Potentiostatically Poised Electrodes Mimic Iron Oxide and Interact with Soil Microbial Communities to Alter the Biogeochemistry of Arctic Peat Soils
Abstract
:1. Introduction
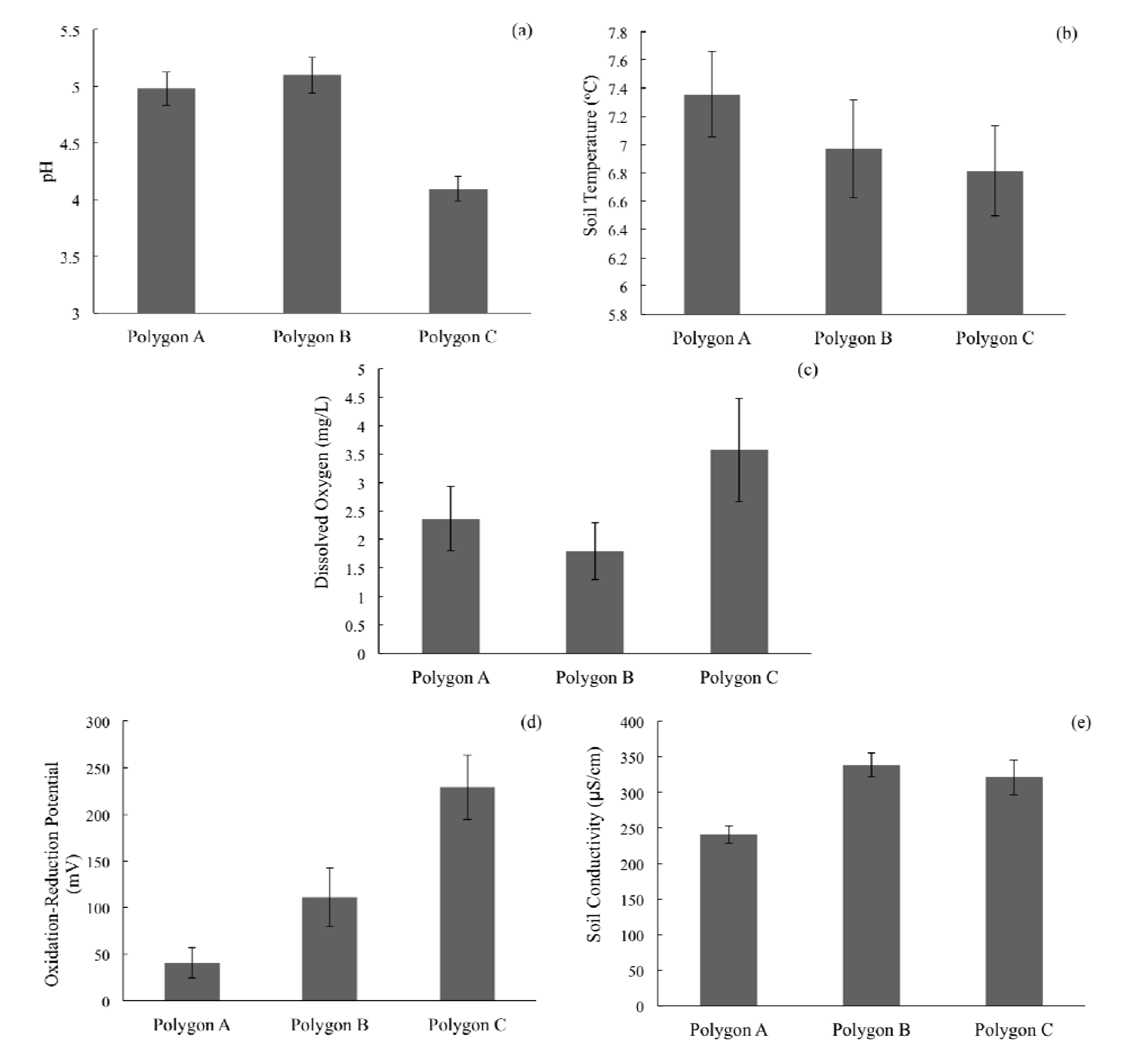
2. Results and Discussion
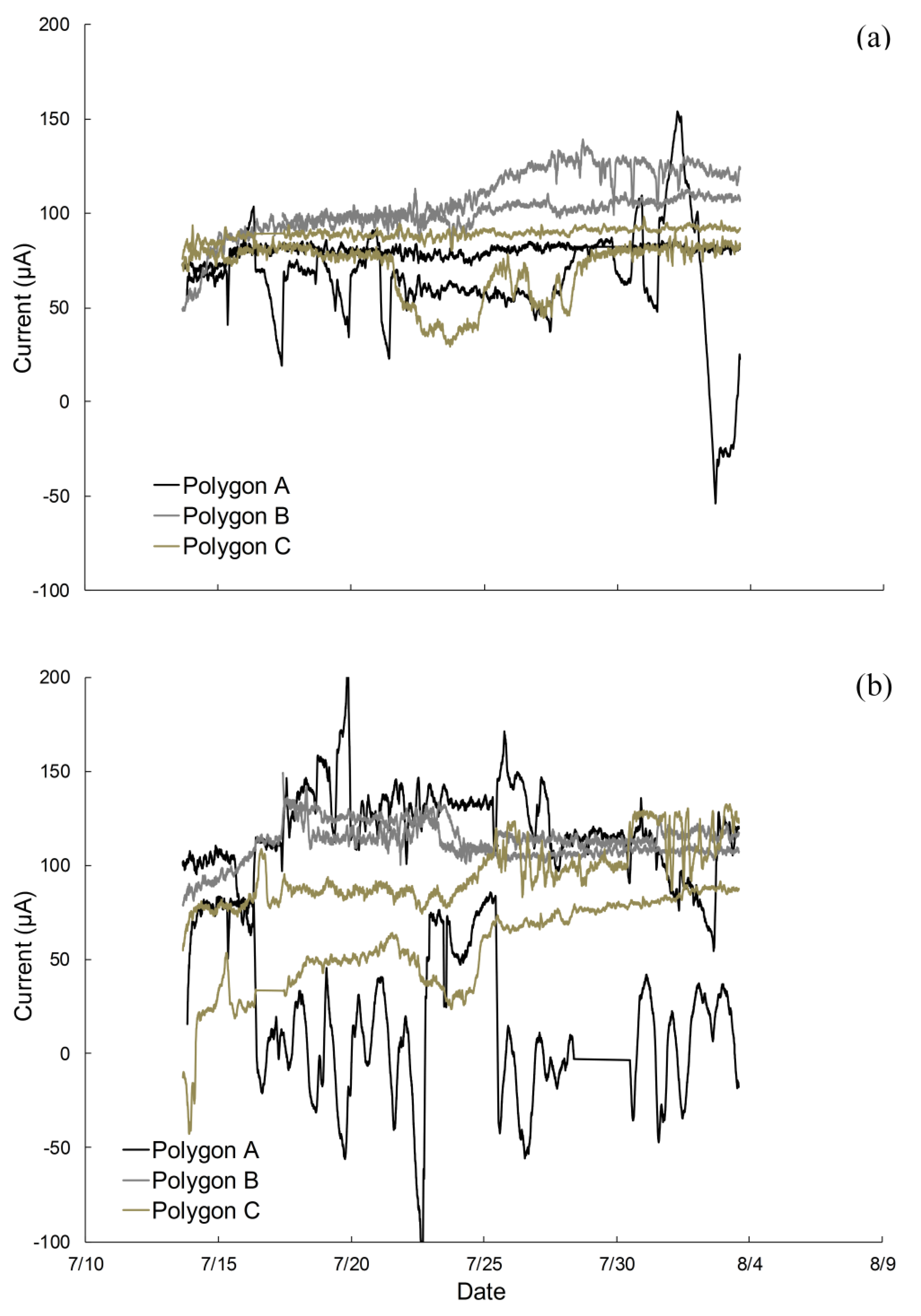
2.1. Electrodes Stimulate Methane Emissions Compared to Soils without Electrodes
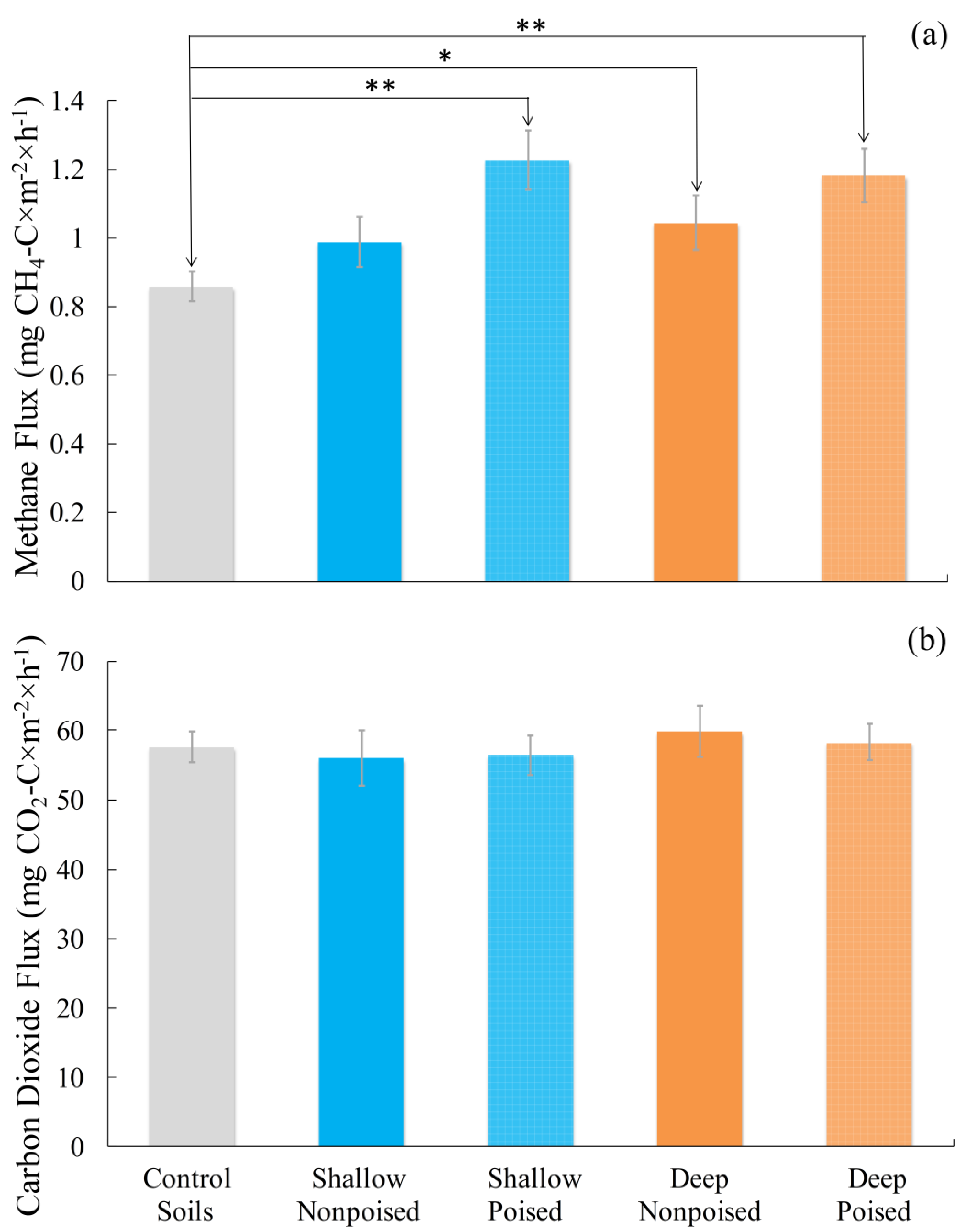
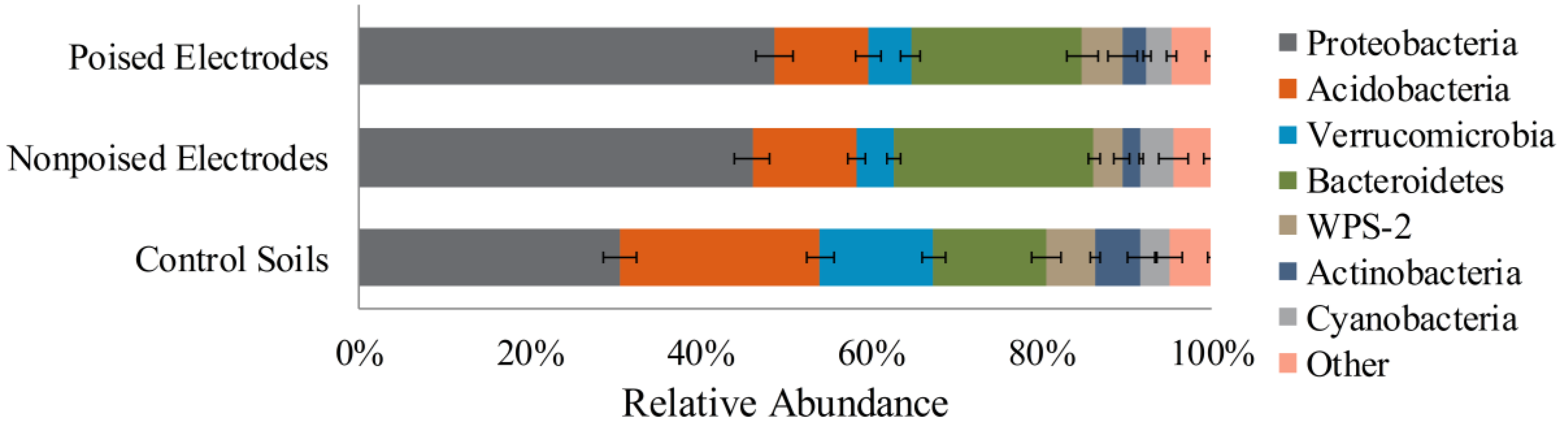
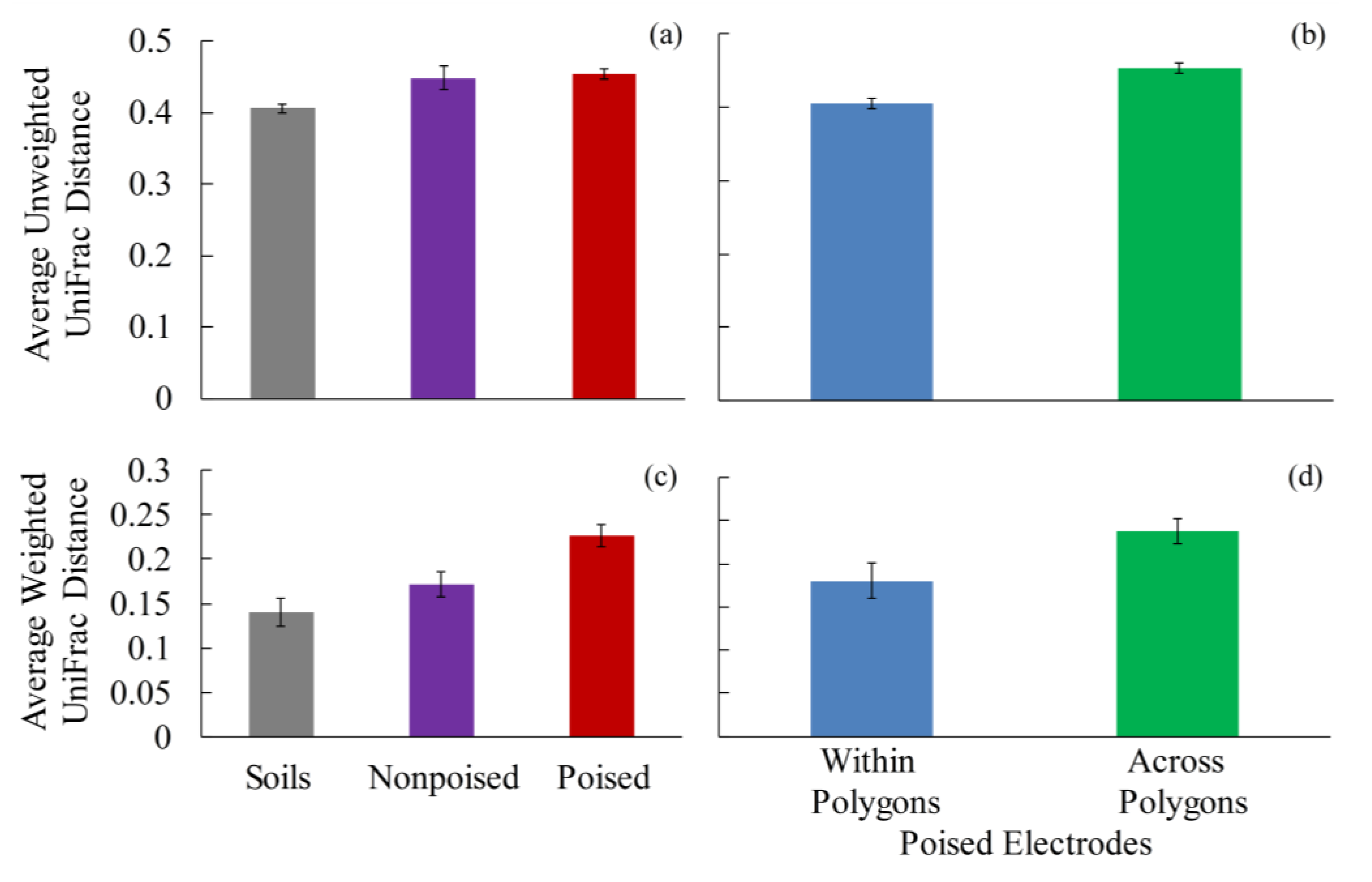
2.2. Electrodes Stimulate a Change in Microbiome

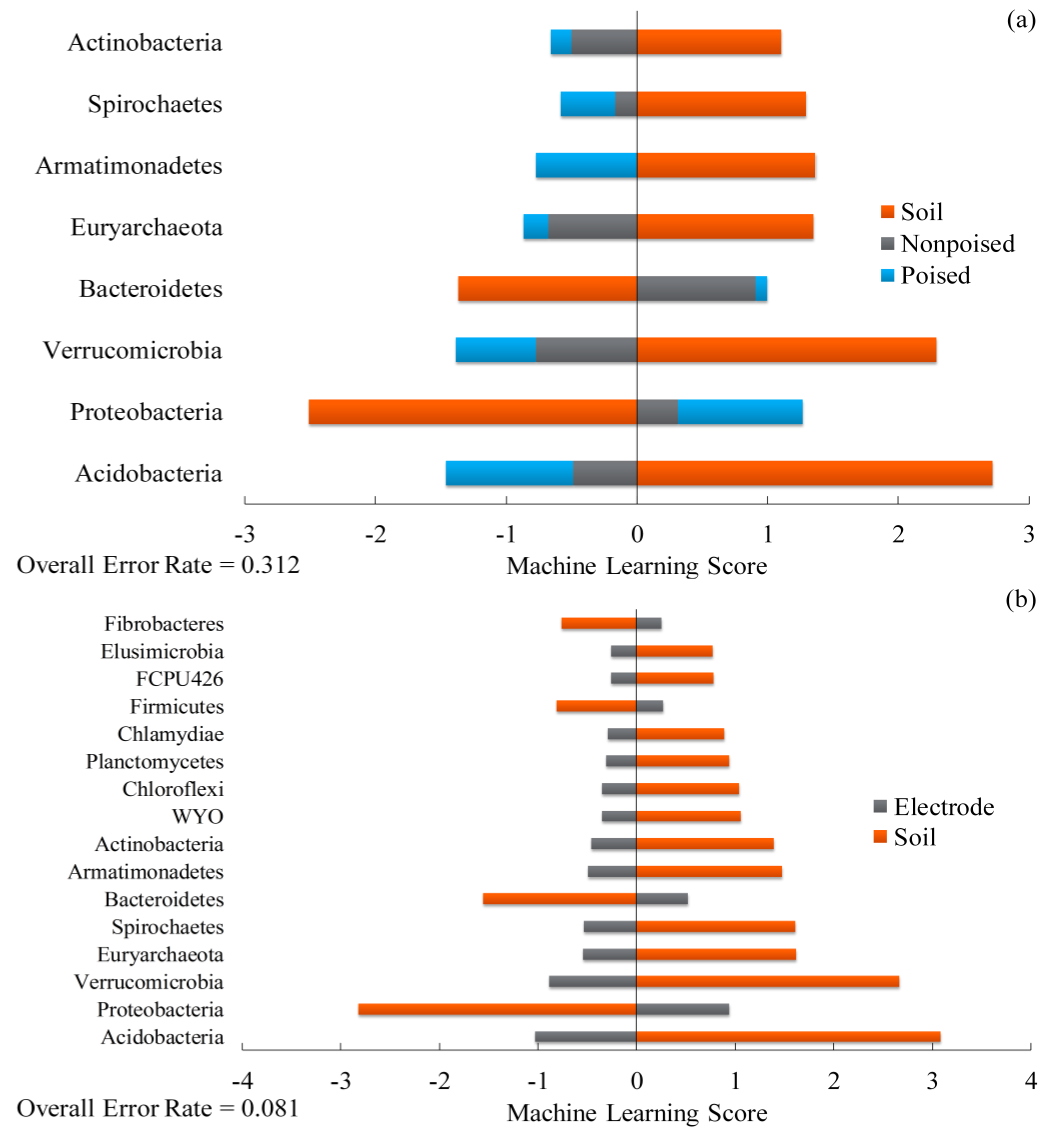
2.3. Changes in Methane Emissions Correspond to Changes in Microbial Communities
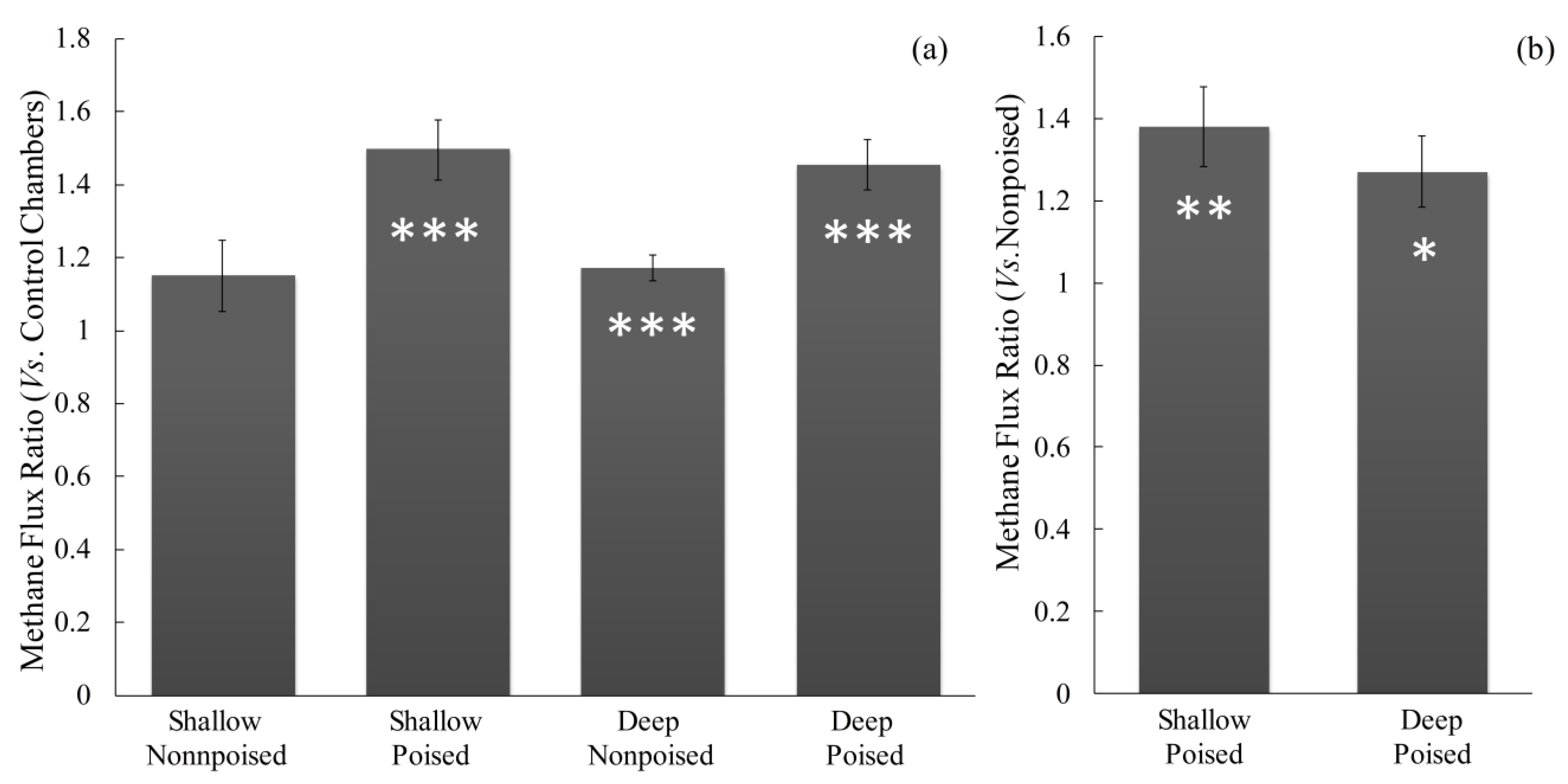
2.4. Poised Electrodes Further Stimulate Methane Emissions with Minimal Changes in Microbiome Structure
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, GB2023. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Bockheim, J.; Canadell, J.G.; Euskirchen, E.; Field, C.B.; Goryachkin, S.V.; Hagemann, S.; Kuhry, P.; Lafleur, P.M.; Lee, H.; et al. Vulnerability of permafrost carbon to climate change: Implications for the global carbon cycle. Bioscience 2008, 58, 701–714. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B.W.; Bowden, W.B.; Brovkin, V.; Camill, P.; Canadell, J.G.; Chanton, J.P.; Chapin, F.S., III; Christensen, T.R.; Ciais, P.; et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Chang. 2013, 119, 359–374. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Natali, S.M.; Schuur, E.A.G.; Rubin, R.L. Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. J. Ecol. 2012, 100, 488–498. [Google Scholar] [CrossRef]
- Lee, H.; Schuur, E.A.G.; Inglett, K.S.; Lavoie, M.; Chanton, J.P. The rate of permafrost carbon release under aerobic and anaerobic conditions and its potential effects on climate. Glob. Chang. Biol. 2012, 18, 515–527. [Google Scholar] [CrossRef]
- Graham, D.E.; Wallenstein, M.D.; Vishnivetskaya, T.A.; Waldrop, M.P.; Phelps, T.J.; Pfiffner, S.M.; Onstott, T.C.; Whyte, L.G.; Rivkina, E.M.; Gilichinsky, D.A.; et al. Microbes in thawing permafrost: The unknown variable in the climate change equation. ISME J. 2012, 6, 709–712. [Google Scholar] [CrossRef]
- Riley, W.J.; Subin, Z.M.; Lawrence, D.M.; Swenson, S.C.; Torn, M.S.; Meng, L.; Mahowald, N.M.; Hess, P. Barriers to predicting changes in global terrestrial methane fluxes: Analyses using CLM4Me, a methane biogeochemistry model integrated in CESM. Biogeosciences 2011, 8, 1925–1953. [Google Scholar] [CrossRef]
- Meng, L.; Hess, P.G.M.; Mahowald, N.M.; Yavitt, J.B.; Riley, W.J.; Subin, Z.M.; Lawrence, D.M.; Swenson, S.C.; Jauhiainen, J.; Fuka, D.R. Sensitivity of wetland methane emissions to model assumptions: Application and model testing against site observations. Biogeosci. Discuss. 2011, 8, 6095–6160. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Bell, T.H.; Callender, K.L.; Whyte, L.G.; Greer, C.W. Microbial competition in polar soils: A review of an understudied but potentially important control on productivity. Biology 2013, 2, 533–554. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B. Climate change: High risk of permafrost thaw. Nature 2011, 480, 32–33. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A., III; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef]
- Nealson, K.H.; Saffarini, D. Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Dollhopf, M.E.; Aller, R.; Stackebrandt, E.; Nealson, K.H. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int. J. Syst. Evol. Bacteriol. 1998, 48, 965–972. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef]
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanal. 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Beccaru, M.; Majone, M. Microbial generation of H2 or CH4 coupled to wastewater treatment in bioelectrochemical systems. Chem. Eng. Trans. 2010, 20, 163–168. [Google Scholar]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.L.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhou, S.G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Strycharz, S.M.; Gannon, S.M.; Boles, A.R.; Franks, A.E.; Nevin, K.P.; Lovley, D.R. Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ. Microbiol. Rep. 2010, 2, 289–294. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Crimi, B.; Pruden, A. Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem. Eng. J. 2009, 146, 161–167. [Google Scholar] [CrossRef]
- Strycharz, S.M.; Woodard, T.L.; Johnson, J.P.; Nevin, K.P.; Sanford, R.A.; Loffler, F.E.; Lovley, D.R. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008, 74, 5943–5947. [Google Scholar] [CrossRef]
- Heitmann, T.; Goldhammer, T.; Beer, J.; Blodau, C. Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob. Chang. Biol. 2007, 13, 1771–1785. [Google Scholar] [CrossRef]
- Keller, J.K.; Takagi, K.K. Solid-phase organic matter reduction regulates anaerobic decomposition in bog soil. Ecosphere 2013, 4. [Google Scholar] [CrossRef]
- Lipson, D.A.; Raab, T.K.; Goria, D.; Zlamal, J. The contribution of Fe(III) and humic acid reduction to ecosystem respiration in drained thaw lake basins of the Arctic Coastal Plain. Glob. Biogeochem. Cycles 2013, 27, 399–409. [Google Scholar] [CrossRef]
- Lipson, D.A.; Jha, M.; Raab, T.K.; Oechel, W.C. Reduction of iron (III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. J. Geophys. Res. Biogeosci. 2010, 115, G00I06. [Google Scholar] [CrossRef]
- Friedman, E.S.; Rosenbaum, M.A.; Lee, A.W.; Lipson, D.A.; Land, B.R.; Angenent, L.T. A cost-effective and field-ready potentiostat that poises subsurface electrodes to monitor bacterial respiration. Biosens. Bioelectron. 2012, 32, 309–313. [Google Scholar] [CrossRef]
- Lonergan, D.J.; Jenter, H.L.; Coates, J.D.; Phillips, E.J.; Schmidt, T.M.; Lovley, D.R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 1996, 178, 2402–2408. [Google Scholar]
- Gault, A.G.; Ibrahim, A.; Langley, S.; Renaud, R.; Takahashi, Y.; Boothman, C.; Lloyd, J.R.; Clark, I.D.; Ferris, F.G.; Fortin, D. Microbial and geochemical features suggest iron redox cycling within bacteriogenic iron oxide-rich sediments. Chem. Geol. 2011, 281, 41–51. [Google Scholar] [CrossRef]
- Kostka, J.E.; Nealson, K.H. Dissolution and reduction of magnetite by bacteria. Environ. Sci. Technol. 1995, 29, 2535–2540. [Google Scholar] [CrossRef]
- Inceoglu, O.; Al-Soud, W.A.; Salles, J.F.; Semenov, A.V.; van Elsas, J.D. Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS One 2011, 6, e23321. [Google Scholar] [CrossRef]
- Hou, S.B.; Makarova, K.S.; Saw, J.H.W.; Senin, P.; Ly, B.V.; Zhou, Z.M.; Ren, Y.; Wang, J.M.; Galperin, M.Y.; Omelchenko, M.V.; et al. Complete genome sequence of the extremely acidophilic methanotroph isolate V4, Methylacidiphilum infernorum, a representative of the bacterial phylum Verrucomicrobia. Biol. Direct 2008, 3, 26. [Google Scholar] [CrossRef]
- Freitas, S.; Hatosy, S.; Fuhrman, J.A.; Huse, S.M.; Welch, D.B.M.; Sogin, M.L.; Martiny, A.C. Global distribution and diversity of marine Verrucomicrobia. ISME J. 2012, 6, 1499–1505. [Google Scholar] [CrossRef]
- Pol, A.; Heijmans, K.; Harhangi, H.R.; Tedesco, D.; Jetten, M.S.M.; Op den Camp, H.J. Methanotrophy below pH1 by a new Verrucomicrobia species. Nature 2007, 450, 874–878. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Tibshirani, R.; Hastie, T.; Narasimhan, B.; Chu, G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc. Natl. Acad. Sci.USA 2002, 99, 6567–6572. [Google Scholar] [CrossRef]
- Erable, B.; Etcheverry, L.; Bergel, A. From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): Maximizing chemical oxygen demand (COD) removal from wastewater. Biofouling 2011, 27, 319–326. [Google Scholar] [CrossRef]
- Hoover, C.M. Field Measurements for Forest Carbon Monitoring: A Landscape Scale Approach; Springer: Berlin, Germany, 2008. [Google Scholar]
- Friedman, E.S. Bioelectrochemical Systems as Tools to Study Subsurface Biogeochemical Processes; Cornell University: Ithaca, NY, USA, 2013. [Google Scholar]
- Callaghan, T.V.; Bjorn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Effects on the function of arctic ecosystems in the short- and long-term perspectives. Ambio 2004, 33, 448–458. [Google Scholar]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Olefeldt, D.; Roulet, N.T. Effects of permafrost and hydrology on the composition and transport of dissolved organic carbon in a subarctic peatland complex. J. Geophys. Res. Biogeosci. 2012, 117, G01005. [Google Scholar] [CrossRef]
- Lee, H.; Schuur, E.A.G.; Vogel, J.G. Soil CO2 production in upland tundra where permafrost is thawing. J. Geophys. Res. Biogeosci. 2010, 115, G01009. [Google Scholar] [CrossRef]
- Hinkel, K.M.; Eisner, W.R.; Bockheim, J.G.; Nelson, F.E.; Peterson, K.M.; Dai, X.Y. Spatial extent, age, and carbon stocks in drained thaw lake basins on the Barrow Peninsula, Alaska. Arct. Antarct. Alp. Res. 2003, 35, 291–300. [Google Scholar] [CrossRef]
- Hinkel, K.M.; Paetzold, F.; Nelson, F.E.; Bockheim, J.G. Patterns of soil temperature and moisture in the active layer and upper permafrost at Barrow, Alaska: 1993–1999. Glob. Planet. Chang. 2001, 29, 293–309. [Google Scholar] [CrossRef]
- Zona, D.; Oechel, W.C.; Kochendorfer, J.; Paw U, K.T.; Salyuk, A.N.; Olivas, P.C.; Oberbauer, S.F.; Lipson, D.A. Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Glob. Biogeochem. Cycles 2009, 23, GB2013. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Meyer, F.; Jansson, J.; Gordon, J.; Pace, N.; Tiedje, J.; Ley, R.; Fierer, N.; Field, D.; Kyrpides, N.; et al. The Earth Microbiome Project: Meeting report of the “1st EMP meeting on sample selection and acquisition” at Argonne National Laboratory October 6th 2010. Stand. Genomic Sci. 2010, 3, 249–253. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Earth Microbiome Project Home Page. Available online: http://www.microbio.me/emp/ (accessed on 18 September 2013).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 18 September 2013).
Appendix
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Friedman, E.S.; Miller, K.E.; Lipson, D.A.; Angenent, L.T. Potentiostatically Poised Electrodes Mimic Iron Oxide and Interact with Soil Microbial Communities to Alter the Biogeochemistry of Arctic Peat Soils. Minerals 2013, 3, 318-336. https://doi.org/10.3390/min3030318
Friedman ES, Miller KE, Lipson DA, Angenent LT. Potentiostatically Poised Electrodes Mimic Iron Oxide and Interact with Soil Microbial Communities to Alter the Biogeochemistry of Arctic Peat Soils. Minerals. 2013; 3(3):318-336. https://doi.org/10.3390/min3030318
Chicago/Turabian StyleFriedman, Elliot S., Kimberley E. Miller, David A. Lipson, and Largus T. Angenent. 2013. "Potentiostatically Poised Electrodes Mimic Iron Oxide and Interact with Soil Microbial Communities to Alter the Biogeochemistry of Arctic Peat Soils" Minerals 3, no. 3: 318-336. https://doi.org/10.3390/min3030318
APA StyleFriedman, E. S., Miller, K. E., Lipson, D. A., & Angenent, L. T. (2013). Potentiostatically Poised Electrodes Mimic Iron Oxide and Interact with Soil Microbial Communities to Alter the Biogeochemistry of Arctic Peat Soils. Minerals, 3(3), 318-336. https://doi.org/10.3390/min3030318




