Identifying the Presence of AMD-Derived Soil CO2 in Field Investigations Using Isotope Ratios
Abstract
:1. Introduction
2. Soil Gas Sampling Methods
3. Materials and Methods
3.1. Study Sites
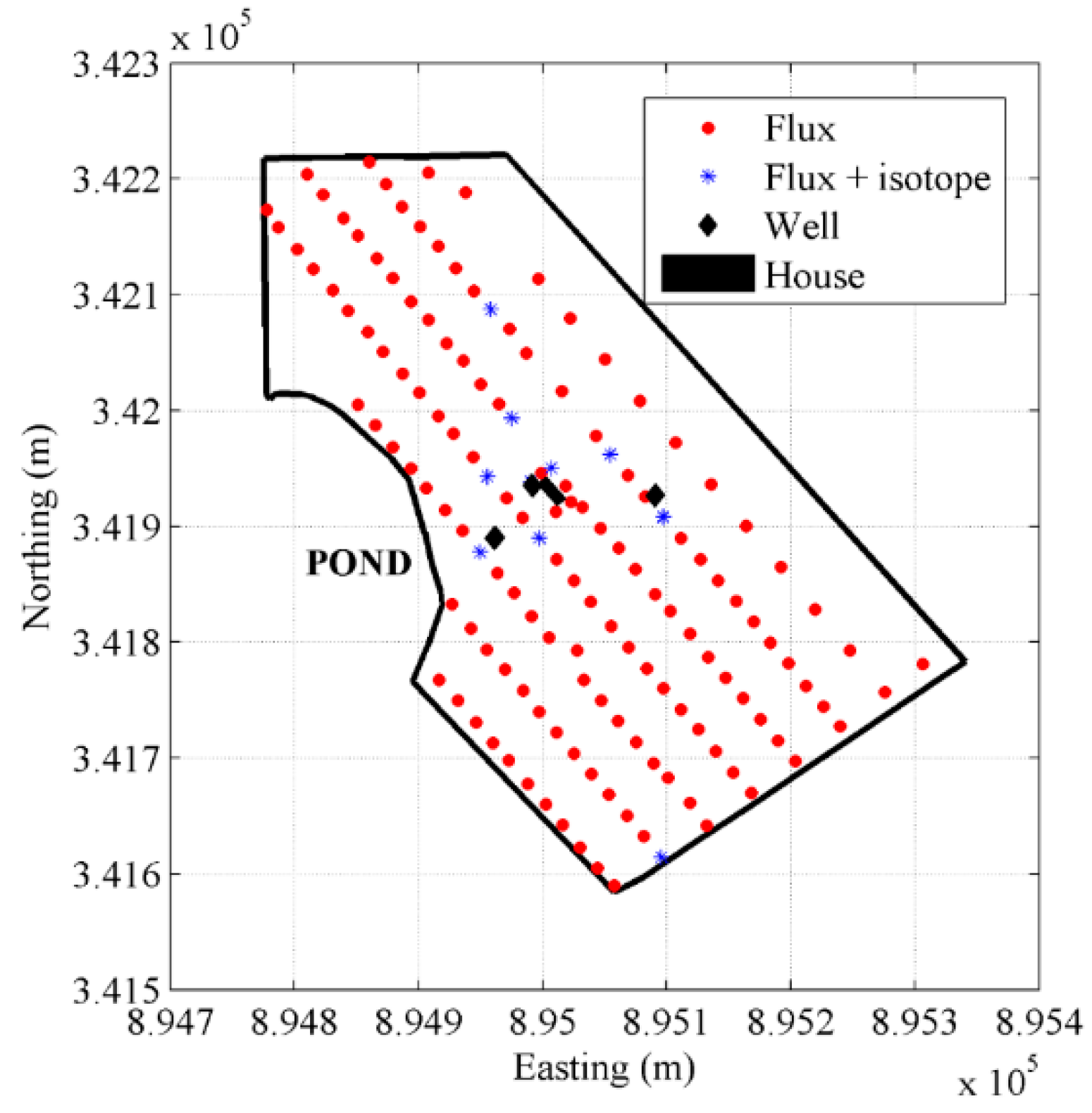
3.1.1. The Hudson Site
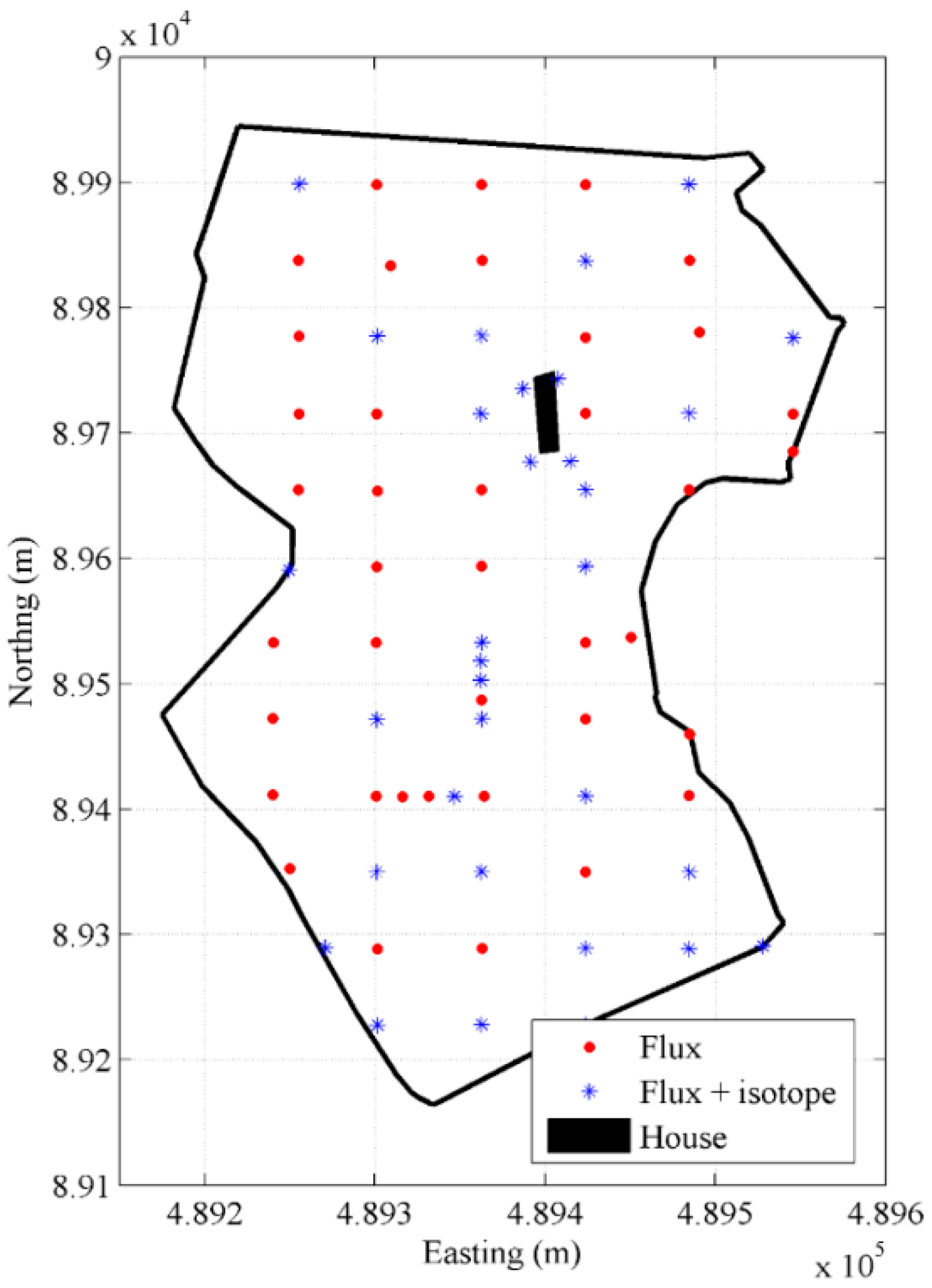
3.1.2. The Godin Site
3.2. Sampling Methods
3.2.1. Accumulation Chamber Technique
3.2.2. Slam Bar and Hand Aspiration Technique
4. Results and Discussions
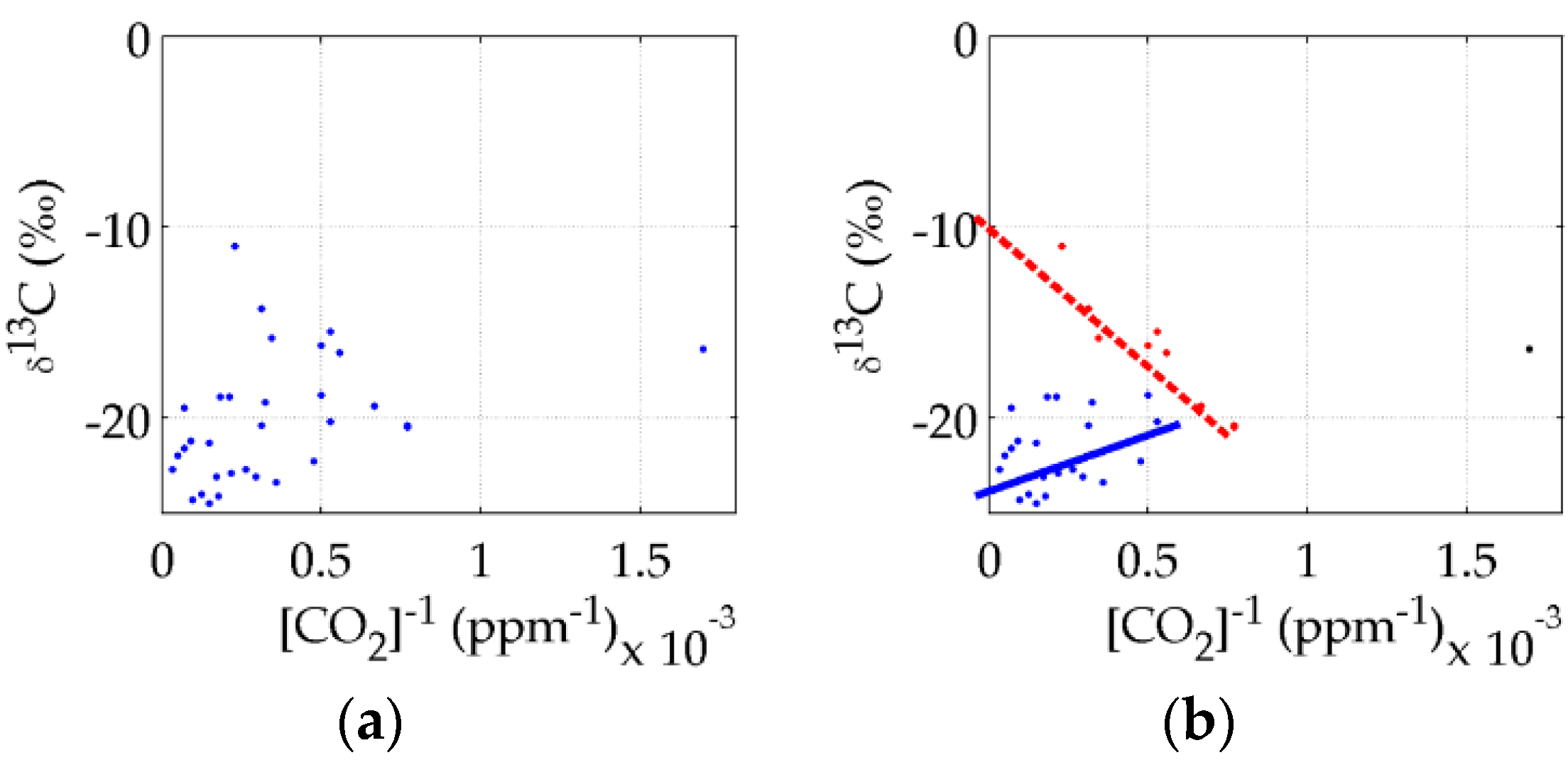
4.1. Accumulation Chamber Results
4.2. Slam Bar Results
4.3. Discussions
5. Conclusions and Recommendations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ehler, W.C. Dangerous atmosphere created by strip mine spoil. In Proceedings of the 24th National Association of Abandoned Mine Lands Programs; National Association of Abandoned Mine Lands Programs: Park City, UT, USA, 2002; pp. 1–16. [Google Scholar]
- Hockley, D.; Walter Kuit, W.; Phillip, M. Sullivan Mine fatalities: Key conclusions and implications for other sites. In Proceedings of the 8th International Conference on Acid Rock Drainage (ICARD), Skelleftea, Sweden, 22–26 June 2009.
- Laughrey, C.D.; Baldassare, F.J. Some applications of isotope geochemistry for determining sources of stray carbon dioxide gas. Environ. Geosci. 2003, 10, 107–122. [Google Scholar] [CrossRef]
- Robinson, B.A. The Occurrence and Mitigation of Carbon Dioxide in Homes Built on Reclaimed Coal Mines; U.S. Geological Survey: Reston, VA, USA, 2010.
- Cravotta, C.A., III; Dugas, D.L.; Brady, K.B.C.; Kovalchuk, T.E. Effects of selective handling of pyritic, acid-forming materials on the chemistry of pore gas and ground water at a reclaimed surface coal mine, Clarion County, PA, USA. In Proceedings of the International Land Reclamation and Mine Drainage Conference and the 3rd International Conference on the Abatement of Acidic Drainage, Pittsburgh, PA, USA, 24–29 April 1994; pp. 365–374.
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany; Heidelberg, Germany, 2009; Volume 46. [Google Scholar]
- Davidson, G.R. The stable isotopic composition and measurement of carbon in soil CO2. Geochim. Cosmochim. Acta 1995, 59, 2485–2489. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Nicot, J.P.; Massmann, J.W. Soil gas movement in unsaturated systems. In Soil Physics Companion; CRC Press: Washington, DC, USA, 2002; pp. 297–341. [Google Scholar]
- ASTM International. ASTM Standard Guide for Soil Gas Monitoring in the Vadose Zone; ASTM International: West Conshohocken, PA, USA, 2006; pp. 1–37. [Google Scholar]
- Awuah-Offei, K.; Que, S.; Mathiba, M. Delineating hazardous CO2 fluxes from acid mine drainage. Environ. Earth Sci. 2015. Accepted. [Google Scholar] [CrossRef]
- Skiba, U.; Smith, K. The control of nitrous oxide emissions from agricultural and natural soils. Chemosphere Glob. Chang. Sci. 2000, 2, 379–386. [Google Scholar] [CrossRef]
- Chiodini, G.; Caliro, S.; Cardellini, C.; Avino, R.; Granieri, D.; Schmidt, A. Carbon isotopic composition of soil CO2 efflux, a powerful method to discriminate different sources feeding soil CO2 degassing in volcanic-hydrothermal areas. Earth Planet. Sci. Lett. 2008, 274, 372–379. [Google Scholar] [CrossRef]
- Venterea, R.T.; Burger, M.; Spokas, K.A. Nitrogen oxide and methane emissions under varying tillage and fertilizer management. J. Environ. Qual. 2005, 34, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). U.S. EPA Standard Operating Procedure 2149; U.S. Environmental Protection Agency: Washington, DC, USA, 1988.
- Devitt, D.A.; Evans, R.B.; Jury, W.A.; Starks, T.P.; Eklund, B.; Gnolson, A.; van Ee, J.J. Soil Gas Sensing for the Detection and Mapping of Volatile Organics; National Ground Water Association: Westerville, OH, USA, 1987. [Google Scholar]
- Rivett, M.O. Soil-gas signatures from volatile chlorinated solvents: Borden field experiments. Groundwater 1995, 33, 84–98. [Google Scholar] [CrossRef]
- Moseley, C.L.; Meyer, M.R. Petroleum contamination of an elementary-school—A case-history involving air, soil-gas, and groundwater monitoring. Environ. Sci. Technol. 1992, 26, 185–192. [Google Scholar] [CrossRef]
- Bertolini, T.; Inglima, I.; Rubino, M.; Marzaioli, F.; Lubritto, C.; Subke, J.; Peressotti, A.; Cotrufo, M. Sampling soil-derived CO2 for analysis of isotopic composition: A comparison of different techniques. Isotopes Environ. Health Stud. 2006, 42, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta 1958, 13, 322–334. [Google Scholar] [CrossRef]
- Keeling, C.D. The concentration and isotopic abundances of carbon dioxide in rural and marine air. Geochim. Cosmochim. Acta 1961, 24, 277–298. [Google Scholar] [CrossRef]
- National Centers for Environmetal Information Data. Available online: http://www.ncdc.noaa.gov/cdo-web/ (accessed on 1 March 2016).
- Natural Resources Conservation Service Web Soil Survey. Available online: http://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 1 March 2016).
- Pataki, D.E.; Bowling, D.R.; Ehleringer, J.R. Seasonal cycle of carbon dioxide and its isotopic composition in an urban atmosphere: Anthropogenic and biogenic effects. J. Geophys. Res. 2003, 108, 1–8. [Google Scholar] [CrossRef]
- Mathiba, M. Spatial Variation of AMD Related CO2 Emissions on Reclaimed Mine Spoil; Missouri University of Science & Technology: Rolla, MO, USA, 2013. [Google Scholar]
| Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) | Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) | Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) |
|---|---|---|---|---|---|---|---|---|
| B11-1 | 621.53 | −15.2 | C15-1 | 1090.55 | −19.7 | D16-1 | 658.19 | −16.7 |
| B11-2 | 899.10 | −18.4 | C15-2 | 1211.28 | −20.4 | D16-2 | 699.67 | −17.6 |
| B11-3 | 1029.50 | −18.9 | C15-3 | 1321.18 | −20.9 | D16-3 | 709.37 | −17.4 |
| B14-1 | 622.32 | −15.7 | C17-1 | 601.74 | −12.6 | E14-1 | 787.78 | −17.4 |
| B14-2 | 826.19 | −18.4 | C17-2 | 715.39 | −13.8 | E14-2 | 1049.69 | −20.0 |
| B14-3 | 981.77 | −20.2 | C17-3 | 751.47 | −14.2 | E14-3 | 1362.63 | −21.6 |
| B21-1 | 638.50 | −15.1 | D13-1 | 791.20 | −17.6 | H3-1 | 556.25 | −12.9 |
| B21-2 | 670.01 | −16.0 | D13-2 | 1036.50 | −19.5 | H3-2 | 627.16 | −14.0 |
| B21-3 | 597.56 | −14.7 | D13-3 | 1355.50 | −21.1 | H3-3 | 654.07 | −14.5 |
| Sample ID | δ13C-CO2 (‰) Samples 1–2 | δ13C-CO2 (‰) Samples 2–3 | δ13C-CO2 (‰) Samples 1–3 |
|---|---|---|---|
| B11 | −25.57 | −22.35 | −24.54 |
| B14 | −26.64 | −29.76 | −27.99 |
| B21 | −34.24 | −26.72 | −20.94 |
| C15 | −26.72 | −26.41 | −26.57 |
| C17 | −20.15 | −22.13 | −20.63 |
| D13 | −25.63 | −26.30 | −26.01 |
| D16 | −31.88 | −2.97 | −26.40 |
| E14 | −27.82 | −26.97 | −23.56 |
| H3 | −22.63 | −26.15 | −23.60 |
| Average | −26.81 | −23.31 | −24.47 |
| Standard Deviation | 4.28 | 7.98 | 2.54 |
| Skewness | −0.35 | 2.52 | 0.38 |
| Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) | Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) | Sample ID | [CO2] (ppm) | δ13C-CO2 (‰) |
|---|---|---|---|---|---|---|---|---|
| A-1 | 2800 | −23.4 | C-7 | 2100 | −22.3 | E-11 | 6800 | −21.3 |
| A-11 | 1900 | −20.2 | C7-100 | 4400 | −11.0 | E-4 | 4600 | −22.9 |
| A-6 | 590 | −16.4 | C7-50 | 5700 | −24.1 | F-11 | 10,400 | −24.3 |
| B-10 | 14,200 | −21.6 | C-8 | 4700 | −18.9 | F-3 | 11,200 | −21.2 |
| B-12 | 5800 | −23.1 | D-11 | 2900 | −15.8 | H-1 | 2000 | −18.8 |
| B-3 | 29,900 | −22.7 | D-12 | 14,000 | −19.5 | H-2 | 3200 | −14.3 |
| B-8 | 1300 | −20.5 | D-2 | 8000 | −24.0 | H-3 | 5500 | −18.9 |
| BC9-150 | 2000 | −16.2 | D-5 | 1800 | −16.6 | H-4 | 19,500 | −22.0 |
| C-10 | 1900 | −15.5 | D-6 | 3200 | −20.4 | Mean | 5947 | −20.1 |
| C-12 | 3100 | −19.2 | D-9 | 1300 | −20.4 | Standard Deviation | 6197 | 3.29 |
| C-3 | 3800 | −22.7 | E-1 | 1500 | −19.4 | Skewness | 2.396 | 0.864 |
| C-4 | 3400 | −23.1 | E-10 | 6800 | −24.5 | - | - | - |
| Site | Description | Depth (m) | [CO2] (%) | δ13C-CO2 (‰) | Source |
|---|---|---|---|---|---|
| Godin | Basement of residence | - | 9.51 | −4.1 | [3] |
| Monitoring well | 21.3 | 8.92 | −4.2 | ||
| Hudson | Monitoring well MW1S | 5.79 | 8.66 | −5.0 | [24] |
| Monitoring well MW2S | 5.79 | 13.30 | −5.3 | ||
| Monitoring well MW3S | 5.79 | 9.40 | −5.3 | ||
| Monitoring well MW1D | 11.58 | 17.58 | −11.4 | ||
| Monitoring well MW2D | 11.58 | 11.24 | −9.5 | ||
| Monitoring well MW3D | 11.58 | 0.52 | −10.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awuah-Offei, K.; Mathiba, M.; Baldassare, F.J. Identifying the Presence of AMD-Derived Soil CO2 in Field Investigations Using Isotope Ratios. Minerals 2016, 6, 18. https://doi.org/10.3390/min6010018
Awuah-Offei K, Mathiba M, Baldassare FJ. Identifying the Presence of AMD-Derived Soil CO2 in Field Investigations Using Isotope Ratios. Minerals. 2016; 6(1):18. https://doi.org/10.3390/min6010018
Chicago/Turabian StyleAwuah-Offei, Kwame, Moagabo Mathiba, and Fred J. Baldassare. 2016. "Identifying the Presence of AMD-Derived Soil CO2 in Field Investigations Using Isotope Ratios" Minerals 6, no. 1: 18. https://doi.org/10.3390/min6010018
APA StyleAwuah-Offei, K., Mathiba, M., & Baldassare, F. J. (2016). Identifying the Presence of AMD-Derived Soil CO2 in Field Investigations Using Isotope Ratios. Minerals, 6(1), 18. https://doi.org/10.3390/min6010018






