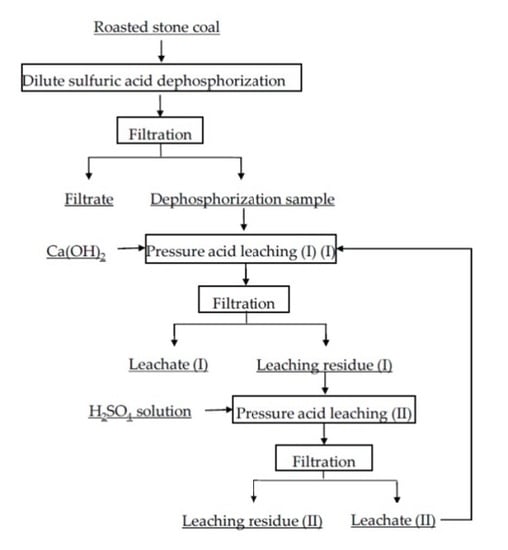
Selective Leaching of Vanadium from Roasted Stone Coal by Dilute Sulfuric Acid Dephosphorization-Two-Stage Pressure Acid Leaching
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Procedure
2.2.1. DSAD Test
2.2.2. Two-Stage PAL Test
2.3. Analysis Methods
- (1)
- The determination of vanadium content was measured in accordance with Test Methods of Vanadium in Coal Standard (GB/T 19226-2003) [16]. Other elements in this study were analyzed by using ICP-AES method which was conducted by an inductively coupled plasma-atomic emission spectrometer (Thermo Scientific) Advantage ER/S inductively coupled plasma-optical emission spectrometer (Thermo Scientific).
- (2)
- The residual sulfuric acid concentration in leachate was determined by titration using 0.1 mol/L Na2CO3 solution with methyl orange as an indicator [17].
- (3)
- XRD analysis was conducted by an Xpertpro X-ray diffractometer (D/MAX 2500PC, Rigaku, Tokyo, Japan) with Cu-Kα radiation, voltage 40 kV, current 30 mA and at the scanning rate of 15° ·min−1 from 5° to 70°.
- (4)
- Detailed mineralogy on the roasted stone coal was done using quantitative evaluation of minerals by QEMSCAN analysis which was conducted by the QEMSCAN analysis system (FEI).
- (5)
- The chemical species was analyzed by using a VG Multilab 2000 X-ray photoelectron spectrometer (Thermo Scientific) and an Al-Kα X-ray source with a solution of 0.47 eV.
3. Results and Discussion
3.1. The DSAD-Two-Stage PAL Result
3.1.1. DSAD Result
3.1.2. Pressure Acid Leaching (I) Result
3.1.3. Pressure Acid Leaching (II) Result
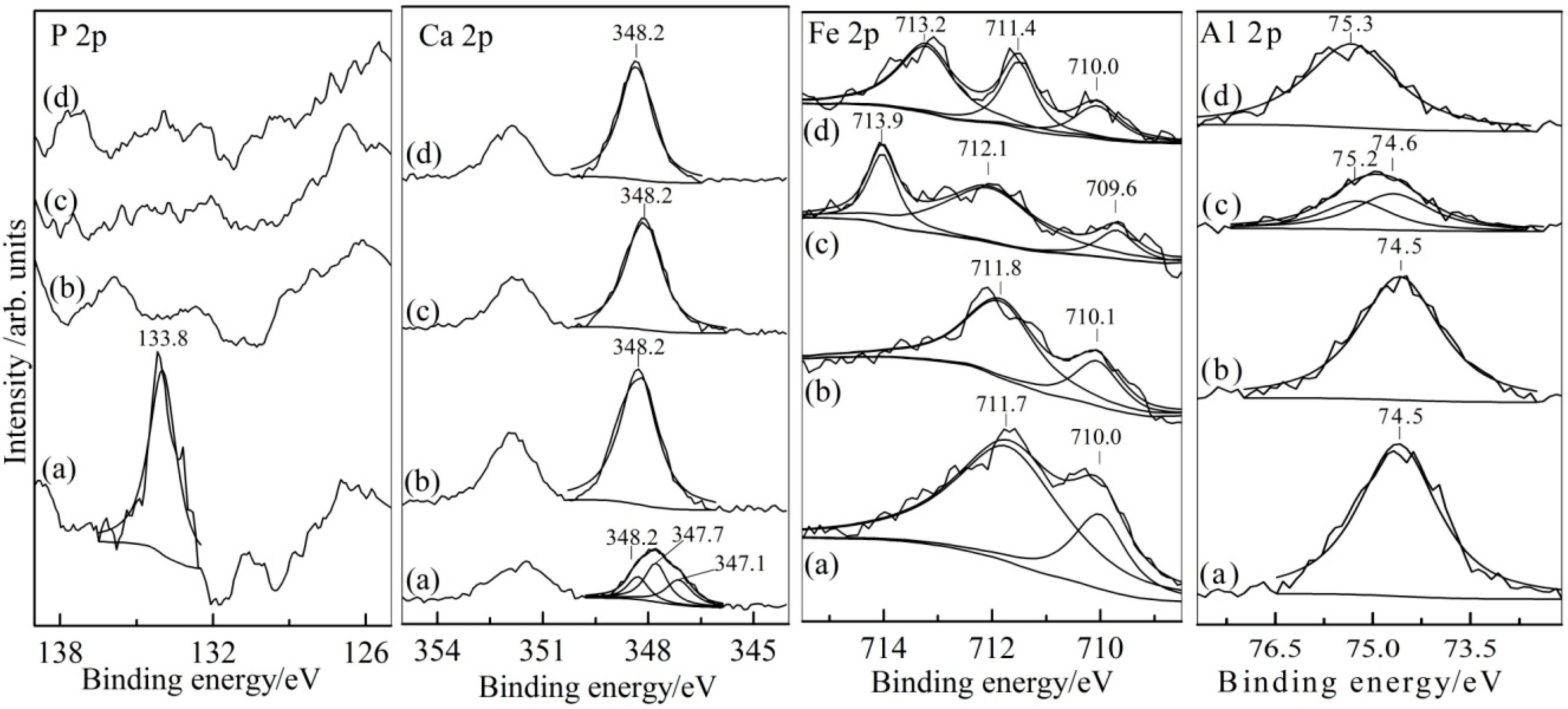
3.2. Analysis of Leaching Residue and Leachate
3.3. Thermodynamics Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology. Minerals 2016, 6, 26. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Liu, R.Q.; Gu, X.C. Flotation recovery of vanadium from low-grade stone coal. Trans. Nonferrous Met. Soc. China 2014, 24, 1145–1151. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, C.L.; Li, J.; Liu, H.Z.; Wu, S.Y. Leaching of vanadium from carbonaceous shale. Hydrometallurgy 2009, 99, 97–99. [Google Scholar] [CrossRef]
- Li, M.T.; Wei, C.; Qiu, S.; Zhou, X.J.; Li, C.X.; Deng, Z.G. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Li, X.B.; Wei, C.; Deng, Z.G.; Li, M.T.; Li, C.X.; Fan, G. Selective solvent extraction of vanadium over iron from stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Wang, X.W.; Wang, M.Y.; Jiang, C.J.; Xiang, X.Y.; Zhang, X.L. Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP. Hydrometallurgy 2015, 153, 38–45. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.M.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131–132, 1–7. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, K.; Hua, W.H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Xia, W.T.; Ren, Z.D.; Gao, Y.F. Removal of phosphorus from high phosphorus iron ores by selective HCl leaching method. J. Iron Steel Res. Int. 2011, 18, 1–4. [Google Scholar] [CrossRef]
- Chen, C.Y.; Misra, V.N.; Clough, J.; Mun, R. Dephosphorisation of western Australian iron ore by hydrometallurgical process. Miner. Eng. 1999, 12, 1083–1092. [Google Scholar] [CrossRef]
- Jin, Y.S.; Jiang, T.; Yang, Y.B.; Li, Q.; Li, G.H.; Guo, Y.F. Removal of phosphorus from iron ores by chemical leaching. J. Cent. South. Univ. Technol. 2006, 13, 673–677. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Topkaya, Y.A. Pressure acid leaching of Çaldağ lateritic nickel ore: An alternative to heap leaching. Hydrometallurgy 2014, 142, 98–107. [Google Scholar] [CrossRef]
- Xu, B.; Zhong, H.; Jiang, T. Recovery of valuable metals from Gacun complex copper concentrate by two-stage countercurrent oxygen pressure acid leaching process. Miner. Eng. 2011, 24, 1082–1083. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhu, X.B.; Liu, T.; Huang, J.; Song, S.X. Effect of colloidal potassium alum formation on vanadium recovery from acid leach solutions of stone coal. Hydrometallurgy 2013, 13, 54–58. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.M.; Huang, J.; Liu, T.; Xue, N.N. Study on Roasting-Pressure Acid Leaching of Vanadium from Stone Coal. Metal Mine 2015, 10, 85–89. (In Chinese) [Google Scholar]
- Li, W.; Zhang, Y.M.; Huang, J.; Zhu, X.B.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Rubisov, D.H.; Papangelakis, V.G. Sulphuric acid pressure leaching of laterites-speciation and prediction of metal solubilities “at temperature”. Hydrometallurgy 2000, 58, 13–26. [Google Scholar] [CrossRef]
- Glorieux, B.; Berjoan, R.; Matecki, M.; Kammouni, A.; Perarnau, D. XPS analyses of lanthanides phosphates. Appl. Surf. Sci. 2007, 253, 3349–3359. [Google Scholar] [CrossRef]
- Cai, Y.H.; Pan, Y.G.; Xue, J.Y.; Sun, Q.F.; Su, G.Z.; Li, X. Comparative XPS study between experimentally and naturally weathered pyrites. Appl. Surf. Sci. 2009, 255, 8750–8760. [Google Scholar] [CrossRef]
- Deng, M.J.; Liu, Q.X.; Xu, Z.H. Impact of gypsum supersaturated solution on surface properties of silica and sphalerite minerals. Miner. Eng. 2013, 46–47, 6–15. [Google Scholar] [CrossRef]
- Kačiulis, S.; Mattogno, G.; Pandolfi, L.; Cavalli, M.; Gnappi, G.; Montenero, A. XPS study of apatite-based coatings prepared by sol-gel technique. Appl. Surf. Sci. 1999, 151, 1–5. [Google Scholar] [CrossRef]
- Du, J.K.; Bao, J.G.; Fu, X.Y.; Lu, C.H.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, M.Y.; Kang, F.F.; Cao, S.S.; Chen, R.F.; Liu, H.; Wei, Y. Formation mechanism of a series of trigonal antiprismatic jarosite-type compounds. J. Cryst. Growth 2015, 429, 49–55. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Legg, B.; Zhang, H.Z.; Gilbert, B.; Yang, R.; Banfield, J.; Waychunas, G.A. Early-stage formation of iron oxyhydroxides during neutralization of simulated acid mine drainage solutions. Environ. Sci. Technol. 2012, 46, 8140–8147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Brown, N.M.D.; McKinley, A. Characterisation of oxygen plasma-modified mica surfaces using XPS and AFM. Appl. Surf. Sci. 1997, 108, 319–332. [Google Scholar] [CrossRef]
- Lim, V.W.L.; Lia, S.; Kanga, E.T.; Neoha, K.G.; Tanb, K.L. In situ XPS study of thermally deposited aluminium on chemically synthesized polypyrrole films. Synth. Met. 1999, 106, 1–11. [Google Scholar] [CrossRef]
- Parsons, R. Atlas of Electrochemical Equilibria in Aqueous Solutions: By Marcel Pourbaix, 2nd ed.; Pergamon Press: Oxford, UK, 1966; pp. 234–626. [Google Scholar]
- Yang, X.W.; He, E.P.; Yuan, B.Z. Thermodynamics Data Calculation Manual of High Temperature Water Solution, 1st ed.; Metallurgical Industry Press: Beijing, China, 1983; pp. 88–648. [Google Scholar]
- Mu, W.Z.; Zhang, T.A.; Dou, Z.H.; Lü, G.Z.; Liu, Y. φ-pH diagram of V-Ti-H2O system during pressure acid leaching of converter slag containing vanadium and titanium. Trans. Nonferrous Met. Soc. China 2011, 21, 2078–2086. [Google Scholar] [CrossRef]
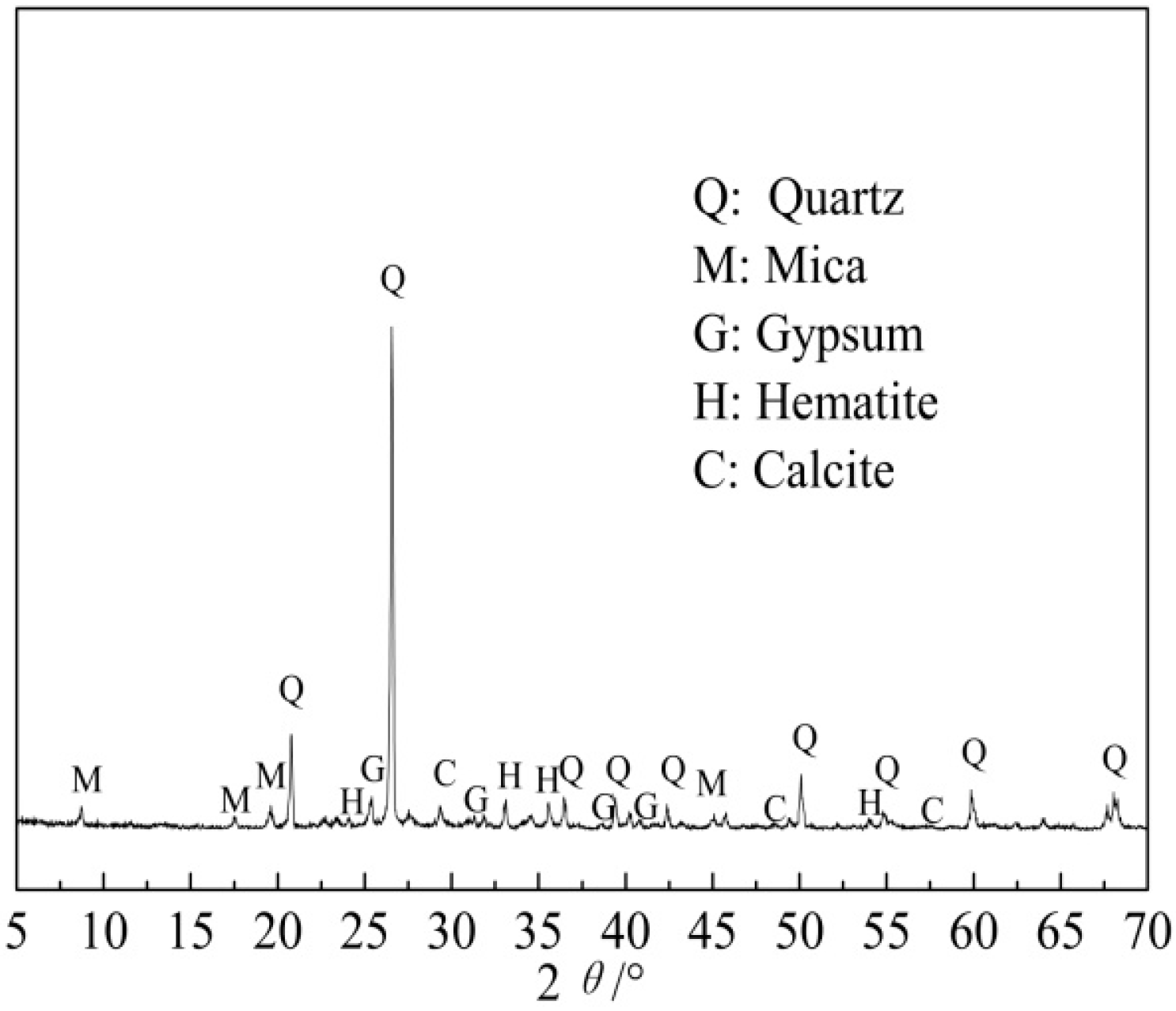
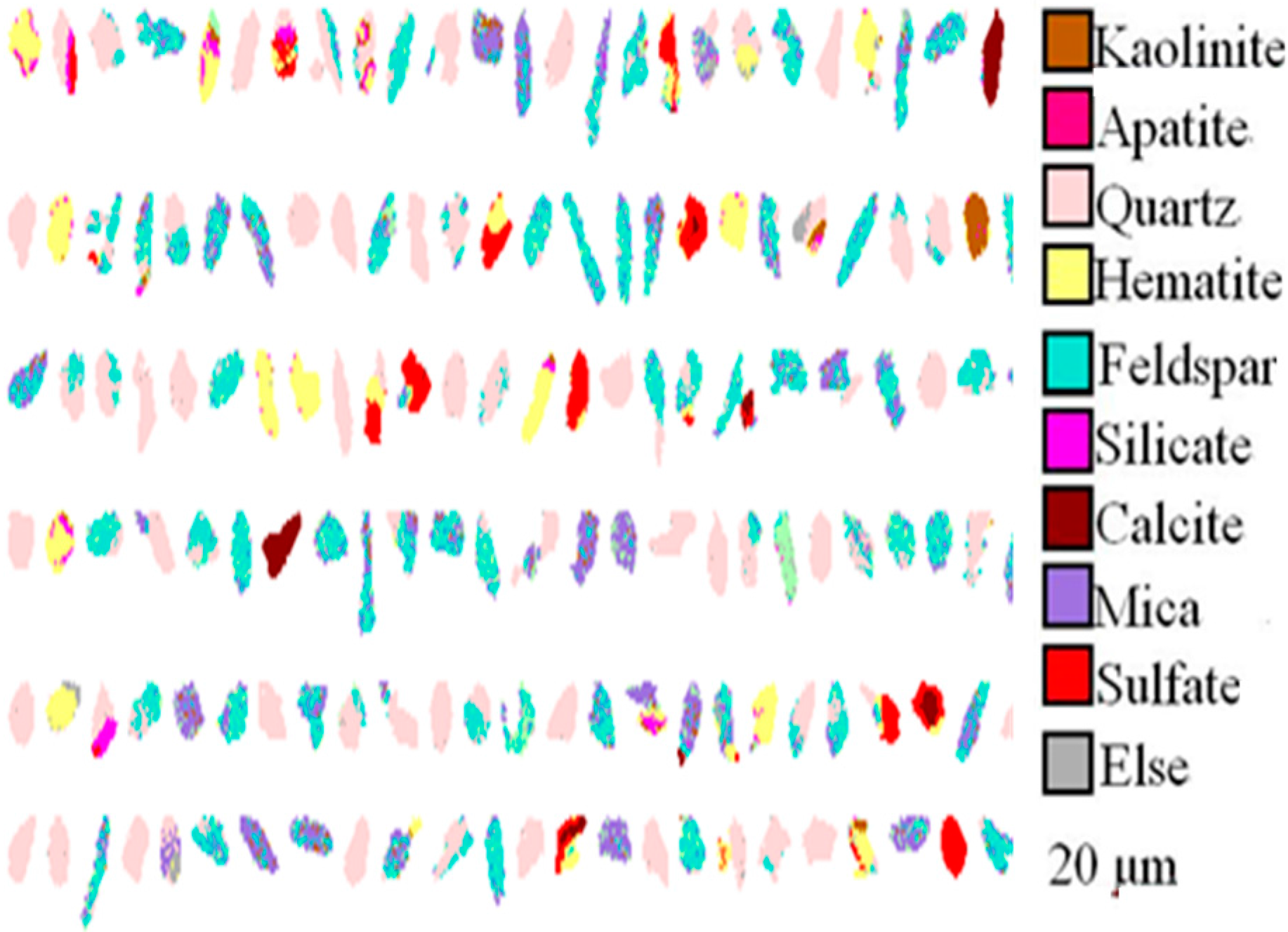
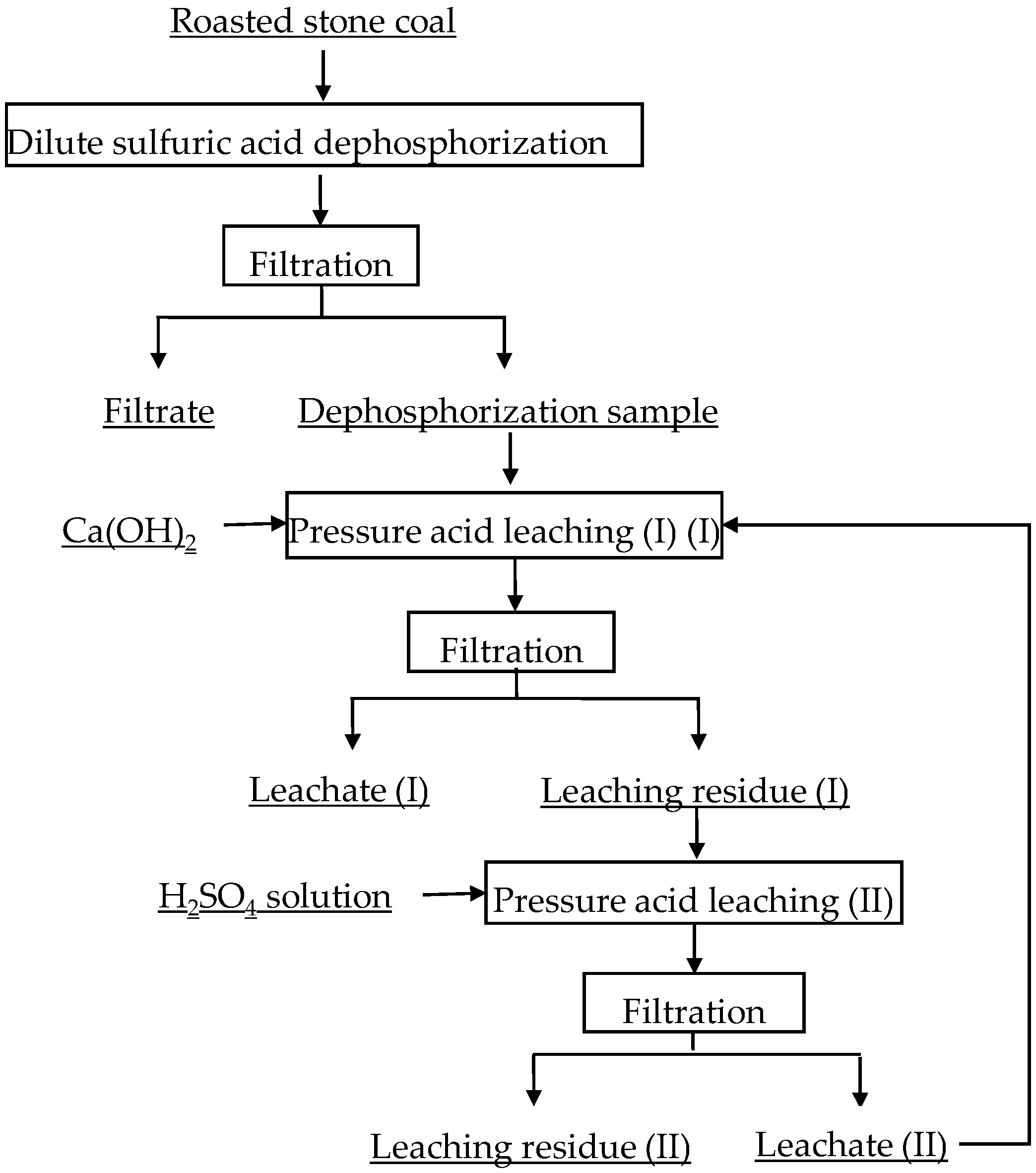
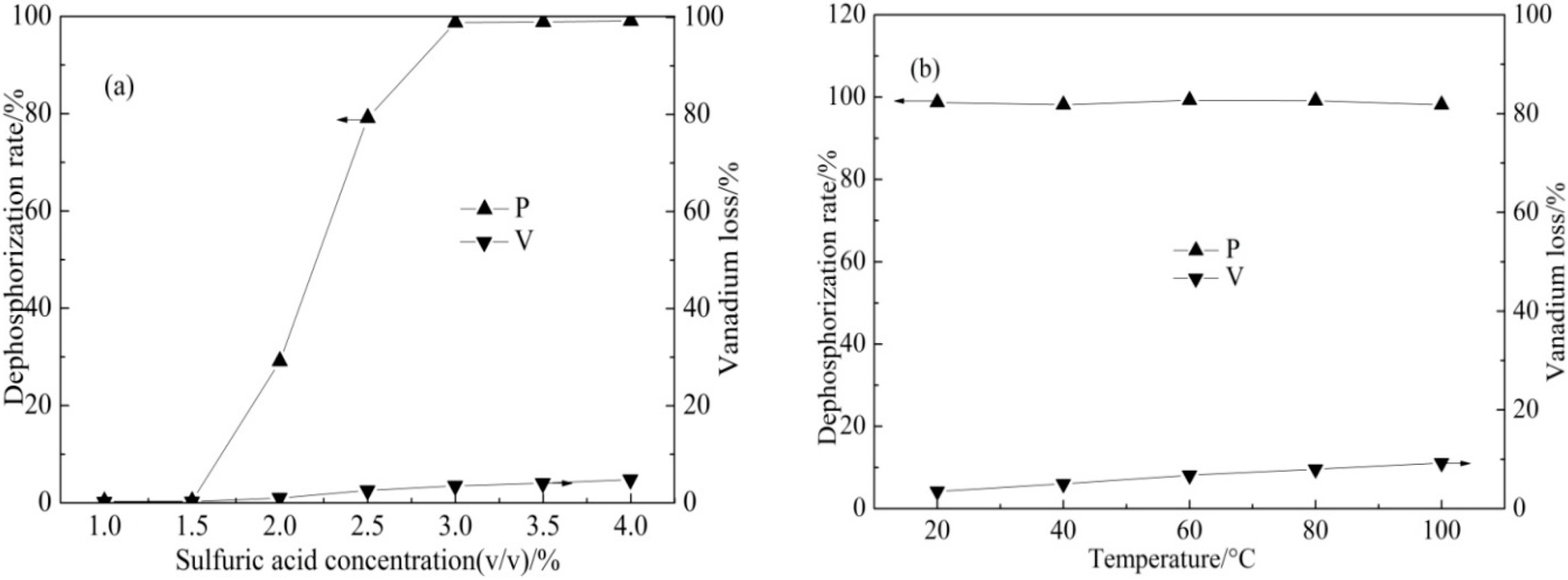
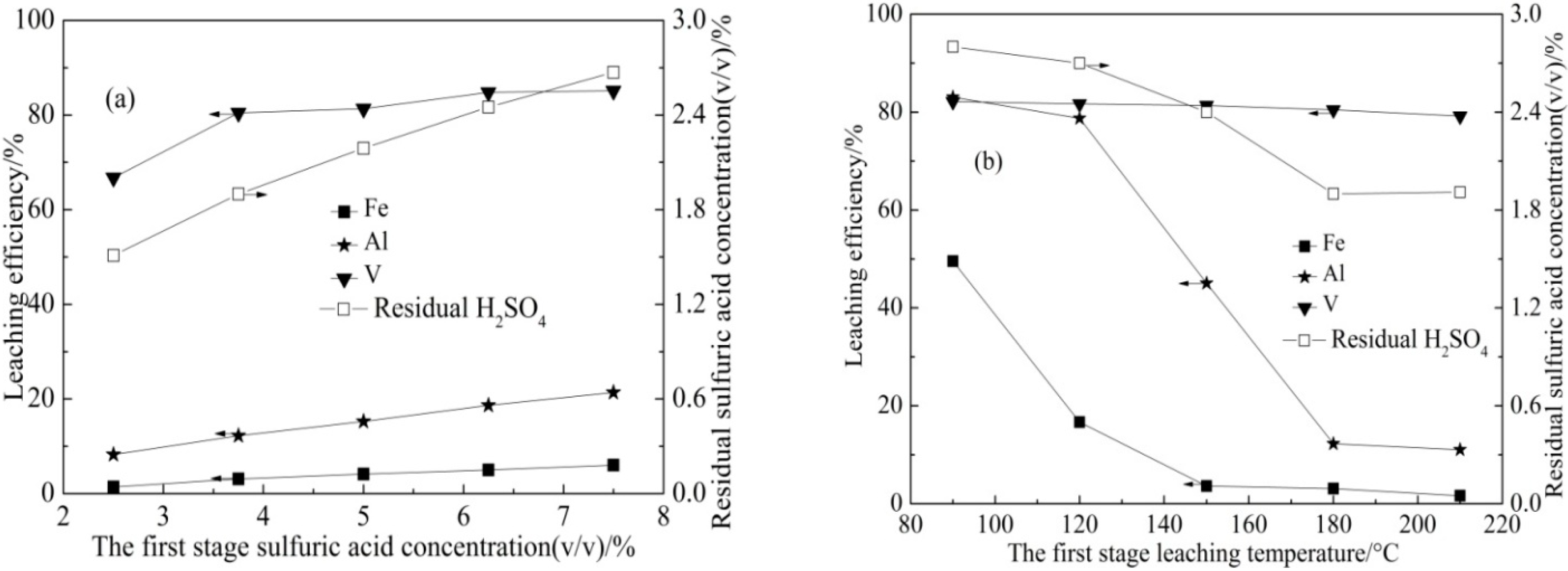
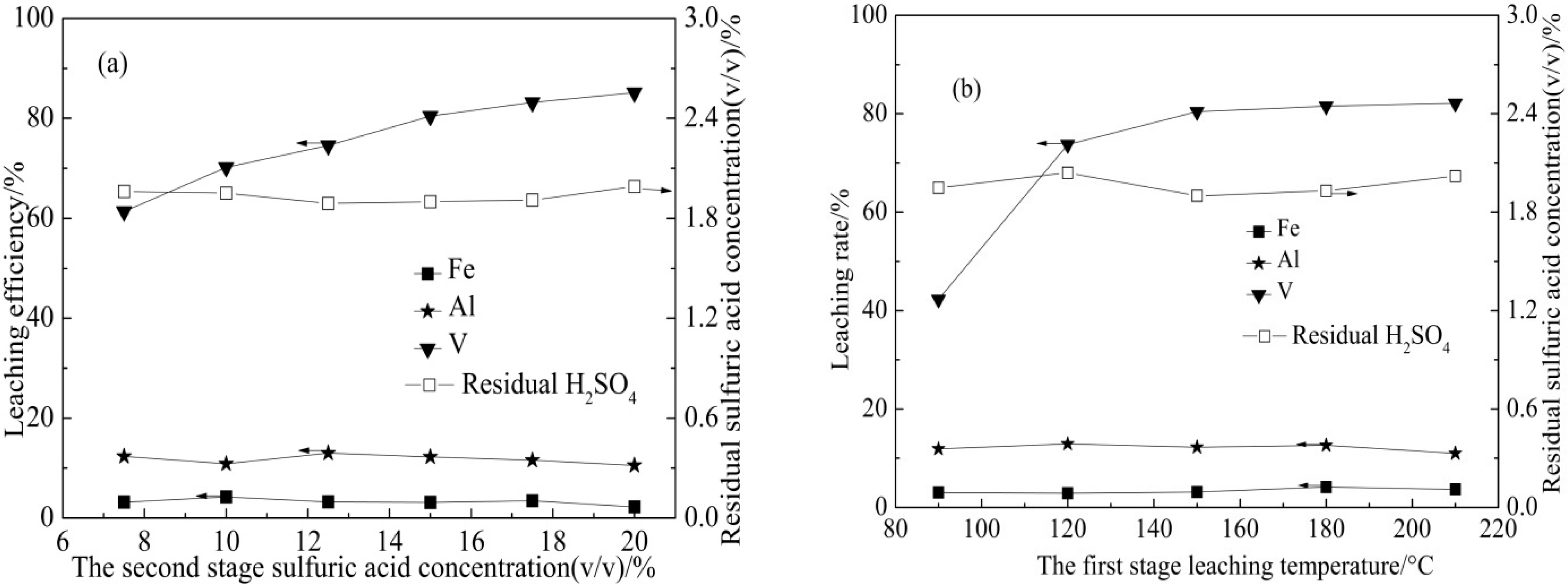
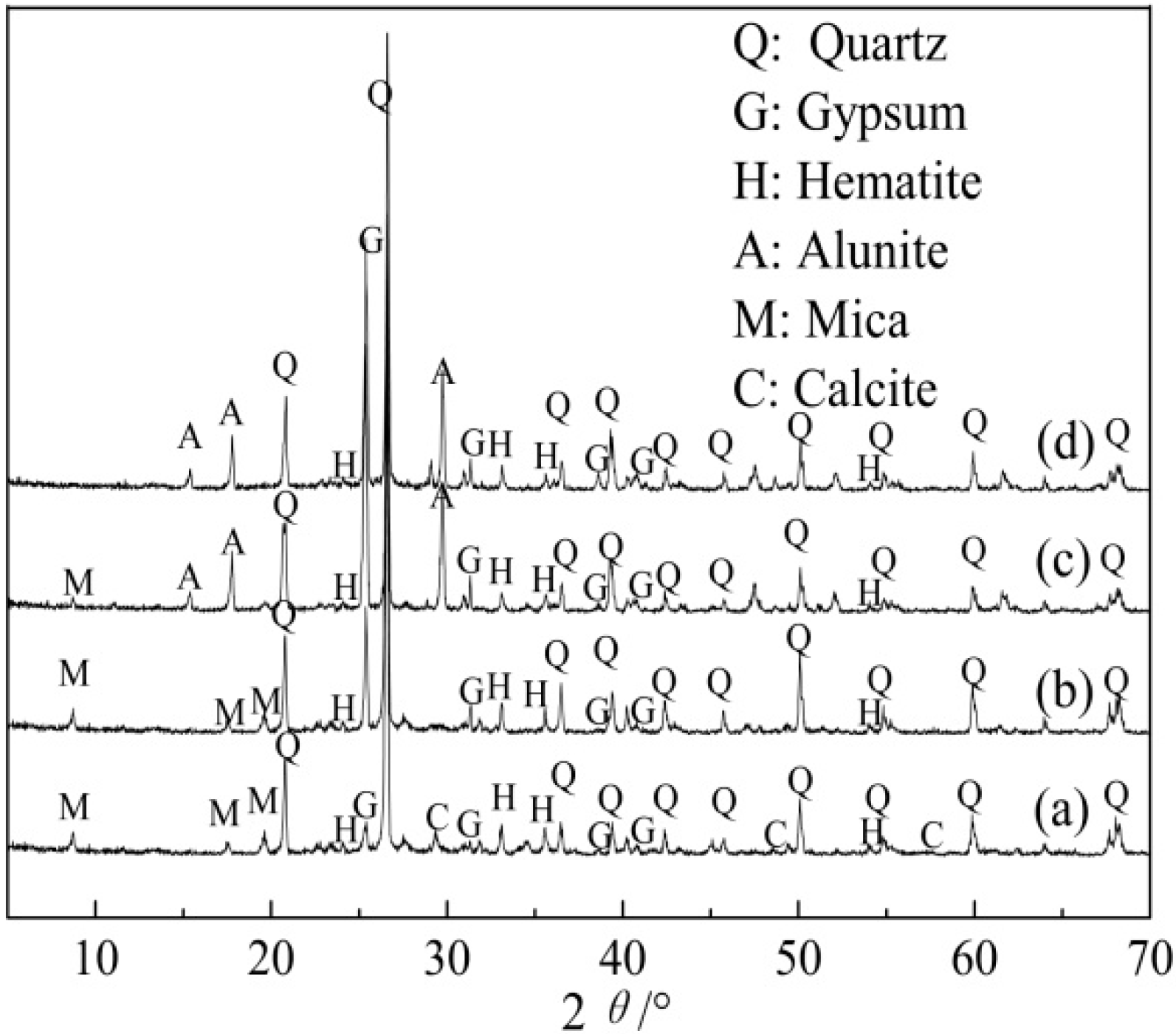
| Element | SiO2 | CaO | Al2O3 | V2O5 | Fe2O3 | K2O | Na2O | MgO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Content | 66.0 | 5.29 | 9.81 | 0.83 | 4.96 | 2.51 | 0.30 | 1.67 | 1.03 |
| Process | V | Fe | Al | P | Residual H2SO4 (v/v) |
|---|---|---|---|---|---|
| a Conventional PAL (i) | 80.51 | 18.92 | 79.25 | 99.21 | 4.39 |
| b Conventional PAL (ii) | 82.13 | 19.96 | 60.48 | 98.20 | 4.29 |
| c DSAD-two-stage PAL | 80.46 | 3.12 | 12.24 | 0.67 | 1.90 |
| Each Stage Filtrate | V | Fe | Al | P |
|---|---|---|---|---|
| Filtrate | 94 | 58 | 120 | 2864 |
| Leachate (I) | 2163 | 623 | 3672 | 19 |
| Leachate (II) | 1487 | 10019 | 20532 | 22 |
| Number | Equilibrium Reaction | |||||
|---|---|---|---|---|---|---|
| 1 | CaCO3 + HSO4− + H+ = CO2↑ + CaSO4↓ + H2O | 13.23 | 12.53 | 12.14 | 11.95 | 11.91 |
| 2 | CaCO3 + SO42− + 2H+ = CO2↑ + CaSO4↓ + H2O | 7.43 | 7.40 | 7.58 | 7.87 | 8.25 |
| 3 | CaCO3 + SO42− + H+ = CaSO4↓ + HCO3− | 7.17 | 6.81 | 6.79 | 6.95 | 7.26 |
| 4 | Fe2O3 + 6H+ = 2Fe3+ + 3H2O | 0.49 | −0.16 | −0.65 | −1.03 | −1.36 |
| 5 | KAl2(AlSi3O10)(OH)2 + 10H+ = 3Al3+ + K+ + 3H4SiO4 | 2.20 | 1.35 | 0.72 | 0.22 | −0.20 |
| 6 | KAl2(AlSi3O10)(OH)2 + 7H+ = 3Al3+ + K+ + 3HSiO3− + 3H2O | −1.90 | −2.65 | −3.30 | −3.89 | −4.46 |
| 7 | KAl3(SO4)2(OH)6 + 8H+ = 3Al3+ + 6H2O + K+ + 2HSO4− | 1.07 | 0.38 | −0.21 | −0.74 | −1.24 |
| 8 | KAl3(SO4)2(OH)6 + 6H+ = 3Al3+ + 6H2O + K+ + 2SO42− | 0.89 | −0.24 | −1.29 | −2.25 | −3.18 |
| 9 | Ca5(PO4)3F + 2H+ + 5HSO4− = 5CaSO4↓ + 3H2PO4− + HF | 6.88 | 5.22 | 3.99 | 3.05 | 2.34 |
| 10 | Ca5(PO4)3F + 5H+ + 5HSO4− = 5CaSO4↓ + 3H3PO4 + HF | 0.63 | 0.41 | 0.50 | 0.67 | 0.92 |
| 11 | Ca5(PO4)3F + H+ + 5SO42− = 5CaSO4↓ + 3PO43− + HF | −37.99 | −36.35 | −35.16 | −34.29 | −33.67 |
| 12 | Ca5(PO4)3F + 4H+ + 5SO42− = 5CaSO4↓ + 3HPO42− + HF | −0.02 | 0.05 | 0.28 | 0.58 | 0.94 |
| 13 | Ca5(PO4)3F + 7H+ + 5SO42− = 5CaSO4↓ + 3H2PO4− + HF | 3.13 | 3.11 | 3.30 | 3.58 | 3.95 |
| 14 | Ca5(PO4)3F + 10H+ + 5SO42− = 5CaSO4↓ + 3H3PO4 + HF | 1.13 | 1.34 | 1.76 | 2.23 | 2.75 |
| 15 | Ca5(PO4)3F + H+ + 5HSO4− = 5CaSO4↓ + 3H2PO4− + F− | 10.79 | 7.12 | 4.28 | 2.03 | 0.21 |
| 16 | Ca5(PO4)3F + 4H+ + 5HSO4− = 5CaSO4↓ + 3H3PO4 + F− | 0.04 | −0.32 | −0.30 | −0.18 | 0.03 |
| 17 | Ca5(PO4)3F + 3H+ + 5SO42− = 5CaSO4↓ + 3HPO42− + F− | −1.02 | −1.04 | −0.86 | −0.58 | −0.24 |
| 18 | Ca5(PO4)3F + 6H+ + 5SO42− = 5CaSO4↓ + 3H2PO4− + F− | 6.30 | 6.16 | 6.46 | 6.99 | 7.71 |
| 19 | Ca5(PO4)3F + 9H+ + 5SO42− = 5CaSO4↓ + 3H3PO4 + F− | 0.92 | 1.12 | 1.54 | 2.02 | 2.56 |
| 20 | V2O4 + 4H+ = 2VO2+ + 2H2O | 2.62 | 1.74 | 1.14 | 0.70 | 0.36 |
| 21 | SO42− + H+ = HSO4− | 1.62 | 2.27 | 3.02 | 3.79 | 4.59 |
| 22 | HSiO3− + H+ + H2O = H4SiO4 | 11.80 | 10.69 | 10.10 | 9.82 | 9.76 |
| 23 | SiO44− + 3H+ = HSiO3− + H2O | 13.68 | 12.90 | 12.72 | 12.95 | 13.43 |
| 24 | HCO3− + H+ = CO2↑ + H2O | 7.68 | 7.99 | 8.36 | 8.78 | 9.24 |
| 25 | CO32− + H+ = HCO3− | 10.62 | 10.17 | 10.09 | 10.22 | 10.51 |
| 26 | F− + H+ = HF | 2.98 | 3.33 | 3.70 | 4.07 | 4.48 |
| 27 | H2PO4− + H+ = H3PO4 | −3.54 | −2.80 | −1.83 | −0.92 | −0.04 |
| 28 | HPO42− + H+ = H2PO4− | 7.32 | 7.19 | 7.32 | 7.58 | 7.95 |
| 29 | PO43− + H+ = HPO42− | 12.64 | 12.19 | 12.09 | 12.21 | 12.48 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, Y.; Huang, J.; Liu, T.; Cai, Z.; Xue, N. Selective Leaching of Vanadium from Roasted Stone Coal by Dilute Sulfuric Acid Dephosphorization-Two-Stage Pressure Acid Leaching. Minerals 2016, 6, 75. https://doi.org/10.3390/min6030075
Huang J, Zhang Y, Huang J, Liu T, Cai Z, Xue N. Selective Leaching of Vanadium from Roasted Stone Coal by Dilute Sulfuric Acid Dephosphorization-Two-Stage Pressure Acid Leaching. Minerals. 2016; 6(3):75. https://doi.org/10.3390/min6030075
Chicago/Turabian StyleHuang, Jun, Yimin Zhang, Jing Huang, Tao Liu, Zhenlei Cai, and Nannan Xue. 2016. "Selective Leaching of Vanadium from Roasted Stone Coal by Dilute Sulfuric Acid Dephosphorization-Two-Stage Pressure Acid Leaching" Minerals 6, no. 3: 75. https://doi.org/10.3390/min6030075
APA StyleHuang, J., Zhang, Y., Huang, J., Liu, T., Cai, Z., & Xue, N. (2016). Selective Leaching of Vanadium from Roasted Stone Coal by Dilute Sulfuric Acid Dephosphorization-Two-Stage Pressure Acid Leaching. Minerals, 6(3), 75. https://doi.org/10.3390/min6030075