Abstract
Several small chromium (Cr) ore bodies are hosted within a unit of tectonically thinned dunite in the retired Ayios Stefanos mine of the western Othris ophiolite complex in Greece. Chromium ores consist of tectonically imprinted bodies of semi-massive to massive, podiform and lenticular chromitites composed of chromian spinel [Cr-spinel] with high Cr# [Cr/(Cr + Al) = 0.51–0.66] and Mg# [Mg/(Mg + Fe2+) = 0.58–0.76], low Fe3+# [Fe3+/(Fe3+ + Fe2+) ≤ 0.43] and low TiO2 (≤0.21 wt %) content. This composition is characteristic of Cr-spinels in equilibrium with melts of intermediate affinity between island-arc tholeiites (IATs) and mid-ocean ridge basalts (MORBs). Several Cr-spinel crystals in these ores exhibit imperfect zones made up of spinel hosting oriented lamellae of Mg-silicates (mostly chlorite) locally overgrown by porous domains along grain boundaries and fractures. From the Cr-spinel core to the lamellae-rich rim Cr#, Mg# and Fe3+# generally increase (0.68–0.87, 0.78–0.88 and 0.55–0.80, respectively), whereas from the core or the spinel zones with oriented lamellae to the porous domains Mg# and Fe3+# generally decrease (0.45–0.74 and ≤0.51, correspondingly). The lamellae-rich rims formed at oxidizing conditions, whereas the porous rims resulted from a later reducing event. Several tiny (≤30 μm), subhedral to anhedral and elongated Zr-bearing silicate mineral grains were discovered mainly along open and healed fractures cutting Cr-spinel. Most of the Zr-bearing silicate minerals (30 out of 35 grains) were found in a chromitite boulder vastly intruded by a complex network of gabbroic dykes. The dominant Zr-bearing silicate phase is by far zircon displaying a homogeneous internal texture in cathodoluminescence (CL) images. Raman spectroscopy data indicate that zircons have experienced structural damage due to self-irradiation. Their trace-element contents suggest derivation from a plagioclase-bearing, low-SiO2 intermediate to mafic source. Combined micro-textural and minerochemical data repeat the possibility of zircon derivation from limited volumes of high-T fluids emanating from the gabbroic intrusions. Once zircon is precipitated in cracks, it may be altered to Ca-rich Zr-bearing silicate phases (i.e., armstrongite, calciocatapleiite). Almost all zircons in these samples show evidence of gains in solvent compounds (CaO, Al2O3 and FeO) possibly due to re-equilibration with late deuteric fluids.
1. Introduction and Rationale of the Study
Depleted sections of old sub-oceanic lithospheric mantle may contain substantial amounts of chromian spinel (hereafter Cr-spinel) in the form of single but sometimes economically valuable deposits of Cr (chrome ores) (e.g., [1]). Chromitites in ophiolites are predominantly dispersed at the transitional boundary between the Earth’s crust and the mantle [2]. Despite their simple mineralogy, the mechanism that governs their genesis remains controversial. Interaction between mafic melts and peridotite wall rocks may change magma composition triggering Cr-spinel precipitation [3]. On the other hand, a growing body of work has concluded that focused flow and mingling of hydrous mafic magmas characterized by varying SiO2-activities within ‘dunite channels’ (e.g., [4]) might be essential for the genesis of chromitites [5]. The inferred parental melts of most chromitites are thought to have arc-related supra subduction zone (SSZ) signatures (e.g., [6]). However, the ‘coincidental’ discovery of chromitite seams in peridotites from ultra-slow mid-ocean ridges (MOR) sends a cautionary message about the possibility of chromitite formation even in typical ‘dry’ environments [7,8]. Furthermore, it has been demonstrated that magmatic assimilation of a hydrated mantle protolith at Moho-level depths may dramatically affect the chemistry of a mid-ocean ridge basalt-type (MORB) melt causing increase of Cr concentration into economic ores [9]. Regardless of their origin, ophiolitic chromitites are widely considered as ‘miniature time capsules’ retaining priceless information on petrological issues of crucial importance such as: (i) the nature of the upper mantle [10]; (ii) mantle melting and subsequent melt extraction and interaction with peridotites [11] and lastly; (iii) recycling of the oceanic lithosphere [12]. Furthermore, Cr-spinel is widely regarded as a sensitive petrotectonic indicator because its composition is controlled by mantle melting processes that are typical of various geotectonic environments (e.g., [1,3,5,6]). The fundamental role of Cr-spinel and chromitites in resolving large scale petrological matters is reflected in their potential use to probe the differentiation processes in our planet’s interior up to the Transition Zone (410–660 km) [13].
An increasing number of studies have revealed that both texture and composition of Cr-spinel in chromitites may be seriously affected by either prograde [14,15,16] or retrograde metamorphism [17,18] leading to a series of alteration products rich in FeOt (and Cr2O3 at a lesser extent) and variably depleted in MgO and Al2O3. These include high reflectivity (under reflected light), Fe2+- and Fe3+-rich phases commonly dubbed in the literature as ferritchromit/ferritchromite [19] or ferrian chromite [20]. A new scenario gaining increasing acceptance postulates that Cr-spinel alteration is a reversible two-stage process that happens during the retrograde evolution of chromitites from eclogite-facies conditions [20].
A major breakthrough in our understanding of the subduction-driven recycling of the oceanic lithosphere was derived from the recovery of zircons (among other uncommon phases) from mantle-hosted podiform chromitites [13,21]. Several studies support that the zircon grains represent xenocrysts derived from the crustal part of a sinking slab [22,23,24]. However, a limited body of work has speculated that zircons may precipitate from fluid-rich metasomatic melts [25,26] or may be introduced to the chromitites via microscopic melt/fluid networks emanating from adjacent magmatic intrusions [27,28]. Hence, diverse processes may account for the discovery of zircon in chromitites but sometimes these processes may be acting in concert in a single mantle section [22].
In the present work we document the mode of occurrence, mineralogy and composition of chromitites from the area of Ayios Stefanos, Othris ophiolite complex, Greece. These observations aid the development of a model delineating the genesis and evolution of chromitites. This study reports our discovery of the existence of zircons in the Greek chromitites. Unraveling the origin of zircons delivers new insights into the nature and sequence of the petrologic processes that operated in the western branch of the Neo-Tethyan mantle. We supplement our investigation with new data on Cr-spinel and zircon alteration in an effort to acquire a more complete image of the processes affecting chromitites in the post-magmatic stage of lithospheric slab evolution.
2. Geological Background and Field Work
2.1. General Geological Framework—The Othris Ophiolites
The collision between Laurasia and Gondwana during the Jurassic and the Cretaceous led to the development of a lengthy suture zone that extends from Europe’s mainland toward southeastern Asia. This suture zone is marked by numerous ophiolites representing remnants of the Neo-Tethys; the Neo-Tethys was a system of oceanic basins opening and spreading between a series of Cimmerian micro-plates during the Mesozoic-Early Paleogene [29]. The ophiolites of continental Greece constitute the southernmost part of the Dinarides-Hellenides sector of the Alpine Neo-Tethyan ophiolitic chain (Figure 1).
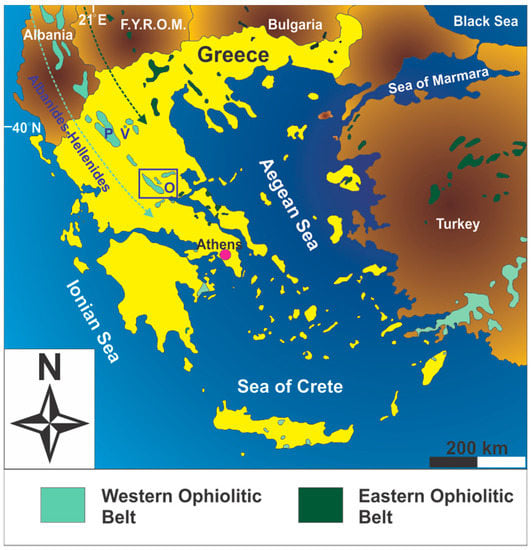
Figure 1.
Distribution of ophiolites in the southernmost part of the Balkan Peninsula (modified after [30]). Key to lettering: V = Vourinos; P = Pindos; O = Othris (also marked by the open square). The initials F.Y.R.O.M. stand for Former Yugoslav Republic of Macedonia.
The Jurassic-aged Othris ophiolite complex (Figure 2) is comprised of a series of imbricated thrust sheets emplaced in reverse lithostratigraphic order onto the Pangean-derived Pelagonian continental margin [31,32]. From the top of the nappe pile to its base, the nappes consist of the following lithospheric sections: (1) plagioclase- and spinel-bearing lherzolites; (2) harzburgites; (3) ‘near petrological Moho’ peridotites including cumulate intrusions; (4) ultramafic and mafic cumulates; (5) sheeted dolerites as well as (6) pillow lavas and flows often including Cu mineralization related to ridgecrest hydrothermal activity. These ophiolitic nappes are obducted over the older (Permo-Triassic) Agrilia volcano-sedimentary sequence associated with rifting and sedimentation along the western margin of the Pelagonian micro-continent [33]. A chaotic lithological mixture of ophiolitic rocks and pelagic sediments in the area of Agoriani represents a remnant accretionary mélange forming part of the Avdella mélange [34]. Amphibolite outcrops near Lamia and Metalleion represent remnants of a sub-ophiolitic sole. Dating of these rocks using the 40Ar/39Ar isotopic pair yielded obduction initiation ages at 169 ± 4 Ma [35].
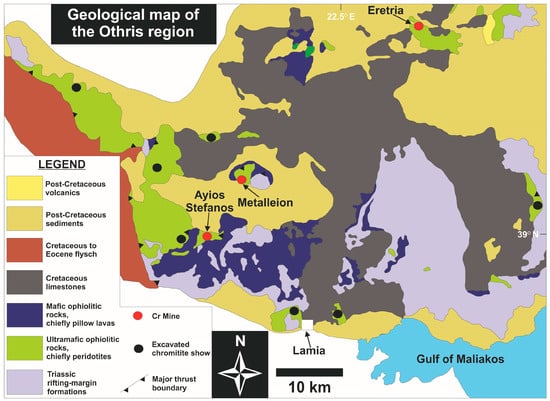
Figure 2.
Geological map of the Othris ophiolite complex [33].
2.2. Study Area, Sampling and Field Observations
Chromitites within Othris are relatively rare in comparison to other Neo-Tethyan ophiolites of Greece. Three inactive chrome (Cr) mines (Ayios Stefanos, Metalleion-Domokos and Tsangli-Eretria) produced approximately 1.5 Mt of ore until the early 1990s.
Ores of the retired Ayios Stefanos mine are almost completely exhausted. A few small (1 × 3 m), massive-textured chromitite bodies remain within a tectonically attenuated and highly serpentinized dunite-harzburgite ductile-brittle thrust zone (Figure 3a,b). This tectonic zone separates the plagioclase-bearing lherzolite-dominant nappe from the underlying harzburgite-dominant nappe that make up the Ayios Stefanos ultramafic unit. Twelve chromitite samples were collected from a chromitite boulder (~2 m3) within the quarry talus and four chromitite specimens were taken from a lenticular ore body (<1 m thick and a few m long; Figure 3c; Table A1 in the Appendix) ‘trapped’ within the reduced dunite-harzburgite deformed zone separating the peridotite nappes. The lenticular ore body is boudinaged and subcordant to the structural fabric (mylonitic foliation) of the hosting ductile-sheared peridotites. The contact between this chromitite body and the surrounding dunite leftover is sharp. The chromitite mine-talus boulder is massively intruded by a complex network of gabbroic dykes (≤15–20 cm-thick) infrequently hosting peridotite xenoliths (Figure 3c). Gabbroic intrusions are absent from the lenticular chrome ore body, but they occur within the tectonically sheared host peridotite within a surrounding zone of ~20 m. Several dunite micro-pods (≤5 cm across) were recognized in the lenticular chromitite. Both chromitites and peridotites appear highly serpentinized but otherwise almost non-weathered in terms of mesoscopic observation. The gabbro dykes in peridotites seem to have experienced variable degrees of rodingitization.
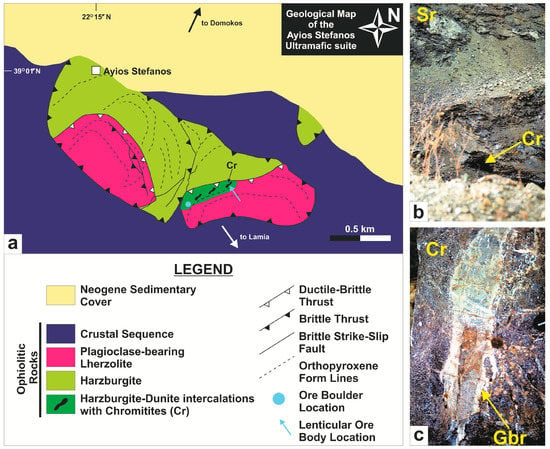
Figure 3.
(a) Geological map of the Ayios Stefanos region illustrating the bowing of structures synchronous to ‘hot’ nappe emplacement [36]; (b) The serpentinized dunite-harzburgite ductile-brittle thrust zone that separates the peridotite nappes in the Ayios Stefanos area; (c) A gabbroic dyke in the chromitite boulder within the quarry talus (this figure also appears in [36]). Abbreviations (in order of appearance): Cr = chromitite gallery (in b) and chromitite (in c); Sr = serpentinite; Gbr = gabbroic dyke.
3. Laboratory Methods
Polished thin sections of chromitites were examined using an OLYMPUS BX51 optical microscope (Olympus Optical Co., Ltd., Tokyo, Japan). A few Zr-bearing silicate mineral grains were discovered using conventional petrographic methods; however, their identification was hampered due to their limited size and low reflectance. For further mineral identification a CARL ZEISS SIGMA field emission-scanning electron microscope (FE-SEM) (ZEISS, Oberkochen, Germany) at the School of Earth Science and Geological Engineering (SESGE), Sun Yat-sen University (SYSU) was employed. Element maps of altered Cr-spinels and qualitative determination of Zr-bearing silicates were performed using energy-dispersive spectroscopy (EDS). The internal texture of the Zr-bearing silicates was studied at cathodoluminescence (CL) images using the same apparatus. To avoid effects of varying experimental conditions each grain was scanned for the same time and CL images were taken with constant signal amplification.
Quantitative analyses, line scanning and element maps of both altered spinel and Zr-bearing silicate mineral grains were performed using a three-detector JEOL JXA-8800R electron micro-probe analyzer (EMPA; JEOL, Tokyo, Japan) operating in the wavelength-dispersive spectroscopy (WDS) mode at the Electron Microprobe Lab, SYSU. Conditions for spinel analyses were 15 kV excitation voltage and 20 nA beam current with a beam diameter of 1 μm. The peak and backgrounds counting times were 10 and 5 s, respectively, for the major elements. Spinels were analyzed using the Kα line for Mg, Si, Ca, Al, Ti, V, Cr, Mn, Fe, Ni and Zn. Natural albite, diopside, Cr-spinel, rhodonite, ilmenite and metallic V, Ni and Zn were used as reference materials. The following diffracting crystals were used: TAP for Al, Mg and Si, PETJ for Ca and Ti, and LIFH for Cr, Fe, Mn, Ni, V and Zn. The detection limits are listed in the following as parts-per-million [(ppm): mg/kg, 10−6]: Mg = 100, Al = 108, Si = 118, Fe = 267, Ca = 174, Ti = 270, V = 199, Cr = 238, Zn = 486, Mn = 193 and Ni = 229. Both Fe3+ and Fe2+ in Cr-spinel were calculated assuming ideal spinel stoichiometry. Data were corrected with a ZAF [atomic number (Z); absorption (A); fluorescence (F)] correction calculation. Representative analyses of ‘pristine’ and altered spinels are given in Table A2 and Table A3 (see Appendix). Analytical conditions for the Zr-bearing silicate minerals were 15 kV excitation voltage with a beam current of 20 nA. The electron beam was focused to a 1 μm spot to eliminate the spurious fluorescence effect from the neighboring phases. Counting times varied between 10 s (peak) and 5 s (background) for the Kα line of Al, Cr, Fe, Mg, Mn, P, Ti, Hf and Si, the Lα line of Ce, Pr, Y and Zr, and the Mα line of Pb, Th and U. The reference materials included synthetic ZrSiO4 (Zr, Si), HfSiO4 (Hf), MgO (Mg), Al2O3 (AI), Y-Al garnet (Y), REE-glasses [rare earth elements (REE)], ThSiO4 (Th) and UO2 (U), natural apatite (P), wollastonite (Ca), rhodonite (Mn) and hematite (Fe). The TAP crystal was used for Al, Mg, Na, P and Si, the PETJ for Ca, Pb, Th, Ti, U, Y and Zr, and the LIFH for Ce, Cr, Fe and Hf. Since most of the analyzed oxides occur in trace amounts in the Zr-bearing silicate minerals, with the selected analytical conditions the following detection limits were achieved: Al2O3 = 139 ppm, FeO = 248 ppm, MgO = 121 ppm, CaO = 206 ppm, Na2O = 130 ppm, Y2O3 = 418 ppm, ZrO2 = 429 ppm, SiO2 = 170 ppm, Ce2O3 = 594 ppm, HfO2 = 1653 ppm, UO2 = 470 ppm, ThO2 = 711 ppm and P2O5 = 184 ppm. The detection limits were automatically calculated by JEOL software taking into account the following parameters: (1) intensity of the characteristic X-ray of the analyzed element; (2) average X-ray intensity and (3) counting time of the background signal; and (4) element content in the standard material. The quantification of minor and especially trace elements by electron micro-probe may lead to analytical uncertainties (e.g., [28]). However, the precision and accuracy of minor and trace element measurements in zircon were evaluated by supplementary analyses performed on synthetic glasses and natural zircon standards. The applied acquisition conditions allowed high internal precision [Standard deviation (SD): ≤3%]. Data were corrected with the CITZAF routine in JEOL software [37]. Representative analyses of the Zr-bearing silicate minerals are given in Table A4 (see Appendix).
The degree of radiation damage in ‘zircons’ Raman spectroscopy analyses was studied using a Renishaw inVia Raman Microscope (RENISHAW, New Mills, Wotton-under-Edge, Gloucestershire, UK) at the Confocal Laser Raman Spectrometry Lab, SYSU. Excitation was achieved using the 514 nm line of an Ar laser through a LEICA DMLM ×50 objective (LEICA MICROSYSTEMS, Wetzlar, Germany). The laser power was 5 mW and the collecting time 20 s. The scattered Raman light was collected in 180° back-scattering geometry and dispersed by a grating of 1800 grooves/mm. Spectra were acquired in the 50–1100 cm−1 range with a resolution of 2 cm−1. Analyses were performed as many μm as possible away from the EMPA spots to avoid biased results due to structural changes caused by the electron beam. The parameters of the Raman bands are given in Table A5 (see Appendix).
4. Petrographic Investigation
4.1. Sample Characteristics
Chromitite within the lenticular body and the ore boulder consists of 60–95 modal % of medium- to coarse-grained Cr-spinel. Chromian spinel grains are smaller than 0.7 cm (in diameter), subhedral to euhedral showing amber to reddish brown color under transmitted light (Figure 4a). Some Cr-spinels show a pull-apart-like texture developed vertically to their long axis (Figure 4b). Chromian spinel fragmentation is common along brecciation zones (≤1 cm thick) within the chromitites. A thin section from the ore boulder was found to contain rutile. Rutile appears as transparent, white to yellowish (under reflected light), plate-like and more commonly rod-shaped ‘inclusions’ within Cr-spinel (Figure 4c). Rutile needles are fine-grained (≤0.4 μm thick, ≤25 μm long) and developed in three crystallographic orientations (Figure 4c,d) indicating that they were exsolved from the Cr-spinel host as topotaxially aligned lamellae.
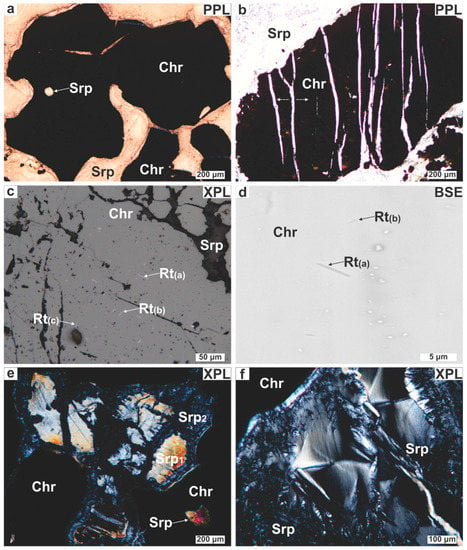
Figure 4.
Photomicrographs illustrating the petrographic characteristics of the Ayios Stefanos chromitites. (a) Reddish lobate Cr-spinel; (b) Chromian spinel displaying pull-apart‒like texture (marked by the white arrows); (c,d) Rutile exsolutions developed in three crystallographic orientations [Rt(a), Rt(b), Rt(c)]; (e) Bastite cut by late serpentine veins; (f) Serpentine showing hourglass texture. Abbreviations (in order of appearance): Chr = Cr-spinel; Srp = serpentine; Rt = rutile; Srp1 = early serpentine; Srp2 = late serpentine; PPL = plane polarized light; XPL = crossed polarized light; BSE = back scattered electron (image).
The interstitial silicate matrix of the chromitites is entirely altered. Their mesostasis is composed of serpentine and chlorite with minor tremolite. Serpentine shows a pseudomorphic mesh texture after olivine. Occasionally bastites after orthopyroxene were found. Some bastites are pervasively cross cut by late serpentine veins (Figure 4e). Non-pseudomorphic serpentine textures (i.e., interpenetrating and hourglass; Figure 4f) were also recognized. Chlorite forms light brown blades intergrown with serpentine or brown to purplish aureoles surrounding Cr-spinel. Chlorite is the dominant filling phase in cataclastic zones and stretching planes of fractured Cr-spinel. Tremolite occurs as elongated grains showing a lepidoblastic texture. Late calcite veins (≤50 μm thick) are superimposed on all aforesaid structures in the chromitite boulder. Chromitites record limited evidence of near-surface weathering expressed in the form of red iddingsite aggregates.
The Ayios Stefanos chromitites contain silicate minerals randomly distributed as inclusions in Cr-spinel. These are euhedral to subhedral and spherical in shape (Figure 4a). Inclusions range in size from several to 250 μm. Silicate mineral inclusions comprise anhydrous (olivine, pyroxene) and hydrous [amphibole (edenitic pargasite) and Na-bearing phlogopite (aspidolite)] minerals; as well as their alteration products (chlorite and serpentine). Composite inclusions made up of orthopyroxene and amphiboles are the most common. A few olivine inclusions appear as negative crystals mimicking the symmetry of Cr-spinel. The negative-crystal faces of these inclusions denote that they might have grown along with the Cr-spinel host.
4.2. Micro-Structural Description of the Alteration Zones in Cr-Spinel
Chromian spinel grains may be partly surrounded by dark grey to black rims (Figure 5a); these rims are paragenetically associated with interpenetrating chlorite blades (≤600 μm; Figure 5b). Reflected light microscopy shows these rims to be more reflective than cores. Comparison of several BSE images of Cr-spinel grains with their equivalent photomicrographs indicates that the dark-colored rims correspond to bright zones in BSE images. The rim thickness ranges between 10 and 300 μm, seldom reaching 500 μm. Rim boundaries with the intact cores are sharp and linear, but few appear irregular and diffuse. In extreme cases, the rim occupies almost the entire grain, leaving just a few isolated ‘islands’ of the original Cr-spinel (Figure 5c), this demonstrates that the rims do not represent overgrowths but products of Cr-spinel replacement. The rims display two internal structures. The first, which is easily discernible under low magnification, consists of porous/spongy spinel with chlorite as the sole pore-filling phase (Figure 5c,d). Infrequently, these pores exhibit a negative euhedral shape or they may be elongated (≤200 μm; Figure 5e,f). The second micro-fabric resembles the oriented chlorite lamellae texture in Cr-spinel described by [38]. Under low magnification this micro-texture appears as disordered aggregates of fine-grained spinel mixed with silicates (Figure 5g) but under high magnification has a net-like to diaplectic morphology. Careful observation shows that this micro-fabric is defined by a series of Mg-silicate lamellae (≤3 μm long) oriented parallel to one of four directions that correspond to the four {111} planes of the Cr-spinel host with lamellae in the fourth orientation appearing as sub-rounded patches (Figure 5h). The spatial recurrence of this structure points toward a growth control through crystallographic planes. Both micro-structures were recognized in the lenticular ore body (Table A1 in the Appendix). When both micro-textures coexist in a single Cr-spinel grain, the outer part of the rimming zone is always porous.
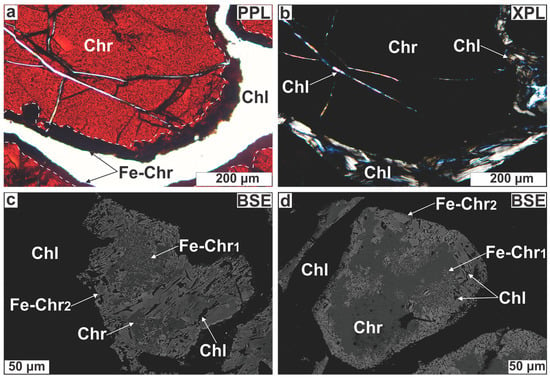
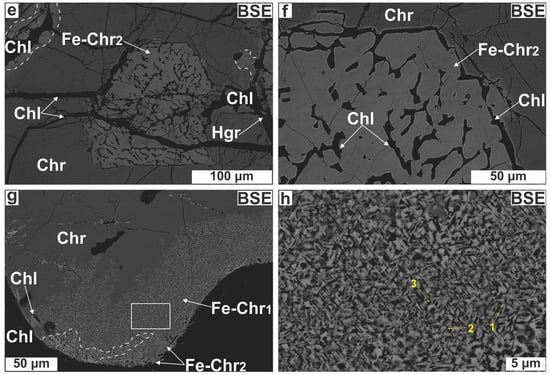
Figure 5.
Photomicrographs illustrating Cr-spinel textural modification. (a) Chromian spinel surrounded by a dark grey rim; (b) The same image in XPL showing that the rim is surrounded by chlorite; (c) Chromian spinel almost entirely replaced by a bright (spinel) phase; (d) Porous spinel for Cr-spinel substitution; (e,f) Spongy spinel displaying elongation of pores; (g) Cr-spinel with a boundary made up of spinel mixed with Mg-silicates; (h) Close up of the white, open square in (g) illustrating the oriented Mg-silicate lamellae micro-texture in Cr-spinel. The intersection of Mg-silicate needles in three directions is also marked. Abbreviations (in order of appearance): Fe-Chr = altered spinel; Chl = chlorite; Fe-Chr1 = lamellae-rich spinel; Fe-Chr2 = porous spinel; Hgr = hydrogrossular; the rest as in Figure 4.
4.3. The Zr-Bearing Silicate Minerals
4.3.1. Distribution, Paragenesis and Micro-Textural Relations
A total of 35 Zr-bearing silicate grains were discovered in 5 chromitite samples (Table A1 in the Appendix). Most of them (22 grains) were found distributed in narrow zones of about 400 × 200 μm2, each grain is smaller than 30 μm across; grain size distribution is lopsided because most particles (80%) range between 10 and 15 μm. Four different zirconosilicate minerals were recognized; namely, zircon, armstrongite, calciocatapleiite and a Zr-bearing Mg-silicate phase. Under transmitted light, the largest Zr-bearing silicate grains appear as dark grey to murky-brown and translucent crystals with low interference colors. They are located within the following structural sites: (i) unaltered Cr-spinel cores commonly along restored fissures or cracks filled with secondary silicates (Figure 6a–c); (ii) altered Cr-spinel boundaries (Figure 6d) and (iii) cataclastic zones cemented by serpentine (Figure 6e,f).
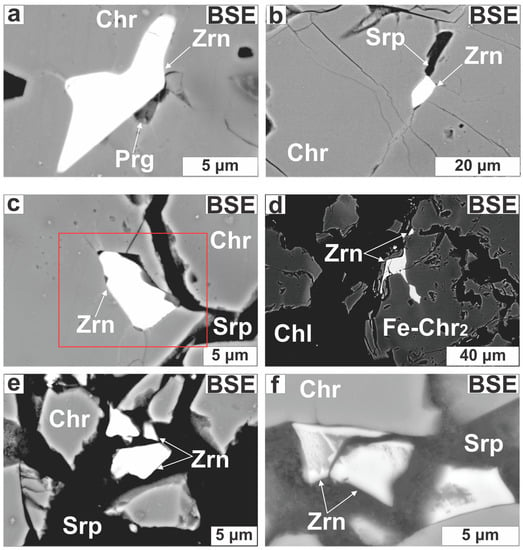
Zircon (30 grains) forms subhedral to anhedral (Figure 7a) and elongated grains (Figure 7b), occurring as single grains or as intergrowths with pargasite (Figure 6a) or Ca-bearing Zr-rich silicates (Figure 7b,c). Some zircons exhibit across-grain thickness variations (1–10 μm; Figure 7b,c). Zircons are located in fractures filled with chlorite and serpentine or placed along annealed micro-cracks (Figure 7c). Zircon in cataclastic zones probably represents wreckages of shattered grains (Figure 6e,f). The Ca-bearing Zr-rich silicates are small (<10 μm), mottled and develop at the expense of zircons transected by open or healed cracks (Figure 7d–f).
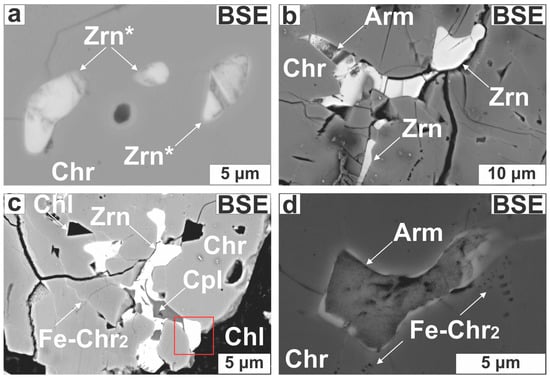
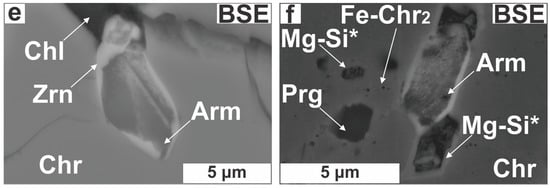
Figure 7.
Photomicrographs illustrating the impact of alteration on zircons. (a) Subhedral, altered zircon in Cr-spinel; (b) Elongated zircon; (c) Intergrowth of zircon with calciocatapleiite; (d–f) Almost complete armstrongite for zircon substitution. Abbreviations (in order of appearance): Zrn* = altered zircon; Arm = armstrongite; Cpl = calciocatapleiite; Mg-Si* = unidentified Zr-bearing Mg-silicate; the rest as in Figure 4, Figure 5 and Figure 6.
4.3.2. Cathodoluminescence Imaging and Element Distribution Maps
In CL images zircons appear to be dark and practically textureless, highly resembling metamict zircon (Figure 8a) [39,40]. Only in a few grains could some complex and irregular internal patterns be discerned (Figure 8b–e). All zircons have a strong BSE signal and suppressed CL emission (Figure 6, Figure 7 and Figure 8). The Ca-bearing Zr-rich silicate grains yielded weak BSE and CL intensities being virtually non-luminescent in accordance with their hydrous nature (Figure 8f) [39].
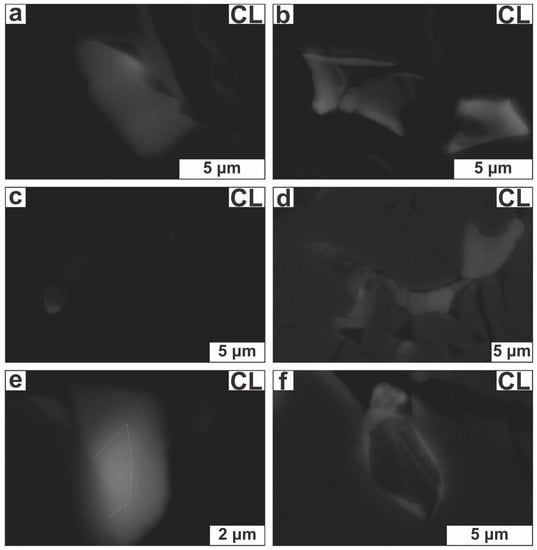
Figure 8.
Cathodoluminescence (CL) images of selected Zr-bearing silicate crystals. (a) Zircon (marked by the red, open square in Figure 6c) with nearly homogeneous texture; (b–e) Zirconium-bearing silicates with irregular texture [originally depicted in Figure 6f and Figure 7a–c (in the last figure the imaged zircon is marked by a red square)]; (f) Armstrongite (originally depicted in Figure 7e) with very weak CL intensity.
Zircons with irregular internal texture commonly have crystal domains with weak CL response but higher Y2O3 and P2O5 contents compared to those with stronger CL emission, suggesting that both Y3+ and P5+ act as possible CL stimulators (Figure 9a,b). We note that various trace elements below the microprobe detection limit may also cause or suppress luminescence (e.g., [40]). Though it is known that the U-content of zircon may be qualitatively predicted via CL imaging [41] no differences in the amount of U were documented between areas of distinct CL intensity (Figure 9c). Element distribution maps and line scans revealed that a few zircon grains are cryptically zoned with decreasing abundances of major elements (Si, Zr and Y) from core to rim (Figure 9d–f); though it was not possible to detect which trace element(s) counterbalance this decrease.
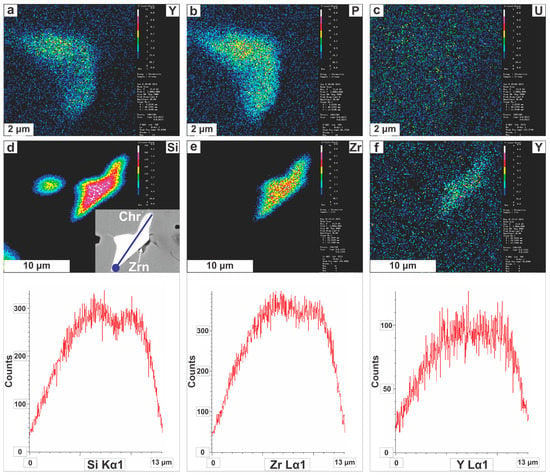
Figure 9.
(a–c) Single-element (WDS) X-ray maps of the zircon grain originally illustrated in Figure 6a; (d–f) Single-element (WDS) X-ray maps of the zircon grain originally illustrated in Figure 7c (marked by the red square). Line scans are also shown for the zircon crystal depicted in Figure 6a, where the beginning of analyses is marked by the dark blue circle. Abbreviations are as in Figure 4 and Figure 6.
5. Mineral Chemistry
5.1. Spinel-Type Minerals
Electron microprobe analyses of Cr-spinels from the Ayios Stefanos ores (excluding Cr-spinel analyses from sample No. 6) show that their composition is rather heterogeneous (42.09–51.76 wt % Cr2O3, 16.55–27.13 wt % Al2O3 (Figure 10a), 12.28–16.91 wt % MgO and 12.32–18.75 wt % FeOt) with Cr# [Cr/(Cr + Al)] values that range from 0.51 to 0.66, Mg# [Mg/(Mg + Fe2+)] values that vary between 0.58 and 0.76 (Figure 10b), moderate Fe3+# [Fe3+/(Fe3+ + Fe2+) = ≤0.43] ratios and quite low TiO2 content (≤0.21 wt %; Figure 10c,d).
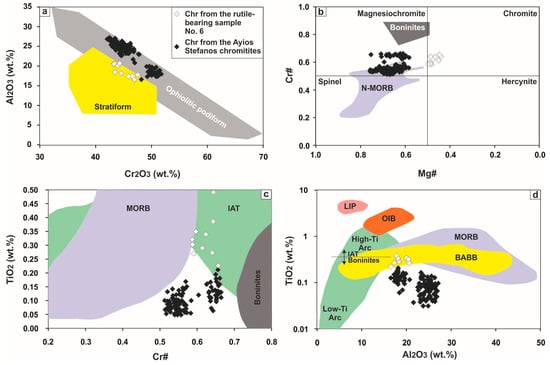
Figure 10.
Pristine Cr-spinel composition in terms of: (a) Al2O3 vs. Cr2O3; (b) Cr# [= Cr/(Cr + Al)] vs. Mg# [= Mg/(Mg + Fe2+)]; (c) TiO2 vs. Cr# and (d) TiO2 vs. Al2O3 (wt %). Fields for podiform and stratiform chromitites are from [42]. Fields for spinel in equilibrium with N-MORBs and boninites in (b) are from [43]. Fields for Cr-spinel in equilibrium with MORBs, IATs and boninites in (c) are from [44]. Compositional fields in (d) are from [45]. Abbreviations: (N-)MORBS = (normal) mid-ocean ridge basalts, IAT = island arc tholeiites, BABB = back-arc basin basalts; OIB = ocean islands basalts, LIP = large igneous province (basalts).
Chromian spinel in sample No. 6 has a quite different composition [Mg#: 0.44–0.50, TiO2: 0.23–0.49 wt % (Figure 10c,d) and V2O3: 0.21–0.31 wt %] compared to Cr-spinel from the rest specimens. Rather than invoking primary magma source variation, this corresponds with the presence of rutile exsolutions from Cr-spinel in this sample; though, care was taken to avoid the exsolved rutile during the stage of microanalyses.
The lamellae-rich zones show limited variation in Cr2O3 (45.20–48.38 wt %), wide variations in Al2O3 (4.49–12.57 wt %), MgO (15.01–17.63 wt %) and Fe2O3 (11.48–19.00 wt %), high Cr# (0.71–0.87), Mg# (0.78–0.88) and Fe3+# (0.55–0.80). The porous-appearing textural zones have a different composition (Cr2O3: 43.71–62.43 wt %, Al2O3: 0.55–21.96 wt. %, MgO: 8.89–16.17 wt %, Fe2O3: ≤16.68 wt %, Cr#: 0.58–0.99, Mg#: 0.45–0.74, Fe3+#: ≤0.51; Figure 11a,b) from the lamellae-rich zones. Selected ‘intact’ and altered Cr-spinel analyses are listed in Table A2 and Table A3 (Appendix). The high Cr# values of the porous spinel zones resemble those of the chromite coronas and inclusions in the Maqsad (Oman) chromitites recently reported by [9] as well as those of Cr-spinel from boninites [43]. However, a striking difference is the very high TiO2 content (up to 0.80 wt %) measured in the porous spinel domains. Furthermore, porous spinel may have much higher Fe2O3 contents than those reported for chromite coronas and inclusions in the Maqsad chromitites [9].
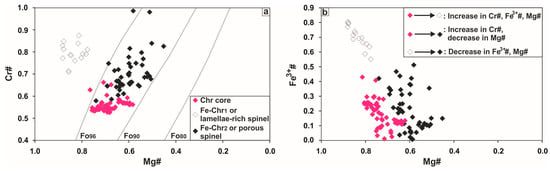
Figure 11.
Variations of altered Cr-spinel in terms of: (a) Cr# vs. Mg#; (b) Fe3+# vs. Mg#. The composition of Cr-spinel is contoured at a nominal T of 1200 °C for olivine compositions from Fo80 to Fo96 [43]. Abbreviations as in Figure 5.
Particularly for the lamellae-rich zones, these variations imply removal of Al (Figure 12a,b) and slight addition of Fe (Figure 12c). The Cr content remains steady (Figure 12d) so that the removal of Al results in the apparent augmentation of the Cr# ratio. The Mg content ‘seems’ unchangeable from the Cr-spinel core toward the alteration rim (Figure 12e). The high SiO2 content is due to the presence of silicates in micro-pore spaces (Figure 12f).
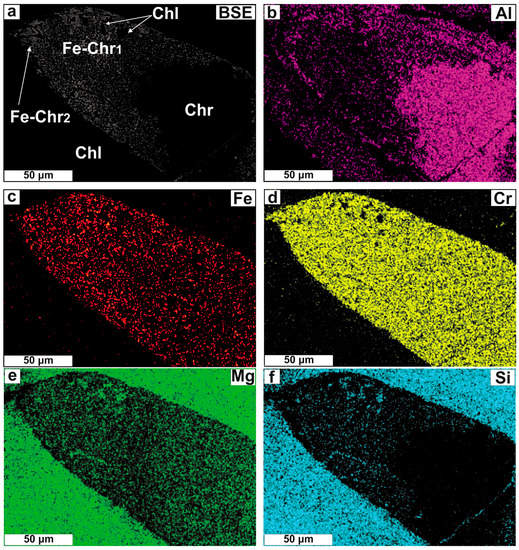
Figure 12.
Single-element (EDS) X-ray maps on part of a Cr-spinel grain that displays oriented lamellae intergrowth texture.
5.2. Zirconium-Bearing Silicates
5.2.1. Zircon (Ideally ZrSiO4)
Zircon contains 52.71 to 64.03 wt % ZrO2 and 34.02 to 37.62 wt % SiO2 (34 spot EMPA-WDS analyses). This composition deviates from the ideal stoichiometric formula as ZrO2 content is low (Figure 13a). These zircon grains contain up to 3.46 wt % HfO2, which is within the range of HfO2 abundance for natural zircon (0.5–5.0 wt %) [46]. They also contain limited amounts of Y2O3 (≤1.21 wt %) and traces of P2O5 (≤0.43 wt %). The UO2 and ThO2 contents are quite low (≤0.28 wt % and ≤0.26 wt %, respectively) and the PbO content even lower (≤0.14 wt %). Common impurities include CaO, Al2O3 and FeOt.
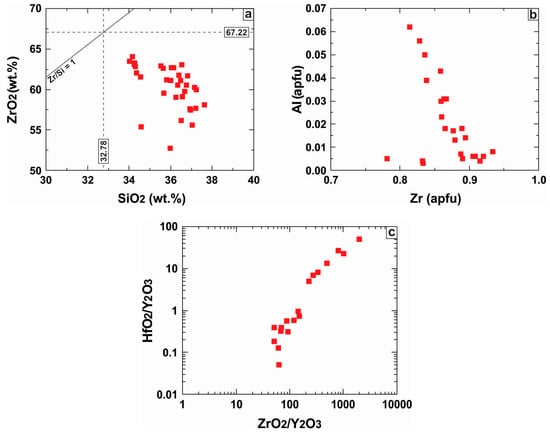
Figure 13.
Plot of major and minor element abundances in zircon in the following variation diagrams: (a) ZrO2 vs. SiO2; (b) Al vs. Zr at. %; (c) HfO2/Y2O3 vs. ZrO2/Y2O3.
The Al3+ proportion in zircon was found to be negatively correlated with that of Zr4+ (Figure 13b). This probably implies that Al3+ for Zr4+ substitution in the dodecahedral site may be possible through a charge balance mechanism. In contrast, the HfO2/Y2O3 ratio is positively correlated with the ZrO2/Y2O3 ratio (Figure 13c) as it is expected when incorporation of Zr, Hf and Y in zircon is dominated by the typical xenotime-type coupled substitution [(Y3+ + REE3+) + P5+ ↔ Zr4+ + Si4+]. Selected zircon analyses are listed in Table A4 (see Appendix).
5.2.2. Ca-Bearing Zr-Rich Silicates: Armstrongite (Ideally CaZrSi6O15·2.5H2O) and Calciocatapleiite (Ideally CaZrSi3O9·2H2O)
Only one armstrongite microprobe analysis was possible (grain in Figure 7d) owing to the small size and complex association of the mineral with zircon. The analyzed armstrongite has: 48.86 wt % SiO2, 25.79 wt % ZrO2, 7.95 wt % CaO, 3.60 wt % MgO, 3.57 wt % Cr2O3, 1.60 wt % FeOt, 0.89 wt % HfO2 and 0.47 wt % Y2O3 (Table A4 in the Appendix). The low analytical total implies the presence of OH−.
A single microprobe analysis of calciocatapleiite (grain in Figure 7c) was carried out owing to the same analytical limitations mentioned above for armstrongite. Calciocatapleiite has the following composition: 45.88 wt % SiO2, 22.36 wt % ZrO2, 14.58 wt % CaO, 4.93 wt % Cr2O3, 3.74 wt % MgO, 2.18 wt % FeOt, 1.03 wt % HfO2, 0.42 wt % Y2O3 (Table A4 in the Appendix). The Zr/Hf ratio (37.78) is higher compared to that of the intergrowing zircon (27.77) indicating considerable loss of Hf from zircon. The imperfect analytical total is probably due to the hydrous nature of the mineral.
6. Confocal Raman Microspectroscopy
According to group and symmetry theory, 12 Raman active modes are expected in zircon [47]. One of the most prominent peaks in Raman spectra from natural zircons is the mode at 1008 cm−1 (B1g-antisymmetric stretching vibration) that is due to internal vibration of the SiO4 tetrahedrons. More internal modes are observed at: 975 cm−1 (A1g), 439 cm−1, 296 cm−1, whereas the external modes are detected at: 393 cm−1, 356 cm−1, 225 cm−1, 214 cm−1 and 202 cm−1 [48]. Care in interpretation is required since a number of studies indicate that Raman modes change at conditions that will damage zircon structure [49].
Several Raman spectra were analyzed over different areas of the zircons. The bands of the Si-O antisymmetric stretching vibration at 1008 cm−1 show a large wavenumber shift at 1002.7–1005.6 cm−1 and their full-widths at half-maximum (FWHM) are at 10.09–14.54 cm−1 (Table A5 in the Appendix). The bands of the symmetric bending vibration and the symmetric rotating vibration are observed at 438.05–439.56 cm−1 and 352.1–355.4 cm−1 (Figure 14a), respectively, and their FWHM are at 8.06–19.38 cm−1 and 9.80–17.17 cm−1 (Table A5 in the Appendix). Both Si-O stretching modes between 970 and 1010 cm−1 can be observed in all Raman spectra. The degree of disorder in zircon is proportional to the FWHM of the v3(SiO4) stretching mode, which can be linked to variations in the Si-O bond lengths [50]. In our case the FWHM values (Table A5 in the Appendix) show limited variation (Figure 14b) around the ~1008 cm−1 peak.
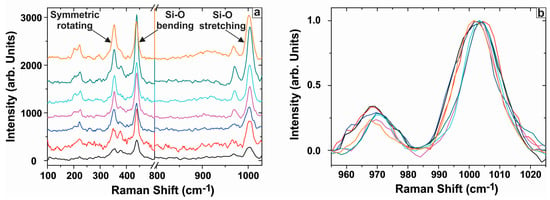
Figure 14.
(a) Raman spectra in the wavenumber range of 100–1025 cm−1 for the studied zircons; (b) Spectra of zircons normalized to the height of the v3(SiO4) stretching mode at ~1008 cm−1.
7. Discussion
7.1. Magmatic Stage of Lithospheric Slab Evolution
7.1.1. Extent of Subsolidus Re-Equilibration and Parental Melt of the Chromitites
The majority of chromitite samples are essentially monomineralic enabling us to consider that Cr-spinel composition remained nearly undisturbed at the post-magmatic stage of evolution after the termination of spinel-spinel re-equilibration (/homogenization). This is corroborated by the chemical similarity of Cr-spinels from the massive-textured samples implying originally high MgO abundance and low Fe2O3 content in Cr-spinel cores. These compositional signatures cannot be explained by subsolidus Fe-Mg exchange between Cr-spinel cores and olivine due to diffusive re-equilibration [51] as it was demonstrated by [9]. Re-equilibration between Cr-spinel and orthopyroxene in our samples should be even less pronounced. This is due to the limited abundance of interstitial orthopyroxene in the studied chromitites marking the point at which saturation of the intercumulus melt with Cr-spinel and olivine terminated. However, the conceivable compositional range of olivine (Fo ≈ 91–96) surrounding Cr-spinel (Figure 11a) implies that if any re-equilibration phenomena occurred these were restricted to the Cr-spinel grain boundaries. We have also tested the extent of subsolidus exchange between Cr-spinel and the gabbroic dykes for the semi-massive chromitite samples taken from the ore boulder. We found out that significant exchange happened between the gabbroic dyke and the chromitite walls of the ore boulder that was intruded by the gabbroic dykes network. We actually noted that Cr-spinel composition in sample No. 6 (low Mg#, high TiO2 and V2O3), which represents part of the ore boulder almost in contact with a gabbroic dyke, deviates from that of Cr-spinel in the rest samples (Figure 10b–d; Table A2 in the Appendix). The presence of magmatic titanite in the dyke means that it was crystallized from a TiO2-rich melt, that is, more likely “MOR” related. Interaction (in the concept of chemical exchange) of this gabbroic melt with the chromitite triggered enrichment of Cr-spinel in TiO2. The TiO2 in excess was expelled from Cr-spinel in the form of rutile exsolutions.
Experimental studies of spinel-liquid equilibrium performed at low P (1 bar) have shown that the Al2O3 content and the FeO/MgO ratio in Cr-spinel depend on the concentrations of these substances in the chromitite-precipitating melt (e.g., [52]). Chromian spinel can be used to compute massive chromitites parental melt composition applying the empirical formulae suggested by [52]:
where (FeO/MgO)Cr-spinel = FeO/MgO ratio in Cr-spinel, Al#Cr-spinel = Al/(Cr + Al + Fe3+) ratio in Cr-spinel and Fe3+#Cr-spinel = Fe3+/(Cr + Al + Fe3+) ratio in Cr-spinel and (FeO/MgO)melt = FeO/MgO ratio in melt. The Al2O3 and TiO2 budget of the parental melt can be estimated using the logarithmic expressions developed by [53]:
where (Al2O3)melt = Al2O3 in melt, (Al2O3)Cr-spinel = Al2O3 in Cr-spinel, and
where (TiO2)melt = TiO2 in melt, (TiO2)Cr-spinel = TiO2 in Cr-spinel Equation (3).
ln(FeO/MgO)Cr-spinel = 0.47–1.07Al#Cr-spinel + 0.64Fe3+#Cr-spinel + ln(FeO/MgO)melt
(Al2O3)melt = 4.1386ln(Al2O3)Cr-spinel + 2.2828
(TiO2)melt = 0.708ln(TiO2)Cr-spinel + 1.6436
Implementation of Equation (1) to Equation (3) shows that the parental melt had FeO/MgO ratio varying from 0.75 to 1.42, 14.08–15.94 wt % Al2O3 and up to 0.55 wt % TiO2. Our results indicate that chromitites crystallized from melts that had an intermediate composition between that of high-Mg island-arc tholeiites (IATs: 11.4–16.4 wt % Al2O3) [54] and mid-ocean ridge basalts (MORBs: ~15 wt % Al2O3) [55]. The inferred parental melts bear resemblance to those reported for the high-Al chromitites from the (shallow) mantle section of Oman (Figure 15) [9,56].
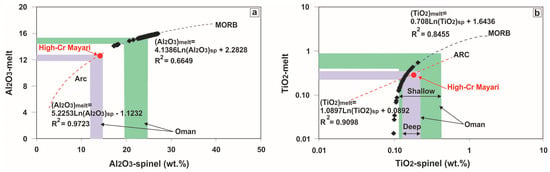
Figure 15.
Plots of the calculated Al2O3 and TiO2 contents of the parental melts in equilibrium with chromitites. The MORB and Arc lines are from [53] using data on spinel-melt inclusions in MORB and arc lavas reported by [45,56]. The range of Cr-spinel and the calculated melt compositions from the chromitites of the shallow and deep mantle section in Oman [56] are shown for comparison. Only data of chromitite samples not associated with gabbroic intrusions were used for computation.
Jurassic basalts with intermediate affinity between MORBs and IATs [known as medium-Ti basalts (MTBs)] have been described from the Agoriani mélange (group 2 volcanic rocks) in Othris [57] and represent an intriguing feature of the western Albanides-Hellenides ophiolite belt [58].
7.1.2. Genetic Mechanism and Geodynamic Setting of the Ayios Stefanos Chromitites
A series of diverse models have been proposed to decipher the enigmatic presence of dunites-chromitites in ophiolites [3,4,5,6,7,8,9]. Chromitites of Ayios Stefanos are sited within a common mantle ‘horizon’ and exhibit sharp contacts with the surrounding attenuated dunite sheath: these appear as artefacts of the ductile deformation within the tectonically-thinned dunite-harzburgite zone. However, the serpentinized dunite clots within the lenticular ore body show that chromitites and dunite share the same origin. This is confirmed by the similarity in the expression of optical properties (i.e., extinction) between the mesh serpentine in the surrounding serpentinite (after dunite) and mesh serpentine in the clots.
The frequent presence of edenitic pargasite and aspidolite as inclusions in Cr-spinel may point toward a volatile-rich zone encountered by the rising melt that formed the chromitites; however, there is no indication for the existence of a volatile-rich zone in the Othris mantle suite. Phase equilibria experiments and thermodynamic considerations show that in the presence of a water-rich fluid phase the stability domain of coexisting pyroxene, amphibole, aspidolite and associated Cr-spinel is confined to P up to 200 MPa and T under 950 °C far below the conditions traditionally invoked to explain the formation of Cr-rich immiscible melts (e.g., [9]). Therefore, the presence of amphibole and phlogopite and/or aspidolite inclusions in Cr-spinel probably indicates the involvement of an alkali-bearing hydrous melt in the precipitation of the chromitites as it has been proposed by previous studies (e.g., [51]).
Chromian spinel analyses straddle the area between the fields of spinel in equilibrium with arc melts and MORBs (Figure 10b–d). Melts of MTB affinity may be produced in the mantle underneath nascent arcs or back-arc basins; however, it is ‘unlikely that the Othris ophiolites formed in a back-arc basin’ [59]. The release of this type of melts in Othris has been ascribed to 8%–10% hydrous melting of rising depleted lherzolitic (/clinopyroxene-bearing harzburgitic) diapirs [60] (i.e., the Ano Agoriani diapir).
We are in favor of a mechanism that involves migrating (ultra-)mafic melts-harzburgite interaction followed by melt-melt mixing to explain the formation of the Ayios Stefanos chromitites (e.g., [44]). The idea that the Ayios Stefanos chromitites crystallized from melts migrating through the mantle is supported by their dunitic envelope relics, which are interpreted to be partly replacive in origin following dissolution of pyroxenes from the harzburgite (e.g., [4]) and partly cumulative after olivine addition (e.g., [3,6]). Furthermore, the (ultra-)mafic nature of the melts is supported by the common finding of negative crystals of olivine inclusions in Cr-spinel, which plausibly share the same origin with the Cr-spinel. We presume that small volumes of poor in Cr and oxidized permeating MTB melts initially interacted with the Ayios Stefanos mantle harzburgites to transform them into dunites and modify their own composition by enhancing their SiO2 content. Subsequently, mixing of these modified melts with the next inputs of (primitive) MTB melts moved the resultant hybrid MTB magmas (enriched in Mg, Cr and H2O) into the liquidus field of Cr-spinel, promoting the precipitation of chromitites (e.g., [3,5,6]). Soon these melts started diversifying and the ‘instant’ changes in their composition were recorded in the wide range of Cr# values (0.51–0.66) in Cr-spinel even from a single ore body. Further evidence of the diversification of the melts could be the limited presence of orthopyroxene (preserved as bastite) merely in those chromitite samples composed of low Cr# (<0.60) Cr-spinel.
We emphasize the heterogeneous nature of the West Othris mantle unit composed of minor dunite, harzburgite and lherzolite and their plagioclase-bearing equivalents [61]. This lithological variability does not require that the western-most branch of Neotethys consisted of numerous small oceanic basins nor does it necessitate a Jurassic left-lateral strike-slip fault juxtaposing ophiolites from different domains [61]. We also highlight the geochemical diversity among the Othris harzburgites, including both abyssal [62] and arc-type harzburgites [63]; though the former are predominant. Judging from this compositional variability and the contrasting geochemical signatures of the parental melts of both chromitites (MTB-type melt) and TiO2-rich gabbro intrusions (MORB-type melt) we cannot accept the customarily adopted model of a well-developed suprasubduction zone (SSZ) as the ideal geotectonic setting for the genesis of the Ayios Stefanos chromitites. It is likely that these chromitites record a stage of magmatism along a near-transform fault of a slow-spreading ridge [59,62]. Our model represents a simpler, perhaps more ‘elegant’, hypothesis explaining the genesis of the Othris chromitites.
7.1.3. An Inductive Reasoning Approach to the Potential Origin of Zircons
Three main scenarios have been proposed to explain the ‘paradoxical’ finding of Zr-rich phases in ophiolitic chromitites: (i) incorporation of ancient zircons in the sub-oceanic mantle by ascending fluids/melts as a result of deep subduction [22]; (ii) precipitation of Zr-dominant phases by rich-in-fluid melts related to mantle metasomatism (e.g., [64,65]) and (iii) introduction of zircon in chromitites through (a network of) intergranular fluids representing late fractions of igneous intrusions in the upper mantle [27,28].
Zirconium-rich minerals have never before been reported from the West Othris peridotites and petrogenetic signatures related to carbonatite metasomatism are missing from the entire massif. We also note that there is no structural imprint related to a phase of carbonatite metasomatism from a subduction channel within the West Othris ophiolite. Zircon is a stable phase in the upper mantle [66] but the petrological history of the West Othris mantle, encompassing various episodes of partial melting and metasomatism [67], would hardly permit zircon storage for a long time. The Ayios Stefanos zircons do not host mantle mineral inclusions, have no inherited cores, and more importantly, do not show signs of corrosion due to incipient reaction with melt. For this reason, it is hard to envision that zircons were entrained from the peridotite wall-rocks by migrating melts.
The zircons we discovered are small, unlike crustal or detrital zircons that can reach much larger sizes. They are neither prismatic nor rounded and appear practically featureless in CL images, lacking any pre-existing texture relics (i.e., oscillatory zoning; Figure 8). Zircons are entirely absent from the mesh-textured serpentinized groundmass; possibly entrapment of old inherited zircons in olivine could effectively protect them from the ambient environment [22]. The zircon-pargasite intergrowths in Cr-spinel are anhedral with a granular texture (Figure 6a) unlike those described by [68]. These features are not indicative of zircons that derive from the sedimentary lid or the cumulate pile of a downgoing slab undergoing amphibolite-facies metamorphism [68]. Xenocrystic zircons have been described from several chromitite occurrences over the globe [21,22,23,24,25,26]. We note that recycled zircons from the Tibetan (Luobusa, Dongqiao), Semail (Oman) and Ray-Iz (Urals) chromitites have complex internal structures and co-occur with various combinations of super-reducing ultrahigh-pressure (SuR-UHP) minerals without showing resetting of their U-Pb ages [22,26]. However, a SuR-UHP mineral assemblage is missing from the Ayios Stefanos chromitites and the studied zircons show metamictization at variable degrees.
Zirconium contents in Cr-spinel rarely exceed a few ppm [69] making it hard for zircon to exsolve from the Cr-spinel lattice. This is further corroborated by zircon distribution and morphology, and is inconsistent with the crystallographically controlled patterns commonly resulting from exsolution.
The solubility of zircon in melt increases with increasing T and decreasing SiO2-activity [70], thus zircon may be a liquidus phase mainly in felsic and intermediate melts. Mafic magmas require an unrealistically high Zr abundance (>5000 ppm) to crystallize zircon [71], whereas they are capable of dissolving zircon upon their release. However, zircon in mafic settings may grow in small and secluded Zr-rich melt volumes far from the liquidus [72]. Recently, it was shown that a population of ‘young’ (100–110 Ma) zircons from the Luobusa chromitites [25] could derive from mixing between juvenile mantle-derived melts and a crustal component [26]. Those zircons show idiomorphic habits with strongly marked, narrow oscillatory zoning and prismatic inclusions of fluorapatite [25] unlike the zircons we discovered.
It is accepted that rodingitization-related fluids can serve as potential carriers of zircon (e.g., [73]). We, however, did not find zircon associated with rodingitization-derived minerals (i.e., hydrogrossular), and the presence of ‘micro-rodingite’ veinlets occurs solely in a single zircon-bearing chromitite sample (1a). This would preclude a metasomatic origin for the examined zircons. In addition, the Ayios Stefanos zircons have no OH− content that is symptomatic of zircon settled by low-T fluids [74].
We found that the majority of Zr-bearing silicate grains (30/35) were located in samples taken from a chromitite boulder that is massively intruded by gabbroic dykes. It is not coincidental that most of these grains are located in open or healed fractures that cut Cr-spinel (Figure 6a–d and Figure 7b–d). Mantle deformation can develop fractures in ‘brittle’ massive-textured chromitites [75] that commonly serve as fluid pathways. At relatively high T conditions such fractures may anneal owing to Cr-spinel grain-boundary migration, promoted during recrystallization and metamorphism [20]. Evidence for this comes from the observation of almost linear trails of zircon or silicate grains (Figure 7a,d,f), which could correspond to former Cr-spinel crystal boundaries or restored fractures. A number of annealed cracks end up in Zr-bearing silicate grains without cutting them (i.e., Figure 6b). This indicates that these grains and the associated secondary silicates were introduced into already solid Cr-spinel by a fluid/melt prior to annealing. The absence of oscillatory zoning in CL images also indicates equilibrium with a volatile-bearing fluid/melt at high T [76].
The zircons in the Ayios Stefanos chromitites have a compositional signature that differs from that of mantle zircons, but is quite similar to the trace-element signature of zircons reported from plagioclase-bearing, low-SiO2 intermediate to mafic sources (Figure 16) (e.g., [77,78]). In the Y vs. U and Y vs. Th diagrams (Figure 16a,b) our zircon analyses plot on the overlapping compositional fields of zircons from mafic lithologies, dolerites, larvikites and granitoids, substantially deviating from the composition of kimberlitic and carbonatitic zircons. As shown on the Hf vs. Y diagram (Figure 16c) they plot in the fields II, III and IV, whereas most analyses plot outside of any known field for zircon composition because of their low Hf contents. Even in this case our zircon analyses bear similarity to those from mafic to intermediate and felsic sources. Most of them are characterized by low HfO2/Y2O3 ratios and low Hf (<1 wt %) contents likewise early to late magmatic zircons [74] from mafic settings (Figure 13c) [27]. Alternatively, it can be said that these zircon analyses define a fractionation trend toward progressively more depleted-in-Hf compositions.
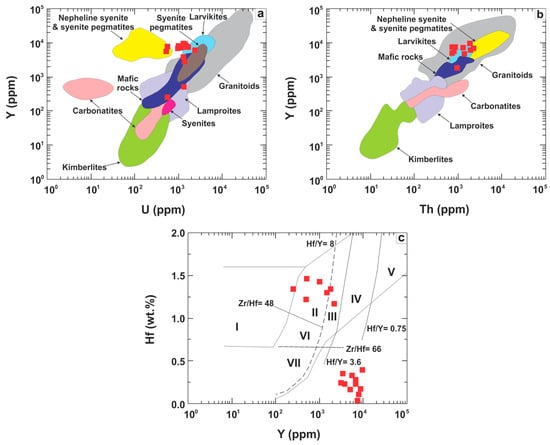
Figure 16.
Trace element correlations for zircons. (a) Y (ppm) vs. U (ppm); (b) Y (ppm) vs. Th (ppm); (c) Hf (wt %) vs. Y (ppm). Fields in (a,b) are from [77]; fields in (c) are from [78] (I = kimberlites, II = ultramafic, mafic and intermediate rocks, III = quartz-bearing intermediate and felsic rocks, IV = felsic rocks with ‘high’ SiO2 content, V = greisens, VI =alkaline rocks and alkaline metasomatites of alkaline complexes, VII = carbonatites).
Though the gabbroic intrusions are missing from the lenticular chromitite, they crop out within a radius of less than 20 m away from this ore body and crack propagation in the mantle is known to provide a robust pathway for the transport of metasomatic fluids and melts over long distances [27,79]. Moreover, all zircons display textural and compositional similarities implying that they share a mutual history. The relatively small thickness of each gabbroic intrusion let us believe that their parental melts were rapidly consumed leaving behind small volumes of high-T fluids. We suggest that the zircons derive from limited proportions of moderately high-T gabbro-related fluids that were introduced into the chromitites via a complex network of micro-fractures, which seems to be pervasive to the Ayios Stefanos mantle suite. Regarding zircon survival, it has been experimentally demonstrated that heat wave propagation from an igneous intrusion will completely dissolve and reprecipitate zircons in Zr-poorer lithologies many diameters of the plutonic body away and often 103–104 years after the intrusion event [80].
A quite similar scenario has been proposed to explain the origin of zircons in the chromitites of the Tumut ophiolite complex in southeast Australia [27] and the Cedrolina chromitites in Brazil [28]. The zircons we studied bear some resemblance to those found in the Early Jurassic Finero alpine chromitites (western Alps) wherein zircon genesis was ascribed to direct crystallization from the melt that formed the Finero chromitites during the Early Jurassic [81,82]; those anhedral to subhedral zircons occur in interstitial positions between Cr-spinel and olivine and/or orthopyroxene [65], forming aggregates of up to four crystals [64]. The Finero zircons are inclusion-free with low CL signals, no internal texture and high Th/U ratios, thus resembling the zircons in the Ayios Stefanos chromitites [64,82]. However, the Finero zircons may be up to 600 μm across and sometimes are found as euhedral inclusions in Cr-spinel and olivine, with low Y contents [64]. The Finero zircons are attributed to formation in relation to carbonatitic metasomatism, a pervasive phenomenon in the Finero phlogopite peridotite complex [65]. This carbonatitic imprint is, as we have noted, missing from the Othris ophiolitic rocks.
A remaining question could be: did the presumed gabbro-derived fluids carry any existing zircons (from the dykes) or did the zircons settle at the stage of chromitites being invaded by the fluids? In the absence of any isotopic data for these zircons it is not possible to provide a direct answer to this question; our textural data cannot rule out either hypothesis at this stage of study.
7.2. Post-Magmatic Stage of Evolution
7.2.1. The Polyphase Cooling History of the Chromitites
In cumulate lithologies, such as chromitites, zoning in Cr-spinel may be due to reaction with the intercumulus melt. However, this scenario is not applicable to the chromitite samples examined herein as zoning in Cr-spinel can be better described as a replacement texture rather than as a typical crystal growth texture. For instance, neither of the two micro-structures recognized in zoned Cr-spinels resembles oscillatory zoning indicating disruption of the melt boundary layer around Cr-spinel and changing growth-rate [83]. On the other hand, none of them has a typical sieved to wormy appearance that are supposed to be characteristic of diffusion-controlled or diffusion-limited (cellular) growth processes due to constitutional supercooling of metals [83,84]. Furthermore, from the ‘pristine’ Cr-spinel core to the reworked rims the Cr# increases, which is against a growth origin or even a magma fractionation trend for both kinds of micro-textures recognized at Cr-spinel grain boundaries. Additional evidence that no reaction between Cr-spinel and a remnant intercumulus melt actually occurred comes from the conceivable forsteritic composition of the adjacent olivine (Fo ≈ 91–96) [43]. Petrographic observations indicate that both structures do not represent diffusion-controlled growth textures [38]. In most cases the oriented Mg-silicates lamellae appear to preferentially develop at the boundaries not in the interior of the Cr-spinel grains, which is against their formation due to diffusion of components from the Cr-spinel lattice. In fact, petrographic evidence suggests that the zoning observed in Cr-spinel is not a primary feature; but a replacement texture obtained as a result of reaction that proceeded from the outside to the inside of the affected Cr-spinel grains [14,16,17,18,20]. A strong relationship exists between zoning in Cr-spinel and the degree of chloritization-tremolitization of the Ayios Stefanos chromitites. Furthermore, the discovery of clinochlore ‘haloes’ around Cr-spinel and the observation that clinochlore blades are cut by serpentine confirm that retrograde metamorphism predated oceanic alteration (e.g., [20]).
The preservation of two alteration micro-textures in Cr-spinel from Ayios Stefanos indicates that chromitites underwent a multi-stage history of lithospheric cooling. Two compositional trends linked to Cr-spinel modification were recognized: (i) a general increase in the Cr#, Mg# and Fe3+# related to the development of the lamellae-rich zones (Figure 11a) and (ii) an increase in the Cr# coupled with a general decrease in the Mg# and Fe3+# related to the formation of the porous texture (Figure 11b). The first trend is inexplicable since the loss of Al3+ is not coupled with Mg2+ loss; hence, it cannot be ascribed to Cr-spinel and olivine replacement by ferrous chromite and chlorite at reducing conditions [20] or to advanced subsolidus re-equilibration (i.e., down to 750–700 °C, PH2O = PTot) [85]. The high Fe3+# values of the lamellae-rich zones favor their formation at oxidizing conditions. The second trend results from Cr-spinel or lamellae-rich spinel reaction with olivine and their replacement by porous spinel and chlorite at reducing conditions as confirmed by the general decrease in the Fe3+# from the cores and lamellae-rich zones to the porous rims (Figure 11b).
Computation performed in the closed system Cr2O3-MgO-Al2O3-SiO2-H2O (CrMASH) rules out the formation of ferrian chromite and chlorite by reaction between Al-spinel and serpentine [20]. Thermodynamic predictions in the fluid-saturated system Cr2O3-MgO-FeO-Al2O3-SiO2-H2O (CrMFASH) do not exclude direct breakdown of Al-spinel to chlorite and ferrian chromite after reaction with MgO- and SiO2-rich fluids [86]. The lamellae-rich zones probably formed in this way according to a modified version of the equation proposed by [87]:
where M stands for Cr, Fe3+, Fe2+ and Mg in spinels, as well as Fe2+ and Mg in the fluid and chlorite. It is likely in such a reaction that the fluid was derived from the adjacent peridotites. We emphasize that oxidation of Fe2+ to Fe3+ rather than diffusion of Fe2+ from olivine to Cr-spinel aided lamellae-rich spinel growth.
MAl2O4 + 4.5MO + 3SiO2 + 4H2O + 0.083O2 → 0.167M3O4 + M5AlSi3AlO10(OH)8
Phase relations for massive chromitites support that Cr-spinel reacts with olivine to produce porous spinel according to the reaction proposed by [86]:
Reaction (2) considers only Mg end-members and proceeds under reducing conditions. A similar reaction can explain porous spinel for lamellae-rich spinel substitution with the reactant spinel having higher Cr/Al ratio and Fe3+ abundance compared to the one used in Reaction (2). The thickness of the chlorite zones produced by Reaction (1) (separating lamellae-rich spinel from olivine,) is not enough to prevent Reaction (2) but once a thin porous rim will form Reaction (2) will stop because the reactants will be isolated from each other. The conversion of lamellae-rich spinel to porous spinel is marked by an increase in the Fe2+ content of the latter plausibly due to the combined effect of Fe3+ reduction owing to: (i) interaction with a reducing fluid and (/or) (ii) the simultaneous breakdown of the serpentine proportion of the Mg-silicate needles in lamellae-rich spinel (as confirmed by average SEM/EDS data), following the very general scheme:
where a, b, c and d stand for the appropriate integers.
4(Mg0.7Fe0.3)CrAlO4 + 4(Mg1.88Fe0.12)SiO4 + 2SiO2aq. + 8H2O → 2(Mg4.61Fe0.31)AlSi3AlO10(OH)8 + 2(Mg0.55Fe0.45)Cr2O4
alamellae-rich spinel + bSerpentine → cPorous spinel + dClinochlore
Phase relations in the system (Fe2+,Mg)Cr2O4-(Fe2+,Mg)Fe3+2O4-(Fe2+,Mg)Al2O4 [88] suggest that porous rims started forming at slightly higher T compared to the lamellae-rich ones (Figure 17). The documented overgrowth of porous rims onto the lamellae-rich zones implies an abrupt change of conditions from oxidizing to reducing. The extremely thin nature of these alteration rims suggests that they formed at a low fluid/chromitite ratio; a greater abundance of porous spinel indicates that the second event was more widespread. We also note that altered Cr-spinel grains were not recognized in all samples, but were most abundant in the sheared (cataclastic) material. Chromian spinel displaying both types of texture was recognized in samples from the subcordant lenticular ore body. We conclude that the metamorphic processes did not operate uniformly; though, were probably controlled by shear deformation.
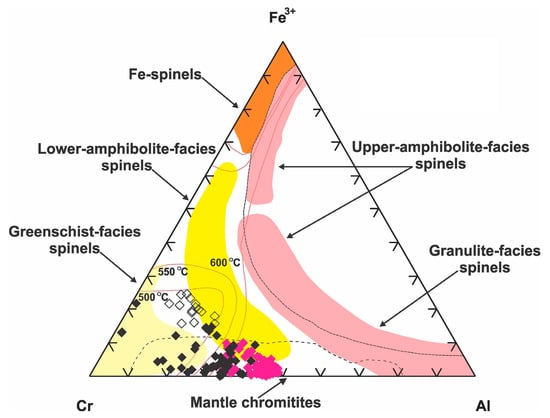
Figure 17.
Compositional changes in spinel phases from the examined chromitites expressed in a triangular Al-Fe3+-Cr diagram with special reference to the fields of the different metamorphic facies defined for Cr-spinels by [89,90,91]. Solvus determined at 600, 550 and 500 °C by [88] for Cr-spinel coexisting with forsteritic olivine (Fo90). Explanation of symbols as in Figure 11.
7.2.2. Zircon Re-Equilibration with Deuteric Fluids
The zircons of Ayios Stefanos were pseudomorphically replaced by Ca-bearing Zr-rich silicate minerals as a result of dissolution-reprecipitation processes [92,93] under reducing conditions prevailing during the post-magmatic stage of evolution. The replacing minerals are commonly mottled in appearance, and porous due to pristine zircon volume decrease (e.g., [39]). The sharp boundaries between the altered and intact areas in the partly replaced zircons (Figure 7b,c) correspond to the stage at which the advancing chemical front stopped. Zircon degradation to armstrongite is best described by the following reaction:
showing that the ingressing fluid had an acidic character [94]. Calciocatapleiite forms in undersaturated-in-H4SiO4 and Ca-bearing settings [95]. Both of these secondary, Ca-bearing Zr-rich silicate phases stabilize at crustal levels (<2.5 km) and T lower than 600 °C [96]. We suggest that zircons were compositionally altered in variable degrees by highly alkaline and Ca-rich fluids released during the serpentinization of peridotites-chromitites [73] or the tremolitization of clinopyroxene in the gabbroic intrusions (Unpubl. data). Another potential origin for these fluids is that they might represent remnant Ca-rich fluids left after the crystallization of zircons in the chromitites (causing auto-metasomatism).
ZrSiO4 + CaCO3 + 5(H4SiO4)aq ↔ CaZrSi6O15∙3H2O + 7H2O + CO2
The detection of non-formula compounds (CaO, Al2O3, FeO) in most of the examined zircons (Table A4) can be explained by: (i) the presence of micro-inclusions or (ii) the incorporation of solvent substances in zircon through a diffusion-reaction process at T above 200 °C [97]. All zircons are free of visible inclusions; hence, the second explanation seems to be more valid. The common discovery of armstrongite pseudomorphs after zircon displaying surface pitting and a spongy internal texture is also in favor of zircon in situ alteration by hydrothermal processes.
Equilibration of zircon with aqueous fluids may be facilitated if the zircon is structurally damaged. Our data show that zircons experienced metamictization. This process is known for drastically quenching the CL emission and resetting the U-Pb system [39]. We note that the time difference between oceanic crust formation and emplacement is short for the neighboring Vourinos and Pindos ophiolites [98]. If the same scenario is applicable for Othris, then the time interval between gabbro intrusion in the chromitites and metamorphism/serpentinization should also be limited. So, it is likely that the examined zircons were modified first and then underwent serpentinization-related metamictization, possibly in fluid-assisted conditions (e.g., [82]).
7.3. Synthesis and Geochronological Implications
Both micro-textural and compositional characteristics of the Ayios Stefanos zircons indicate that they were introduced into the chromitites via a network of high-T fluids emanating from the gabbroic dykes that were syn-kinematically emplaced into the thrust (contact) zone between ultramafic mantle nappes. The ages of these zircons should mostly indicate the time of intrusion of their protolith, and that of incipient obduction rather than the time of mantle-hosted chromitite genesis. In the absence of isotopic data it is hard to reach a conclusion about the exact age of the gabbroic intrusions. We can, however, use our data to constrain the relative sequence of magmatic, tectonic and post-magmatic events in the study area. The common occurrence of deformed chromitites along the plagioclase-bearing lherzolite-harzburgite nappe contact implies that the latter served as a potential ‘structural trap’ for the ore bodies [33]. The intrusion of gabbroic dykes, showing a tectonic pattern in agreement with that of the deformation in the tectonically thinning zone, within the chromitites quite obviously illustrates that the formation of the ore bodies predated the intrusion of gabbros. The exact role of focused constrictive deformation in Cr-spinel metamorphic alteration is not yet completely understood; it could be envisaged that the thrust zone also served as a localized permeability path enabling the passage of post-magmatic fluids that modified Cr-spinel texture and composition during ophiolite obduction. Petrographic observations (i.e., serpentine cutting clinochlore) document that serpentinization (and rodingitization) occurred after Cr-spinel post-magmatic modification, and were related to oceanic alteration that commonly accompanies ophiolite emplacement.
If the above geochronological assumptions are correct, then dating of the Ayios Stefanos zircons may serve as a potential way to date the initial stages of obduction in Othris as the ophiolitic slab was coming off the ridgecrest.
8. Concluding Remarks
The present study has led to the following conclusions:
- The Ayios Stefanos Al-rich chromitites precipitated from an alkali-rich melt of medium-Ti basalt (MTB) affinity produced at a near-transform boundary of a slow-spreading ridge.
- A few samples show limited Cr-spinel replacement by spinel with oriented lamellae of Mg-silicates (predominantly chlorite) ascribed to the infiltration of oxidizing fluids in the chromitites. Most specimens display common porous spinel for Cr-spinel substitution linked to Cr-spinel (or lamellae-rich spinel) breakdown after reaction with olivine in a reducing environment.
- Chromitites host numerous tiny, subhedral to anhedral and elongated Zr-bearing silicate phase that are commonly placed in open and restored fractures in Cr-spinel. Textural associations and compositional data echo the possibility that zircons originate from high-T fluids emanating from the gabbroic dykes that intrude both chromitites and peridotites in the study area.
- Zircon pseudomorphic replacement by Ca-bearing Zr-rich silicates and enrichment in solvent cation oxides (CaO, Al2O3 and FeO) provide evidence for zircon re-equilibration with deuteric fluids.
- This study highlights that a clear understanding of the origin of zircons in ophiolitic chromitites is essential for the interpretation of the sequence of synchronological petrologic and deformation events recorded by upper-mantle rocks, including their obduction, emplacement and subsequent alteration.
Supplementary Materials
The following are available online at www.mdpi.com/2075-163X/6/4/124/s1, Table S1: Pristine chromian spinel analyses, Table S2: Altered spinel analyses, Table S3: Zr-bearing silicate mineral analyses.
Acknowledgments
Research was partly supported by Sun Yat-sen University Special Fund Project in the form of a Young Faculty’s Research Start-up Grant (No. 32110-31121401) and the National Natural Science Foundation of China (NNSFC) Youth Science Fund Project (No. 41402065) both awarded to Argyrios Kapsiotis. Special thanks are due to the Guest Editor of the Special Issue “Mineral Deposit Genesis and Exploration” M. Economou-Eliopoulos. P. Pagé, A.Y. Borisova and five anonymous reviewers are acknowledged for their constructive criticism and valuable comments that improved the manuscript. Acknowledgments are also extended to the Editorial staff of Minerals.
Author Contributions
Argyrios Kapsiotis collected the samples, performed all types of analyses, processed the data and wrote the paper. Annie Ewing Rassios provided part of the geological and field information and co-wrote the paper. Aspasia Antonelou interpreted the Raman data. Evangelos Tzamos processed part of the analytical data. All authors contributed to the elaboration and interpretation of the data.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Appendix A

Table A1.
Summary of the main petrographic characteristics of the studied chromitites. Abbreviations: Cal = calcite; Chl = chlorite; Hgr = hydrogrossular; Ser = serpentine; Spn = Spinel; Tr = tremolite; Wo = wollastonite. The symbols: + and -, denote the accessory presence and absence of zircon, respectively.
| Sample Number | Morphology | Chr Texture | Groundmass Texture | Secondary Phases | Zr-Si Phases | Other Features |
|---|---|---|---|---|---|---|
| 1a | Boulder | Massive | Mesh | Srp, Chl, Tr, Hgr, Porous Spn | + | Hgr microveins |
| 1b | Boulder | Massive | Interpenetrating | Srp, Chl, Tr, Porous Spn | + | Chr brecciation |
| 2a | Boulder | Massive | Interlocking, bastite | Srp, Chl, Tr, Wo, Hgr | - | - |
| 2b | Boulder | Massive | Interpenetrating, bastite | Srp, Chl, Wo | - | - |
| 3a | Boulder | Massive | Mesh | Srp, Chl | - | Pull apart texture in Chr |
| 3b | Boulder | Massive | Mesh | Srp, Chl, Hgr | - | Pull apart texture in Chr |
| 3c | Boulder | Massive | Interpenetrating | Srp, Chl, Hgr | - | Microfolds |
| 3d | Boulder | Massive | Interpenetrating | Srp, Chl, Hgr, Cal | - | Hgr-Cal intergrowths |
| 4a | Boulder | Massive | Mesh | Srp, Chl, Wo | - | Lobate Chr |
| 4b | Boulder | Semi-massive | Mesh | Srp, Chl | - | Pull apart texture in Chr |
| 5 | Boulder | Massive | Interpenetrating | Srp, Chl, Porous Spn | - | Chr brecciation |
| 6 | Boulder | Semi-massive | Mesh, bastite | Srp, Chl, Tr, Wo | + | Rt exsolutions |
| 7a | Lenticular | Semi-massive | Interpenetrating, hourglass | Srp, Chl, Tr, Porous & Lamellae-rich Spn | - | Lobate Chr |
| 7b | Lenticular | Massive | Interpenetrating, hourglass | Srp, Chl, Tr, Porous & Lamellae-rich Spn | - | Chl intersection by Srp |
| 7c | Lenticular | Massive | Interpenetrating, mesh | Srp, Chl, Tr, Porous Spn | + | Chr brecciation |
| 8 | Lenticular | Semi-massive | Interpenetrating, mesh | Srp, Chl, Tr, Porous Spn | + | - |

Table A2.
Electron-microprobe analyses and structural formulae (on the basis of 4 atoms of O) of Cr-spinel. Fe2O3(rec.) is not included in the Total of each analysis. Abbreviations: bdl = below detection limit; na = not analyzed.
| Sample/Analysis Number | SiO2 (wt %) | TiO2 | Al2O3 | Cr2O3 | V2O3 | FeOt | Fe2O3 (rec.) | MnO | MgO | NiO | CaO | ZnO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-a/1 | bdl | 0.05 | 26.31 | 43.61 | 0.17 | 13.28 | 1.28 | 0.20 | 15.27 | 0.20 | bdl | 0.10 | 99.17 |
| 2-a/5 | bdl | bdl | 24.79 | 46.00 | 0.18 | 14.16 | 0.68 | 0.20 | 14.42 | 0.11 | bdl | 0.06 | 99.92 |
| 2-d/5 | bdl | 0.10 | 24.96 | 46.29 | 0.15 | 14.46 | 0.58 | 0.21 | 14.28 | 0.16 | bdl | 0.10 | 100.71 |
| 3-c/17 | bdl | 0.08 | 26.45 | 43.03 | 0.20 | 15.05 | 2.14 | 0.24 | 14.62 | 0.09 | 0.41 | bdl | 100.16 |
| 3-d/3 | bdl | bdl | 26.42 | 43.35 | 0.16 | 14.22 | 2.15 | 0.18 | 15.38 | 0.17 | bdl | 0.14 | 100.01 |
| 4-b/10 | bdl | 0.13 | 19.66 | 50.06 | 0.16 | 15.81 | 1.22 | 0.18 | 13.00 | 0.11 | bdl | bdl | 99.08 |
| 5/4 | bdl | bdl | 23.77 | 44.89 | 0.21 | 14.70 | 3.01 | 0.15 | 15.21 | 0.16 | bdl | bdl | 99.08 |
| 6/1 | bdl | 0.29 | 18.38 | 43.47 | 0.26 | 27.87 | 8.32 | 0.25 | 9.37 | 0.10 | bdl | 0.07 | 100.07 |
| 7-a/20 | bdl | 0.10 | 26.47 | 43.58 | 0.14 | 14.89 | 2.37 | 0.13 | 15.35 | 0.12 | bdl | 0.07 | 100.86 |
| 7-b/1 | bdl | 0.08 | 25.49 | 44.33 | 0.17 | 13.07 | 3.17 | 0.14 | 16.82 | 0.08 | bdl | bdl | 100.18 |
| 7-c/3 | bdl | 0.07 | 24.96 | 45.99 | 0.13 | 12.89 | 1.14 | 0.16 | 15.57 | 0.11 | bdl | bdl | 99.90 |
| 8/9 | 0.03 | 0.16 | 17.60 | 50.91 | 0.21 | 16.62 | 4.52 | 0.38 | 14.41 | na | bdl | 0.08 | 100.39 |
| Apfu | Si | Ti | Al | Cr | V | Fe3+ | Fe2+ | Mn | Mg | Ni | Ca | Zn | Total |
| 1-a/1 | - | - | 0.93 | 1.04 | - | 0.03 | 0.31 | 0.01 | 0.68 | 0.01 | - | - | 3.01 |
| 2-a/5 | - | - | 0.88 | 1.10 | - | 0.02 | 0.34 | 0.01 | 0.65 | - | - | - | 3.00 |
| 2-d/5 | - | - | 0.88 | 1.10 | - | 0.01 | 0.35 | 0.01 | 0.64 | - | - | - | 2.99 |
| 3-c/17 | - | - | 0.93 | 1.02 | 0.01 | 0.05 | 0.33 | 0.01 | 0.65 | - | 0.01 | - | 3.01 |
| 3-d/3 | - | - | 0.93 | 1.02 | - | 0.05 | 0.31 | - | 0.68 | - | - | - | 2.99 |
| 4-b/10 | - | - | 0.73 | 1.24 | - | 0.03 | 0.39 | 0.01 | 0.61 | - | - | - | 3.01 |
| 5/4 | - | - | 0.85 | 1.08 | 0.01 | 0.07 | 0.30 | - | 0.69 | - | - | - | 3.00 |
| 6/1 | - | 0.01 | 0.69 | 1.10 | 0.01 | 0.20 | 0.54 | 0.01 | 0.45 | - | - | - | 3.01 |
| 7-a/20 | - | - | 0.92 | 1.02 | - | 0.05 | 0.32 | - | 0.68 | - | - | - | 2.99 |
| 7-b/1 | - | - | 0.89 | 1.04 | - | 0.07 | 0.25 | - | 0.74 | - | - | - | 2.99 |
| 7-c/3 | - | - | 0.88 | 1.09 | - | 0.03 | 0.30 | - | 0.70 | - | - | - | 3.00 |
| 8/9 | - | - | 0.64 | 1.24 | 0.01 | 0.11 | 0.32 | 0.01 | 0.66 | - | - | - | 2.99 |

Table A3.
Electron-microprobe analyses and structural formulae (on the basis of 4 atoms of O) of porous spinel and lamellae-rich spinel. All analyses correspond to porous spinel except for analyses 7-a/7, 7-b/2 and 7-b/21, which represent lamellae-rich spinel. Analyses from samples 7-a and 7-b represent pair analyses. Fe2O3(rec.) is not included in the Total of each analysis. Abbreviations: bdl = below detection limit; na = not analyzed.
| Sample/Analysis Number | SiO2 (wt %) | TiO2 | Al2O3 | Cr2O3 | V2O3 | FeO | Fe2O3 (rec.) | MnO | MgO | NiO | CaO | ZnO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-a/6 | bdl | 0.16 | 17.25 | 50.67 | 0.13 | 18.35 | 3.20 | 0.20 | 12.32 | 0.06 | bdl | bdl | 99.14 |
| 1-b/6 | bdl | 0.10 | 17.36 | 51.11 | 0.19 | 17.11 | 3.97 | 0.31 | 13.69 | na | bdl | 0.06 | 99.93 |
| 1-b/8 | bdl | 0.11 | 15.91 | 51.68 | 0.18 | 18.42 | 5.08 | 0.36 | 13.37 | na | bdl | bdl | 100.03 |
| 5/17 | bdl | 0.12 | 18.30 | 49.63 | 0.17 | 18.74 | 2.51 | 0.33 | 11.52 | 0.10 | bdl | 0.21 | 99.12 |
| 7-a/7 | 0.56 | 0.11 | 8.57 | 48.38 | 0.17 | 21.35 | 15.52 | 0.18 | 16.11 | 0.09 | bdl | bdl | 95.52 |
| 7-a/8 | bdl | 0.11 | 14.32 | 48.52 | 0.20 | 24.14 | 10.19 | 0.20 | 12.56 | 0.20 | bdl | bdl | 100.25 |
| 7-b/2 | 0.61 | bdl | 12.57 | 45.63 | 0.14 | 16.63 | 12.67 | 0.22 | 17.37 | 0.03 | 0.04 | 0.10 | 93.34 |
| 7-b/3 | 0.01 | 0.06 | 12.04 | 49.12 | 0.14 | 25.83 | 12.08 | 0.24 | 12.17 | 0.19 | bdl | 0.19 | 99.99 |
| 7-b/21 | 8.30 | bdl | 8.98 | 45.52 | 0.10 | 18.13 | 11.81 | 0.18 | 17.04 | 0.09 | bdl | bdl | 98.34 |
| 7-b/22 | 0.06 | 0.07 | 0.55 | 56.11 | 0.21 | 29.38 | 16.68 | 0.26 | 11.19 | bdl | bdl | bdl | 97.83 |
| 7-c/2 | 0.06 | 0.10 | 7.80 | 60.48 | 0.20 | 20.74 | 3.45 | 0.31 | 9.97 | 0.11 | bdl | bdl | 99.77 |
| 7-c/4 | bdl | 0.06 | 8.36 | 58.96 | 0.21 | 22.61 | 3.72 | 0.30 | 8.89 | 0.14 | bdl | bdl | 99.53 |
| 8/6 | bdl | 0.54 | 8.27 | 54.43 | 0.26 | 23.20 | 9.70 | 0.49 | 11.94 | na | 0.03 | bdl | 99.16 |
| Apfu | Si | Ti | Al | Cr | V | Fe3+ | Fe2+ | Mn | Mg | Ni | Ca | Zn | Total |
| 1-a/6 | - | - | 0.65 | 1.27 | - | 0.08 | 0.41 | 0.01 | 0.58 | - | - | - | 3.00 |
| 1-b/6 | - | - | 0.64 | 1.26 | 0.01 | 0.09 | 0.35 | 0.01 | 0.64 | - | - | - | 3.00 |
| 1-b/8 | - | - | 0.59 | 1.28 | 0.01 | 0.12 | 0.36 | 0.01 | 0.63 | - | - | - | 3.00 |
| 5/17 | - | - | 0.69 | 1.25 | - | 0.06 | 0.44 | 0.01 | 0.55 | - | - | 0.01 | 3.01 |
| 7-a/7 | 0.02 | - | 0.33 | 1.26 | 0.01 | 0.38 | 0.20 | 0.01 | 0.79 | - | - | - | 3.00 |
| 7-a/8 | - | - | 0.54 | 1.22 | 0.01 | 0.24 | 0.40 | 0.01 | 0.59 | 0.01 | - | - | 3.02 |
| 7-b/2 | 0.02 | - | 0.48 | 1.18 | - | 0.31 | 0.14 | 0.01 | 0.85 | - | - | - | 2.99 |
| 7-b/3 | - | - | 0.46 | 1.25 | - | 0.29 | 0.40 | 0.01 | 0.58 | 0.01 | - | 0.01 | 3.01 |
| 7-b/21 | 0.26 | - | 0.33 | 1.13 | - | 0.28 | 0.20 | 0.01 | 0.80 | - | - | - | 3.01 |
| 7-b/22 | - | - | 0.02 | 1.53 | 0.01 | 0.43 | 0.42 | 0.01 | 0.58 | - | - | - | 3.00 |
| 7-c/2 | - | - | 0.31 | 1.60 | 0.01 | 0.09 | 0.49 | 0.01 | 0.50 | - | - | - | 3.01 |
| 7-c/4 | - | - | 0.33 | 1.57 | 0.01 | 0.09 | 0.54 | 0.01 | 0.45 | - | - | - | 3.00 |
| 8/6 | - | 0.01 | 0.32 | 1.42 | 0.01 | 0.24 | 0.40 | 0.01 | 0.59 | - | - | - | 3.00 |

Table A4.
Electron-microprobe analyses of Zr-bearing silicate phases from the studied chromitites. All analyses correspond to zircon except for 40 and 41 which represent calciocatapleiite and armstrongite, respectively. Structural formulae for zircon were calculated on the basis of 4 atoms of O, whereas those of calciocatapleiite and armstrongite were calculated on the basis of 9 and 15 atoms of O. Analyses 35–37 correspond to zircon texturally associated with porous spinel and chlorite. The full range of detected major oxides for analyses 40 and 41 is given in the Supplementary files. Abbreviations: An. = analysis number; D.L. = detection limit; bdl = below detection limit.
| An. | SiO2 (wt %) | Al2O3 | FeO | MgO | CaO | Na2O | Y2O3 | ZrO2 | Ce2O3 | HfO2 | UO2 | ThO2 | P2O5 | Total |
| 4 | 35.63 | 0.12 | bdl | bdl | 0.05 | 0.02 | 0.98 | 62.63 | bdl | bdl | 0.06 | bdl | bdl | 99.49 |
| 5 | 35.66 | 1.25 | bdl | 0.29 | 2.06 | 0.05 | 0.86 | 59.53 | bdl | 0.33 | bdl | 0.26 | bdl | 100.29 |
| 6 | 37.14 | 0.86 | bdl | 0.24 | 0.82 | bdl | 0.92 | 60.24 | bdl | bdl | 0.12 | 0.15 | bdl | 100.49 |
| 12 | 36.00 | 0.51 | bdl | 0.15 | 0.32 | 0.02 | 1.16 | 61.08 | bdl | 0.00 | 0.16 | bdl | bdl | 99.40 |
| 13 | 36.57 | 0.65 | bdl | 0.30 | 0.67 | 0.04 | 0.86 | 59.12 | 0.12 | 0.28 | 0.11 | bdl | bdl | 98.72 |
| 16 | 36.54 | 0.19 | bdl | 0.20 | 0.20 | 0.07 | 0.53 | 63.05 | bdl | bdl | bdl | bdl | bdl | 100.78 |
| 21 | 36.41 | 0.40 | bdl | 0.08 | 0.00 | 0.04 | 0.71 | 61.77 | bdl | 0.39 | 0.06 | 0.09 | bdl | 99.95 |
| 23 | 37.62 | 1.12 | bdl | 0.32 | 0.17 | 0.00 | 0.63 | 58.07 | bdl | 0.20 | bdl | 0.08 | bdl | 98.21 |
| 25 | 36.15 | 0.16 | bdl | 0.09 | 0.06 | 0.00 | 0.44 | 62.70 | bdl | 0.41 | bdl | bdl | bdl | 100.01 |
| 35 | 35.97 | 0.14 | 1.27 | 3.62 | 0.21 | 0.03 | 0.07 | 52.71 | 0.08 | 1.73 | 0.15 | bdl | 0.07 | 96.05 |
| 36 | 34.28 | bdl | 0.32 | 0.03 | 0.07 | 0.03 | 0.13 | 63.25 | 0.15 | 1.68 | bdl | bdl | 0.36 | 100.30 |
| 37 | 34.17 | bdl | 0.37 | 0.05 | 0.04 | bdl | 0.19 | 64.03 | 0.09 | 1.53 | bdl | bdl | 0.43 | 100.90 |
| 40 | 45.88 | 0.19 | 2.18 | 3.74 | 14.58 | 0.04 | 0.42 | 22.36 | 0.09 | 1.03 | 0.21 | 0.08 | 0.15 | 90.95 |
| 41 | 48.86 | 0.15 | 1.60 | 3.60 | 7.95 | 0.05 | 0.47 | 25.79 | 0.00 | 0.89 | 0.08 | 0.21 | 0.10 | 89.75 |
| Apfu | Si | Al | Fe2+ | Mg | Ca | Na | Y | Zr | Ce | Hf | U | Th | P | Total |
| 4 | 1.07 | - | - | - | - | - | 0.02 | 0.92 | - | - | - | - | - | 2.01 |
| 5 | 1.06 | 0.04 | - | 0.01 | 0.07 | - | 0.01 | 0.86 | - | - | - | - | - | 2.05 |
| 6 | 1.09 | 0.03 | - | 0.01 | 0.03 | - | 0.01 | 0.86 | - | - | - | - | - | 2.03 |
| 12 | 1.07 | 0.02 | - | 0.01 | 0.01 | - | 0.02 | 0.89 | - | - | - | - | - | 2.02 |
| 13 | 1.09 | 0.02 | - | 0.01 | 0.02 | - | 0.01 | 0.86 | - | - | - | - | - | 2.01 |
| 16 | 1.08 | 0.01 | - | 0.01 | 0.01 | - | 0.01 | 0.91 | - | - | - | - | - | 2.03 |
| 21 | 1.08 | 0.01 | - | - | - | - | 0.01 | 0.89 | - | - | - | - | - | 1.99 |
| 23 | 1.11 | 0.04 | - | 0.01 | 0.01 | - | 0.01 | 0.84 | - | - | - | - | - | 2.02 |
| 25 | 1.08 | 0.01 | - | - | - | - | 0.01 | 0.91 | - | - | - | - | - | 2.01 |
| 35 | 1.09 | 0.01 | 0.03 | 0.16 | 0.01 | - | - | 0.78 | - | 0.01 | - | - | - | 2.09 |
| 36 | 1.03 | - | 0.01 | - | - | - | - | 0.93 | - | 0.01 | - | - | 0.01 | 1.99 |
| 37 | 1.03 | - | 0.01 | - | - | - | - | 0.94 | - | 0.01 | - | - | 0.01 | 2.00 |
| 40 | 2.98 | 0.01 | 0.12 | 0.36 | 1.02 | - | 0.01 | 0.71 | - | 0.02 | - | - | 0.01 | 5.24 |
| 41 | 5.25 | 0.02 | 0.14 | 0.58 | 0.92 | 0.01 | 0.03 | 1.35 | - | 0.03 | - | 0.01 | 0.01 | 8.35 |

Table A5.
Parameters of the Raman bands of representative zircon grains from the studied chromitites, where v is in cm−1 and I is in counts.
| Raman Analysis Number | Eg (Tetrahedron Rotation) | A1g (Symmetric Bending, v2) | B1g (Antisym. Stetching, v3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| v | FWHM | I | v | FWHM | I | v | FWHM | I | |
| 15.12.078 | 353.4 | 12.40 | 319.50 | 438.90 | 8.06 | 257.75 | 1003.7 | 10.09 | 373.74 |
| 15.12.080 | 352.1 | 17.17 | 531.07 | 438.05 | 19.38 | 735.50 | 1002.7 | 11.30 | 417.48 |
| 15.12.084 | 354.1 | 10.11 | 475.50 | 438.60 | 13.80 | 989.50 | 1005.1 | 10.90 | 816.26 |
| 15.12.087 | 353.4 | 12.70 | 381.29 | 438.99 | 16.16 | 780.10 | 1003.7 | 11.90 | 624.87 |
| 15.12.091 | 353.1 | 14.70 | 198.52 | 438.96 | 14.86 | 504.21 | 1003.6 | 12.10 | 310.05 |
| 15.12.096 | 353.9 | 12.80 | 202.29 | 438.67 | 14.14 | 454.63 | 1003.7 | 12.60 | 315.24 |
| 15.12.099 | 355.4 | 11.30 | 402.32 | 438.78 | 15.38 | 809.33 | 1003.9 | 13.34 | 738.46 |
| 15.12.102 | 355.2 | 16.08 | 1535.80 | 439.27 | 16.44 | 787.89 | 1004.3 | 13.60 | 694.56 |
| 15.12.105 | 355.1 | 15.14 | 1927.50 | 439.56 | 15.52 | 976.13 | 1004.9 | 13.32 | 935.55 |
| 15.12.110 | 353.4 | 12.34 | 436.40 | 439.24 | 13.42 | 1210.50 | 1004.1 | 14.54 | 1098.04 |
| 15.12.117 | 353.5 | 13.54 | 779.04 | 438.25 | 15.78 | 766.12 | 1004.5 | 13.08 | 700.00 |
| 15.12.126 | 354.8 | 9.80 | 151.80 | 439.40 | 14.86 | 599.60 | 1005.6 | 14.86 | 249.70 |
References
- Ahmed, A.H.; Arai, S. Unexpectedly high-PGE chromitite from the deeper mantle section of the northern Oman ophiolite and its tectonic implications. Contrib. Mineral. Petrol. 2002, 143, 263–278. [Google Scholar] [CrossRef]
- Leblanc, M.; Violette, J.F. Distribution of aluminium-rich and chromium-rich chromite pods in ophiolite peridotites. Econ. Geol. 1983, 78, 293–301. [Google Scholar] [CrossRef]
- Arai, S.; Yurimoto, H. Podiform chromitites of the Tari-Mikasa ultramafic complex, Southwest Japan, as mantle-melt interaction products. Econ. Geol. 1994, 89, 1279–1288. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Shmizu, N.; Salters, V.J.M. Extraction of mid-ocean ridge basalt from the upwelling mantle by focused melt in dunite channels. Nature 1995, 375, 747–753. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Griffin, W.L.; Proenza, J.A.; Gervilla, F.; O’Reilly, S.Y.; Akbulut, M.; Pearson, N.J.; Arai, S. Chromitites in ophiolites: How, where, when, why? Part II. The crystallization of chromitites. Lithos 2014, 189, 140–158. [Google Scholar] [CrossRef]
- Pagé, P.; Barnes, S.-J. Using trace elements in chromites to constrain the origin of podiform chromitites in the Thetford Mines ophiolite, Québec, Canada. Econ. Geol. 2009, 104, 997–1018. [Google Scholar] [CrossRef]
- Payot, B.D.; Arai, S.; Dick, H.J.B.; Abe, N.; Ichiyama, Y. Podiform chromitite formation in a low-Cr/high-Al system: An example from the Southwest Indian Ridge (SWIR). Mineral. Petrol. 2014, 108, 553–549. [Google Scholar] [CrossRef]
- Arai, S.; Miura, M. Podiform chromitites do form beneath mid-ocean ridges. Lithos 2015, 232, 143–149. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Ceuleneer, G.; Kamenetsky, V.S.; Arai, S.; Béjina, F.; Abily, B.; Bindeman, I.N.; Polvé, M.; De Parseval, P.; Aigouy, T.; et al. A new view on the petrogenesis of the Oman ophiolite chromitites from microanalyses of chromite-hosted inclusions. J. Petrol. 2012, 53, 2411–2440. [Google Scholar] [CrossRef]
- Yamamoto, S.; Komiya, T.; Hirose, K.; Maruyama, S. Coesite and clinopyroxene exsolution lamellae in chromites: In situ ultrahigh-pressure evidence from podiform chromitites in the Luobusa ophiolite, southern Tibet. Lithos 2009, 109, 314–322. [Google Scholar] [CrossRef]
- González-Jimenéz, J.M.; Proenza, J.A.; Gervilla, F.; Melgarejo, J.C.; Blanco-Moreno, J.A.; Ruiz-Sánchez, R.; Griffin, W.L. High-Cr and High-Al chromitites from the Sagua de Tánamo district, Mayarí-Cristal ophiolitic massif (eastern Cuba): Constraints on their origin from mineralogy and geochemistry of chromian spinel and platinum-group elements. Lithos 2011, 125, 101–121. [Google Scholar] [CrossRef]
- Arai, S. Conversion of low-pressure chromitites to ultra-high-pressure chromitites by deep recycling: A good inference. Earth Planet. Sci. Lett. 2013, 379, 81–87. [Google Scholar] [CrossRef]
- Xu, X.Z.; Yang, J.S.; Robinson, P.T.; Xiong, F.H.; Zhu, B.; Guo, G.L. Ultra high pressure, highly reduced and crustal-type minerals from podiform chromitite and mantle peridotite of the Luobusa ophiolite, Tibet. Gondwana Res. 2015, 2, 686–700. [Google Scholar] [CrossRef]
- Barnes, S.J. Chromite in komatiites, II. Modification during greenschist to mid-amphibolite facies metamorphism. J. Petrol. 2000, 41, 387–409. [Google Scholar] [CrossRef]
- Burkhard, D.J.M. Accessory chromium spinels: Their coexistence and alteration in serpentinites. Geochim. Cosmochim. Acta 1993, 57, 1297–1306. [Google Scholar] [CrossRef]
- Merlini, A.; Grieco, G.; Diella, V. Ferritchromite and chromian-chlorite formation in mélange-hosted Kalkan chromitite (Southern Urals, Russia). Am. Mineral. 2009, 94, 1459–1467. [Google Scholar] [CrossRef]
- Grieco, G.; Merlini, A. Chromite alteration processes within Vourinos ophiolite. Int. J. Earth Sci. 2012, 101, 1523–1533. [Google Scholar] [CrossRef]
- Colás, V.; González-Jimenéz, J.M.; Griffin, W.L.; Fanlo, I.; Gervilla, F.; O’Reilly, S.Y.; Pearson, N.J.; Kerestedjian, T.; Proenza, J.A. Fingerprints of metamorphism in chromite: New insights from minor and trace elements. Chem. Geol. 2014, 389, 137–152. [Google Scholar] [CrossRef]
- Spangenberg, K. Die chromitlagerstätte von Tampadel in Zobten. Z. Prakt. Geol. 1943, 51, 13–35. [Google Scholar]
- Gervilla, F.; Padron-Navarta, J.A.; Kerestedjian, T.; Sergeeva, I.; Gonzalez-Jimenez, J.M.; Fanlo, I. Formation of ferrian chromite in podiform chromitites from the Golyamo Kamenyane serpentinite, Eastern Rhodopes, SE Bulgaria: A two stage process. Contrib. Mineral. Petrol. 2012, 164, 643–657. [Google Scholar] [CrossRef]
- Savelieva, G.N.; Suslov, P.V.; Larionov, A.N. Vendian tectono-magmatic events in mantle ophiolitic complexes of the Polar Urals: U-Pb dating of zircon from chromitite. Geotectonics 2007, 41, 105–113. [Google Scholar] [CrossRef]
- Robinson, P.T.; Trumbull, R.B.; Schmitt, A.; Yang, J.-S.; Li, J.-W.; Zhou, M.-F.; Erzinger, J.; Dare, S.; Xiong, F. The origin and significance of crustal minerals in ophiolitic chromitites and peridotites. Gondwana Res. 2015, 27, 486–506. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Locmelis, M.; Belousova, E.; Griffin, W.L.; Gervilla, F.; Kerestedjian, T.; O’Reilly, S.Y.; Sergeeva, I.; Pearson, N.J. Genesis and tectonic implications of podiform chromitites in the metamorphosed Ultramafic Massif of Dobromirtsi (Bulgaria). Gondwana Res. 2015, 27, 555–574. [Google Scholar] [CrossRef]
- Stern, R.J.; Ali, K.A.; Liegois, J.P.; Jhonson, P.R.; Kozdrojs, W.; Kattan, F.H. Distribution and significance of pre-Neoproterozoic zircons in juvenile Neoproterozoic igneous rocks of the Arabian-Nubian shield. Am. J. Sci. 2010, 130, 791–811. [Google Scholar] [CrossRef]
- Yamamoto, S.; Komiya, T.; Yamamoto, H.; Kaneko, Y.; Terabayashi, M.; Katayama, I.; Iizuka, T.; Maruyama, S.; Yang, J.; Kon, Y.; et al. Recycled crustal zircons from podiform chromitites in the Luobusa ophiolite, southern Tibet. Island Arc 2013, 22, 89–103. [Google Scholar] [CrossRef]
- McGowan, N.; Griffin, W.L.; González-Jiménez, J.M.; Belousova, E.; Afonso, J.C.; Shi, R.; McCammon, C.A.; Pearson, N.J.; O’Reilly, S.Y. Tibetan chromitites: Excavating the slab graveyard. Geology 2015, 43, 179–182. [Google Scholar] [CrossRef]
- Belousova, E.A.; González-Jiménez, J.M.; Graham, I.; Griffin, W.L.; O’Reilly, S.Y.; Pearson, N.; Martin, L.; Craven, S.; Talavera, C. The enigma of crustal zircons in upper-mantle rocks: Clues from the Tumut ophiolite, southeast Australia. Geology 2015, 43, 119–122. [Google Scholar] [CrossRef]
- Portella, Y.M.; Zaccarini, F.; Luvizotto, G.L.; Garuti, G.; Bakker, R.J.; Angeli, N.; Thalhammer, O. The Cedrolina chromitite, Goiás State, Brazil: A metamorphic puzzle. Minerals 2016, 6, 91. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Clift, P.D.; Degnan, P.J.; Jones, G. Palaeogeographic and palaeotectonic evolution of the Eastern Mediterranean Neotethys. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 87, 289–343. [Google Scholar] [CrossRef]
- Vérgely, P. Origine “vardarienne”, chevauchement vers l’Ouest et rétrocharriage vers l’Est des ophiolites de Macédoine (Grèce) au cours du Jurassique supérieur-Eocrétacé. CR Acad. Sci. Paris 1976, 280, 1063–1066. (In French) [Google Scholar]
- Smith, A. Othris, Pindos and Vourinos ophiolites and the Pelagonian zone. In Proceedings of the 6th Colloquium on the Geology of the Aegean Region, Athens, Greece, 14–20 September 1977; pp. 1369–1374.
- Smith, A.; Rassios, A. The evolution of ideas for the origin and emplacement of the western Hellenic ophiolites. In Ophiolite Concept and the Evolution of Geological Thought; GSA Special Papers; Dilek, Y., Newcomb, S., Eds.; GSA Special Publication: Boulder, CO, USA, 2003; Volume 373, pp. 337–350. [Google Scholar]
- Rassios, A.; Konstantopoulou, G. Emplacement tectonism and the position of chrome ores in the Mega Isoma peridotites, SW Othris, Greece. Bull. Geol. Soc. Greece 1993, 28, 463–474. [Google Scholar]
- Ferrière, J.; Bertrand, J.; Simantov, J.; De Wever, P. Comparaison entre les formations volcano-detritiques (Mélanges) du malm des Hellenides internes (Othrys, Eubee): Implications geodynamics. Bull. Geol. Soc. Greece 1988, 20, 223–235. (In French) [Google Scholar]
- Spray, J.G.; Bébien, J.; Rex, D.C.; Roddick, J.C. Age constraints on the igneous and metamorphic evolution of the Hellenic-Dinaric ophiolites. In The Geological Evolution of the Eastern Mediterranean; Dixon, J.E., Robertson, A.H.F., Eds.; The Geological Society Special Publications: London, UK, 1984; Volume 17, pp. 619–627. [Google Scholar]
- Rassios, A.; Dilek, Y. Rotational deformation in the Jurassic Mesohellenic Ophiolites, Greece, and its tectonic significance. Lithos 2009, 108, 207–223. [Google Scholar] [CrossRef]
- Armstrong, J.T. CITZAF: A package of correction programs for the quantitative electron microbeam X-ray analysis of thick polished materials, thin films, and particles. Microbeam Anal. 1995, 4, 177–200. [Google Scholar]
- Fleet, M.E.; Angeli, N.; Pan, Y. Oriented chlorite lamellae in chromite from the Pedra Branca Mafic-Ultramafic Complex, Ceará, Brazil. Am. Mineral. 1993, 78, 68–74. [Google Scholar]
- Nasdala, L.; Kronz, A.; Wirth, R.; Váczi, T.; Pérez-Soba, C.; Willner, A.; Kennedy, A.K. The phenomenon of deficient electron microprobe totals in radiation-damaged and altered zircon. Geochim. Cosmochim. Acta 2009, 73, 1637–1650. [Google Scholar] [CrossRef]
- Nasdala, L.; Lengauer, C.L.; Hanchar, J.M.; Kronz, A.; Wirth, R.; Blanc, P.; Kennedy, A.K.; Seydoux-Guillaume, A.-M. Annealing radiation damage and the recovery of cathodoluminescence. Chem. Geol. 2002, 191, 121–140. [Google Scholar] [CrossRef]
- Sommerauer, J. Trace elements distribution patterns and mineralogical stability of zircons—An application for combined electron microprobe techniques. Electron Microsc. Soc. S. Afr. Proc. 1974, 4, 71–72. [Google Scholar]
- Bonavia, F.F.; Diella, V.; Ferrario, A. Precambrian podiform chromitites from Kenticha Hill, Southern Ethiopia. Econ. Geol. 1993, 88, 198–202. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib. Mineral. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Arai, S. Control of wall-rock composition on the formation of podiform chromitites as a result of magma/peridotite interaction. Resour. Geol. 1997, 47, 177–187. [Google Scholar]
- Kamenetsky, V.S.; Crawford, A.J.; Meffre, S. Factors controlling chemistry of magmatic spinel: An empirical study of associated olivine, Cr-spinel and melt inclusions from primitive rocks. J. Petrol. 2001, 42, 655–671. [Google Scholar] [CrossRef]
- Claiborne, L.L.; Miller, C.F.; Walker, B.A.; Wooden, J.L.; Mazdab, F.K.; Bea, F. Tracking magmatic processes through Zr/Hf ratios in rocks and Hf and Ti zoning in zircons: An example from the Spirit Mountain batholith, Nevada. Mineral. Mag. 2006, 70, 517–543. [Google Scholar] [CrossRef]
- Dawson, P.; Hargreave, M.M.; Wilkinson, G.R. The vibrational spectrum of zircon (ZrSiO4). J. Phys. C Solid State Phys. 1971, 4, 240–255. [Google Scholar] [CrossRef]
- Nasdala, L.; Zhang, M.; Kempe, U.; Panczer, G.; Gaft, M.; Andrut, M.; Plötze, M. Spectroscopic methods applied to zircon. Rev. Mineral. Geochem. 2003, 53, 427–467. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Farnan, I.; Graeme-Barber, A.; Daniel, P.; Ewing, R.C.; Clark, A.M.; Leroux, H. Metamictization of zircon: Raman spectroscopic study. J. Phys. Condens. Matter 2000, 12, 1915–1925. [Google Scholar] [CrossRef]
- Palenik, C.S.; Nasdala, L.; Ewing, R.C. Radiation damage in zircon. Am. Mineral. 2003, 88, 770–781. [Google Scholar] [CrossRef]
- Johan, Z.; Dunlop, H.; Le Bel, Robert, J.L.; Volfinger, M. Origin of chromite deposits in ophiolitic complexes: Evidence for a volatile- and sodium-rich reducing fluid phase. Fortschr. Mineral. 1983, 61, 105–107. [Google Scholar]
- Maurel, C.; Maurel, P. Étude expérimentale de la distribution de l’aluminium entre bain silicaté basique et spinelle chromifère. Implications pétrogénétiques: Teneur en chrome des spinelles. Bull. Minéral. 1982, 105, 197–202. (In French) [Google Scholar]
- Zaccarini, F.; Garuti, G.; Proenza, J.A.; Campos, L.; Thalhammer, O.A.R.; Aiglsperger, T.; Lewis, J. Chromite and platinum-group-elements mineralization in the Santa Elena ophiolitic ultramafic nappe (Costa Rica): Geodynamic implications. Geol. Acta 2011, 9, 407–423. [Google Scholar]
- Augé, T. Chromite deposits in the northwestern Oman ophiolite: Mineralogical constraints. Miner. Deposita 1987, 22, 1–10. [Google Scholar] [CrossRef]
- Wilson, M. Igneous Petrogenesis; Unwin Hyman: London, UK, 1989; p. 466. [Google Scholar]
- Rollinson, H. The geochemistry of mantle chromitites from the northern part of the Oman ophiolite: Inferred parental melt compositions. Contrib. Mineral. Petrol. 2008, 156, 273–288. [Google Scholar] [CrossRef]
- Photiades, A.; Saccani, E.; Tassinari, R. Petrogenesis and tectonic setting of volcanic rocks from the Subpelagonian ophiolitic mélange in the Agoriani area (Othrys, Greece). Ofioliti 2003, 28, 121–135. [Google Scholar]
- Saccani, E.; Beccaluva, L.; Coltorti, M.; Siena, F. Petrogenesis and tectono-magmatic significance of the Albanide-Hellenide subpelagonian ophiolites. Ofioliti 2004, 29, 77–95. [Google Scholar]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Drury, M.R. The Othris Ophiolite, Greece: A snapshot of subduction initiation at a mid-ocean ridge. Lithos 2008, 100, 234–254. [Google Scholar] [CrossRef]
- Saccani, E.; Beccaluva, L.; Photiades, A.; Zeda, O. Petrogenesis and tectono-magmatic significance of basalts and mantle peridotites from the Albanian-Greek ophiolites and sub-ophiolitic mélanges. New constraints for the Triassic-Jurassic evolution of the Neo-Tethys in the Dinaride sector. Lithos 2011, 124, 227–242. [Google Scholar] [CrossRef]
- Dijkstra, A.H.; Drury, M.R.; Vissers, R.L.M. Structural petrology of plagioclase-peridotites in the West Othris Mountains (Greece): Melt impregnation in mantle lithosphere. J. Petrol. 2001, 42, 5–24. [Google Scholar] [CrossRef]
- Dijkstra, A.H.; Barth, M.G.; Drury, M.R.; Mason, P.R.D.; Vissers, R.L.M. Diffuse porous melt flow and melt-rock reaction in the mantle lithosphere at a slow-spreading ridge: A structural petrology and LA-ICP-MS study of the Othris Peridotite Massif (Greece). Geochem. Geophys. Geosyst. 2003, 4, 8613. [Google Scholar] [CrossRef]
- Bizimis, M.; Salters, V.J.M.; Bonatti, E. Trace and REE content of clinopyroxenes from supra-subduction zone peridotites. Implications for melting and enrichment processes in island arcs. Chem. Geol. 2000, 165, 67–85. [Google Scholar] [CrossRef]
- Grieco, G.; Ferrario, A.; Von Quadt, A.; Koeppel, V.; Mathez, E.A. The zircon-bearing chromitites of the phlogopite peridotite of Finero (Ivrea Zone, Southern Alps): Evidence and geochronology of a metasomatized mantle slab. J. Petrol. 2001, 42, 89–101. [Google Scholar] [CrossRef]
- Zaccarini, F.; Stumpfl, E.; Garuti, G. Zirconolite and Zr-Th-U minerals in chromitites of the Finero complex, western Alps, Italy: Evidence for carbonatite-type metasomatism in a subcontinental mantle plume. Can. Mineral. 2004, 42, 1825–1845. [Google Scholar] [CrossRef]
- Tange, Y.; Takahashi, E. Stability of the high-pressure polymorph of zircon (ZrSiO4) in the deep mantle. Phys. Earth Planet. Inter. 2004, 143–144, 223–229. [Google Scholar] [CrossRef]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Dijkstra, A.H.; Drury, M.R. Geochemistry of the Othris Ophiolite, Greece: Evidence for refertilization? J. Petrol. 2003, 44, 1759–1785. [Google Scholar] [CrossRef]
- Zhou, M.-F.; Robinson, P.T.; Su, B.-X.; Gao, J.-F.; Li, J.-W.; Yang, J.-S.; Malpas, J. Compositions of chromite, associated minerals, and parental magmas of podiform chromite deposits: The role of slab contamination of asthenospheric melts in suprasubduction zone environments. Gondwana Res. 2014, 26, 262–283. [Google Scholar] [CrossRef]
- Chen, Z.; Doherty, W.; Gregoire, D.C. Application of laser sampling microprobe inductively-coupled plasma-mass spectrometry to the in-situ trace-element analysis of selected geological-materials. J. Anal. At. Spectrom. 1997, 12, 653–659. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Boehnke, P.; Watson, E.B.; Trail, D.; Harrison, T.M.; Schmitt, A.K. Zircon saturation re-revisited. Chem. Geol. 2013, 351, 324–334. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Ireland, T.R. Rare earth element chemistry of zircon and its use as a provenance indicator. Geology 2000, 28, 627–630. [Google Scholar] [CrossRef]
- Dubińska, E.; Bylina, P.; Kozlowski, A.; Dorr, W.; Nejbert, K.; Schastok, J.; Kulicki, C. U–Pb dating of serpentinization: Hydrothermal zircon from a metasomatic rodingite shell (Sudetic ophiolite, SW Poland). Chem. Geol. 2004, 203, 183–203. [Google Scholar] [CrossRef]
- Pettke, T.; Audétat, A.; Schaltegger, U.; Heinrich, C.A. Magmatic-to-hydrothermal crystallization in the W–Sn mineralized Mole Granite (NSW, Australia) Part II: Evolving zircon and thorite trace element chemistry. Chem. Geol. 2005, 220, 191–213. [Google Scholar] [CrossRef]
- Leblanc, M.; Nicolas, A. Ophiolitic chromitites. Int. Geol. Rev. 1992, 34, 653–686. [Google Scholar] [CrossRef]
- Corfu, F.; Hanchar, J.M.; Hoskin, P.W.O.; Kinny, P. Atlas of zircon textures. Rev. Mineral. Geochem. 2003, 53, 469–500. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Igneous zircon: Trace element composition as an indicator of source rock type. Contrib. Mineral. Petrol. 2002, 143, 602–622. [Google Scholar] [CrossRef]
- Shnukov, S.E.; Andreev, A.V.; Savenok, S.P. Admixture Elements in Zircons and Apatites: A Tool for Provenance Studies of Terrigenous Sedimentary Rocks; European Union of Geosciences (EUG 9): Strasburg, Germany, 1997. [Google Scholar]
- O’Reilly, S.Y.; Griffin, W.L. Rates of magma ascent: Constraints from mantle-derived xenoliths. In Timescales of Magmatic Processes: From Core to Atmosphere; Dosseto, A., Turner, S., van Orman, J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 116–124. [Google Scholar]
- Bindeman, I.N.; Melnik, O.E. Zircon Survival, Rebirth and Recycling during Crustal Melting, Magma Crystallization, and Mixing Based on Numerical Modelling. J. Petrol. 2016, 57, 437–460. [Google Scholar] [CrossRef]
- Badanina, I.Y.; Malitch, K.N. Timing of metasomatism in a subcontinental mantle: Evidence from zircon at Finero (Italy). In Proceedings of the EGU General Assembly, Vienna, Austria, 22–27 April 2012.
- Zanetti, A.; Giovanardi, T.; Langone, A.; Tiepolo, M.; Wu, F.-Y.; Dallai, L.; Mazzucchelli, M. Origin and age of zircon-bearing chromitite layers from the Finero phlogopite peridotite (Ivrea–Verbano Zone, Western Alps) and geodynamic consequences. Lithos 2016, 262, 58–74. [Google Scholar] [CrossRef]
- Roeder, P.L.; Poustovetov, A.; Oskarsson, N. Growth forms and composition of chromian spinel in MORB magma: Diffusion-controlled crystallization of chromian spinel. Can. Mineral. 2001, 39, 397–416. [Google Scholar] [CrossRef]
- Zagrtdenov, N.; Borisova, A.Y.; Toplis, M.; Duployer, B.; Tenailleau, C. Preliminary experimental investigation of chromite dissolution in mid-ocean ridge basalt melt. In Proceedings of the Goldschmidt Conference, Prague, Czech Republic, 16–21 August 2015.
- Candia, M.A.F.; Gaspar, J.C. Chromian spinels in metamorphosed ultramafic rocks from Mangabal I and II complexes, Goiás, Brazil. Mineral. Petrol. 1997, 60, 27–40. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Reich, M.; Camprubí, A.; Gervilla, F.; Griffin, W.; Colás, V.; O’Reilly, S.Y.; Proenza, J.A.; Pearson, N.J.; Centeno-García, E. Thermal metamorphism of mantle chromites and the stability of noble-metal nanoparticles. Contrib. Mineral. Petrol. 2015, 170. [Google Scholar] [CrossRef]
- Mellini, M.; Rumori, C.; Viti, C. Hydrothermally reset magmatic spinels in retrogade serpentinites: Formation of “ferritchromit” rims and chlorite aureoles. Contrib. Mineral. Petrol. 2005, 149, 266–275. [Google Scholar] [CrossRef]
- Shack, R.O.; Ghiorso, M.S. Chromian spinels as petrogenetic indicators: Thermodynamic and petrological applications. Am. Mineral. 1991, 76, 827–847. [Google Scholar]
- Purvis, A.C.; Nesbitt, R.W.; Hallberg, J.A. The geology of part of the Carr Boyd rocks complex and its associated nickel mineralization, Western Australia. Econ. Geol. 1972, 67, 1093–1113. [Google Scholar] [CrossRef]
- Evans, B.W.; Frost, B.R. Chrome-spinel in progressive metamorphism-a preliminary analysis. Geochim. Cosmochim. Acta 1975, 39, 959–972. [Google Scholar] [CrossRef]
- Suita, M.T.F.; Streider, A.J. Cr-spinel from Brazilian mafic-ultramafic complexes: Metamorphic modifications. Int. Geol. Rev. 1996, 38, 245–267. [Google Scholar] [CrossRef]
- Putnis, C.V.; Tsukamoto, K.; Nishimura, Y. Direct observation of pseudomorphism: Compositional and textural evolution at a fluid-solid interface. Am. Mineral. 2005, 90, 1909–1912. [Google Scholar] [CrossRef]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of zircon in aqueous fluids and melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Kynicky, J.; Chakhmouradian, A.R.; Xu, C.; Krmicek, L.; Galiova, M. Distribution and evolution of zirconium mineralization in peralkaline granites and associated pegmatites of the Khan Bogd complex, southern Mongolia. Can. Mineral. 2011, 49, 947–965. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Zirconosilicate phase relations in the Strange Lake (Lac Brisson) pluton, Québec–Labrador, Canada. Am. Mineral. 1995, 80, 1031–1040. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Alteration, HFSE mineralisation and hydrocarbon formation in peralkaline igneous systems: Insights from the Strange Lake Pluton, Canada. Lithos 2006, 91, 19–34. [Google Scholar] [CrossRef]
- Geisler, T.; Rashwan, A.A.; Rahn, M.K.W.; Poller, U.; Zwingmann, H.; Pidgeon, R.T.; Schleicher, H.; Tomaschek, F. Low-temperature hydrothermal alteration of natural metamict zircons from the Eastern Desert, Egypt. Mineral. Mag. 2003, 67, 485–508. [Google Scholar] [CrossRef]
- Liati, A.; Gebauer, D.; Fanning, C.M. The age of ophiolitic rocks of the Hellenides (Vourinos, Pindos, Crete): First U-Pb ion microprobe (SHRIMP) zircon ages. Chem. Geol. 2004, 207, 171–188. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).