Sn-Bearing Minerals and Associated Sphalerite from Lead-Zinc Deposits, Kosovo: An Electron Microprobe and LA-ICP-MS Study
Abstract
:1. Introduction
2. Geological Background
2.1. Stan Terg Deposit
2.2. Artana Deposit
2.3. Kizhnica Deposit
2.4. Drazhnje Deposit
3. Methods
4. Results
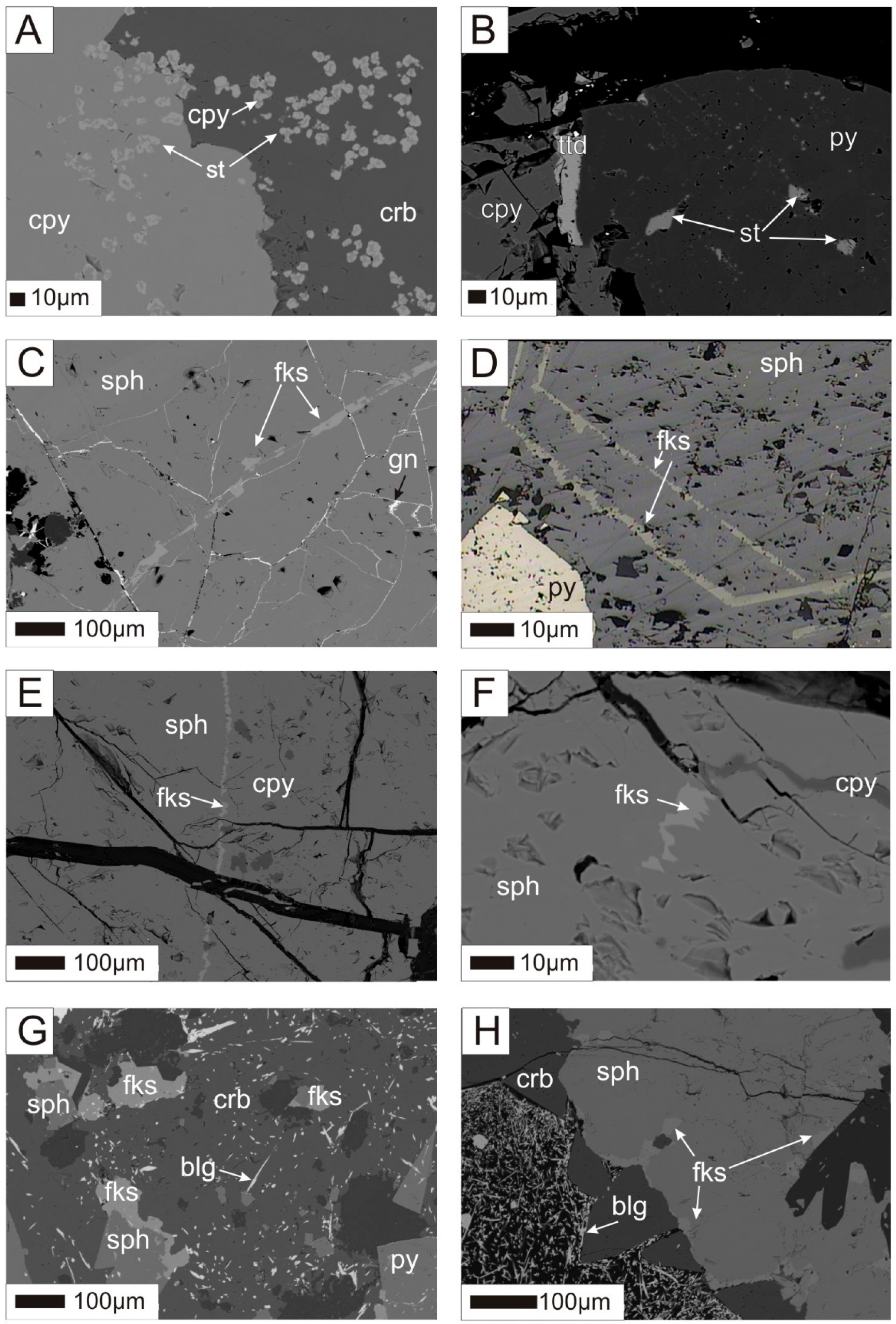
4.1. Mineralogy
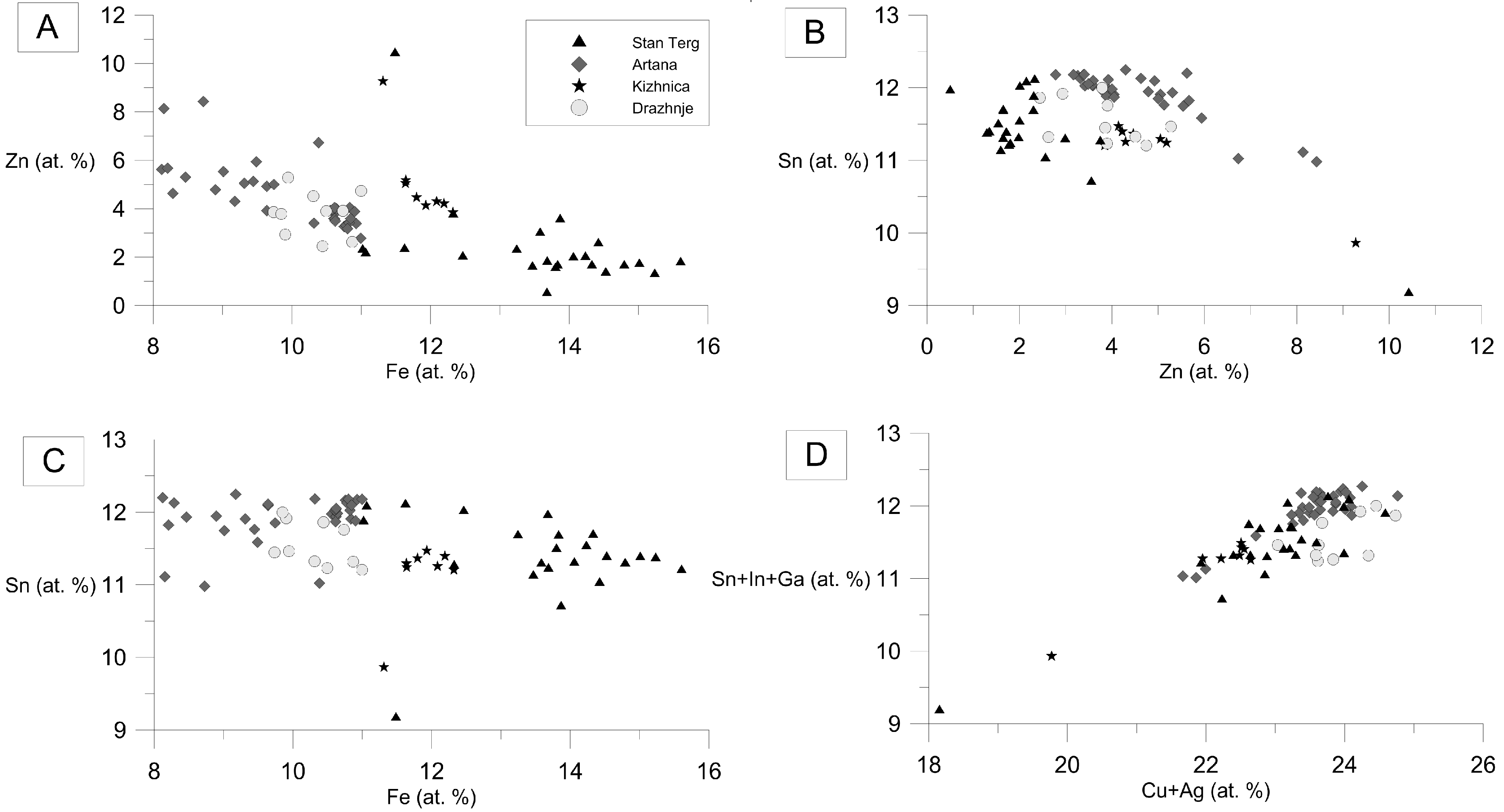
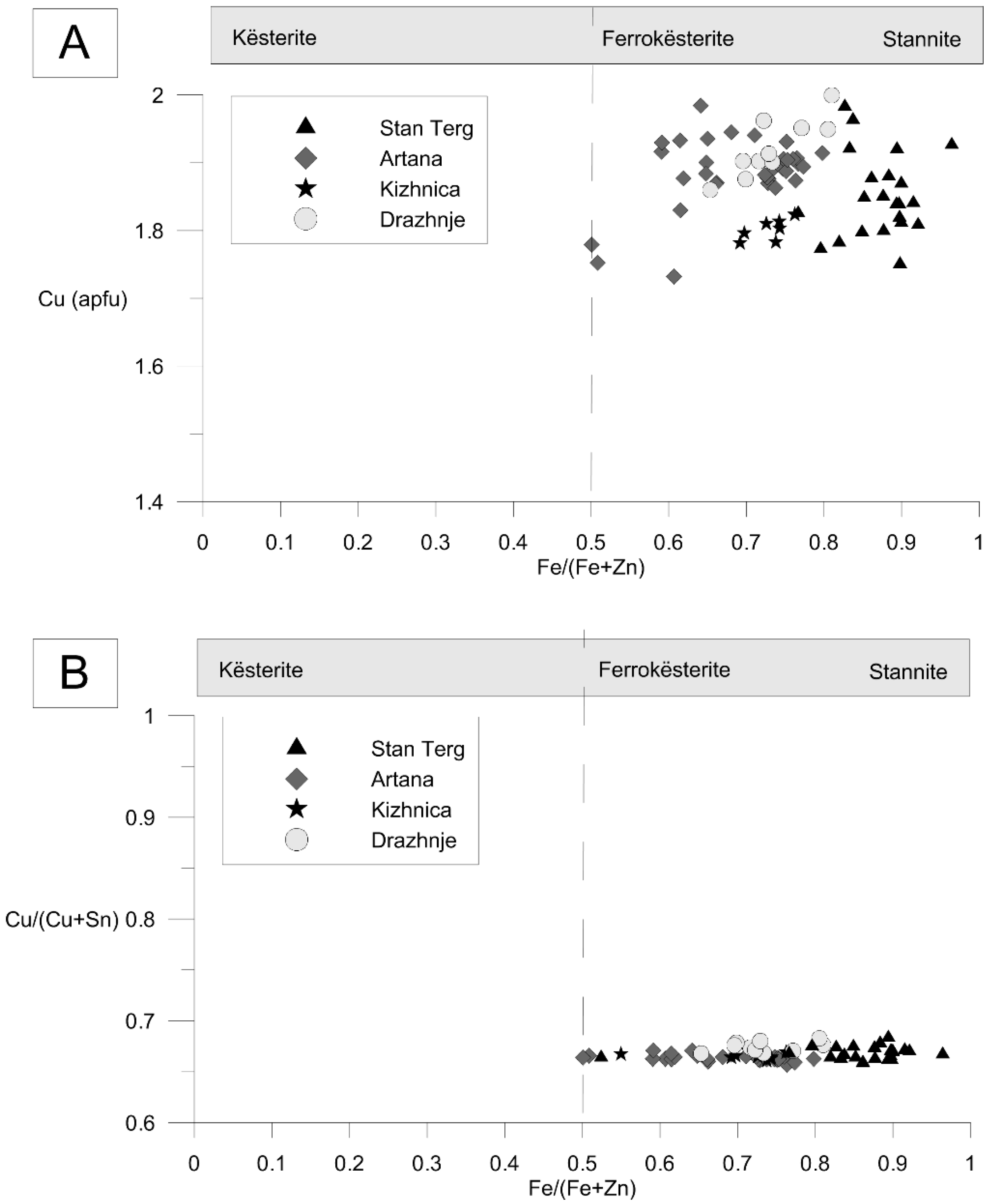
4.2. Chemical Composition of Stannite Group Minerals
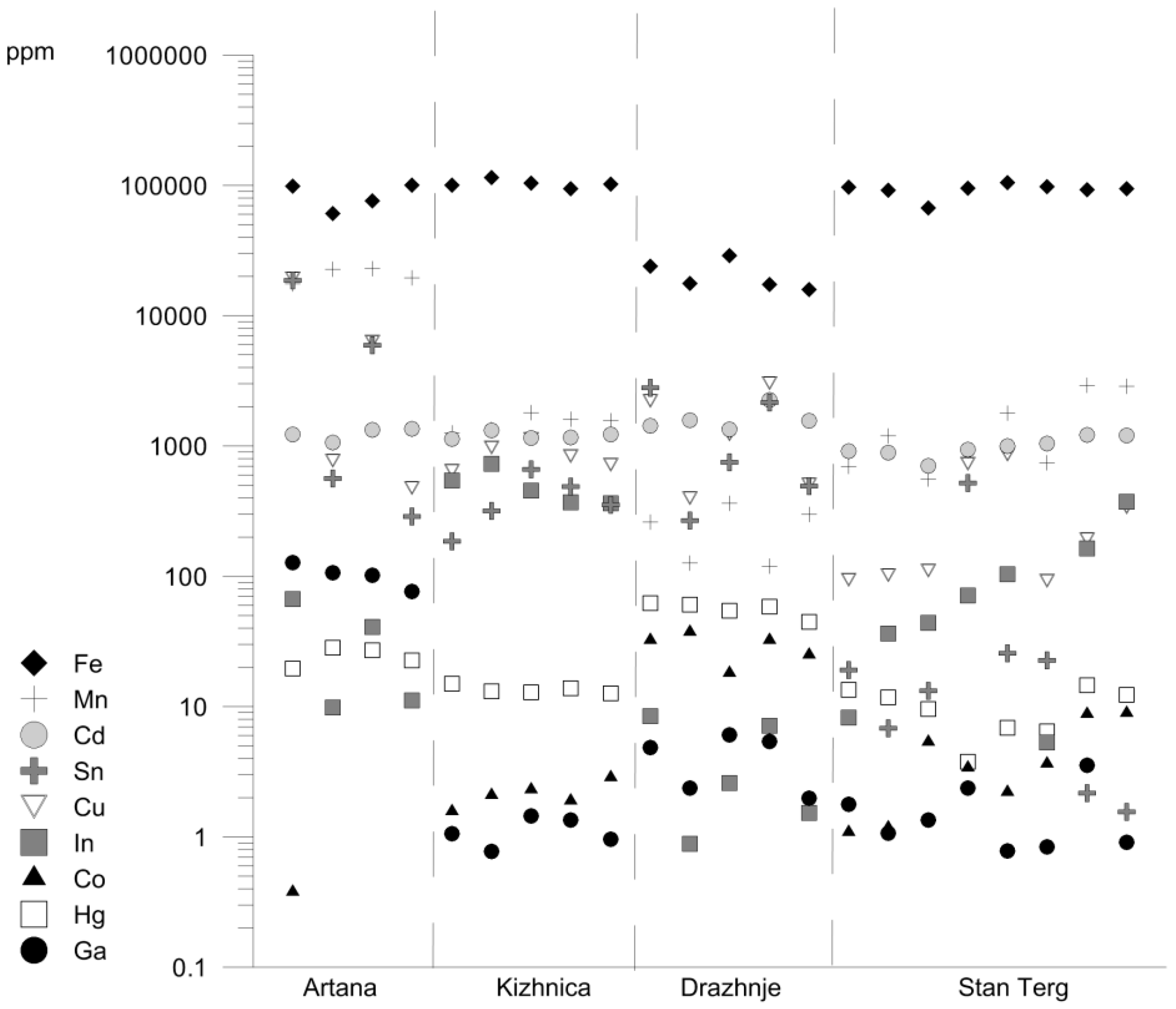
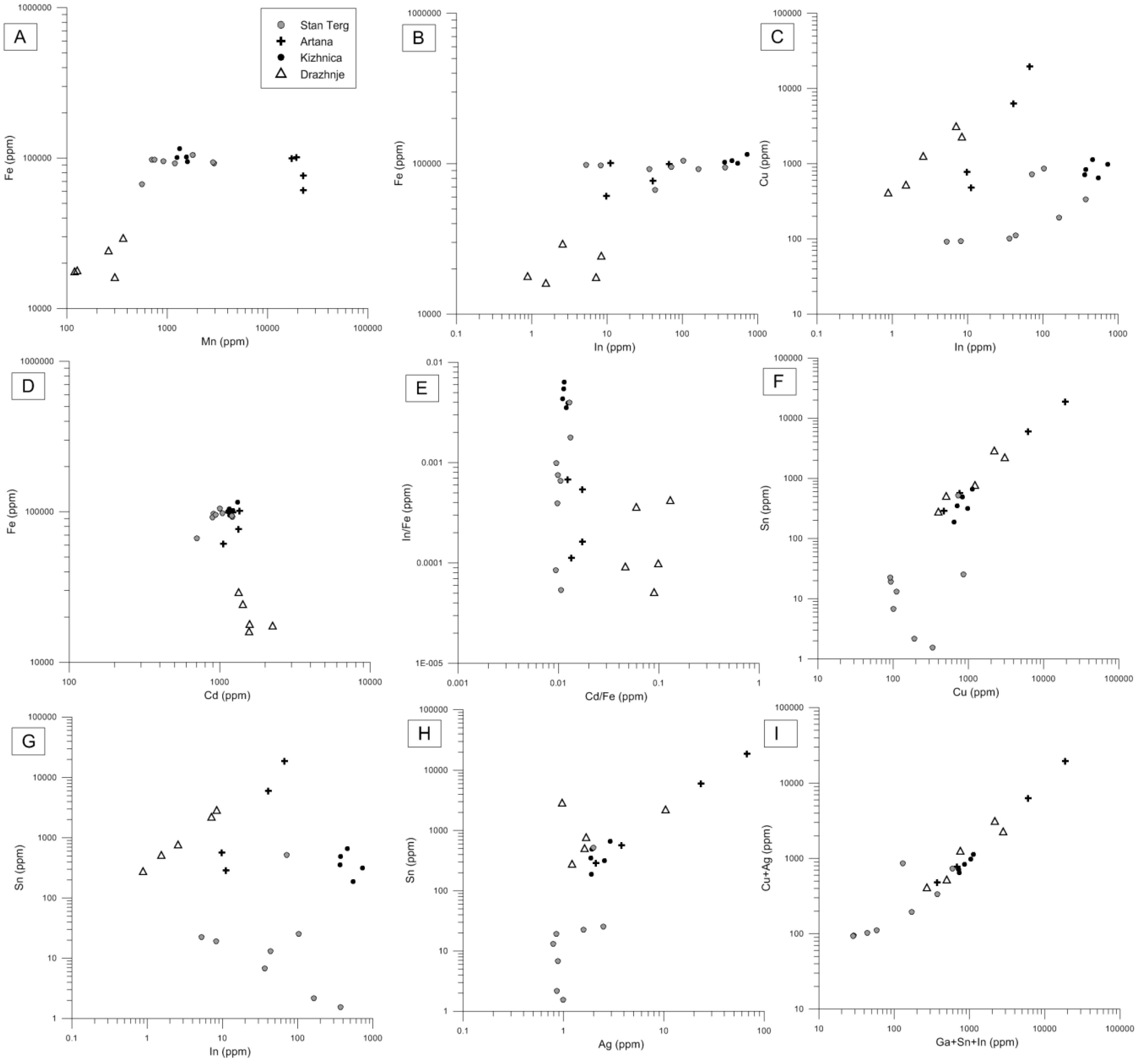
4.3. Chemical Composition of Sphalerite
5. Discussion
5.1. Stannite-Kësterite Solid Solution
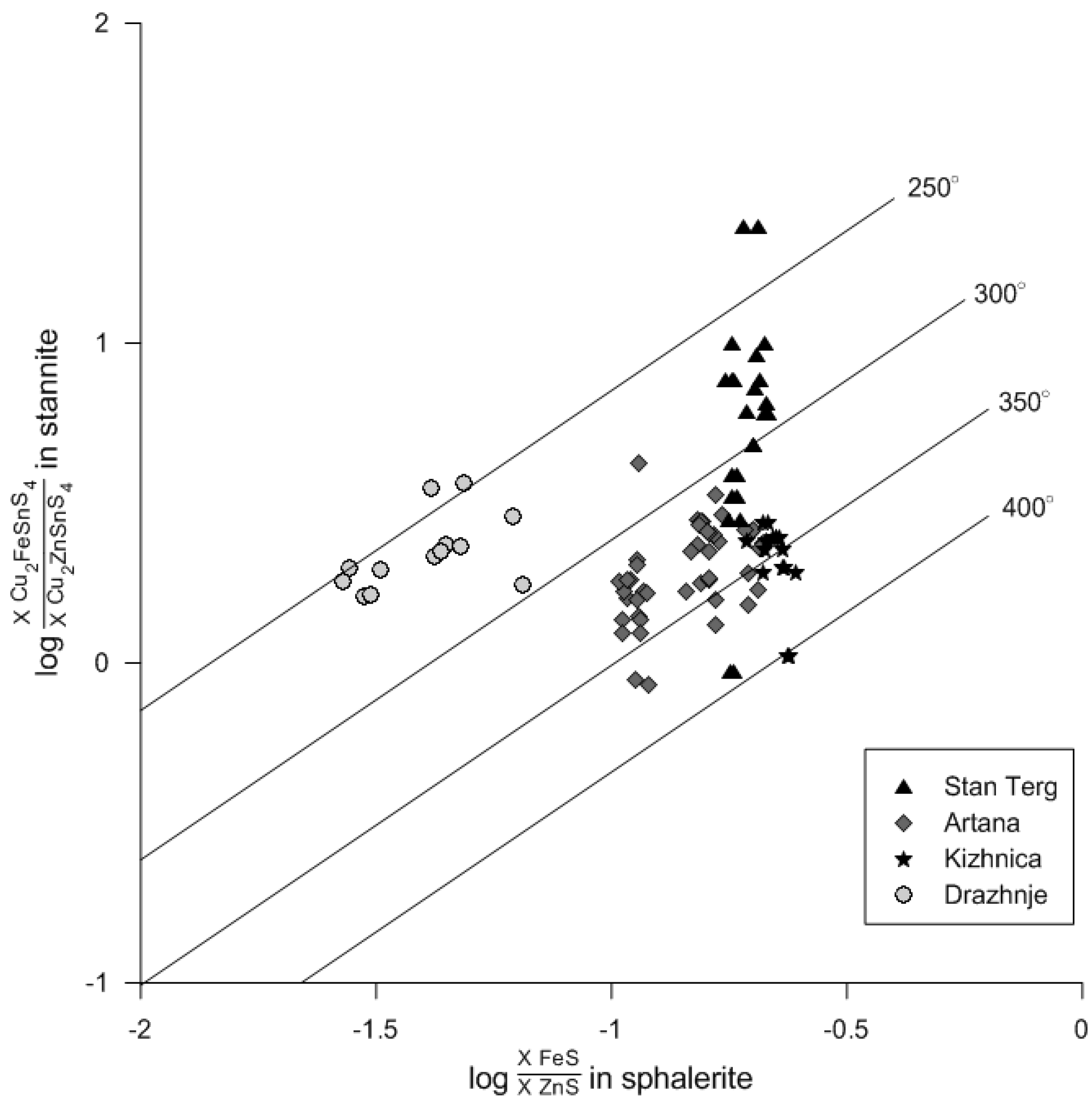
5.2. Implication for Ore Precipitation Conditions
5.3. Partioning of Sn, Zn, and Fe into Sphalerite and Sn-Bearing Sulfide
6. Concluding Remarks
- Tin mineralization is very similar in the Trepça Mineral Belt among different deposits. Only members of stannite-kësterite solid solution were found, and there is no evidence of presence other Sn-minerals enriched in Cu, Ag, Cd, In, or Ge. In a few samples from Stan Terg a phase enriched in In (0.02 apfu) was found and it could fit petrukite.
- The chemical composition of stannite group minerals indicates extensive mutual substitution between Fe and Zn. In the coexisting sphalerites correlations between Sn, Cu, and In, suggest either incorporation of these elements in the structrure, and/or the presence of stannite, kuramite, roquesite, and petrukite micro-inclusions in sphalerite.
- Estimated temperatures on the basis of coexisting sphalerite-stannite group minerals, indicate a wide range of ore precipitation in each deposit: 240–390 °C (Stan Terg), 240–370 °C (Artana), >340 °C (Kizhnica), and 245–295 °C (Drazhnje). Although sphalerite and Sn-minerals precipitated simultaneously during cooling of the hydrothermal fluids, oscillatory zoning in sphalerite (as evidenced by ferrokësterite growth zones) suggests fluctuation in physico-chemical conditions, and short-time intervals of magmatic pulses.
- Tin-bearing minerals and sphalerite at Kosovo contain small amounts of In, Ga, and Ge, and may be considered as potential carriers of critical metals in the district with implication for future exploration.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SGM | Stannite Group Minerals |
| TMB | Trepça Mineral Belt |
| LA-ICP-MS | Laser ablation inductively coupled plasma mass spectrometry |
References
- Kesler, S.E.; Wilkinson, B.H. Tectonic-diffusion estimates of global minerals resources: Extending the method: Granitic tin deposits. In Ore Deposits in an Evolving Earth; Jenkin, G.R.T., Lusty, P.A.J., McDonald, I., Smith, M.P., Boyce, A.J., Wilkinson, J.J., Eds.; Geological Society: London, UK, 2013; Volume 393, pp. 277–290. [Google Scholar]
- Ishibashi, M. A Sn-Te-Bi-Sb paragenesis in ores from the suttsu mine, Hokkaidô. J. Fac. Sci. Hokkaido Univ. 1952, 8, 97–106. [Google Scholar]
- Wenyuan, L.; Cook, N.J.; Ciobanu, C.L.; Yu, L.; Xiaoping, Q.; Yuchuan, C. Mineralogy of tin-sulfides in the Zijinshan porphyry–epithermal system, Fujian Province, China. Ore Geol. Rev. 2016, 72, 682–698. [Google Scholar] [CrossRef]
- Novák, F.; Blüml, A.; Tacl, A. The origin of stannite by replacement of cassiterite in the Turkaňk zone of the Kutná Hora ore deposit. Mineral. Mag. 1962, 33, 339–342. [Google Scholar] [CrossRef]
- Kettaneh, Y.A.; Badham, J.P.N. Mineralization and paragenesis at the Mount Wellington Mine, Cornwall. Econ. Geol. 1978, 73, 486–495. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Pinto, A.; Migeon, H.; Wagner, F.E. Tin and indium mineralogy within selected samples from the Neves Corvo ore deposit (Portugal): A multidisciplinary study. Miner. Eng. 2003, 16, 1291–1302. [Google Scholar] [CrossRef]
- Brill, B.A. Trace-element contents and partitioning of elements in ore minerals from the CSA Cu–Pb–Zn deposit, Australia. Can. Mineral. 1989, 27, 263–274. [Google Scholar]
- Catchpole, H.; Kouzmanov, K.; Fontboté, L. Copper-excess stannoidite and tennantite-tetrahedrite as proxies for hydrothermal fluid evolution in a zoned Cordilleran base metal district, Morococha, central Peru. Can. Mineral. 2012, 50, 719–743. [Google Scholar] [CrossRef]
- Kase, K. Tin, Arsenic, Zinc and Silver vein mineralization in the Besshi mine, central Shikoku. Min. Geol. 1988, 38, 407–418. [Google Scholar]
- Petruk, W. Tin sulphides from the deposit of Brunswick Tin Mines Limited. Can. Mineral. 1973, 12, 46–54. [Google Scholar]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Bonsall, T.A.; Tarkian, M.; Solomos, Ch. Carbonate-replacement Pb–Zn–Ag ± Au mineralization in the Kamariza area, Lavrion, Greece: Mineralogy and thermochemical conditions of formation. Mineral. Petrol. 2008, 94, 85–106. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.G.; Moritz, R.; Papavassiliou, K.; Falalakis, G. Mineralogy and geochemical environment of formation of the Perama Hill high-sulfidation epithermal Au-Ag-Te-Se deposit, Petrota Graben, NE Greece. Mineral. Petrol. 2011, 103, 79–100. [Google Scholar] [CrossRef]
- Repstock, A.; Voudouris, P.; Kolitsch, U. New occurrences of watanabeite, colusite, “arsenosulvanite” and Cu-excess tetrahedrite-tennantite at the Pefka high-sulfidation epithermal deposit, northeastern Greece. Neues Jahrb. Mineral. Abh. 2015, 192, 135–149. [Google Scholar] [CrossRef]
- Zarić, P.; Janković, S.; Radosavljević, S.; Dordević, D. Tin minerals and tin mineralization of the Pb-Zn-Sb-Ag ore deposits of the Srebrenica orefield. In Proceedings of the International Symposium Geology and Metallogeny of the Dinarides and the Vardar Zone; Academy of Sciences and Arts of the Republic of Srpska: Banja Luka, Bosnia and Herzegovina, 2000; pp. 425–434. [Google Scholar]
- Nekrasov, I.Y.; Sorokin, V.I.; Osadchii, E.G. Fe and Zn partitioning between stannite and sphalerite and its application in geothermometry. Phys. Chem. Earth 1979, 11, 739–742. [Google Scholar] [CrossRef]
- Springer, G. The pseudobinary system Cu2FeSnS,-Cu2ZnSnS4 and its mineralogical significance. Can. Mineral. 1972, 11, 535–541. [Google Scholar]
- Nakamura, Y.; Shima, H. Fe and Zn partitioning between sphalerite and stannite. In Proceedings of the Joint Meeting of Society of Mining Geologists of Japan; The Japanese Association of Mineralogists, Petrologists and Economic Geologists and the Mineralogical Society of Japan: Sendai, Japan, 1982. (In Japanese) [Google Scholar]
- Shimizu, M.; Shikazono, N. Iron and zinc partitioning between coexisting stannite and sphalerite: A possible indicator of temperature and sulfur fugacity. Miner. Depos. 1985, 20, 314–320. [Google Scholar] [CrossRef]
- Wagner, T.; Mlynarczyk, M.S.; Williams-Jones, A.E.; Boyce, A.J. Stable isotope constraints on ore formation at the San Rafael tin-copper deposit, Southeast Peru. Econ. Geol. 2009, 104, 223–248. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoshino, K.; Myint, K.K.; Miyazaki, K.; Nishido, H. Stannite from the Otoge kaolin-pyrophyllite deposits, Yamagata Prefecture, NE Japan and its genetical significance. Resour. Geol. 1994, 44, 439–444. [Google Scholar]
- Ohta, E. Occurrence and chemistry of indium-containing minerals from the Toyoha mine, Hokkaido, Japan. Min. Geol. 1989, 39, 355–372. [Google Scholar]
- Radosavljevic, S.A.; Rakic, S.M.; Stojanovic, J.N.; Radosavljevic-Mihajlovic, A.S. Occurrence of Petrukite in Srebrenica Orefield, Bosnia and Herzegovina. Neues Jahrb. Mineral. Abh. 2005, 181, 21–26. [Google Scholar] [CrossRef]
- Féraud, J.; Deschamps, Y. French Scientific Cooperation 2007–2008 on the Trepça Lead-Zinc-Silver Mine and the Gold Potential of Novo Brdo/Artana Tailings (Kosovo); BRGM Report No RP-57204-FR; BRGM: Orléans, France, 2009; p. 92. [Google Scholar]
- Strmić Palinkaš, S.; Palinkaš, L.A.; Renac, C.; Spangenberg, J.E.; Lüders, V.; Molnar, F.; Maliqi, G. Metallogenic model of the Trepča Pb-Zn-Ag Skarn deposit, Kosovo: Evidence from fluid inclusions, rare earth elements, and stable isotope data. Econ. Geol. 2013, 108, 135–162. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Pršek, J.; Melfos, V.; Voudouris, P.C.; Maliqi, F.; Kozub-Budzyń, G. Bismuth minerals from the Stan Terg deposit (Trepça, Kosovo). Neues Jahrb. Mineral. Abh. 2015, 192, 317–333. [Google Scholar]
- Lehmann, S.; Barcikowski, J.; Von Quadt, A.; Gallhofer, D.; Peytcheva, I.; Heinrich, C.A.; Serafimovski, T. Geochronology, geochemistry and isotope tracing of the Oligocene magmatism of the Buchim–Damjan–Borov Dol ore district: Implications for timing, duration and source of the magmatism. Lithos 2013, 180–181, 216–233. [Google Scholar] [CrossRef]
- Hyseni, M.; Durmishaj, B.; Fetahaj, B.; Shala, F.; Berisha, A.; Large, D. Trepça Ore Belt and Stan Terg mine—Geological overview and interpretation, Kosovo (SE Europe). Geologija 2010, 51, 87–92. [Google Scholar] [CrossRef]
- Forgan, C.B. Ore deposits at the Stan Terg lead-zinc mine International Geological Congress. In The Geology, Paragenesis, and Reserves of Ores of Lead and Zinc; Co-operative Wholesale Society Ltd.: Reddish, UK, 1948; Volume 7, pp. 290–307. [Google Scholar]
- Smejkal, S. Structure, Mineralization, Mineralogical Parageneses and Genesis of Lead-Zinc Deposits of Kopaonic District. Ph.D. Thesis, Faculty of Mining and Geology University of Belgrade, Beograd, Serbia, 1960. [Google Scholar]
- Jankovic, S. The isotopic composition of lead in some Tertiary lead-zinc deposits within the Serbo-macedonian metallogenic province (Yugoslavia). Ann. Geol. Pénins. Balk. 1978, 42, 507–525. [Google Scholar]
- Jankovic, S. The principal metallogenic features of the Kopaonik District. In Proceedings of the Geology and Metallogeny of the Kopaonik Mt Symposium, Belgrade, Serbia, 19–22 June 1995; pp. 79–101.
- Simić, M. Metallogeny of the Dražnja-Propaštica-Novo Brdo ore field in the Vardar Zone. In Proceedings of the International Symposium Geology and Metalogeny of the Dinarides and the Vardar Zone; Academy of Sciences and Arts of the Republic of Srpska: Banja Luka, Bosnia and Herzegovina, 2000; Volume 1, pp. 409–424. [Google Scholar]
- Dangić, A. Minor element distribution between galena and sphalerite as a geothermometer—Application to two lead-zinc areas in Yugoslavia. Econ. Geol. 1985, 80, 180–183. [Google Scholar] [CrossRef]
- Durmishaj, B.; Tashko, A.; Sinojmeri, A.; Neziraj, A. Chemical composition of mineral phases form Hajvalja, Badovc and Kizhnica (Kosovo). Bul. Shk. Gjeol. 2006, 2, 91–100. (In Albanian) [Google Scholar]
- Popović, R. Distribution of base and precious metals in the Lece volcano-intrusive massif (Vardar Zone). In Proceedings of the International Symposium Geology and Metalogeny of the Dinarides and the Vardar Zone; Academy of Sciences and Arts of the Republic of Srpska: Banja Luka, Bosnia and Herzegovina, 2000; Volume 1, pp. 443–452. [Google Scholar]
- Guillong, M.; Meier, D.L.; Allan, M.M.; Heinrich, C.A.; Yardley, B.W.D. Appendix A6: SILLS: A MATLAB-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course 40; Mineralogical Association of Canada: Vancouver, BC, Canada, 2008; pp. 328–333. [Google Scholar]
- Nekrasov, I.Y.; Sorokin, V.I.; Osadchii, E.G. Partition of iron and zinc between sphalerite and stannite at T = 300 to 500 °C and P = 1 kb. Dokl. Earth Sci. 1976, 226, 136–138. [Google Scholar]
- Bortnikov, N.S.; Zaozerina, O.N.; Genkin, A.D.; Muravitskaya, G.N. Stannite-sphalerite intergrowths—Possible indicators of conditions of ore deposition. Int. Geol. Rev. 1990, 32, 1132–1144. [Google Scholar] [CrossRef]
- Smejkal, S. Lead-zinc deposits of the Tetriary metallogeny in the Kopaonik area. In Excursion Guide. Savez Geoloških Društava FNRJ; Savjetovanje Geologa FNRJ: Beograd, Serbia, 1962; pp. 51–58. [Google Scholar]
- Kissin, S.A.; Owens, D.R. New data on stannite and related tin sulfide minerals. Can. Mineral. 1979, 17, 125–135. [Google Scholar]
- Kissin, S.A. A reinvestigation of the stannite (Cu2FeSnS4)–kësterite (Cu2ZnSnS4) pseubinary system. Can. Mineral. 1989, 27, 689–697. [Google Scholar]
- Bonazzi, P.; Bindi, L.; Bernardini, G.; Menchetti, S. A model for the mechanism of incorporation of Cu, Fe and Zn in the stannite–kësterite series, Cu2FeSnS4–Cu2ZnSnS4. Can. Mineral. 2003, 41, 639–647. [Google Scholar] [CrossRef]
- Hall, S.R.; Szymanski, J.T.; Stewart, J.M. Kësterite, Cu2(Zn,Fe)SnS4 and stannite, Cu2 (Fe,Zn) SnS4, structurally similar but distinct minerals. Can. Mineral. 1978, 16, 131–137. [Google Scholar]
- Di Benedetto, F.; Bernardini, G.P.; Borrini, D.; Lottermoser, W.; Tippelt, G.; Amthauer, G. 57Fe- and 119Sn-Mössbauer study on stannite (Cu2FeSnS4)–kesterite (Cu2ZnSnS4) solid solution. Phys. Chem. Miner. 2005, 31, 683–690. [Google Scholar] [CrossRef]
- Rusakov, V.S.; Chistyakova, N.I.; Burkovsky, I.A.; Gapochka, A.M.; Evstigneeva, T.L.; Schorr, S. Mössbauer study of isomorphous substitutions in Cu2Fe1−xCuxSnS4 and Cu2Fe1−xZnxSnS4 series. J. Phys. 2010, 217. [Google Scholar] [CrossRef]
- Springer, G. Electronprobe analyses of stannite and related tin minerals. Miner. Mag. 1968, 36, 1045–1051. [Google Scholar] [CrossRef]
- Moore, F.; Howie, R.A. Tin-bearing sulphides from St Michael’s Mount and Cligga Head, Cornwall. Miner. Mag. 1984, 48, 389–396. [Google Scholar] [CrossRef]
- Evstigneeva, T.L.; Rusakov, V.S.; Kabalov, Y.K. Isomorphism in the minerals of the stannite family. New Data Miner. 2003, 38, 65–70. [Google Scholar]
- Schorr, S.; Hoebler, H.J.; Tovar, M. A neutron diffraction study of the stannite-kesterite solid solution series. Eur. J. Mineral. 2007, 19, 65–73. [Google Scholar] [CrossRef]
- Stoiber, R.E. Minor elements in sphalerite. Econ. Geol. 1940, 35, 501–519. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Ye, L.; Cook, N.J.; Ciobanu, C.L.; Liu, Y.P.; Zhang, Q.; Gao, W.; Yang, Y.L.; Danyushevsky, L.V. Trace and minor elements in sphalerite from base metal deposits in South China: A LA-ICPMS study. Ore Geol. Rev. 2011, 39, 188–217. [Google Scholar] [CrossRef]
- Evrard, C.; Fouquet, Y.; Moëlo, Y.; Rinnert, E.; Etoubleau, J.; Langlade, J.A. Tin concentration in hydrothermal sulphides related to ultramafic rocks along the Mid-Atlantic Ridge: A mineralogical study. Eur. J. Mineral. 2015, 27, 627–638. [Google Scholar] [CrossRef]
- Ono, S.; Hirai, K.; Matsueda, H.; Kabashima, T. Polymetallic mineralization at the Suttsu vein-type deposit, southwestern Hokkaido, Japan. Resour. Geol. 2004, 54, 453–464. [Google Scholar] [CrossRef]
- Dobrovol’skaya, M.G.; Genkin, A.D.; Bortnikov, N.S.; Golovanova, T.I. Unusual sphalerite, chalcopyrite, and stannite intergrowths at tin deposits. Geol. Ore Depos. 2008, 50, 75–85. [Google Scholar] [CrossRef]
- Oen, I.S.; Kager, P.; Kieft, C. Oscillatory zoning of a discontinuous solid-solution series: Sphalerite-stannite. Am. Mineral. 1980, 65, 1220–1232. [Google Scholar]

| Elements | Stan Terg | Artana | Kizhnica | Drazhnje | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Cu | 27.09 | 26.81 | 28.57 | 27.46 | 28.09 | 28.26 | 26.01 | 28.20 | 26.08 | 27.94 | 27.12 | 26.32 | 26.95 | 27.38 | 27.70 | 27.92 | 27.66 | 26.92 |
| Fe | 15.73 | 12.83 | 14.10 | 15.09 | 11.32 | 9.19 | 9.13 | 9.72 | 11.01 | 8.38 | 12.47 | 12.75 | 12.56 | 13.10 | 11.04 | 11.43 | 10.63 | 10.21 |
| Zn | 1.57 | 4.57 | 1.96 | 1.64 | 3.37 | 5.79 | 10.32 | 6.17 | 8.35 | 6.77 | 5.52 | 5.31 | 5.10 | 4.78 | 4.70 | 5.77 | 5.44 | 6.34 |
| Sn | 24.93 | 24.90 | 24.74 | 25.12 | 26.75 | 26.24 | 24.23 | 26.40 | 24.83 | 25.66 | 25.54 | 25.22 | 25.66 | 25.32 | 25.67 | 24.75 | 24.78 | 25.02 |
| S | 28.75 | 29.83 | 29.79 | 29.46 | 29.46 | 29.62 | 29.86 | 29.69 | 30.18 | 29.14 | 30.19 | 30.48 | 30.16 | 30.46 | 29.36 | 29.34 | 29.57 | 29.49 |
| In | bd | 0.06 | 0.43 | 0.65 | 0.27 | 0.09 | 0.07 | 0.04 | 0.01 | 0.10 | 0.08 | 0.05 | 0.05 | 0.03 | 0.02 | 0.04 | bd | bd |
| Mn | 0.34 | 0.02 | bd | 0.13 | 0.41 | 0.31 | 0.29 | 0.31 | 0.59 | 0.42 | 0.03 | 0.03 | bd | 0.02 | 0.17 | 0.19 | 0.28 | 0.25 |
| Ag | 0.29 | bd | bd | bd | 0.10 | 0.13 | 0.04 | 0.15 | 0.09 | 0.10 | 0.02 | 0.05 | 0.01 | 0.02 | bd | bd | bd | 0.01 |
| Hg | bd | bd | 0.03 | bd | bd | bd | bd | bd | 0.01 | 0.03 | 0.02 | bd | bd | bd | bd | bd | bd | 0.04 |
| Ga | 0.04 | 0.02 | bd | bd | 0.02 | bd | bd | bd | 0.01 | bd | bd | bd | bd | 0.05 | bd | 0.02 | bd | bd |
| Total | 98.74 | 99.04 | 99.62 | 99.55 | 99.79 | 99.63 | 99.95 | 100.68 | 101.16 | 98.54 | 101.76 | 100.21 | 100.49 | 101.16 | 98.66 | 99.46 | 98.36 | 98.28 |
| Chemical formula based on four cations | ||||||||||||||||||
| Cu | 1.81 | 1.83 | 1.92 | 1.84 | 1.91 | 1.94 | 1.75 | 1.90 | 1.73 | 1.93 | 1.81 | 1.78 | 1.81 | 1.82 | 1.90 | 1.88 | 1.90 | 1.86 |
| Fe | 1.18 | 0.98 | 1.06 | 1.13 | 0.87 | 0.71 | 0.69 | 0.73 | 0.82 | 0.65 | 0.93 | 0.97 | 0.95 | 0.98 | 0.85 | 0.86 | 0.82 | 0.79 |
| Zn | 0.10 | 0.30 | 0.13 | 0.11 | 0.22 | 0.38 | 0.67 | 0.40 | 0.53 | 0.45 | 0.35 | 0.34 | 0.33 | 0.30 | 0.31 | 0.37 | 0.36 | 0.42 |
| Sn | 0.88 | 0.89 | 0.88 | 0.89 | 0.96 | 0.95 | 0.87 | 0.94 | 0.87 | 0.93 | 0.90 | 0.90 | 0.91 | 0.89 | 0.93 | 0.88 | 0.90 | 0.91 |
| S | 3.74 | 3.96 | 3.91 | 3.83 | 3.92 | 3.96 | 3.92 | 3.90 | 3.91 | 3.93 | 3.93 | 4.03 | 3.96 | 3.96 | 3.93 | 3.85 | 3.97 | 3.97 |
| In | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.03 | 0.00 | 0.00 | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.04 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.02 | 0.02 |
| Ag | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ga | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| XFe/XZn | 11.80 | 3.27 | 8.15 | 10.27 | 3.95 | 1.87 | 1.03 | 1.83 | 1.55 | 1.44 | 2.66 | 2.85 | 2.88 | 3.27 | 2.74 | 2.32 | 2.28 | 1.88 |
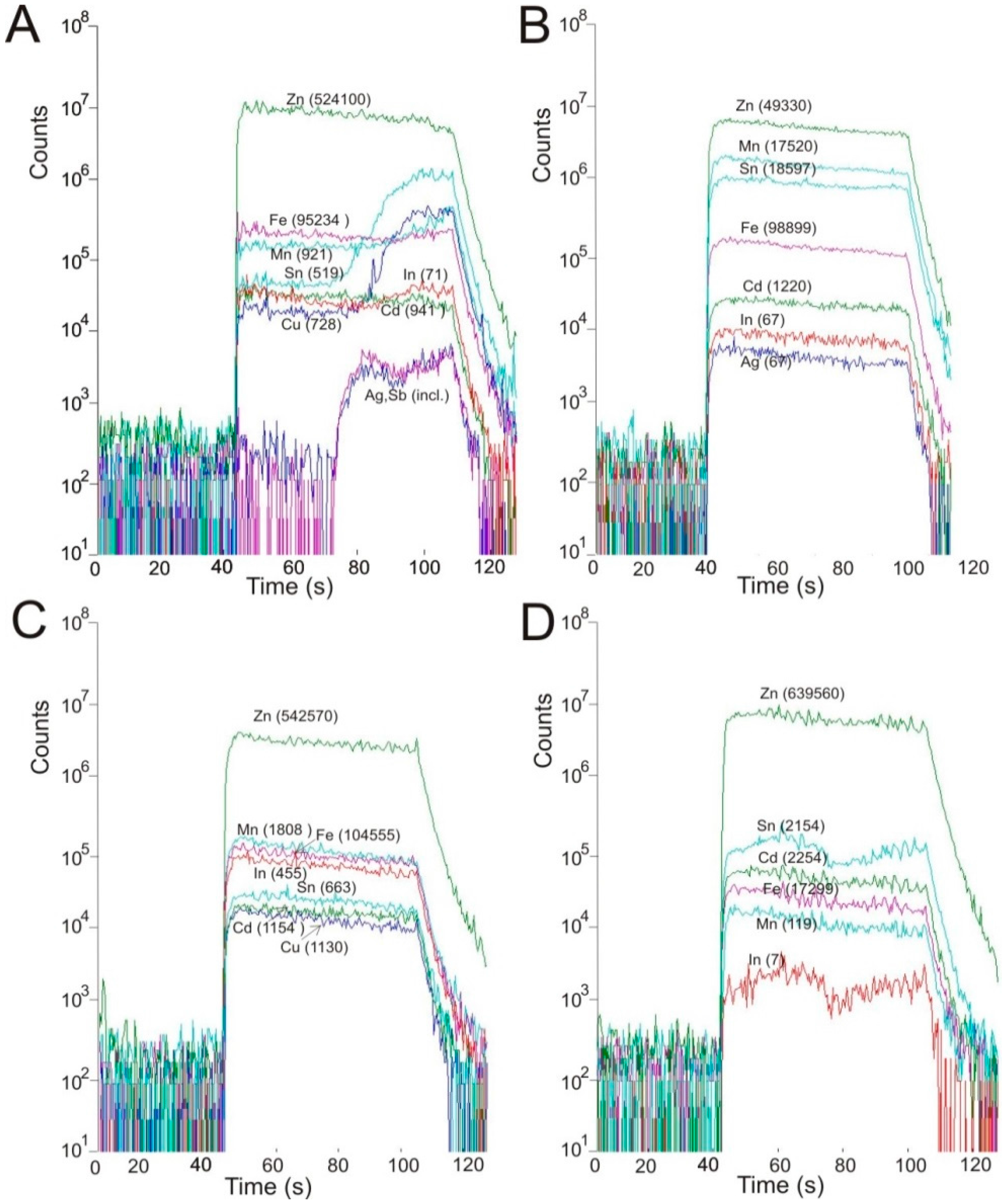
| Elements | Stan Terg | EPMA | Artana | EPMA | Kizhnica | EPMA | Drazhnje | EPMA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | 524,100 | 547,800 | 53.42 | 543,380 | 563,160 | 55.80 | 544,690 | 542,570 | 53.94 | 645,600 | 654,430 | 63.97 |
| Fe | 95,234 | 92,029 | 11.37 | 76,359 | 100,781 | 11.31 | 115,454 | 104,555 | 11.88 | 23,947 | 17,635 | 2.59 |
| Mn | 699 | 1,196 | 0.16 | 22,879 | 19,515 | 1.97 | 1,337 | 1,808 | 0.18 | 260 | 126 | 0.21 |
| Cd | 921 | 892 | 0.06 | 1,331 | 1,351 | 0.06 | 1,313 | 1,154 | 0.04 | 1,428 | 1,575 | 0.14 |
| Sn | 519 | 6.8 | 0.21 | 5,936 | 286 | 1.66 | 316 | 663 | 0.03 | 2,787 | 267 | 0.05 |
| Cu | 728 | 101 | 0.85 | 6,288 | 474 | 1.86 | 973 | 1,130 | 0.08 | 2,215 | 398 | 0.14 |
| In | 71 | 36 | 0.03 | 40 | 11 | bd | 730 | 455 | 0.05 | 8.4 | 0.88 | bd |
| Co | 3.4 | 1.2 | n.a. | <0.29 | <0.20 | n.a. | 2.1 | 2.3 | n.a. | 32.3 | 37 | n.a. |
| Hg | 3.8 | 11 | bd | 26 | 22 | bd | 13 | 13 | bd | 62 | 6 | 0.02 |
| Ga | 2.4 | 1.1 | bd | 101 | 76 | bd | 0.78 | 1.4 | bd | 4.8 | 2.5 | bd |
| Ag | 2.0 | 0.88 | bd | 23 | 2.1 | bd | 2.5 | 2.9 | bd | 0.97 | 1.2 | bd |
| S | n.a. | n.a. | 32.91 | n.a. | n.a. | 33.34 | n.a. | n.a. | 33.84 | n.a. | n.a. | 32.44 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejczyk, J.; Pršek, J.; Voudouris, P.; Melfos, V.; Asllani, B. Sn-Bearing Minerals and Associated Sphalerite from Lead-Zinc Deposits, Kosovo: An Electron Microprobe and LA-ICP-MS Study. Minerals 2016, 6, 42. https://doi.org/10.3390/min6020042
Kołodziejczyk J, Pršek J, Voudouris P, Melfos V, Asllani B. Sn-Bearing Minerals and Associated Sphalerite from Lead-Zinc Deposits, Kosovo: An Electron Microprobe and LA-ICP-MS Study. Minerals. 2016; 6(2):42. https://doi.org/10.3390/min6020042
Chicago/Turabian StyleKołodziejczyk, Joanna, Jaroslav Pršek, Panagiotis Voudouris, Vasilios Melfos, and Burim Asllani. 2016. "Sn-Bearing Minerals and Associated Sphalerite from Lead-Zinc Deposits, Kosovo: An Electron Microprobe and LA-ICP-MS Study" Minerals 6, no. 2: 42. https://doi.org/10.3390/min6020042
APA StyleKołodziejczyk, J., Pršek, J., Voudouris, P., Melfos, V., & Asllani, B. (2016). Sn-Bearing Minerals and Associated Sphalerite from Lead-Zinc Deposits, Kosovo: An Electron Microprobe and LA-ICP-MS Study. Minerals, 6(2), 42. https://doi.org/10.3390/min6020042








