Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results and Discussion
3.1. Roasting Process
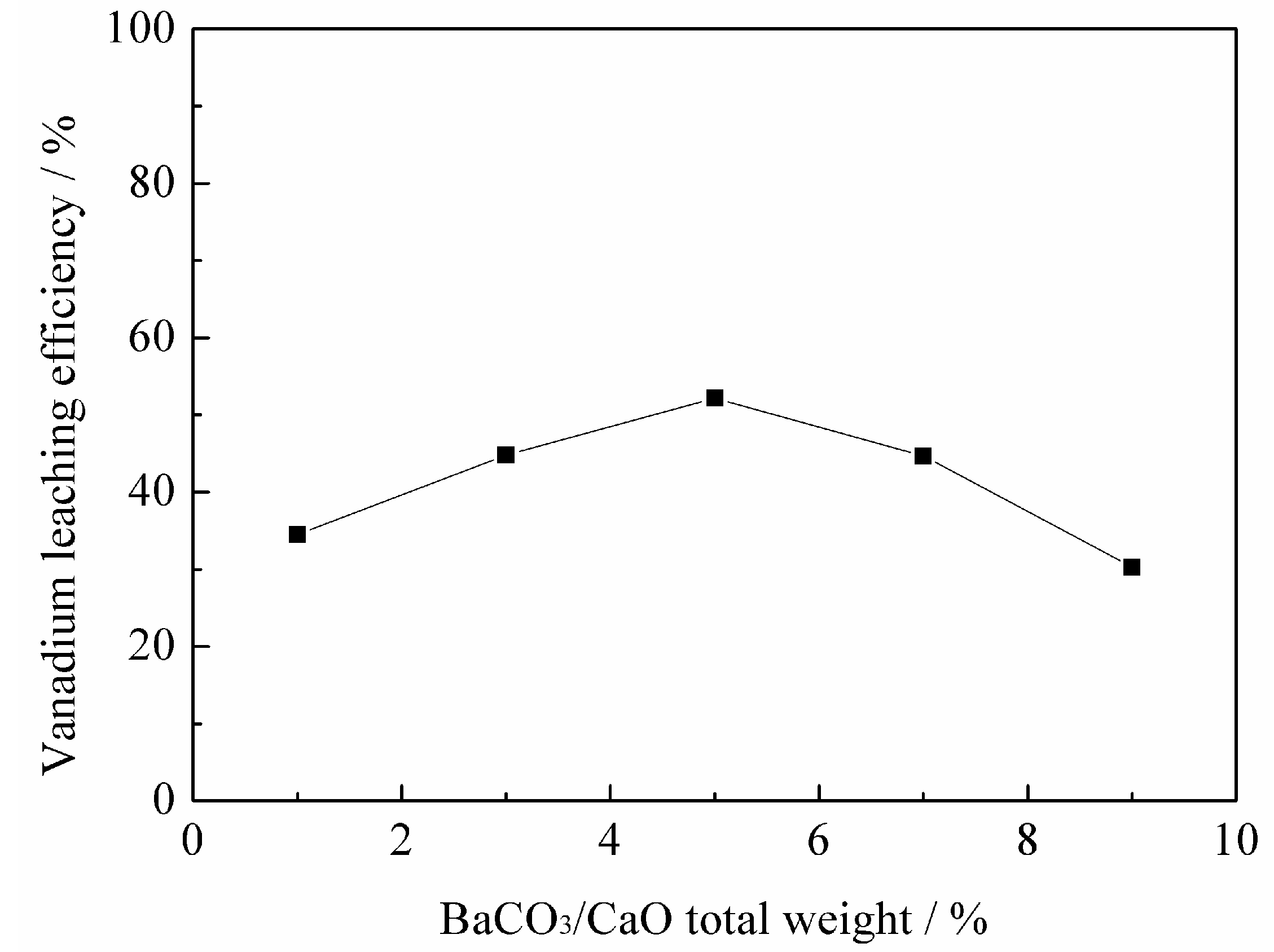
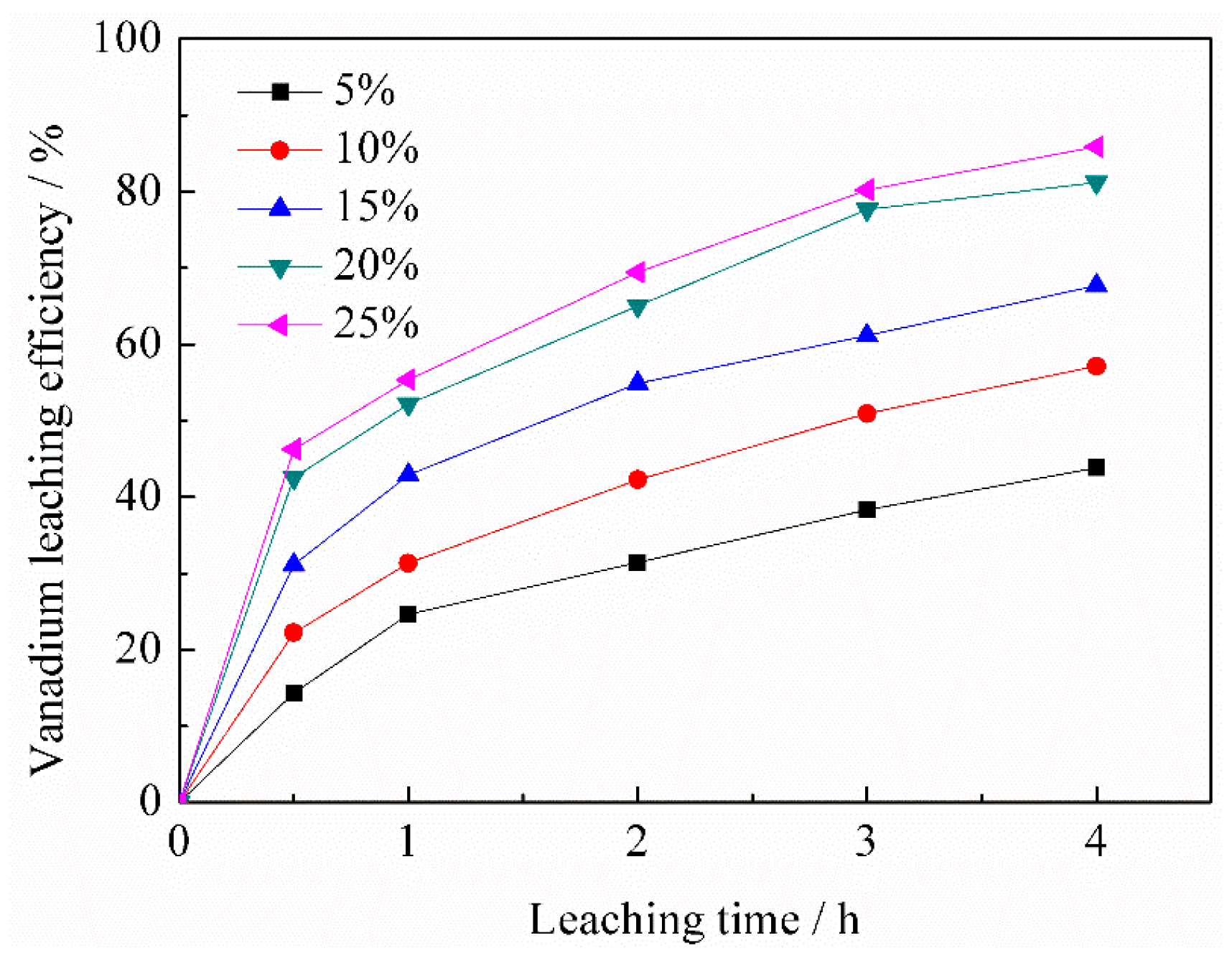
3.1.1. Effect of BaCO3/CaO Total Weight on Vanadium Leaching Efficiency
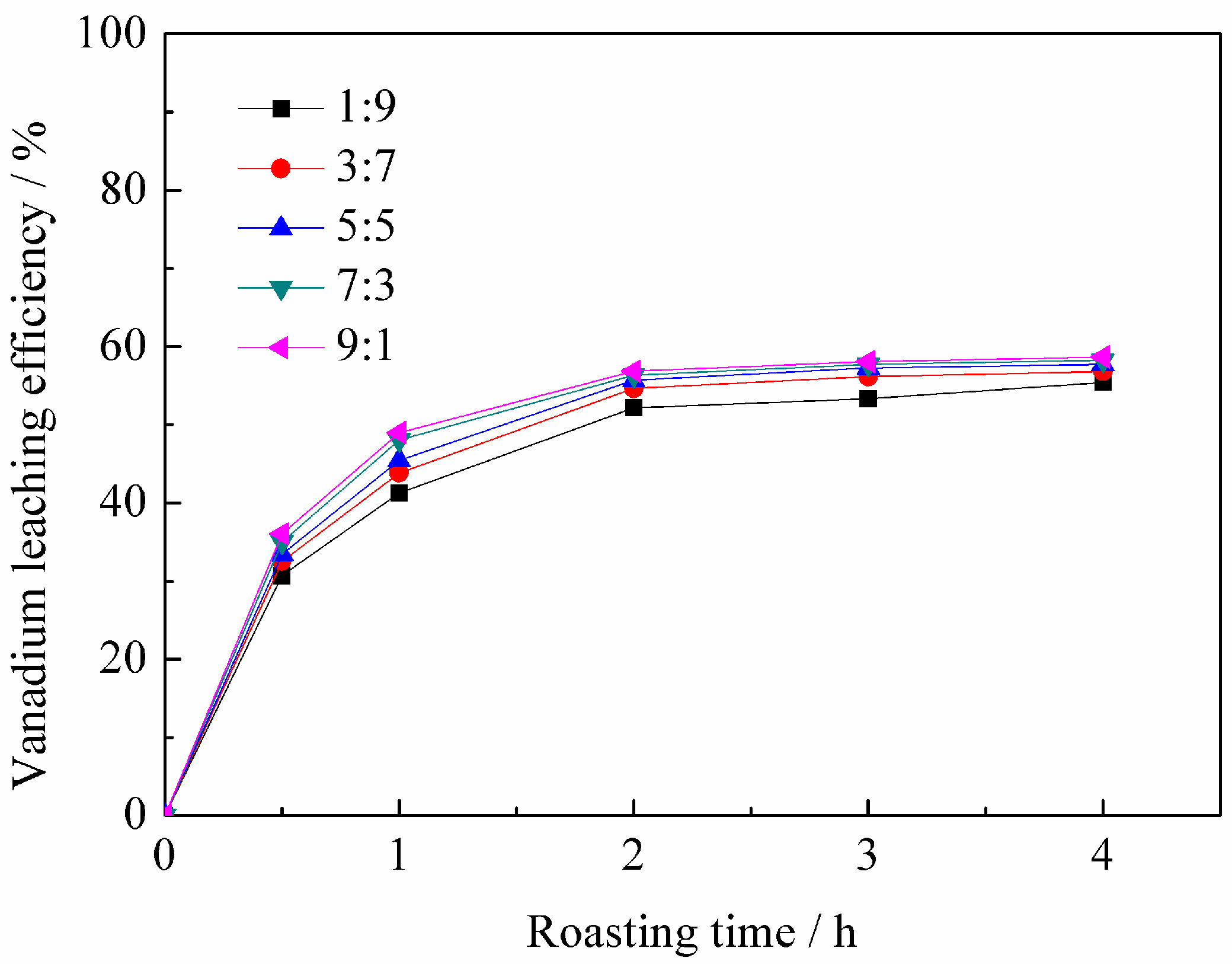
3.1.2. Effect of Mass Ratio of BaCO3 to CaO on Vanadium Leaching Efficiency
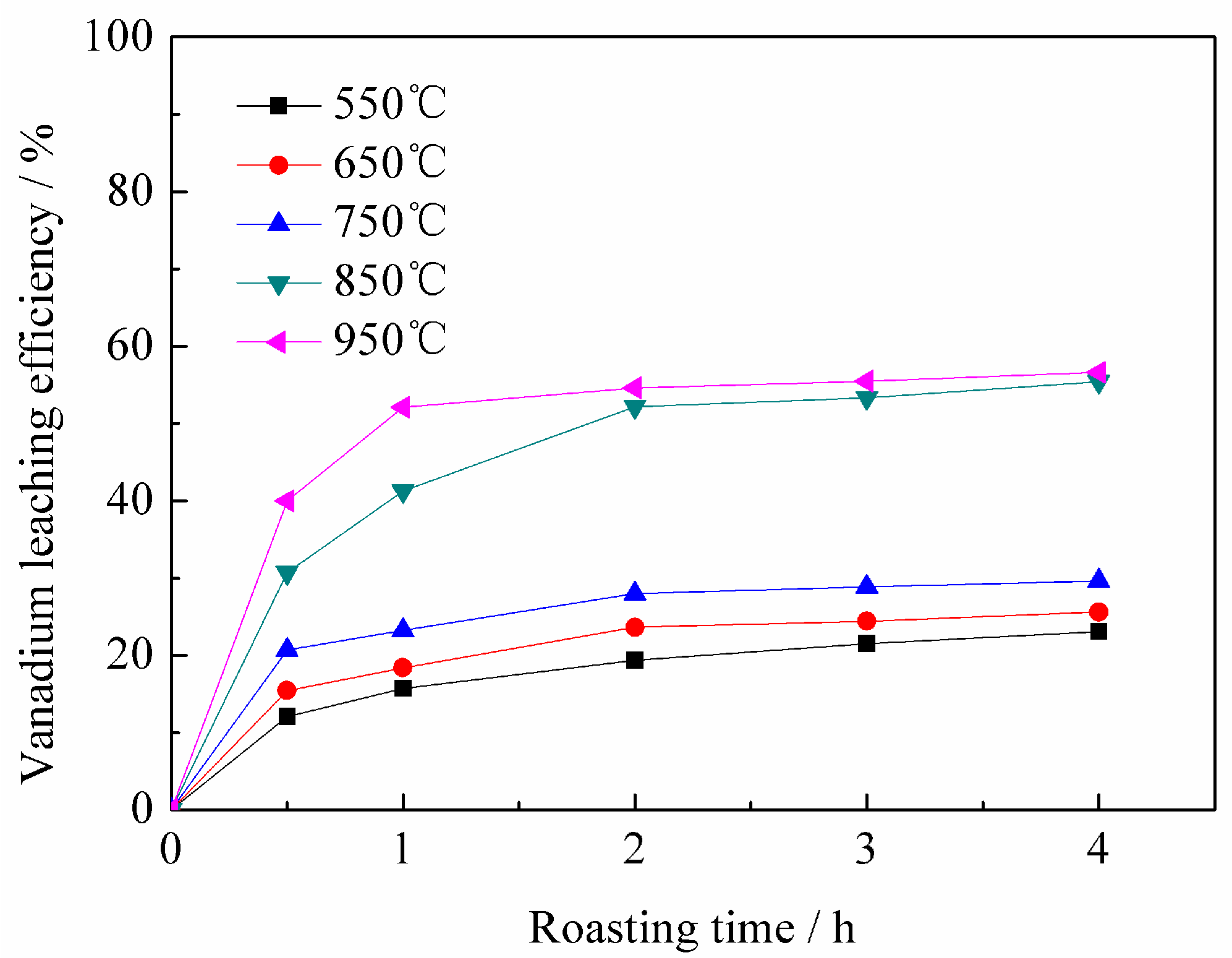
3.1.3. Effect of Roasting Temperature on Vanadium Leaching Efficiency
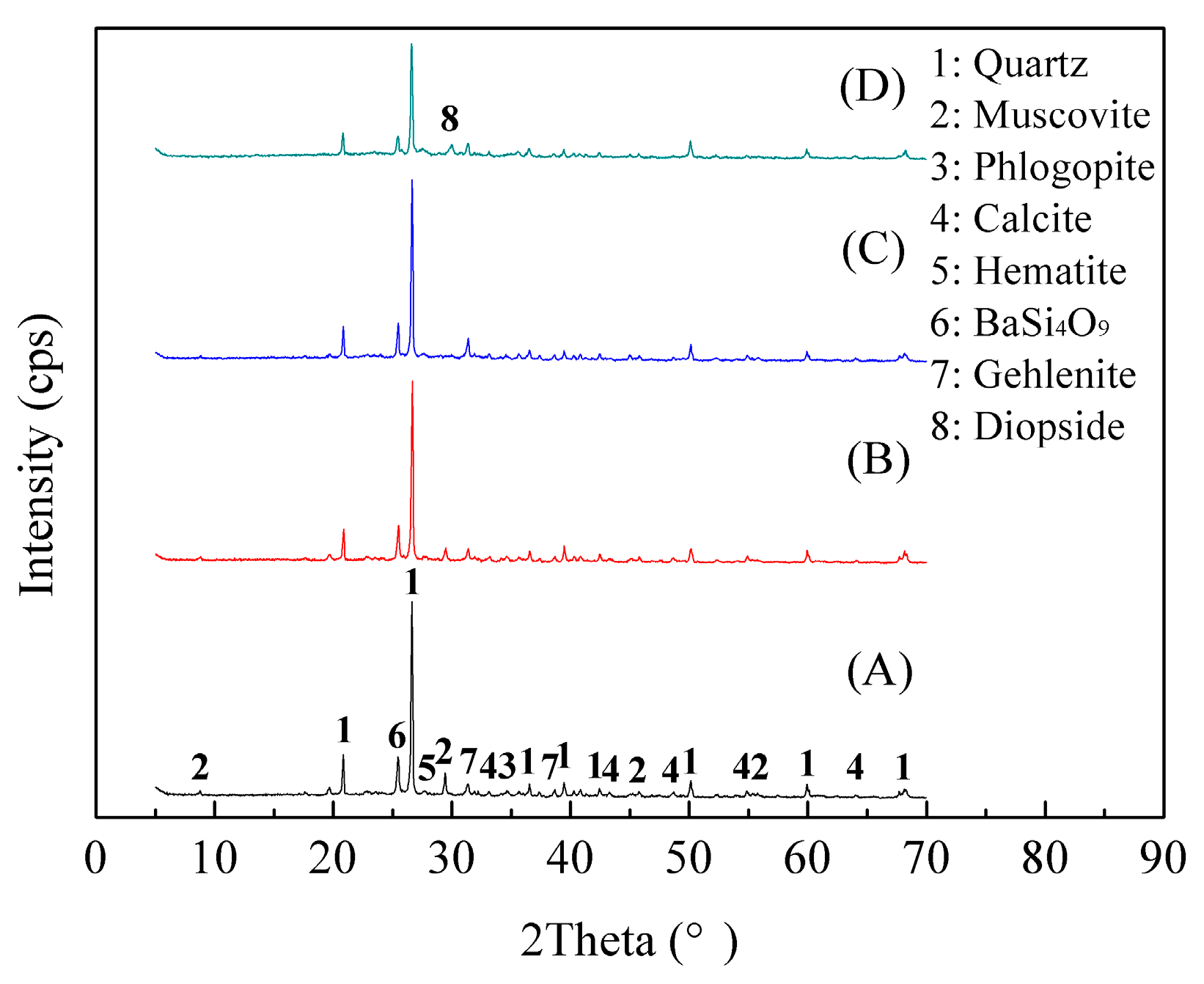
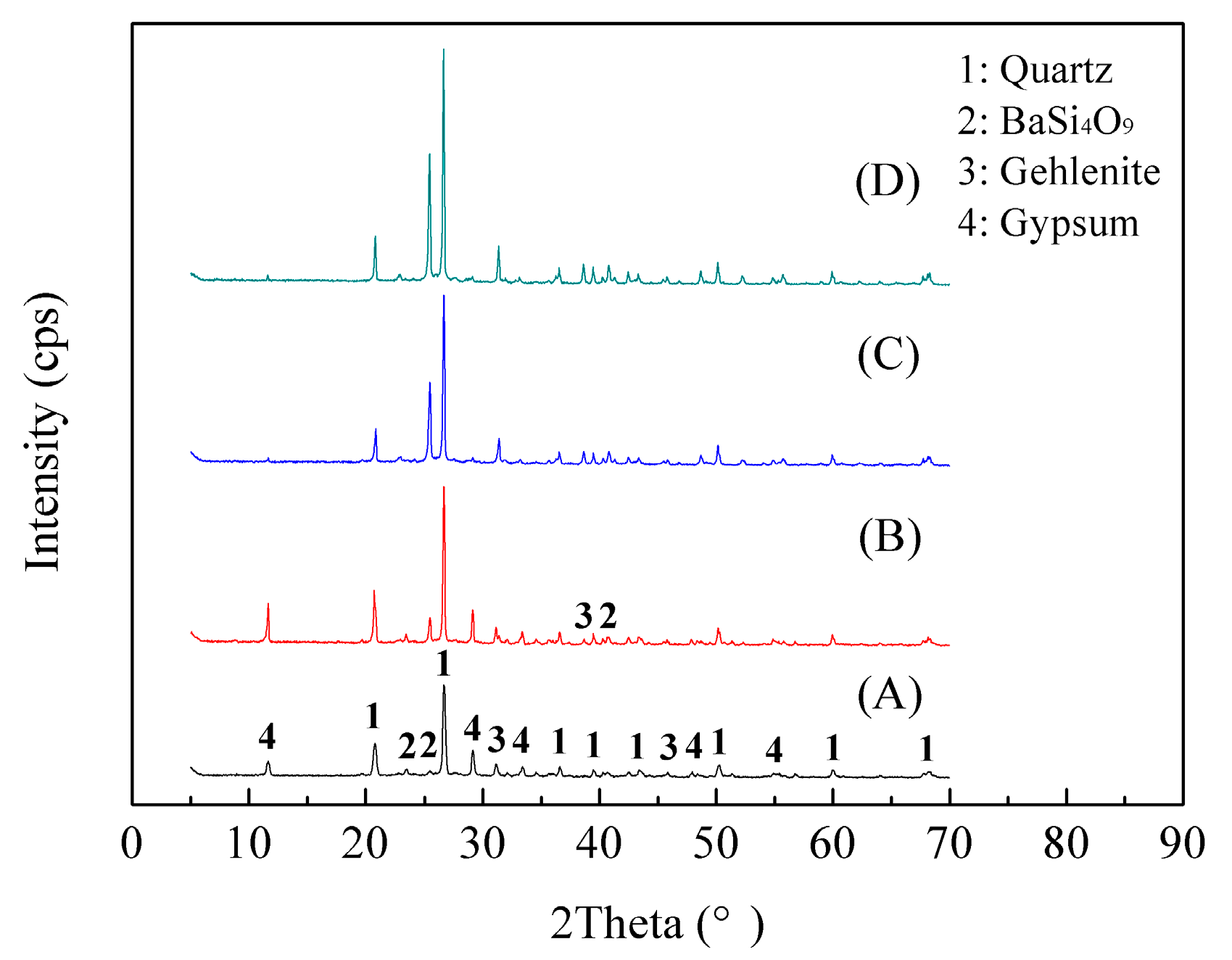
3.1.4. Phase Transformation of Stone Coal during the Roasting Process
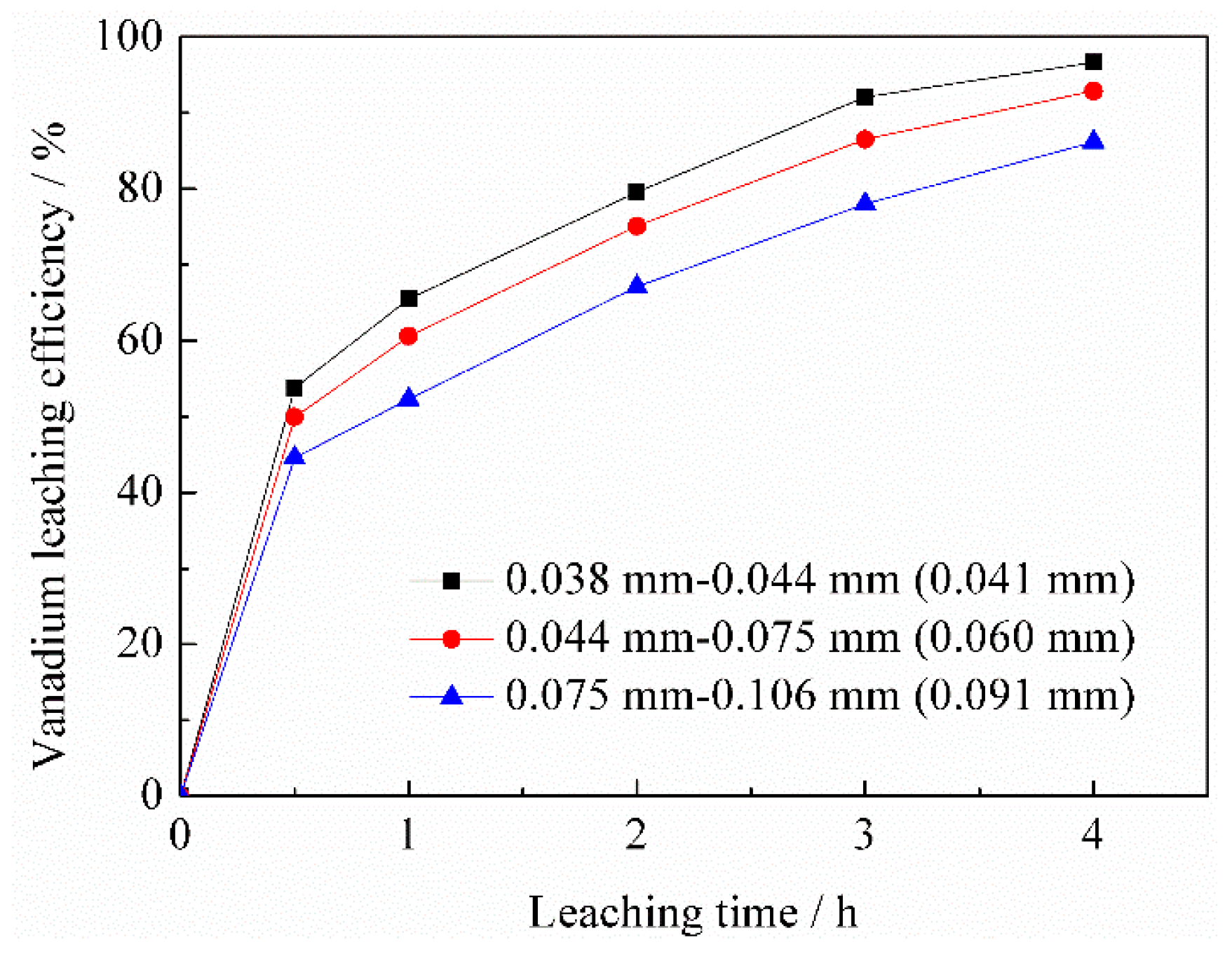
3.2. Leaching Process
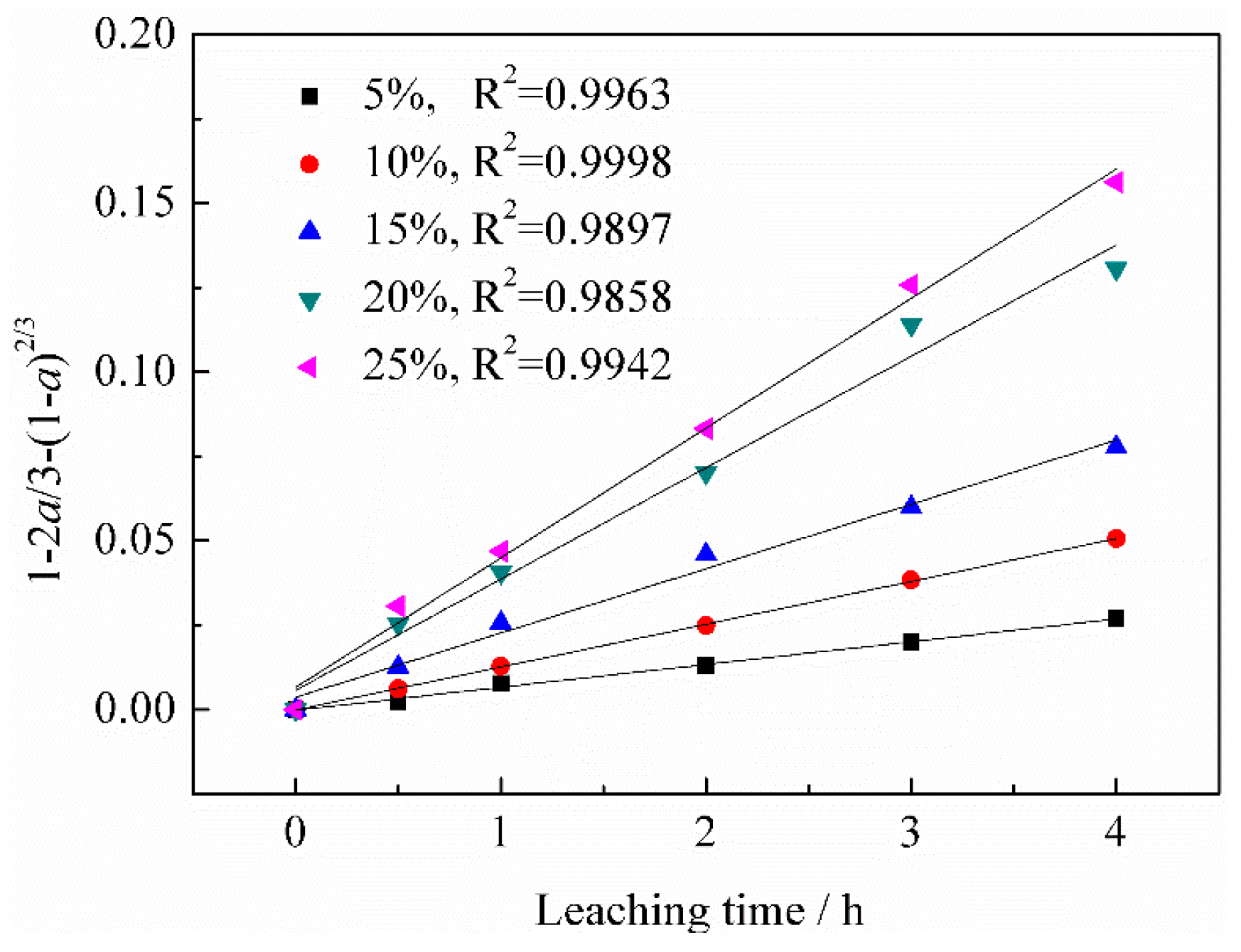
3.2.1. Effect of Sulfuric Acid Concentration on Vanadium Leaching Efficiency
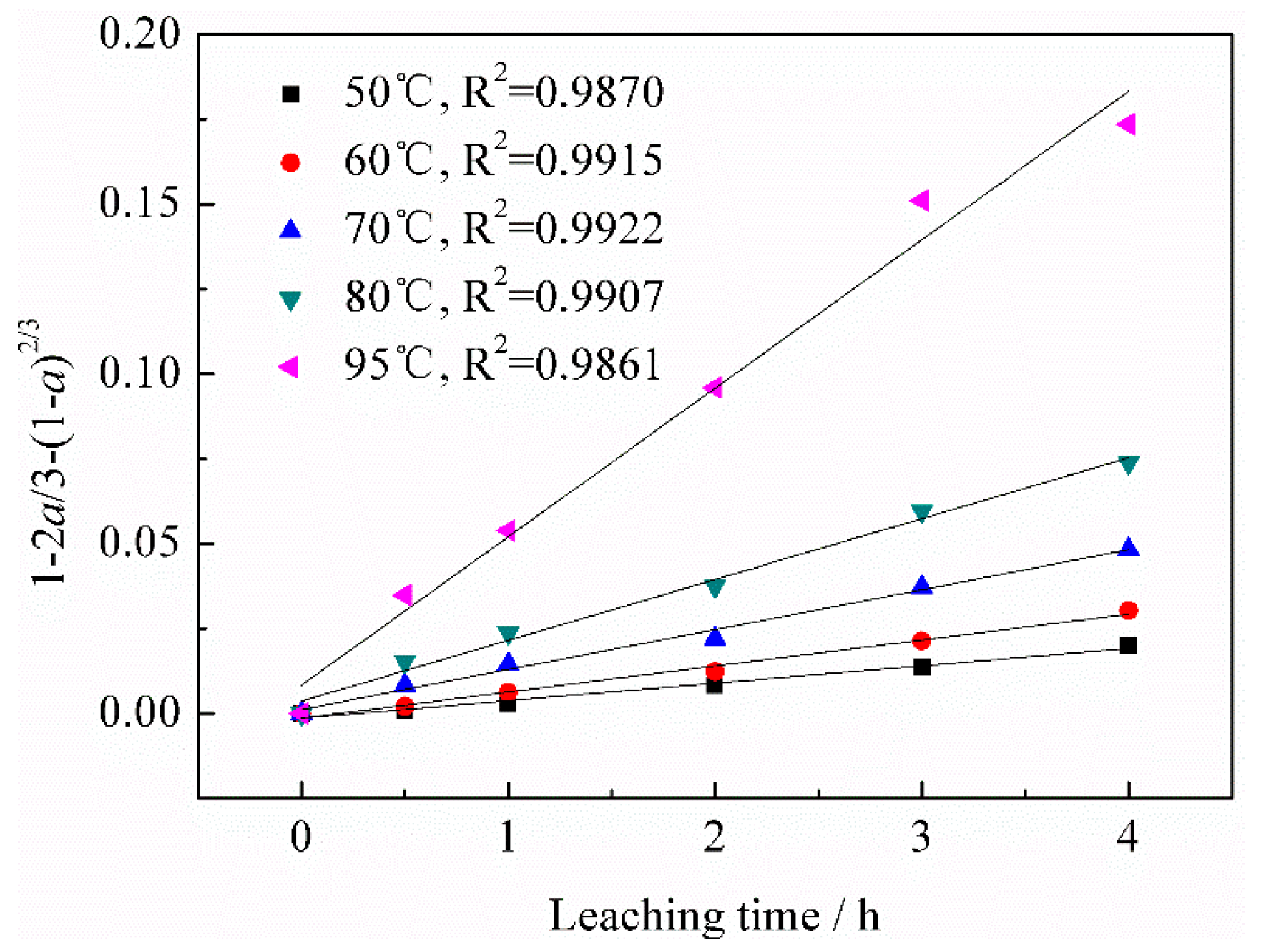
3.2.2. Effect of Leaching Temperature on Vanadium Leaching Efficiency
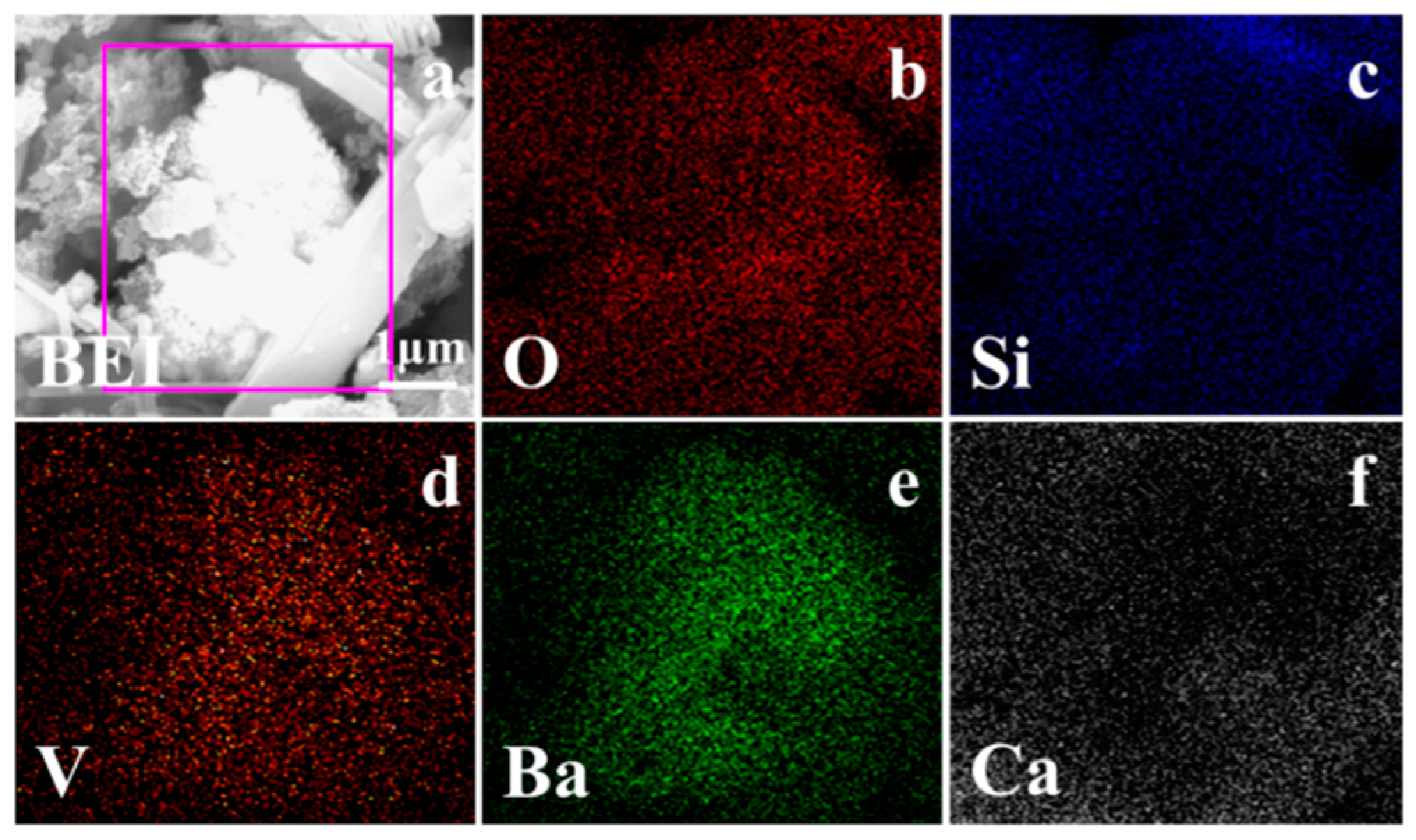
3.2.3. Phase Transformation of Roasting Samples during the Leaching Process
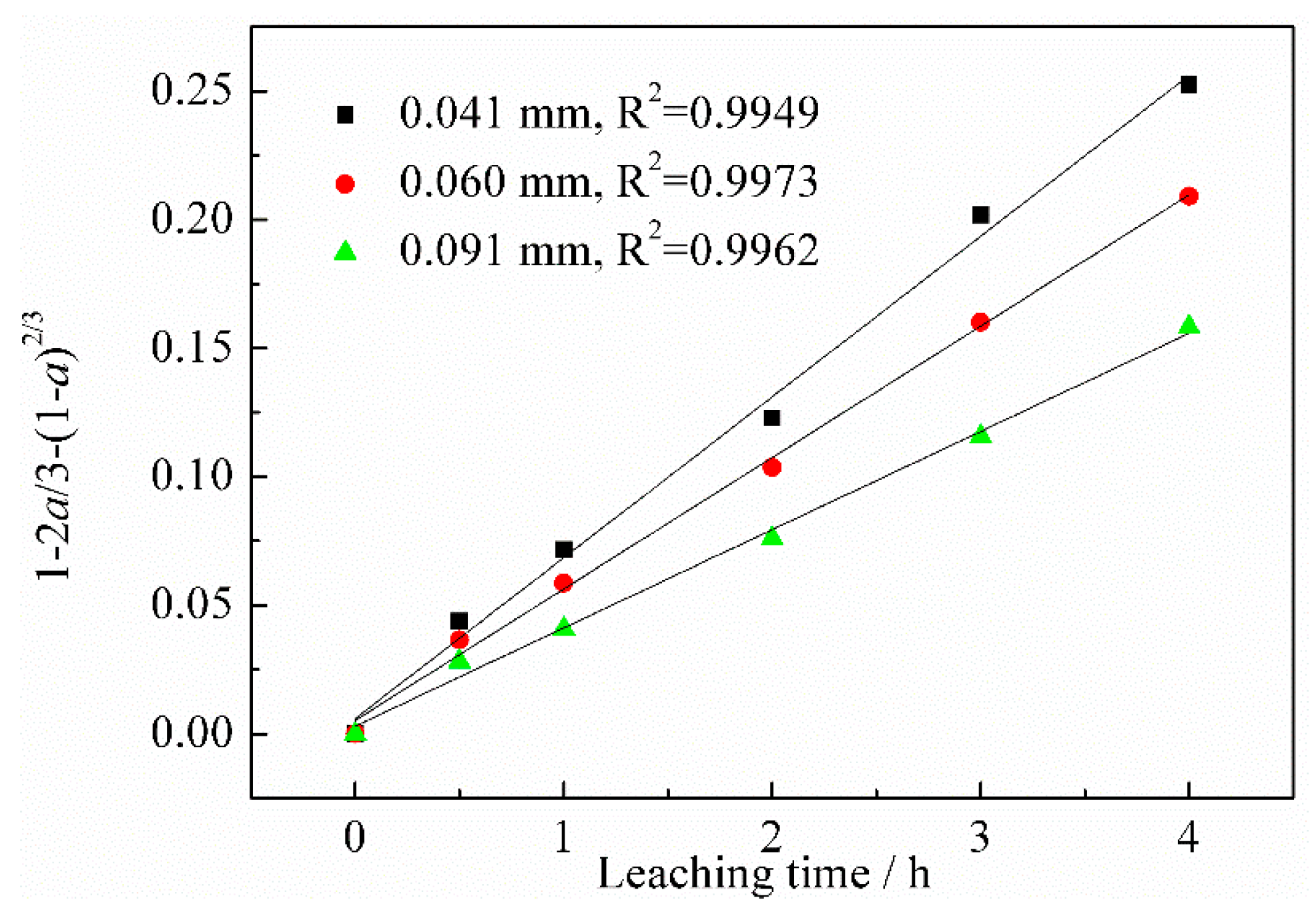
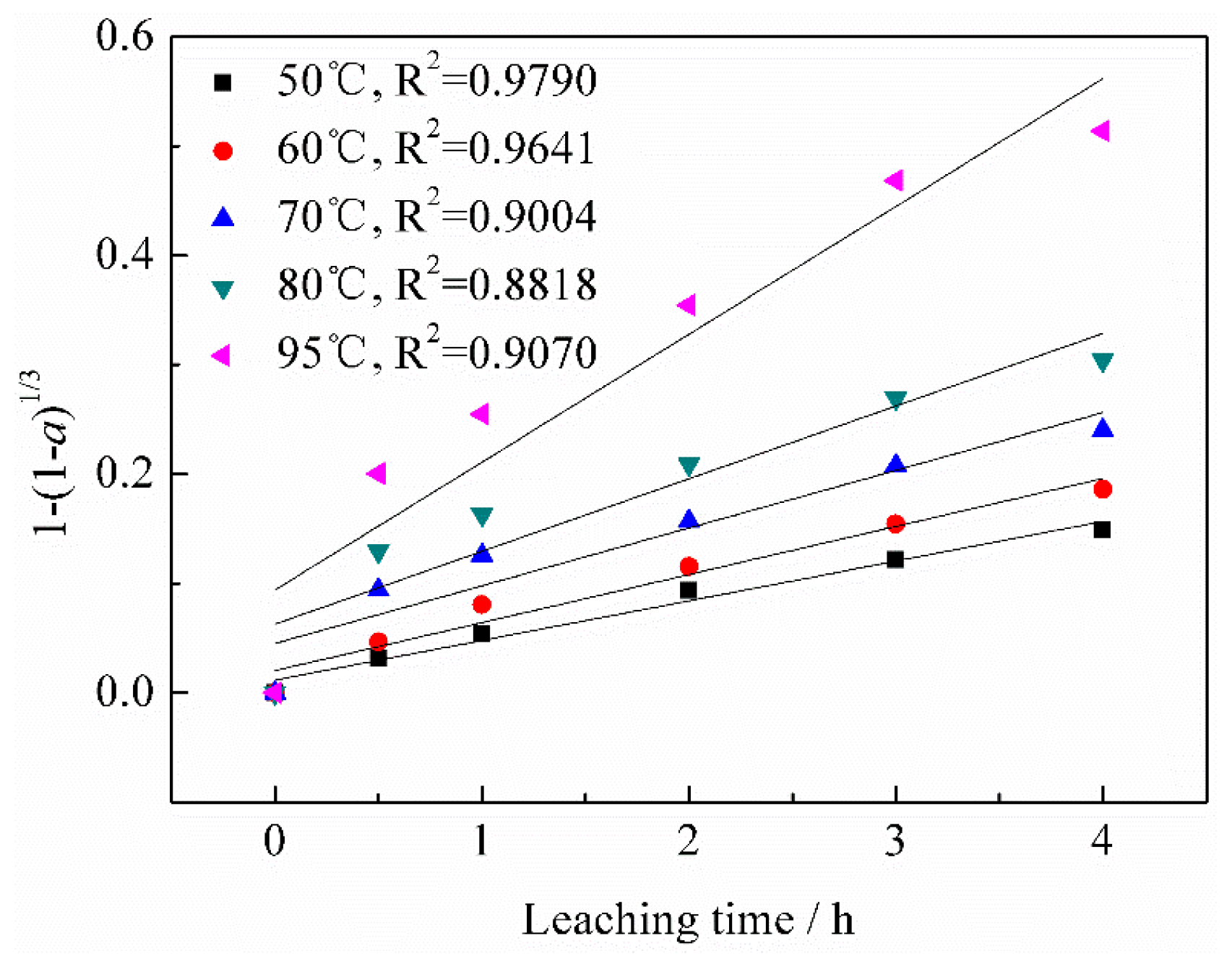
3.3. Kinetic Analysis
3.3.1. Calculation of Reaction Orders
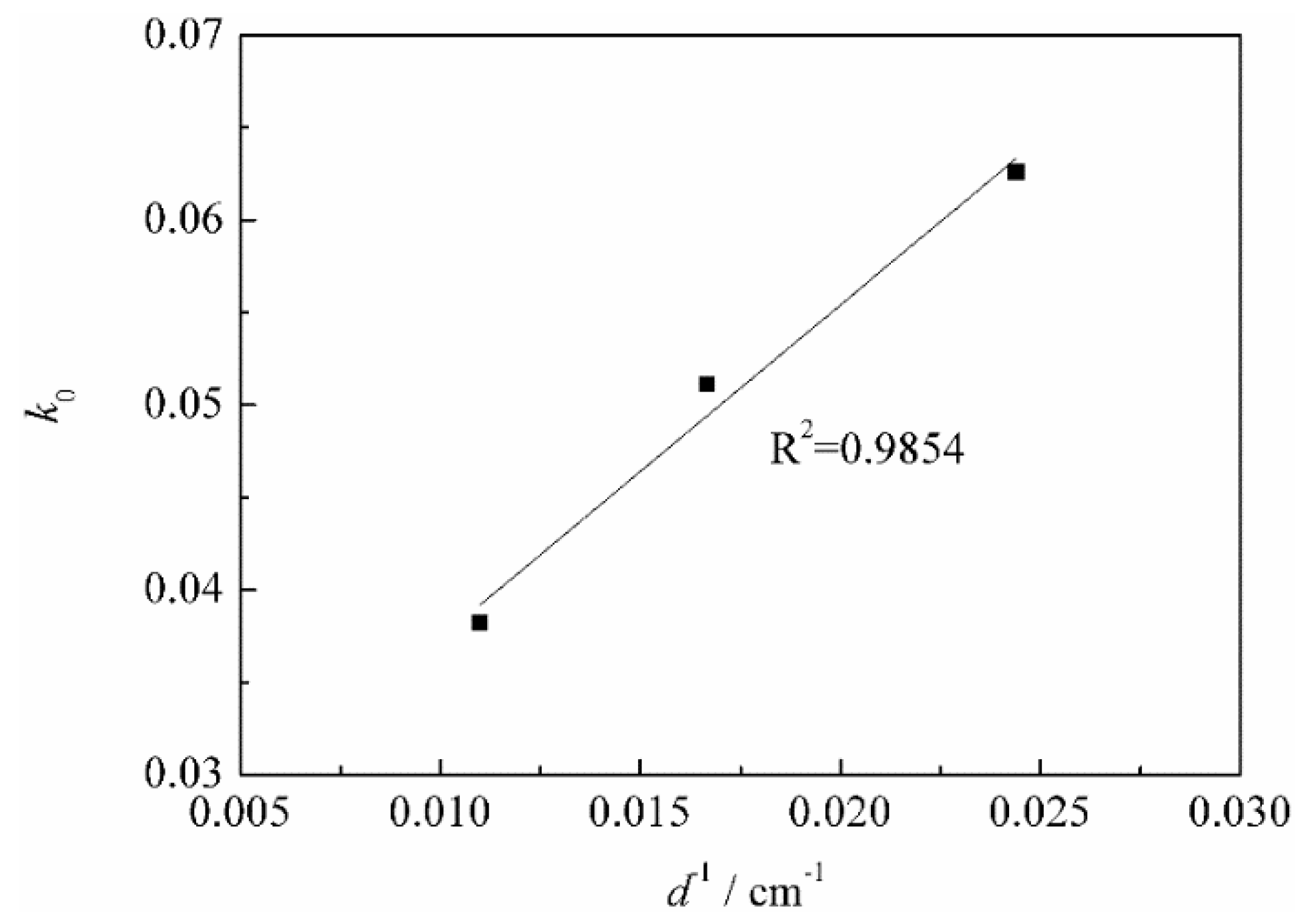
3.3.2. Calculation of Apparent Activation Energy
4. Conclusions
- The novel BaCO3/CaO composite additive roasting and acid leaching technology was proved to be feasible for the vanadium recovery from refractory stone coal.
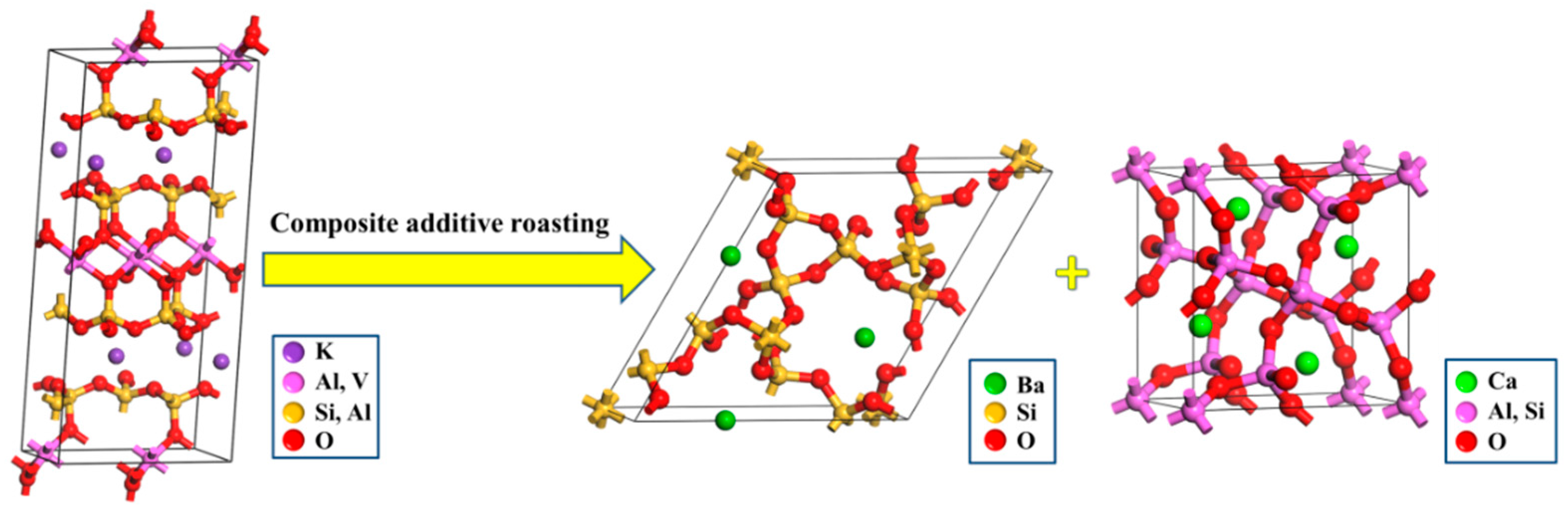
- According to the phase transformation analysis, the monoclinic crystalline structure of muscovite (K(Al,V)2[Si3AlO10](OH)2) was converted into the hexagonal crystalline structure of BaSi4O9 and the tetragonal crystalline structure of Gehlenite (Ca2Al2SiO7) during the composite additive BaCO3/CaO roasting process, which could, therefore, facilitate the release and extraction of vanadium. Vanadium in leaching residue was probably in the form of vanadate or pyrovanadate of barium and calcium, which were hardly extracted during the sulfuric acid leaching process.
- According to the vanadium leaching kinetic analysis, the process was controlled by the diffusion through a product layer. The apparent activation energy could be achieved as 46.51 kJ/mol. The reaction order with respect to the sulfuric acid concentration was 1.1059. The kinetic model of vanadium recovery from stone coal using novel composite additive BaCO3/CaO could be finally established.
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Li, W.; Zhang, Y.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131–132, 1–7. [Google Scholar] [CrossRef]
- Gouda, G.M.; Nagendra, C.L. Preparation and characterization of thin film thermistors of metal oxides of manganese and vanadium (Mn-VO). Sens. Actuators Phys. 2013, 190, 181–190. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, W.; Tang, Q.H.; Guo, Z.; Yang, Y.H.; Su, F.B. Aerobic oxidation of benzyl alcohol over activated carbon supported manganese and vanadium catalysts: Effect of surface oxygen-containing groups. Catal. Lett. 2011, 141, 149–157. [Google Scholar] [CrossRef]
- Li, X.B.; Wei, C.; Deng, Z.G.; Li, M.T.; Li, C.X.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting–water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Queneau, P.B.; Hogsett, R.F.; Beckstead, L.W.; Barchers, D.E. Processing of petroleum coke for recovery of vanadium and nickel. Hydrometallurgy 1989, 22, 3–24. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, H.; Zhao, B.; Zhu, J.; Liu, D. Dynamics study on vanadium extraction technology from chloride leaching steel slag. Rare Met. Mater. Eng. 2013, 42, 696–699. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, M.; Zhang, M. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method. J. Hazard. Mater. 2015, 286, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-T.; Wei, C.; Fan, G.; Li, C.-X.; Deng, Z.-G.; Li, X.-B. Pressure acid leaching of black shale for extraction of vanadium. Trans. Nonferrous Met. Soc. China 2010, 20, S112–S117. [Google Scholar] [CrossRef]
- Li, C.-X.; Wei, C.; Deng, Z.-G.; Li, M.-T.; Li, X.-B.; Fan, G. Recovery of vanadium from black shale. Trans. Nonferrous Met. Soc. China 2010, 20, S127–S131. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.; Liu, J. A mechanism of calcium fluoride-enhanced vanadium leaching from stone coal. Int. J. Miner. Process. 2015, 145, 87–93. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, K.; Tian, X.; Qin, W. Vanadium leaching from carbonaceous shale using fluosilicic acid. Int. J. Miner. Process. 2011, 100, 184–187. [Google Scholar] [CrossRef]
- Wang, M.; Xiang, X.; Zhang, L.; Xiao, L. Effect of vanadium occurrence state on the choice of extracting vanadium technology from stone coal. Rare Met. 2008, 27, 112–115. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Zhang, H.; Cui, L.; Yu, J.; Xu, G. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor. Fuel 2015, 142, 180–188. [Google Scholar] [CrossRef]
- Aarabi-Karasgani, M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Vanadium extraction from refractory stone coal using novel composite additive. JOM 2015, 67, 2629–2634. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Qiu, S.; Zhou, X.; Li, C.; Deng, Z. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Ju, Z.-J.; Wang, C.-Y.; Yin, F. Dissolution kinetics of vanadium from black shale by activated sulfuric acid leaching in atmosphere pressure. Int. J. Miner. Process. 2015, 138, 1–5. [Google Scholar] [CrossRef]
- Shirin, E.; Fereshteh, R.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Vida, S.; Gilnaz, A.; Fereshteh, R.; Navid, M. A shrinking particle–shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar]
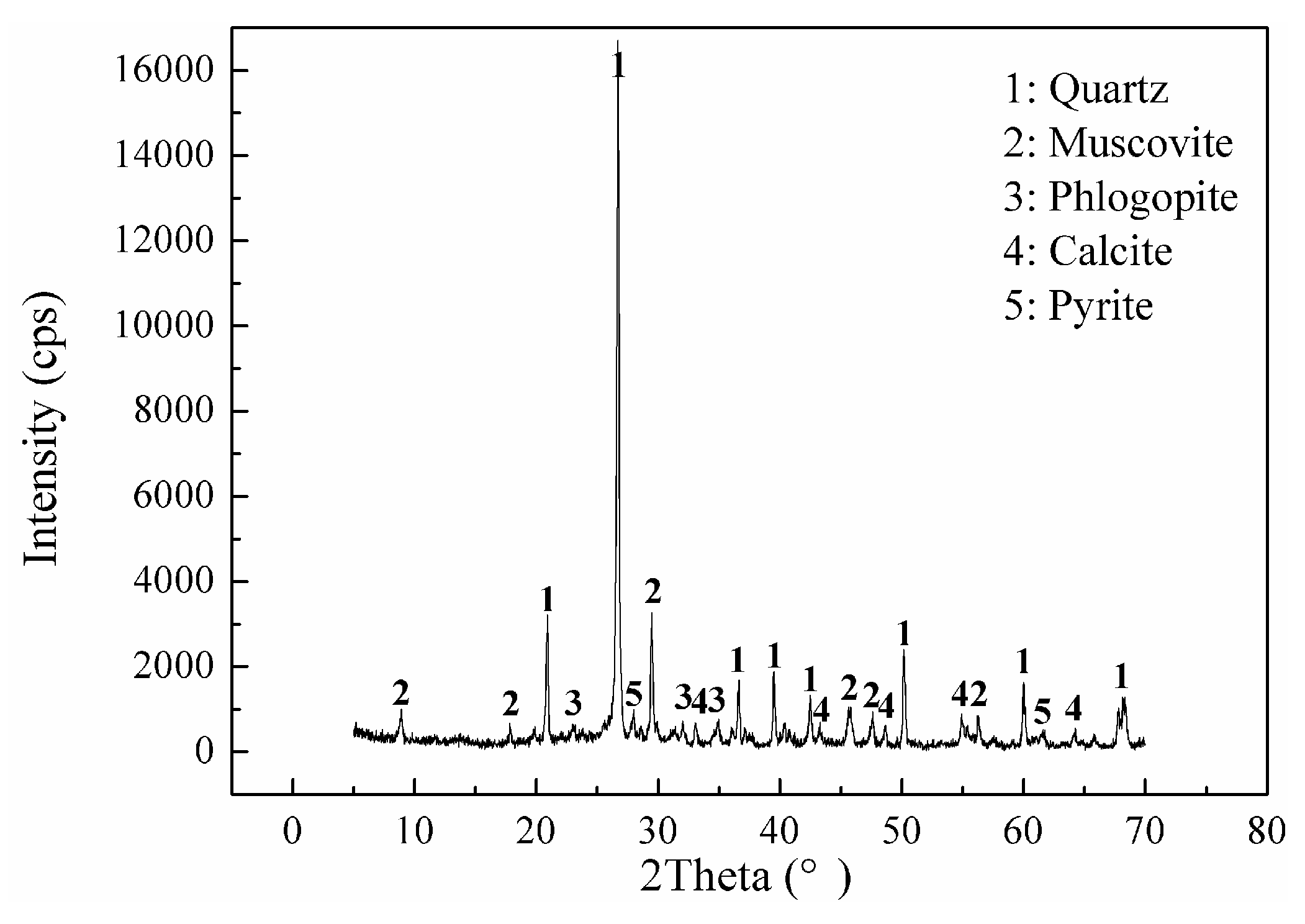
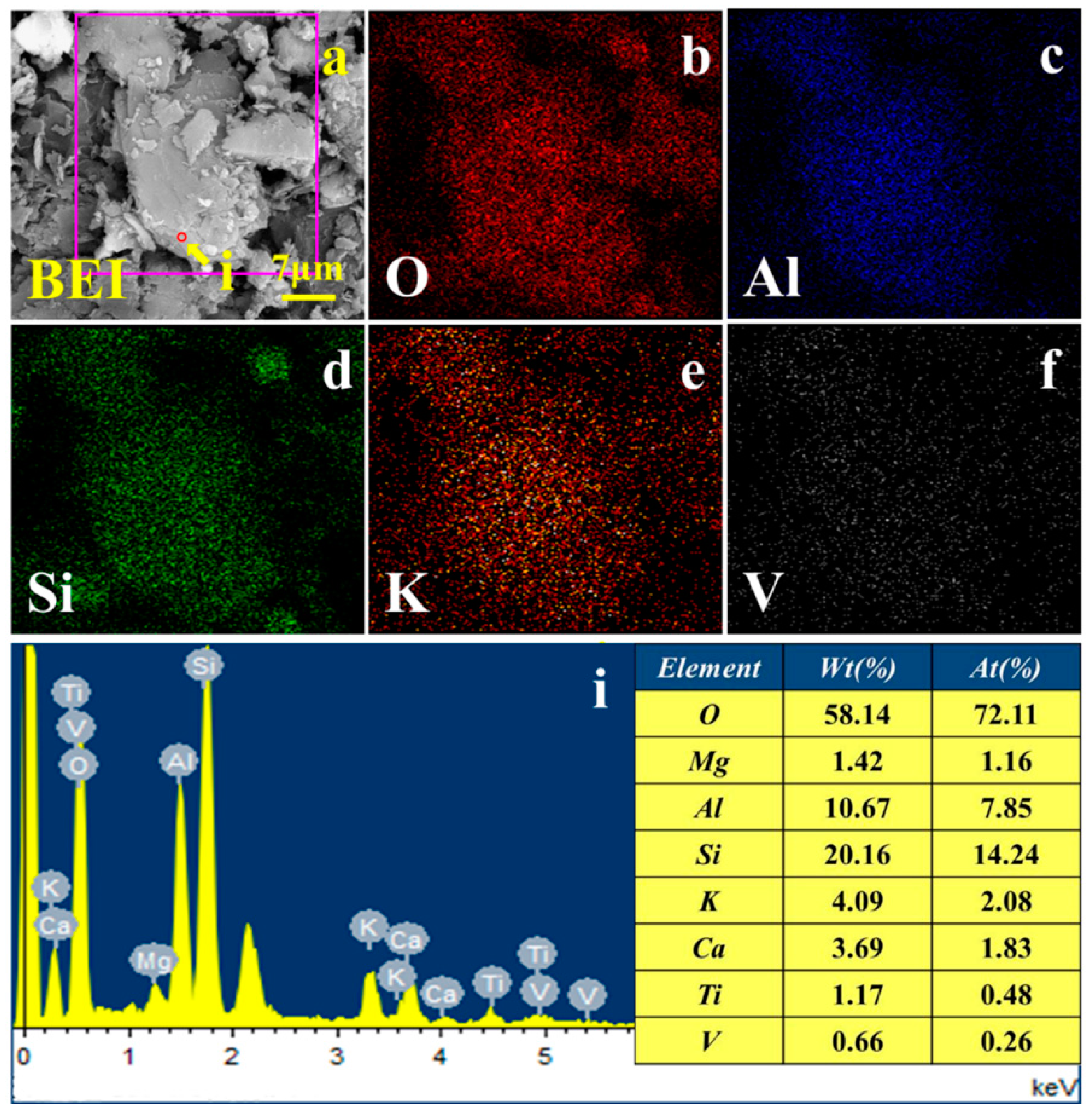



| Element | V2O5 | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SO3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.77 | 51.15 | 9.08 | 8.33 | 2.44 | 1.97 | 1.82 | 0.45 | 3.55 | 1.29 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Zhang, Y.; Liu, T.; Huang, J. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals 2016, 6, 26. https://doi.org/10.3390/min6020026
Cai Z, Zhang Y, Liu T, Huang J. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals. 2016; 6(2):26. https://doi.org/10.3390/min6020026
Chicago/Turabian StyleCai, Zhenlei, Yimin Zhang, Tao Liu, and Jing Huang. 2016. "Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology" Minerals 6, no. 2: 26. https://doi.org/10.3390/min6020026
APA StyleCai, Z., Zhang, Y., Liu, T., & Huang, J. (2016). Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals, 6(2), 26. https://doi.org/10.3390/min6020026





