Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
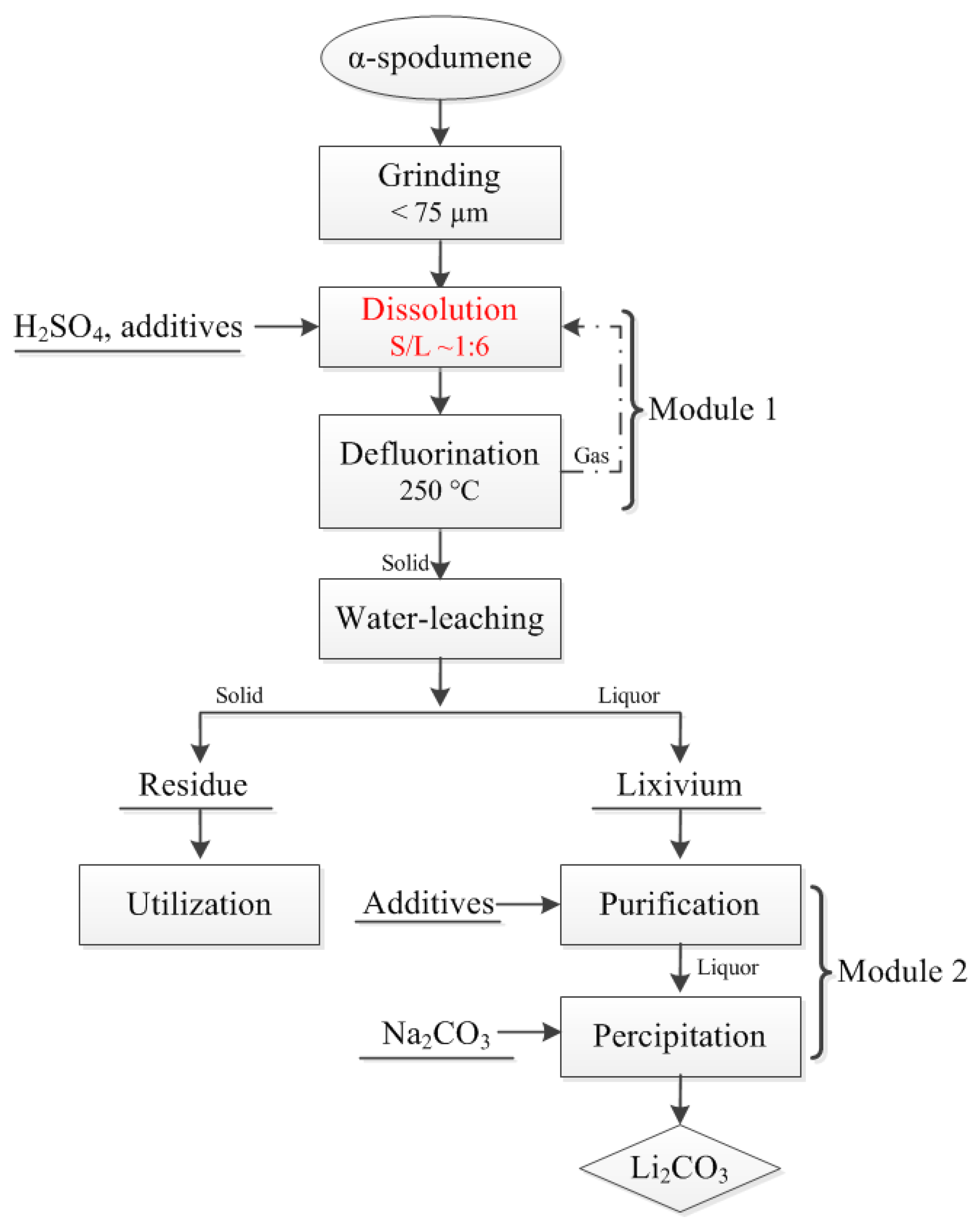
2.2. Experimental Procedures
2.3. Analytical Methods and Charaterization
3. Results and Discussion
3.1. Chemical Analysis and Characterization of Ore Samples
3.2. Thermodynamic Analysis
3.3 Investigation of Dissolution Conditions
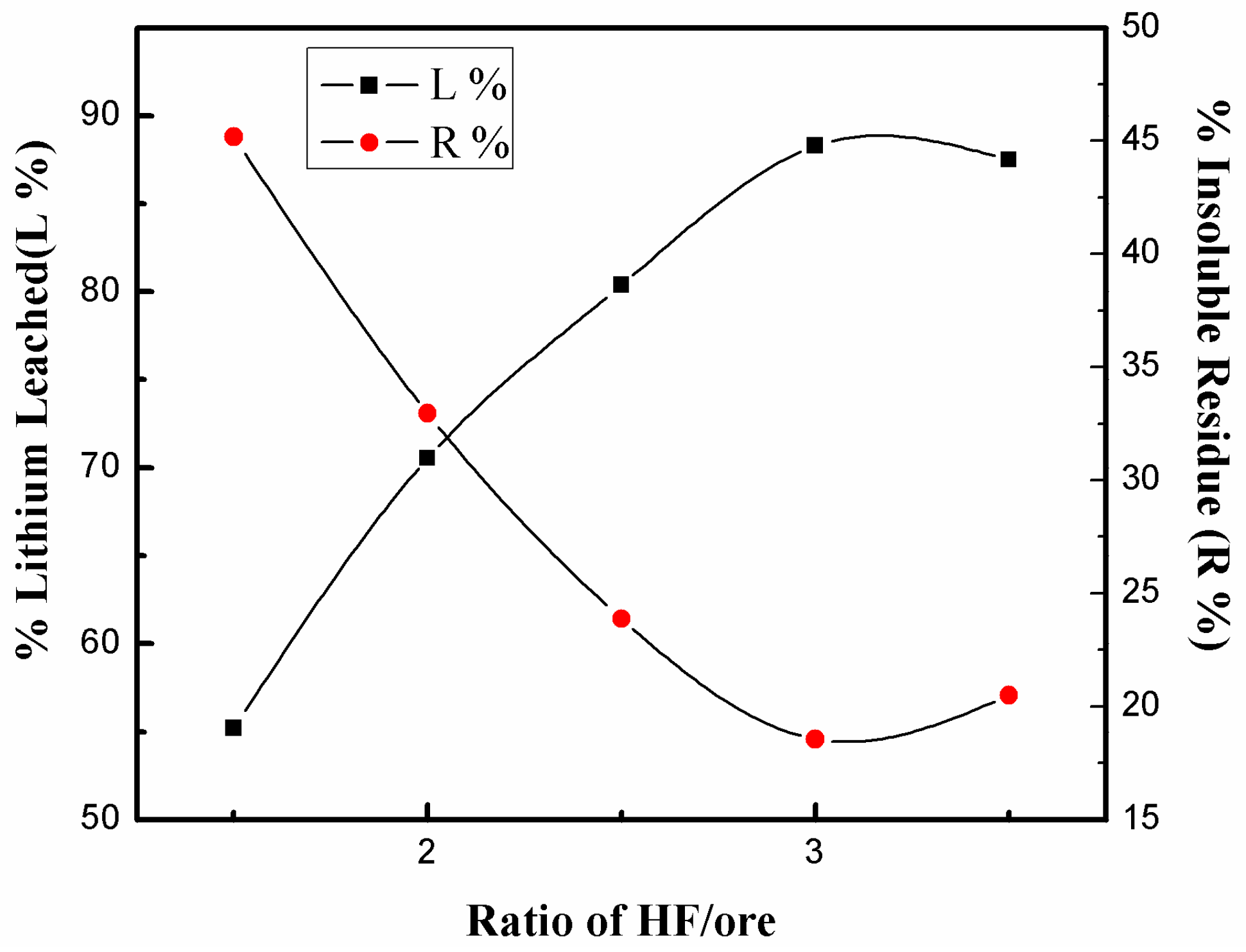
3.3.1. Effect of HF/Ore Ratio
3.3.2. Effect of Dissolution Temperature
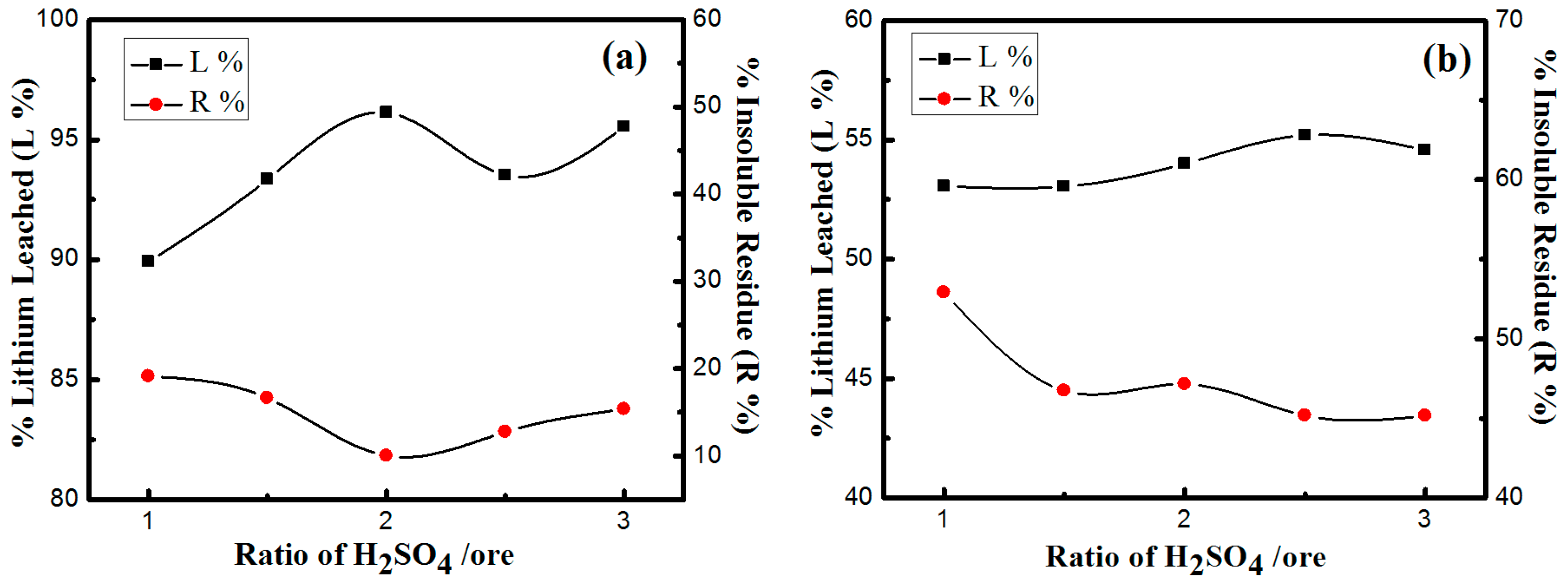
3.3.3. Effects of H2SO4/Ore Ratio
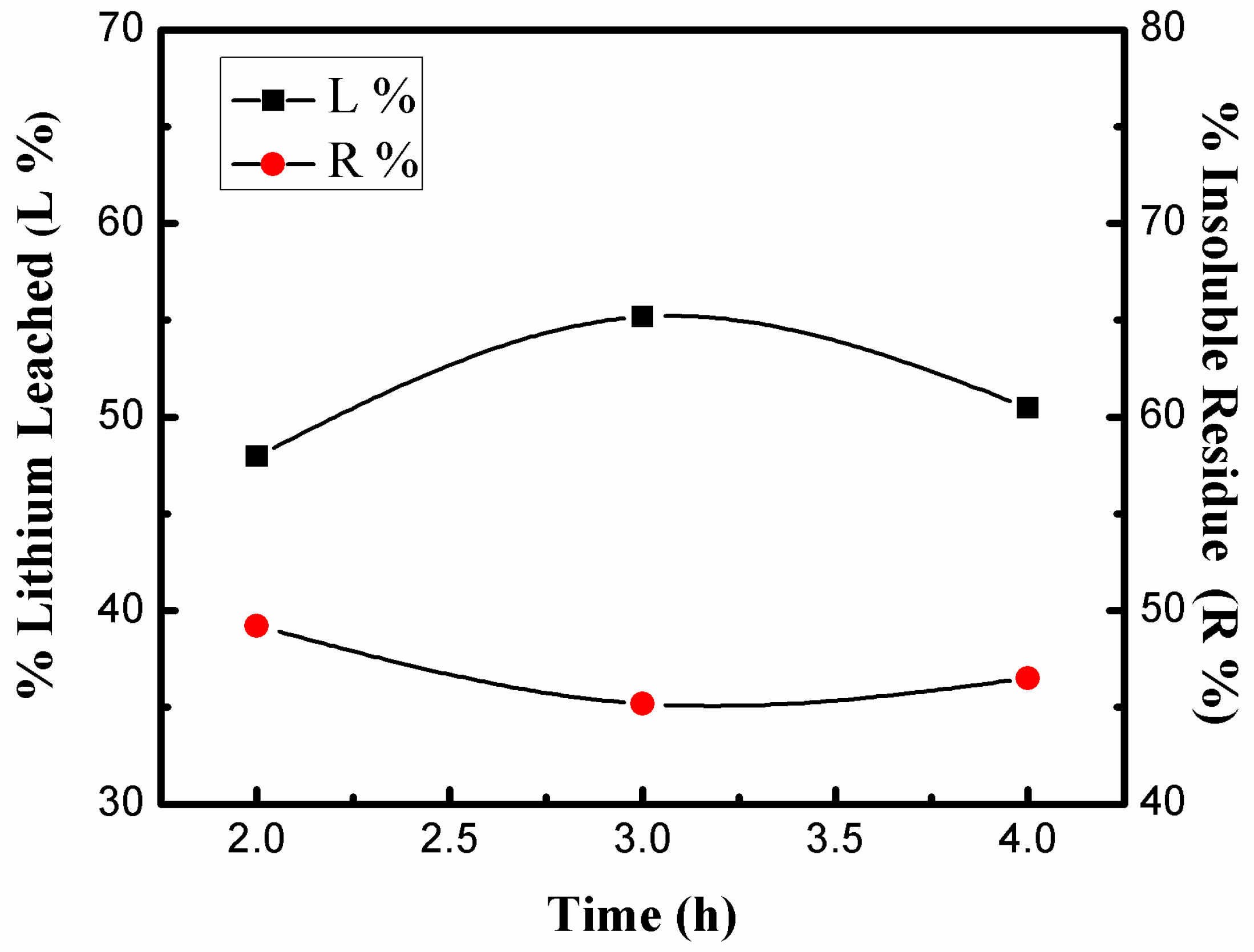
3.3.4. Effects of Dissolution Time
3.4. Dissolution Kinetics
3.5. Characterization of Solids
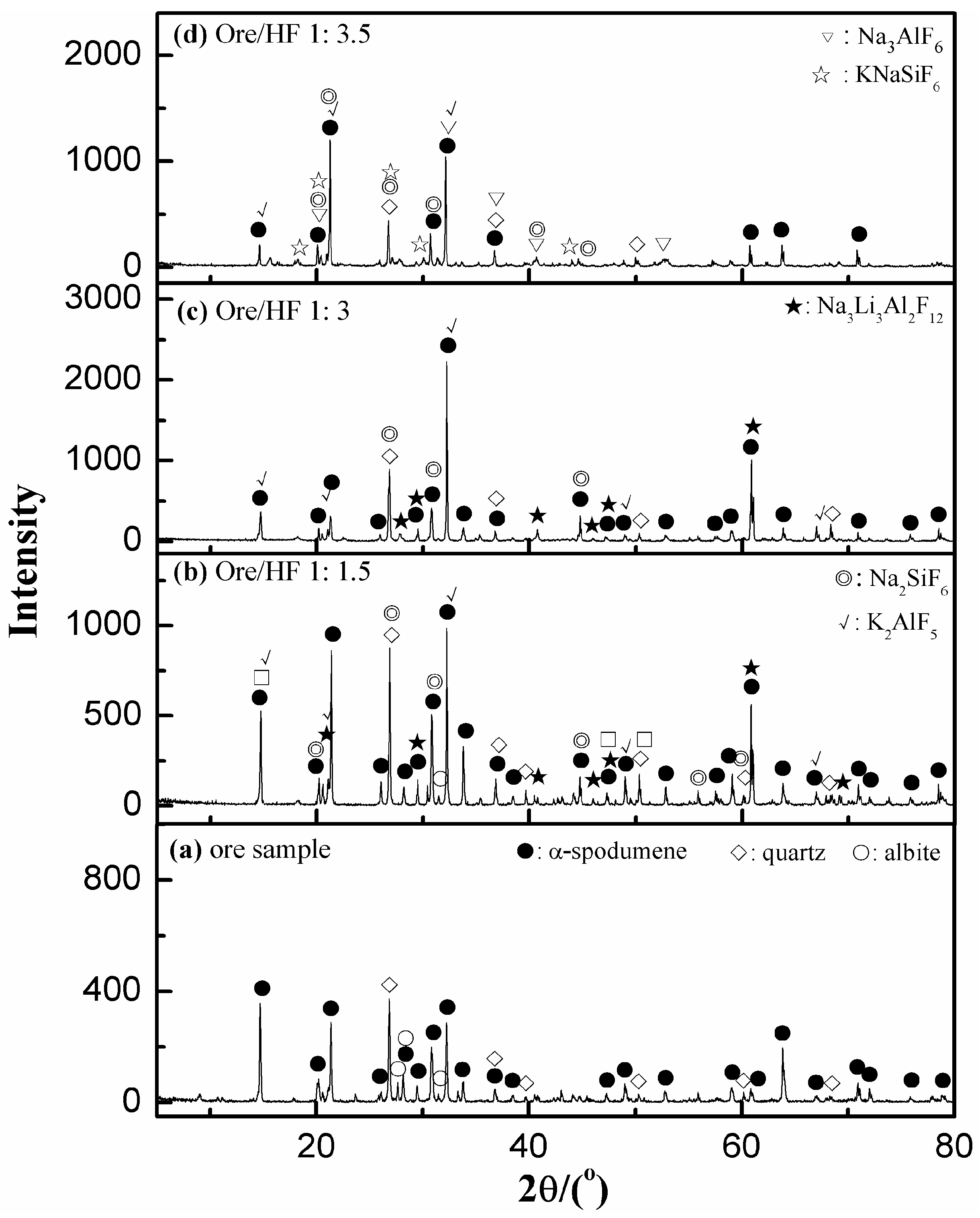
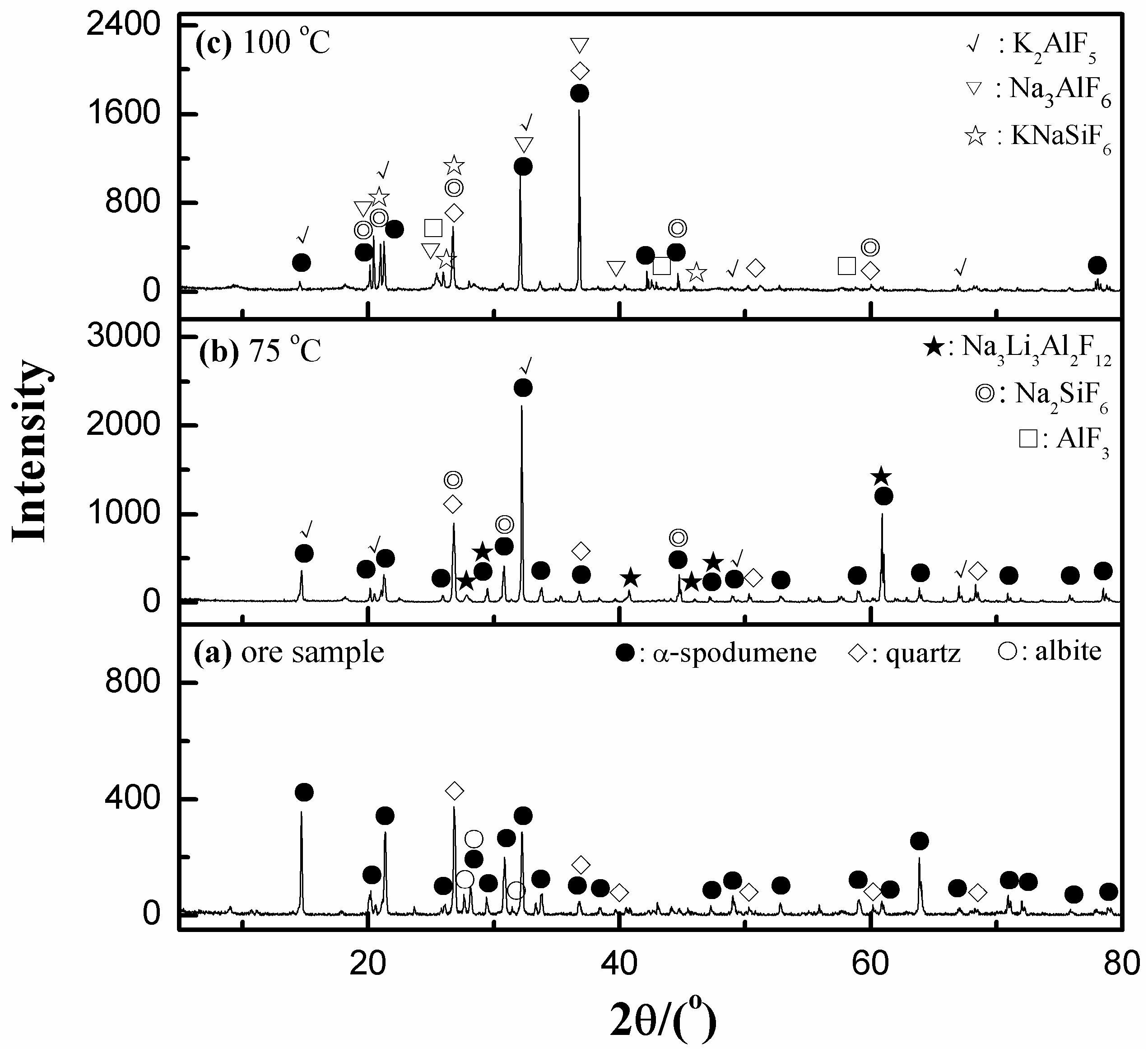
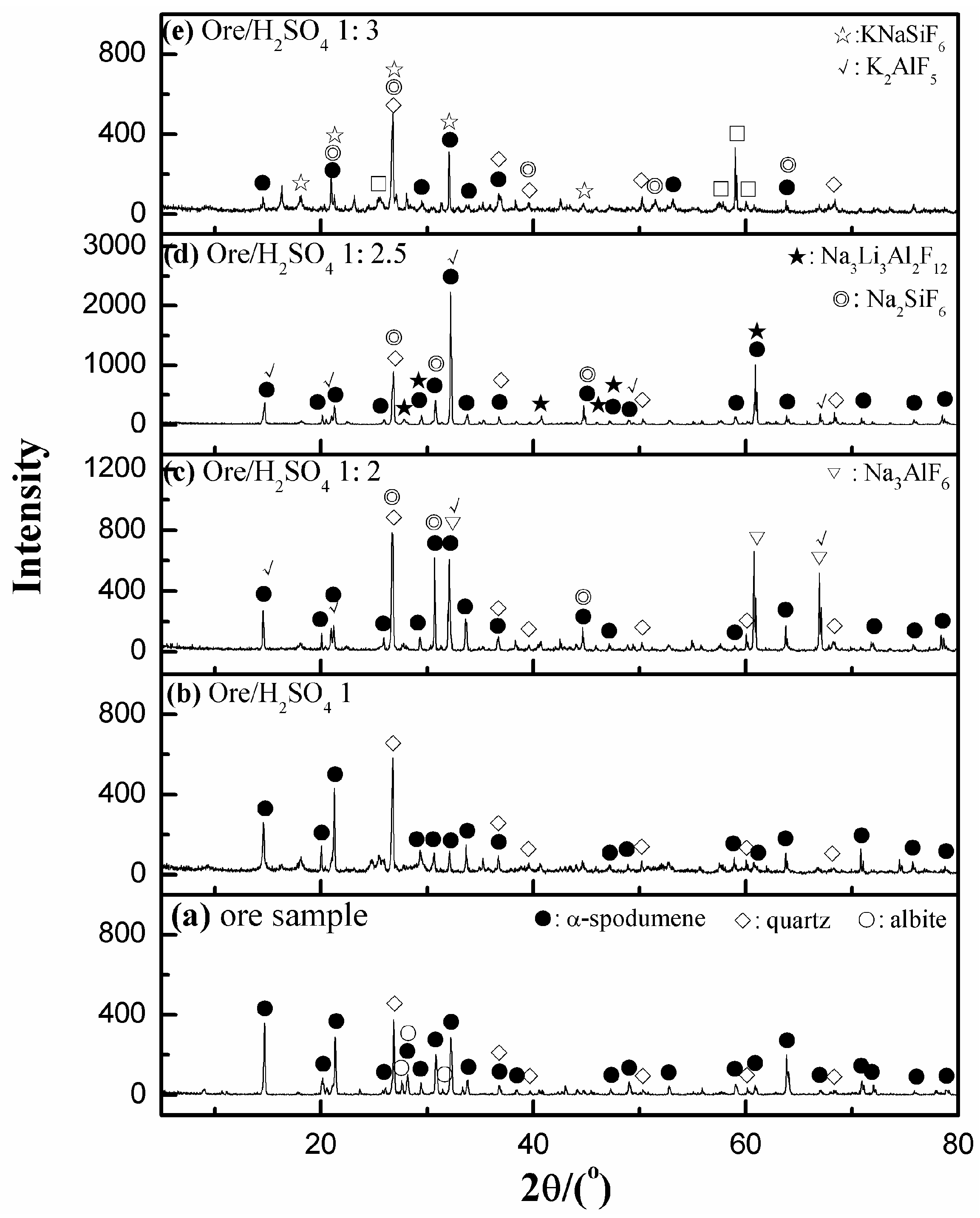
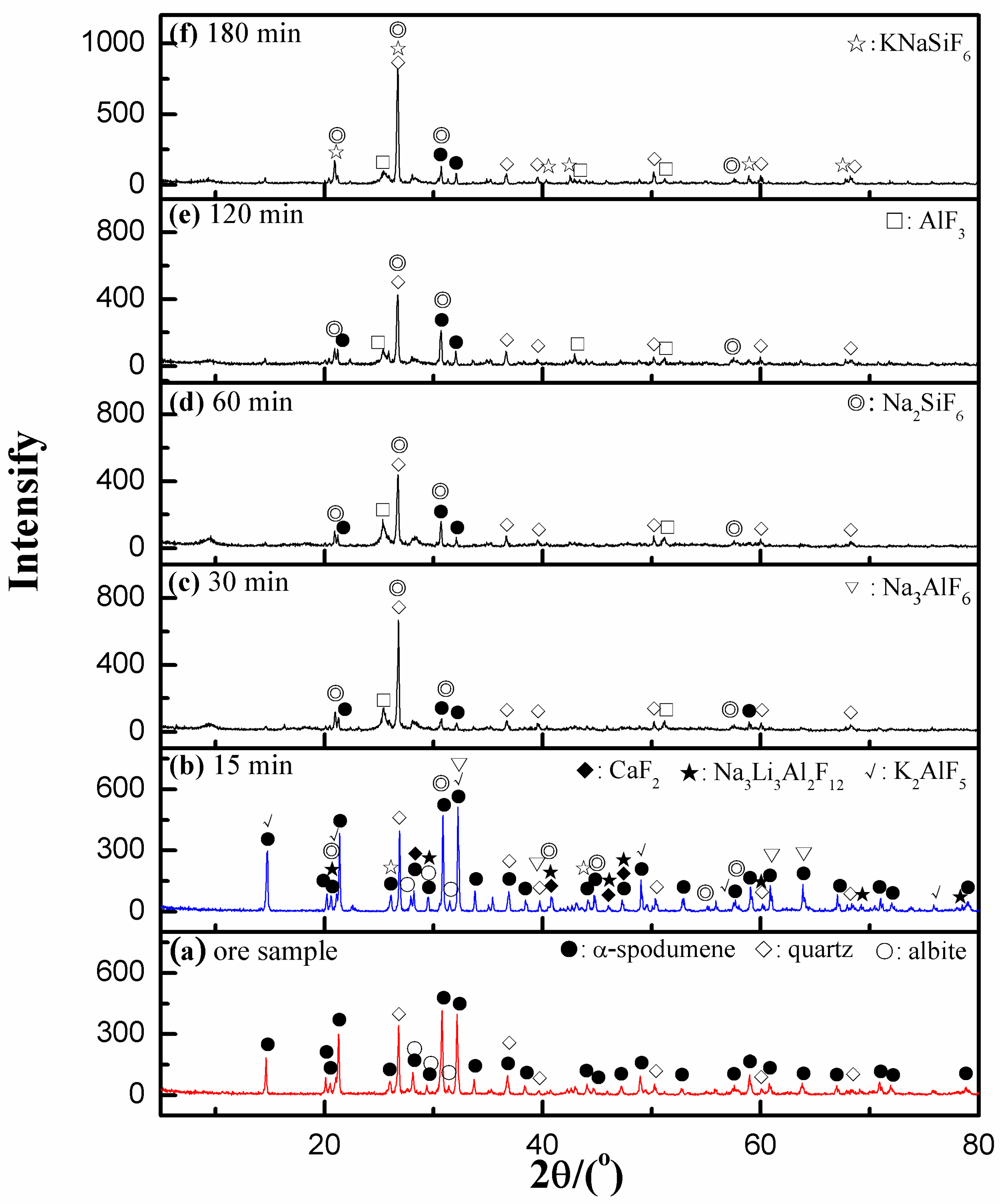
3.5.1. XRD Analyses
3.5.2. XPS Analyses
3.5.3. SEM-EDX Analyses
3.5.4. TG-DSC Analyses
3.6. Chemical Analysis under Optimal Conditions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| symbol | phase |
| ● | α-Spodumene, LiAlSi2O6, JCPDS 33-0786 |
| ◇ | Albite, NaAlSi3O8, JCPDS 09-0466 |
| ○ | Quartz, SiO2, JCPDS 65-0466 |
| ★ | Cryolithionite, Na3Li3Al2F12, JCPDS 22-0416 |
| ▽ | Cryolite, Na3AlF6, JCPDS 82-0216 |
| √ | K2AlF5, JCPDS 52-0735 |
| □ | AlF3, JCPDS 44-0231 |
| ☆ | KNaSiF6, JCPDS 43-1313 |
| ◎ | Na2SiF6, JCPDS 72-1115 |
| ◆ | CaF2, JCPDS 48-9812 |
References
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Eversond, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- An, J.W.; Kang, J.D.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 2012, 117, 64–70. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.S.; Srivastava, R.R.; Lee, J.C.; Lee, J.Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Kwang, S.M.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Barbosa, L.I.; González, J.A.; del Carmen Ruiz, M. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Rosales, G.D.; del Carmen Ruiz, M.; Rodriguez, M.H. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147, 1–6. [Google Scholar] [CrossRef]
- Suarez, D.S.; Pinna, E.G.; Rosales, G.D.; Rodriguez, M.H. Synthesis of Lithium Fluoride from Spent Lithium Ion Batteries. Minerals 2017, 7, 81. [Google Scholar] [CrossRef]
- Monk, D.J.; Soane, D.S.; Howe, R.T. A review of the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications. Thin Solid Films 1993, 232, 1–12. [Google Scholar] [CrossRef]
- Dhar, S.; Seitz, O.; Halls, M.D.; Choi, S.; Chabal, Y.J.; Feldman, L.C. Chemical Properties of Oxidized Silicon Carbide Surfaces upon Etching in Hydrofluoric Acid. J. Am. Chem. Soc. 2009, 131, 16808–16813. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Fogler, H.S.; Lund, K.; McCune, C.C. Acidization III—The kinetics of the dissolution of sodium and potassium feldspar in HF/HCl acid mixtures. Chem. Eng. Sci. 1975, 30, 1325–1332. [Google Scholar] [CrossRef]
- Kline, W.E.; Fogler, H.S. Dissolution kinetics: The nature of the particle attack of layered silicates in HF. Chem. Eng. Sci. 1981, 36, 871–884. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.B.; Li, J.; Zhang, Q.; Feng, Y.; Liu, P. Kinetic mechanics of the reactions between HCl/HF acid mixtures and sandstone minerals. J. Nat. Gas Sci. Eng. 2016, 34, 792–802. [Google Scholar] [CrossRef]
- Kuang, G.; Chen, Z.; Guo, H.; Li, M. Lithium Extraction Mechanism from α-Spodumene by Fluorine Chemical Method. Adv. Mater. Res. 2012, 524, 2011–2016. [Google Scholar] [CrossRef]
- HSC Chemistry Software, version 6.0; Outokumpu Technology Company: Espoo, Finland, 2006.
- Ye, D.L.; Hu, J.H. Thermochemical Properties of Inorganic Substances; Metallurgy Industrial Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Miki, N.; Maeno, M.; Maruhashi, K.; Ohmi, T. Vapor-Liquid Equilibrium of the Binary System HF-H2O Extending to Extremely Anhydrous Hydrogen Fluoride. J. Electrochem. Soc. 1990, 137, 787–790. [Google Scholar] [CrossRef]
- Luo, W.; Feng, Q.; Ou, L.; Zhang, G.; Chen, Y. Kinetics of saprolitic laterite leaching by sulphuric acid at atmospheric pressure. Miner. Eng. 2010, 23, 458–462. [Google Scholar] [CrossRef]
- Hess, A.; Kemnitz, E.; Lippitz, A.; Unger, W.E.S.; Menz, D.H. Esca, XRD, and IR characterization of aluminum oxide, hydroxyfluoride, and fluoride surfaces in correlation with their catalytic activity in heterogeneous halogen exchange reactions. J. Catal. 1994, 148, 270–280. [Google Scholar] [CrossRef]
- Hasha, D.; Saldarriaga, L.S.D.; Saldarriaga, C.; Hathaway, P.E.; Cox, D.F.; Davis, M.E. Studies of silicoaluminophosphates with the sodalite structure. J. Am. Chem. Soc. 1988, 110, 2127–2135. [Google Scholar] [CrossRef]
- Morgan, W.E.; Wazer, J.R.V.; Stec, W.J. Inner-orbital photoelectron spectroscopy of the alkali metal halides, perchlorates, phosphates, and pyrophosphates. J. Am. Chem. Soc. 1973, 95, 751–755. [Google Scholar] [CrossRef]
- Wagner, C.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 32–65. [Google Scholar]
- Ro, C.; Linton, R.W. Characterization of LiF using XPS. Surf. Sci. Spectr 1992, 1, 277–283. [Google Scholar] [CrossRef]
- Murch, G.E.; Thorn, R.J. Relation between orbital binding energies and ionicities in alkali and alkaline earth flourides. J. Phys. Chem. Solids 1980, 41, 785–791. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Buslaev, Y.A.; Kokunov, Y.V. X-ray electron spectroscopy of alkali metal fluorides and alkaline earth metal fluorides. Zhurnal Neorg. Khim. 1974, 19, 1166–1169. (In Russian) [Google Scholar]
- Rueda, F.; Mendialdua, J.; Rodriguez, A.; Casanova, R.; Barbaux, Y.; Gengembre, L.; Jalowiecki, L. Characterization of venezuelan laterites by X-ray photoelectron spectroscopy. J. Electron. Spectrosc. Relat. Phenom. 1996, 82, 135–143. [Google Scholar] [CrossRef]
- Frayret, J.; Castetbon, A.; Trouve, G.; Potin-Gautier, M. Solubility of (NH4)2SiF6, K2SiF6, and Na2SiF6 in acidic solutions. Chem. Phys. Lett. 2006, 427, 356–364. [Google Scholar] [CrossRef]
- Menz, D.H.; Zacharias, A.; Kolditz, L. A comparison of the thermal behaviour of α-AIF3, and aluminium fluoride hydrates. J. Therm. Anal. Calorim. 1988, 33, 811–815. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Yang, J.X.; Hu, S. Fundamental research on a new process to remove Al3+, as potassium alum during lithium extraction from lepidolite. Metall. Mater. Trans. B 2016, 47, 3557–3564. [Google Scholar] [CrossRef]
- Kuang, G.; Li, H.; Hu, S.; Jin, R.; Liu, S.; Guo, H. Recovery of aluminium and lithium from gypsum residue obtained in the process of lithium extraction from lepidolite. Hydrometallurgy 2015, 157, 214–218. [Google Scholar] [CrossRef]



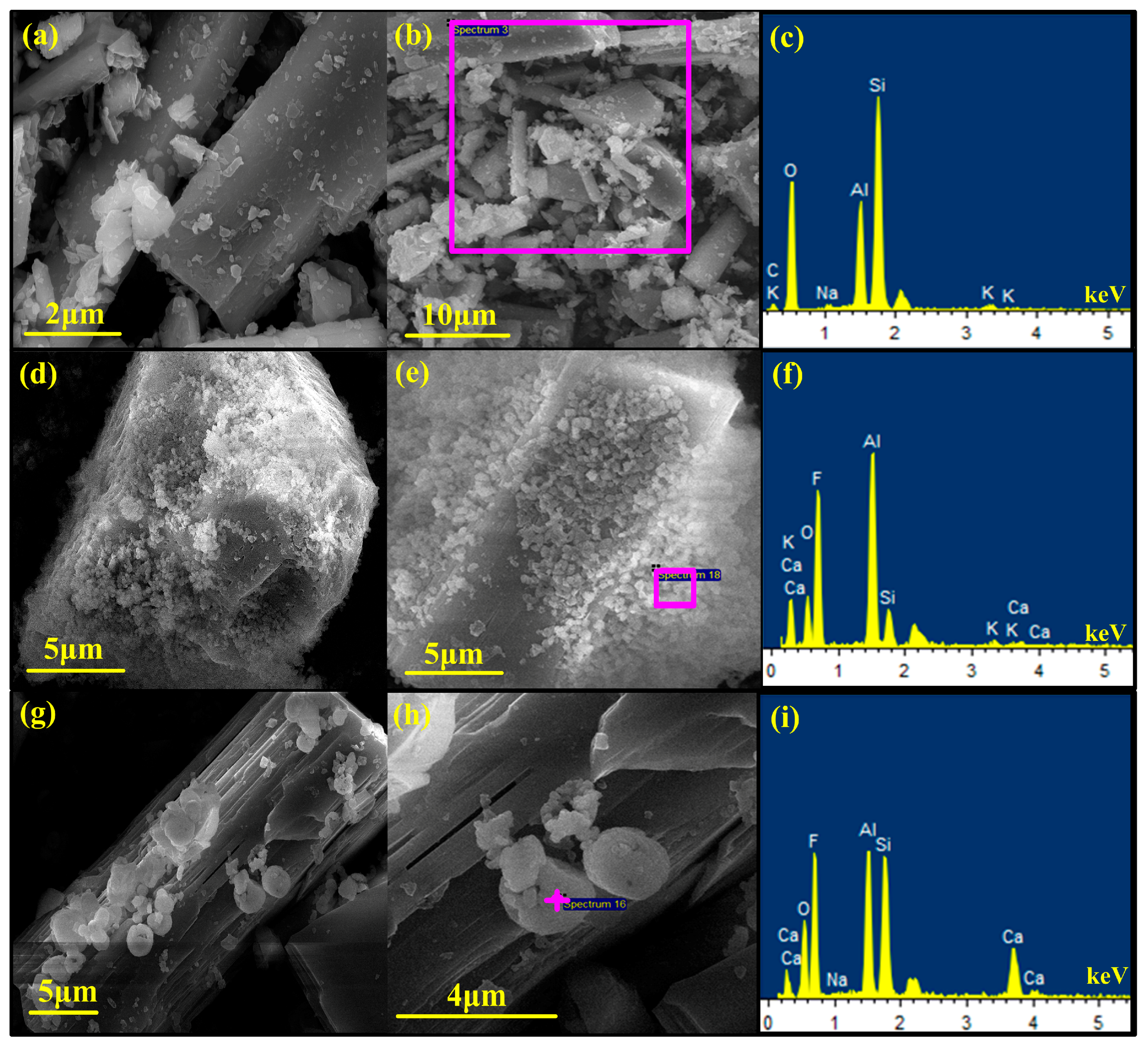
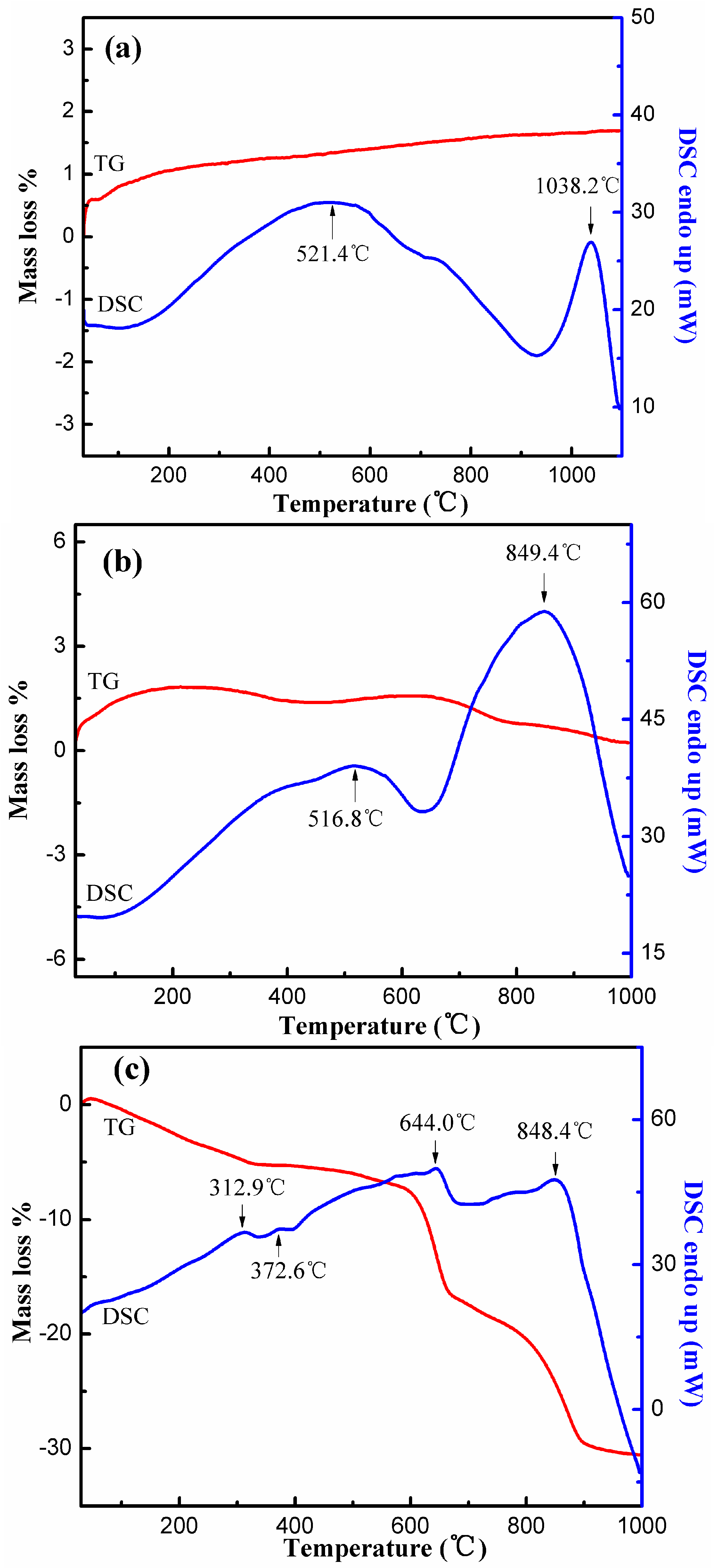
| Element | Si | Al | Li | O | Na | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|
| Content, wt % | 32.63 | 11.43 | 2.56 | 41.04 | 0.35 | 0.68 | 0.45 | 0.54 |
| T, °C | ∆H°, kJ | ∆S°, J/K | ∆G°, kJ |
|---|---|---|---|
| 0 | −572.534 | 715.044 | −767.848 |
| 25 | −547.462 | 802.860 | −786.835 |
| 50 | −521.889 | 885.214 | −807.946 |
| 75 | −495.764 | 963.072 | −831.058 |
| 100 | −468.996 | 1037.314 | −856.069 |
| 125 | −441.461 | 1108.725 | −882.900 |
| Element | Residues, wt % | Lixivium | |
|---|---|---|---|
| Concentration, g/L * | Leaching Efficiency a, % | ||
| Si | 20.32 | ||
| Al | 15.39 | 7.83 | 89 |
| Li | 1.51 | 96 | |
| O | 23.82 | ||
| Na | 0.12 | 0.21 | 91 |
| K | 0.36 | 0.58 | 95 |
| Ca | 0.19 | ||
| Ore Sample | Lixivium | Residues | |
|---|---|---|---|
| Lithium content, g | 0.2566 | 0.2214 | 0.0319 |
| Deviation, % | 1.3% | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Kuang, G.; Wang, H.; Yu, H.; Zhao, X. Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals 2017, 7, 205. https://doi.org/10.3390/min7110205
Guo H, Kuang G, Wang H, Yu H, Zhao X. Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals. 2017; 7(11):205. https://doi.org/10.3390/min7110205
Chicago/Turabian StyleGuo, Hui, Ge Kuang, Haidong Wang, Haizhao Yu, and Xiaokang Zhao. 2017. "Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid" Minerals 7, no. 11: 205. https://doi.org/10.3390/min7110205
APA StyleGuo, H., Kuang, G., Wang, H., Yu, H., & Zhao, X. (2017). Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals, 7(11), 205. https://doi.org/10.3390/min7110205




