Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
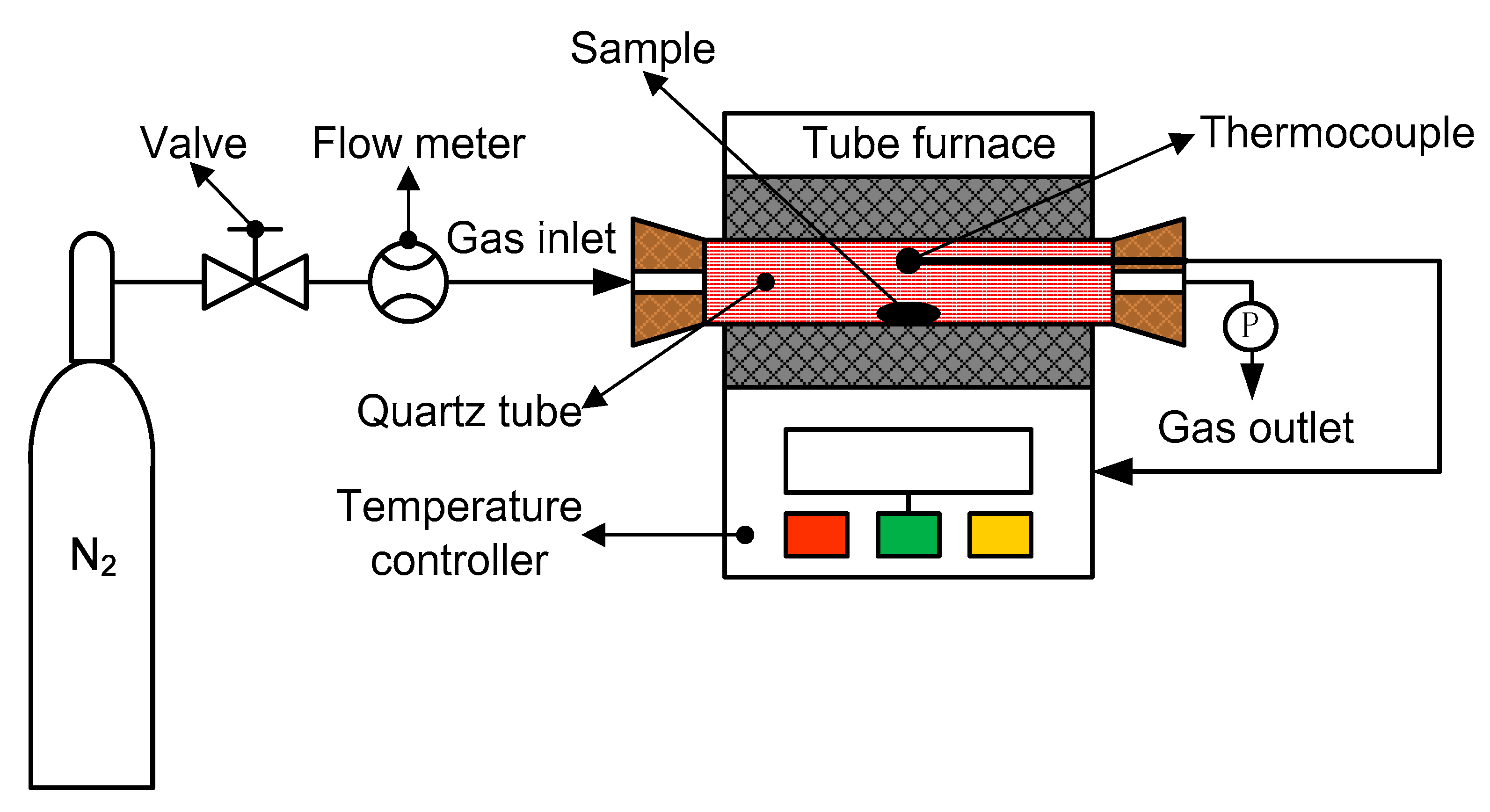
2.2.1. Pyrolysis Behavior Analysis of Siderite
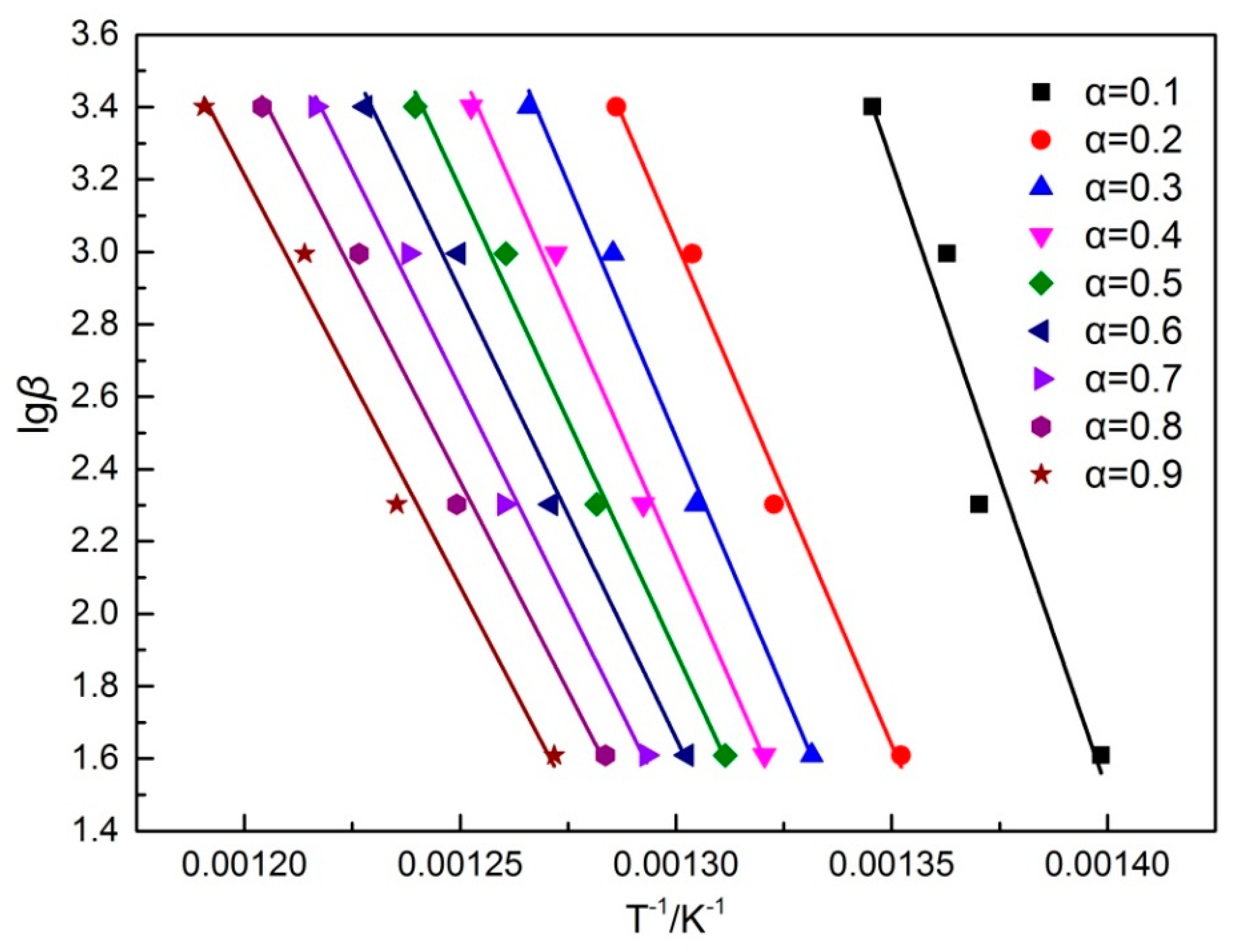
2.2.2. Pyrolysis Kinetics Characteristics of Siderite at Different Heating Rates
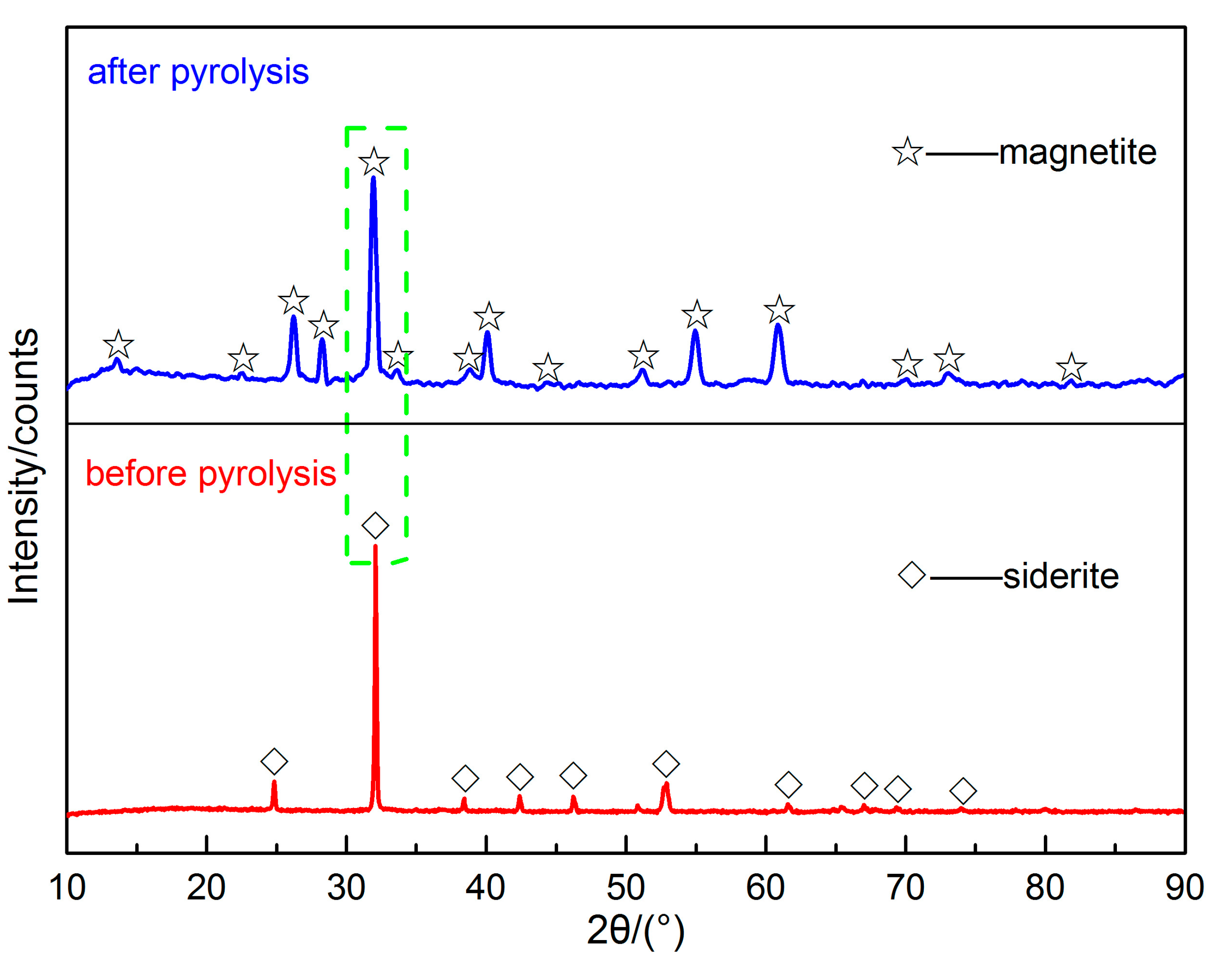
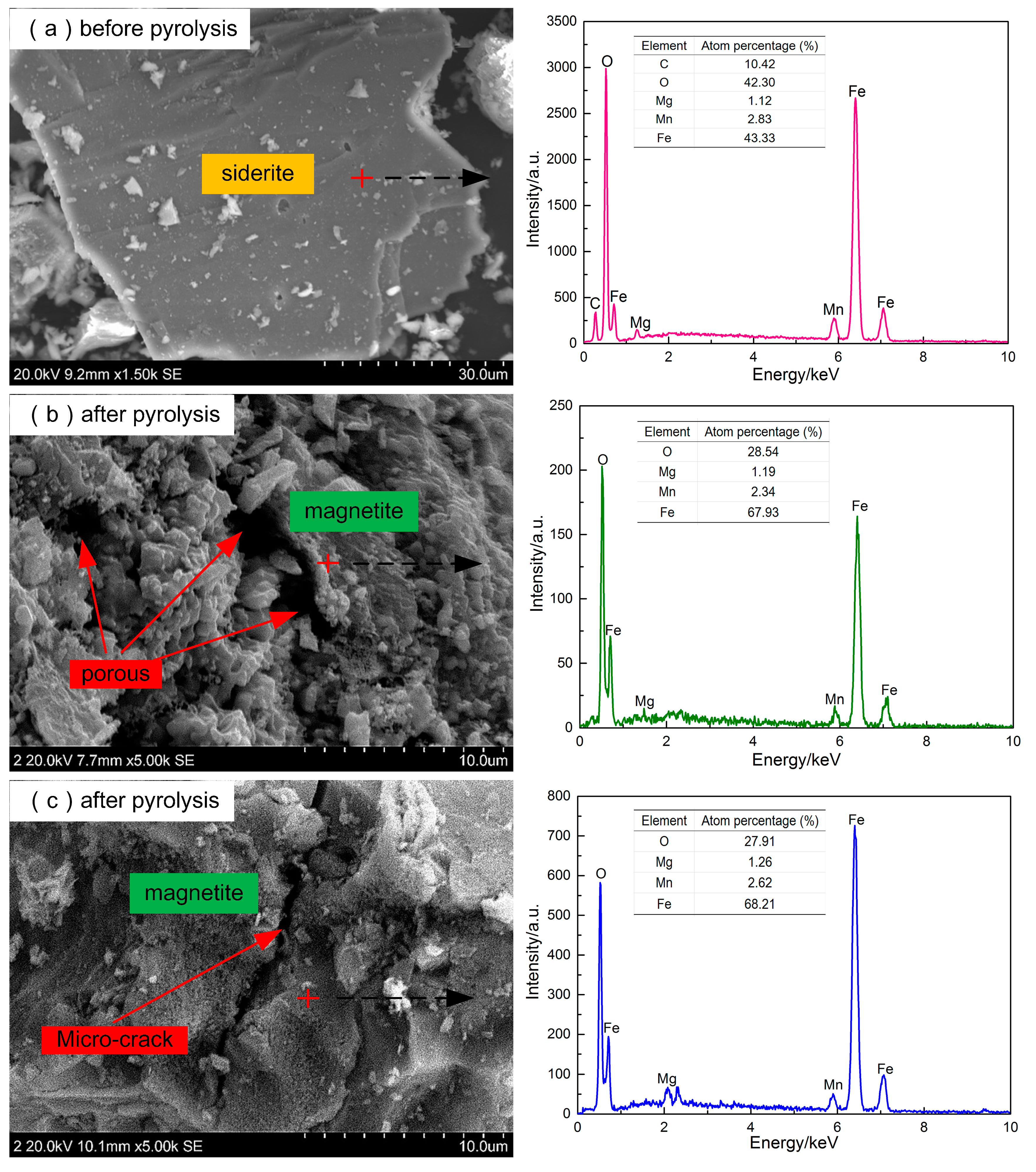
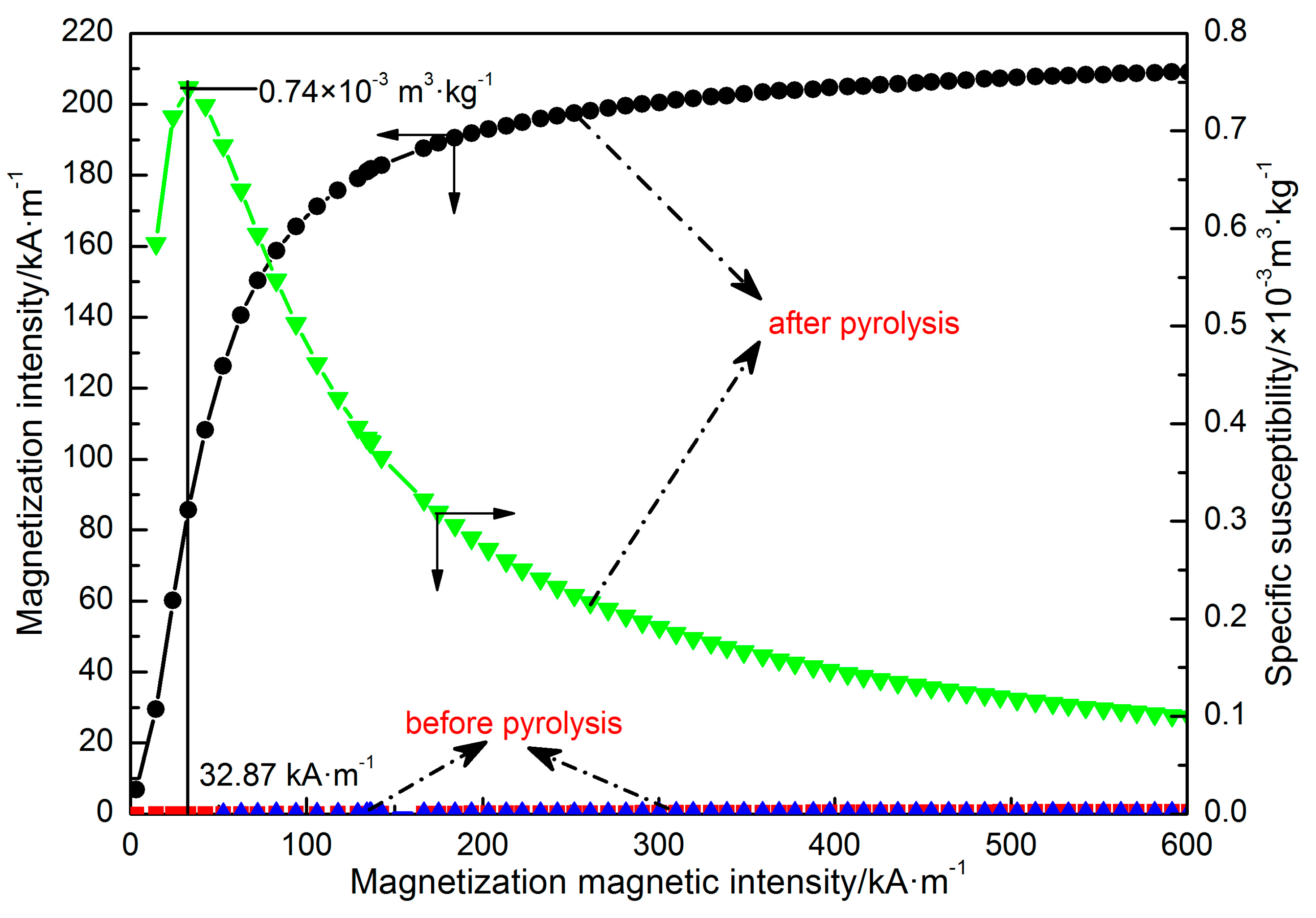
2.2.3. The Characteristics of Siderite both before and after Pyrolysis
3. Results and Discussion
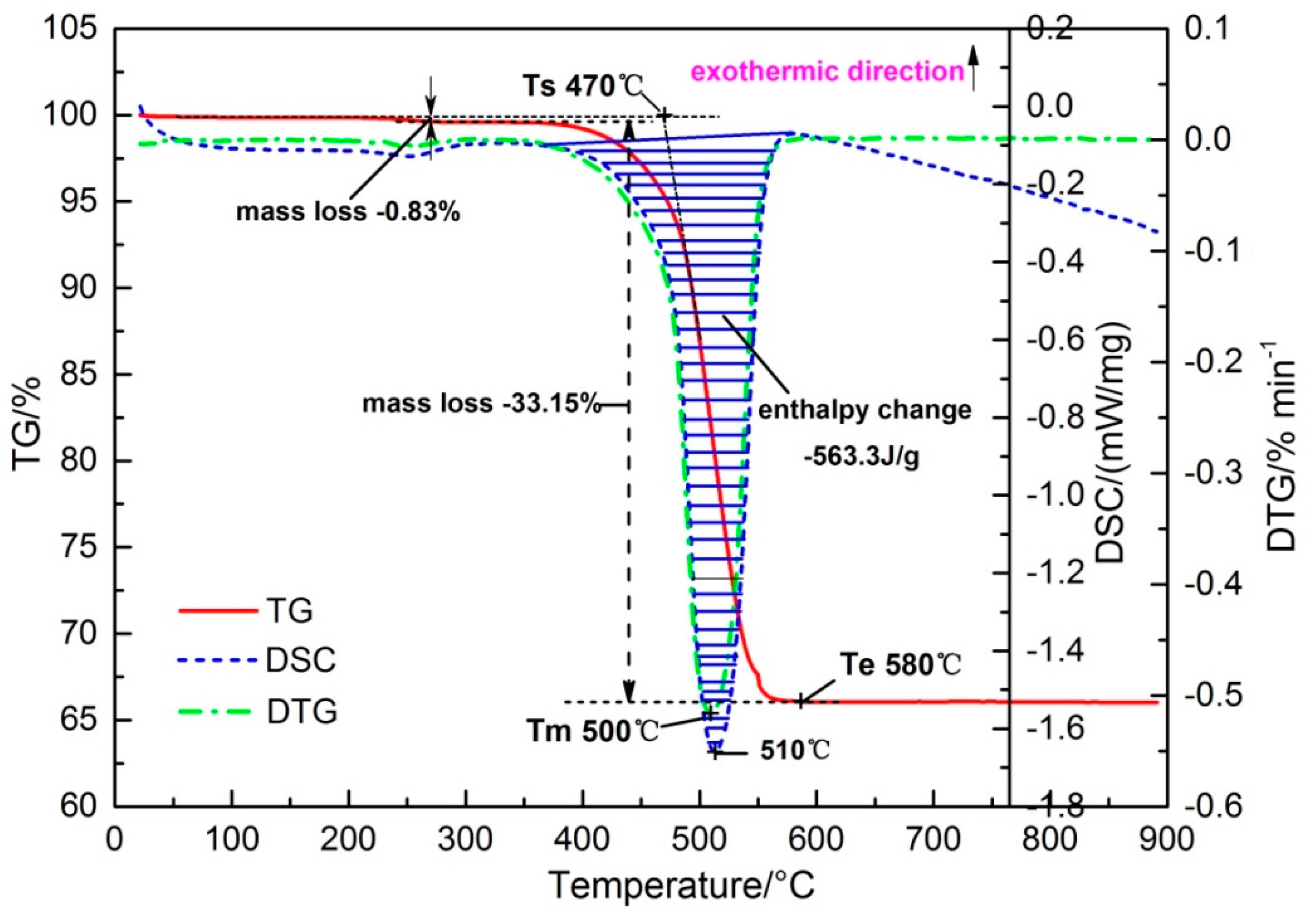
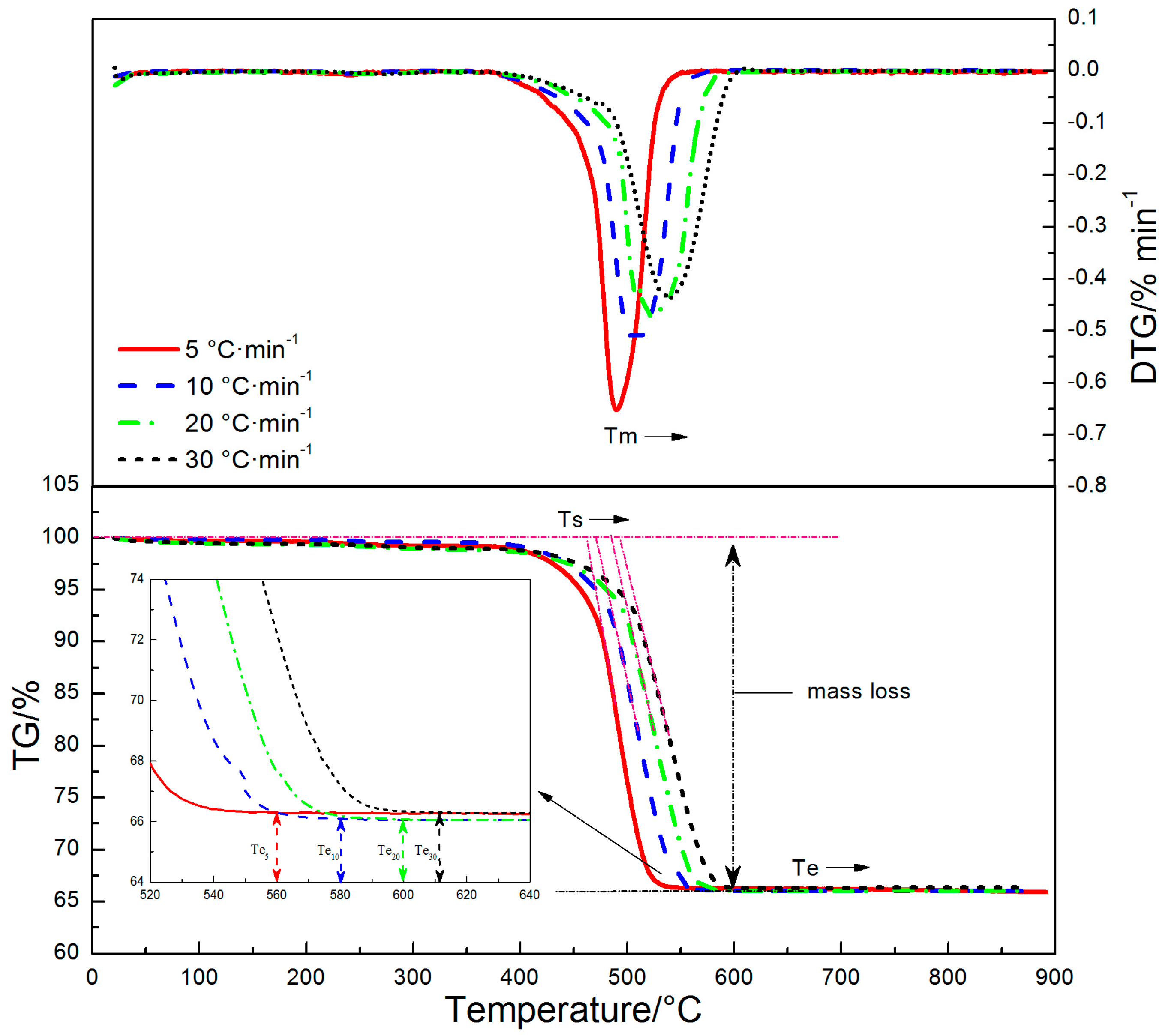
3.1. Decomposition Behavior of Siderite
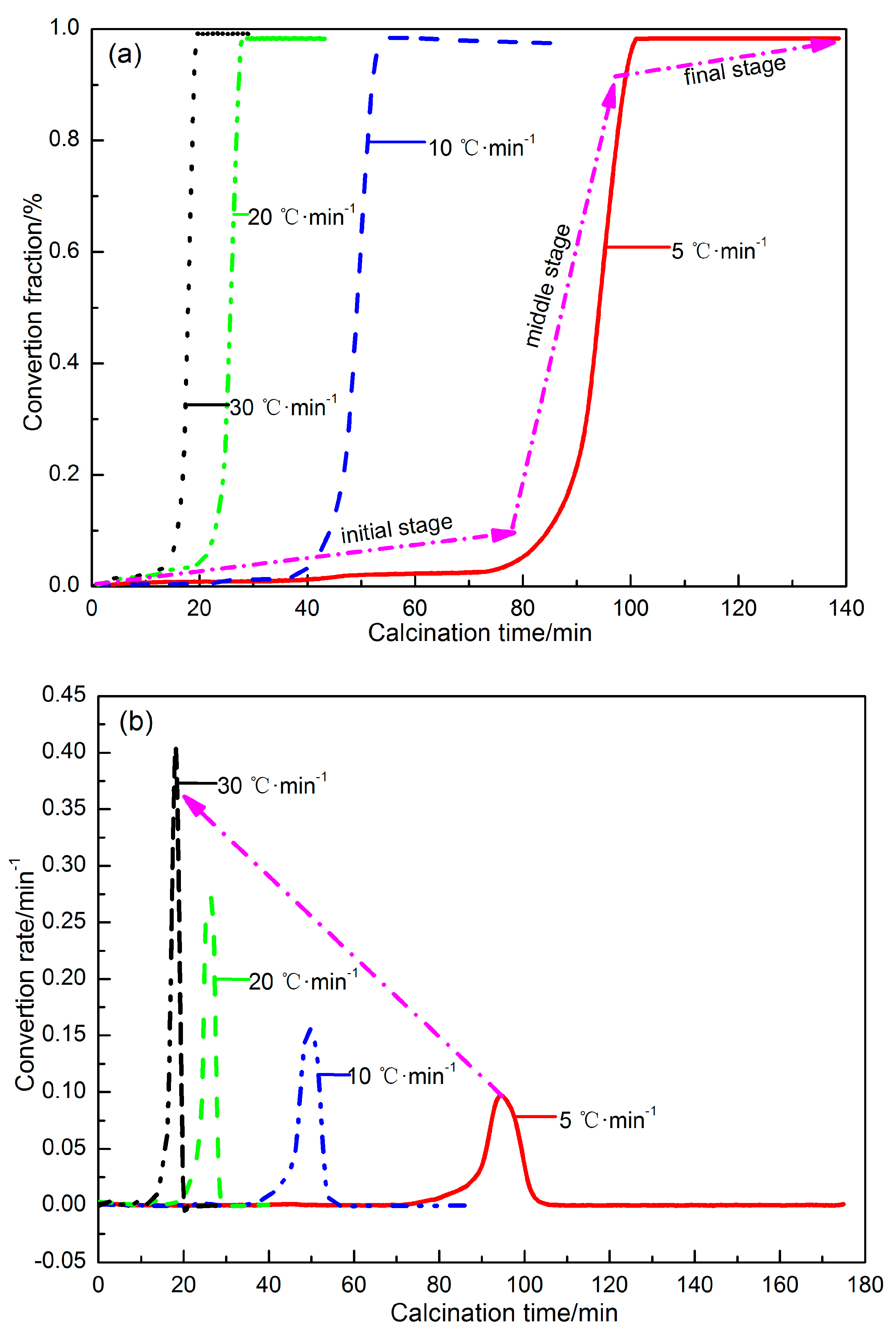
3.2. Pyrolysis Kinetics Characteristics of Siderite at Different Heating Rates
3.3. The Characteristics of Siderite both before and after Pyrolysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ponomar, V.P.; Dudchenko, N.O.; Brik, A.B. Phase transformations of siderite ore by the thermomagnetic analysis data. J. Magn. Magn. Mater. 2017, 423, 373–378. [Google Scholar] [CrossRef]
- Zhu, D.; Zhao, Q.; Qiu, G. Magnetizing roasting magnetic separation of limonite ores from Anhui province in east China. J. Univ. Sci. Technol. B 2010, 32, 713–718. [Google Scholar]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, U.; Kawatra, S.K. Strategies for processing low-grade iron ore minerals. Miner. Process. Extr. Metall. Rev. 2009, 30, 361–371. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, L.; Peng, J.; Hu, T.; Yang, L. Microwave roasting of siderite and the catalytic combustion effects on anthracite. Appl. Therm. Eng. 2017, 117, 668–674. [Google Scholar] [CrossRef]
- Xing, B.; Chen, T.; Cheng, S.; Liu, H.; Xie, Q.; Xie, J. Structural characteristic of natural siderite during thermal treatment. J. Chin. Ceram. Soc. 2016, 44, 1207–1212. [Google Scholar]
- Vusikhis, A.S.; Leont’Ev, L.I.; Kudinov, D.Z.; Gulyakov, V.S. Metallization of siderite ore in reducing roasting. Russ. Metall. 2016, 2016, 404–408. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, X.; Pan, J.; Luo, Y. Direct reduction and beneficiation of a refractory siderite lump. Miner. Process. Extr. Metall. Rev. 2014, 123, 246–250. [Google Scholar] [CrossRef]
- Bai, S.J.; Meng, W.; Chao, L.; Wen, S.M. Microstructure Characteristic and Phase Evolution of Refractory Siderite Ore during Sodium-carbonate-added Catalyzing Carbothermic Reduction. J. Iron Steel Res. 2016, 23, 891–899. [Google Scholar] [CrossRef]
- Luo, Y.H.; Zhu, D.Q.; Pan, J.; Zhou, X.L. Thermal decomposition behaviour and kinetics of Xinjiang siderite ore. Miner. Process. Extr. Metall. Rev. 2016, 125, 17–25. [Google Scholar] [CrossRef]
- Gallagher, P.K.; West, K.W.; Warne, S.S.J. Use of the Mössbauer effect to study the thermal decomposition of sidewire. Therm. Acta 1981, 50, 41–47. [Google Scholar] [CrossRef]
- Chang, Y.A.; Ahmad, N. Thermodynamic data on metal carbonates and related oxides. Metall. Soc. AIME 1982, 21, 94–100. [Google Scholar]
- Dhupe, A.P.; Gokarn, A.N. Studies in the thermal decomposition of natural siderites in the presence of air. Int. J. Miner. Process. 1990, 28, 209–220. [Google Scholar] [CrossRef]
- Geva, S.; Farren, M.; John, D.H.S.; Hayes, P.C. The effects of impurity elements on the reduction of wustite and magnetite to iron in CO/CO2, and H2/H2O gas mixtures. Metall. Mater. Trans. B 1990, 21, 743–751. [Google Scholar] [CrossRef]
- Goldin, D.M.; Kulikova, G.V. On the dissociation mechanism of carbonates and their isomorphous mixture. J. Therm. Anal. Calorim. 1984, 29, 139–145. [Google Scholar] [CrossRef]
- Pan, Y.X.; Lin, M.; Hao, J.Q. Rock-magnetic properties related to thermal mineral alterations in siderite samples. Chin. J. Geophys. 1999, 42, 756–763. [Google Scholar]
- Chen, C.; Zhang, Y.S.; Yang, Q. Thermokinetic study on magnetic roasting of refractory iron ore. Conserv. Util. Miner. Resour. 2013, 23, 31–34. [Google Scholar]
- Dubrawski, J.V. Thermal decomposition of some siderite-magnesite minerals using DSC. J. Therm. Anal. Calorim. 1991, 37, 1213–1221. [Google Scholar] [CrossRef]
- Gallagher, P.K.; Warne, S.S.J. Application of thermomagnetometry to the study of siderite. Mater. Res. Bull. 1981, 16, 141–144. [Google Scholar] [CrossRef]
- Jagtap, S.B.; Pande, A.R.; Gokarn, A.N. Kinetics of thermal decomposition of siderite: Effect of particle size. Int. J. Miner. Process. 1992, 36, 113–124. [Google Scholar] [CrossRef]
- Gotor, F.J.; Macías, M.; Ortega, A.; Criado, J.M. Comparative study of the kinetics of the thermal decomposition of synthetic and natural siderite samples. Phys. Chem. Miner. 2000, 27, 495–503. [Google Scholar] [CrossRef]
- He, W. The Basic Research on Magnetizing Roasting-Magnetic Separation of Siderite. Ph.D. Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Feng, Z.L.; Yu, Y.F.; Liu, G.F.; Chen, W.; Lv, G. Thermal decomposition kinetics of siderite ore in suspension condition. Chin. J. Process. Eng. 2012, 12, 427–432. [Google Scholar]
- Huang, M.X.; Zhou, C.R.; Han, X.W. Investigation of thermal decomposition kinetics of taurine. J. Therm. Anal. Calorim. 2013, 113, 589–593. [Google Scholar] [CrossRef]
- Otero, M.; Calvo, L.F.; Gil, M.V.; Garc´ıa, A.I.; Mora´n, A. Co-combustion of different sewage sludge and coal: A non-isothermal thermogravimetric kinetic analysis. Bioresour. Technol. 2008, 99, 6311–6319. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.; Adnađević, B.; Mentus, S. The kinetic analysis of non-isothermal nickel oxide reduction in hydrogen atmosphere using the invariant kinetic parameters method. Thermochim. Acta 2007, 456, 48–55. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Ribeiro, C.A.; Crespi, M.S.; Veronezi, A.M. Tin(II) EDTA complex: Kinetic of thermal decomposition by non-isothermal procedures. Thermochim. Acta 2004, 414, 35–42. [Google Scholar] [CrossRef]
- Jankovic, B.; Mentus, S. Model-fitting and model-free analysis of thermal decomposition of palladium acetylacetonate [Pd (acac)2]. J. Therm. Anal. Calorim. 2008, 94, 395–403. [Google Scholar] [CrossRef]
- Wu, D.; Liu, G.; Chen, S.; Sun, R. An experimental investigation on heating rate effect in the thermal behavior of perhydrous bituminous coal during pyrolysis. J. Therm. Anal. Calorim. 2015, 119, 2195–2203. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Wei, X.; Gao, P. Non-isothermal reduction kinetics of oolitic iron ore in ore/coal mixture. J. Therm. Anal. Calorim. 2016, 123, 703–715. [Google Scholar] [CrossRef]
- Polli, H.; Pontes, L.A.M.; Araujo, A.S. Application of model-free kinetics to the study of thermal degradation of polycarbonate. J. Therm. Anal. Calorim. 2005, 79, 383–387. [Google Scholar] [CrossRef]
- Lin, Y.K.; Li, Q.S.; Li, X.F.; Ji, K.; Zhang, H.P.; Yu, Y.M.; Song, Y.H.; Fu, Y.; Sun, L.Y. Pyrolysates distribution and kinetics of Shenmu long flame coal. Energy Convers. Manag. 2014, 86, 428–434. [Google Scholar] [CrossRef]
- Doyle, C.D. Series approximations to the equations of thermogravimetric data. Nature 1965, 207, 290–291. [Google Scholar] [CrossRef]
- Škvára, F.; Šesták, J. Computer calculation of the mechanism and associated kinetic data using a non-isothermal integral method. J. Therm. Anal. 1975, 8, 477–489. [Google Scholar] [CrossRef]
- Hu, R.Z.; Shi, Q.Z. Thermal Analysis Kinetics, 2nd ed.; Science Press: Beijing, China, 2008. [Google Scholar]
- Li, P.; Yu, Q.B.; Xie, H.Q.; Qin, Q.; Wang, K. CO2 gasification rate analysis of Datong coal using slag granules as heat carrier for heat recovery from blast furnace slag by using a chemical reaction. Energy Fuels 2013, 27, 4810–4817. [Google Scholar] [CrossRef]
- Vlaev, L.T.; Markovska, I.G.; Lyubchev, L.A. Non-isothermal kinetics of pyrolysis of rice husk. Thermochim. Acta 2003, 406, 1–7. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.B.; Qin, Q.; Lei, W. Kinetics of CO2/coal gasification in molten blast furnace slag. Ind. Eng. Chem. Res. 2012, 51, 15872–15883. [Google Scholar] [CrossRef]
| Fetotal | FeO | SiO2 | Al2O3 | CaO | MgO | Na | MnO | P | S | Loss of Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| 44.21 | 58.05 | 0.55 | 0.13 | 0.30 | 1.19 | 0.01 | 2.53 | undetected | 0.34 | 36.04 |
| Iron Phases | Iron Carbonate | Magnetite | Hematite | Iron Sulfide | Ferrosilite |
|---|---|---|---|---|---|
| Iron % | 39.45 | 0.12 | 3.69 | 0.85 | 0.10 |
| Mineral % | 96.23 | 0.27 | 2.35 | 0.92 | 0.23 |
| Code | Reaction Model | Mechanism | Integral G(α) | Differential f(α) |
|---|---|---|---|---|
| A1/3 | Avrami–Erofeev (n = 1/3) | Nucleation and growth | [−ln(1 − α)]3 | 1/3(1 − α)[−ln(1 − α)]−2 |
| A1/2 | Avrami–Erofeev (n = 1/2) | [−ln(1 − α)]2 | 1/2(1 − α)[−ln(1 − α)]−1 | |
| A2/3 | Avrami–Erofeev (n = 2/3) | [−ln(1 − α)]3/2 | 2/3(1 − α)[−ln(1 − α)]−1/2 | |
| A1 | Avrami–Erofeev (n = 1) | −ln(1 − α) | 1 − α | |
| A4/3 | Avrami–Erofeev (n = 4/3) | [−ln(1 − α)]3/4 | 4/3(1 − α)[−ln(1 − α)]1/4 | |
| A3/2 | Avrami–Erofeev (n = 3/2) | [−ln(1 − α)]2/3 | 3/2(1 − α)[−ln(1 − α)]1/3 | |
| A2 | Avrami–Erofeev (n = 2) | [−ln(1 − α)]1/2 | 2(1 − α)[−ln(1 − α)]1/2 | |
| A5/2 | Avrami–Erofeev (n = 5/2) | [−ln(1 − α)]2/5 | 5/2(1 − α)[−ln(1 − α)]3/5 | |
| A3 | Avrami–Erofeev (n = 3) | [−ln(1 − α)]1/3 | 3(1 − α)[−ln(1 − α)]2/3 | |
| A4 | Avrami–Erofeev (n = 4) | [−ln(1 − α)]1/4 | 4(1 − α)[−ln(1 − α)]3/4 | |
| D1 | One-dimensional diffusion | α2 | 1/2α−1 | |
| D2 | Valensi | Two-dimensional diffusion | α + (1 − α) ln(1 − α) | [−ln(1 − α)]−1 |
| D3 | Jander | Three-dimensional diffusion | [1 − (1 − α)1/2]2 | (1 − α)1/2[1 − (1 − α)1/2]−1 |
| D4 | Jander (n = 2) | [1 − (1 − α)1/3]2 | 3/2(1 − α)2/3[1 − (1 − α)1/3]−1 | |
| D5 | Jander (n = 1/2) | [1 − (1 − α)1/3]1/2 | 6(1 − α)2/3[1 − (1 − α)1/3]1/2 | |
| D6 | anti-Jander | [(1 + α)1/3 − 1]2 | 3/2(1 + α)2/3[(1 + α)1/3 − 1]−1 | |
| D7 | Z-L-T | [(1 − α)−1/3 − 1]2 | 3/2(1 − α)4/3[(1 − α)−1/3 − 1]−1 | |
| D8 | Ginstling–Brounshtein | 1 − 2α/3 − (1 − α)2/3 | 3/2[(1 − α)−1/3 − 1]−1 | |
| R1/2 | Contraction sphere (n = 1/2) | Phase boundary reaction | 1 − (1 − α)2 | 1/2(1 − α)−1 |
| R1/3 | Contraction sphere (n = 1/3) | 1 − (1 − α)3 | 1/3(1 − α)−2 | |
| R1/4 | Contraction sphere (n = 1/4) | 1 − (1 − α)4 | 1/4(1 − α)−3 | |
| R2 | Contraction sphere (n = 2) | 1 − (1 − α)1/2 | 2(1 − α)1/2 | |
| R3 | Contraction sphere (n = 3) | 1 − (1 − α)1/3 | 3(1 − α)2/3 | |
| R4 | Contraction sphere (n = 4) | 1 − (1 − α)1/4 | 4(1 − α)3/4 | |
| P2/3 | Mampel power law (n = 2/3) | α3/2 | 2/3α−1/2 | |
| P1 | Mampel power law (n = 1) | α | 1 | |
| P2 | Mampel power law (n = 2) | α1/2 | 2α1/2 | |
| P3 | Mampel power law (n = 3) | α1/3 | 3α2/3 | |
| P4 | Mampel power law (n = 4) | α1/4 | 4α3/4 | |
| C1 | Reaction order (n = 2) | Chemical reaction | (1 − α)−1 − 1 | (1 − α)2 |
| C2 | Reaction order (n = 3/2) | (1 − α)−1/2 | 2(1 − α)3/2 | |
| C3 | Reaction order (n = 3) | (1 − α)−2 | 1/2(1 − α)3 |
| Conversion Fraction/α | E/kJ·mol−1 a | R b |
|---|---|---|
| 0.1 | 532.65 | 0.978 |
| 0.2 | 516.05 | 0.988 |
| 0.3 | 510.97 | 0.989 |
| 0.4 | 492.93 | 0.990 |
| 0.5 | 466.63 | 0.989 |
| 0.6 | 450.20 | 0.988 |
| 0.7 | 436.79 | 0.989 |
| 0.8 | 421.32 | 0.987 |
| 0.9 | 413.92 | 0.982 |
| Average | 471.27 | 0.987 |
| Heating Rate (°C·min−1) | Code | Intercept | Slope | R | Activation Energy Es a (kJ·mol−1) | Pre-Exponential Factor A b (min−1) |
|---|---|---|---|---|---|---|
| 5 | A1/2 | −24506.71 | 20.54 | 0.9980 | 446.13 | 6.67 × 10−18 |
| 10 | −25768.30 | 20.98 | 0.9993 | 469.10 | 1.75 × 10−19 | |
| 20 | −26800.51 | 22.01 | 0.9990 | 487.89 | 1.80 × 10−20 | |
| 30 | −27750.87 | 23.15 | 0.9995 | 505.19 | 2.40 × 10−21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Han, Y.; Li, Y.; Sun, Y. Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite. Minerals 2017, 7, 211. https://doi.org/10.3390/min7110211
Zhang X, Han Y, Li Y, Sun Y. Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite. Minerals. 2017; 7(11):211. https://doi.org/10.3390/min7110211
Chicago/Turabian StyleZhang, Xiaolong, Yuexin Han, Yanjun Li, and Yongsheng Sun. 2017. "Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite" Minerals 7, no. 11: 211. https://doi.org/10.3390/min7110211
APA StyleZhang, X., Han, Y., Li, Y., & Sun, Y. (2017). Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite. Minerals, 7(11), 211. https://doi.org/10.3390/min7110211




