Iron Recovery from Discarded Copper Slag in a RHF Direct Reduction and Subsequent Grinding/Magnetic Separation Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Theoretical Analysis
2.2.1. Reduction Mechanism of Fayalite
2.2.2. Recovery Mechanism of Zn
2.2.3. Reaction Mechanism of Additives
2.3. Experimental Procedure
3. Results and Discussions
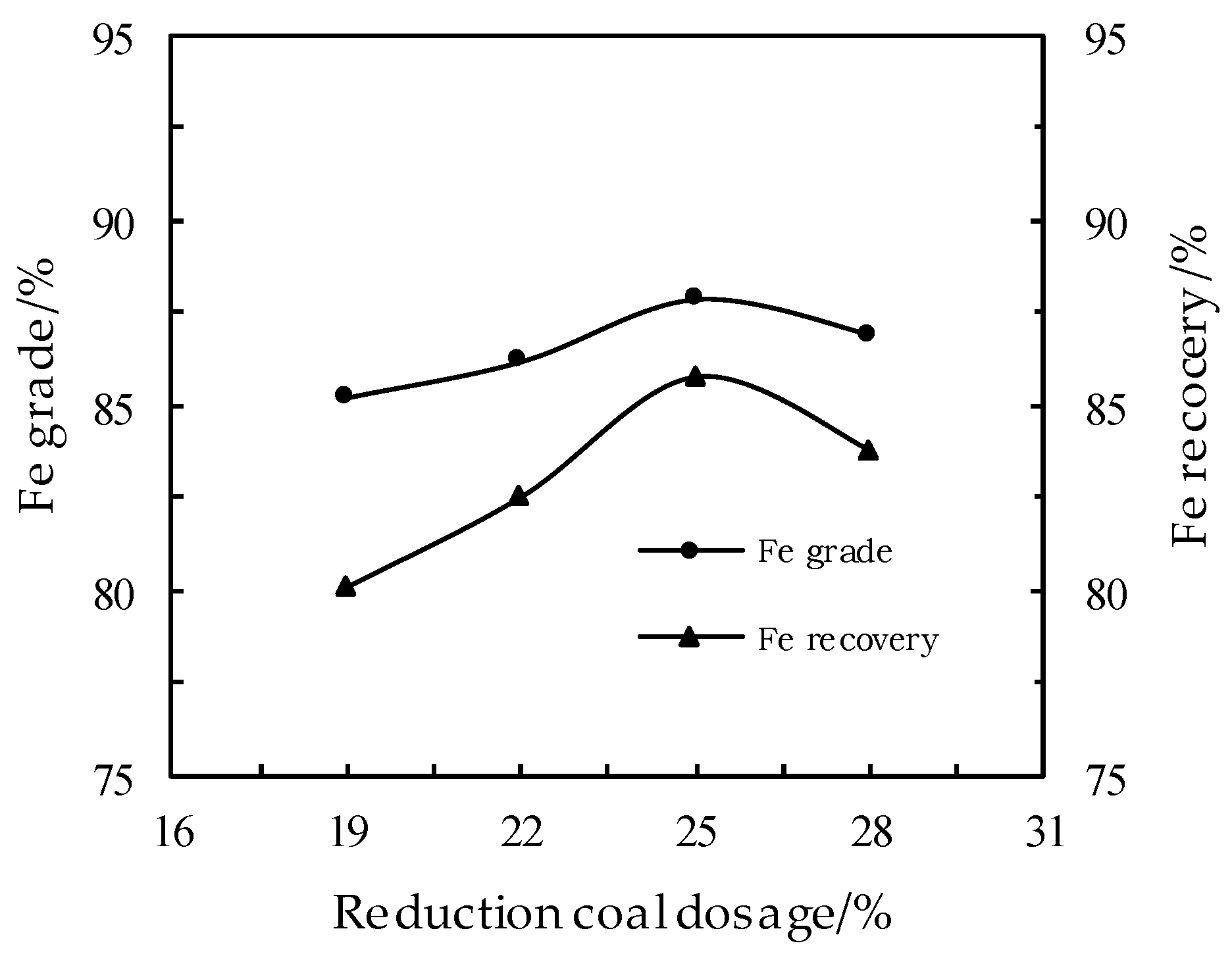
3.1. Effect of Reduction Coal Dosage on the DR Process
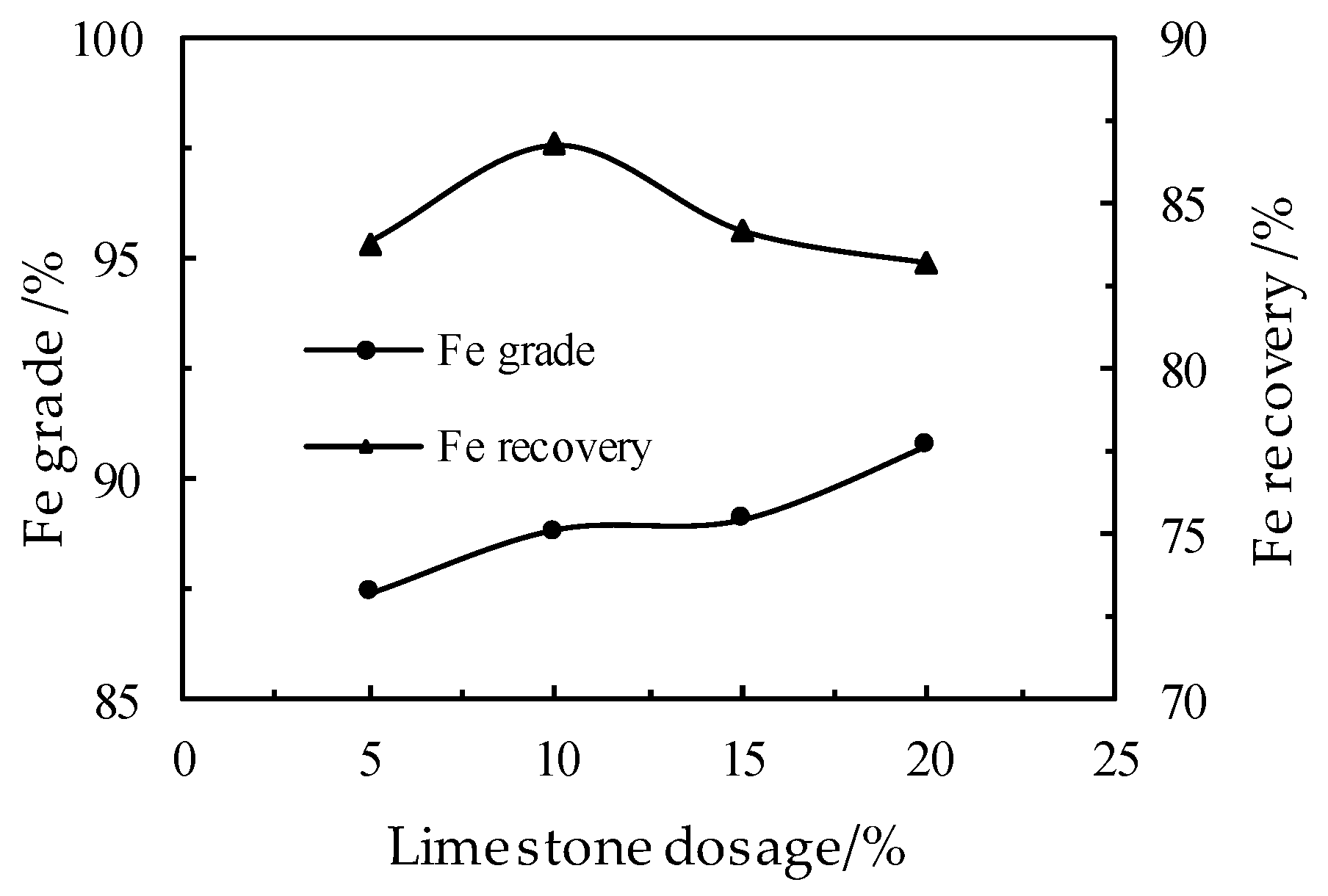
3.2. Effect of the Limestone Dosage on the DR Process
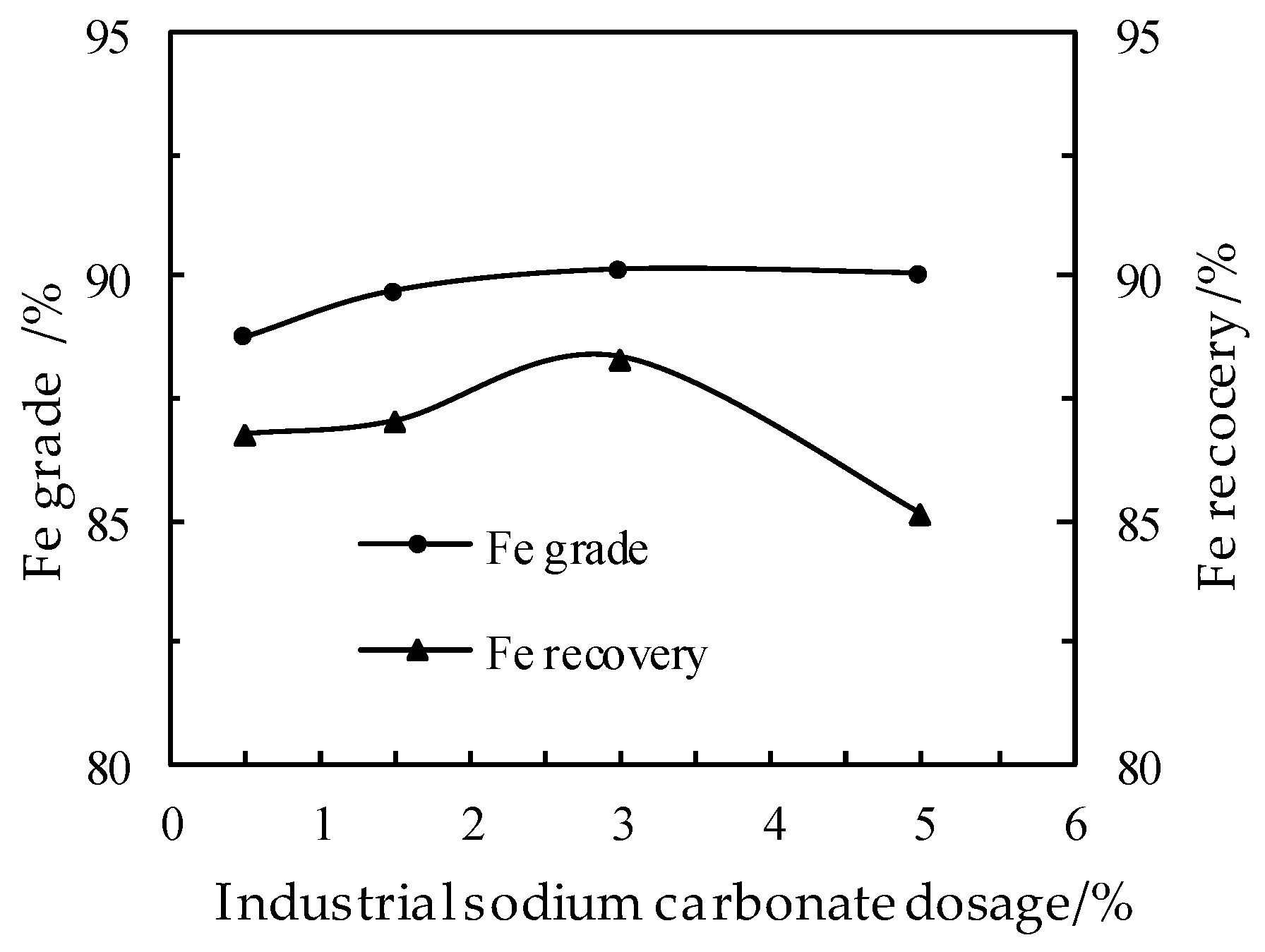
3.3. Effect of the Industrial Sodium Carbonate Dosage on the DR Process
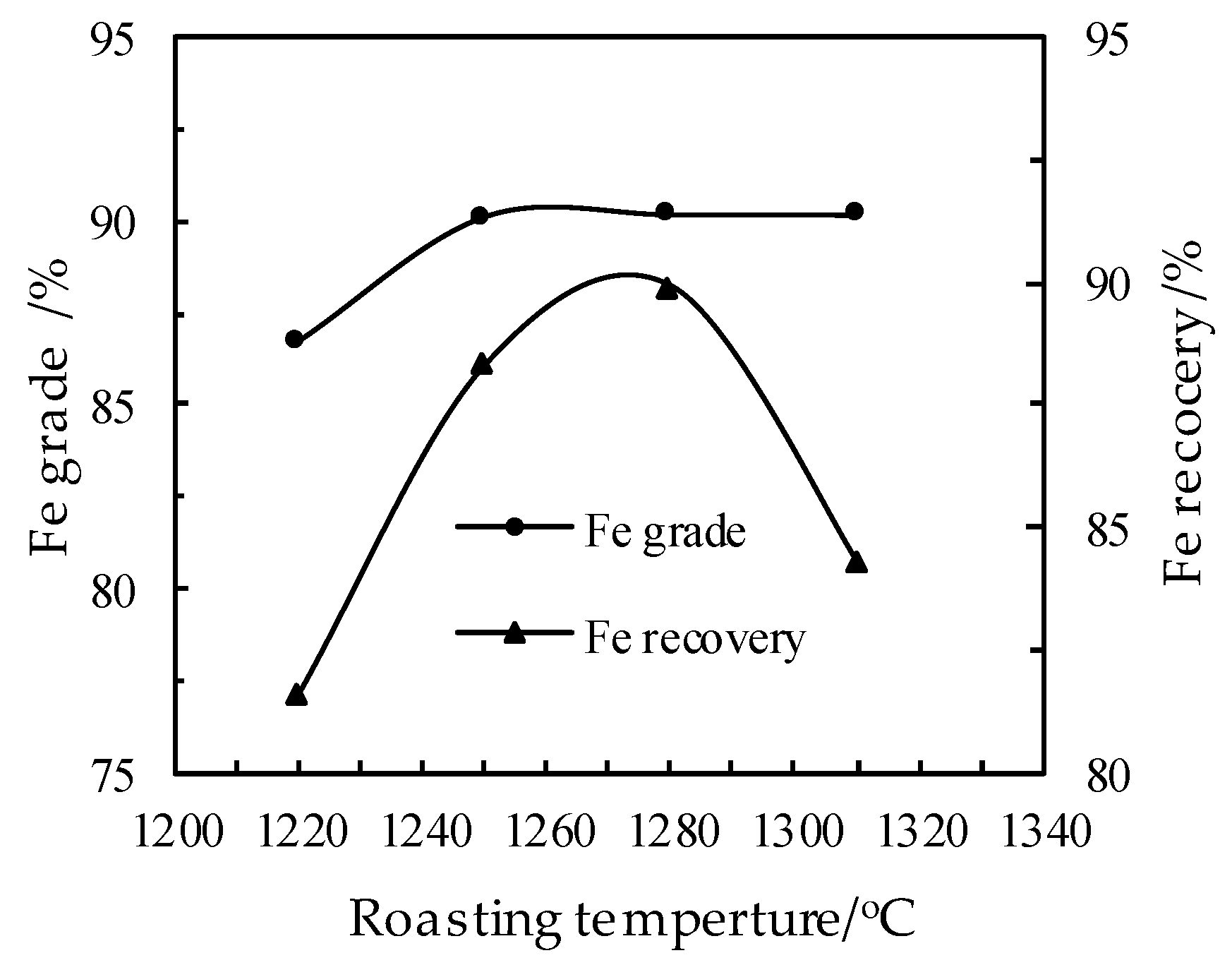
3.4. Effect of the Roasting Temperature on the DR Process
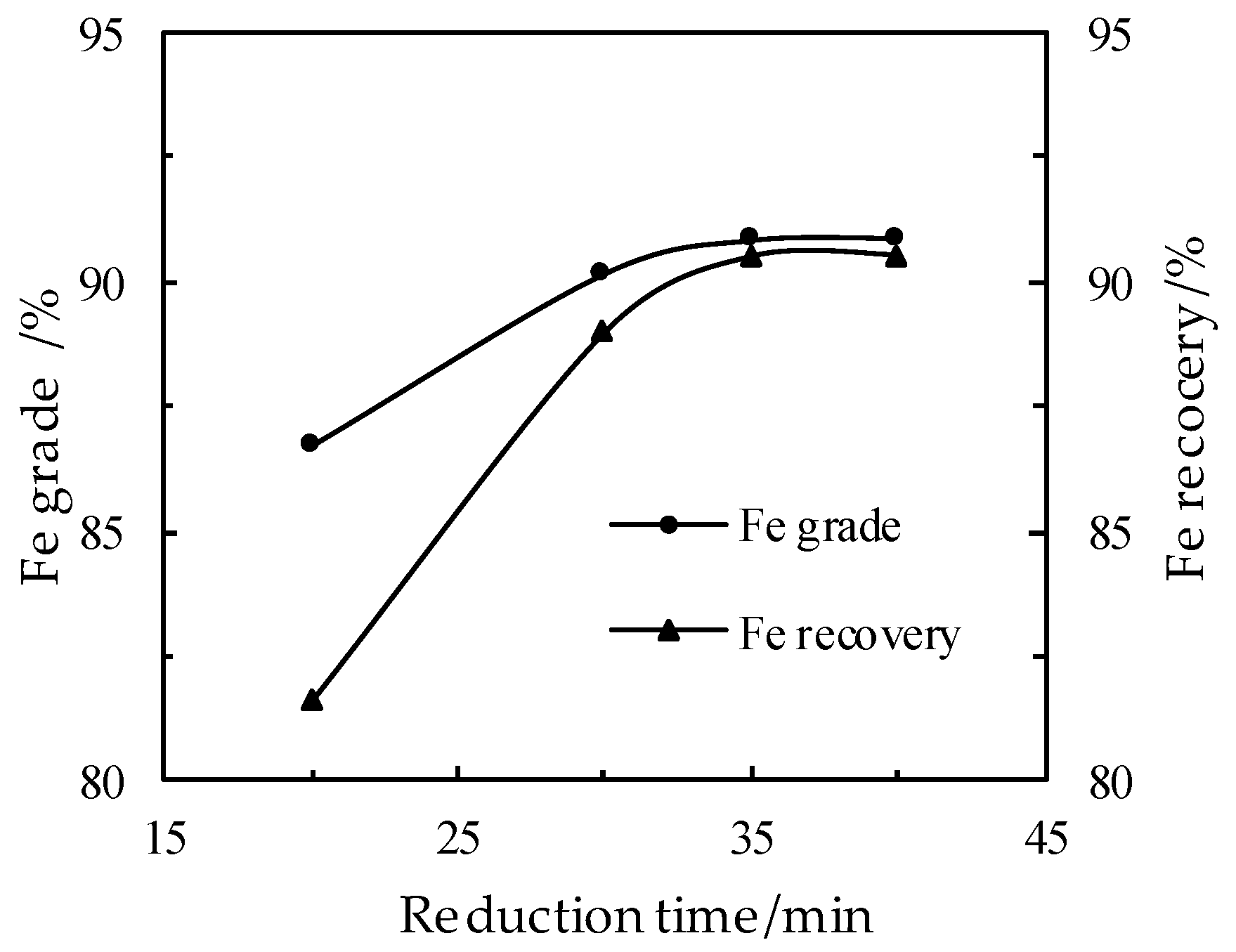
3.5. Effect of the Reduction Time on the DR Process
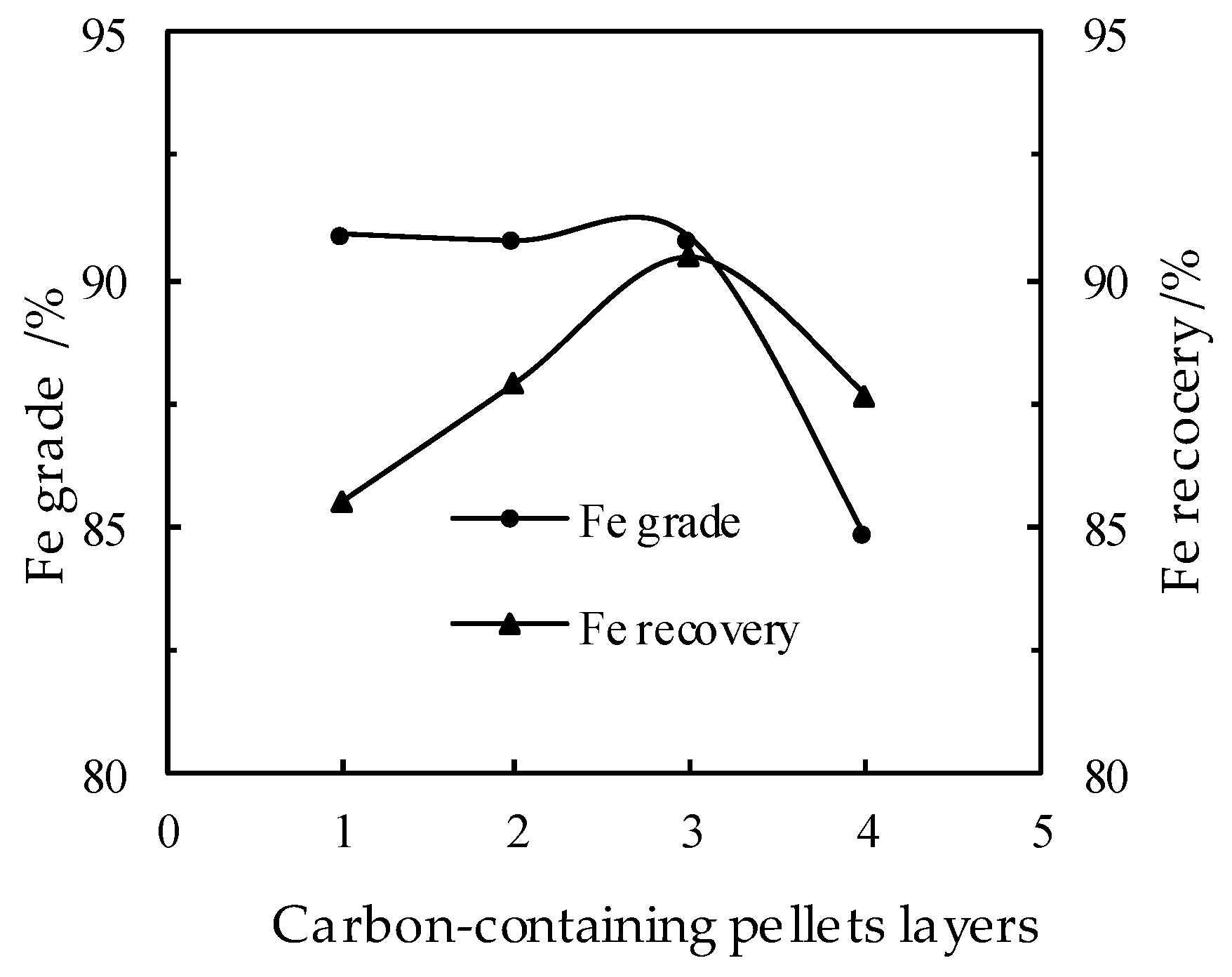
3.6. Effect of the Layers of the Carbon-Bearing Pellets on the DR Process
4. The Pilot-Scale Tests via RHF
5. Studies of Mechanisms and Product Analysis
5.1. Analysis of the Mechanism of the Distribution Thickness (Pellets Layers)
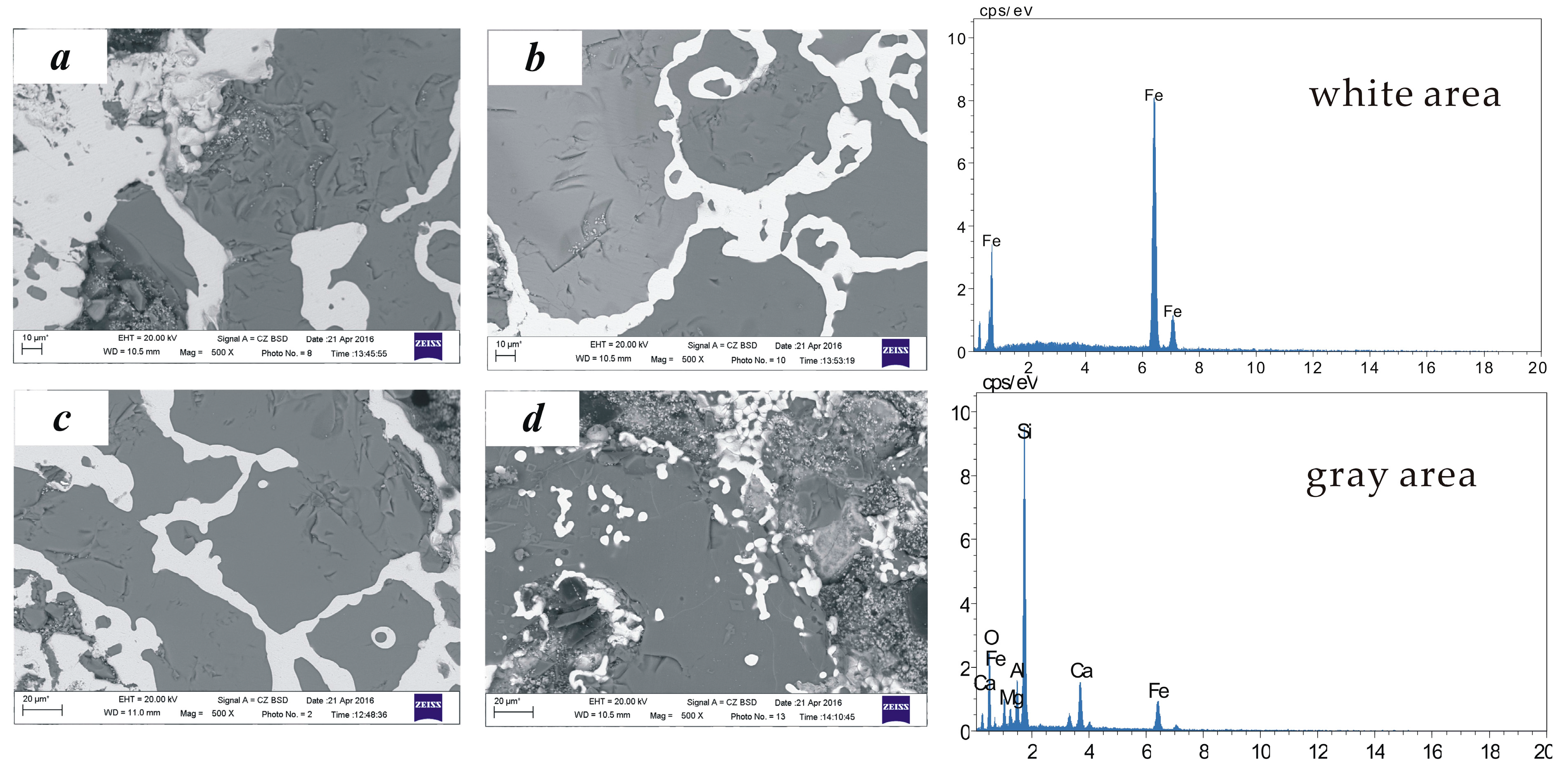
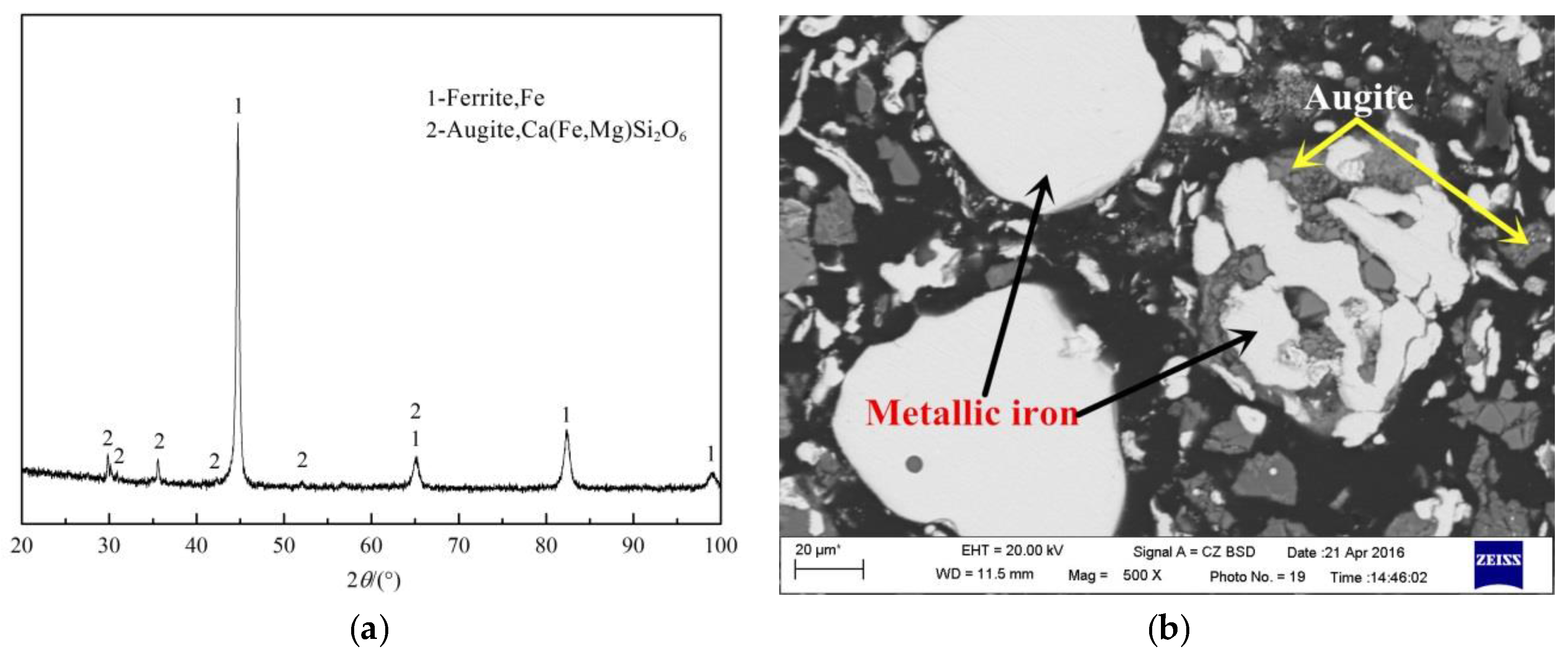
5.2. Product Analysis
6. Conclusions
- (1)
- Basic tests and pilot scale plant tests have been conducted on the discarded copper slag from a Chinese copper smelting plant, by adopting a direct reduction in a RHF and a subsequent grinding/magnetic separation process, from which optimal conditions are acquired as follows: the proportion of discarded copper slag, reduction coal, limestone and industrial sodium carbonates of 100:25:10:3, the reduction temperature of 1280 °C for the reduction time of 35 min, and the pellets in three layers (about 42 mm thick). The pilot-scale tests in a RHF have been carried out under such conditions and the obtained iron powder products have an iron grade of 90.35% with an iron recovery rate of 89.70%.
- (2)
- Analysis of the mechanism shows that fayalite and magnetite in the copper slag are reduced into metallic Fe in the direct reduction process, and the mass and heat transfer become stronger from the bottom layer to the top layer, exhibiting a higher diffraction peak. In addition, the iron in the metallization pellets turns into iron crystal chains from a dispersion state, resulting in a rising iron recovery rate.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, P.G.; Wu, P.F.; Hu, X.J.; Zhou, G.Z. Current state and research of comprehensive utilization of copper slag, and proposal of new technology. China Min. Mag. 2015, 25, 76–77. [Google Scholar]
- Zhao, K.; Chen, X.L.; Qi, Y.H.; Zhen, C.L.; Shuai, X.F. Study on impact factors in recovering iron and copper from copper slag by carbon reduction. Environ. Eng. 2012, 30, 76–79. [Google Scholar]
- Zeng, J.L.; Xiao, K.M. Research on using dispersant agent to iron recovery in furnace slag. Nonferr. Met. Sci. Eng. 2011, 2, 71–73. [Google Scholar]
- Hu, G.; Xu, S.P.; Li, S.G. Steam gasification of apricot stones with olivine and dolomite as downstream catalysts. Fuel Process. Technol. 2006, 87, 375–382. [Google Scholar] [CrossRef]
- Yang, T.; Hu, J.H.; Wang, H.; Li, L. Enrichment of Fe3O4 by roasting the dilution copper slag from the electric furnace smelting. Chin. J. Process. Eng. 2011, 11, 613–619. [Google Scholar]
- Yuan, S.Q.; Dong, J.; Wang, C.; Wang, Z.J. Study on processes for comprehensive treatment of extractive copper tailings and nickel smelting slag. Chin. J. Rare Met. 2014, 38, 108–114. [Google Scholar]
- Mansoor, B.; Keneths, C. Electrical and electronic conductivity of CaO-SiO2-FeOx slags at various oxygen potentials: Part 1. Experimental results. Met. Mater. Trans. 2006, 37, 41–49. [Google Scholar]
- Li, Z.K.; Wen, Y.X.; Su, J. Directive reducing of iron minerals from copper slag with anthracite as reductant. Inorg. Chem. Ind. 2014, 46, 51–55. [Google Scholar]
- Wang, Y.; Zhu, R.; Guo, Y.G.; Zhou, M.; Guo, M.W.; Zhou, C.F. Experimental study on the copper slag reduction process. Nonferr. Met. Sci. Eng. 2014, 5, 61–67. [Google Scholar]
- Yang, H.F.; Jing, L.L.; Dang, C.G. Iron recovery from copper-slag with lignite-based direct reduction followed by magnetic separation. Chin. J. Nonferr. Met. 2011, 21, 1165–1170. [Google Scholar]
- Wang, S.; Ni, W.; Wang, C.L.; Li, D.Z.; Wang, H.Y. Study of deep reduction process for iron recovery from copper slag. Met. Mine 2014, 453, 156–160. [Google Scholar]
- Ge, X.F.; Xu, A.J.; He, D.F.; Wang, H.B.; Tian, N.Y. Experimental research on the tunnel kiln process for processing stainless steel dust. J. Univ. Sci. Technol. Beijing 2012, 34, 859–866. [Google Scholar]
- Wei, R.F.; Li, J.X.; Long, H.M.; Wang, P.; Shi, S.Q. Quantitative study on bonding behavior during reduction of carbon bearing pellets. J. Iron Steel Res. Int. 2014, 26, 13–16. [Google Scholar]
- Gao, J.T.; Zhou, C.F.; Zhu, R.; Liu, R.Z. Research on the heat supply of different sections in a rotary hearth furnace. J. Univ. Sci. Technol. Beijing 2014, 4, 110–116. [Google Scholar]
- She, X.F.; Wang, J.S.; Han, Y.H.; Zhang, X.X.; Xue, Q.G. Comprehensive mathematical model for direct reduction processes in rotary hearth furnaces. J. Univ. Sci. Technol. Beijing 2014, 35, 1580–1587. [Google Scholar]


| Elements | TFe | FeO | Cu | CaO | MgO | SiO2 | Al2O3 | Na2O | K2O | Pb | Zn | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 40.78 | 38.52 | 0.25 | 1.74 | 2.08 | 33.20 | 2.29 | 0.40 | 0.61 | 0.51 | 1.35 | 0.19 |
| Elements | Tfe | Mfe | CaO | MgO | SiO2 | Al2O3 | Cu | C | Mn | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 91.03 | 90.20 | 0.14 | 0.67 | 0.40 | 1.87 | 0.35 | 0.61 | 0.07 | 0.02 | 0.08 |
| Elements | TFe | CaO | MgO | SiO2 | Al2O3 | K2O | Na2O | PbO | ZnO | C | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 1.84 | 0.84 | 3.21 | 1.47 | 0.60 | 0.54 | 0.17 | 14.21 | 68.53 | 0.11 | 3.54 | 0.13 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Sun, T.; Xue, X.; Liu, Z. Iron Recovery from Discarded Copper Slag in a RHF Direct Reduction and Subsequent Grinding/Magnetic Separation Process. Minerals 2016, 6, 119. https://doi.org/10.3390/min6040119
Cao Z, Sun T, Xue X, Liu Z. Iron Recovery from Discarded Copper Slag in a RHF Direct Reduction and Subsequent Grinding/Magnetic Separation Process. Minerals. 2016; 6(4):119. https://doi.org/10.3390/min6040119
Chicago/Turabian StyleCao, Zhicheng, Tichang Sun, Xun Xue, and Zhanhua Liu. 2016. "Iron Recovery from Discarded Copper Slag in a RHF Direct Reduction and Subsequent Grinding/Magnetic Separation Process" Minerals 6, no. 4: 119. https://doi.org/10.3390/min6040119
APA StyleCao, Z., Sun, T., Xue, X., & Liu, Z. (2016). Iron Recovery from Discarded Copper Slag in a RHF Direct Reduction and Subsequent Grinding/Magnetic Separation Process. Minerals, 6(4), 119. https://doi.org/10.3390/min6040119





