Mechanism of Enhancing Extraction of Vanadium from Stone Coal by Roasting with MgO
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure and Methods
3. Results and Discussion
3.1. Effect of Roasting Temperature on Vanadium Leaching Efficiency
3.2. Effect of Roasting Time on Vanadium Leaching Efficiency
3.3. Effect of MgO Dosage on Vanadium Leaching Efficiency
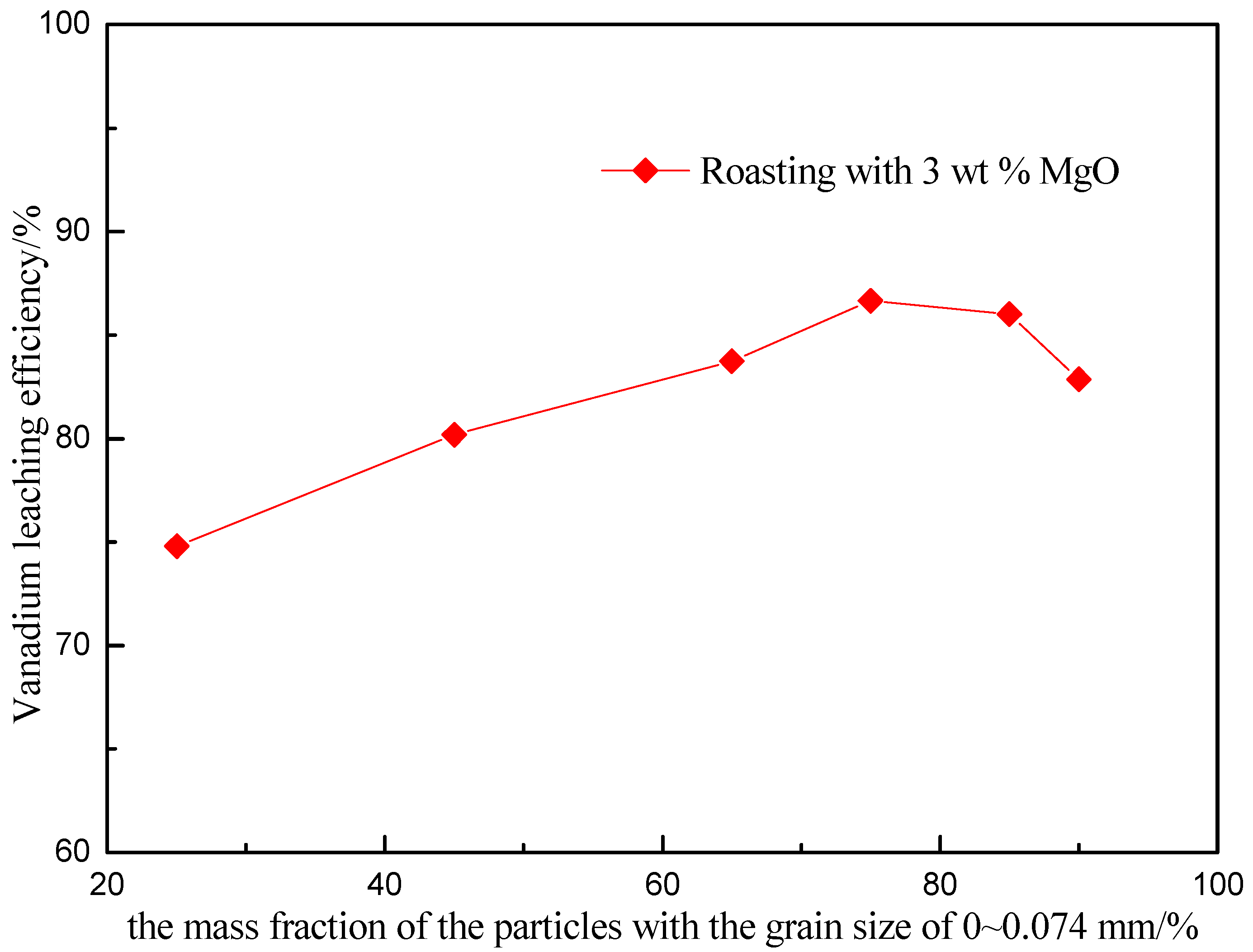
3.4. Effect of the Particle Size of Raw Ore on Vanadium Leaching Efficiency
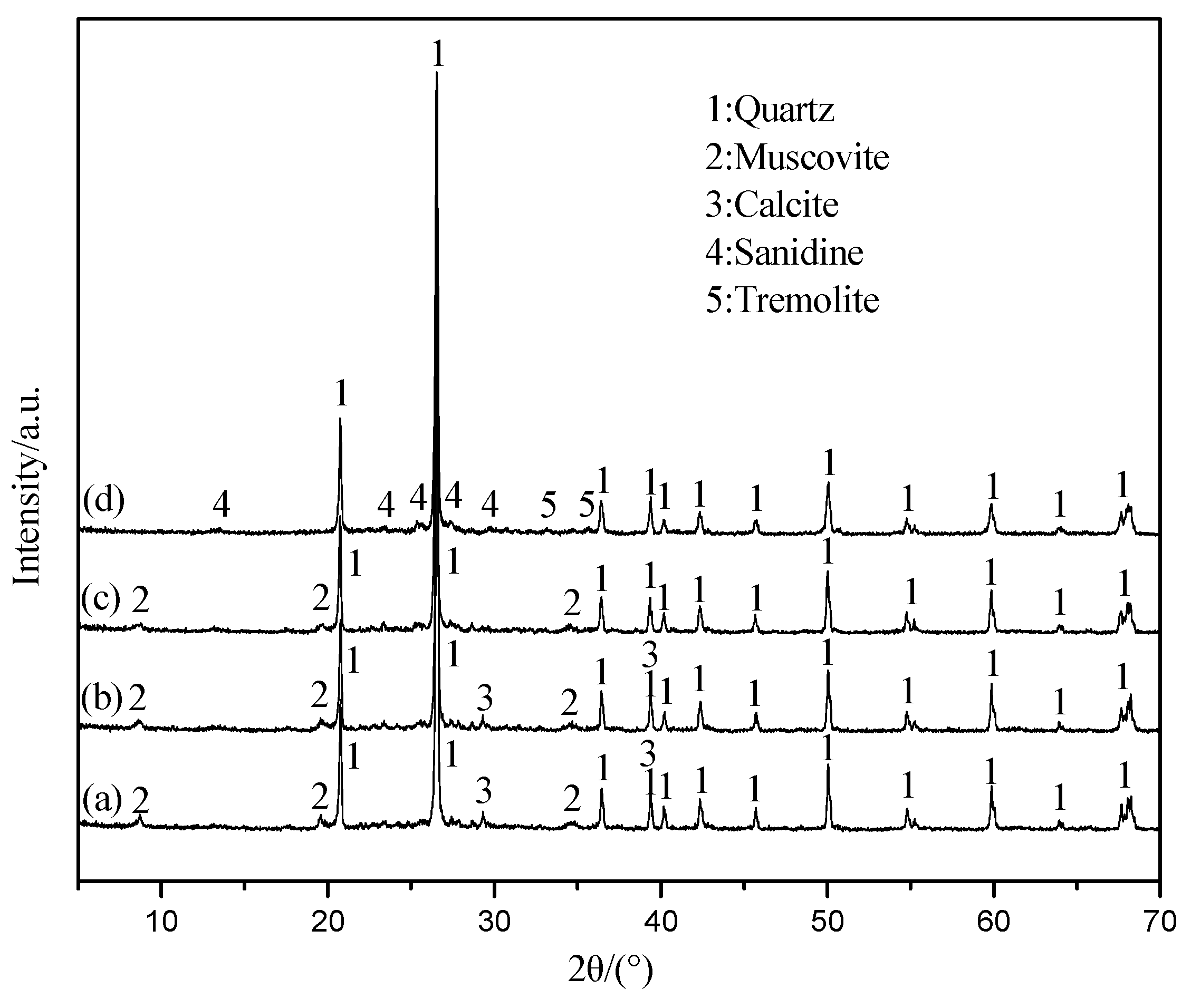
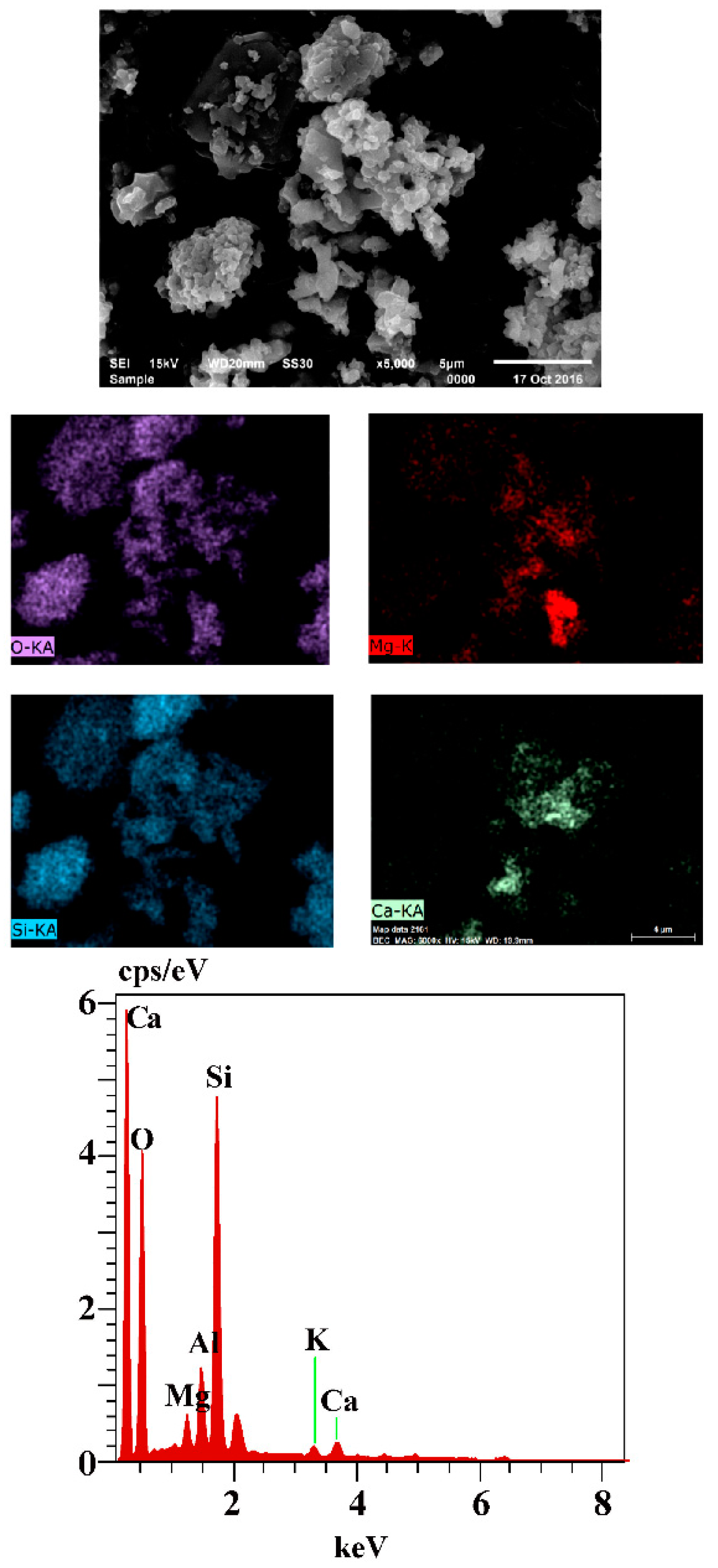
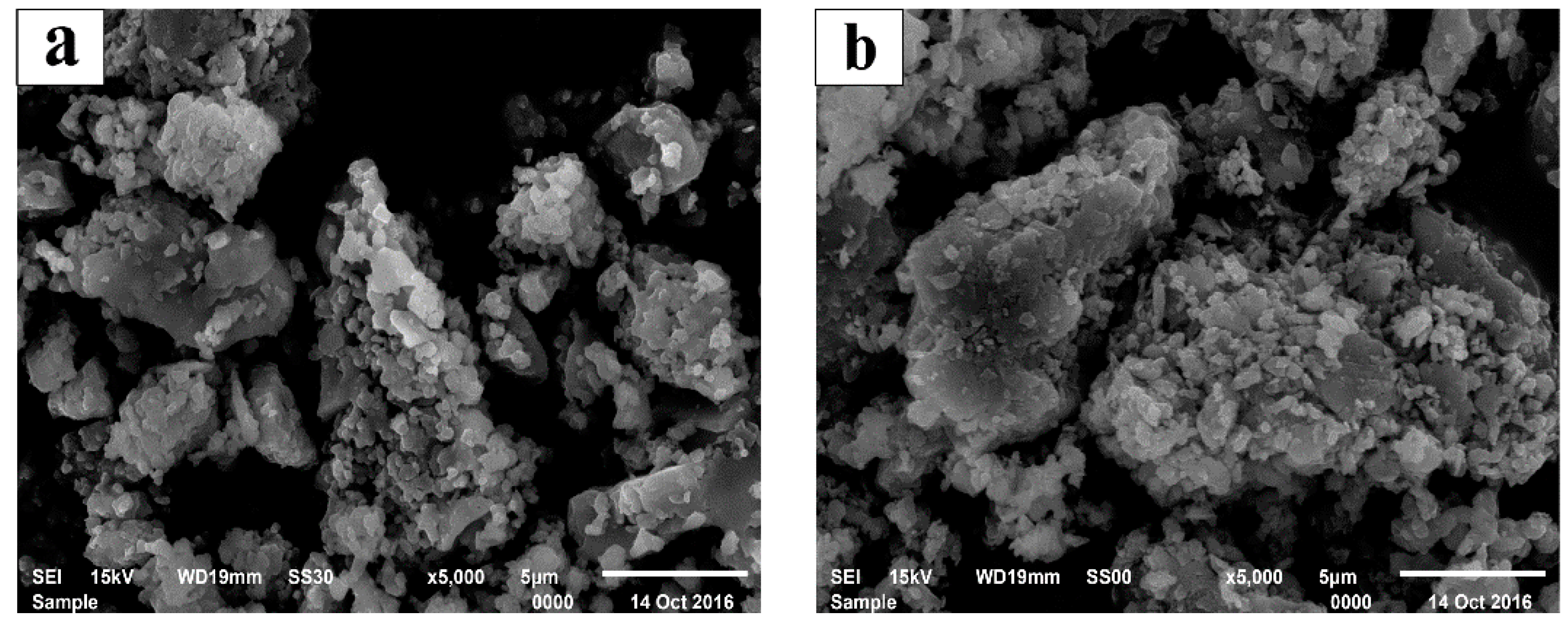
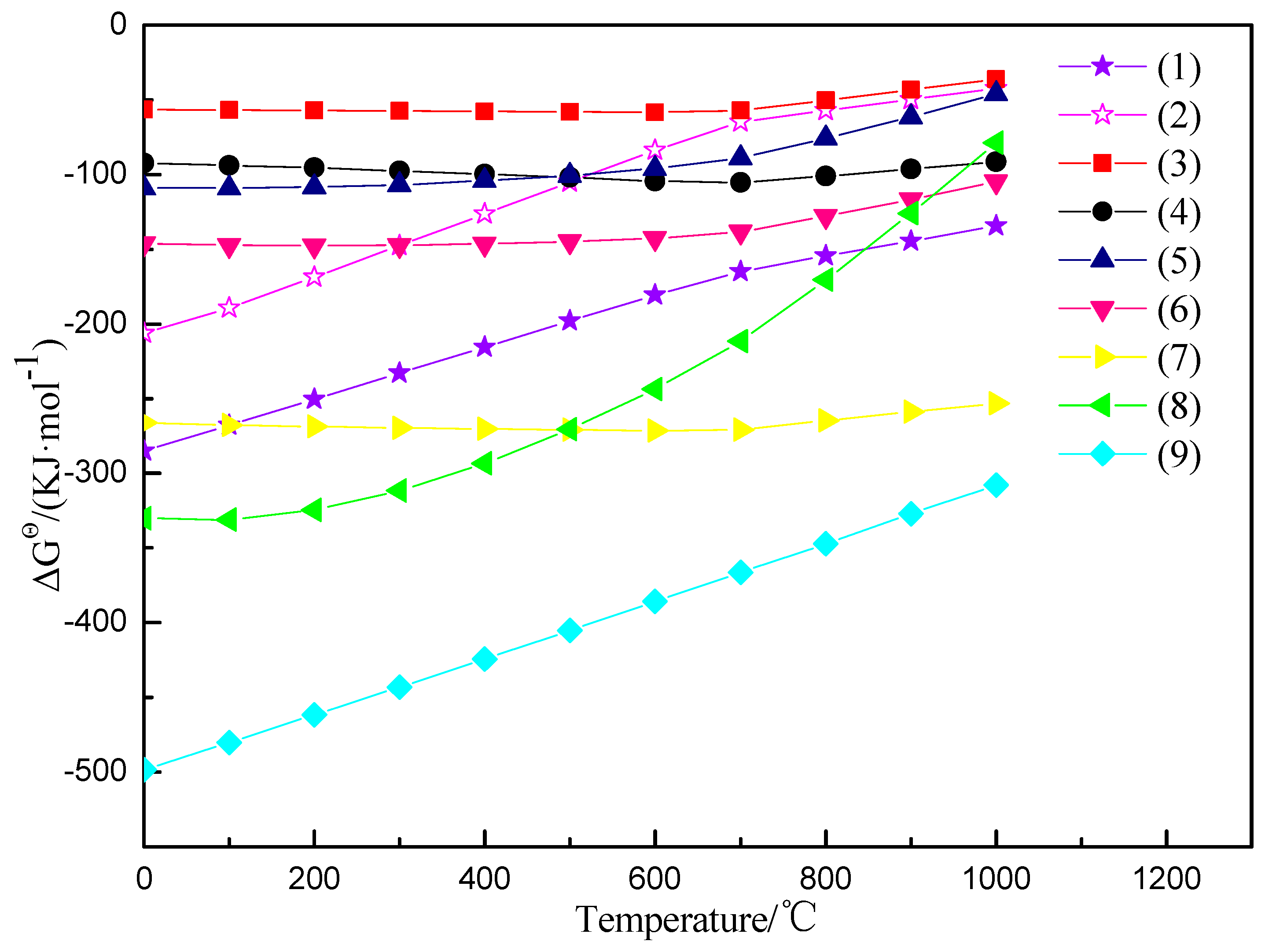
3.5. The Mechanism Analysis of Stone Coal Roasting with MgO
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skyllaskazacos, M.; Cao, L.Y.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemInform 2016, 47, 1521–1543. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate Toxicology and Pharmacological Activities: V10 or V1, Both or None? Oxid. Med. Cell. Longev. 2016, 2016, 6103457. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.M.; Bao, S.X. Separation and recovery of sulfuric acid from acidic vanadium leaching solution of stone coal via solvent extraction. J. Environ. Chem. Eng. 2016, 4, 1399–1405. [Google Scholar] [CrossRef]
- Bin, Z.Y. Progress of the research on extraction of vanadium pentoxide from stone coal and the market of the V2O5. Hunan Nonferr. Met. 2006, 22, 16–20. [Google Scholar]
- Shang, G.H.; Zhang, G.Q.; Gao, C.J.; Fu, W.; Zeng, L. A novel nanofiltration process for the recovery of vanadium from acid leach solution. Hydrometallurgy 2013, 142, 94–97. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J.; Liu, H.; Chen, F. Mechanism of vanadium extraction from stone coal via hydrating and hardening of anhydrous calcium sulfate. Hydrometallurgy 2016, 166, 48–56. [Google Scholar] [CrossRef]
- Ye, G.H.; Zhang, S.; He, W.; Tong, X.; Wu, N. Process mineralogy characteristics of stone coal and its relationship to vanadium extraction. Chin. J. Rare Met. 2014, 38, 146–157. [Google Scholar]
- Hu, P.C.; Zhang, Y.M.; Liu, T.; Huang, J.; Yuan, Y.Z.; Zheng, Q.S. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. J. Ind. Eng. Chem. 2016, 45, 241–247. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.B.; Liu, J. Comparison of direct acid leaching process and blank roasting acid leaching process in extracting vanadium from stone coal. Int. J. Miner. Process. 2014, 128, 40–47. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J. Study of the dissolution behavior of muscovite in stone coal by oxygen pressure acid leaching. Metall. Mater. Trans. B 2016, 47, 694–701. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, X.W. Research status and prospect of vanadium leaching processes from stone coal. Chin. J. Rare Met. 2010, 34, 90–97. [Google Scholar]
- Wang, M.Y.; Xian, P.F.; Wang, X.W.; Li, B.W. Extraction of vanadium from stone coal by microwave assisted sulfation roasting. JOM 2015, 67, 369–374. [Google Scholar] [CrossRef]
- He, D.S.; Feng, Q.M.; Zhang, G.F.; Ou, L.M.; Lu, Y.P. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Deng, Z.G.; Wei, C.; Fan, G.; Li, M.T.; Li, C.X.; Li, X.B. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferr. Met. Soc. China 2010, 20, S118–S122. [Google Scholar] [CrossRef]
- Wang, T.Y.; Xu, L.J.; Liu, C.L.; Zhang, Z.D. Calcified roasting-acid leaching process of vanadium from low-grade vanadium-containing stone coal. Acta Geochim. 2014, 33, 163–167. [Google Scholar] [CrossRef]
- Zhang, X.G.; Gao, Y.B.; Xu, Q.; Liu, D.J.; Long, H.; Tang, Y. Vanadium anhydride extracted from stone coal by calcified roasting and NaOH leaching. Appl. Chem. Ind. 2013, 42, 1026–1028. [Google Scholar]
- Zeng, Y.Y.; Hua, J.; Yan, W.B.; Gao, F.; Cai, J. Leaching vanadium and silicon from stone coal by alkaline process. J. Jishou Univ. Nat. Sci. Ed. 2015, 36, 59–62. [Google Scholar]
- Jin, X.J.; Yang, C.P.; Zeng, G.M.; He, H.J.; Li, C.L.; Luo, Z.B.; Luo, S.X. Vanadium extraction technology from stone coal by oxidizing roasting-alkaline leaching method. Chin. J. Nonferr. Met. 2014, 24, 3177–3184. [Google Scholar]
- Wang, P.; Feng, Y.L.; Li, H.R.; Zhang, P.; Liu, X.W. Extracting vanadium from high-carbon stone coal by oxidizing roasting-acid leaching method. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 2917–2921. [Google Scholar]
- Zhao, Q.; Ning, S.M.; She, Z.H.; Huang, Z.G. Extracting vanadium from stone coal with composite additive. Chin. J. Rare Met. 2013, 37, 961–967. [Google Scholar]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology. Minerals 2016, 6, 26–39. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.B.; Liu, J. A mechanism of calcium fluoride-enhanced vanadium leaching from stone coal. Int. J. Miner. Process. 2015, 145, 87–93. [Google Scholar] [CrossRef]
- Hu, Y.J.; Zhang, Y.M.; Bao, S.X.; Liu, T. Effects of the mineral phase and valence of vanadium on vanadium extraction from stone coal. Int. J. Miner. Metall. Mater. 2012, 19, 893–898. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.M.; Liu, T.; Yang, D. Sintering action of NaCl calcination during extracting vanadium from stone coal. Chin. J. Rare Met. 2009, 33, 894–897. [Google Scholar]
- Zhu, X.B.; Zhang, Y.M.; Liu, T. Experiment and Mechanism of Vanadium Extraction from Stone Coal by Roasting with Activators. Chin. J. Rare Met. 2013, 37, 283–288. [Google Scholar]
- Joung, M.R.; Kim, J.S.; Song, M.E.; Nahm, S.; Paik, J.H. Formation and microwave dielectric properties of the Mg2V2O7 ceramics. J. Am. Ceram. Soc. 2009, 92, 3092–3094. [Google Scholar] [CrossRef]
- Zubkov, V.G.; Leonidov, I.A.; Poeppelmeier, K.R.; Kozhevnikov, V.L. Subsolidus-phase equilibria in the system MgO–V2O5–MoO3. J. Solid State Chem. 1994, 111, 197–201. [Google Scholar] [CrossRef]
- Ji, C.L.; Zhan, Q.L.; Zeng, G.Y. Solubility of magnesium vanadates in water—A basic study on extraction of vanadium from iron ore concentrate pellets with non-sodium salt additive. Nonferr. Met. 1984, 36, 60–66. [Google Scholar]
- He, D.S. Theoretical Investigation of Roasting and Leaching Process of Stone Coal-Type Vanadium Ore. Ph.D. Thesis, Central South University, Changsha, China, 2009. [Google Scholar]
- Ronie, A. Outokumpu HSC Chemistry for Windows: Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database; Outokumpu Research OY: Pori, Finland, 2006. [Google Scholar]
- Li, W.B.; Yuan, Z.F.; Liu, J.X.; Xu, C.; Wei, Q.S. Thermodynamics on the carbochlorination of titanium-bearing ores. Chin. J. Process Eng. 2004, 4, 121–123. [Google Scholar]
- Wang, Y.K. Application of HSC chemistry software in university chemical scientific research. J. Henan Inst. Educ. 2013, 22, 28–30. [Google Scholar]
- Wang, Y.; Thomson, W.J. The effects of steam and carbon dioxide on calcite decomposition using dynamic X-ray diffraction. Chem. Eng. Sci. 1995, 50, 1373–1382. [Google Scholar] [CrossRef]
- Spinolo, G.; Anselmi-Tamburini, U. Mechanism of low temperature calcite decomposition. Solid State Ion. 1989, 32, 413–419. [Google Scholar] [CrossRef]
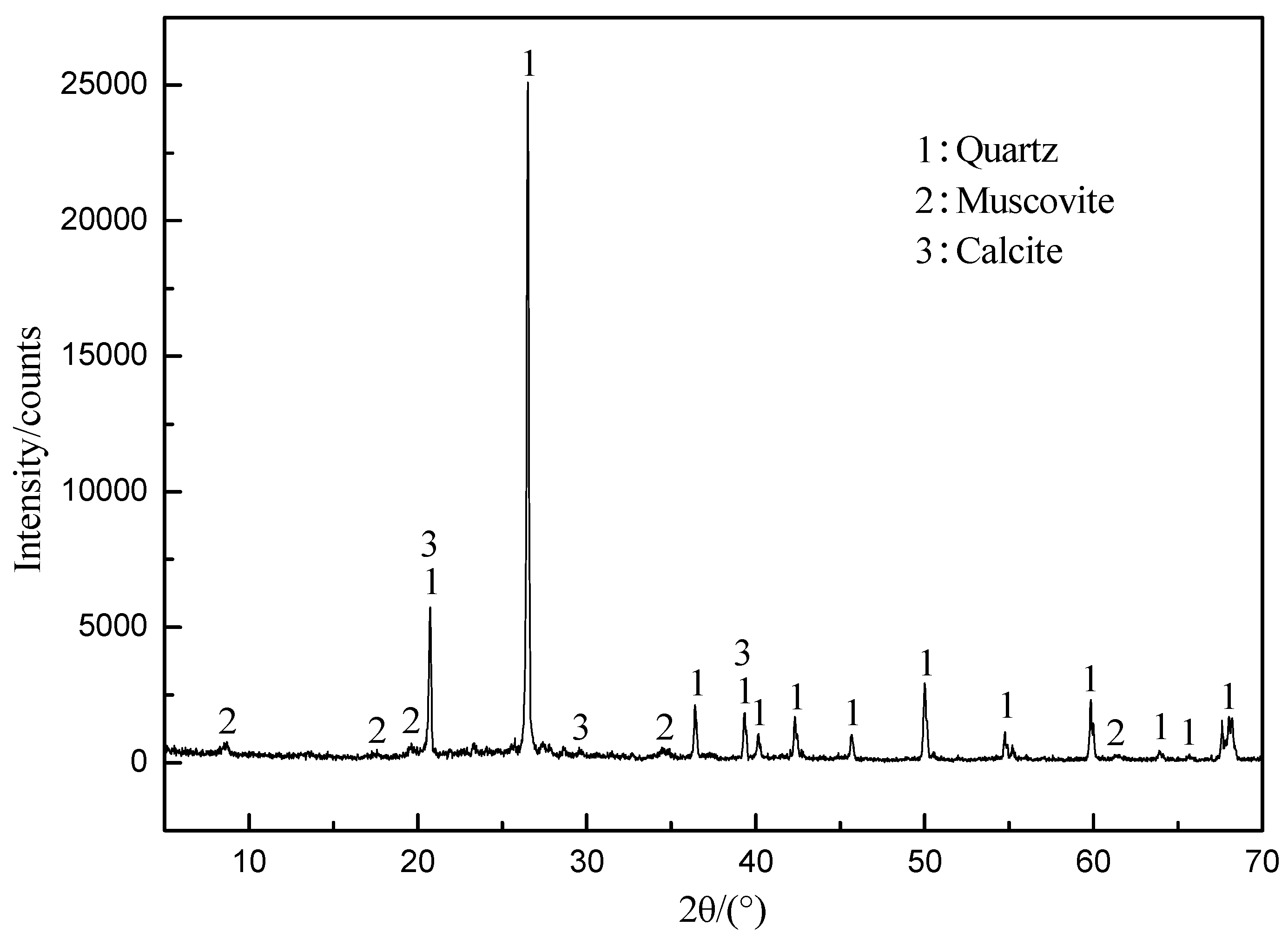
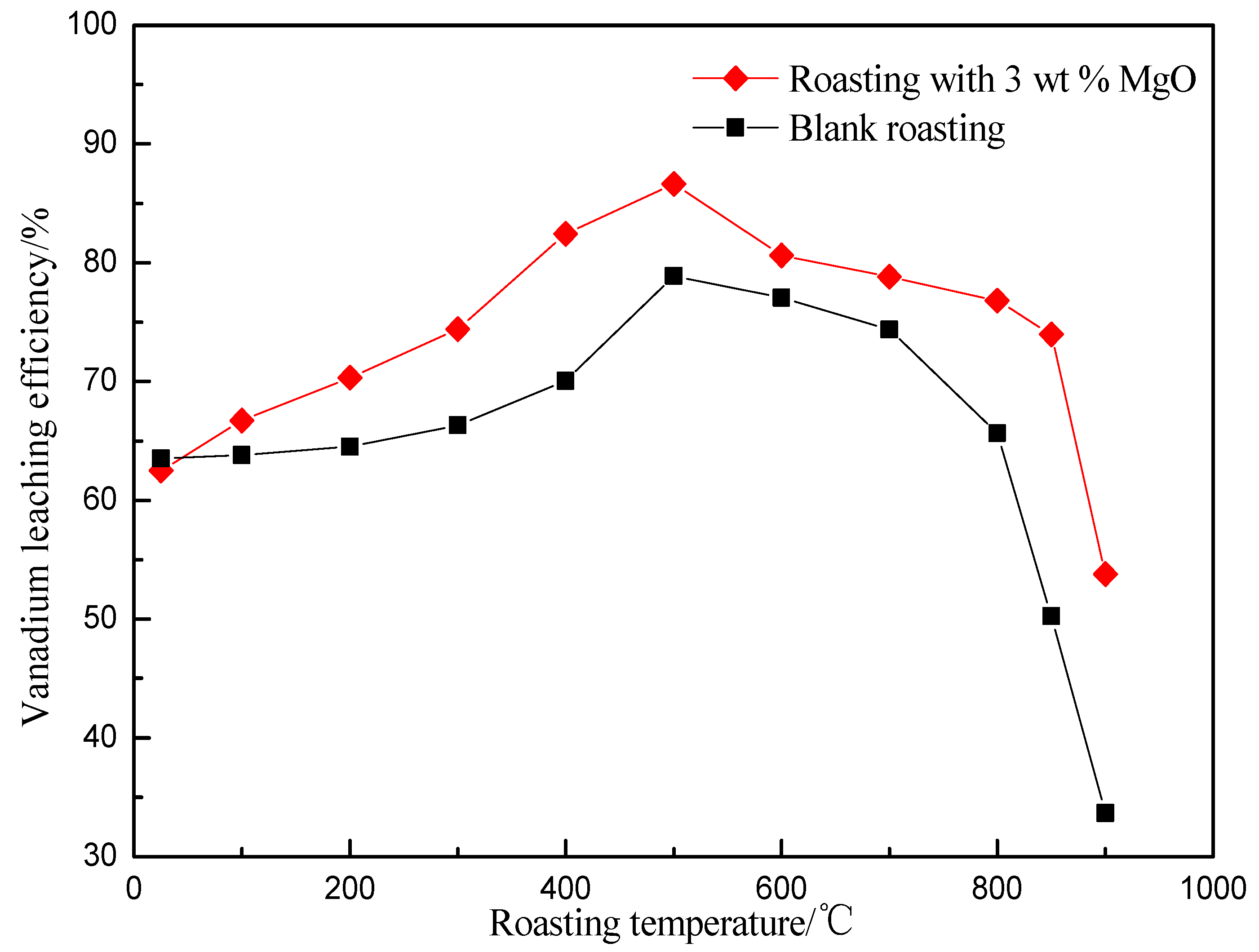
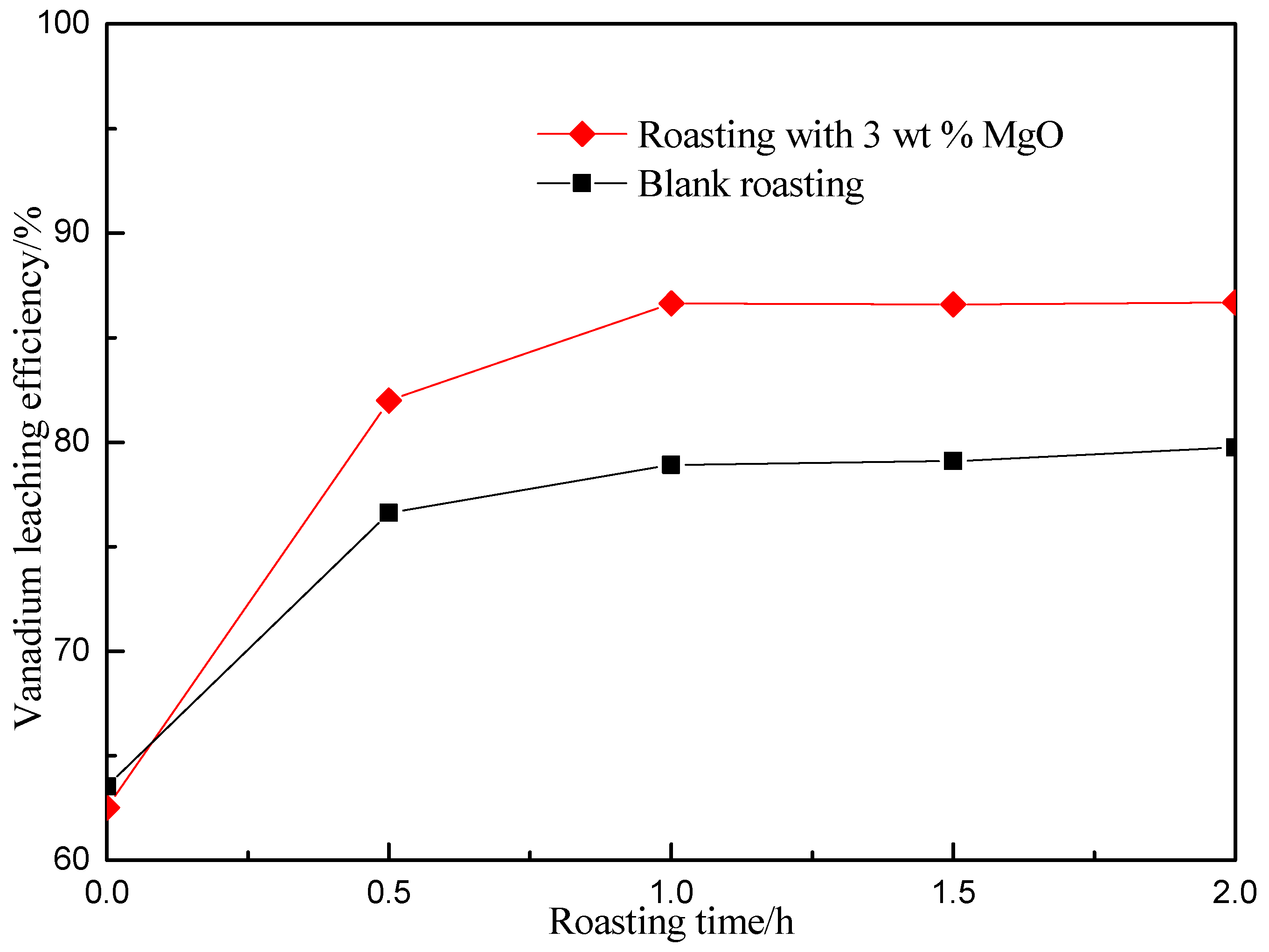
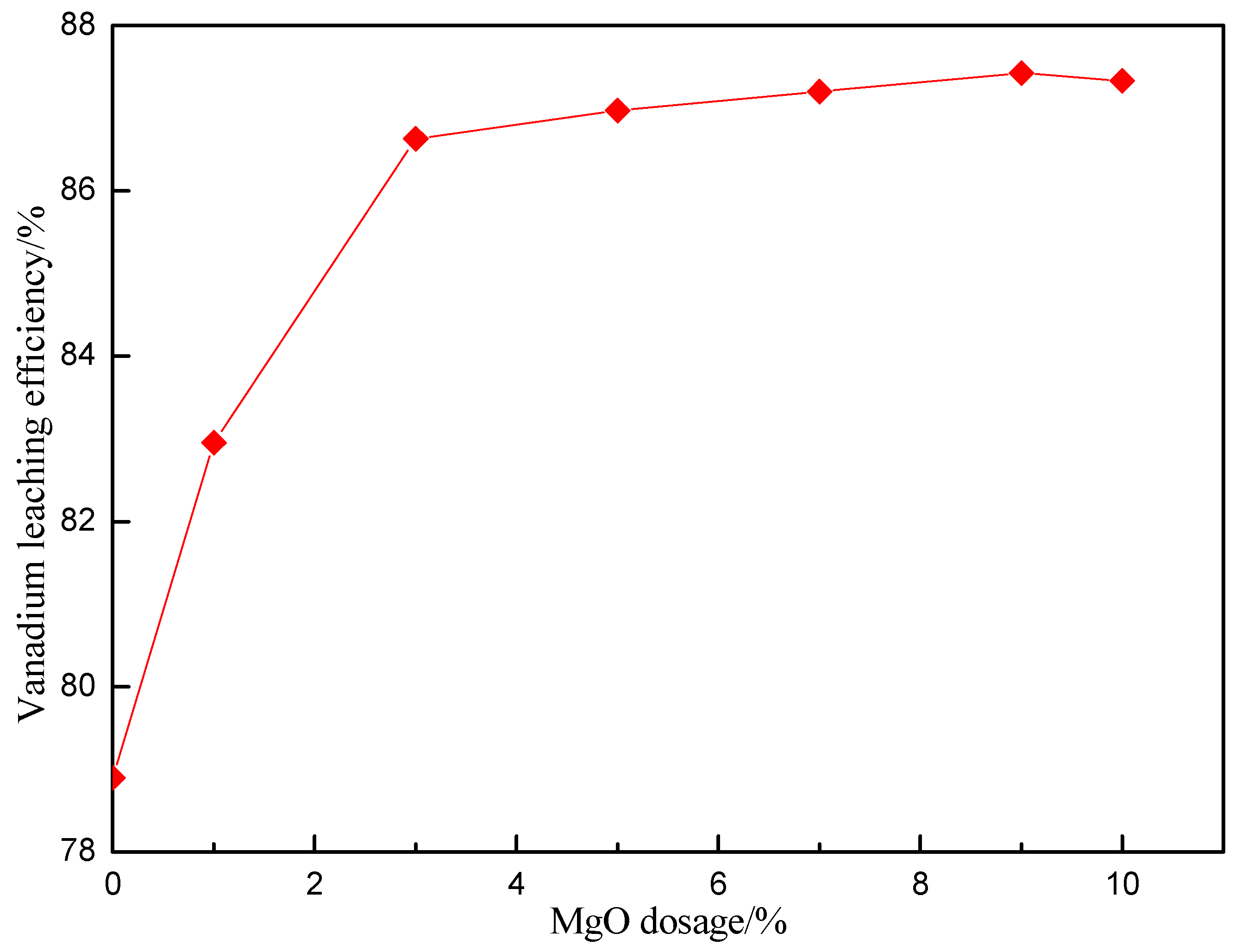

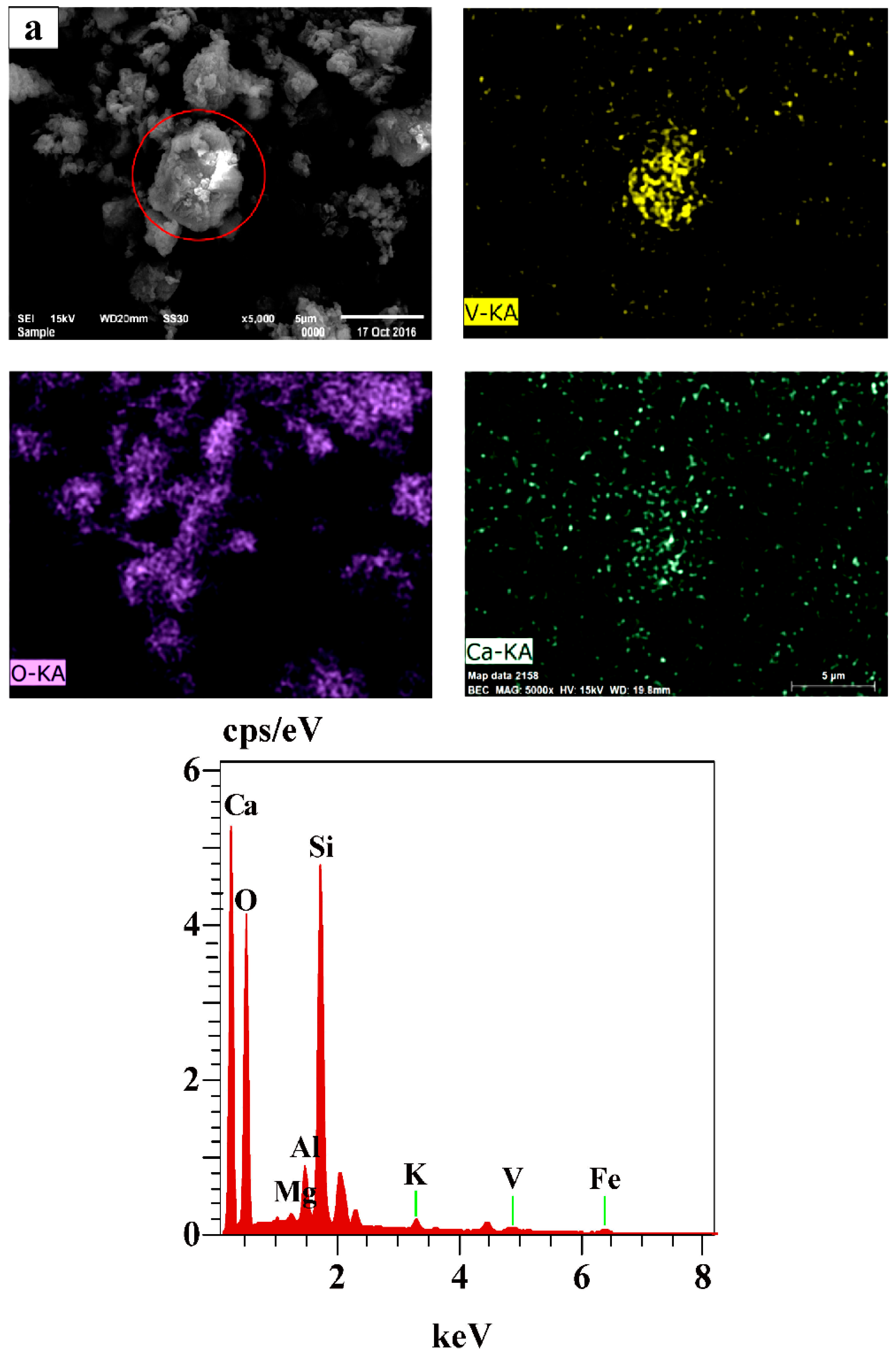
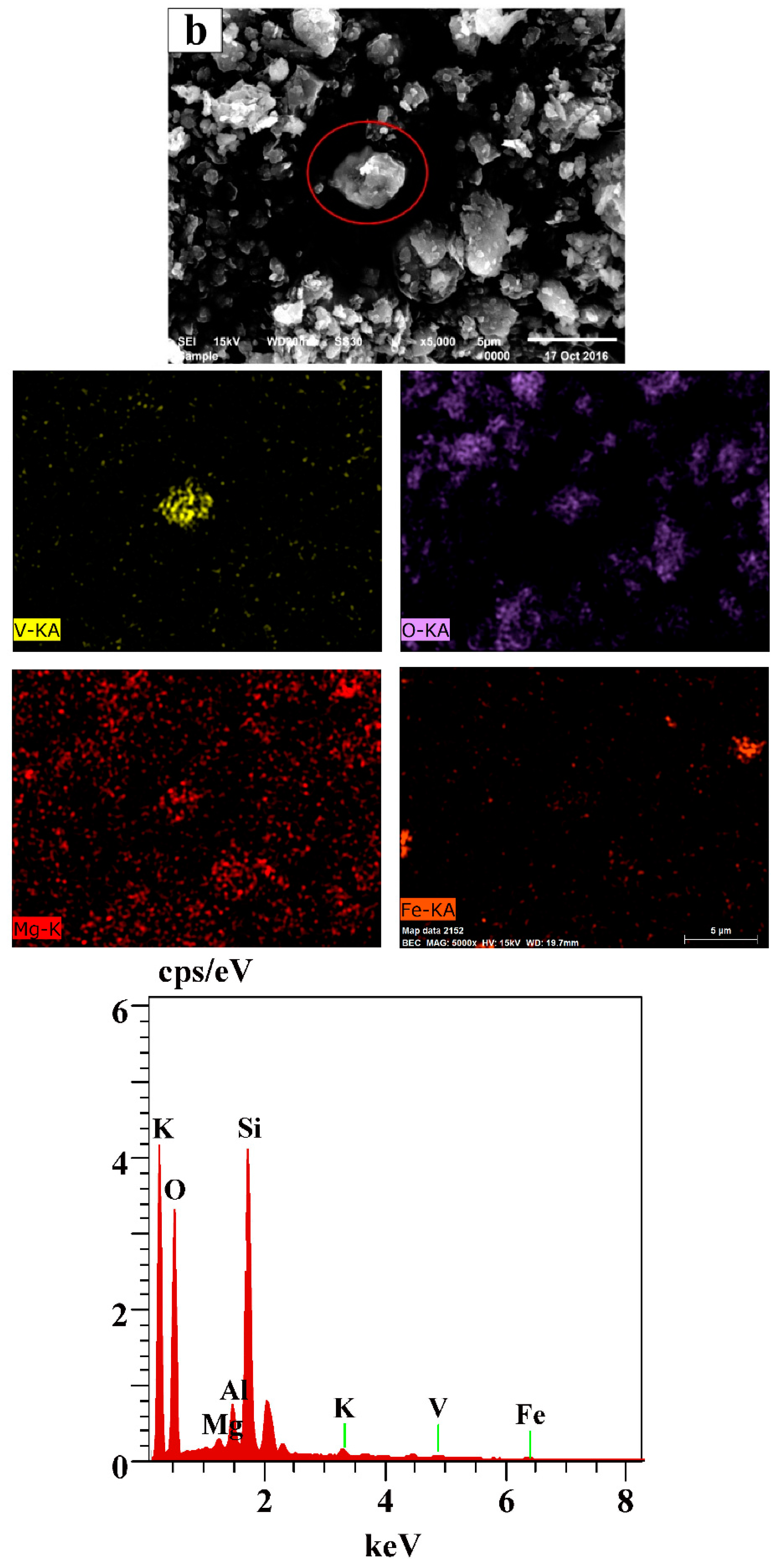
| Element | V2O5 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | C | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.80 | 65.00 | 6.78 | 3.75 | 3.29 | 1.53 | 1.73 | 0.58 | 9.21 | 0.87 |
| Minerals | V | Si | Al | Fe | Ca | Mg | K | O |
|---|---|---|---|---|---|---|---|---|
| Quartz | 0.00 | 42.91 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 49.07 |
| Muscovite | 0.97 | 26.57 | 3.66 | 0.35 | 0.03 | 0.67 | 1.71 | 35.06 |
| Calcite | 0.01 | 0.71 | 0.31 | 0.00 | 38.53 | 0.32 | 0.25 | 16.81 |
| Category | Total Vanadium | Vanadium Phase | Valence State | ||||
|---|---|---|---|---|---|---|---|
| Bound to Free Oxide | Silicoaluminate | Bound to Organics | V(III) | V(IV) | V(V) | ||
| Percentage | 100 | 7 | 89 | 4 | 73 | 27 | 0 |
| No. | Chemical Reactions |
|---|---|
| (1) | V2O3 + O2 = V2O5 |
| (2) | 2V2O4 + O2 = 2V2O5 |
| (3) | MgO + V2O5 = MgV2O6 |
| (4) | 2MgO + V2O5 = Mg2V2O7 |
| (5) | 3MgO + V2O5 = Mg3(VO4)2 |
| (6) | CaO + V2O5 = CaV2O6 |
| (7) | 2CaO + V2O5 = Ca2V2O7 |
| (8) | 3CaO + V2O5 = Ca3V2O8 |
| (9) | 2CaO + 5MgO + 8SiO2 + H2O = Ca2Mg5Si8O22(OH)2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N. Mechanism of Enhancing Extraction of Vanadium from Stone Coal by Roasting with MgO. Minerals 2017, 7, 33. https://doi.org/10.3390/min7030033
Chen F, Zhang Y, Huang J, Liu T, Xue N. Mechanism of Enhancing Extraction of Vanadium from Stone Coal by Roasting with MgO. Minerals. 2017; 7(3):33. https://doi.org/10.3390/min7030033
Chicago/Turabian StyleChen, Fang, Yimin Zhang, Jing Huang, Tao Liu, and Nannan Xue. 2017. "Mechanism of Enhancing Extraction of Vanadium from Stone Coal by Roasting with MgO" Minerals 7, no. 3: 33. https://doi.org/10.3390/min7030033
APA StyleChen, F., Zhang, Y., Huang, J., Liu, T., & Xue, N. (2017). Mechanism of Enhancing Extraction of Vanadium from Stone Coal by Roasting with MgO. Minerals, 7(3), 33. https://doi.org/10.3390/min7030033





