Formation of Carbonate Nanoglobules by a Mixed Natural Culture under Hypersaline Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Site and Enrichment of Halophilic Culture
2.3. Culture Media
2.4. Carbonate Precipitation Experiments
2.5. Mineralogical and Morphological Examination of the Precipitates
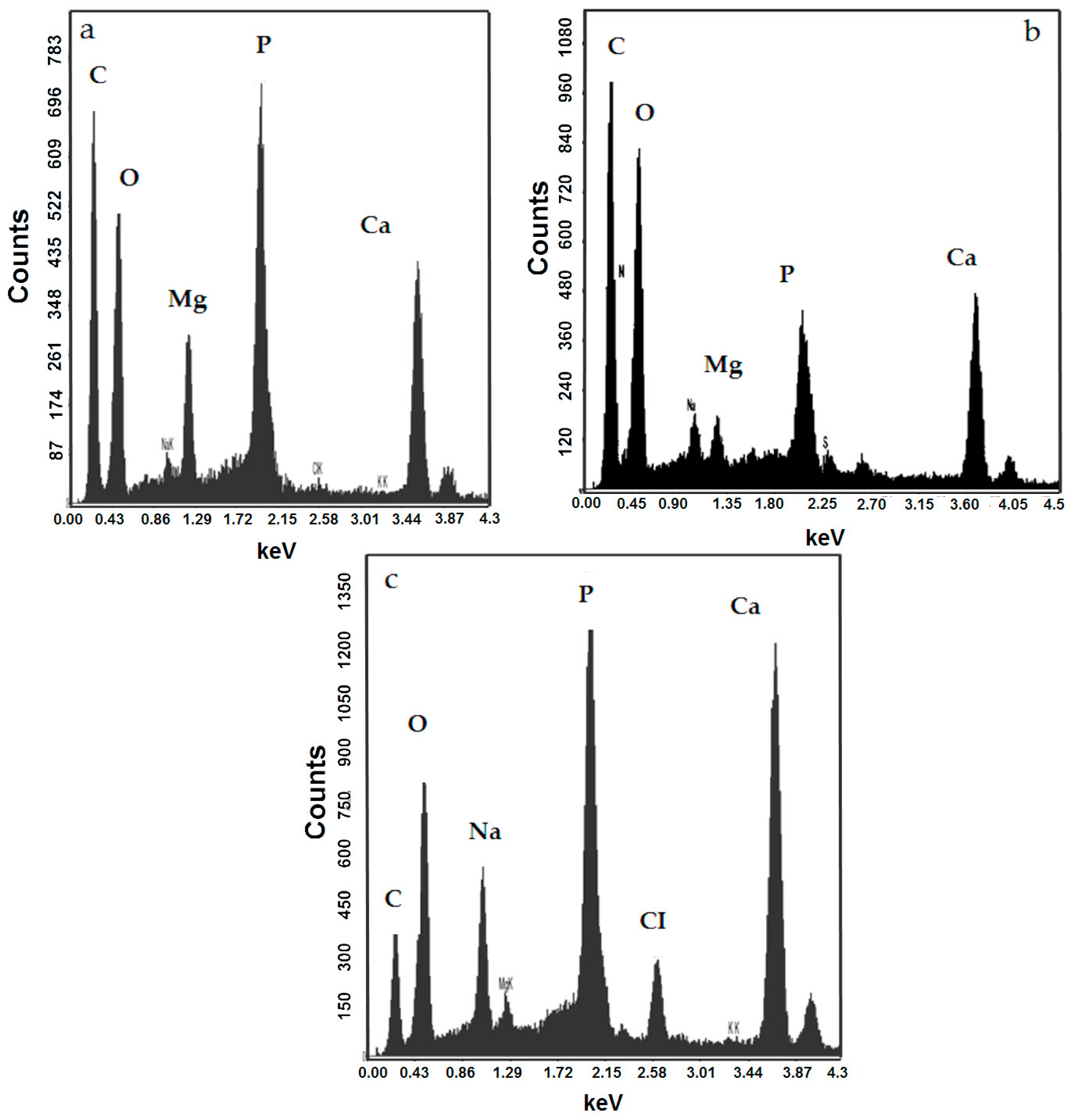
2.6. Geochemical Study
3. Results
3.1. Changes in Solution Chemistry during Precipitation
3.2. Mineralogy of the Bioliths
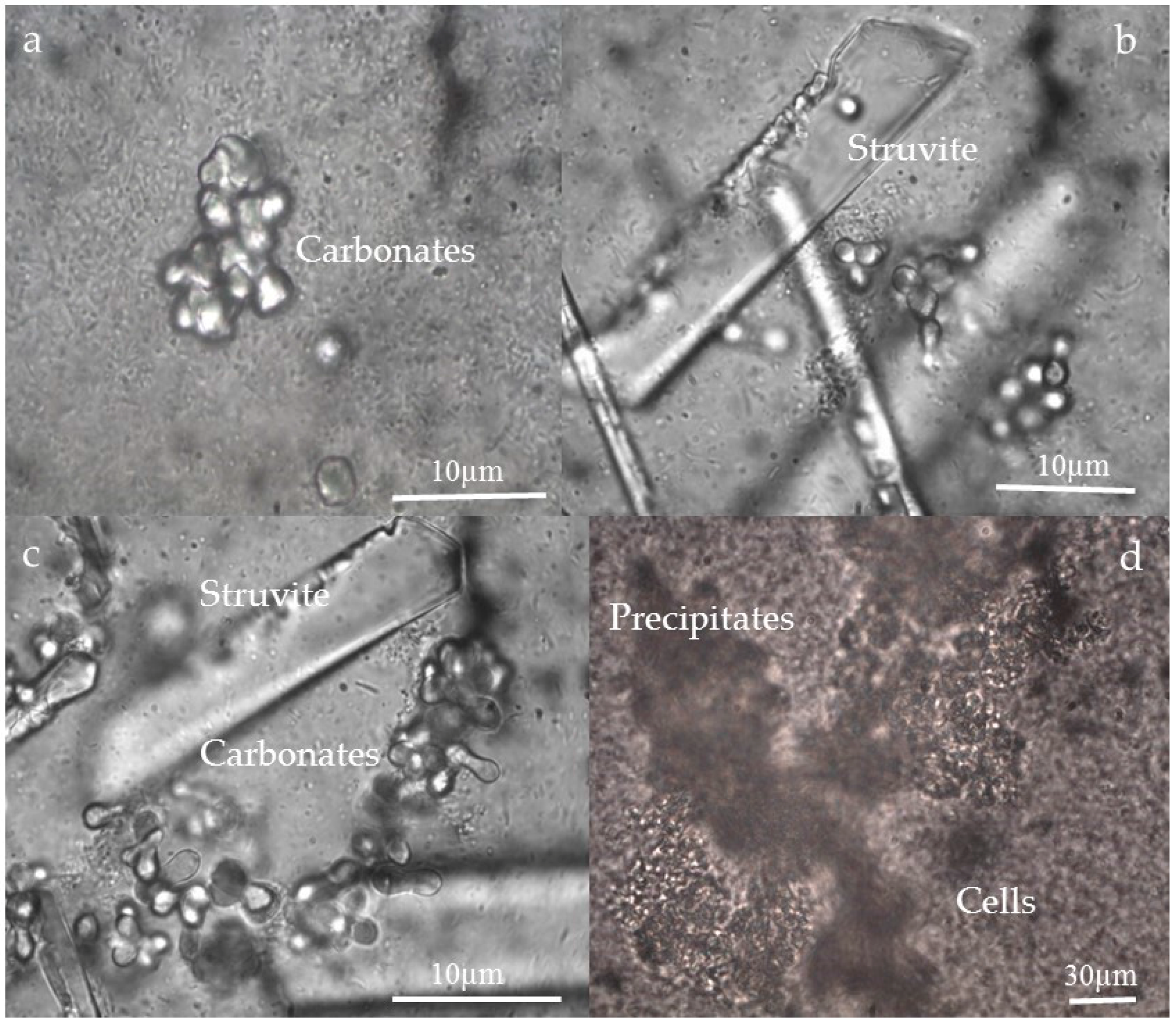
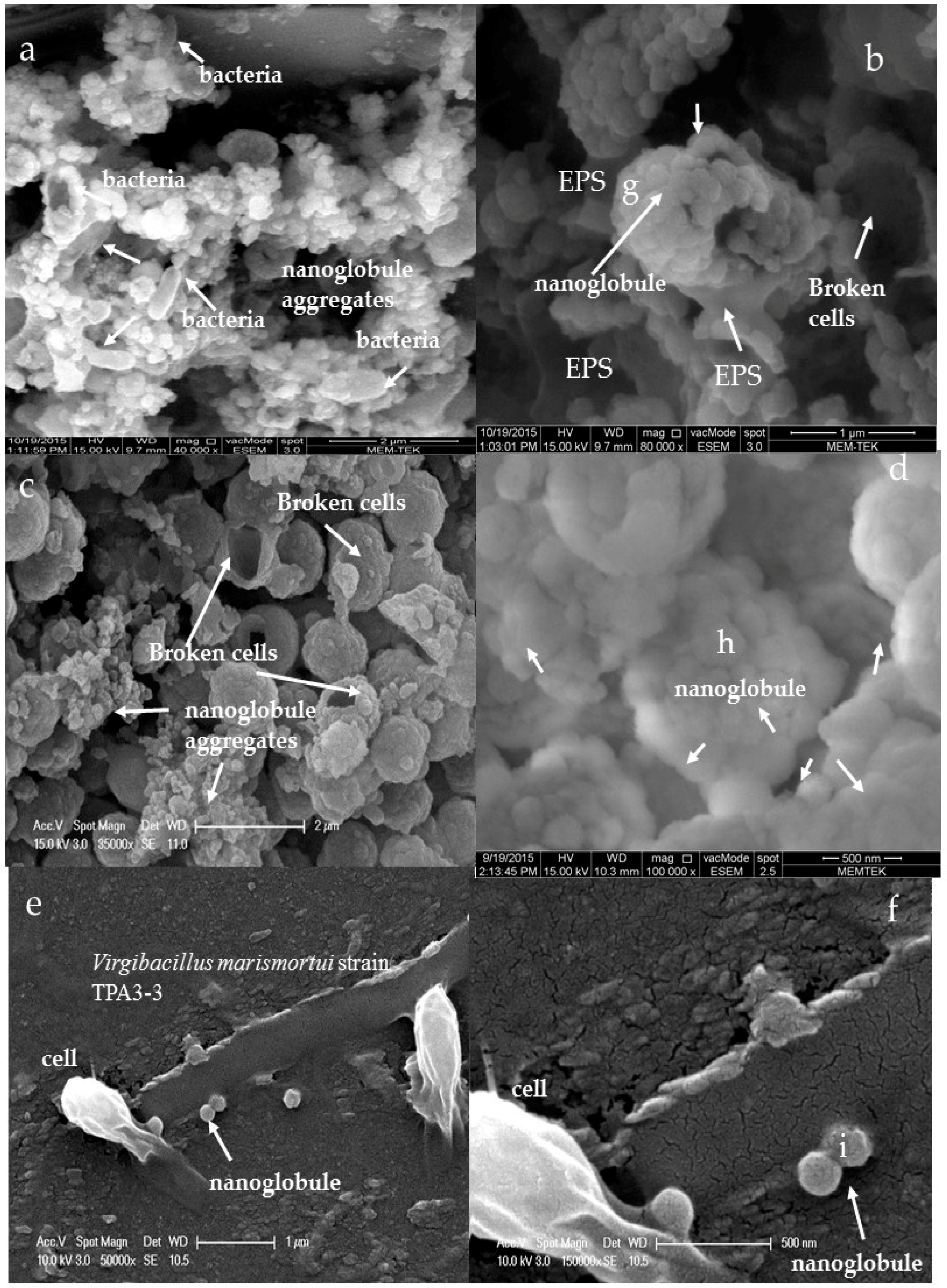
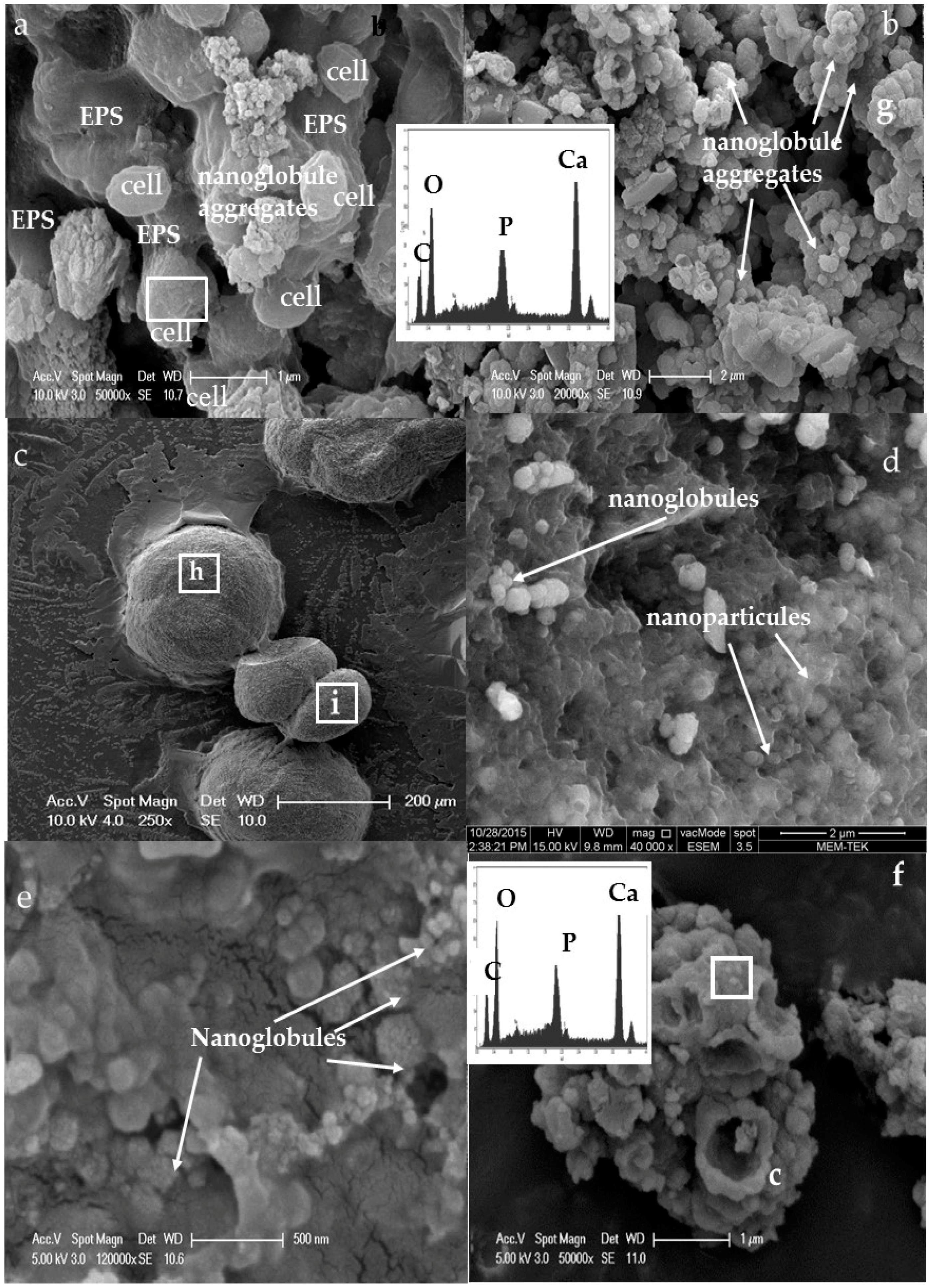
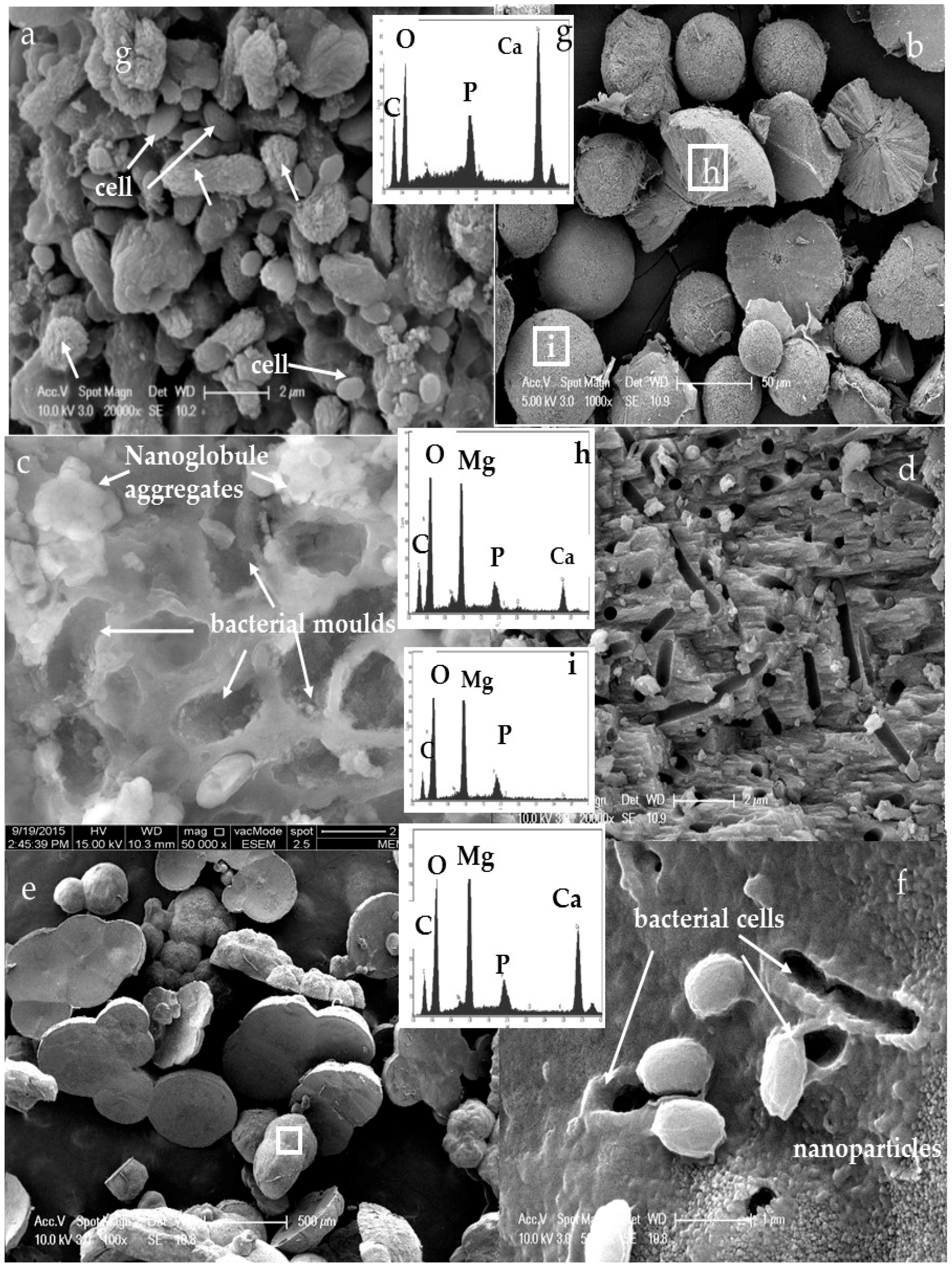
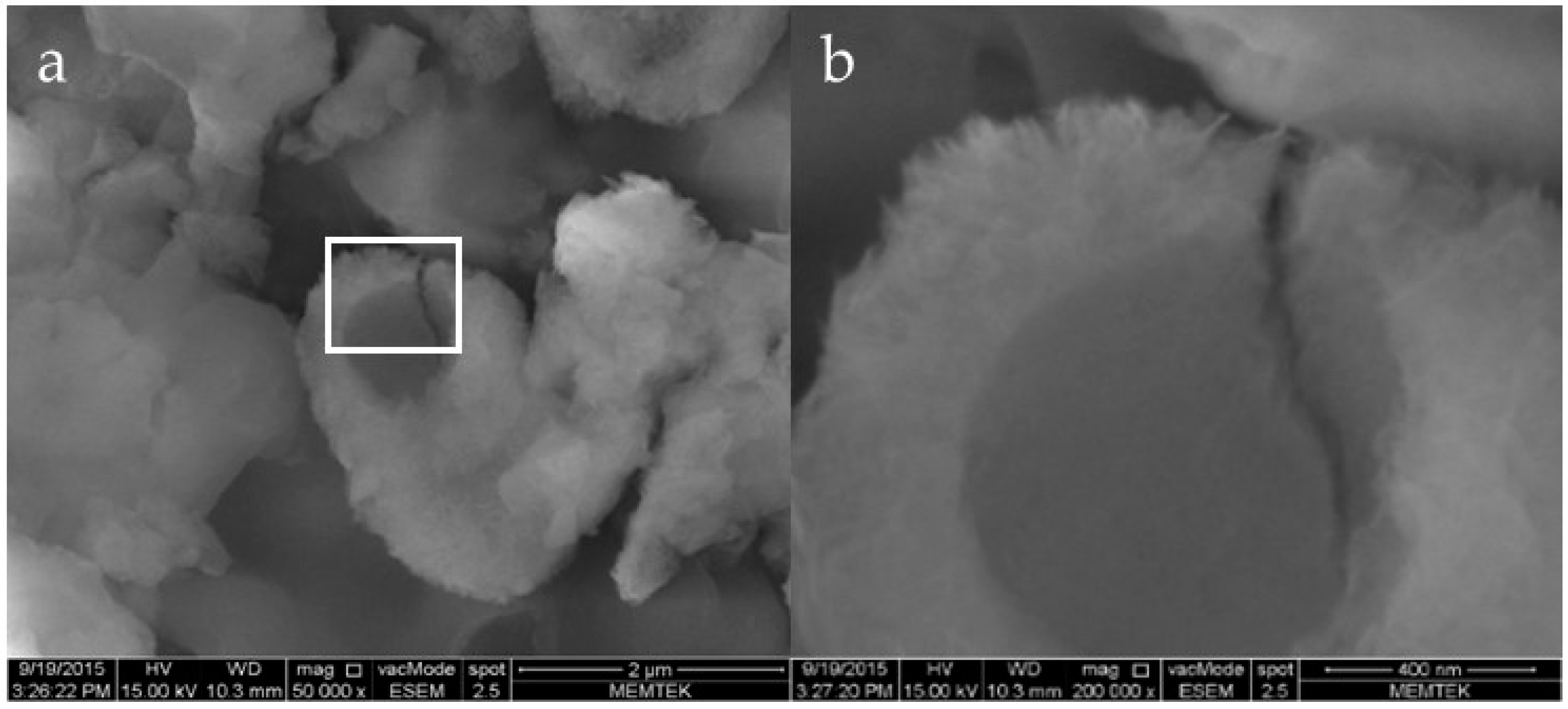
3.3. Morphology and Texture of Bioliths
4. Discussion
4.1. Bacterial Nucleation and Carbonate Precipitation
4.2. Influence of Salinity and Mg+2/Ca+2 Ratios on Bacterial Nanoglobule Formation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kastner, M. Control of dolomite formation. Nature 1984, 311, 410–411. [Google Scholar] [CrossRef]
- Ferrer, M.R.; Quevedo-Sarmiento, J.; Rivadeneyra, M.A.; Béjar, V.; Delgado, R.; Ramos-Cormenzana, A. Calcium carbonate precipitation by two groups of moderately halophilic microorganisms at different temperatures and salts concentrations. Curr. Microbiol. 1988, 17, 221–227. [Google Scholar] [CrossRef]
- Castanier, S.; Me’tayer-Levrel, G.L.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Vásconcelos, C.; McKenzie, J.A. Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Microbial fossilization in carbonate sediments: A result of the bacterial surface involvement in carbonate precipitation. Sedimentology 2003, 50, 237–245. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello, W.A.; Rivadeneyra, M.A.; Vásconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth Planet. Sci. Lett. 2009, 285, 131–139. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vásconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: Fifth Edition; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009; p. 589. [Google Scholar]
- Balci, N.; Menekşe, M.; Karagüler, N.; Sönmez, M.Ş.; Meister, P. Reproducing Authigenic Carbonate Precipitation in the Hypersaline Lake Acıgöl (Turkey) with Microbial Cultures. Geomicrobiol. J. 2016. [Google Scholar] [CrossRef]
- Balci, N.C. Effect of bacterial acivitiy on trace metals release from oxidation of sphalerite at low pH (<3) and implications for AMD environment. Environ. Earth Sci. 2010, 60, 485–493. [Google Scholar]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Precipitation of carbonates by Deleya halophila in liquid media: Pedological implications in saline soils. Arid Soil Res. Rehabil. 1997, 11, 35–49. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–286. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Paraga, J.; Delgado, G.; Ramos-Coemenzana, A.; Delgado, G. Biomineralization of carbonates by Halobacillus trueperi in solid and liquid media with different salinities. FEMS Microbiol. Ecol. 2004, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, M.A.; Martín-Algarra, A.; Sánchez-Román, M.; Sánchez-Navas, A.; Martín-Ramos, D. Amorphous Ca phosphate precursors for Ca-carbonate biominerals mediated by Chromohalobacter marismortui. ISME J. 2010, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, M.A.; Martín-Algarra, A.; Sánchez-Navas, A.; Martín-Ramos, D. Carbonate and phosphate precipitation by Chromohalobacter marismortui. Geomicrobiol. J. 2006, 23, 1–13. [Google Scholar] [CrossRef]
- Bosak, T.; Newman, D.K. Microbial nucleation of calcium carbonate in the Precambrian. Geology 2003, 31, 577–580. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A.; Berrnasconi, S.; Grujic, D.; Tien, A.J. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Warthmann, R.; van Lith, Y.; Vásconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vásconcelos, C.; McKenzie, J.A. Carbonate and phosphate precipitation by halophilic bacteria: Influence of Ca and Mg ions. FEMS Microbiol. Ecol. 2007, 6, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.; Dupraz, C.; Bernasconi, S.M.; McKenzie, J.A. Microbes produce nanobacteria-like structures, avoiding cell entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Aloisi, G.; Gloter, A.; Krüger, M.; Wallmann, K.; Guyot, F.; Zuddas, P. Nucleation of calcium carbonate on bacterial nanoglobules. Geology 2006, 34, 1017–1020. [Google Scholar] [CrossRef]
- Sanchez-Roman, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Bundeleva, I.A.; Shirokova, L.; Pokrovsky, O.S.; Bénézeth, P.; Ménez, B.; Gérard, E.; Balor, S. Experimental modeling of calcium carbonate precipitation by cyanobacterium Gloeocapsa sp. Chem. Geol. 2014, 374–375, 44–60. [Google Scholar] [CrossRef]
- Bundeleva, I.A.; Shirokova, L.; Bénézeth, P.; Pokrovsky, O.S.; Kompantseva, E.I.; Balor, S. Calcium carbonate precipitation by anoxygenic phototrophic bacteria. Chem. Geol. 2012, 291, 116–131. [Google Scholar] [CrossRef]
- Roberts, J.A.; Bennett, P.C.; Gonzáles, L.A.; Macpherson, G.L.; Milliken, K.L. Microbial precipitation of dolomite in methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldsteın, R.H.; Gonzalez, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Helvacı, C.; Alçiçek, C.; Gündoğan, İ.; Gemici, U. Tectonosedimentary Development and Palaeoenvironmental Changes in the Acıgöl Shallow-Perennial Playa-Lake Basin, SW Anatolia, Turkey. Turkish J. Earth Sci. 2012, 21, 1–20. [Google Scholar]
- Mutlu, H.; Kadir, S.; Akbulut, A. Mineralogy and water chemistry of the Lake Acıgöl (Denizli), Turkey. Carbonates Evaporites 1999, 14, 91–99. [Google Scholar]
- Schneegurt, M.A. Media and conditions for the growth of halophilic and halotolerant bacteria and archaea. In Advances in Understanding the Biology of Halophilic Microorganisms; Vreeland, R.H., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 35–58. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis. Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 894–895. [Google Scholar]
- Hillier, S. Accurate quantitative analysis of clay and other minerals in sandstones by XRD: Comparison of a Rietveld and a reference intensity ratio (RIR) method and the importance of sample preparation. Clay Miner. 2000, 35, 291–302. [Google Scholar] [CrossRef]
- Parkhurst, D.; Appelo, C.A.J. User’s Guide to PhreeqC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report; 99-4259; U.S. Department of the Interior: Denver, CO, USA, 1999.
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; San Martin-Uriz, P. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Román, M.; Puente-Sánchez, F.; Parro, V.; Amils, R. Nucleation of Fe-rich phosphates and carbonates on microbial cells and exopolymeric substances. Front. Microbial. 2015. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.W. The kinetics of calcium carbonate dissolution and precipitation. In Reviews in Mineralogy: Carbonates-Mineralogy and Chemistry; Reeder, R.J., Ed.; Mineralogical Society of America: Chelse, MA, USA, 1983; pp. 227–264. [Google Scholar]
- Moore, C.H. The nature of carbonate depositional systems-comparison of carbonates and silici clastics. In Developments in Sedimentology; Moore, C.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 1–19. [Google Scholar]
- Rivadeneyra, M.A.; Delgado, G.; Soriano, M.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Marinococcus albus and Marinococcus halophilus isolated from the Salar de Atacama (Chile). Curr. Microbiol. 1999, 39, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. Growth and form of spherulites. Phys. Rev. Lett. E 2005, 72, 011605. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, J.P.; Flaten, E.M.; Beck, R.; Lewis, A.E. Investigations of spherulitic growth in industrial crystallization. Chem. Eng. Res. Des. 2010, 88, 1163–1168. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in water-alcohol mixtures: Spherulitic growth, polymorph stabilization and morphology change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallisation of monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. A route for the direct crystallization of dolomite. Am. Mineral. 2015, 100, 1172–1181. [Google Scholar] [CrossRef]
- Cisar, J.O.; Xu, D.-Q.; Thompson, J.; Swaim, W.; Hu, L.; Kopecko, D.J. An alternative interpretation of nanobacteria-induced biomineralization. Proc. Natl. Acad. Sci. USA 2000, 97, 11511–11515. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Menguy, N.; Guyot, F.; Skouri, F.; de Luca, G.; Barakat, M.; Heulin, T. Biologically controlled precipitation of calcium phosphate by Ramlibacter tataouinensis. Earth Planet. Sci. Lett. 2004, 228, 439–449. [Google Scholar] [CrossRef]
- Krause, S.; Liebetrau, V.; Goeb, S.; Sánchez-Román, M.; McKenzie, J.A.; Treude, T. Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern sea water salinity: New insight into an old enigma. Geology 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Folk, R.L. Nanobacteria and the precipitation of carbonates in unusual environments. Sediment. Geol. 1999, 126, 47–56. [Google Scholar] [CrossRef]
- Folk, R.L.; Chafetz, H.S. Bacterially induced microscale and nanoscale carbonate precipitates. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin, Germany, 2000; pp. 40–49. [Google Scholar]
- Dupraz, C.; Visscher, P.T.; Baumgartner, L.K.; Reid, R.P. Microbe–mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2004, 51, 745–765. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; López-García, P.; Yoon, T.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E. Nanoscale detection of organic signatures in carbonate microbiolites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
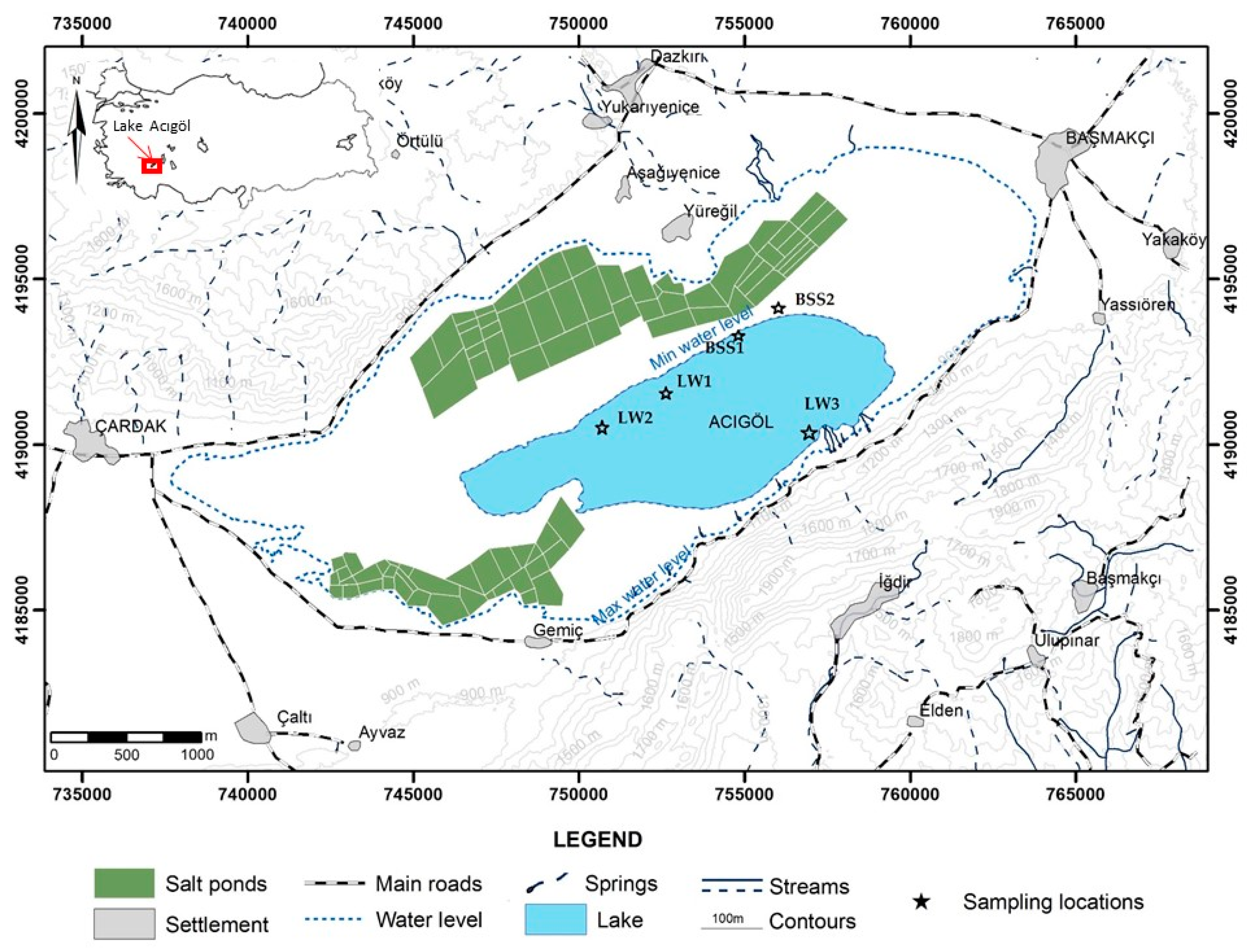
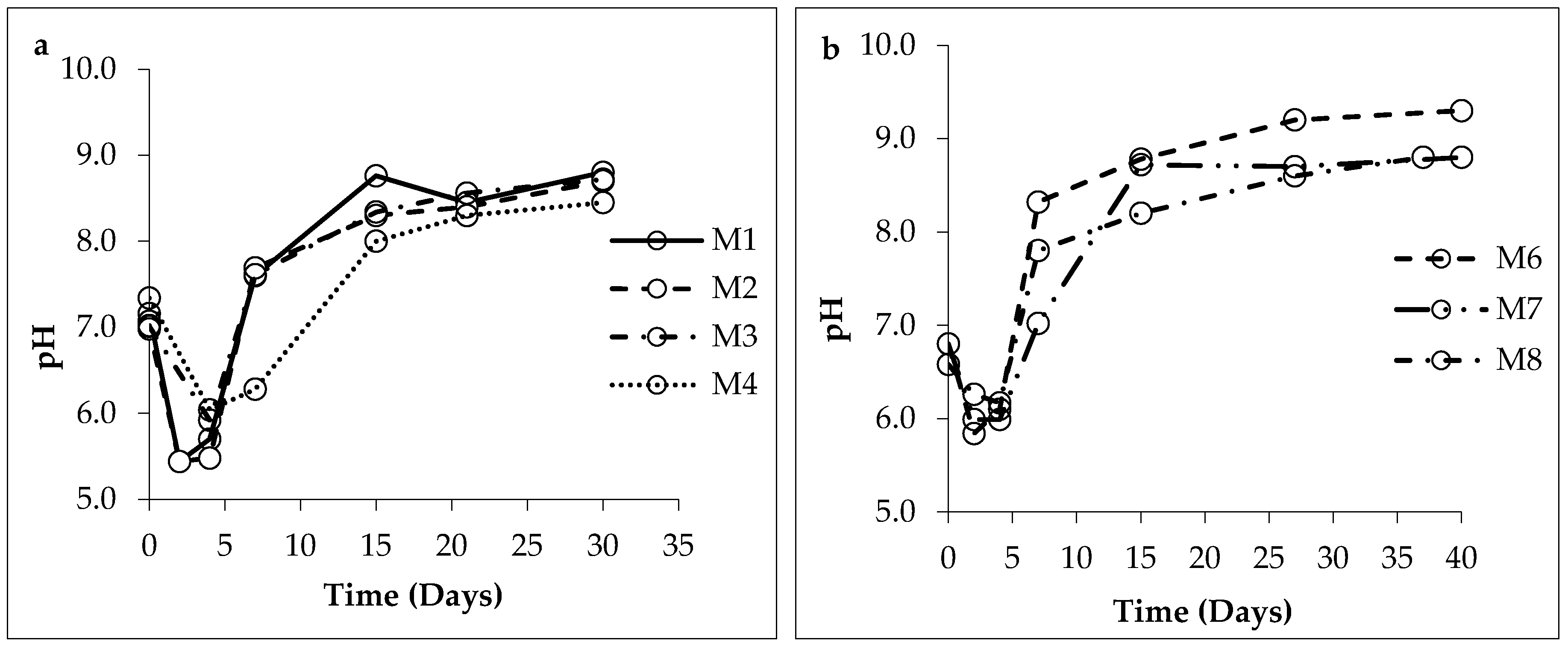
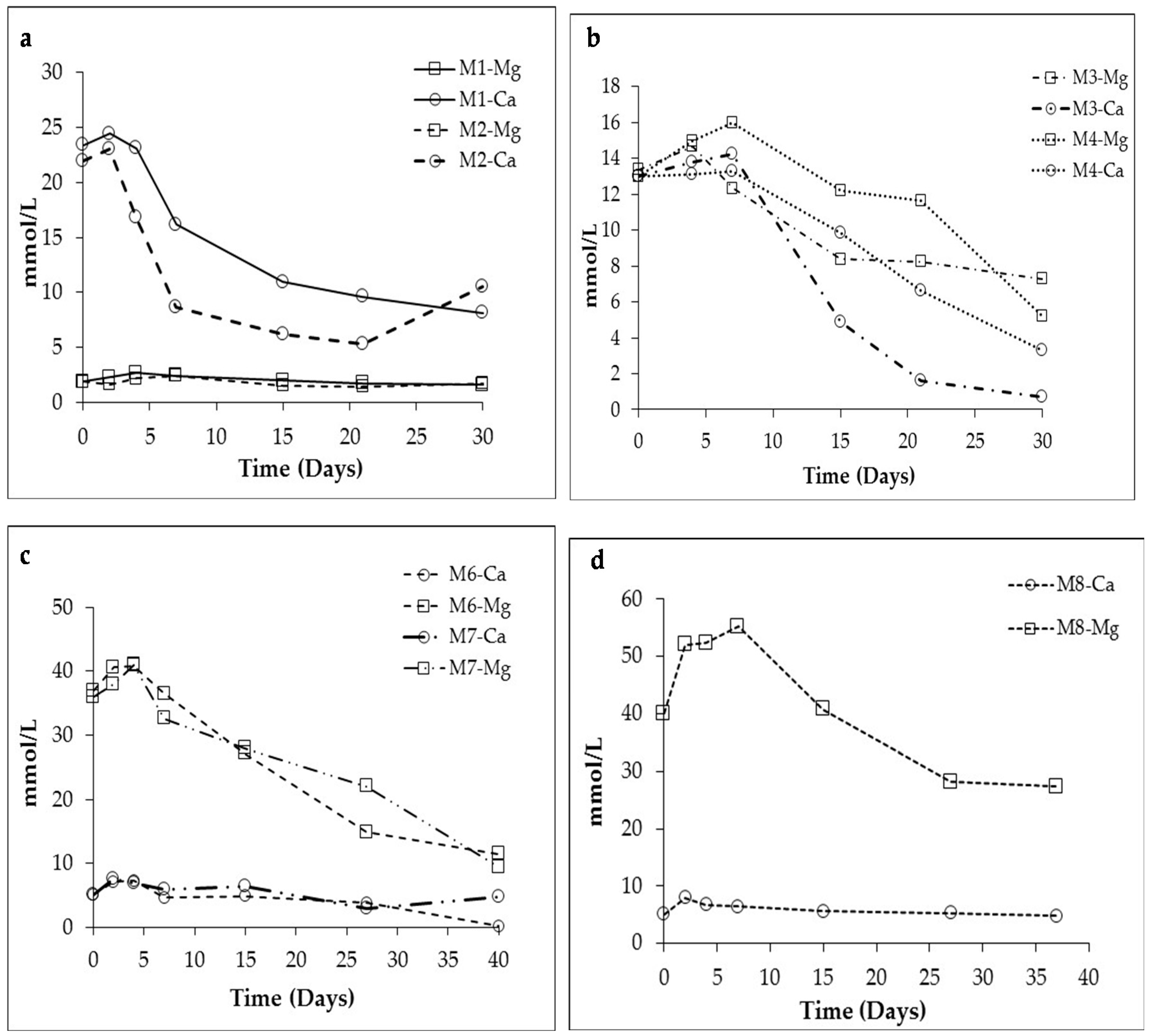
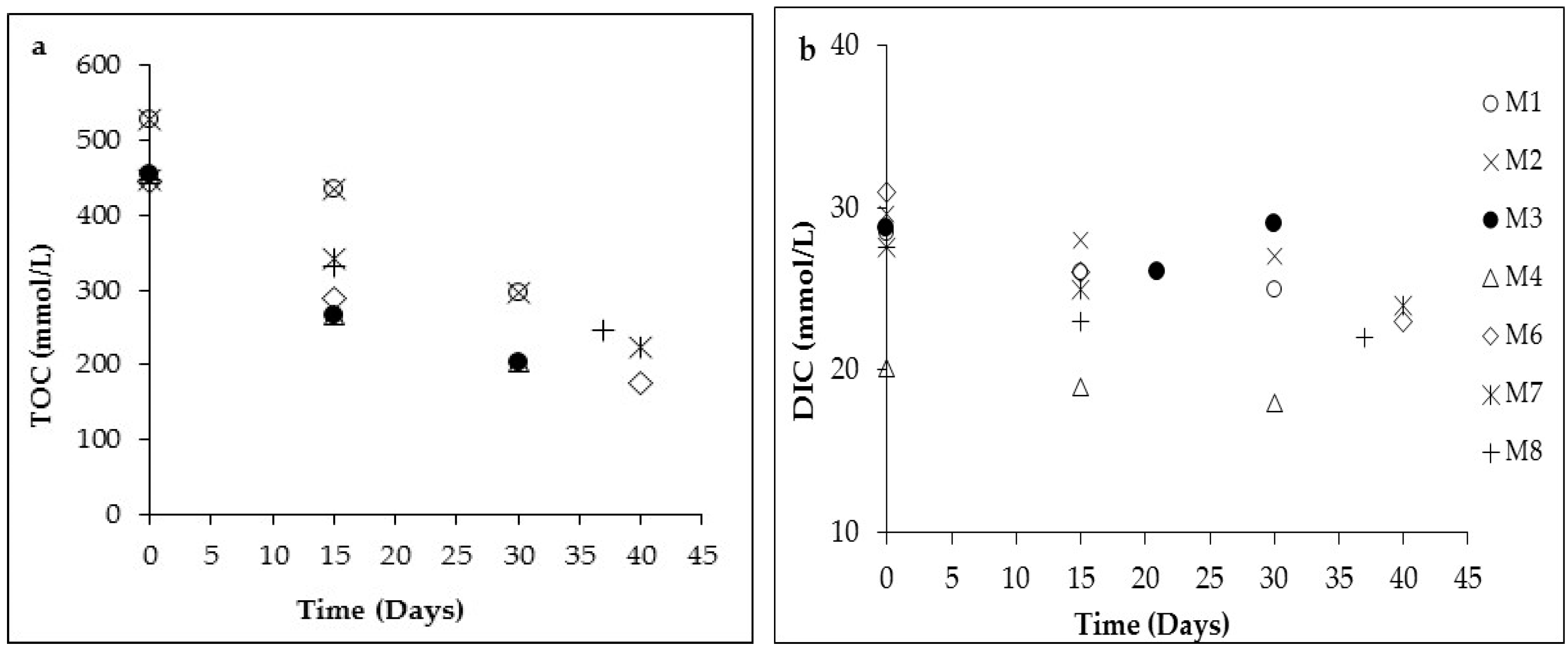
| Sample | LW1 a | LW2 | LW3 | BSS1 b | BSS2 |
|---|---|---|---|---|---|
| pH | 8.2 | 8.5 | 8.6 | 8.3 | 8.7 |
| T (°C) | 22 | - | 29 | 24 | 32 |
| EC (µS/cm) | 62,100 | 58,200 | 85,200 | 95,230 | 123,532 |
| CI− (mmol/L) | 1131.4 | 672 | 1214.2 | 4071.4 | 1578.5 |
| SO42− (mmol/L) | 100 | 89.58 | 130.2 | 820.9 | 891.6 |
| Mg+2 (mmol/L) | 138.5 | 57.3 | 123.3 | 120.4 | 135.4 |
| Na+ (mmol/L) | 963.6 | 511.3 | 1165 | 1361.9 | 840.9 |
| K+ (mmol/L) | 22.1 | 13.6 | 24.6 | 11.5 | 22.82 |
| Ca+2 (mmol/L) | 17.25 | 14 | 30 | 13.25 | 18.7 |
| Salinity (g/L) | 70 | n.d. | 75 | n.d | 82 |
| CO32− (mmol/L) | - | 1.8 | - | - | - |
| HCO3− (mmol/L) | n.d. | 12.4 | 19.2 | n.d | n.d |
| Sample | Ca+2 (mmol/L) | Mg+2 (mmol/L) | Mg+2/Ca+2 | Ca+2 +Mg+2 (total) (mmol/L) | pH | Salinity (g/L) | T (°C) |
|---|---|---|---|---|---|---|---|
| M1 | 23 | 2 | 0.1 | 25 | 7.2 | 80 | 30 |
| M2 | 23 | 2 | 0.1 | 25 | 7.2 | 150 | 30 |
| M3 | 13 | 13 | 1.0 | 26 | 7.2 | 80 | 30 |
| M4 | 13 | 13 | 1.0 | 26 | 7.2 | 150 | 30 |
| M5 * | 10 | 40 | 4.0 | 50 | 7.2 | 80 | 10 |
| M6 | 5 | 36 | 7.2 | 41 | 7.2 | 80 | 30 |
| M7 | 5 | 36 | 7.2 | 41 | 7.2 | 150 | 30 |
| M8 | 5 | 40 | 8.0 | 45 | 7.2 | 80 | 30 |
| Experiment | Identified Minerals |
|---|---|
| M1 | CAP (83 *) |
| M2 | CAP (84) |
| M3 | C (12), D (2), S (80.3) |
| M4 | C (10), D (6), S (84) |
| M5 | No data |
| M6 | C (40.2), D (14.7), MHC (31.2), S (4.4) |
| M7 | C (14), D (12), HM (33.7), S (36.7) |
| M8 | MC (7.3), D (23), HM (38), S (21) |
| Mineral Phase | M1 | M2 | M3 | M4 | M6 | M7 | M8 |
|---|---|---|---|---|---|---|---|
| Aragonite | 0.38 | 0.57 | −1.9 | 0.34 | 0.06 | −0.56 | −0.02 |
| Calcite | 0.52 | 0.71 | −1.76 | −0.48 | −0.23 | −0.39 | 0.15 |
| Dolomite(disordered) | 0.02 | 0.03 | −0.32 | −0.37 | 1.07 | 0.87 | 1.11 |
| Halite | −1.65 | −0.96 | −4.58 | −0.96 | −1.36 | −0.34 | −1.35 |
| Huntite | −4.65 | −3.71 | −11.45 | −1.42 | 0.01 | −0.65 | 0.3 |
| Hydromagnesite | −15.75 | −14.63 | −21.96 | −10.05 | −7.27 | −4.96 | −6.57 |
| Hydroxyapatite | 14.23 | 14.9 | 1.51 | 14.42 | 11.43 | 8.45 | 11.23 |
| Magnesite | −0.87 | −0.63 | −0.48 | −0.60 | 0.07 | 0.49 | 0.19 |
| Monohydrocalcite | −0.44 | −0.56 | −0.68 | −0.98 | −1.19 | −1.24 | −1.14 |
| Nesquehonite | −3.36 | −3.19 | −4.8 | −2.35 | −2.66 | −2.32 | −2.54 |
| Struvite | −0.05 | 0.05 | −0.42 | −0.36 | −0.56 | −0.61 | −0.74 |
| Experiment | Media Composition | Microbial Strains | Nanoglobules | Precipitated Minerals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current Study | Ca2+ mmol/L | Mg2+ mmol/L | Mg+2/Ca+2 mmol/L | Na+ mmol/L | K+ mmol/L | SO42− mmol/L | PO43− mmol/L | Salt (%) | pH | T (°C) | Oxic/anoxic | Liquid | Agar | |||
| Halomonas saccharevitans strain AJ275 | ||||||||||||||||
| M1 | 23 | 2 | 0.1 | 8 | 7.2 | 30 | O | X | X | Idiomarina sp. TBZ29, 98% Idiomarina seosensis strain CL-SP19 | No | Capatite | ||||
| M2 | 23 | 2 | 0.1 | 15 | 7.2 | 30 | O | X | X | Virgibacillus marismortui | No | Capatite | ||||
| M3 | 13 | 13 | 1 | 8 | 7.2 | 30 | O | X | X | Bacillus selenitireducens strain M1S6-17 | Yes | Aragonite, Calcite, Dolomite, Struvite | ||||
| M4 | 13 | 13 | 1 | 15 | 7.2 | 30 | O | X | Halomonas saccharevitans strain AJ275 | Yes | Calcite, Dolomite, Struvite | |||||
| M5* | 10 | 40 | 4 | 8 | 7.2 | 10 | O | X | Salinicoccus roseus strain DSM 5351 | no data | no data | |||||
| M6 | 5 | 36 | 7.2 | 8 | 7.2 | 30 | O | X | X | Halomonas alimentaria strain L7B | Yes | Calcite, Dolomite, Monohydrocalcite, Struvite | ||||
| M7 | 5 | 36 | 7.2 | 15 | 7.2 | 30 | O | X | X | Planococcus maritimus isolate LLQ | Yes | Calcite, Dolomite, Hydromagnesite, Struvite | ||||
| M8 | 5 | 56 | 11.2 | 8 | 7.2 | 30 | O | X | X | Planococcus rifietoensis strain M8 | Yes | Magnesian Calcite, Dolomite Hydromagnesite, Struvite | ||||
| Krause et al. [48] | 10 | 54 | 5 | 470 | 10 | 28 | 35 | 21 | A | X | Desulfobulbus mediterraneus | Yes | Dolomite | |||
| Sánchez-Román et al. [34] | 0.25 | 6.0 | 30 | A | X | Tessaracoccuslapidicaptus CECT8385 | Yes | Siderite and Vivianite | ||||||||
| Sánchez-Román et al. [33] | 3.5 | 35 | A | X | X | Acidiphilium sp. PM | Yes | Siderite | ||||||||
| Sánchez-Román et al. [22] | 7.2 | 25-35 | O | X | Halomonas meridiana ACAM 246 | Yes | Dolomite, Hydromagnesite | |||||||||
| Virgibacillus marismortui AJ009793 | ||||||||||||||||
| Alois et al. [21] | 13 | 80 | 6.2 | 737 | 7.2 | 10 | 0.9 | 8 | 30 | A | X | Desulfonatronum lacustre | Yes | Calcite | ||
| 0 | 0.49 | 0.49 | 312 | 7.2 | 35.2 | 1.6 | 8 | 30 | A | X | Desulfonatronum lacustre | No | ||||
| Roberts et al. [25] | 3.71 | 1.34 | 0.43 | 0.3 | 0.3 | 0.01 | 6.9 | A | X | Methanogenic species | Yes | Dolomite | ||||
| Benzerara et al. [46] | 3.63 | 30 | O | X | Ramlibacter tataouinensis, a B-proteobacterium | Yes | Calcium phosphate | |||||||||
| Warthmann et al. [18] | 10 | 8.0 | O | SRB LVform6 | ||||||||||||
| Desulfonatronovibrio hydrogenovorans | Yes | Dolomite | ||||||||||||||
| Vasconcelos et al. [17] | Lagoa Vermelha natural sediment incubation | 8 | 30 | A | X | Desulfovibrio group | Yes | Dolomite | ||||||||
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balci, N.; Demirel, C. Formation of Carbonate Nanoglobules by a Mixed Natural Culture under Hypersaline Conditions. Minerals 2016, 6, 122. https://doi.org/10.3390/min6040122
Balci N, Demirel C. Formation of Carbonate Nanoglobules by a Mixed Natural Culture under Hypersaline Conditions. Minerals. 2016; 6(4):122. https://doi.org/10.3390/min6040122
Chicago/Turabian StyleBalci, Nurgul, and Cansu Demirel. 2016. "Formation of Carbonate Nanoglobules by a Mixed Natural Culture under Hypersaline Conditions" Minerals 6, no. 4: 122. https://doi.org/10.3390/min6040122
APA StyleBalci, N., & Demirel, C. (2016). Formation of Carbonate Nanoglobules by a Mixed Natural Culture under Hypersaline Conditions. Minerals, 6(4), 122. https://doi.org/10.3390/min6040122





