Abstract
At present, the vesuvianite group of minerals consists of eight members, six of which are distinguished by the dominant cation in the Y1(A,B) five-coordinated site. We investigated two vesuvianite samples from the type locality by electron microprobe analysis, Mössbauer and infrared spectroscopy, TGA/DSC, MAS NMR, single-crystal and powder X-ray diffraction. The crystal structures of these samples (# 27844 and 51062 from the Vesuvius collection, Fersman Mineralogical Museum, Moscow) have been refined to R1 = 0.027 and R1 = 0.035, respectively. Both samples have the space group P4/nnc; a = 15.5720(3) and 15.5459(3), c = 11.8158(5) and 11.7988(4), respectively. In both samples low-occupied T1 and T2 sites are populated by minor B and Al, which agrees with their high-temperature origin. According to our experimental results, the general revised crystal-chemical formula of vesuvianite can be written as VII−IXX19VY1VIY12(Z2O7)4(ZO4)10(W)10, where X are seven- to nine-coordinated sites of Ca with minor Na, K, Fe2+ and REE impurities; VY has a square pyramidal coordination and is occupied predominantly by Fe3+ with subordinate Mg, Al, Fe2+ and Cu2+; VIY has octahedral coordination and is predominantly occupied by Al with subordinate Mg, Fe2+, Fe3+, Mn2+, Mn3+, Ti, Cr and Zn; ZO4 = SiO4, sometimes with subordinate AlO4 and/or (OH)4, and W = OH, F, with minor O and Cl. The idealized charge-balanced formula of the vesuvianite end-member without subordinate cations is Ca19Fe3+(Al10Me2+2)(Si2O7)4(SiO4)10O(OH)9, where Ме = Fe2+, Mg2+, Mn2+.
1. Introduction
The vesuvianite-group minerals are widespread in different contact rocks (including skarns formed during contact or regional metamorphism of limestones; in garnetized gabbros, mafic and ultramafic rocks, and serpentinites) of metamorphic, volcanic and hydrothermal origin [1]. They crystallize in a wide range of PT conditions (0–8 kbar and 200–800 °C) at the greenschist up to granulite facies of metamorphism and can be considered as a geothermometer [2]. Because of their crystal structure flexibility, vesuvianite-group minerals can contain variable amounts of di- and trivalent cations, and they are stable under reducing and oxidizing conditions [3].
First found by Kappeler [4] at the Somma-Vesuvius volcanic complex, vesuvianite was initially confused with garnets, schorl, and even obsidian [5]. It was established later as a mineral species with its present name by Werner [6]. Many authors tried to determine its formula on the basis of the chemical data [7,8,9,10], but only structural investigations made it possible to propose the first rational formula for vesuvianite, Ca10Al4(Mg,Fe)2Si9O34(OH)4 [11]. Another formula provided by Machatschki [12] is X19Y13Z18(O,OH)76, where X = Ca (partly with minor Na, K, Mn), Y = Al, Fe3+, Fe2+, Mg, Ti, Zn, Mn, and Z = Si, which is comparable with the improved formula, Ca19(Mg,Fe,Al,Ti,Mn)5Al8(O,OH)10(SiO4)10(Si2O7)4 [13], determined on the basis of accurate single-crystal XRD studies. Boron incorporation into additional T sites (T1 with tetrahedral coordination and T2 with triangular coordination) of the vesuvianite structure is reflected in the following formula: X19Y13Z18T0–5O68W10 [14], where X = Ca, Na, REE3+, Pb2+ and Sb3+; Y = Al, Mg, Fe3+, Fe2+, Ti4+, Mn, Cu and Zn; Z = Si; T = B; W = (OH, F, O). Over the past 50 years, numerous substitution schemes have been established that significantly expand the crystal-chemical diversity of the vesuvianite group:
| Т1B3+ + YMg2+ + 2O10,11O2−↔T1□ + YAl3+ + 2O10,11(OH)− and T2B3+ + 2O10,11O2−↔T2□ + O10,11(OH)− in wiluite [15] |
| Y1Mn3+↔Y1Fe3+ in manganvesuvianite [16] |
| Z1,2(SiO4)4−↔Z1,2(H4O4)4− [17] |
| O10OH− + O11OH−↔O10F− + O11F− in fluorvesuvianite [18] |
| O10OH−↔O10Cl− [19] |
| X3Bi3+ + Y2,3Mg2+↔X3Ca2+ + Y2,3Al3+ [20] |
| Y2Mg2+ + Y3Ti4+↔Y2Al3+ + Y3Al3+ [21] |
| X1,4Сa2+ + Y2,3Al3+↔X1,4Na+ + Y2,3Ti4+ in “natrovesuvianite” [22] |
| Y1Cu2+ + Y2,3Mn3+↔Y1Fe3+ + Y2,3Mg2+ in cyprine [23] |
| Y1Mg2+ + Y3Al3+↔Y1Fe3+ + Y3Mg2+ in magnesiovesuvianite [24] |
| Y1Fe3+↔Y1Al3+ in alumovesuvianite [25] |
At present, the vesuvianite group consists of seven mineral species (Table 1), distinguished by the dominant component at five-coordinated Y1 site, as well as T1 and T2 and anionic W positions. Taking into account cation ordering in the octahedral positions and the incorporation of additional B, Na and (H4O4)4− into the structure, the general formula of the vesuvianite-group minerals can be written as follows (Z = 2): X16X12X4Y1Y24Y38T0–5(Z2O7)4[(ZO4)10−x(H4O4)x](W)9O1–3, where x < 3, X are seven- to nine-coordinated sites (Ca, Na, K, Fe2+, REE), X4 has square antiprismatic coordination (Ca, Na), Y1 has square pyramidal coordination (Fe3+, Mg, Al, Fe2+, Cu2+), Y2 and Y3 have octahedral coordination (Al, Mg, Zn, Fe2+, Fe3+, Mn2+, Mn3+, Ti, Cr, Zn), T is the additional site with triangular or tetrahedral coordination (B, Fe), ZO4 [SiO4, □, (OH)4, AlO4] and Z2O7 (Si2O7) and the additional anionic position W can be occupied by OH, F, or minor O, Cl [12,13,15,17,19,21,26,27,28,29].

Table 1.
Dominant components in crystallographic sites of vesuvianite-group minerals 1.
The crystal structure of vesuvianite-group minerals contains half-populated cation sites arranged along the fourfold axis in the Y1–X4–X4–Y1 sequence with the Y1–X4 and X4–X4 distances less than 1.3 Å and 2.5 Å, respectively [28]. Cation ordering at the Y1 and X4 sites produces different ordering schemes and leads to the lower symmetry P4/nnc → P4/n and P4/nnc → P4nc [13,30], which results in the appearance of subsites (for example Y1A and Y1B instead Y1, X4A and X4B instead X4, etc.).
However, the general formula of vesuvianite given in the International Mineralogical Association (IMA) List of Minerals (http://ima-cnmnc.nrm.se/imalist.htm), (Ca,Na)19(Al,Mg,Fe)13(SiO4)10(Si2O7)4(OH,F,O)10 [36], does not explicitly show the occupancies of the Y1 site. This simplified formula is now out of date since it refers to five vesuvianite-group minerals: vesuvianite sensu stricto, manganvesuvianite, cyprine, magnesiovesuvianite and alumovesuvianite. For this reason, we report the results of a multimethodological study of two vesuvianite samples from the type locality of this mineral, i.e., the Somma-Vesuvius complex, Campania, Italy. The studied samples nos. 27844 and 51062 originate from the Vesuvius collection of the Fersman Mineralogical Museum, Moscow. The aim of this work is to establish a correct formula of vesuvianite, which does not contradict the IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) guidelines [37].
2. Occurrence
Vesuvianite from the Somma-Vesuvius volcanic complex (Campania region, Southern Italy), typically occurs in various kinds of xenolithic rocks, commonly associated with various Plinian eruptives and found as ejecta in pyroclastic deposits [38,39,40,41].
The typical occurrence of vesuvianite is related to xenoliths of skarn, a largely mineralogically zoned, coarse-grained Ca-Mg-Fe(Mn)-rich silicate rocks [42]. At Somma-Vesuvius, these rocks are very complex in mineralogical and textural aspects. Most of skarns at Somma-Vesuvius complex show a sequence of mono- or bimineral zones dominated by Ca- and/or Mg-silicates, such as clinopyroxene, phlogopite, wollastonite, meionite, olivine, or clinohumite, and other minerals (perovskite, spinel, calcite, etc.) commonly with a central cavity containing euhedral crystals of vesuvianite, garnet, wollastonite, gehlenite, or anorthite. Mono- or bimineral skarns, as well as composite ejecta (consisting of two or more rock types, such as skarn-marble, skarn-hornfels, cumulate-skarn or cumulate skarn-marble are common and document the close spatial association of at least some of these rock types at depth) with sharp contacts of skarns to marbles or hornfelses are common [42,43,44]. The mineralogical composition of skarn ejecta generally includes highly variable amounts of vesuvianite, wollastonite, clinopyroxene (diopside, hedenbergite, low-iron augite, i.e., “fassaite”), anorthite, phlogopite, clinoamphibole, garnet (mainly of the grossular-andradite series), forsterite, and humite-group minerals. Other minerals locally observed as minor to accessory phases include melilite (gehlenite), feldspathoids (leucite, nepheline, sodalite, haüyne, nosean, scapolite, meionite, davyne, balliranoite), fluorapatite, fluorite, cuspidine, zircon, perovskite, calcite, baddeleyite, spinels (spinel sensu stricto, magnetite), REE-minerals, sulfides, etc. [41,42]. The vesuvianite-bearing calc-silicate ejecta are most likely related to the Plinian eruptions of Avellino (3800 BC) and Pompeii (79 AD) [44]. The investigations of fluid inclusions as well as the isotope compositions of Pb, Nd and Sr have been carried out, among others, on vesuvianite crystals as reported in [42]. According to [42], the formation of skarns can be related to assimilation of carbonate-wall rocks by the alkaline magma at moderate depths (<5 km), and consequent exsolution of CO2-rich vapor and complex saline melts from the contaminated magma that reacted with the carbonate rocks to form skarns. According to fluid inclusion studies [42], the estimated temperature of the formation skarn minerals at Somma-Vesuvius complex is in the range 800–900 °C.
Together with the skarn occurrence, vesuvianite has been also observed in thermally metamorphosed carbonate nodules (“marbles” [5]), interpreted as the result of recrystallization and in part, devolatilization without significant metasomatic interaction with aqueous fluids or silicate melts [42]. In these calcitic marbles, vesuvianite is variably associated with (i) wollastonite, clinopyroxene, sodalite and humite; (ii) clinopyroxene, sodalite, cuspidine, forsterite, nepheline and davyne; or (iii) sanidine, fluorite, fluorapatite and sulfides [5].
In addition, vesuvianite occurs as an accessory mineral in syenitic K-feldspar-rich nodules [5,39]. In this rock, it is locally observed in vugs and cavities as grains (<1 mm) among the tabular sanidine crystals, in association with garnet, clinoamphibole, clinopyroxene, davyne, titanite, or with fluorite, zircon and wöhlerite-group minerals [5]. In [39], accessory vesuvianite crystals (~200–500 µm) were observed in the K-feldspar syenitic ejecta together with other minor to trace phases that can be alternatively represented by clinoamphibole, clinopyroxene, biotite, plagioclase, sodalite, nosean, nepheline, hematite, magnetite, fluorite and titanite. Vesuvianite also occurs in sanidinite ejecta found at the San Sebastiano valley at Vesuvius in association with pyrochlore, magnetite, wöhlerite group minerals, baddeleyite, zircon, fluorite, ferrohornblende, phlogopite and nepheline [45].
In samples 51062 and 27844 (Figure 1), euhedral vesuvianite occurs in association with grossular and clinozoisite or as a monomineralic aggregate, respectively; the large sizes of the crystals indicate their skarn provenance. In particular, the association of vesuvianite with garnet, wollastonite, gehlenite or anorthite in the first sample is typical for zoned skarns of the Somma-Vesuvius complex [42]. It is worth nothing that the occurrence of clinozoisite in the sample 51062 represents, to the authors’ knowledge, the first record of this mineral at Somma-Vesuvius.
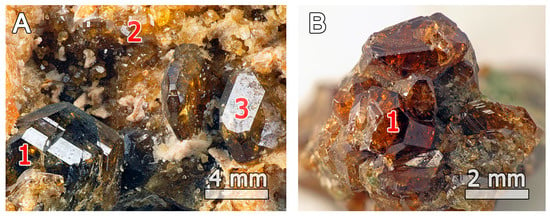
Figure 1.
Short-prismatic vesuvianite crystals (1) associated with grossular (2), and clinozoisite (3), in samples (A) 51062 and (B) 27844.
3. Materials and Methods
Both vesuvianite samples studied in this work (27844 and 51062) form well-shaped short-prismatic crystals up to 6 mm long (Figure 1) with the main prismatic forms {100} and {110} terminated by the pyramidal {101} and pinacoidal {001} faces. The crystals are lustrous and semi-transparent. Their color varies from greenish brown (27844) to brownish green and orange-brown in the marginal zone (51062).
Chemical analyses of both samples (Table 2, each analysis is the mean from 4 to 7 points) were obtained using a HITACHI S-3400N scanning electron microscope (Tokyo, Japan) equipped with INCA Wave 500 WDS spectrometer. The system was operated at 20 kV and 10 nA, and the electron beam was focused to a 5 µm spot. The following standards were used: rutile (Ti), wollastonite (Si, Ca), forsterite (Mg), fluorite (F), cryolite (Na), halite (Cl), spessartine (Mn, Al), hematite (Fe), danburite (B), synthetic Ce-glass (Ce), synthetic Nd-glass (Nd). The content of boron was measured at the Faculty of Geology, University of Warsaw, by a CAMECA SX100 instrument operated at 6 kV and 80 nA for 50–100 s counting at each point (Kα line, PC2 crystal (Ni/C-LSM), standard deviation 0.25 wt % B2O3). No other elements having atomic numbers above eight were found above their detection limits. The content of H2O in 27844 was determined by means of thermogravimetric analysis using NETZSCH STA 449 F3 Jupiter thermoanalyzer (Exton, PA, USA) during 10 °C/min heating of a 68.4 mg sample from room temperature to 1100 °C in a dynamic argon atmosphere, with aluminum oxide standard.

Table 2.
Single-crystal data, data collection and structure refinement parameters.
The 27Al nuclear magnetic resonance (NMR) spectrum was obtained at room temperature by means of a Bruker Avance III 400 WB spectrometer (Billerica, MA, USA) at 104.24 MHz. The magic-angle spinning (MAS) spin rate of the rotor was 20 kHz. For these investigations, a single-pulse sequence with a pulse length of 86 kHz was used, with the recycle delay of 1 s and 4096 scans. The 1H NMR spectrum was obtained at room temperature using a Bruker Advance III 400 WB spectrometer operating at 400.23 MHz. The MAS spin rate of rotor was 20 kHz. A single-pulse sequence was used with a pulse length of 100 kHz, recycle delay of 20 s and 32 scans.
The Fe2+/Fe3+ ratio is given in accordance with the Mössbauer spectrometry data. Gamma-resonance studies of 27844 and 51062 were carried out using a commercial Mössbauer spectrometer (WissEl Wissenschaftliche Elektronik GmbH, Starnberg, Germany) in order to clarify the valence state of iron and the coordination environment(s) of the Fe. The spectrometer was equipped with a standard radioactive source, 57Co, in a rhodium matrix (Ritverc). The absorption-mode measurements have been performed at room temperature. The samples were crushed in an agate mortar, distributed homogeneously within the punch-holder and packed in the form of a tablet with a density of 112 mg/cm2 in order to optimize the conditions for measurement. The spectral fitting of the experimental Mössbauer spectrum was carried out by the method of least squares under the assumption of Lorentz-shaped spectral lines. Isomer shifts were measured relative to metallic iron. The mathematical analysis was performed with the commercial Mössbauer software SpectRelax Version 2.1 (Moscow State University, Moscow, Russia).
A FTIR spectrum of 27844 was collected using a Bruker ALPHA spectrometer at room temperature in the range 360–3800 cm−1 (KBr pellet, resolution 4 cm−1, 16 scans). A pure KBr pellet was used as a reference.
Powder X-ray diffraction data (Table A1) were collected with a Bruker Phaser D2 diffractometer in the 2θ range of 10–80° (CuKα; 1.5418 Å), with a scanning step of 0.02° (1 s per step) in 2θ.
Single-crystal X-ray diffraction experiments were carried out using an Agilent Technologies Xcalibur Eos diffractometer (Agilent Technologies, Santa Clara, CA, USA) operated at 50 kV and 40 mA. A hemisphere of three-dimensional data was collected at 293 K for each sample using monochromatic Mo Kα radiation, with frame widths of 1° and 20 s count for each frame. The crystal-to-detector distance was 45 mm. For the investigated samples, P4/nnc was chosen as the most probable space group. Only 79 reflections violating the absence conditions in the range 2σ(I) < I < 188σ(I) with maximum Fo2 = 881 were found for 27844, whereas only 15 such reflections with 2σ(I) < I < 382σ(I) and maximum Fo2 = 2021 were observed for 51062. Refinements of both structures in space groups P4/n and P4nc did not indicate any reasons for the symmetry lowering from the P4/nnc space group.
The crystal structures of 27844 and 51062 were refined to R1 = 0.027 and R1 = 0.035 for 1653 and 1622 unique observed reflections with |Fo| ≥ 4σF, respectively, using the SHELX program [46]. Empirical absorption corrections were applied in the CrysAlisPro program complex [47] using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The final difference Fourier map showed no features higher than 0.53/0.56 e− for 27844 and 51062, respectively. The experimental details and crystallographic parameters are given in Table 2. Crystallographic information files (CIFs) are deposited in the electronic Supplementary Materials.
4. Results
4.1. Chemical Composition
The crystal-chemical formulae of the investigated samples (Table 3) were calculated on the basis of 19 X-cations (Ca + Na + REE). Both compositions are in good agreement with the previous data for vesuvianite from the Monte-Somma-Vesuvius complex [38,48].

Table 3.
Chemical composition of 27844 and 51062 1.
4.2. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA/DSC)
The TGA curve (Figure 2) of 27844 contains one major step of weight loss, accompanied by two endothermic effects at 980 °C and 1030 °C in the DSC-curve, which can be assigned to separated dehydroxylation processes for the O(11)H and O(10)H groups [23,49]. According to [49,50] one-step weight loss in the temperature range of 820–1090 °C is characteristic for low-temperature vesuvianite. Weight loss in the range of 990–1150 °C responds to the high-symmetry vesuvianite. The total weight loss for 27844 is 1.60 wt %, which corresponds to 5.22 OH-groups per formula unit. As a result of dehydroxylation, vesuvianite transforms into the mixture of wollastonite, grossular, quartz and an amorphous glass-like substance.
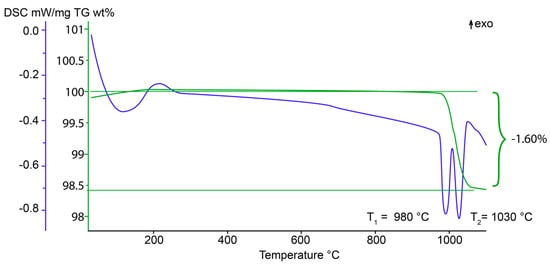
Figure 2.
Thermogravimetric (wt %, green line) and differential scanning calorimetry (blue line) curves for 27844.
4.3. Solid State Magic-Angle Spinning Nuclear Magnetic Resonance (MAS NMR)
The 27Al MAS NMR spectrum (Figure 3) of 27844 contains one broad peak, which is centered at −2.5 ppm and can be assigned to the octahedrally coordinated Al at the Y2 and Y3 sites [41,42,43,44,45,46,47,48,49,50,51,52]. Similarly to wiluite, broadening of the peak at 60 ppm can be assigned to a trace amount of Al in the 4-coordinated T1 site (<1 apfu) [29]. The weak symmetric peaks observed near ±190 ppm belong to spinning sidebands.
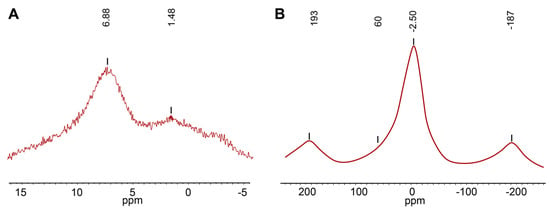
Figure 3.
(A) 1H MAS NMR and (B) 27Al MAS NMR spectra of 27844.
The 1H MAS NMR spectrum (Figure 3) of 27844 contains two peaks centered at 6.9 and 1.5 ppm, respectively. Calculations of the O⋯O distances using the equation δiso(ppm) = 79.05 − 0.255d (O–H⋯O)(pm) [53] provided the values of 2.83 Å and 3.04 Å. The most intense peak at 6.9 ppm corresponds to the O11–H1a⋯O7 and O10–H2⋯O10 hydrogen interactions, which is in agreement with the distances 2.784(4) and 2.74–2.82 Å reported by other authors [54,55,56,57,58,59]. A low intense line at 1.5 ppm can be assigned to the H1b site, since its chemical shift agrees well with the O11–H1⋯O11 distances of 3.01–3.04 Å [54,57].
4.4. Mössbauer Measurenments
The Mössbauer spectra of 27844 and 51062 can be described as a superposition of five and four symmetric doublets, respectively (Figure 4). The assignments based on the crystal-structure refinement and Mössbauer parameters of these doublets (isomer shifts IS, quadrupole splittings QS, line widths and areas under resonance doublets S) according to [21,60,61] are listed in Table 4. Trivalent iron dominates in both samples: C doublets with IS = 0.38, 0.38, QS = 0.49, 0.52 and S = 54, 60% are assigned to Fe3+ at Y3 octahedra, whereas E doublet with IS = 0.48, 0.41, QS = 1.08, 1.31 and S = 12.8, 16.3 corresponds to Fe3+ at the five-coordinated Y1 site. D doublets with IS = 0.82, 0.84, QS = 0.40, 0.43 and S = 18, 15% correspond to divalent iron at the octahedral Y3 site. The B doublets with IS = 1.02, 1.03, QS = 2.92, 2.92 and S = 18, 15%, similar to doublets reported in [21,61,62,63], correspond to minor amounts of Fe2+ at the Y1 site. The G doublet with IS = 0.92 and QS = 2.40 observed in the Mössbauer spectra of 27844 and wiluite [29] can be assigned to the octahedrally coordinated Fe2+ incorporated into the Y2 site.
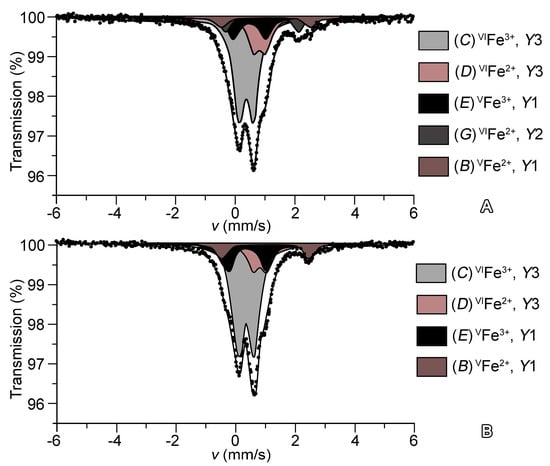
Figure 4.
Mössbauer spectra of (A) 27844 and (B) 51062.

Table 4.
Parameters of the Mössbauer spectra of 27844 and 51062 at 293 K.
4.5. X-ray Crystallography
The unit-cell dimensions of 27844 were determined from the X-ray powder-diffraction pattern (Figure 5) by Rietveld refinement using the program Topas 4.2 [64] and are in a good agreement with the single-crystal XRD data: a = 15.5779(6) Å, c = 11.8156(6) Å, V = 2867.3(4) Å3.
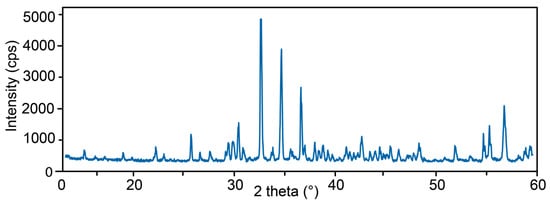
Figure 5.
Powder diffraction pattern of 27844. All reflections refer to vesuvianite (Table A1).
Structural models of 27844 and 51062 are close to that reported in [48], with site designations according to [15]. We pay particular attention to the occupancy of the Y1 site that plays a key role in the diversity of the vesuvianite-group minerals.
In the crystal structure of vesuvianite, the five-coordinated Y1 site (Table 1 and Figure 6) can be occupied by Fe3+, Fe2+, Mn3+, Mn2+, Cu, Mg and Al [16,23,30,51,52,55,65]. In most cases, including previously studied vesuvianite from the Somma-Vesuvius volcanic complex, Fe3+ dominates in the Y1 site [48]. In accordance with the 27Al MAS NMR data, five-coordinated Al3+ in 27844 is absent, and the final occupancy of the Y1 site is determined by Fe and Mg. In the structures of 27844 and 51062, iron is predominant at the Y1 site: (Fe0.72Mg0.28)1.00 and (Fe0.73Mg0.27), respectively, or, taking into account Mössbauer data, Fe3+0.50Mg0.28Fe2+0.22 and Fe3+0.47Mg0.27Fe2+0.26, respectively. Scattering of the Y2 site in both samples is close to 13 ē, and the average <Y2–O> bond lengths are equal to 1.896 and 1.895 Å for 27844 and 51062, respectively, which agrees well with the site occupancy by Al atoms only. The octahedral Y3 site has minor admixtures of iron (mainly Fe3+), which is confirmed by a slightly higher mean length of the <Y3–O> bonds: 1.966 and 1.961 Å, respectively. The total refined Y3 site occupancies are (Al0.90Fe0.10)1.00 and (Al0.91Fe0.09), respectively. Both the contents and oxidation states of iron have been confirmed by the Mössbauer data.
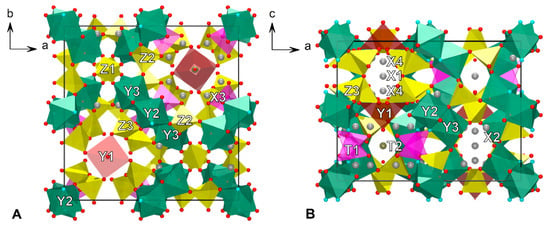
Figure 6.
The crystal structure of 27844 projected along (A) c axis and (B) b axis. T-sites have low occupation: T1 ≈ 10% and T2 ≈ 25%. The O11 site with mixed (O0.75F0.25) occupancy represent by blue circles, oxygen atoms are red circles.
The mean <Z − O> bond lengths and scattering factors of the tetrahedral Z1, Z2 and Z3 sites are in agreement with the full occupancies of these sites by Si atoms only.
In the refinement model, the 7- to 9-coordinated X1, X2, X3 and X4 sites are completely populated by Ca. The X sites have full occupancies except for the eightfold-coordinated X4 position that is half-populated and is situated in the structure channels. According to [66], REEs incorporate into the X3 site, but their low content (1.5% of the X3 site occupancy) cannot be precisely determined by the structural analysis. Similar reasoning applies also to the admixture of Na in the X4 site [22].
Incorporation of small amounts of boron into the vesuvianite structure does not lead to the splitting of the O7 site and the appearance of the O12 site as it does in wiluite [67]. The low-occupied T2 site (Figure 7) is populated by about 25% boron, which prevented us from the determination of its coordination. However, the presence of 3-coordinated boron is confirmed by FTIR. The observed scattering factors of the T1 site of 1.4 and 1.0 e− correspond to the refined occupancies Al0.106 and Al0.072, respectively. Due to the low occupancies of the T1 site, the mean <T1–O> bond lengths, 1.827 and 1.830 Å, are significantly longer than the theoretical one for tetrahedrally coordinated Al with full occupancy. At the same time, the presence of small amounts (~0.45 apfu) of tetrahedrally coordinated boron at the T1 site cannot be excluded.
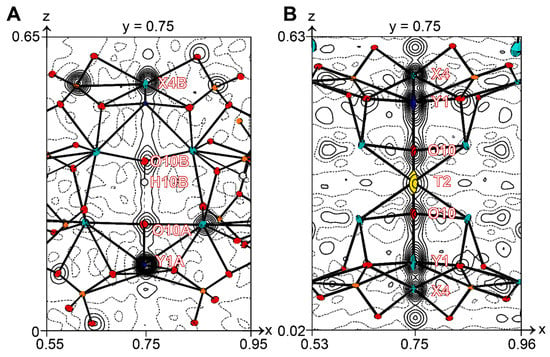
Figure 7.
Graphs of observed electron density along the cation rods (fourfold axis is centered at 0.75; 0.75; z) in the crystal structures of: (A) low-symmetry P4/n vesuvianite from Akhmatovskaya Pit and (B) 51062. Projections are onto the (010) plane, contour intervals are 0.1 eÅ−3. Displacement ellipsoids are drawn at the 50% probability level.
All oxygen sites have been refined as fully occupied by oxygen atoms, excluding the O7 site that possesses anomalously high displacement parameters. In the final model, the O7 site was refined as (O0.75F0.25)1.00 with plausible displacement parameters. Small amounts of Cl determined by the electron microprobe (0.05 and 0.04 apfu for 27844 and 51062, respectively) are probably situated in the O10 site [19].
Taking into account all of the data given above, the final crystal-chemical formulae of the 27844 and 51062 samples can be written, as follows: X1(Ca)2.00X2(Ca)8.00X3(Ca)8.00X4(Ca0.97Na0.03)1.00Y1 (Fe3+0.50Mg0.28Fe2+0.22)Σ1.00Y2(Al3.85Fe2+0.15)Σ4.00Y3(Al5.26Mg1.83Fe3+0.54Fe2+0.26Mn0.11)Σ8.00Z1(Si)2.00Z2(Si)8.00Z3(Si)8.00(O)68.00T1+T2(Al0.44B0.25□4.31)Σ5.00W(OH5.65F2.00O1.30Cl0.05)Σ9.00 and X1(Ca)2.00X2(Ca)8.00X3(Ca7.90Ce0.06Nd0.04)8.00 X4(Ca)1.00Y1(Fe3+0.47Mg0.27Fe2+0.26)Σ1.00Y2(Al)Σ4.00Y3(Al5.01Mg2.10Fe3+0.53Fe2+0.13Ti0.12Mn0.11)Σ8.00Z1(Si)2.00Z2(Si)8.00Z3(Si)8.00(O)68.00T1+T2(Al0.29B0.25□4.46)Σ5.00W(OH5.88F2.00O1.08Cl0.04)Σ9.00. The Fe content in each Y site was assigned according to the crystal-structure data, whereas the Fe2+/Fe3+ ratio is given in accordance with the Mössbauer spectroscopy data.
4.6. Infrared Spectroscopy
The IR spectrum of 27844 (Figure 8) is typical for high-symmetry vesuvianite-group minerals. It contains strong bands of Si–O stretching in the Si2O7 (at 1018 and 976 cm−1) and SiO4 groups (at 914 cm−1 with shoulders at 900 cm−1 and 870 cm−1), as well as Si–O–Si and O–Si–O bending vibrations, partly mixed with the M–O stretching vibrations, where M = Al, Mg, Fe, Ti, Mn (below 700 cm−1). The band at 799 cm−1 is assigned to bending vibrations of the M⋯O–H groups.
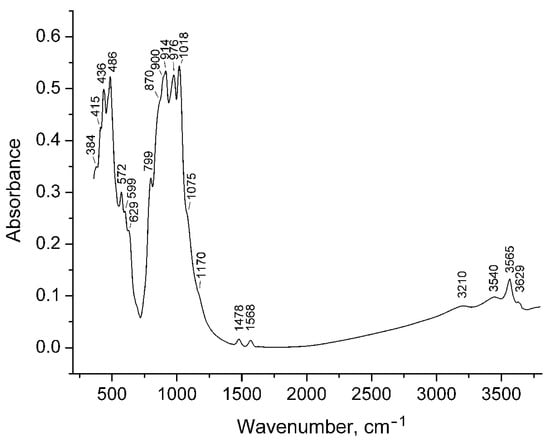
Figure 8.
Infrared spectrum of 27844.
The region of O–H stretching vibrations is dominated by the peak at 3565 cm−1 (D band according to [68], which corresponds to the vibrations of the O11–H1 bond in the presence of F in the neighboring O11 site [69]. The weak bands at 3450 cm−1 and 3629 cm−1 are related to the OH groups coordinated by Ti and to the O11–H1⋯O7 fragment with the angle <120°, respectively [69]. The weak band at 3210 cm−1 (J band) corresponds to the OH groups oriented along the c-axis [68] forming strong hydrogen bonds.
Two weak bands at 1568 and 1478 cm−1 are assigned to the BO33− anions (the T2-centered triangle) with shortened (as compared to wiluite) B–O bonds. The shoulders at 1170 cm−1 and 1075 cm−1 are tentatively assigned to boron atoms having 4-fold coordination.
5. Discussion
Generalization of the vesuvianite formula is an important step for the development of the nomenclature for this group of minerals. The tentative vesuvianite formula, (Ca,Na)19(Al,Mg,Fe)13(SiO4)10(Si2O7)4(OH,F,O)10, does not specify the occupancies of the Y1(A,B) site [36], which are responsible for the observed species diversity of the vesuvianite-group minerals [16,21,29]. According to the IMA CNMNC guidelines for compositional criteria [37], at least one structural site in the potential new mineral should be predominantly occupied by a different chemical component than that which occurs in the equivalent site in an existing mineral species [16]. In this context, recently approved members of the vesuvianite group with Mn3+, Al3+, Mg2+, Cu2+ as predominant cations at the Y1 site can be confused with vesuvianite itself, according to the present formula of this mineral. Therefore, it is crucially important to determine a new vesuvianite formula, which would be in accordance with the above-mentioned IMA rule.
In most cases, vesuvianite with the P4/nnc symmetry has only Fe and Mg at the Y1 site [55]. There are only three publications available that report vesuvianite with the Fe2+/Fe3+ ratio determined by direct chemical or spectroscopic methods (Figure 9) [21,29,63]. Our data demonstrate the predominance of Fe3+ at the Y1 site for both vesuvianite samples from the type locality, which is consistent with previous conclusions [38,48]. According to our experimental results, the new formula of vesuvianite can be written as VII−IXX19VY1VIY12(ZO7)4(ZO4)10(W)10, where X are seven- to nine-coordinated sites of Ca with minor Na, K, Fe2+ and REE impurities; VY has square pyramidal coordination of dominant Fe3+ and subordinate Mg, Al, Fe2+ and Cu2+; VIY has octahedral coordination and is dominantly occupied by Al with subordinate Mg, Fe2+, Fe3+, Mn2+, Mn3+, Ti, Cr and Zn; ZO4 = SiO4, sometimes with subordinate AlO4, (O4H4)4−, and W = OH, F, Cl and minor O [12,13,15,17,19,21,22,23,24,25,26,27,28,29]. Since the sum of occupancies of the T1 and T2 sites is below 1 apfu, their populations are not taken into account. The simplified vesuvianite formula may be written as Ca19Fe3+(Al,Mg,Fe)12(SiO4)10(Si2O7)4(OH,F,O)10. The idealized charge-balanced formula of the vesuvianite end-member without subordinate cations is Ca19Fe3+(Al10Me2+2)(Si2O7)4(SiO4)10O(OH)9, where Me = Fe2+, Mg2+, Mn2+.
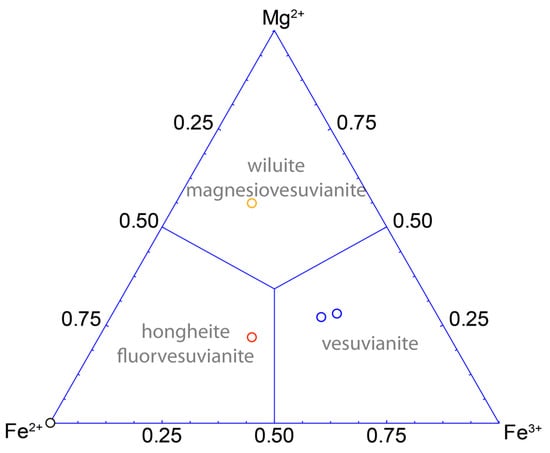
Figure 9.
Triangular compositional diagram of the Y1 site in the P4/nnc vesuvianite based on single-crystal X-ray diffraction with Fe2+/Fe3+ ratio obtained from Mössbauer data [63] (black circle), [21] (red circle), [29] (orange circle) and present study (blue circles)).
6. Conclusions
By revising vesuvianite-group of minerals [16,21,29] two vesuvianite samples 27844 and 51062 from type locality were investigated by electron microprobe analysis, Mössbauer and infrared spectroscopy, TGA/DSC, MAS NMR, single-crystal and powder X-ray diffraction.
Present studies reveal square-pyramidal Y1 position as a key site in the diversity of vesuvianite-group minerals. On the basis of this approach we determine vesuvianite formula as Ca19Fe3+(Al10Me2+2)(Si2O7)4(SiO4)10O(OH)9, which would not contradict present CNMNC guidelines for compositional criteria.
The vesuvianite from the Somma-Vesuvius complex demonstrate disorder inside the structural channels and overall P4/nnc symmetry, which partially connected with the incorporation of boron at additional T1, T2 sites and agrees with the estimated temperature of crystallization at 800–900 °C.
Supplementary Materials
The following are available online at http://www.mdpi.com/2075-163X/7/12/248/s1.
Acknowledgments
This work was supported by the President of Russian Federation grant for leading scientific schools (to S.V.K.). Authors are grateful to Sergey Aksenov, Piotr Dzierżanowski and Evgeny Galuskin for their contribution to the experimental studies and useful discussions. Anonymous referees, handling editor are thanked for critical reviews and helpful comments.
Author Contributions
Taras L. Panikorovskii, Nikita V. Chukanov, Gregory Yu. Ivanyuk, Giuseppina Balassone and Sergey V. Krivovichev wrote the paper. Taras L. Panikorovskii conceived and designed the single-crystal, powder diffraction, TGA/DSC experiments. Nikita V. Chukanov performed and analyzed infrared data. Vyacheslav S. Rusakov performed and analyzed Mössbauer data. Vladimir V. Shilovskikh performed microprobe measurements. Anton S. Mazur performed MAS NMR measurements. Gregory Yu. Ivanyuk prepared the photo of vesuvianite species.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Powder X-ray diffraction data for 27844 1.
Table A1.
Powder X-ray diffraction data for 27844 1.
| dmeas Å | Imeas | dcalc Å | Icalc | hkl | dmeas Å | Imeas | dcalc Å | Icalc | hkl |
|---|---|---|---|---|---|---|---|---|---|
| 10.83 | 4 | 10.84 | 2 | 110 | 2.081 | 5 | 2.076 | 8 | 623 |
| 5.85 | 4 | 5.85 | 2 | 002 | 2.060 | 4 | 2.060 | 3 | 543 |
| 4.673 | 4 | 4.667 | 6 | 202 | 2.038 | 9 | 2.047 | 13 | 730 |
| 4.000 | 6 | 4.027 | 4 | 222 | 2.022 | 4 | 2.023 | 6 | 642 |
| 3.857 | 2 | 3.845 | 2 | 400 | 2.008 | 2 | 2.001 | 1 | 731 |
| 3.463 | 15 | 3.460 | 15 | 322 | 1.9941 | 8 | 1.9957 | 12 | 633 |
| 3.234 | 4 | 3.229 | 6 | 402 | 1.9602 | 6 | 1.6712 | 6 | 651 |
| 3.062 | 5 | 3.065 | 5 | 313 | 1.9281 | 2 | 1.9265 | 5 | 116 |
| 3.038 | 8 | 3.031 | 6 | 510 | 1.9171 | 2 | 1.9098 | 2 | 713 |
| 2.995 | 9 | 2.995 | 14 | 501 | 1.9029 | 4 | 1.9030 | 2 | 206 |
| 2.939 | 18 | 2.933 | 45 | 511 | 1.8823 | 7 | 1.8898 | 11 | 216 |
| 2.897 | 5 | 2.895 | 6 | 323 | 1.8647 | 2 | 1.8635 | 2 | 515 |
| 2.744 | 100 | 2.748 | 100 | 440 | 1.7960 | 1 | 1.7950 | 2 | 831 |
| 2.659 | 3 | 2.654 | 2 | 530 | 1.7614 | 9 | 1.7587 | 18 | 714 |
| 2.586 | 58 | 2.587 | 60 | 531 | 1.7158 | 2 | 1.7149 | 3 | 910 |
| 2.522 | 6 | 2.523 | 5 | 314 | 1.6768 | 17 | 1.6756 | 11 | 734 |
| 2.492 | 1 | 2.483 | 1 | 611 | 1.6603 | 22 | 1.6631 | 26 | 436 |
| 2.454 | 45 | 2.451 | 48 | 620 | 1.6215 | 30 | 1.6226 | 31 | 526 |
| 2.433 | 7 | 2.432 | 2 | 324 | 1.5853 | 2 | 1.5854 | 1 | 824 |
| 2.367 | 9 | 2.368 | 7 | 541 | 1.5712 | 5 | 1.5712 | 4 | 327 |
| 2.345 | 5 | 2.345 | 3 | 404 | 1.5580 | 7 | 1.5579 | 6 | 616 |
| 2.320 | 8 | 2.319 | 7 | 105 | 1.5397 | 2 | 1.5362 | 2 | 735 |
| 2.293 | 5 | 2.296 | 5 | 334 | 1.5318 | 1 | 1.5312 | 2 | 626 |
| 2.269 | 2 | 2.268 | 2 | 631 | 1.5235 | 4 | 1.5237 | 5 | 942 |
| 2.193 | 5 | 2.194 | 9 | 710 | 1.5089 | 2 | 1.5084 | 1 | 1021 |
| 2.177 | 5 | 2.169 | 5 | 701 | 1.5028 | 1 | 1.5029 | 1 | 1002 |
| 2.158 | 3 | 2.164 | 3 | 711 | 1.4979 | 8 | 1.4979 | 5 | 636 |
| 2.141 | 2 | 2.143 | 2 | 305 | 1.4753 | 1 | 1.4752 | 2 | 1022 |
| 2.121 | 11 | 2.122 | 14 | 315 |
1 The eight strongest lines are highlighted bold.
References
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Orthosilicates, Vol 1A, 1st ed.; Geological Society: London, UK, 1982; pp. 701–719. [Google Scholar]
- Gnos, E.; Armbruster, T. Relationship among metamorphic grade, vesuvianite “rod polytypism”, and vesuvianite composition. Am. Mineral. 2006, 91, 862–870. [Google Scholar] [CrossRef]
- Olesch, M. Natürliche und synthetische Fe-haltige Vesuviane. Fortschr. Mineral. 1979, 57, 114–115. (In German) [Google Scholar]
- Kappeler, M.A. Prodromus Crystallographiae de Crystallis Improprie sic Dictis Commentarium; Heinrich, R.W., Ed.; HR Wyssing: Lucerna, Switzerland, 1723; pp. 1–43. [Google Scholar]
- Russo, M.; Punzo, I. I Minerali del Somma-Vesuvio, 1st ed.; AMI: Cremona, Italy, 2004; pp. 1–317. [Google Scholar]
- Werner, A.G. Klaproth’s Beiträge: Über Vesuvian; Klaproth, M.H., Ed.; Becker und Compagnie: Berlin, Germany, 1795; pp. 1–34. [Google Scholar]
- Clarke, F. The constitution of the silicates. Bull. U. S. Geol. Surv. 1895, 125, 109–110. [Google Scholar]
- Sjögren, H. Analyser pa tvenne vesuvian-varieter och vesuvianens kemiska constitution I Allmänhet. Geol. I Stockh. Forh. 1895, 17, 267–271. [Google Scholar] [CrossRef]
- Weibull, M. Studien über vesuvian. Z. Krystallogr. 1896, 35, 26–35. [Google Scholar] [CrossRef]
- Jannasch, P.; Weingarten, P. Uber die chemische Zusammensetzung und Konstitution des Vesuvians und des Wiluits. Z. Anorg. Allg. Chem. 1896, 11, 40–48. [Google Scholar] [CrossRef]
- Warren, B.E.; Modell, D.I. The structure of vesuvianite Ca10Al4(Mg,Fe)2Si9O34(OH)4. Z. Kristallogr. 1931, 78, 422–432. [Google Scholar] [CrossRef]
- Machatschki, F. Zur Formel des Vesuvian. Z. Kristallogr. 1932, 81, 148–152. [Google Scholar] [CrossRef]
- Coda, A.; Giusta, D.A.; Isetti, G.; Mazzi, F. On the structure of vesuvianite. Atti Accad. Sci. Torino 1970, 105, 1–22. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S.; Grice, J.D. Wiluite, Ca19(Al,Mg,Fe,Ti)13(B,Al,◻)5Si18O68(O,OH)10, a new mineral species isostructural with vesuvianite, from the Sakha Republic, Russian Federation. Can. Mineral. 1998, 36, 1301–1304. [Google Scholar] [CrossRef]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S. The chemistry of vesuvianite. Can. Mineral. 1992, 33, 19–48. [Google Scholar]
- Armbruster, T.; Gnos, E.; Dixon, R.; Gutzmer, J.; Hejny, C.; Döbelin, N.; Medenbach, O. Manganvesuvianite and tweddillite, two new Mn3+-silicate minerals from the Kalahari manganese fields, South Africa. Mineral. Mag. 2002, 66, 137–150. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Sitarz, M.; Stadnicka, K. Si-deficient, OH-substituted, boron-bearing vesuvianite from the Wiluy River, Yakutia, Russia. Can. Mineral. 2003, 41, 833–842. [Google Scholar] [CrossRef]
- Britvin, S.N.; Antonov, A.A; Krivovichev, S.V.; Armbruster, T.; Burns, P.C.; Chukanov, N.V. Fluorvesuvianite, Ca19(Al,Mg,Fe 2+)13[SiO4]10[Si2O7]4O(F,OH)9, a new mineral species from Pitkäranta, Karelia, Russia: Description and crystal structure. Can. Mineral. 2003, 41, 1371–1380. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Dzierżanowski, P. Chlorine in vesuvianites. Miner. Pol. 2005, 36, 51–61. [Google Scholar]
- Hålenius, U.; Bosi, F.; Gatedal, K. Crystal structure and chemistry of skarn-associated bismuthian vesuvianite. Am. Mineral. 2013, 98, 566–573. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Chukanov, N.V.; Rusakov, V.S.; Panikorovskii, T.L.; Gainov, R.R.; Vagizov, F.G.; Rastsvetaeva, R.K.; Lyssenko, K.A.; Belakovskiy, D.I. Towards a revisitation of vesuvianite-group nomenclature: The crystal structure of Ti-rich vesuvianite from Alchuri, Shigar valley, Pakistan. Acta Crystallogr. B 2016, 72, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Panikorovskii, T.L.; Krivovichev, S.V.; Yakovenchuk, V.N.; Shilovkikh, V.V.; Mazur, A.S. Crystal chemistry of Na-bearing vesuvianite from fenitized gabbroid of the Western Keivy (Kola peninsula, Russia). Zap. Ross. Mineral. Obsh. 2016, 145, 83–95. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Shilovskikh, V.V.; Avdontseva, E.Y.; Zolotarev, A.A.; Pekov, I.V.; Britvin, S.N.; Hålenius, U.; Krivovichev, S.V. Cyprine, Ca19Cu2+(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group mineral from the Wessels mine, South Africa. Eur. J. Mineral. 2017, 29, 295–307. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Shilovskikh, V.V.; Avdontseva, E.Y.; Zolotarev, A.A.; Karpenko, V.Y.; Mazur, A.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Pekov, I.V. Magnesiovesuvianite, Ca19Mg(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group mineral. J. Geosci. 2017, 1, 25–36. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Chukanov, N.V.; Aksenov, S.M.; Mazur, A.S.; Avdontseva, E.Y.; Shilovskikh, V.V.; Krivovichev, S.V. Alumovesuvianite, Ca19Al(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group member from the Jeffrey mine, Asbestos, Estrie Region, Québec, Canada. Mineral. Petrol. 2017, 111, 833–842. [Google Scholar] [CrossRef]
- Dyrek, K.; Platonov, A.N.; Sojka, Z.; Żabinski, W. Optical absorption and EPR study of Cu2+ ions in vesuvianite (“cyprine”) from Sauland, Telemark, Norway. Eur. J. Mineral. 1992, 4, 1285–1289. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Krivovichev, S.V.; Zolotarev, A.A.; Antonov, A.A. Crystal chemistry of low-symmetry (P4nc) vesuvianite from the Kharmankul’ Cordon (South Urals, Russia). Zap. Ross. Mineral. Obsh. 2016, 145, 94–104. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Krivovichev, S.V; Galuskin, E.V.; Shilovskikh, V.V.; Mazur, A.S.; Bazai, A.V. Si-deficient, OH-substituted, boron-bearing vesuvianite from Sakha-Yakutia, Russia: A combined single-crystal, 1H MAS-NMR and IR spectroscopic study. Eur. J. Mineral. 2016, 28, 931–941. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Mazur, A.S.; Bazai, A.V.; Shilovskikh, V.V.; Galuskin, E.V.; Chukanov, N.V.; Rusakov, V.S.; Zhukov, Y.M.; Avdontseva, E.Y.; Aksenov, S.M.; et al. X-ray diffraction and spectroscopic study of wiluite: Implications for the vesuvianite-group nomenclature. Phys. Chem. Mineral. 2017, 44, 577–593. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Mazzi, F. The crystal structure of a vesuvianite with P4/n symmetry. Tsch. Mineral. Petrol. 1983, 31, 277–288. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Fan, G.; Ge, X.; Zhu, X.; Shen, G. Hongheite, IMA 2017-027. CNMNC Newsletter No. 39, October 2017, page 1283. Mineral. Mag. 2017, 81, 1279–1286. [Google Scholar]
- Veblen, D.R.; Wiechmann, M.J. Domain structure of low-symmetry vesuvianite from Crestmore, California. Am. Mineral. 1991, 76, 397–404. [Google Scholar]
- Allen, F.M.; Burnham, C.W. A comprehensive structure-model for vesuvianite: Symmetry variation and crystal growth. Can. Mineral. 1992, 30, 1–18. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S.; Putnis, A. The symmetry of vesuvianite. Can. Mineral. 1993, 31, 617–635. [Google Scholar]
- Tanaka, T.; Akizuki, M.; Hudoh, Y. Optical properties and crystal structure of triclinic growth sectors in vesuvianite. Mineral. Mag. 2002, 66, 261–274. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Leavens, P.B.; Rossman, G.R.; Yap, G.P.A.; Rose, T. Vesuvianite from Pajsberg, Sweden, and the role of Be in the vesuvianite structure. Can. Mineral. 2016, 54, 1525–1537. [Google Scholar] [CrossRef]
- Nickel, E.H.; Grice, J.D. The IMA Commission on New Minerals and Mineral Names: Procedures and guidelines on mineral nomenclature, 1998. Can. Mineral. 1998, 36, 913–926. [Google Scholar] [CrossRef]
- Balassone, G.; Talla, D.; Beran, A.; Mormone, A.; Altomare, A.; Moliterni, A.; Mondillo, N.; Saviano, M.; Petti, C. Vesuvianite from Somma-Vesuvius volcano (Southern Italy): Chemical, X-ray diffraction and single-crystal polarized FTIR investigations. Period. Mineral. 2011, 80, 369–384. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Mormone, A.; Biagioni, C.; Pasero, M.; Petti, C.; Mondillo, N.; Fameli, G. Sodalite-group minerals from the Somma-Vesuvius volcanic complex, Italy: A case study of K-feldspar-rich xenoliths. Mineral. Mag. 2012, 76, 191–212. [Google Scholar] [CrossRef]
- Balassone, G.; Kahlenberg, V.; Altomare, A.; Mormone, A.; Rizzi, R.; Saviano, M.; Mondillo, M. Nephelines from the Somma-Vesuvius volcanic complex (Southern Italy): Crystal chemical, structural and genetic investigations. Mineral. Petrol. 2014, 108, 71–90. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Ottolini, L.; Mormone, A.; Petti, C.; Ghiara, M.R.; Altomare, A.; Saviano, M.; Rizzim, R.; D’oraziom, L. Sodalite-group minerals from Somma-Vesuvius volcano (Naples, Italy): A combined EPMA, SIMS and FTIR crystal chemical study. Can. Mineral. 2016, 54, 583–604. [Google Scholar] [CrossRef]
- Gilg, A.H.; Lima, A.; Somma, R.; Belkin, H.E.; De Vivo, B.; Ayuso, R.A. Isotope geochemistry and fluid inclusion study of skarns from Vesuvius. Mineral. Petrol. 2001, 73, 145–176. [Google Scholar] [CrossRef]
- Hermes, O.D.; Cornell, W.C. Petrochemical significance of xenolithic nodules associated with potash-rich lavas of Somma-Vesuvius volcano. In NSF Final Technical Report; University Rhode Island: Kingston, RI, USA, 1978; p. 58. [Google Scholar]
- Joron, J.L.; Metrich, N.; Rosi, M.; Santacroce, R.; Sbrana, A. Chemistry and petrography. CNR Quad. Ric. Sci. 1987, 114, 105–174. [Google Scholar]
- Gruppo Mineralogico Geologico Napoletano. Available online: http://www.gmgn.it/index.html (accessed on 1 November 2017).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Elmi, C.; Brigattim, M.F.; Pasqualim, L.; Montecchi, M.; Laurora, A.; Malferrari, D.; Nannarone, S. High-temperature vesuvianite: Crystal chemistry and surface considerations. Phys. Chem. Miner. 2011, 38, 459–468. [Google Scholar] [CrossRef]
- Żabiński, W.; Wactawska, Z.; Paluszkiewicz, C. Thermal decomposition of vesuvianite. J. Therm. Anal. 1996, 46, 1437–1447. [Google Scholar] [CrossRef]
- Foldvari, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; pp. 1–180. [Google Scholar]
- Phillips, B.L.; Allen, F.M.; Kirkpatrick, R.J. High-resolution solid-state 27Al NMR spectroscopy of Mg-rich vesuvianite. Am. Mineral. 1987, 72, 1190–1194. [Google Scholar]
- Olejniczak, Z.; Żabiński, W. 27Al NMR study of white vesuvianite from Piz Lunghin, Switzerland. Miner. Pol. 1996, 27, 41–45. [Google Scholar]
- Yesinowski, J.P.; Eckert, H.; Rossman, G.R. Characterization of hydrous species in minerals by high-speed 1H MAS-NMR. J. Am. Chem. Soc. 1988, 110, 1367–1375. [Google Scholar] [CrossRef]
- Lager, G.A.; Xie, Q.; Ross, F.K.; Rossman, G.R.; Armbruster, T.; Rotella, F.J.; Schultz, A.J. Hydrogen-atom position in P4/nnc vesuvianite. Can. Mineral. 1999, 37, 763–768. [Google Scholar]
- Ohkawa, M.; Yoshiasa, A.; Takeno, S. Crystal chemistry of vesuvianite: Site preferences of square-pyramidal coordinated sites. Am. Mineral. 1992, 77, 945–953. [Google Scholar]
- Ohkawa, M.; Armbruster, T.; Galuskin, E. Structural investigation of low symmetry vesuvianite collected from Tojyo, Hiroshima, Japan: Implications for hydrogarnet-like substitution. J. Mineral. Petrol. Sci. 2009, 104, 69–76. [Google Scholar] [CrossRef]
- Pavese, A.; Prencipe, M.; Tribaudino, M.; Aagaard, S.S. X-ray and neutron single-crystal study of P4/n vesuvianite. Can. Mineral. 1998, 36, 1029–1037. [Google Scholar]
- Armbruster, T.; Gnos, E. “Rod” polytypism in vesuvianite: Crystal structure of a low-temperature P4nc with pronounced octahedral cation ordering. Schweiz. Mineral. Petrogr. 2000, 80, 109–116. [Google Scholar]
- Galuskin, E.V. Vesuvianite-Group Minerals from Achtarandite Rocks (Wiluy River, Yakutia), 1st ed.; University of Silesia: Katowice, Poland, 2005; pp. 123–137. (In Polish) [Google Scholar]
- Kraczka, J.; Żabiński, W. Mössbauer study of iron in some vesuvianites. Mineral. Pol. 2003, 34, 37–44. [Google Scholar]
- Rusakov, V.S.; Kovalchuk, R.V.; Borovikova, E.Y.; Kurazhkovskaya, V.S. State of iron atoms in high vesuvianites according to Mössbauer spectroscopy data. Zap. Ross. Mineral. Obsh. 2006, 135, 91–100. (In Russian) [Google Scholar]
- Manning, P.G.; Tricker, M.J. Optical absorption and Mössbauer spectral studies of iron and titanium site-populations in vesuvianites. Can. Mineral. 1975, 13, 259–265. [Google Scholar]
- Groat, L.A.; Evans, R.J.; Cempírek, J.; McCammon, C.; Houzar, S. Fe-rich and As-bearing vesuvianite and wiluite from Kozlov, Czech Republic. Am. Mineral. 2013, 98, 1330–1337. [Google Scholar] [CrossRef]
- Bruker AXS. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS GmbH: Karlsruhe, Germany, 2009. [Google Scholar]
- Groat, L.A.; Evans, R.J. Crystal chemistry of Bi- and Mn-bearing vesuvianite from Langban, Sweden. Am. Mineral. 2012, 97, 1627–1634. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Leavens, P.B.; Rheingold, A.L.; Nelen, J.A. Crystal structure of a REE-bearing vesuvianite from San Benito County, California. Am. Mineral. 1987, 72, 625–628. [Google Scholar]
- Galuskin, E.V.; Galuskina, I.O.; Stadnicka, K.; Armbruster, T.; Kozanecki, M. The crystal structure of Si-deficient, OH-substituted, boron-bearing vesuvianite from the Wiluy River, Sakha-Yakutia, Russia. Can. Mineral. 2007, 45, 239–248. [Google Scholar] [CrossRef]
- Groat, L.A.; Hawthorne, F.C.; Rossman, G.R.; Scott, T.E. The infrared spectroscopy of vesuvianite in the OH region. Can. Mineral. 1995, 33, 609–626. [Google Scholar]
- Chukanov, N.V.; Panikorovskii, T.L.; Chervonnyi, A.D. On the relationships between crystal-chemical characteristics of vesuvianite-group minerals and their IR spectra. Zap. Ross. Mineral. Obsh. 2017. in print (In Russian) [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).