1. Introduction
Fluorides and fluorinated materials appear in various aspects of modern life. The strategic role of fluoride materials involves diverse research fields and applications in areas such as energy production, microelectronics and photonics, catalysis, pigments, textiles, cosmetics, plastics, domestic wares, automotive technology and the construction industry.
One of the most commonly used lithium salts is LiF, which is utilized as a flux in the ceramics and glass industries, as well as in light metals welding [
1,
2]. It is especially used in the manufacture of optical components for analysis equipments (IR and UV spectroscopies). Also, it has recently been used in the fabrication of new cathodes for lithium-ion batteries, in the LiF-Fe ones [
3]. A potentially important use of LiF is represented by atomic fusion, where it would be employed as a source of
6Li isotopes [
4,
5].
The fluorometalates of K, and particularly fluorosilicates, have applications in the field of Al brazing. Firstly, the corresponding reaction gives rise to the formation of elemental Si and K fluoroaluminate, which act as a fluxing agent. The elements formed combine and alloy with Al, diffusing through the base material so as to reach the corresponding eutectics mixtures, thereby locally lowering the melting point of the Al and thus acting as a metal fillet or clad, which forms the joint [
6].
Synthetic cryolite is a very important fluoroaluminate regarding to its industrial applications. Probably its most important and best-known application is in the production and refining of aluminum in combination with other fluorides, the so-called Hall-Heroult process [
7]. Cryolite is also employed in grinding applications as an abrasive aid in the polymeric matrix of phenolics resins [
4]. Other uses, dealing with lower volumes, include solid lubricant for brakes in heavy-duty applications, and in the production of welding agents, pyrotechnics and metal surface treatments.
Lepidolite (KLiAl
2Si
3O
10(OH,F)
3) is a mica with a complex and variable formula. Its Li concentration ranges from 1.39% (3.0% Li
2O) to a theoretical maximum of 3.58% Li (7.7% Li
2O). The major commercial deposits of lepidolite are in: Bikita, Zimbabwe; Bernie Lake, Manitoba, Canada; Karibib, Namibia; and Mina Gerais, Brazil [
1]. In Argentina, the main deposits of lepidolite are found in the San Luis, Salta and Catamarca provinces [
8,
9].
The most significant processes for the extraction of Li from lepidolite are the “sulfate acid” and “lime” methods. Acid digestion is carried out with concentrated sulphuric acid at temperatures higher than 250 °C, whereas the lime method is carried out with CaCO
3 at 1040 °C. The products obtained through these methods are Li
2CO
3 and LiOH, respectively [
1,
2].
Relevant findings about the dissolution of lepidolite with a combination of both pyro and hydrometallurgical routes have been published. In such processes, lepidolite is firstly calcined together with Na
2SO
4, K
2SO
4, FeSO
4 or FeS, at temperatures higher than 800 °C. Then, the obtained mixture is leached with water [
10,
11,
12,
13,
14]. In the whole process mentioned before, the only valuable metal recovered is Li. Al and Si are lost with the waste generated during the Li recovery process.
The aim of this paper is to describe a process to recover Li, Si and Al from lepidolite and to highlight the controlling parameters of the process, including the best operational conditions.
2. Materials and Methods
The leaching agent was HF (40% w/w), with analytical grade. The reagents used for the recovery assays were KOH and NaOH, both of analytical grades. The employed mineral was lepidolite, extracted from the mine “Las Cuevas”, located in the department of San Martín, San Luis, Argentina. The ore was concentrated by hand sorting, then grounded in a ring mill and sieved to a particle size of <45 μm.
Characterization of the ore and products was performed by X-ray fluorescence (XRF) on a Philips PW 1400 instrument (Philips, Amsterdam, The Netherlands) and by X-ray diffraction (XRD) in a Rigaku D-Max III C diffractometer (Rigaku, Osaka, Japan), operated at 35 kV and 30 mA. The Kα radiation of Cu and the filter of Ni, λ = 0.15418 nm were used. Morphological analysis was done by scanning electron microscopy (SEM), in an equipment LEO 1450 VP (Zeiss, Jena, Germany) which was equipped with an EDAX Genesis 2000 X-ray dispersive spectrometer (EDAX, Mahwah, NJ, USA), used to determine the semi-quantitative composition of the residues obtained through the leaching of the minerals by electron probe microanalysis (EPMA).
Determination of lithium content in the ore was performed by atomic absorption spectroscopy (AAS) using a Varian SpectrAA 55 spectrometer (Palo Alto, Santa Clara, CA, USA) with a hollow-cathode lamp (analytical error 1.5%). Previously, the sample (lepidolite) was dissolved using a concentrated mixture of sulfuric acid and hydrofluoric acid, according to the method of Brumbaugh and Fanus, 1954 [
15]. The bulk composition of the ore is shown in
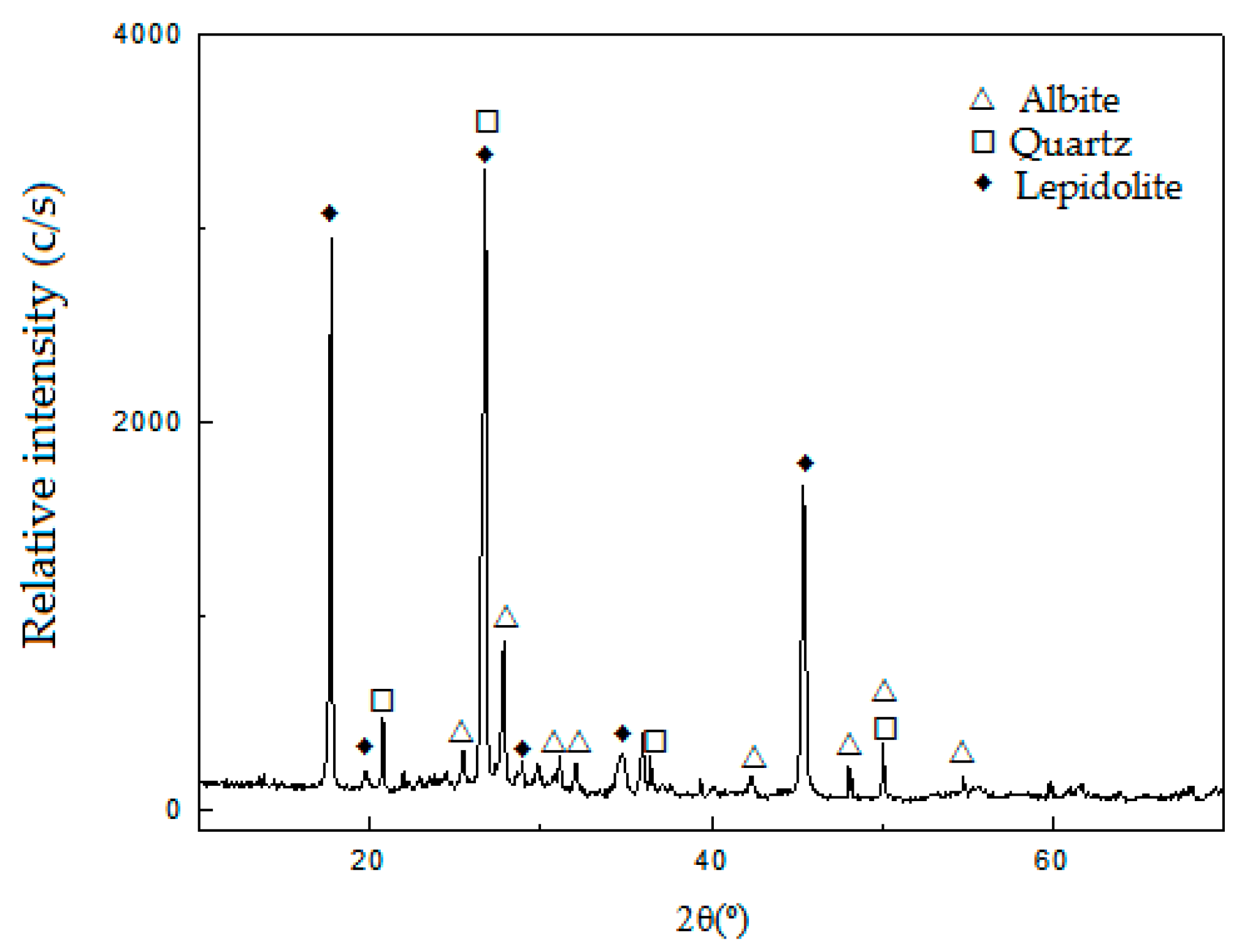
Table 1, determined by AAS (Li and Na) and XRF (Si, Al, Fe, Ca, Mg, K and Ti). The results of the characterization of the ore by XRD are shown in
Figure 1.
The XRD patterns in
Figure 1 show that the sample is mainly composed of lepidolite (ICDD 01-085-0398), with the presence of albite (ICDD 96-900-1631) and quartz (JCPDS 33-1161) as gangue.
2.1. Experimental Equipment and Procedure
2.1.1. Leaching Assays
The experimental tests were performed in a steel closed vessel of 500 mL coated with Teflon and equipped with magnetic stirring and temperature control systems.
For each test, a mass of the ore and a volume of distilled water were placed into the reactor. The mixture was subsequently heated in an oil bath, with stirring until the final work temperature was reached. Once the desired temperature was achieved, an appropriate amount of HF was added to the mixture, so as to obtain different acid concentrations. From that moment, the reaction time was calculated. Once the experiment was finished, the solid was filtered, dried at 75 °C, and then weighed.
Li was analyzed by atomic absorption to calculate the extraction percentage in all the experiments by the following equation:
where: Li
m is the initial amount of lithium in the mineral and Li
s is the amount of the element in the leach liquor [
16,
17].
The experimental study of this work was performed by univariate analysis. In order to evaluate the experimental error, each test was replicated three times. The average extraction efficiency and standard deviation were calculated for each parameter studied.
The operational studied parameters were: temperature, reaction time and HF concentration. The following parameters were kept constant: particle size, <45 μm; solid-liquid ratio, 1.82% (w/v); and stirring speed, 330 rpm.
2.1.2. Recovery Assays
The residual Si and Al in the liquor obtained after the leaching of the mineral were removed as the compounds K
2SiF
6 and Na
3AlF
6, by using KOH and NaOH, respectively. The reactions proposed for obtaining these compounds are the following [
16,
17,
18]:
The recovery of Li from the resulting solution after the precipitation of Al and Si can be carried out by one of the many known methods. In this case, lithium was precipitated as LiF, evaporating the solutions above the solubility product constant (Ksp) of LiF [
16,
17,
19,
20,
21,
22,
23]. The recovery efficiency of each element was calculated by gravimetric analysis.
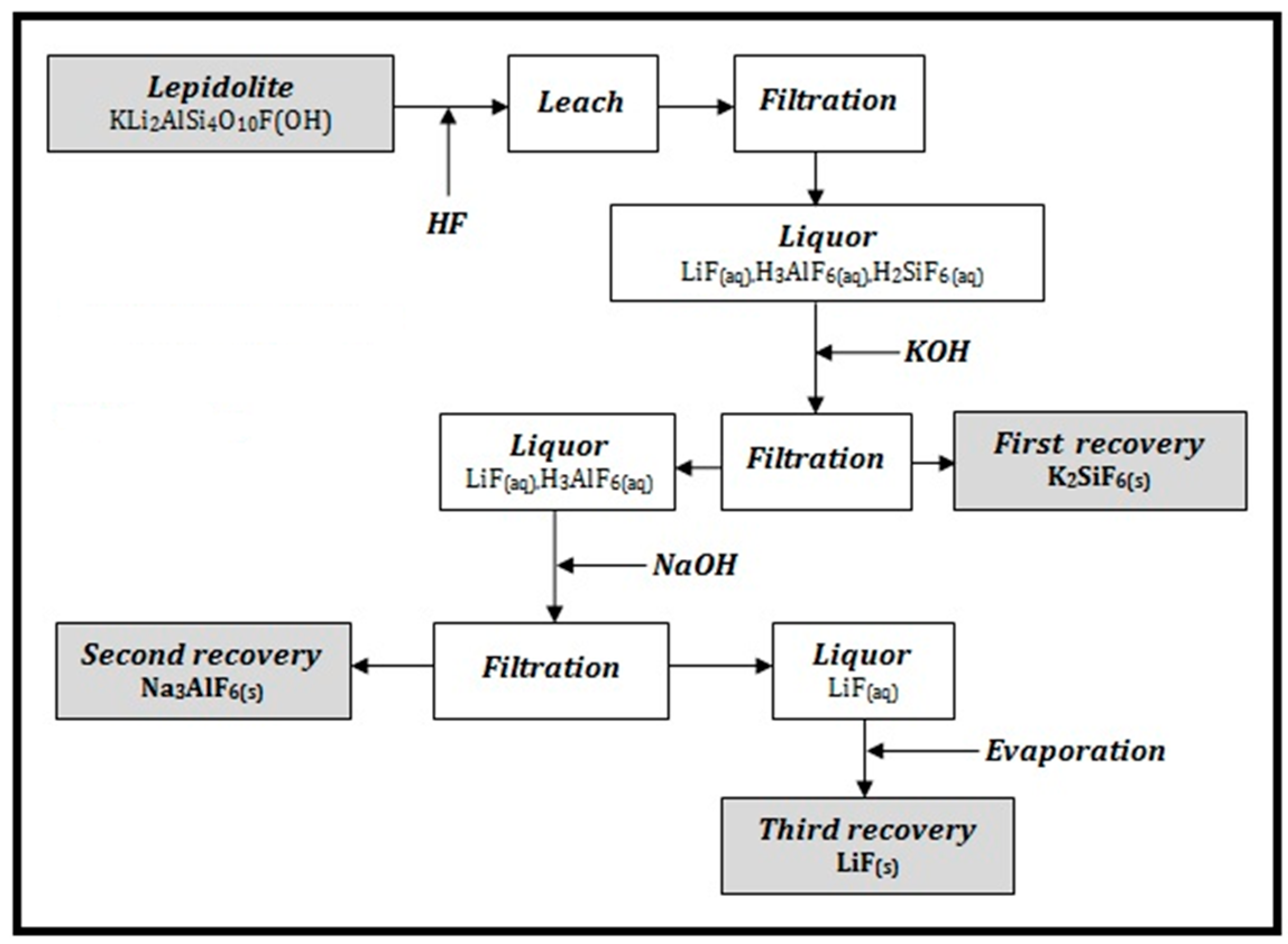
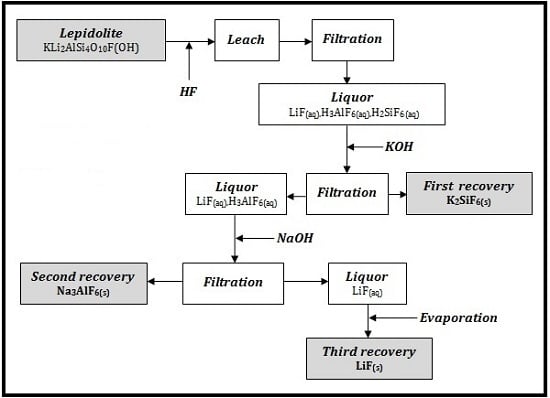
In
Figure 2 it is presented the process flow sheet for Li, Si and Al recovery.
3. Results
3.1. Leaching of Li from Lepidolite
The lepidolite dissolution with HF can be represented by the following reaction [
16,
17,
18]:
3.1.1. Effect of Temperature
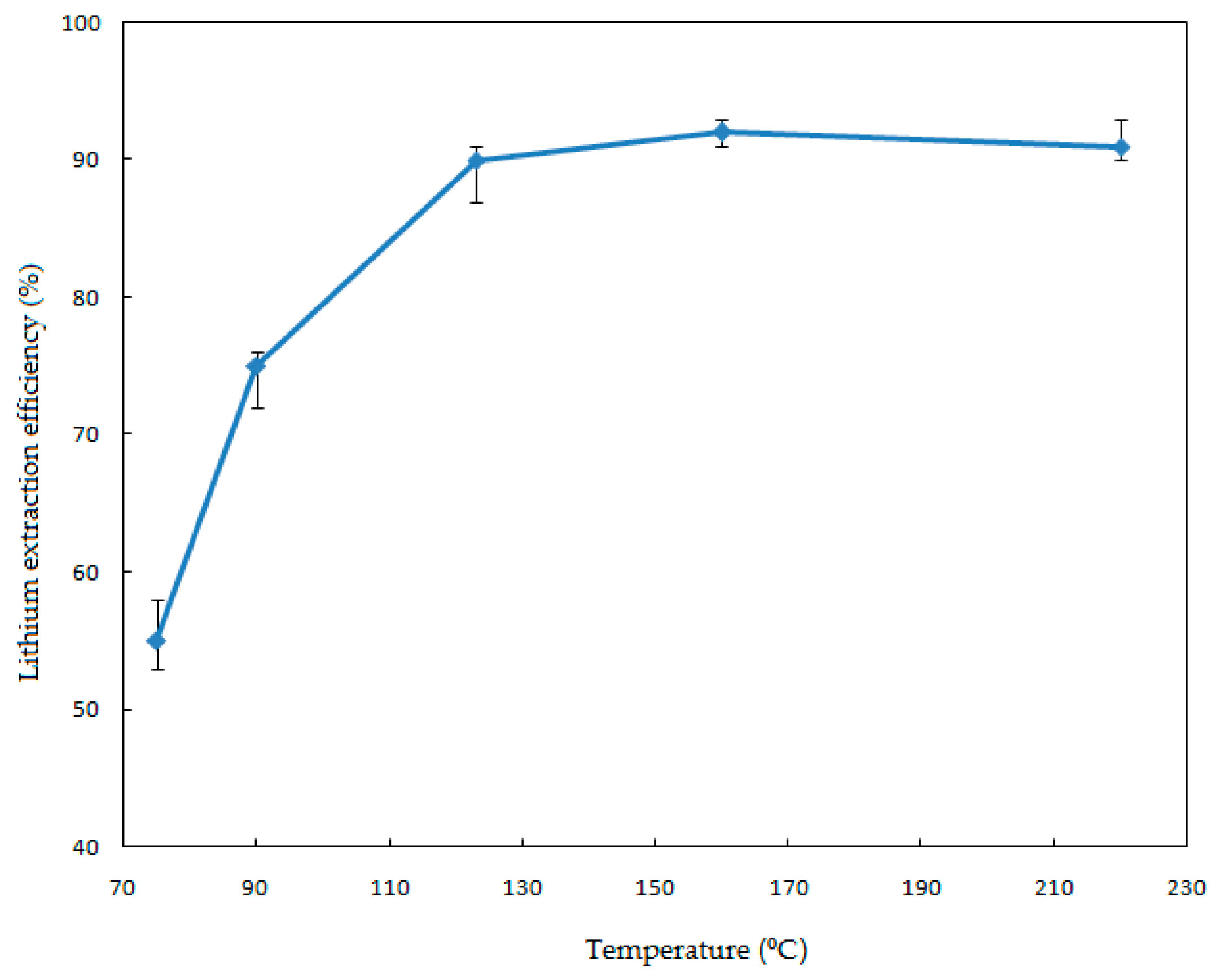
In order to investigate the effect of the leaching temperature on the Li extraction, a series of leaching experiments were performed from 75 to 220 °C. Conditions of the leaching process were as follows: solid-liquid ratio, 1.82% (
w/v); HF concentration, 7% (
v/v); stirring speed, 330 rpm; and reaction time, 120 min. The results are presented in
Figure 3 from which it can be appreciated from the error bars that the largest standard deviation in the experimental data is about ±2.5%.
Figure 3 illustrates that the reaction temperature has an obvious effect on the leaching process. The extraction efficiency increases significantly when the leaching temperature is increased from 75 to 123 °C. This coincides with the results of Rosales et al. (2014 and 2016), who determined that the dissolution of lithium aluminosilicates (β-spodumene) with HF is strongly dependent on the temperature [
17,
18]. The extraction efficiency was 90% when the reaction temperature was 123 °C. However, a further increase of the temperature did not result in a clear increase of the extraction efficiency (92%). The optimum temperature was chosen to be 123 °C for the economic and industrial applications.
3.1.2. Effect of Reaction Time
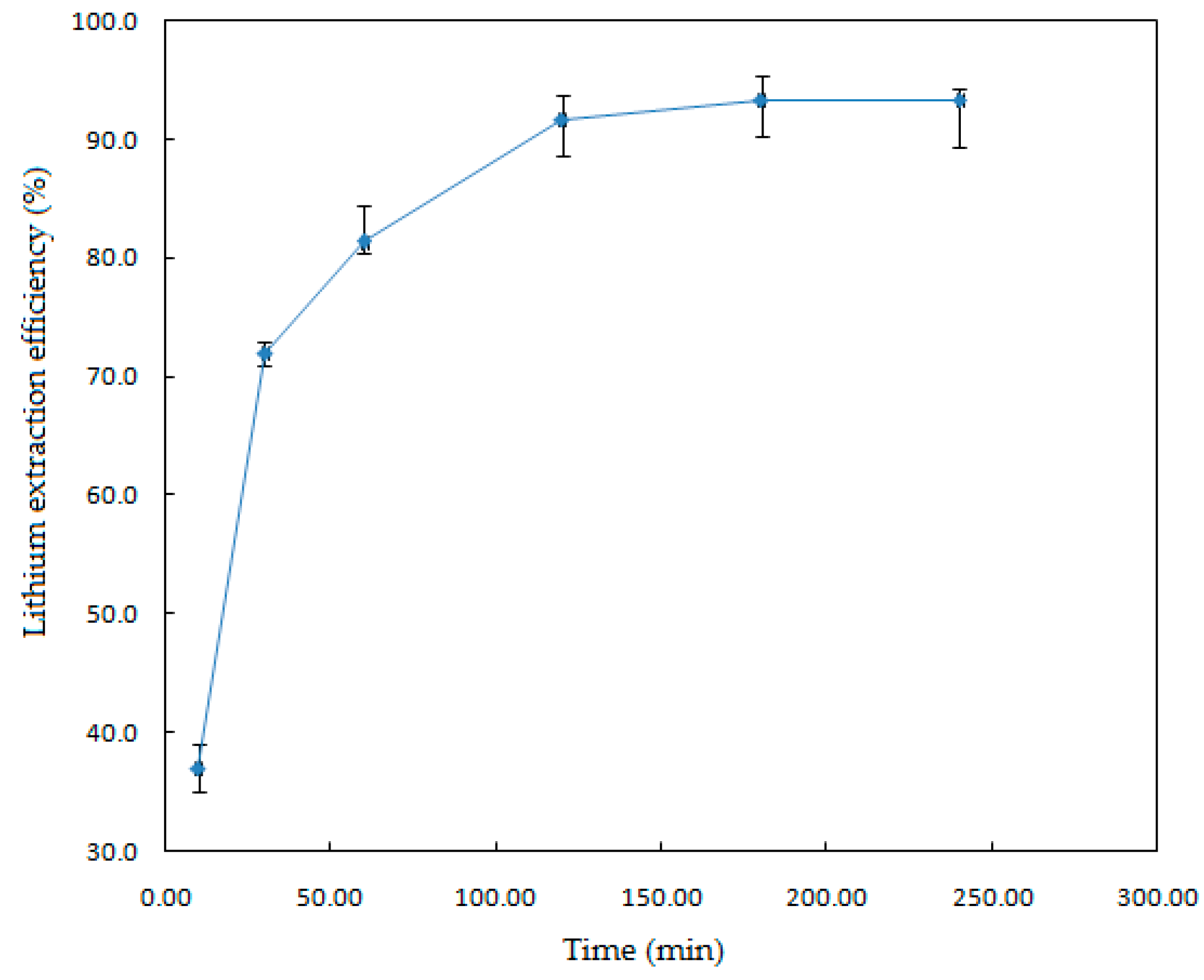
To study the effect of the reaction time on the Li extraction, experiments were conducted at conditions of solid-liquid ratio, 1.82% (w/v); HF concentration, 7% (v/v); temperature, 123 °C; and stirring speed, 330 rpm.
The results are plotted in
Figure 4, which shows that leaching time has a remarkable effect on the dissolution of the mineral. As the time increased from 10 to 240 min, the lithium extraction efficiency increased from 37% to 95%, due to the reaction time favoring the contact between the leaching agent and the mineral, improving the extraction efficiency. As the reaction time increased up to 120 min, the extraction percentage of Li remained almost constant. Statistical analysis of the results indicates that there is a standard deviation of 1.6%.
3.1.3. Effect of HF Concentration
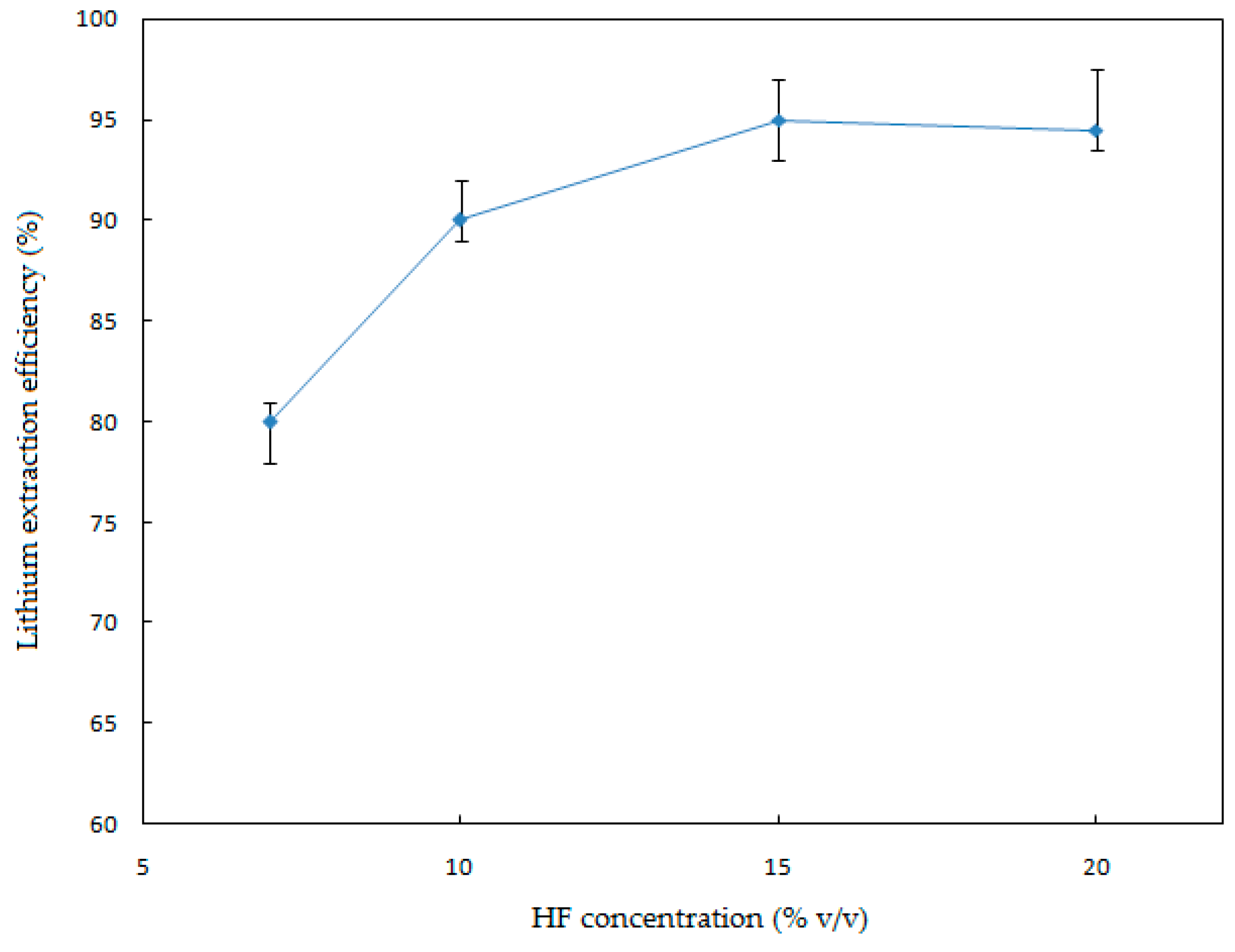
In order to investigate the effect of the HF concentration on the Li extraction, a series of leaching experiments were performed ranging from 7% to 20% (
v/v) of HF. The conditions of the leaching process were as follows: solid-liquid ratio, 1.82% (
w/v); stirring speed, 330 rpm; temperature, 123 °C; and reaction time, 60 min. The results are plotted in
Figure 5.
Figure 5 illustrates that the HF concentration has a notorious effect on the leaching process. The extraction efficiency increased significantly when the HF concentration was increased from 7% to 20% (
v/v). The maximum extraction value of Li (95%) was achieved by working with HF 15% (
v/v). Above 15% (
v/v) of HF, the Li extraction percentage decreased, since high concentrations of HF produce a precipitation of Li alkali fluorides (e.g., Li
3Na
3Al
2F
12) [
17].
3.1.4. Characterization of the Residue
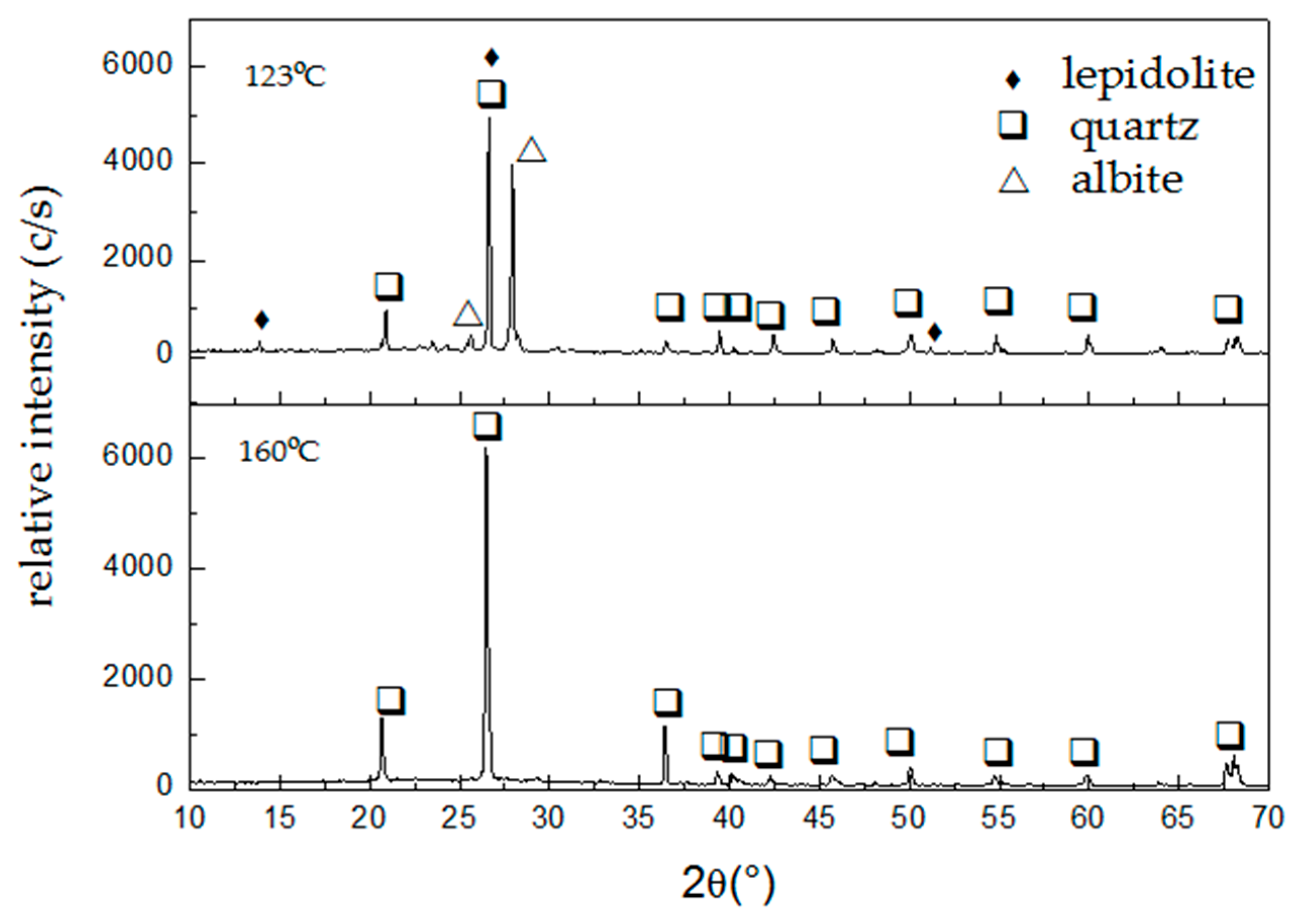
Figure 6 presents the diffractograms of the leaching residues obtained at 123 and 160 °C with extractions of 90% and 95% of Li, respectively, under the following experimental conditions: HF, 7% (
v/v) and time, 120 min.
From
Figure 6 it can be inferred that the dissolution of the lepidolite present in the ore occurs, and even more, it enhances the intensity of the diffraction lines of albite and quartz. This indicates that, working at 123 °C, the dissolution reaction is preferential for lepidolite. Meanwhile, the dissolution of lepidolite and albite is achieved at 160 °C, leaving quartz in the leaching residue.
3.2. Recovery of Si, Al and Li
3.2.1. Recovery of Silicon as K2SiF6
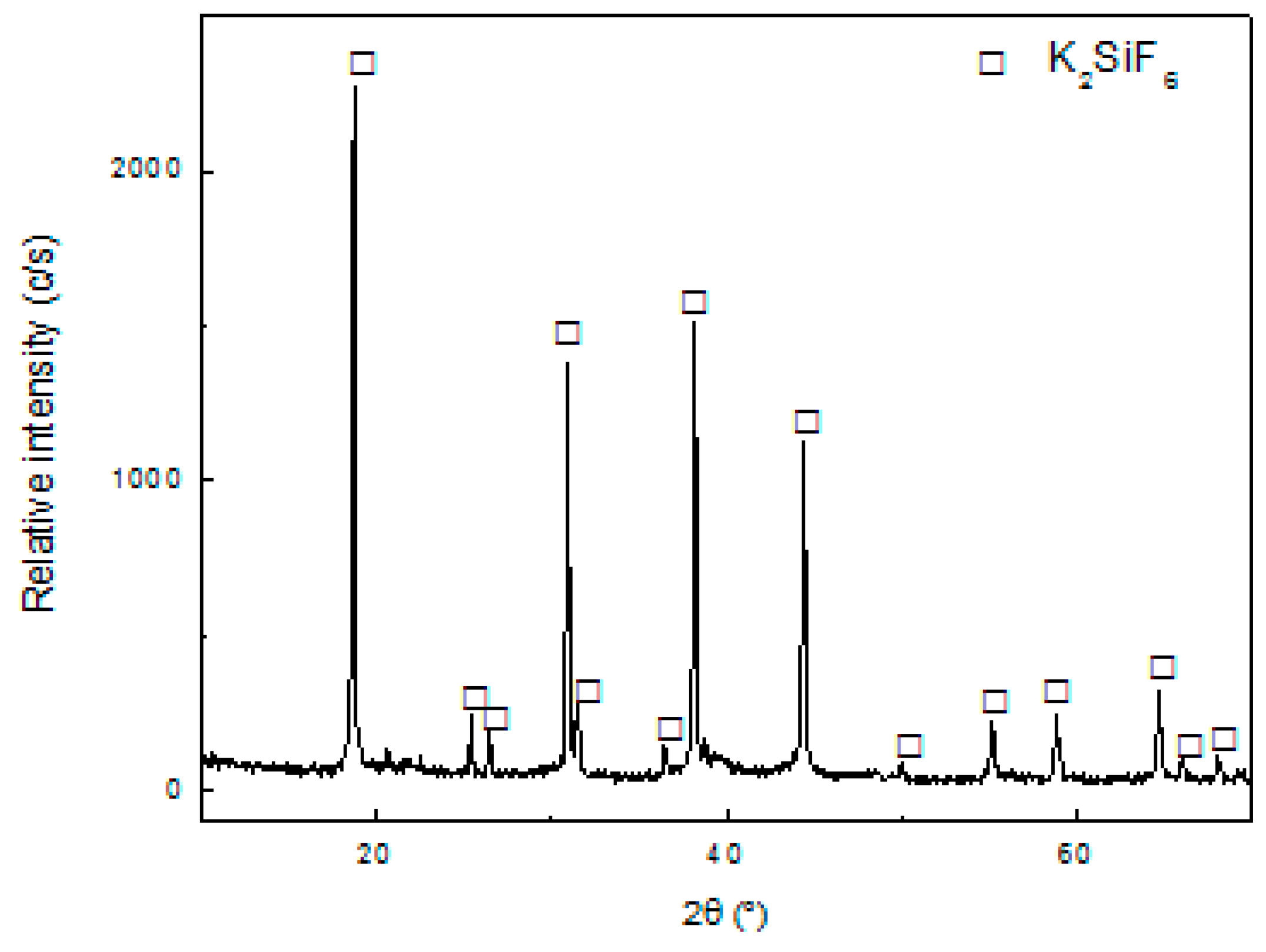
The leach liquor obtained after the first filtration was treated with KOH to precipitate the residual Si according to Equation (2). The amount of KOH used was equal to the stoichiometric value calculated from Equation (2). The result of the XRD characterization of the obtained solid is shown in
Figure 7.
Figure 7 shows the appearance of diffraction lines that correspond to the structure of K
2SiF
6 (JCPDS 7-217), which is in agreement with the proposed Equation (2). By gravimetry, it was determined that the recovery of Si was 93%.
The K
2SiF
6 precipitate was analyzed by microanalysis EDS and XRF to determine the purity of the precipitate.
Table 2 shows the purity of the compound.
The results obtained from
Figure 7 and
Table 2 indicate that the precipitation reaction was highly selective to Si, seeing that there was no formation of any Al and Li compound.
3.2.2. Recovery of Aluminum as Na3AlF6
To precipitate Al as Na
3AlF
6, the liquor obtained after the recovery of Si was treated with NaOH according to Equation (3) (Rosales et al., [
16,
17]). The amount of NaOH used was equal to the stoichiometric value calculated from Equation (3). After the addition of NaOH, the appearance of a white precipitate corresponding to Na
3AlF
6 was observed. The solid was filtered and dried for characterization.
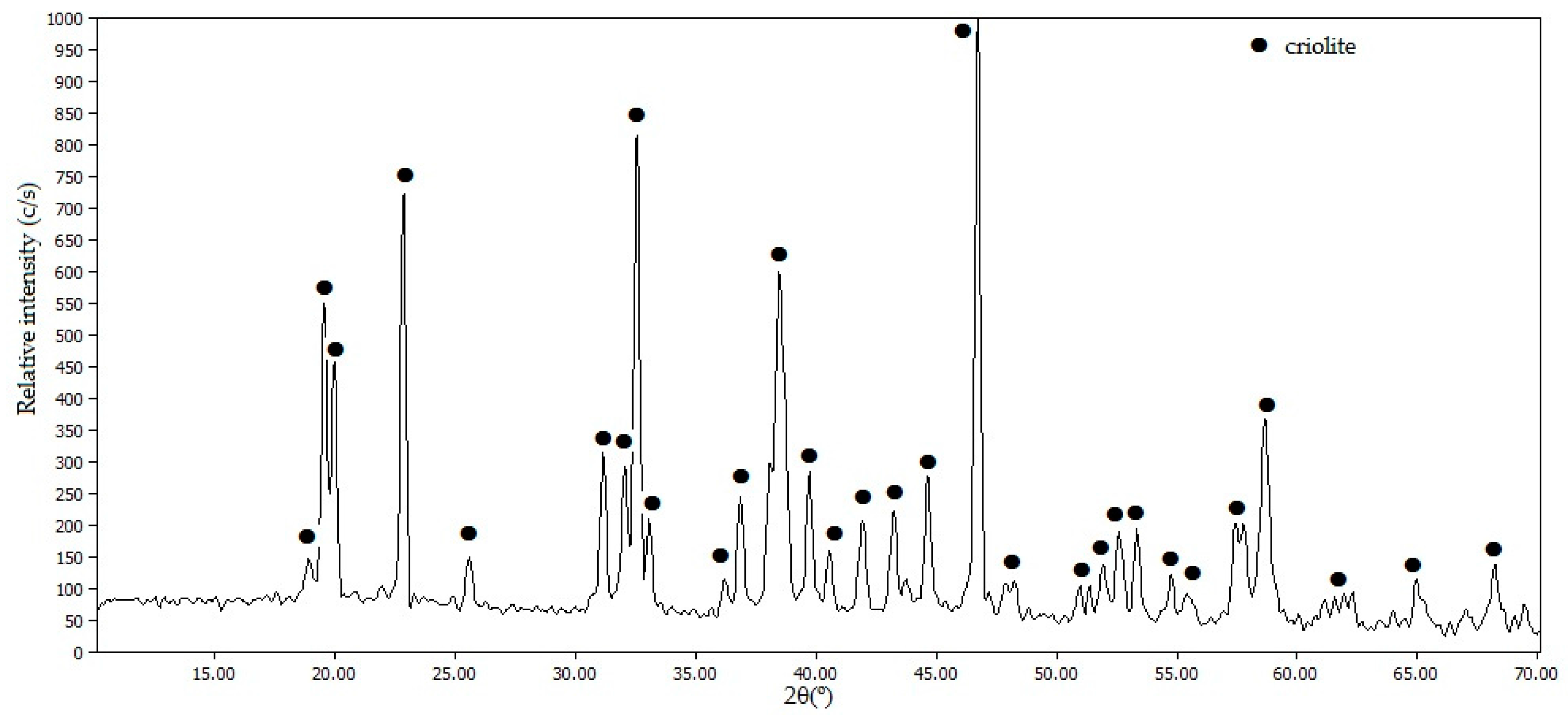
Figure 8 shows the diffractogram of the solid obtained after the addition of NaOH.
The diffractogram in
Figure 8 indicates that the formation of solid Na
3AlF
6 has taken place according to Equation (3), without other phases detected as impurities.
Table 3 shows the purity of the compound Na
3AlF
6, obtained by microanalysis EDS and XRF.
The appearence of Na
2SiF
6 in the diffractogram of
Figure 8 is not observed, but the presence of Si (2.5%) by EDS analysis is detected. This indicates that the Si, which was not recovered in the previous step, was precipitated as Na
2SiF
6 in amounts below the detection limit of XRD. For further purification the solid can be washed with solutions at different pH values [
20]. The achieved recovery percentage of Al was 95%.
3.2.3. Recovery of Lithium as LiF
The liquor obtained after the recovery of Al and Si was evaporated (at 75 °C) until the appearance of a gelatinous white precipitate that corresponded to LiF [
23]. The obtained solid was washed and dried at 75 °C for further characterization. The total lithium precipitation, obtained by gravimetric analisys, was about 92%.
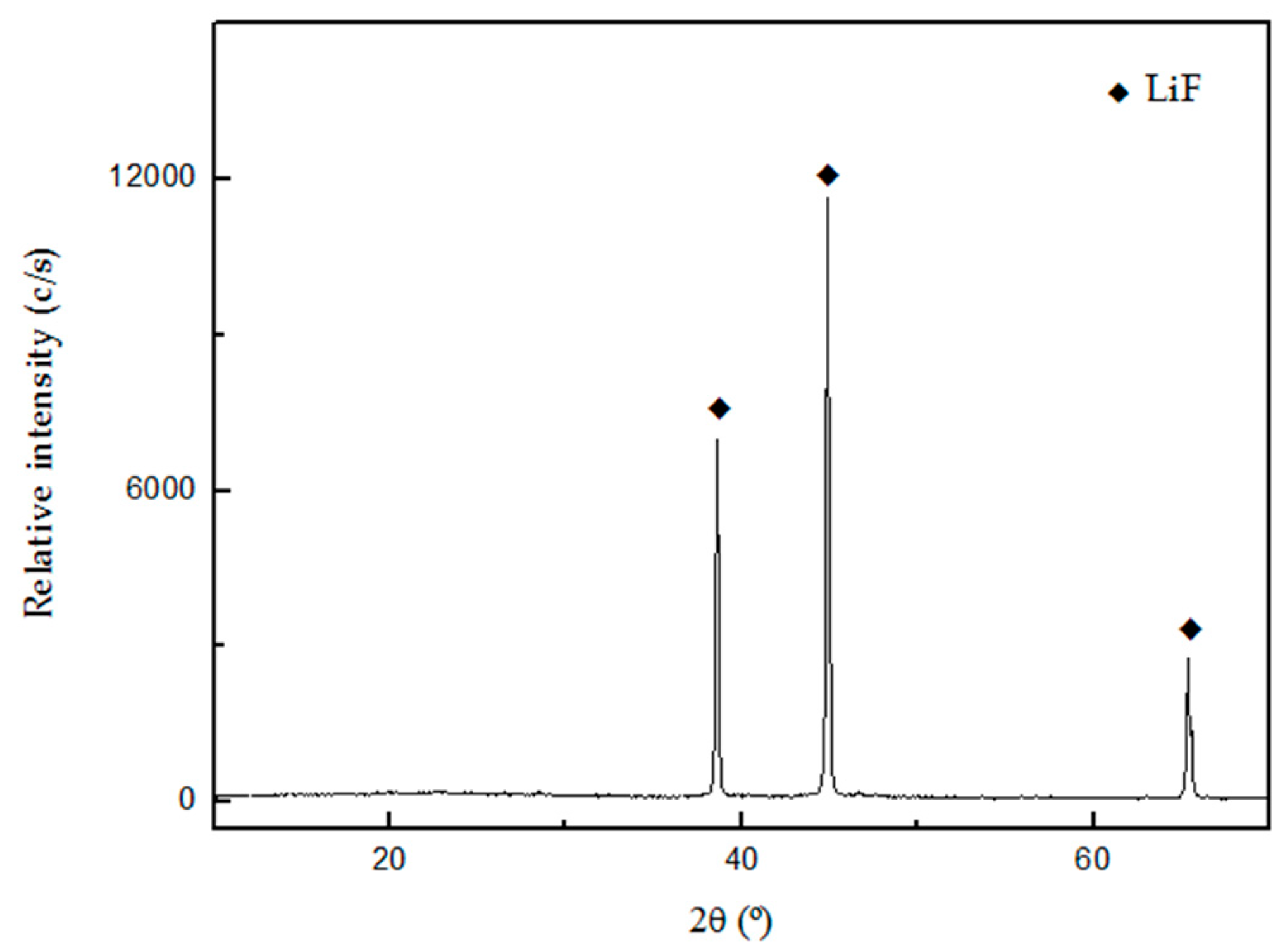
Figure 9 and
Figure 10 show the diffractogram and the SEM micrograph of the obtained LiF, respectively.
Figure 9 shows that the compound LiF was obtained without other phases as impurities. Frontino et al. (2008), obtained a similar diffractogram for the precipitation of LiF from spent Li-ion batteries [
23].
In
Figure 10 it can be seen that the LiF particles have a well-defined crystal structure and coincided with the cubic shape of LiF particles found in the literature.
The resulting LiF was analyzed by AAS and EDS to determine the concentration of major impurities in the sample. The results are presented in
Table 4.
Table 4 indicates that the purity of the LiF precipitated was 99.1%.
4. Conclusions
In summary, a process that includes leaching with HF was employed to extract Li, Al and Si from lepidolite. The experimental results indicate that the optimal conditions to achieve a Li extraction higher than 90% are: solid-liquid ratio, 1.82% (w/v); temperature, 123 °C; HF concentration, 7% (v/v); stirring speed, 330 rpm; and reaction time, 120 min. The compounds K2SiF6 and Na3AlF6 can be obtained as subproducts of the process, with a recovery of 93% and 95%, respectively. The Li dissolved can be separated by chemical precipitation as LiF, with a recovery value of 92% and a purity of 99.1%. Furthermore, Al and Si are recovered as valuable subproducts, diminishing the generation of residues during the process. The results of the current work can provide a simple, economic and effective way to recover Li from lepidolite.
Acknowledgments
The financial support from the Universidad Nacional de Cuyo, Secretaría de Ciencia, Técnica y Posgrado, is gratefully acknowledged. Two anonymous referees provided constructive and helpful reviews, corrections and comments. Many thanks are due to the editorial staff of Minerals.
Author Contributions
Gustavo D. Rosales, Eliana G. Pinna, Daniela S. Suarez and Mario H. Rodriguez conceived and designed the experiments; Gustavo D. Rosales, Eliana G. Pinna and Mario H. Rodriguez performed the experiments and analyzed the data; Mario H. Rodriguez contributed reagents, materials and analysis tools; Gustavo D. Rosales, Eliana G. Pinna, Daniel S. Suarez and Mario H. Rodriguez wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garret, D.E. Part 1—Lithium. In Handbook of Lithium and Natural Calcium Chloride; Elsevier Ltd.: London, UK, 2004. [Google Scholar]
- Habashi, F. Alkali Metals—Lithium. In Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997; Volume IV. [Google Scholar]
- Das, B.; Pohl, A.; Chakravadhanula, V.S.K.; Kübel, C.; Fichtner, M. LiF/Fe/V2O5 nanocomposite as high capacity cathode for lithium ion batteries. J. Power Sources 2014, 267, 203–211. [Google Scholar] [CrossRef]
- Tressaud, A. Functionalized Inorganic Fluorides Synthesis: Characterization and Properties of Nanostructured Solids; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Fasel, D.; Tran, M.Q. Availability of lithium in the context of the future D-T fusion reactors. Fusion Eng. Des. 2005, 75–79, 1163–1168. [Google Scholar] [CrossRef]
- Lovering, D.G. Molten Salt Technology; Plenum Press: New York, NY, USA, 1982. [Google Scholar]
- Byrns, A.C. Production of Cryolite. U.S. Patent 2,994,582, 1 August 1961. [Google Scholar]
- Galliski, M. La Provincia Pegmatítica Pampeana II: Tipología y distribución de sus distritos económicos. Rev. Asoc. Geol. Argent. 1994, 49, 99–112. (In Spanish) [Google Scholar]
- Galliski, M. La Provincia Pegmatítica Pampeana II: Metalogénesis de sus distritos económicos. Rev. Asoc. Geol. Argent. 1994, 49, 113–122. (In Spanish) [Google Scholar]
- Hien-Dinh, T.T.; Luong, V.T.; Gieré, R.; Tran, T. Extraction of lithium from lepidolite via iron sulphide roasting and water leaching. Hydrometallurgy 2015, 153, 154–159. [Google Scholar] [CrossRef]
- Kuang, G.; Li, H.; Hu, S.; Jin, R.; Liu, S.; Guo, H. Recovery of aluminum and lithium from gypsum residue obtained in the process of lithium extraction from lepidolite. Hydrometallurgy 2015, 157, 214–218. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110–111, 1–5. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Yin, Z.; Wang, Z.; Guo, H.; Peng, W.; Hu, Q. A novel process for extracting lithium from lepidolite. Hydrometallurgy 2012, 121–124, 54–59. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Guo, H.; Hu, Q.; Peng, W.; Wang, J. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117–118, 116–118. [Google Scholar] [CrossRef]
- Brumbaugh, R.; Fanus, W. Determination of lithium in spodumene by flame photometry. Anal. Chem. 1954, 26, 463–465. [Google Scholar] [CrossRef]
- Rosales, G.; Ruiz, M.C.; Rodriguez, M. Alkaline metal fluorides synthesis as subproduct of β-spodumene leaching. Hydrometallurgy 2013, 139, 73–78. [Google Scholar] [CrossRef]
- Rosales, G.; Ruiz, M.C.; Rodriguez, M.H. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147–148, 1–6. [Google Scholar] [CrossRef]
- Rosales, G.; Ruiz, M.C.; Rodriguez, M. Study of the extraction kinetics of lithium by leaching β-spodumene with hydrofluoric acid. Minerals 2016, 6, 98. [Google Scholar] [CrossRef]
- Kumar, M.; Mankhand, T.R.; Murthy, D.S.R.; Mukhopadhyay, R.; Prasad, P.M. Refining of a low-grade molybdenite concentrate. Hydrometallurgy 2006, 86, 56–62. [Google Scholar] [CrossRef]
- Kumar, M.; Nani, M.; Mankhand, T.; Pandey, B. Precipitation of sodium silicofluoride (Na2SiF6) and cryolite (Na3AlF6) from HF/HCl leach liquors of alumino-silicates. Hydrometallurgy 2010, 104, 304–307. [Google Scholar] [CrossRef]
- Lisbona, D.F.; Steel, K.M. Recovery of fluoride values from spent pot-lining: Precipitation of an aluminum hydroxyfluoride hydrate product. Sep. Purif. Technol. 2008, 61, 182–192. [Google Scholar] [CrossRef]
- Burriel Martí, F.; Lucena Conde, F.; Arribas Jimeno, S. Química Analítica Cualitativa; Parainfo: Madrid, Spain, 1998. (In Spanish) [Google Scholar]
- Frontino, P.J.; Giovanini, N.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).