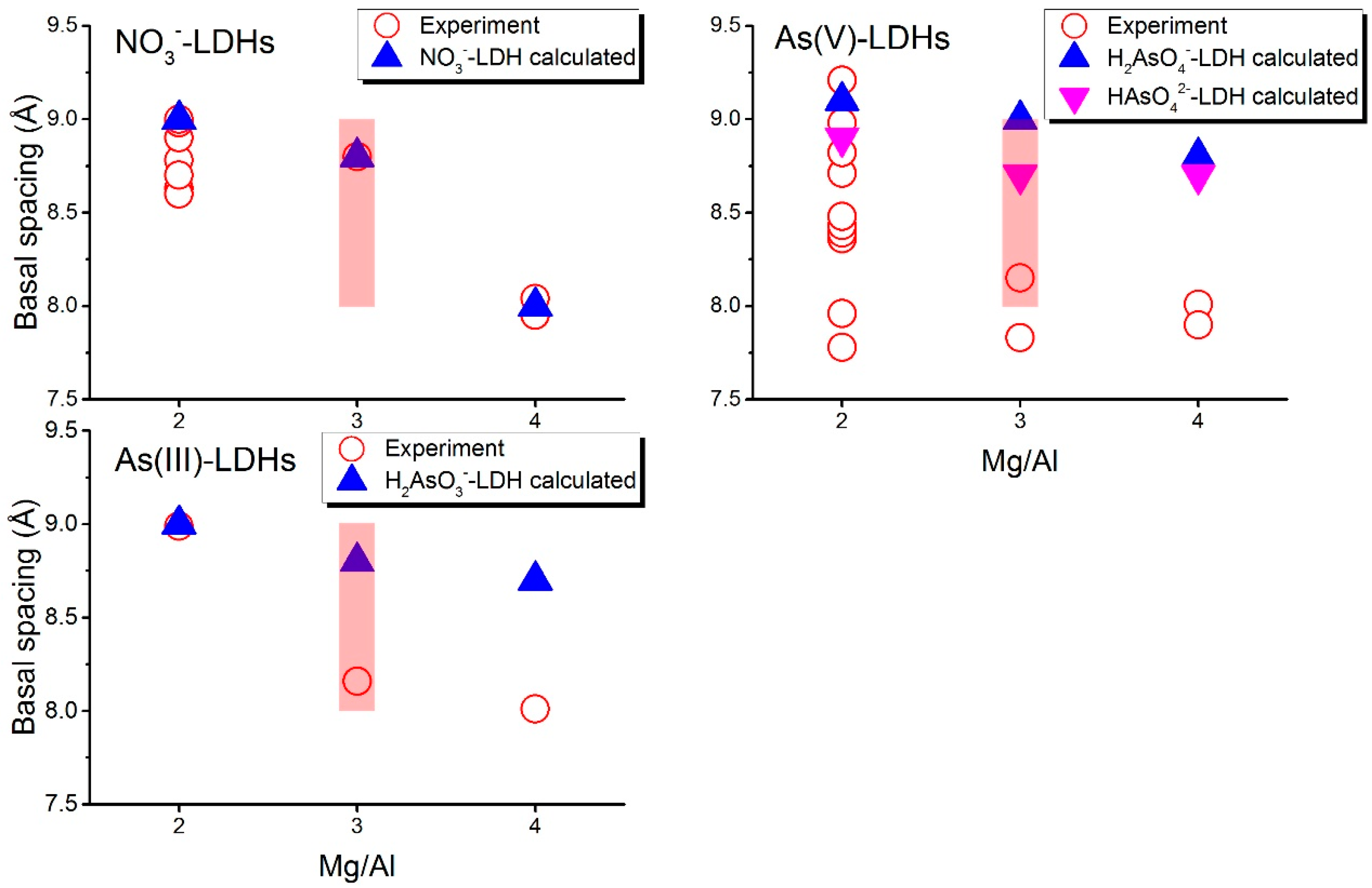
3.1. Basal Spacing
3.2. Interlayer Structures
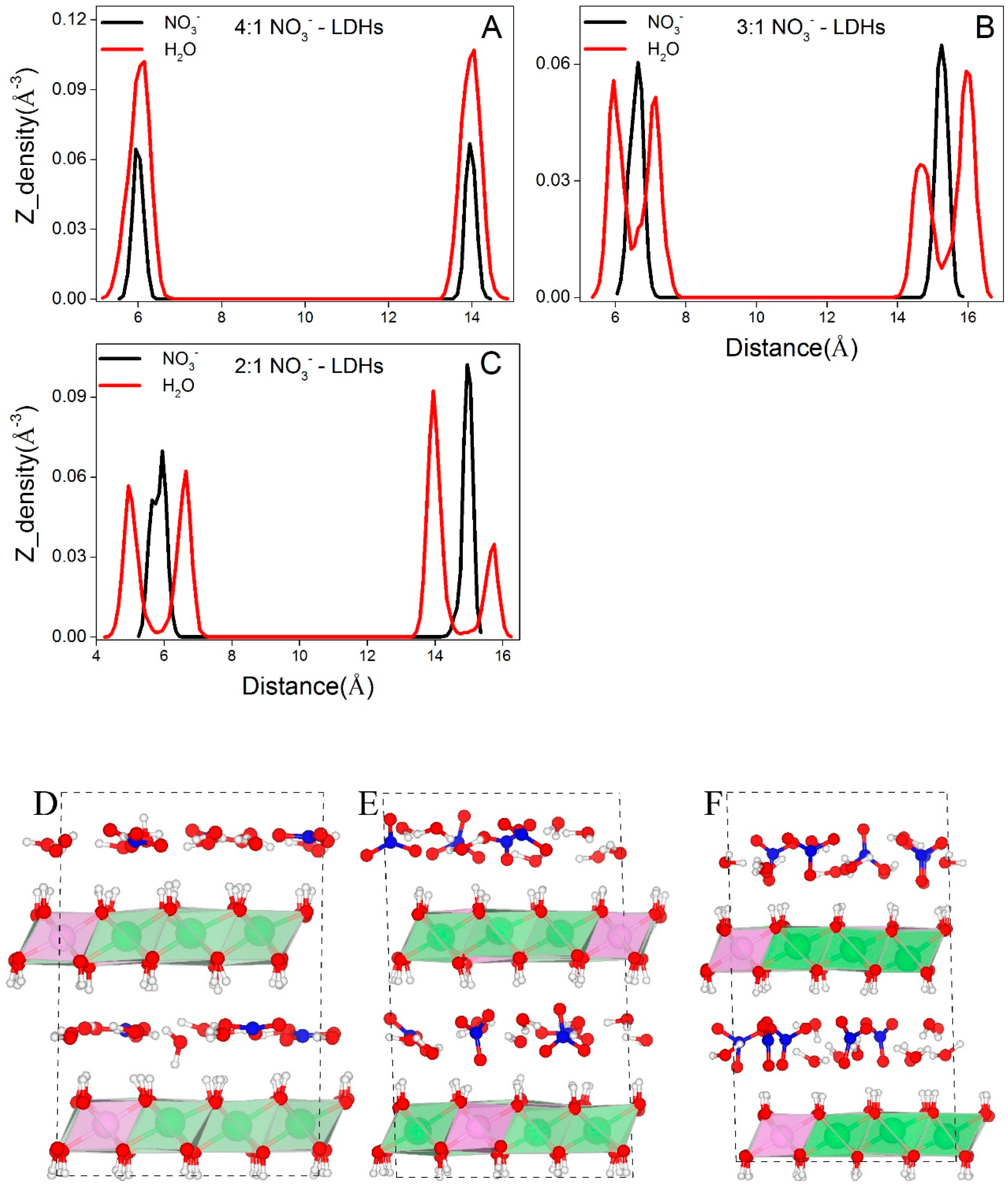
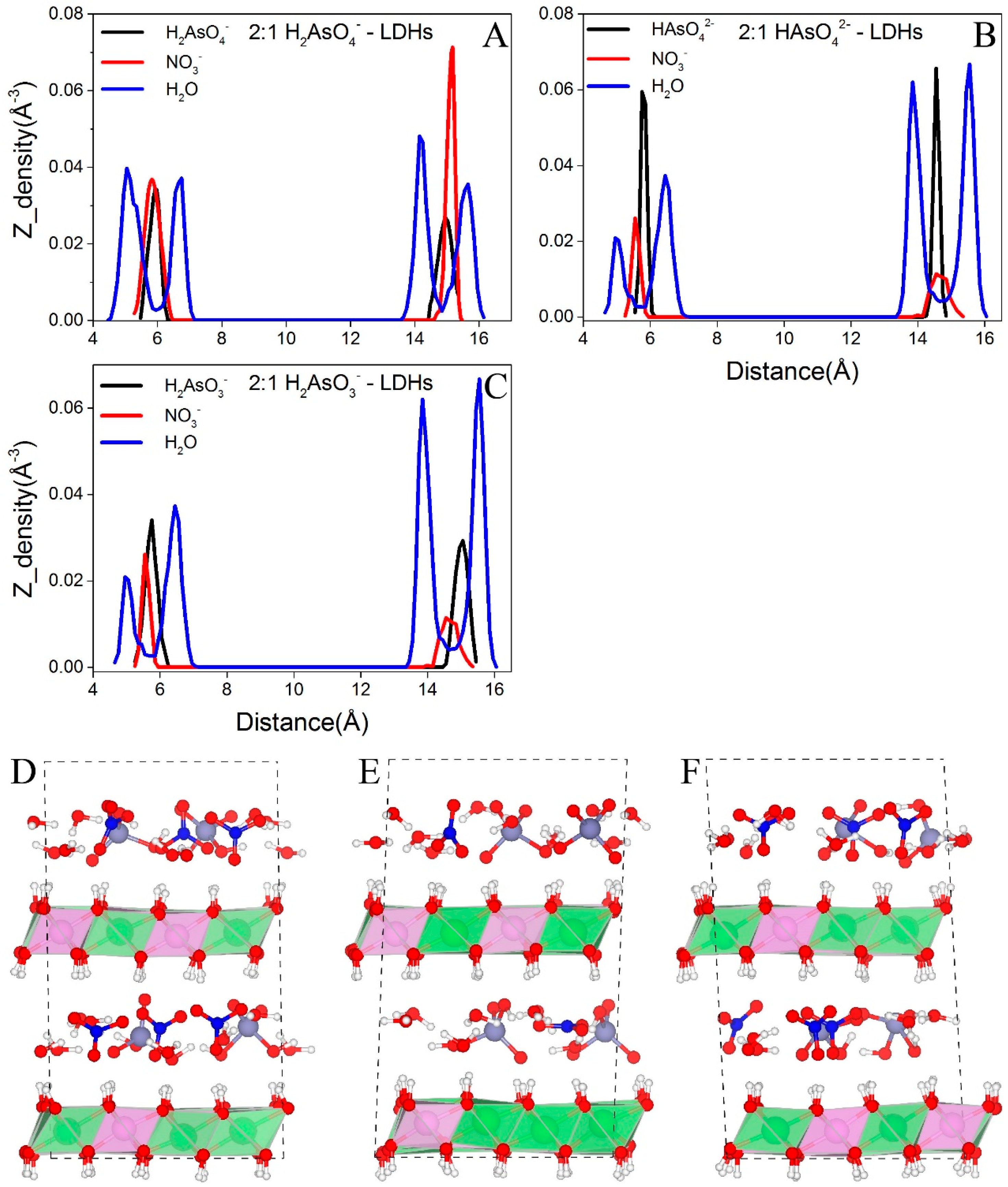
3.2.1. Distributions of Interlayer Species
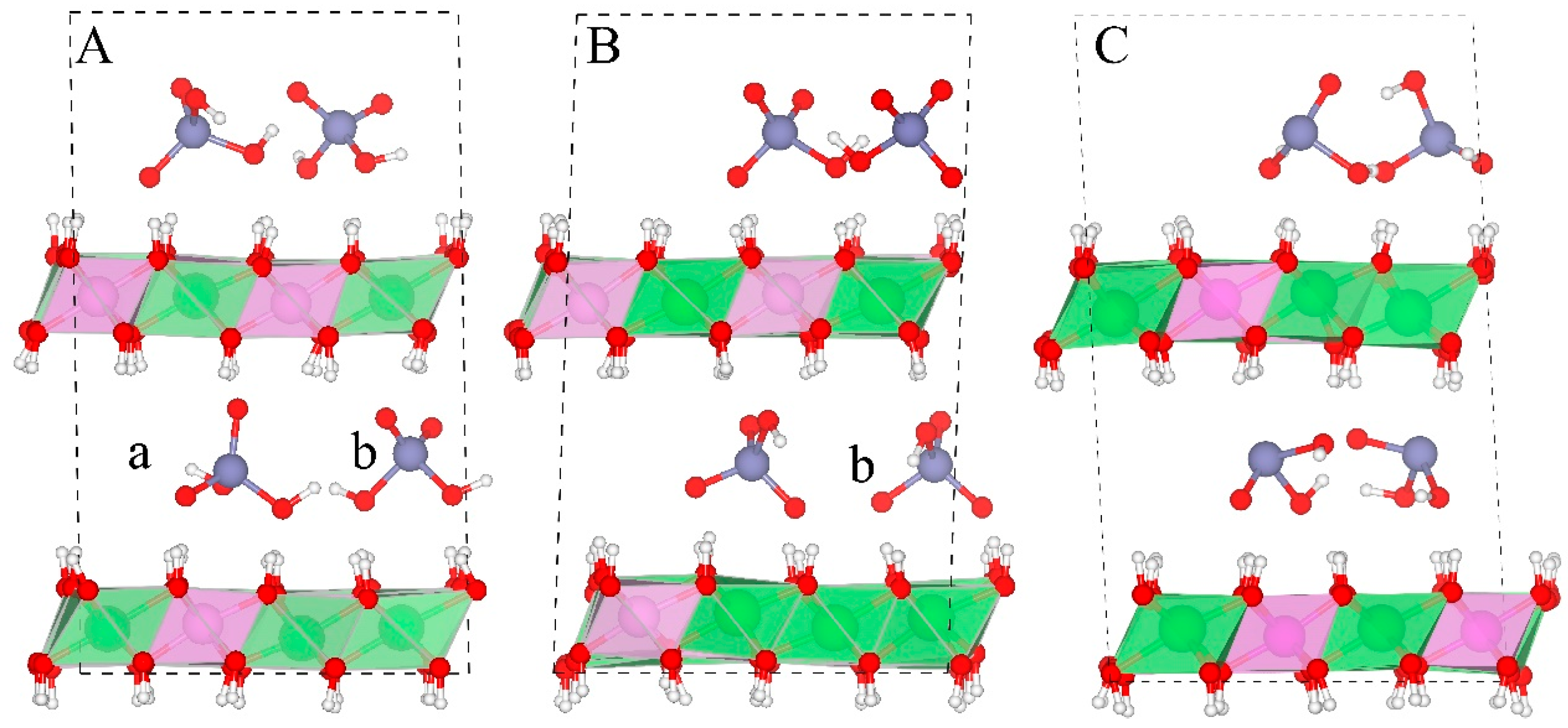
3.2.2. Orientations of Interlayer Species
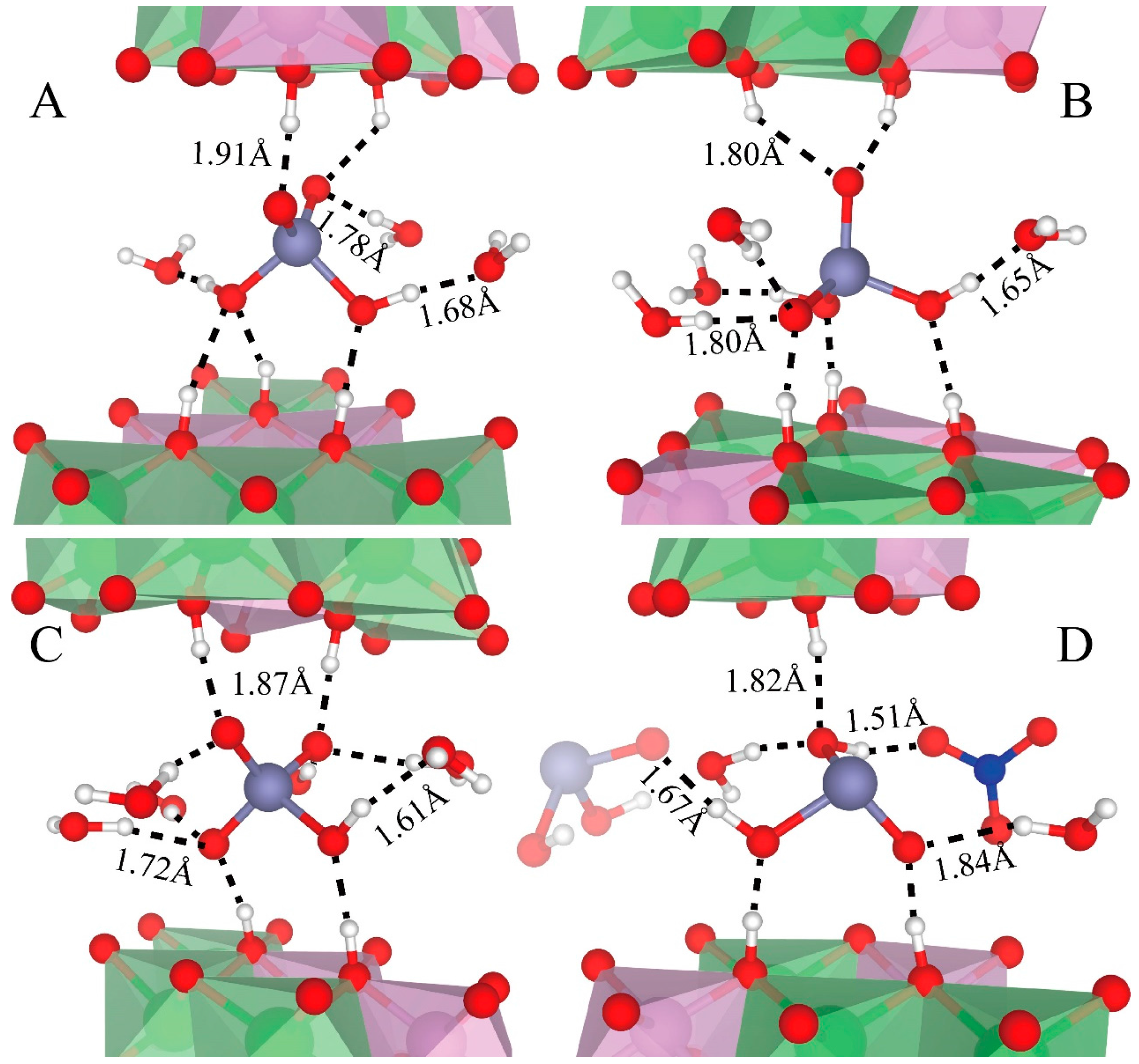
3.3. Hydrogen Bonding of As Species
3.4. Mobility of Interlayer Species
4. Conclusions
- Comparisons between computed and experimental basal spacings indicate that arsenite and arsenate can enter the gallery spaces of LDHs with a Mg/Al ratio of 2:1, but may not enter the gallery spaces of LDHs with Mg/Al of 3:1 or 4:1.
- All interlayer species show layering distributions. As species and NO3− form single layer distributions while water molecules form single layer distributions at low layer charge densities and double layer distributions at high layer charge densities.
- H2AsO4− has two orientations in the interlayer regions, i.e., one with its three fold axis normal to the layer sheets and another with its two fold axis normal to the layer sheets, while only the latter one is observed for HAsO42−. H2AsO3− orientates in a tilt-lying way.
- It is found that the mobility of water and NO3− increases with the layer charge densities. All As species show very low mobility (of the order 10−13 m2/s) in LDHs.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2001, 17, 517–568. [Google Scholar] [CrossRef]
- Frankenberger, W.T., Jr. Environmental Chemistry of Arsenic; CRC Press: Baco Raton, FL, USA, 2001. [Google Scholar]
- Andreae, M.O. Distribution and speciation of arsenic in natural waters and some marine algae. Deep Sea Res. Part II Top. Stud. Oceanogr. 1978, 25, 391–402. [Google Scholar] [CrossRef]
- Andreae, M.O.; Andreae, T.W. Dissolved arsenic species in the schelde estuary and watershed, Belgium. Estuar. Coast. Shelf Sci. 1989, 29, 421–433. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G.; Huq, I.; Luo, Z.; Nicolli, H.B. International perspective on naturally occurring arsenic problems in groundwater. In Arsenic Exposure and Health Effects IV; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9–25. [Google Scholar]
- Smedley, P.L.; Zhang, M.; Zhang, G.; Luo, Z. Arsenic and Other Redox-Sensitive Elements in Groundwater from the Huhhot Basin, Inner Mongolia; A A Balkema Publishers: Leiden, IL, USA, 2001; pp. 581–584. [Google Scholar]
- Wang, S.L.; Cheng, H.L.; Ming, K.W.; Chuang, Y.H.; Chiang, P.N. Arsenate adsorption by Mg/Al–NO3 layered double hydroxides with varying the Mg/Al ratio. Appl. Clay Sci. 2009, 43, 79–85. [Google Scholar] [CrossRef]
- Goldberg, S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 2002, 66, 413–421. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M. Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci. Soc. Am. J. 2002, 66, 1788–1796. [Google Scholar] [CrossRef]
- Dousova, B.; Lhotka, M.; Grygar, T.; Machovic, V.; Herzogova, L. In situ co-adsorption of arsenic and iron/manganese ions on raw clays. Appl. Clay Sci. 2011, 54, 166–171. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Jung, W.; Halajnia, A.; Lakzian, A.; Kabra, A.N.; Jeon, B.H. Removal of arsenate and arsenite from aqueous solution by adsorption on clay minerals. Geosyst. Eng. 2015, 18, 302–311. [Google Scholar] [CrossRef]
- Na, P.; Jia, X.M.; Bin, Y.; Li, Y.; Na, J.; Chen, Y.C.; Wang, L.S. Arsenic adsorption on Ti-pillared montmorillonite. J. Chem. Technol. Biotechnol. 2010, 85, 708–714. [Google Scholar] [CrossRef]
- Lin, Z.; Puls, R.W. Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ. Geol. 2000, 39, 753–759. [Google Scholar] [CrossRef]
- Saada, A.; Breeze, D.; Crouzet, C.; Cornu, S.; Baranger, P. Adsorption of arsenic (V) on kaolinite and on kaolinite-humic acid complexes—Role of humic acid nitrogen groups. Chemosphere 2003, 51, 757–763. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; van Renterghem, W.; van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Wainipee, W.; Cuadros, J.; Sephton, M.A.; Unsworth, C.; Gill, M.G.; Strekopytov, S.; Weiss, D.J. The effects of oil on As(V) adsorption on illite, kaolinite, montmorillonite and chlorite. Geochim. Cosmochim. Acta 2013, 121, 487–502. [Google Scholar] [CrossRef]
- Manning, B.; Fendorf, S.; Goldberg, S. Surface structures and stability of arsenic(III) on goethite: Spectroscopic evidence for inner-sphere complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Farquhar, M.L.; Charnock, J.M.; Livens, F.R.; Vaughan, D.J. Mechanisms of arsenic uptake from aqueous solution by interaction with goethite, lepidocrocite, mackinawite, and pyrite: An X-ray absorption spectroscopy study. Environ. Sci. Technol. 2002, 36, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.M.; Randall, S.R. Surface complexation of arsenic(V) to iron(III) (hydr)oxides: Structural mechanism from Ab initio molecular geometries and exafs spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 4223–4230. [Google Scholar] [CrossRef]
- Tu, Y.J.; You, C.F.; Chang, C.K.; Wang, S.L. Xanes evidence of arsenate removal from water with magnetic ferrite. J. Environ. Manag. 2013, 120, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Root, R.A.; Farrell, J.; Ela, W.; Chorover, J. Effect of silicic acid on arsenate and arsenite retention mechanisms on 6-l ferrihydrite: A spectroscopic and batch adsorption approach. Appl. Geochem. 2013, 38, 110. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, A.C.Q.; Ciminelli, V.S.T.; Duarte, H.A.; Alves, M.C.M.; Ramos, A.Y. Mechanism of anion retention from exafs and density functional calculations: Arsenic (V) adsorbed on gibbsite. Geochim. Cosmochim. Acta 2001, 65, 1211–1217. [Google Scholar] [CrossRef]
- Onanguema, G.; Morin, G.; Wang, Y.; Foster, A.L.; Juillot, F.; Calas, G.; Brown, G.E. Xanes evidence for rapid arsenic(III) oxidation at magnetite and ferrihydrite surfaces by dissolved O2 via Fe2+-mediated reactions. Environ. Sci. Technol. 2010, 44, 5416–5422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Paul, K.W.; Kubicki, J.D.; Sparks, D.L. Quantum chemical study of arsenic (III, V) adsorption on mn-oxides: Implications for arsenic(III) oxidation. Environ. Sci. Technol. 2009, 43, 6655. [Google Scholar] [CrossRef] [PubMed]
- Watts, H.D.; Tribe, L.; Kubicki, J.D. Arsenic adsorption onto minerals: Connecting experimental observations with density functional theory calculations. Minerals 2014, 4, 208–240. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Kwon, A.K.D.; Paul, A.K.W.; Sparks, D.L. Surface complex structures modelled with quantum chemical calculations: Carbonate, phosphate, sulphate, arsenate and arsenite. Eur. J. Soil Sci. 2007, 58, 932–944. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H. Characterization of adsorbed arsenate on amorphous and nano crystalline mgfe-layered double hydroxides. J. Nanopharm. Res. 2011, 13, 887–894. [Google Scholar] [CrossRef]
- Caporale, A.G.; Pigna, M.; Dynes, J.J.; Cozzolino, V.; Zhu, J.; Violante, A. Effect of inorganic and organic ligands on the sorption/desorption of arsenate on/from Al–Mg and Fe–Mg layered double hydroxides. J. Hazard. Mater. 2011, 198, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Violante, A.; Pucci, M.; Cozzolino, V.; Zhu, J.; Pigna, M. Sorption/desorption of arsenate on/from Mg–Al layered double hydroxides: Influence of phosphate. J. Colloid Interface Sci. 2009, 333, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.V.; Kamath, P.V. Synthesis and characterization of arsenate-intercalated layered double hydroxides (ldhs): Prospects for arsenic mineralization. J. Colloid Interface Sci. 2009, 331, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.V.; Kamath, P.V. Chromate uptake characteristics of the pristine layered double hydroxides of mg with al. Solid State Sci. 2008, 10, 260–266. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Rao, R.A.; Kamath, P.V. Layered double hydroxides as potential chromate scavengers. J. Colloid Interface Sci. 2007, 304, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.V.; Kamath, P.V.; Shivakumara, C. Synthesis and characterization of layered double hydroxides (ldhs) with intercalated chromate ions. Mater. Res. Bull. 2007, 42, 1028–1039. [Google Scholar] [CrossRef]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Peng, C.; Hong, Z.; Chong, W.; Ma, H.; Hu, J.; Pu, Y.; Peng, L. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg–Al–Co 3 layered double hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108. [Google Scholar]
- Xiang, C.; Huang, X.; Wang, X.; Zhao, B.; Chen, A.; Sun, D. Phosphate adsorption from sewage sludge filtrate using zinc–aluminum layered double hydroxides. J. Hazard. Mater. 2009, 169, 958–964. [Google Scholar]
- Wang, S.L.; Cheng, C.Y.; Tzou, Y.M.; Liaw, R.B.; Chang, T.W.; Chen, J.H. Phosphate removal from water using lithium intercalated gibbsite. J. Hazard. Mater. 2007, 147, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Seida, Y.; Nakano, Y. Removal of phosphate by layered double hydroxides containing iron. Water Res. 2002, 36, 1306–1312. [Google Scholar] [CrossRef]
- He, J.; Wei, M.; Li, B.; Kang, Y.; Evans, D.G.; Duan, X. Preparation of layered double hydroxides. In Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 89–119. [Google Scholar]
- Yang, L.; Dadwhal, M.; Shahrivari, Z.; Ostwal, M.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. Adsorption of arsenic on layered double hydroxides: Effect of the particle size. Ind. Eng. Chem. Res. 2006, 45, 4742–4751. [Google Scholar] [CrossRef]
- Meyn, M.; Beneke, K.; Lagaly, G. Anion-exchange reactions of layered double hydroxides. Inorg. Chem. 1990, 29, 5201–5207. [Google Scholar] [CrossRef]
- Constantino, V.R.; Pinnavaia, T.J. Basic properties of Mg2+1−xAl3+x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Gago, S.; Pillinger, M.; Valente, A.A.; Santos, T.M.; João Rocha, A.; Gonçalves, I.S. Immobilization of oxomolybdenum species in a layered double hydroxide pillared by 2,2′-bipyridine-5,5′-dicarboxylate anions. Inorg. Chem. 2004, 43, 5422–5431. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Zeng, H.C. Abrupt structural transformation in hydrotalcite-like compounds Mg1−xAlx(OH)2(NO3)x·nH2O as a continuous function of nitrate anions. J. Phys. Chem. B 2001, 105, 1743–1749. [Google Scholar] [CrossRef]
- Li, H.; Jing, M.; Evans, D.G.; Zhou, T.; Li, F.; Xue, D. Molecular dynamics modeling of the structures and binding energies of α-nickel hydroxides and nickel−aluminum layered double hydroxides containing various interlayer guest anions. Chem. Mater. 2006, 18, 4405–4414. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, P.C. In situ xrd and atr-ftir study on the molecular orientation of interlayer nitrate in Mg/Al-layered double hydroxides in water. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 131–138. [Google Scholar] [CrossRef]
- Cygan, R.T.; Greathouse, J.A.; Heinz, H.; Kalinichev, A.G. Molecular models and simulations of layered materials. J. Mater. Chem. 2009, 19, 2470–2481. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kalinichev, A.G.; Kirkpatrick, R.J. Molecular dynamics simulation of the energetics and structure of layered double hydroxides intercalated with carboxylic acids. J. Phys. Chem. C 2007, 111, 13517–13523. [Google Scholar] [CrossRef]
- Pisson, J.; Morel, J.P.; Moreldesrosiers, N.; Taviotguého, C.; Malfreyt, P. Molecular modeling of the structure and dynamics of the interlayer species of znalcl layered double hydroxide. J. Phys. Chem. B 2008, 112, 7856–7864. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Kang, H.; Zhang, H.; Yuan, S. Molecular simulation studies for intercalation of photoactive dyes into layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2012, 402, 108–116. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Lu, X.; Zhu, R.; He, H.; Zhu, J. Jumping diffusion of water intercalated in layered double hydroxides. J. Phys. Chem. C 2016, 120, 12924–12931. [Google Scholar] [CrossRef]
- Newman, S.P.; Williams, S.J.; Coveney, P.V.; Jones, W. Interlayer arrangement of hydrated MgAl layered double hydroxides containing guest terephthalate anions: Comparison of simulation and measurement. J. Phys. Chem. B 1998, 102, 6710–6719. [Google Scholar] [CrossRef]
- Liu, X.; He, M.; Lu, X.; Wang, R. Structures and acidity constants of arsenite and thioarsenite species in hydrothermal solutions. Chem. Geol. 2015, 411, 192–199. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Sprik, M.; Lu, X.; Wang, R. Understanding surface acidity of gibbsite with first principles molecular dynamics simulations. Geochim. Cosmochim. Acta 2013, 120, 487–495. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Meijer, E.J.; Wang, R.; Zhou, H. Atomic-scale structures of interfaces between phyllosilicate edges and water. Geochim. Cosmochim. Acta 2012, 81, 56–68. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Sprik, M.; Cheng, J.; Meijer, E.J.; Wang, R. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 2013, 117, 180–190. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Sprik, M.; Lu, X.; Wang, R. Surface acidity of 2:1-type dioctahedral clay minerals from first principles molecular dynamics simulations. Geochim. Cosmochim. Acta 2014, 140, 410–417. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Lu, X.; He, M.; Meijer, E.J.; Wang, R. Surface complexation of heavy metal cations on clay edges: Insights from first principles molecular dynamics simulation of Ni (II). Geochim. Cosmochim. Acta 2017, 203, 54–68. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Olivier Clause, A.; Lynch, J.; Bazin, D.; Elkaïm, E. A reexamination of hydrotalcite crystal chemistry. J. Phys. Chem. B 1996, 100, 8527–8534. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. CP2K: Atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Lippert, B.G.; Hutter, J.; Parrinello, M. A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. Int. J. Interface Chem. Phys. 1997, 92, 477–487. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B Condens. Matter 1995, 54, 1703–1710. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Marry, V.; Rotenberg, B.; Turq, P. Structure and dynamics of water at a clay surface from molecular dynamics simulation. Phys. Chem. Chem. Phys. 2008, 10, 4802–4813. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.-H.; Kwak, S.-Y.; Park, J.-S.; Jeong, Y.-J. Cellular uptake behavior of [γ-32p] labeled ATP–LDH. J. Mater. Chem. 2001, 11, 1671–1674. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W. Comparative study of some layered hydroxide salts containing exchangeable interlayer anions. J. Solid State Chem. 1999, 148, 26–40. [Google Scholar] [CrossRef]
- Han, J.B.; Lu, J.; Wei, M.; Wang, Z.L.; Duan, X. Heterogeneous ultrathin films fabricated by alternate assembly of exfoliated layered double hydroxides and polyanion. Chem. Commun. 2008, 41, 5188–5190. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.H.; Jung, J.S.; Oh, J.M.; Man, P.; Jeong, J.; Kang, Y.K.; Han, O.J. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials 2004, 25, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Bujdosó, T.; Patzkó, Á.; Galbács, Z.; Dékány, I. Structural characterization of arsenate ion exchanged mgal-layered double hydroxide. Appl. Clay Sci. 2009, 44, 75–82. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Zhang, Z.; Jin, R.; Zhang, X.; Yan, X.; Umar, A.; Guo, Z.; Wang, Q. Synthesis of polypropylene/Mg3Al–X (X = CO32−, NO3−, Cl−, SO42−) LDH nanocomposites using a solvent mixing method: Thermal and melt rheological properties. J. Mater. Chem. A 2013, 1, 9928–9934. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Grover, K.; Komarneni, S.; Katsuki, H. Synthetic hydrotalcite-type and hydrocalumite-type layered double hydroxides for arsenate uptake. Appl. Clay Sci. 2010, 48, 631–637. [Google Scholar] [CrossRef]
- Grover, K.; Komarneni, S.; Katsuki, H. Uptake of arsenite by synthetic layered double hydroxides. Water Res. 2009, 43, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, L.H.; Zheng, Z.; Kang, M.L.; Li, C.; Liu, C.L. Molecular dynamics simulation of the diffusion of uranium species in clay pores. J. Hazard. Mater. 2013, 244–245, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yi, H.; Zhao, Y.; Min, F.; Song, S. Study on the differences of Na- and Ca-montmorillonites in crystalline swelling regime through molecular dynamics simulation. Adv. Powder Technol. 2016, 27, 779–785. [Google Scholar] [CrossRef]
- Myshakin, E.M.; Saidi, W.A.; Romanov, V.N.; Cygan, R.T.; Jordan, K.D. Molecular dynamics simulations of carbon dioxide intercalation in hydrated Na-montmorillonite. J. Phys. Chem. C 2013, 117, 141–155. [Google Scholar] [CrossRef]
- Boek, E.S. Molecular dynamics simulations of interlayer structure and mobility in hydrated Li-, Na- and K-montmorillonite clays. Mol. Phys. 2014, 112, 1472–1483. [Google Scholar] [CrossRef]

| Mg/Al | Systems | Formulae |
|---|---|---|
| 2:1 | H2AsO4− | Mg11Al5(OH)32(NO3)3(H2AsO4)2·(H2O)6 |
| HAsO42− | Mg11Al5(OH)32(NO3)(HAsO4)2·(H2O)8 | |
| H2AsO3− | Mg11Al5(OH)32(NO3)3(H2AsO3)2·(H2O)6 | |
| NO3− | Mg11Al5(OH)32(NO3)5·(H2O)8 | |
| 3:1 | H2AsO4− | Mg12Al4(OH)32(NO3)3(H2AsO4)·(H2O)7 |
| HAsO42− | Mg12Al4(OH)32(NO3)2(HAsO4)·(H2O)8 | |
| H2AsO3− | Mg12Al4(OH)32(NO3)3(H2AsO3)·(H2O)7 | |
| NO3− | Mg12Al4(OH)32(NO3)4·(H2O)8 | |
| 4:1 | H2AsO4− | Mg38Al10(OH)96(NO3)9(H2AsO4)·(H2O)25 |
| HAsO42− | Mg38Al10(OH)96(NO3)8(HAsO4)·(H2O)26 | |
| H2AsO3− | Mg38Al10(OH)96(NO3)9(H2AsO3)·(H2O)25 | |
| NO3− | Mg13Al3(OH)32(NO3)3·(H2O)9 |
| Mg/Al | H2O | NO3− | H2AsO4− | HAsO42− | H2AsO3− |
|---|---|---|---|---|---|
| 2:1 | 12.1 | 13.6 | 0.8 | 1.7 | 3.9 |
| 3:1 | 15.4 | 10.8 | - | - | - |
| 4:1 | 3.4 | 1.4 | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Zhang, C.; He, M.; Lu, X. Interlayer Structures and Dynamics of Arsenate and Arsenite Intercalated Layered Double Hydroxides: A First Principles Study. Minerals 2017, 7, 53. https://doi.org/10.3390/min7040053
Zhang Y, Liu X, Zhang C, He M, Lu X. Interlayer Structures and Dynamics of Arsenate and Arsenite Intercalated Layered Double Hydroxides: A First Principles Study. Minerals. 2017; 7(4):53. https://doi.org/10.3390/min7040053
Chicago/Turabian StyleZhang, Yingchun, Xiandong Liu, Chi Zhang, Mengjia He, and Xiancai Lu. 2017. "Interlayer Structures and Dynamics of Arsenate and Arsenite Intercalated Layered Double Hydroxides: A First Principles Study" Minerals 7, no. 4: 53. https://doi.org/10.3390/min7040053
APA StyleZhang, Y., Liu, X., Zhang, C., He, M., & Lu, X. (2017). Interlayer Structures and Dynamics of Arsenate and Arsenite Intercalated Layered Double Hydroxides: A First Principles Study. Minerals, 7(4), 53. https://doi.org/10.3390/min7040053






