Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores
Abstract
:1. Introduction
2. Characterization of the Raw Ore
2.1. Composition and Relative Concentration
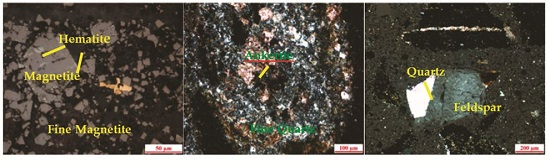
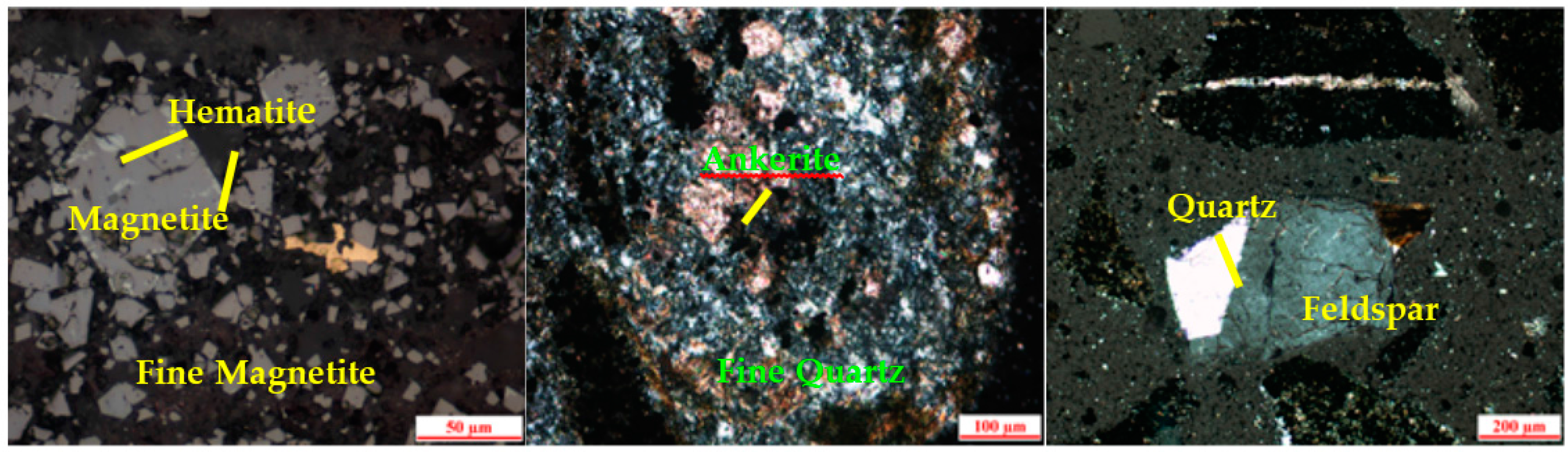
2.2. Dissemination of Main Minerals
2.3. Test Methods
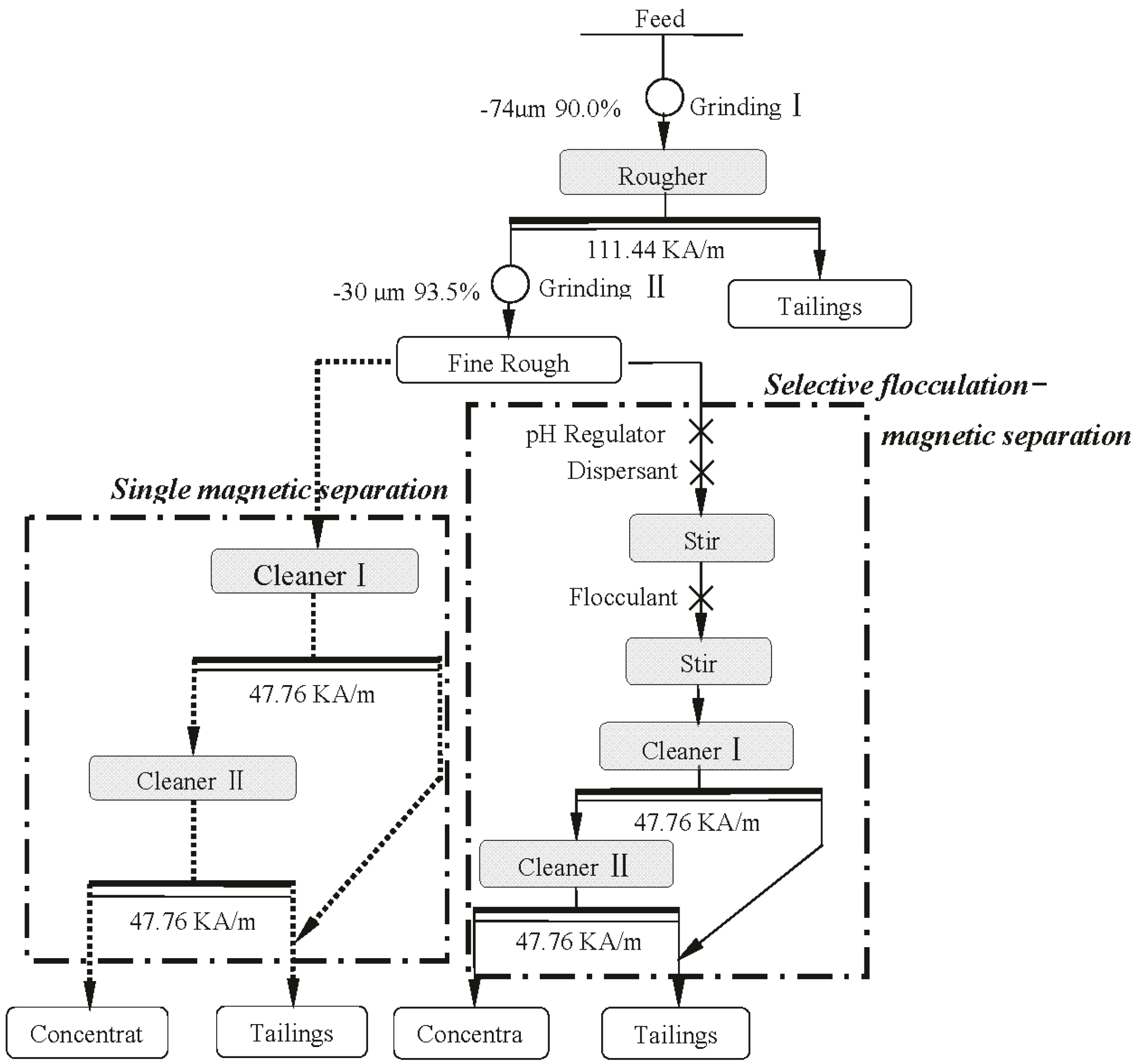
2.3.1. Simple Magnetic Separation Process
2.3.2. Selective Flocculation-Magnetic Separation Process
3. Test Results and Analysis
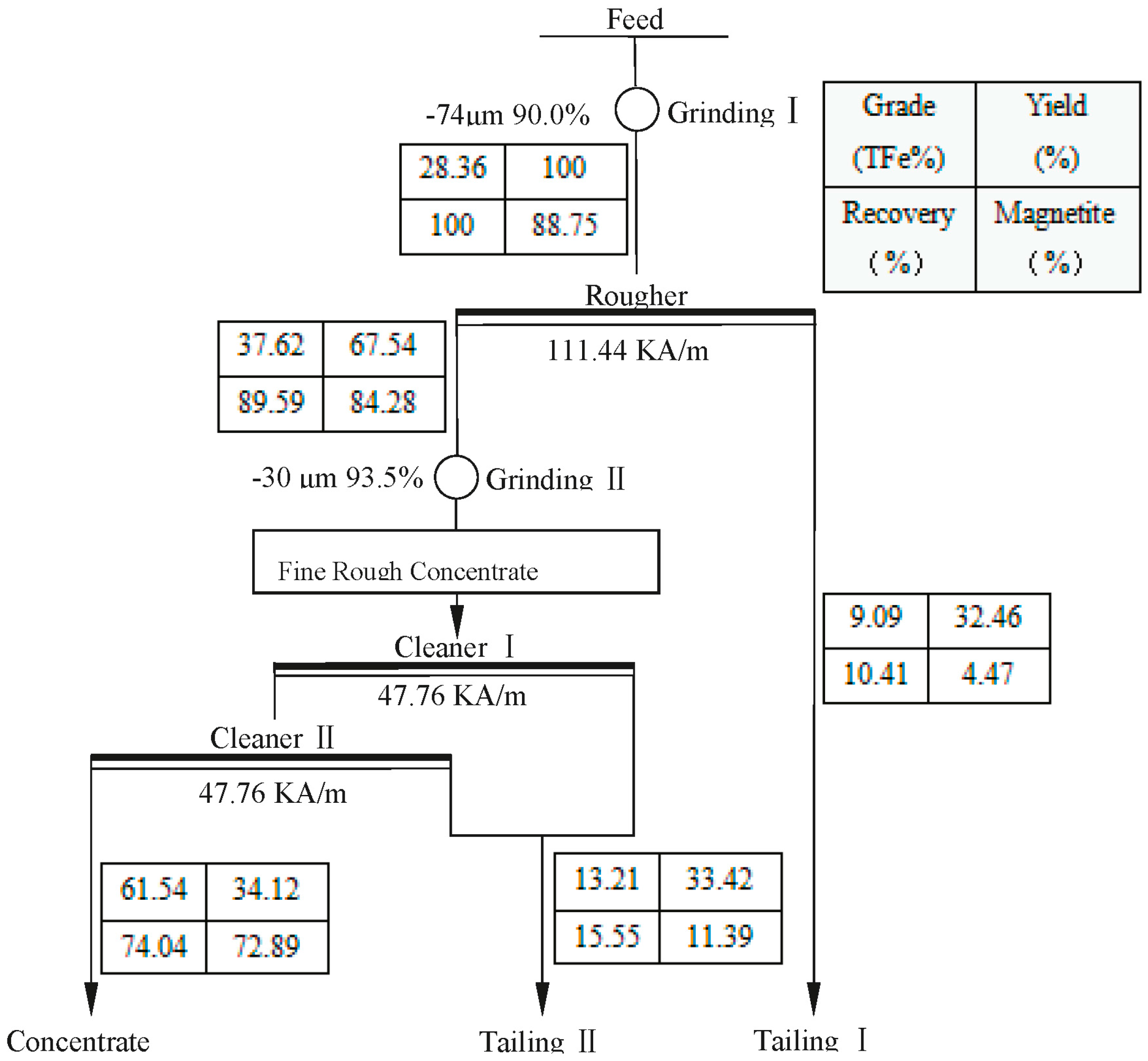
3.1. Simple Magnetic Separation Tests
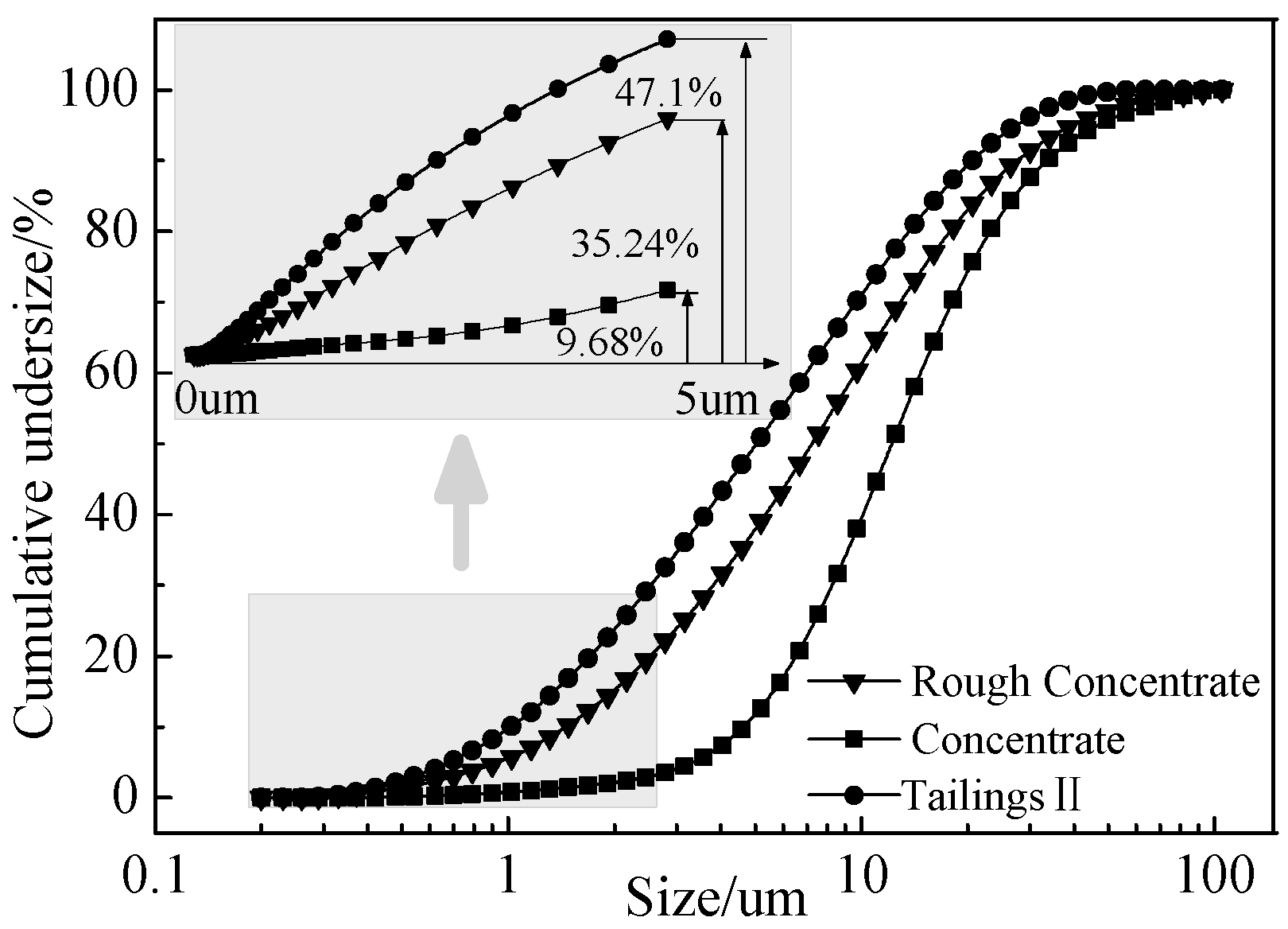
3.2. Selective Flocculation-Magnetic Separation Tests
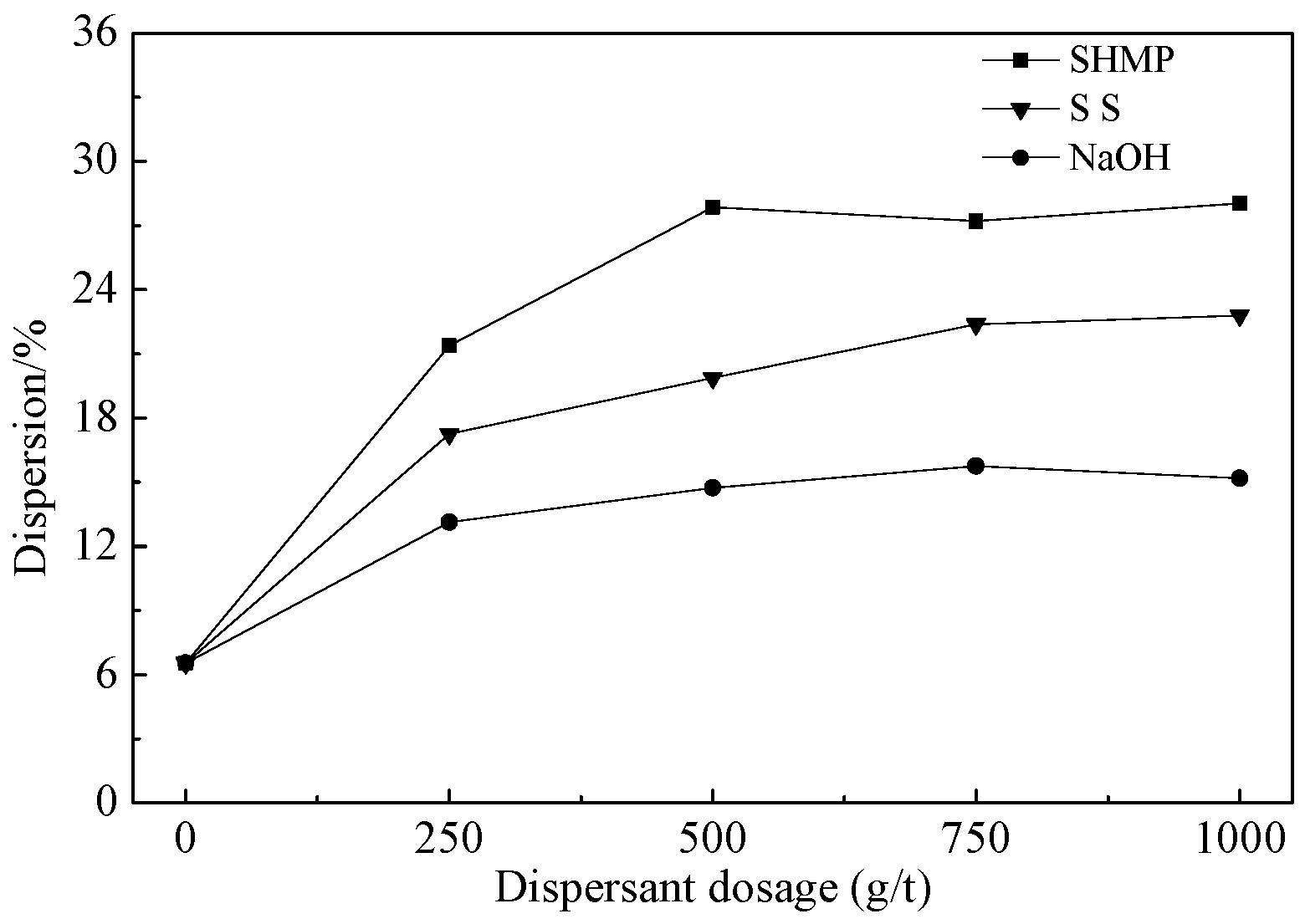
3.2.1. Dispersion Tests
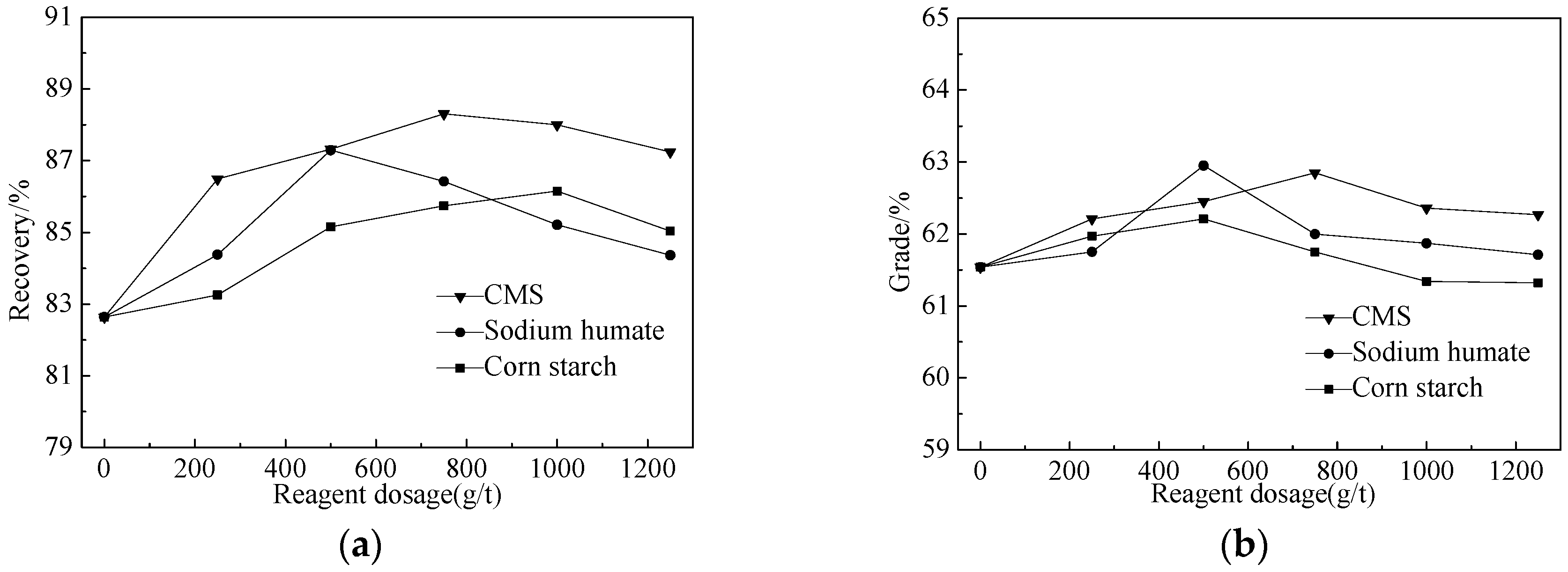
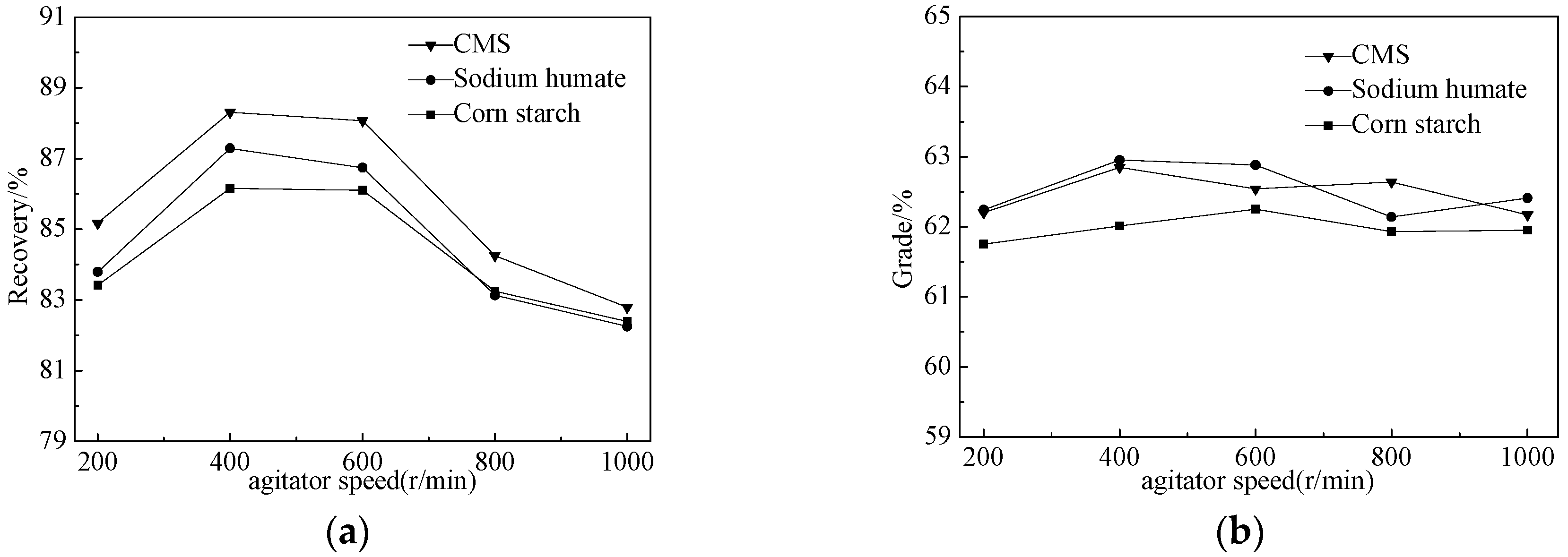
3.2.2. Flocculation Tests
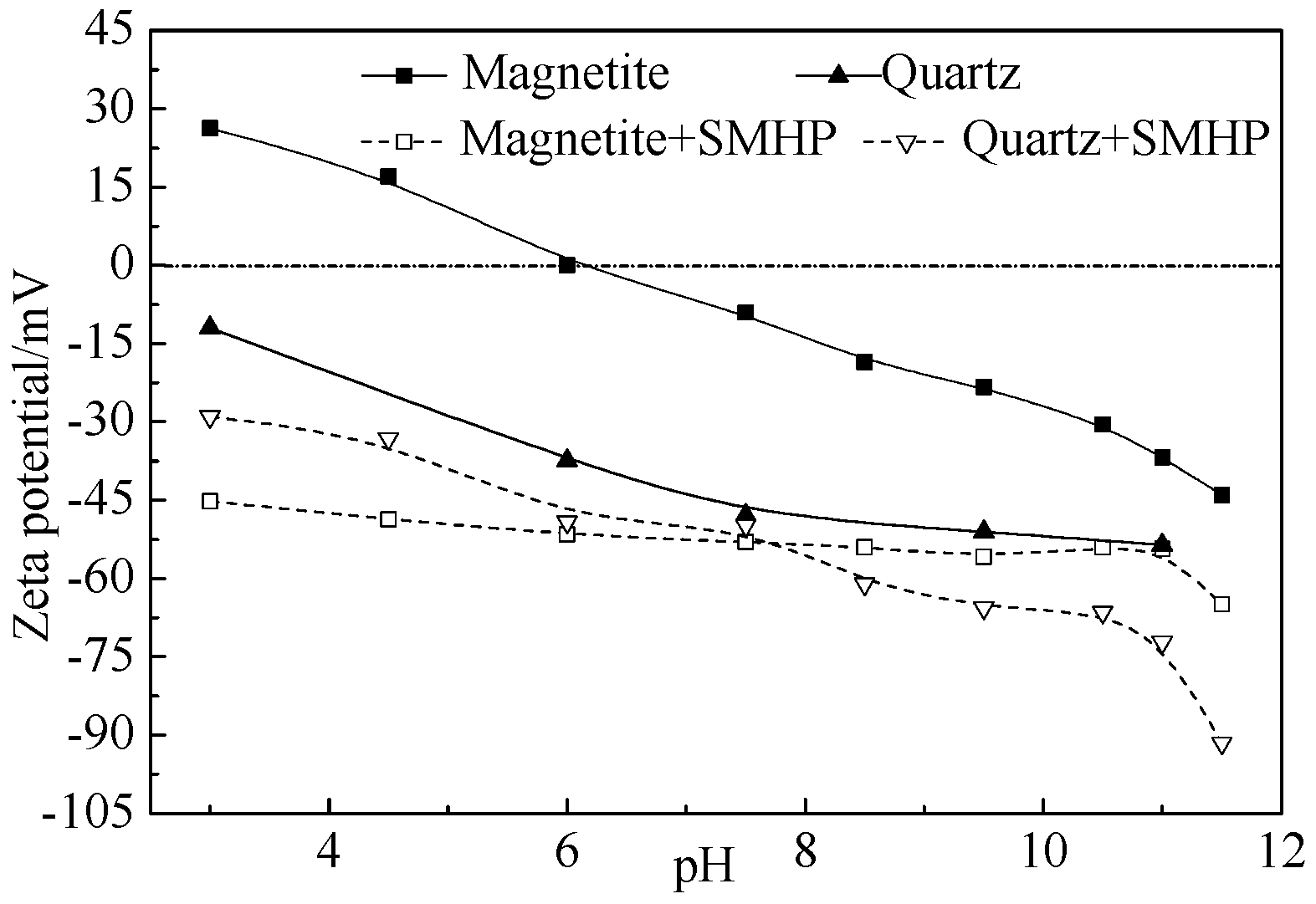
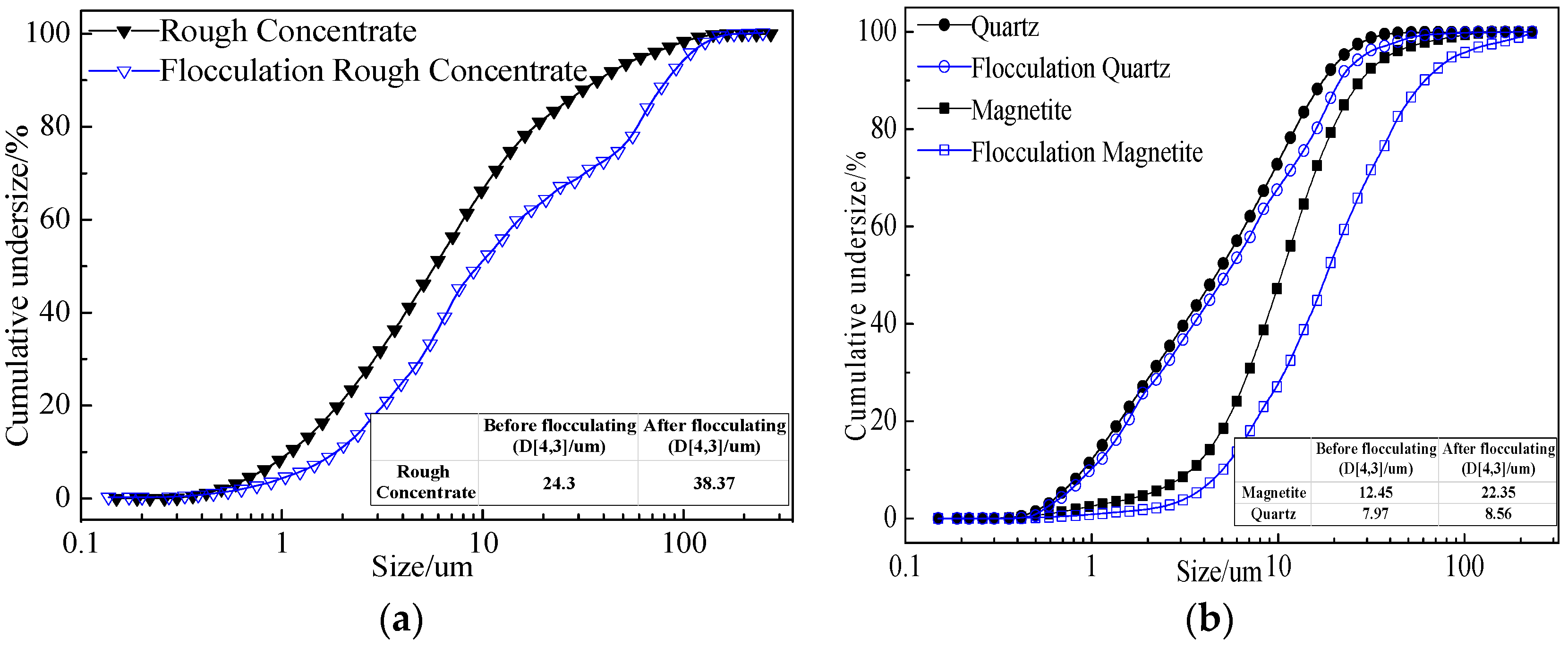
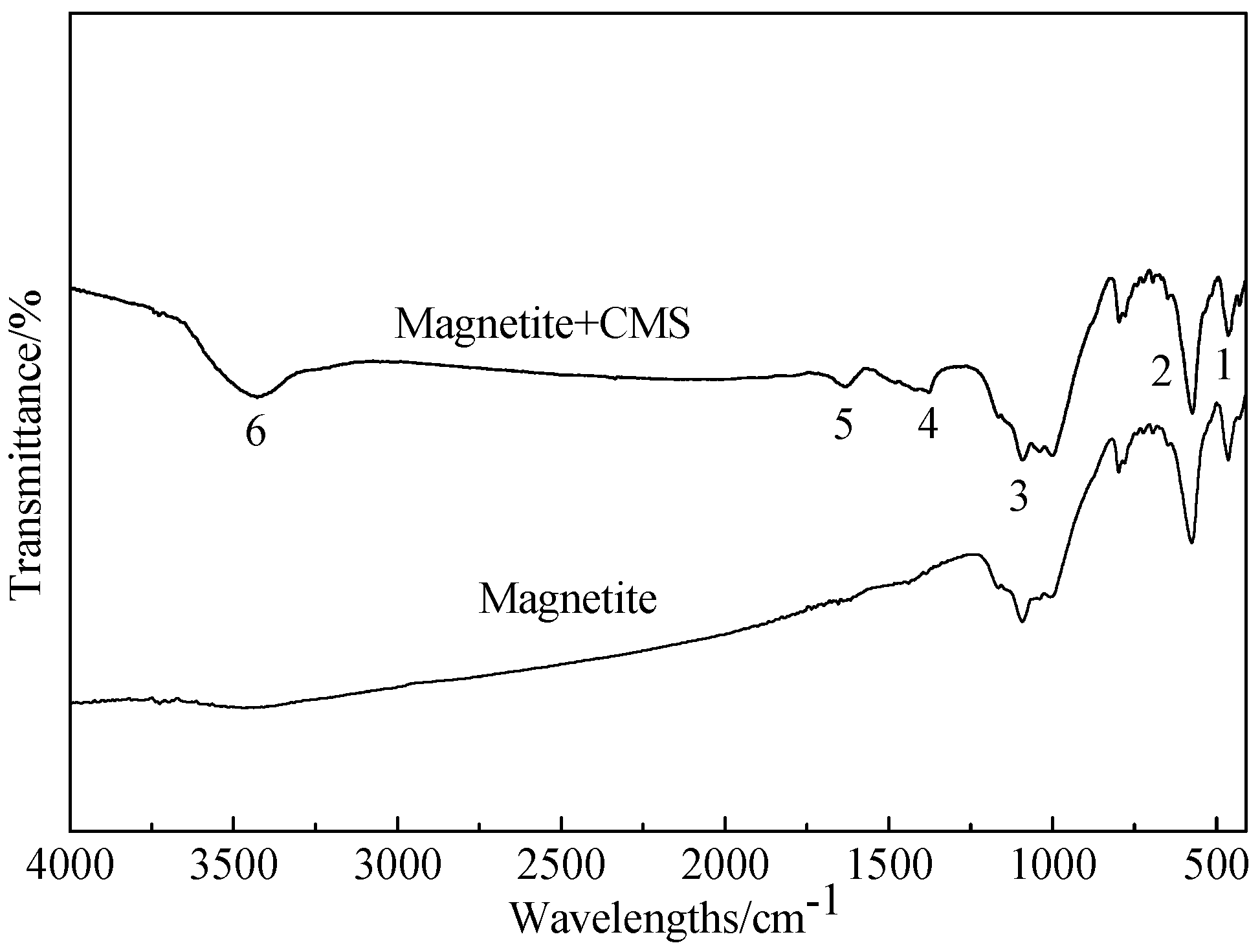
3.2.3. Analysis of Flocculation Mechanism
4. Conclusions
- (1)
- The tested magnetite ore from Gansu has a complex composition. It has a total iron grade of 28.36%, with magnetite as its main ferrous mineral, and quartz and feldspar as the main gangue minerals. The magnetite occurs as ultrafine particles, in dissemination or dense dissemination between gangue minerals, with relatively little singular scattered occurrence. The dissemination fineness is −0.038 mm at 90%.
- (2)
- Through tests, we developed a multi-stage grinding-dispersion-selective flocculation-weak magnetic separation process. The optimum conditions are: 500 g/t sodium hexametaphosphate (SHMP) as dispersant, 750 g/t carboxymethyl starch (CMS) as flocculant, agitated at the speed of 400 rpm for 10 min, using slurry pH of 11, and final grinding fineness −0.03 mm at 93.45%. The tests resulted in a concentrate with iron grade 62.82%, operational recovery rate 88.31%, and total recovery rate 79.12%. Compared to simple magnetic separation, the concentrate’s iron grade had been increased by 1.26 percentage points, and the recovery rate by 5.08%.
- (3)
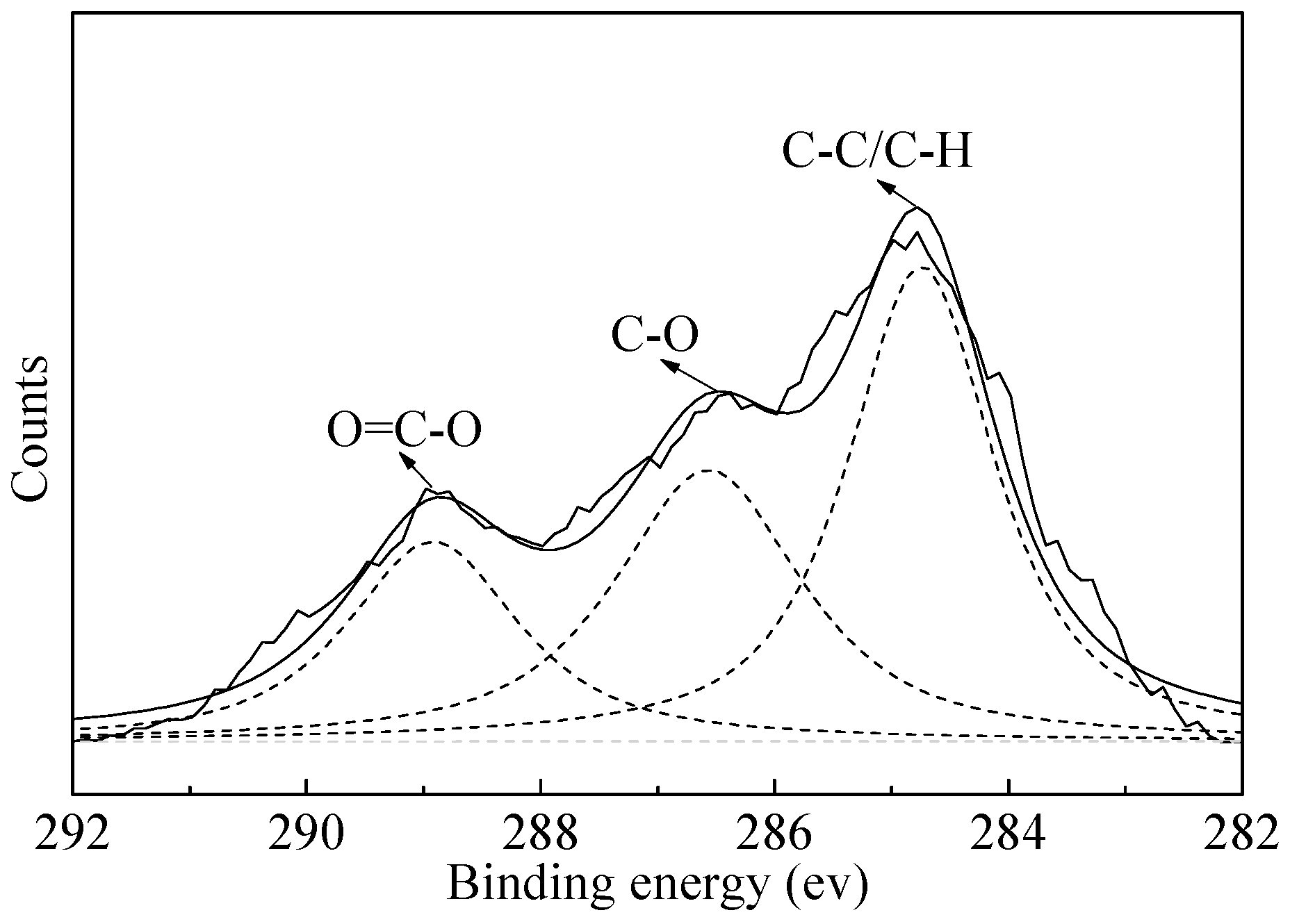
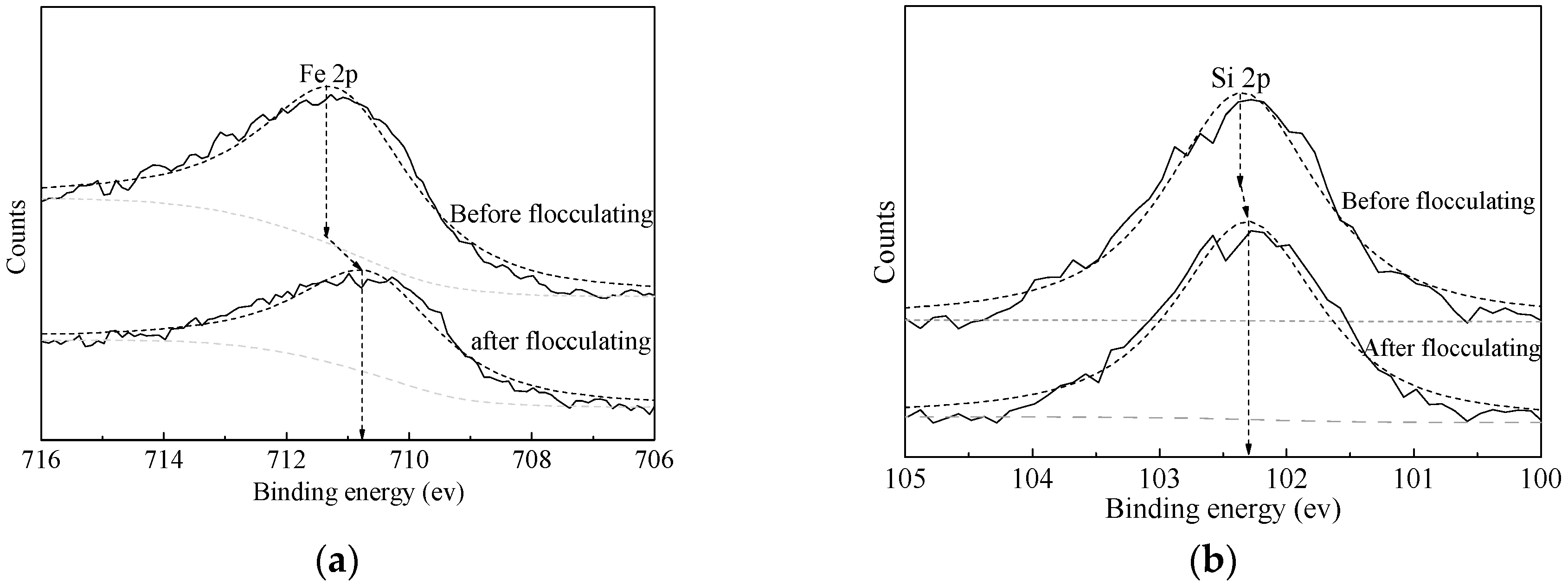
- FTIR and XPS analyses show that the negative CMS ions allow selective adsorption on magnetite particle surfaces through hydrogen bonding and electrostatic forces on the surfaces, causing a significant shift of the Fe binding energy; the final particle sizes of post-flocculation concentrate had increased from 24.30 to 38.37 μm. This process has accomplished the goal of selective flocculation, and increased the separation indices.
Author Contributions
Conflicts of Interest
Abbreviations
| SHMP | Sodium Hexametaphosphate |
| SS | Sodium Silicate |
| CMS | Carboxymethyl Starch |
| BE | Binding Energy |
References
- Wu, P.; Lv, X.; Qiu, J. Study on the mineral processing of a super low grade and microgranular magnetite ore. J. Mineral. Petrol. 2015, 35, 7–12. [Google Scholar]
- Tang, X.; Yu, Y.; Chen, W. Study on rational beneficiation technology of lean iron ore with fine disseminated minerals. Min. Metall. Eng. 2010, 1, 41–43. [Google Scholar]
- Su, T.; Chen, T.J.; Zhang, Y.M. The separation study on micro-fine disseminated and low-grade magnetite. Min. Res. Dev. 2015, 35, 38–42. [Google Scholar]
- Wei, S.; Sonsie, R.; Forbes, E.; Franks, G.V. Flocculation/flotation of hematite fines with, anionic temperature responsive polymer acting as a selective flocculant and collector. Miner. Eng. 2015, 77, 64–71. [Google Scholar]
- Santana, R.C.; Ribeiro, J.A.; Santos, M.A. Flotation of fine apatitic ore using micro bubbles. Sep. Purif. Technol. 2012, 98, 402–409. [Google Scholar] [CrossRef]
- Ravishankar, S.A.; Pradip; Khosla, N.K. Selective flocculation of iron oxide from its synthetic mixtures with cays: A comparison of polyacrylic acid and starch polymers. Int. J. Miner. Process. 1995, 43, 235–247. [Google Scholar] [CrossRef]
- Weissenborn, P.K.; Warren, L.J.; Dunn, J.L. Selective flocculation of ultrafine iron ore 2. Mechanism of selective flocculation. Colloids Surf. A Physicochem. Eng. Asp. 1995, 99, 29–43. [Google Scholar] [CrossRef]
- Abro, M.I.; Pathan, A.G.; Memon, A.R.; Sirajuddin. Dual polymer flocculation approach to overcome activation of gangue minerals during beneficiation of complex iron ore. Powder Technol. 2013, 245, 281–291. [Google Scholar] [CrossRef]
- Dogu, I.; Arol, A.I. Separation of dark-colored minerals from feldspar by selective flocculation using starch. Powder Technol. 2004, 139, 258–263. [Google Scholar] [CrossRef]
- Liu, C.J. Experiment study on the beneficiation of a micro-fine magnetite ore from Qinghai. Metal Mine 2009, 6, 52–55. [Google Scholar]
- Li, S.C.; Zhang, J.X.; Li, Z.H. Experiment study on process mineralogy and beneficiation of micro-fine magnetite ore from Liaoning. Min. Metall. Eng. 2012, 32, 312–315. [Google Scholar]
- Arol, A.I.; Aydogan, A. Recovery enhancement of magnetite fines in magnetic separation. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 151–154. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Wang, W. A facile disposal of Bayer red mud based on selective flocculation desliming with organic humics. J. Hazard. Mater. 2016, 301, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.H.; Guan, C.X.; Hou, D.Y. Study on IR characteristics of carboxylic acid and their salts. J. Anshan Teach. Coll. 2001, 3, 95–98. [Google Scholar]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y. Cryogenic XPS study of fast-frozen sulfide minerals: Flotation-related adsorption of N-butyl xanthate and beyond. J. Electron Spectrosc. Relat. Phenom. 2016, 206, 65–73. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, Y.; Xue, J. Comparative XPS study between experimentally and naturally weathered pyrites. Appl. Surf. Sci. 2009, 255, 8750–8760. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Feng, W. Synchrotron radiation photoelectron spectroscopy study of dextrant-coated Fe3O4 magnetic nanoparticles. Nucl. Technol. 2009, 32, 251–255. [Google Scholar]
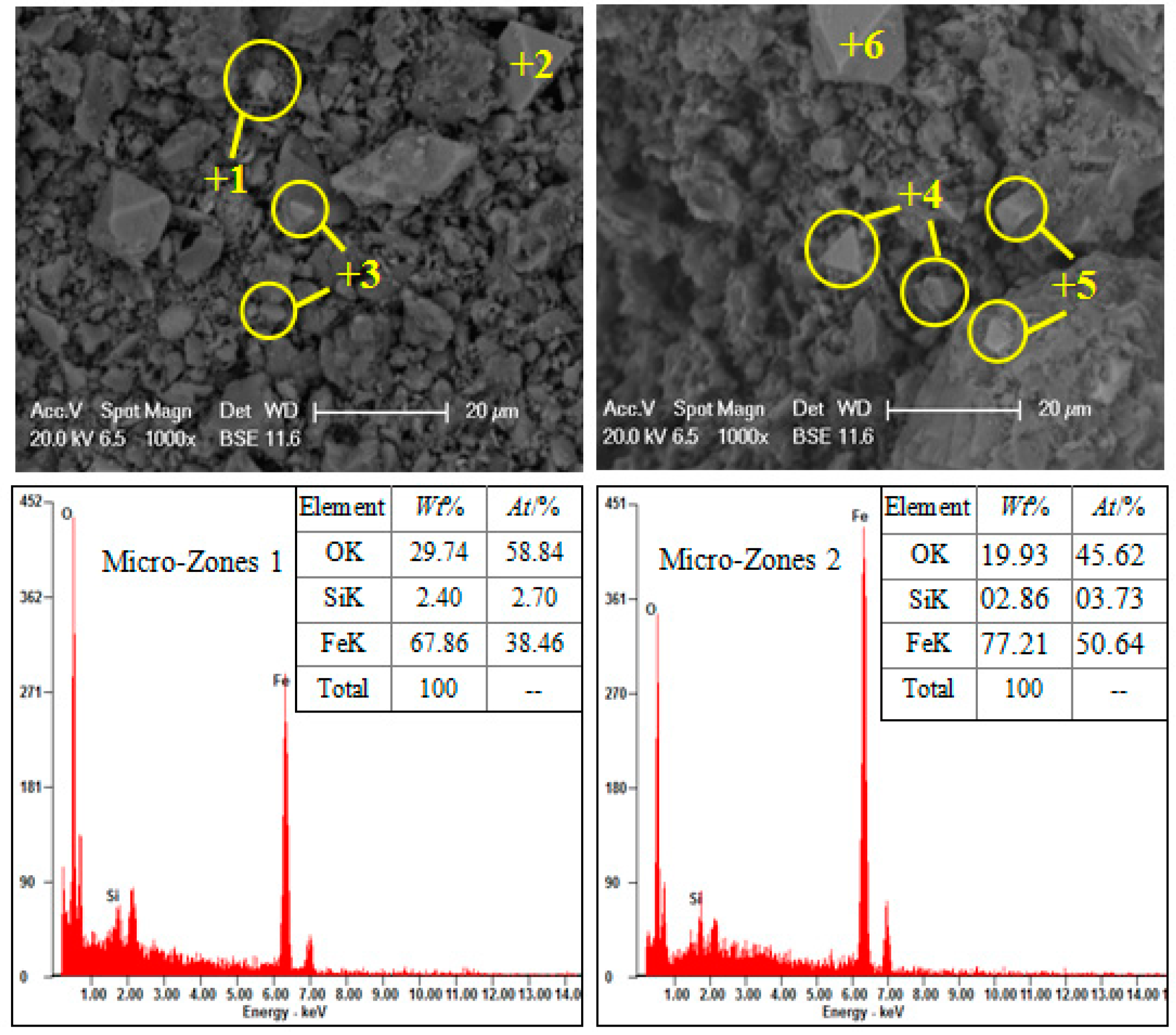

| Component | TFe | SiO2 | Al2O3 | FeO | CaO | MgO | S | P |
|---|---|---|---|---|---|---|---|---|
| Amount % | 28.36 | 43.02 | 5.51 | 13.45 | 1.67 | 2.03 | 0.02 | 0.26 |
| Mineral | Magnetite | Siderite | Hematite | Quartz | Feldspar | Dolomite | Sericite | Ankerite | Calcite |
|---|---|---|---|---|---|---|---|---|---|
| Amount % | 33.8 | 2.4 | 1.5 | 28.7 | 10.4 | 7.8 | 3.2 | 3.7 | 2.7 |
| Chemical State | C | ||
|---|---|---|---|
| C–C/C–H | C–O | O=C–O | |
| BE (eV) | 284.73 | 286.54 | 288.85 |
| Species | Fe | Si |
|---|---|---|
| Fe3O4 | SiO2 | |
| Binding energy (BE) (eV) a | 711.35 | 102.37 |
| BE (eV) b | 710.78 | 102.29 |
| Shift (eV) | 0.57 | 0.08 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.; Chen, T.; Zhang, Y.; Hu, P. Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores. Minerals 2016, 6, 86. https://doi.org/10.3390/min6030086
Su T, Chen T, Zhang Y, Hu P. Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores. Minerals. 2016; 6(3):86. https://doi.org/10.3390/min6030086
Chicago/Turabian StyleSu, Tao, Tiejun Chen, Yimin Zhang, and Peiwei Hu. 2016. "Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores" Minerals 6, no. 3: 86. https://doi.org/10.3390/min6030086
APA StyleSu, T., Chen, T., Zhang, Y., & Hu, P. (2016). Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores. Minerals, 6(3), 86. https://doi.org/10.3390/min6030086