1. Introduction
The management of mine tailings, predominantly composed of fine particles (e.g., kaolinite and quartz, among others), faces a critical challenge: the high colloidal stability of these particles in aqueous suspension, attributed to their negative surface charges, which inhibit their natural sedimentation. This phenomenon, enhanced by the small size and high specific surface area of solids, intensifies electrostatic repulsions between them and prolongs settling times [
1], demanding chemical strategies to destabilize the system. Consequently, chemical conditioning strategies are required to destabilize the colloidal system and accelerate solid–liquid separation. The synergy between coagulants (charge neutralizers) and flocculants (polymer bridge formers) has emerged as a key technical solution, significantly improving sedimentation efficiency and aggregate formation in complex colloidal media. In addition to increasing the sedimentation rate, the combined use of coagulants and flocculants contributes to more efficient water and tailings management, reducing the environmental impact of mining operations [
2,
3]. In industrial practice, optimizing tailings clarification and settling is crucial to maximize water recirculation and reduce dependence on fresh sources of supply [
4]. This reduces the mine’s water footprint and helps comply with increasingly stringent environmental regulations on the quality of clarified water before discharge or reuse.
The initial aggregation of particles in clayey tailings suspensions typically begins with electrostatic neutralization. The zeta potential of the system is strongly dependent on the pH and ionic composition of the medium. Studies have shown that, in the presence of divalent cations (Ca
2+, Mg
2+), the electrical double layer is compressed more effectively than with monovalent cations (Na
+), decreasing the absolute value of the zeta potential and favoring flocculation. It has been observed that, under conditions dominated by divalent ions, the electrokinetic behavior of kaolinite suspensions and fine tailings becomes practically independent of pH [
5]. Nieto et al. [
6] reported that mixed quartz/kaolinite suspensions show faster sedimentation and generate larger flocs in freshwater, while systems rich in sodium montmorillonite achieve greater flocculating efficiency in seawater. This contrast was attributed to the reduction in clay swelling in a saline environment, along with the formation of cation bridges that promoted more stable agglomeration. Notably, in freshwater, they observed the coexistence of non-flocculated particles alongside the aggregates, a phenomenon not present when seawater was used. These findings reinforce how water chemistry (pH, ionic strength, and type of cations) decisively modulates the effectiveness of flocculation–coagulation in tailings.
Inorganic coagulants (metal salts of Al
3+ or Fe
3+) are traditionally used to treat fine suspensions; their mechanism is based on charge neutralization and the precipitation of metal hydroxides that trap the particles. However, the effectiveness of these salts depends on a narrow pH range, and their dosage usually consumes the alkalinity of the medium, producing abundant, low-density chemical sludge. Synthetic cationic polymers have emerged as a superior alternative in complex industrial waters, where metal ion hydrolysis can limit coagulant performance. These compounds, designed with a high positive charge density, neutralize the negative surfaces of particles more efficiently than inorganic salts, facilitating the formation of dense microflocs [
7]. According to Bolto and Gregory [
8], low to medium molecular weight polymeric coagulants, such as polydiallyldimethylammonium chloride (PDADMAC), possess a high charge density that allows them to act effectively by charge neutralization and the formation of compact microflocs. Furthermore, these polymers can induce flocculation through the “electrostatic patch” mechanism, in which localized regions of positive charge adsorbed on the surface of a particle create zones of attraction towards other negatively charged particles, even when the overall surface charge has been neutralized. This phenomenon is especially relevant in high alkalinity systems, where electrostatic repulsive forces are more intense and can hinder conventional aggregation, requiring more potent coagulants.
Coagulants and flocculants act complementarily in tailings treatment systems to improve sedimentation and water clarity. Coagulants (particularly low molecular weight cationic flocculants) induce initial destabilization by charge neutralization. In contrast, high molecular weight anionic flocculants (e.g., polyacrylamides) generate polymeric bridges that agglomerate particles into larger flocs, accelerating their settling [
7,
9]. This synergy requires sequential and controlled dosing: typically, the coagulant is added during a rapid pre-mixing stage, and the flocculant is dosed later (e.g., in the feedwell of a thickener), avoiding redispersion of particles due to unwanted interactions between reactants. Saritha et al. [
9] showed that operating parameters such as coagulant dosage, pH, and agitation speed significantly influence turbidity removal efficiency, without critically altering the treated water’s alkalinity or other physicochemical parameters. This reinforces the need to adjust reagent type and mixing conditions to achieve stable flocculation and efficient tailings dewatering. A recent review by Khazaie et al. [
10] consistently highlighted multiple successful cases of sequential coagulant–flocculant application in coal slurry dewatering, underlining the versatility of this dual strategy in different fine mining wastes.
Several recent studies have highlighted the potential of adding coagulants to improve aggregate densification and supernatant clarity in tailings sedimentation. Wang et al. [
11] demonstrated that the co-application of a cationic polymeric coagulant based on PDMDAC (Magnafloc
® FL 4540) and an anionic polyacrylamide flocculant (SNF-6255) in fluorspar tailings achieved a significant reduction in supernatant turbidity, reaching suspended solids concentrations of ~186 mg/L, which meets stringent effluent discharge standards. The remaining turbidity in the water was mainly attributed to the high proportion of ultrafine montmorillonite particles present, characterized by intense negative charges and high hydration capacity. The observed flocculation mechanism involved electrostatic neutralization of particles by the coagulant and the formation of hydrogen bonds between the flocculant and the colloidal surfaces, favoring larger and more stable flocs. Likewise, Paoli et al. [
7] reported that a natural tannin-based coagulant (Tanfloc) can substantially improve the sedimentation of iron tailings (from the Fundão dam), reducing the turbidity of the supernatant water under optimal pH (~6–8) and dosage (≈15 g/L) conditions. This organic coagulant allowed charge neutralization and the formation of effective flocs without adding metals to the system, demonstrating the efficacy of alternative coagulants in systems where traditional reagents have limitations. In a complementary study, Li et al. [
12] evaluated the synergy between a fly ash-derived magnetic coagulant (FAMC) and a polyacrylamide (PAM) in the sedimentation of ultrafine tailings with high clay content. The FAMC released Fe
3+ and Al
3+ ions capable of neutralizing negative charges, reducing the zeta potential from −34.1 mV to −22.6 mV. Additionally, the FAMC magnetic nanoparticles adsorbed on the colloidal surfaces, inducing a paramagnetic behavior that promoted the agglomeration and sedimentation of the solids. The flocculant, acting as a bridging agent, favored the formation of larger flocs (average diameter ~380 µm). Under optimal conditions (200 mL/t FAMC and 30 g/t PAM), this dual combination achieved a ~90% decrease in supernatant turbidity and substantially improved tailings thickening rate.
Despite the advances mentioned above, knowledge gaps persist regarding the simultaneous effect of polymeric coagulants and anionic flocculants on the microstructure of tailings aggregates, particularly under the adverse ionic conditions (high pH, high salinity) typical of industrial water used in mining plants. While recent studies have demonstrated the operational benefits of combining coagulants and flocculants in tailings, the literature has explored little how the mineralogical composition of the tailings (e.g., different proportions of kaolinitic clay vs. quartz) influences the internal structure of the flocs formed and their sedimentary and rheological behavior under these conditions. In this context, the present study aims to evaluate how the interaction between a high molecular weight anionic flocculant (SNF® 604, anionic polyacrylamide) and a synthetic cationic coagulant (Magnafloc® 1727) influences the structural properties of aggregates formed in clayey tailings suspensions, using industrial water under high alkalinity conditions. Furthermore, the effect of the relative proportion of kaolinite and quartz in the tailings on the structural characteristics of the flocs and their settling behavior, quality of the resulting clarified water, and rheology of the suspensions is examined. Based on the results obtained, this work seeks to provide a comprehensive understanding of the coagulant–flocculant–tailings system under representative operating conditions, generating applied knowledge to address specific challenges in tailings management. Likewise, it is expected to contribute to meeting more stringent environmental standards and promote more efficient and sustainable treatment practices in the mining industry, optimizing both sedimentation processes and water recovery during the waste disposal and management stages.
2. Materials and Methods
2.1. Materials
The quartz particles were purchased from Donde Capo (Santiago, Chile), and their purity was verified by X-ray diffraction (XRD) using a Bruker D8 Advance (Karlsruhe, Germany) equipment and TOPAS 3.0 software, obtaining a purity greater than 99% by weight. The kaolinite samples were supplied by Ward’s Science (Clay Spur, WY, USA) and also characterized by XRD (
Figure 1), confirming a majority composition of illite and kaolinite, with a minor fraction of quartz. Both minerals presented an apparent density of 2.6 g/cm
3.
The diffractogram presented in
Figure 1a confirms the presence of mostly kaolinite and illite, while
Figure 1b confirms the presence of quartz.
Aggregate size characterization was performed using a ParticleTrack G400 FBRM system (Mettler Toledo – Northpointe Parkway Lutz, FL, USA). The probe was located 10 mm above the stirrer and 20 mm from the vessel axis. Each measurement lasted 5 min, and real-time chord length data were collected. Both unweighted and square-weighted distributions were analyzed, allowing simultaneous evaluation of the fine and coarse fractions of the aggregates. Percentile analysis revealed that 10% of the particles had sizes smaller than d10 = 11.2 µm for kaolin and 19.9 µm for quartz, while 90% of the particles were smaller than d90 = 47.6 µm for kaolin and 78.0 µm for quartz (
Figure 2).
The flocculant used was a high molecular weight anionic polyacrylamide (SNF 604®, SNF Chile S.A., Santiago, Chile), while the coagulant was a medium molecular weight cationic polymer (Magnafloc 1727®, BASF Chile S.A., Santiago, Chile). Stock solutions were prepared at 1 g/L, and working solutions at 0.1 g/L for the flocculant and 0.2 g/L for the coagulant.
Synthetic industrial water (IW) was prepared by dissolving 0.005 M CaCl
2 and 0.01 M NaCl in distilled water, representing typical recycled mining water ionic conditions. The pH was adjusted to 11 by adding lime (CaO), simulating operating conditions in industrial alkaline systems. The water properties were characterized with a HI9829 multiparameter meter (Hanna Instruments, Woonsocket, RI, USA) and a DMA 35 densitometer (Anton Paar, Graz, Austria), as summarized in
Table 1.
2.2. Sedimentation Tests with Coagulant and Flocculant Addition
Mineral suspensions were prepared at a solid concentration of 10% (w/w) using industrial water as the medium, consisting of a mixture of 80% quartz and 20% kaolin. The flocculant dosage was kept constant at 20 g/t. This dosage was selected based on values commonly used in copper tailings thickening processes, where high molecular weight anionic polyacrylamides are applied. The coagulant dosage was varied between 0 and 150 g/t. After homogenizing the suspension with the coagulant, the flocculant was added with gentle stirring (200 rpm) for 30 s. Subsequently, 250 mL of suspension was transferred to graduated cylinders to measure the sedimentation rate by video recording for 60 min. Each experimental condition was tested in triplicate (n = 3) to ensure reproducibility. The turbidity of the supernatant was measured using a HI98713 turbidimeter (Hanna Instruments) in quintuplicate (n = 5) for each condition, and the average was reported.
2.3. Aggregate Characterization
The flocculated aggregates were characterized using an FBRM probe, submerged vertically in the suspension at a depth of 10 mm above the PTFE stirrer and 20 mm from the vessel’s centerline. Each measurement lasted 5 min. The suspension pH was pre-adjusted to pH 11 by adding lime, and the flocculant was dosed 60 s after data acquisition began. Analyses were performed using a Mettler-Toledo Particle Track G400 (Mettler Toledo—Northpointe Parkway Lutz, FL, USA) with FBRM technology.
The FBRM system employs a focused laser beam that passes through a sapphire window, scanning the suspension along a circular path at a tangential speed of 2 m/s. This technique allows real-time monitoring of particle size and number changes by measuring chord length, an indirect parameter associated with floc morphology and dimensions. The chord length distribution (CLD) can be analyzed in unweighted form, which helps characterize fine particles, or square-weighted, emphasizing the contribution of larger aggregates. The data were processed using iC FBRM™ 4.4 software.
2.4. Fractal Dimension and Aggregate Density
The fractal dimension (Df) is a key parameter for characterizing the internal structure of aggregates formed in flocculated sedimentation systems. Its value, typically between 1 and 3, is associated with the aggregate morphology: values close to 1 indicate linear or highly branched structures, while values close to 3 correspond to more compact aggregates with an approximately spherical geometry. In the present study, the calculation of Df was based on the model proposed by Heath et al. [
13], which establishes a relationship between the hydrodynamic diameter of the aggregates and the sedimentation velocity in the hindered sedimentation regime, incorporating the effects of porosity through a representation based on fractal geometry.
The squared weighted average chord length, determined using the FBRM technique, was used to estimate the characteristic diameter of the aggregates. This approach was carefully adapted to the specific experimental conditions of this study, allowing a representative characterization of the flocculated structure in each test.
In Equation (1), represents the hindered settling rate (m/s); and correspond to the diameters of the aggregate and the primary particle, respectively (m), and both approximate the weighted mean square chord length obtained by the FBRM technique. The parameter is the gravitational acceleration (9.81 m/s2); and are the densities of the solid (2600 kg/m3) and the liquid (IW: 1027 kg/m3), respectively; is the viscosity of the liquid (0.001084 N∙s/m2); and represents the volume fraction of solids (0.042 v/v). The fractal dimension () characterizes the internal structure of the aggregate. In general, the parameters , , , , , are considered constant and known in all tests, while and depend on the hydrodynamic conditions imposed. The determination of was performed by means of a least squares adjustment between the experimental and calculated values of , which allowed the structural compactness of the aggregates to be estimated quantitatively in a reliable manner.
The apparent density of flocculated aggregates was estimated using the equation derived by Kranenburg [
14], which relates the densities of the solid (
), liquid (
) and aggregate (
), together with the primary particle (
) and aggregate diameters (
), and the fractal dimension (
) determined previously. This relationship, derived from principles of fractal geometry, allows for more accurate inferences about sedimentation behavior. By incorporating structural and compositional parameters, this approach provides a basis for interpreting aggregate settlement dynamics in complex media.
2.5. Rheological Characterization
Flocculated suspensions composed of quartz and kaolin mixtures with a total mass of 100 g were prepared, adjusting the percentages of solids by weight to 45% and 50%. The coagulant dosage varied between 0 and 150 g/t, keeping the quartz/kaolin ratio and the flocculant dosage constant. The pH of the suspensions was adjusted to 11 by adding lime.
For each experiment, 60 mL aliquots of suspension were used individually and not reused; the remainders were discarded. Rheological measurements were carried out using an Anton Paar MCR 102 rheometer (ANAMIN Group, Santiago, Chile), and the data obtained were processed with RheoCompass 1.3 software from the same manufacturer. A vane-in-cup geometry was used to minimize errors due to wall slip, employing a 2.2 cm diameter vane and a 4.2 cm diameter cup.
The yield strength was determined as the average value between the minimum stress required to initiate suspension flow and that associated with the maximum deformation before the onset of plasticity (critical strain). This limit represents the elastic response range of the suspension to applied stresses before reaching irreversible plastic deformation. The yield strength was identified from logarithmic graphs of strain versus shear stress by intersecting the tangent curves corresponding to the elastic and plastic regions of the suspension’s rheological behavior.
2.6. Zeta Potential
The zeta potential was determined in 1% by weight solids suspensions (80% Qz–20% Kao) with the addition of coagulant in the range 0–150 g/t. Measurements were performed using a Litesizer 500 (Anton Paar, Austria) using the Smoluchowski model, with three independent measurements per sample (n = 3). The pH was controlled at 11 ± 0.1 for all samples.
3. Results
3.1. Sedimentation in the Presence of Coagulant
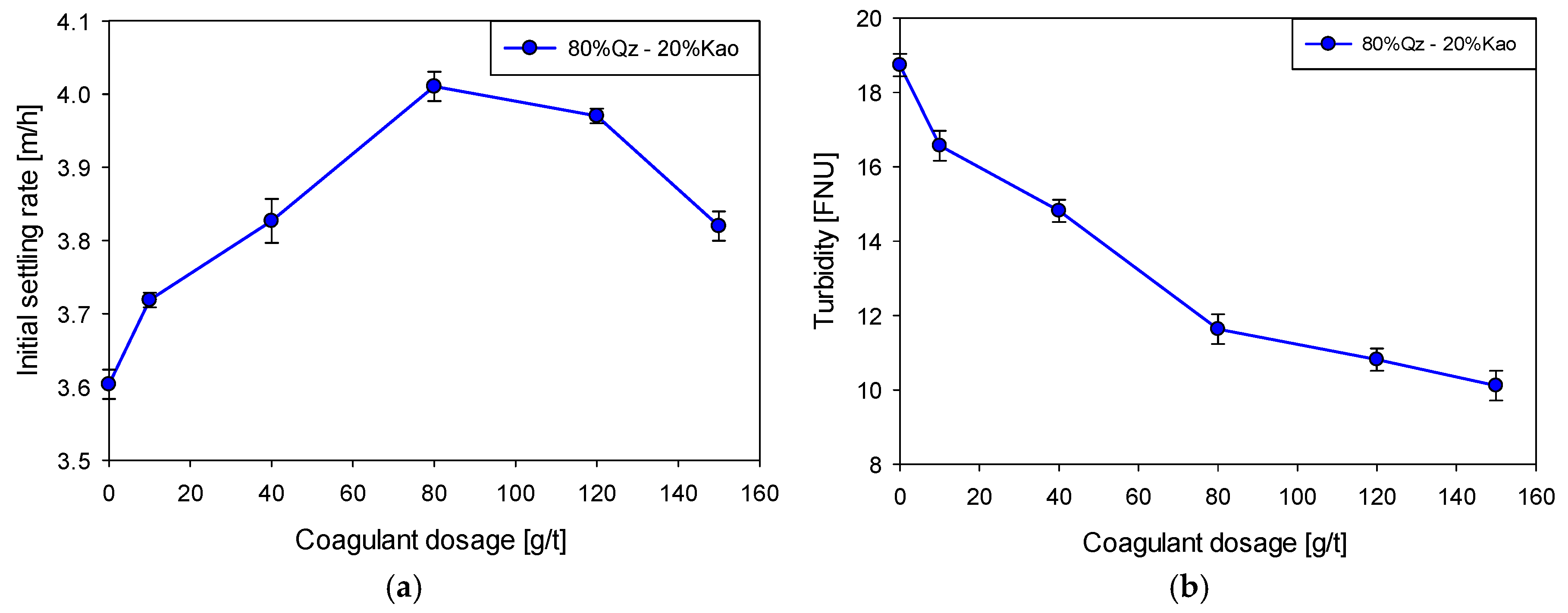
Figure 3a shows the evolution of the sedimentation velocity of Qz–Kao (80:20) suspensions flocculated with 20 g/t of SNF 604
® flocculant as a function of the Magnafloc 1727
® coagulant dosage at pH 11. The sedimentation velocity increases progressively from 3.6 m/h in the absence of coagulant to a maximum value of 4.0 m/h at a dosage of 80 g/t, representing an increase of approximately 11%. This initial behavior is attributed to the progressive neutralization of the negative charges on the particles, which reduces electrostatic repulsions and favors the formation of larger, denser aggregates, accelerating their gravitational sedimentation.
However, increasing the coagulant dosage to 150 g/t recorded a slight decrease in the sedimentation velocity to 3.8 m/h. This phenomenon suggests that excessive coagulant doses can induce supersaturation of colloidal surfaces, causing electrostatic overload, surface charge reversal, and potential floc fragmentation under mechanical shear. As a result, the effective mass of the aggregates is reduced, negatively affecting their settling velocity.
Figure 3b shows the evolution of supernatant turbidity under the same experimental conditions. A continuous decrease in turbidity is evident as the coagulant dose increases, from 18.7 FNU in the absence of coagulant to 10.1 FNU at 150 g/t, corresponding to a 46% reduction. Unlike the settling velocity, turbidity does not deteriorate at high doses, indicating that the capture of ultrafine particles by charge neutralization remains efficient, even when macrofloc formation begins to be impaired. These results are consistent with those reported in the literature for similar systems [
2,
11,
12], where the progressive addition of cationic coagulants promotes colloidal destabilization and improves the sedimentation rate and supernatant quality, until an optimal dosage threshold is reached. Beyond this threshold, re-stabilization or aggregate fragmentation can partially compromise sedimentological efficiency.
From a practical perspective, identifying the optimal coagulant dosage range (80–120 g/t) is critical to maximize sedimentation efficiency and clarified water quality in mining operations. The fact that turbidity continues to decrease even at high dosages suggests that, in scenarios where recovered water quality is a priority, a moderate overdosage could be tolerated at the expense of a slight decrease in thickening rates. Furthermore, carefully controlling the coagulant dosage could represent a strategy for optimizing reagent costs and operational efficiency.
3.2. Kinetics of Flocculated Tailings in the Presence of Magnafloc 1727® Coagulant
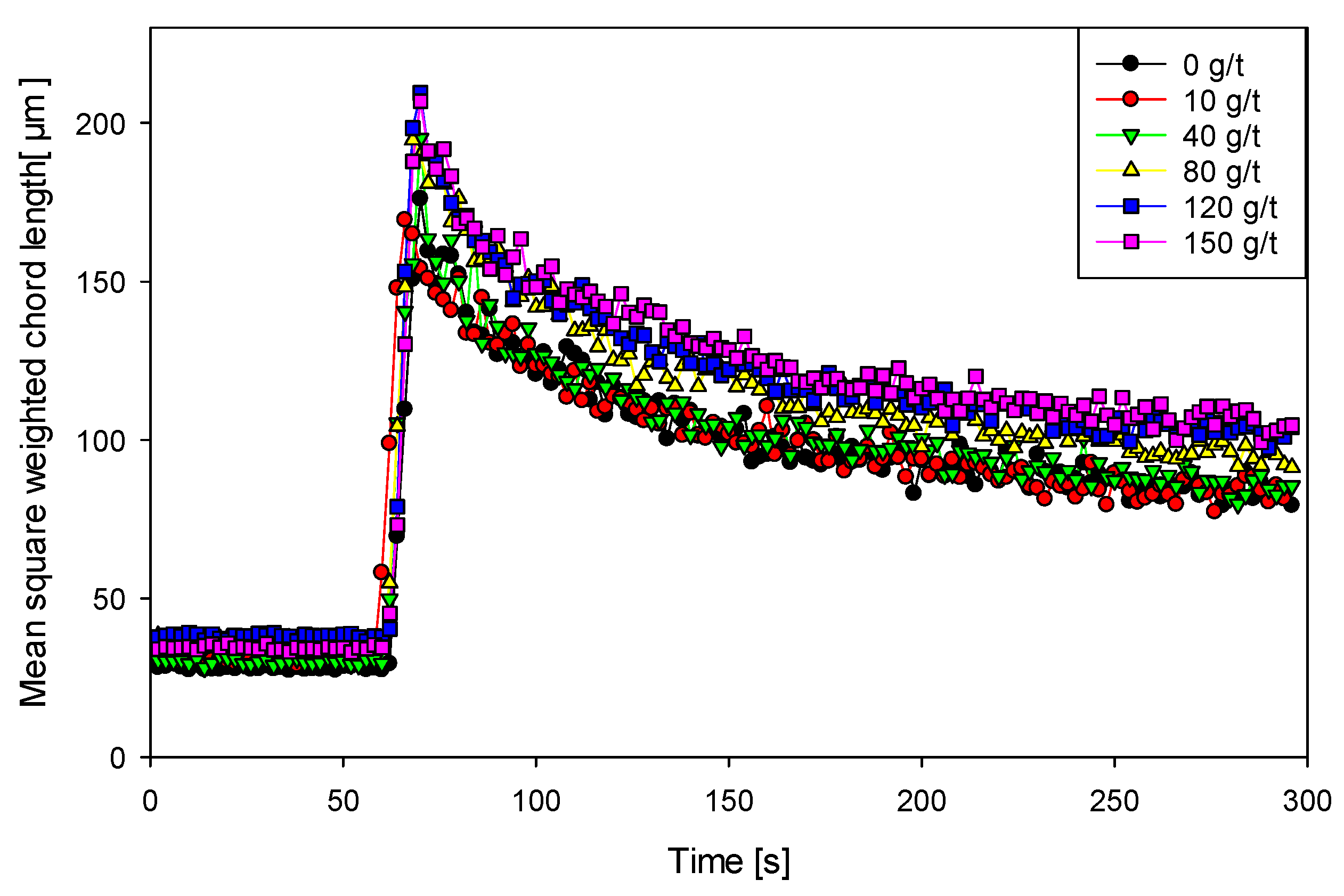
Figure 4 shows the temporal evolution of aggregate size during the coagulation–flocculation process, assessed using FBRM technology. Initially, after adding Magnafloc 1727
® coagulant to the Qz–Kao suspension (80:20), a progressive growth of microflocs was observed, reaching average diameters of 29 to 40 µm in the first minute of agitation. This initial growth reflects the successful electrostatic neutralization of the particles, facilitating their convergence and formation of primary macroaggregates.
Subsequently, upon adding the anionic flocculant SNF 604® at 20 g/t, a sudden increase in aggregate size was recorded, reaching maximum values of 180 to 210 µm in seconds. This phenomenon is attributed to the establishment of polymeric bridges between previously formed microflocs, promoting the generation of larger macroflocs with lower specific density. However, after reaching maximum size, a slight decrease in the average floc size is observed over the following minutes. This reduction is consistent with fragmentation phenomena induced by mechanical shear in the stirred suspension. As the flocs grow, their structure becomes more fragile and susceptible to breaking under shear stresses, establishing a dynamic equilibrium between aggregate formation and rupture.
This kinetic behavior is typical of coagulation–flocculation systems where the coagulant acts first as a primary particle destabilizer and the flocculant as a secondary aggregation agent [
15]. Comparisons with previous work, such as that of Wang et al. [
9], show similar trends, where a stabilized fragmentation stage follows the initial aggregate growth. Therefore, the efficiency of the process depends on the initial quality of the microflocs formed and the robustness of the generated polymeric bonds.
3.3. Chord Length Distributions
Chord length is a fundamental metric for characterizing the size of suspended particles or aggregates in both flocculated and non-flocculated systems. It is represented by chord length distributions (CLDs), expressed in unweighted and quadratic weighted forms. The unweighted CLD provides detailed information on the fine fraction of the system, allowing a more precise analysis of the growth and fragmentation processes of small aggregates. In contrast, the square-weighted CLD provides information on the larger aggregates, is more sensitive to changes in the coarse fraction, and provides a structural view of the system [
16,
17].
3.3.1. In the Absence of Flocculant
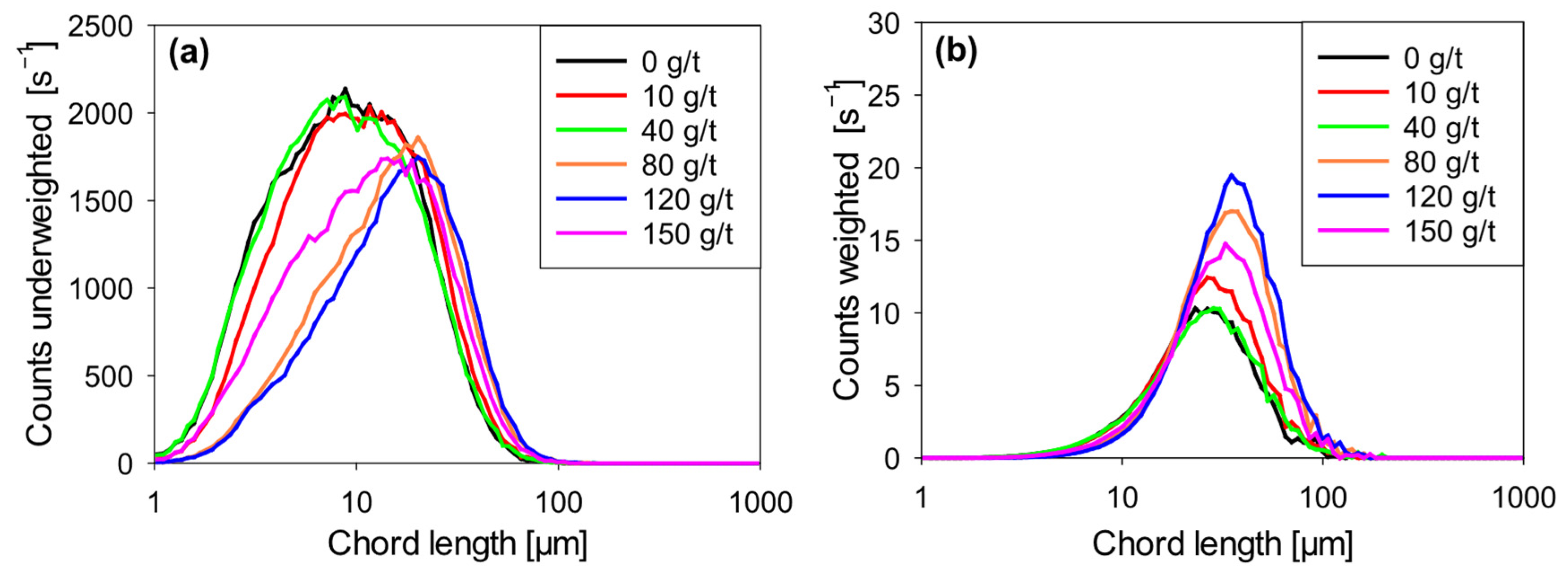
Figure 5a,b show the evolution of floc size distribution in Qz–Kao (80:20) suspensions flocculated solely with Magnafloc 1727
® coagulant at different dosages.
Figure 5a (unweighted chord length distribution) shows that the fine particle count (chord lengths less than 10 µm) progressively decreases from 2137 s
−1 in the absence of coagulant to 1742 s
−1 at a 120 g/t dosage. This trend indicates a gradual capture of fine particles as the coagulant dosage increases, corresponding to more effective electrostatic destabilization and the initial formation of microflocs.
However, increasing the dosage to 150 g/t showed a slight increase in the fine particle count (1748 s−1), suggesting a phenomenon of redispersion or aggregate fragmentation due to the supersaturation of positive charges on the surfaces, which reestablishes electrostatic repulsions between particles.
Figure 5b (squared weighted distribution) shows that the average floc size increases with coagulant dosage up to 120 g/t, shifting toward larger diameters in the weighted distribution, consistent with forming larger aggregates. At 150 g/t, however, a slight reduction in the maximum size reached is evident, in line with the hypothesis of redispersion or weakening of aggregates.
These results confirm that the coagulant can promote the formation of dense microflocs at moderate doses, but excessive doses can compromise aggregate stability. This behavior has been previously reported by Wang et al. [
11] in fine tailings systems, where charge reversal and surface supersaturation with cationic polymers reduced coagulation efficiency at high dosages.
Effectively reducing the fine particle content through precoagulation is critical to maximizing the overall efficiency of the subsequent flocculation process. By decreasing the concentration of particles smaller than 10 µm, competition between ultrafine particles and larger aggregates for the flocculant’s active sites is reduced, favoring the formation of robust and stable macroflocs. Furthermore, limiting the amount of free fines in the system minimizes the likelihood of steric interference and polymer chain blockages, which optimizes the efficiency of the flocculant bridging mechanism.
3.3.2. In the Presence of Flocculant
Figure 6a,b show the floc size distributions when the suspension is treated with a combination of 20 g/t of SNF 604
® flocculant and different doses of Magnafloc 1727
® coagulant.
In
Figure 6a (unweighted chord length distribution), the fine particle count decreases much more steeply than in the absence of flocculant. At 0 g/t of coagulant, the fine particle count is approximately 500–620 s
−1; however, as the coagulant dose increases to 120 g/t, this count drops dramatically to 2.3 s
−1. This significant decrease demonstrates the high efficiency of the combined coagulant–flocculant system in capturing ultrafine particles through simultaneous charge neutralization and polymer bridging mechanisms.
Figure 6b (squared weighted distribution) shows that aggregates reach maximum diameters of around 451 µm at coagulant dosages between 80 and 120 g/t, much larger than the sizes obtained without flocculant. However, at 150 g/t of coagulant, the maximum size reached decreases to 393 µm, again indicating possible macrofloc fragmentation at high dosages.
A direct comparison between the results with and without flocculant reveals that the use of SNF 604
® flocculant not only promotes the capture of fine particles but also facilitates the formation of significantly larger and more stable macroflocs, in line with polymeric bridging mechanisms described in the literature [
18,
19]. However, as in systems flocculated exclusively with coagulant, there is a critical dosage threshold beyond which the additional benefit diminishes or is reversed. From an operational perspective, these results suggest that combining a cationic coagulant with a high molecular weight anionic flocculant can substantially improve the clarification and settling efficiency of clayey tailings suspensions, provided that adequate dosage is maintained to prevent overloading and fragmentation. This synergistic approach is particularly promising for optimizing water recovery and tailings consolidation under high industrial alkalinity conditions.
3.4. Evolution of Fractal Dimension and Aggregate Density
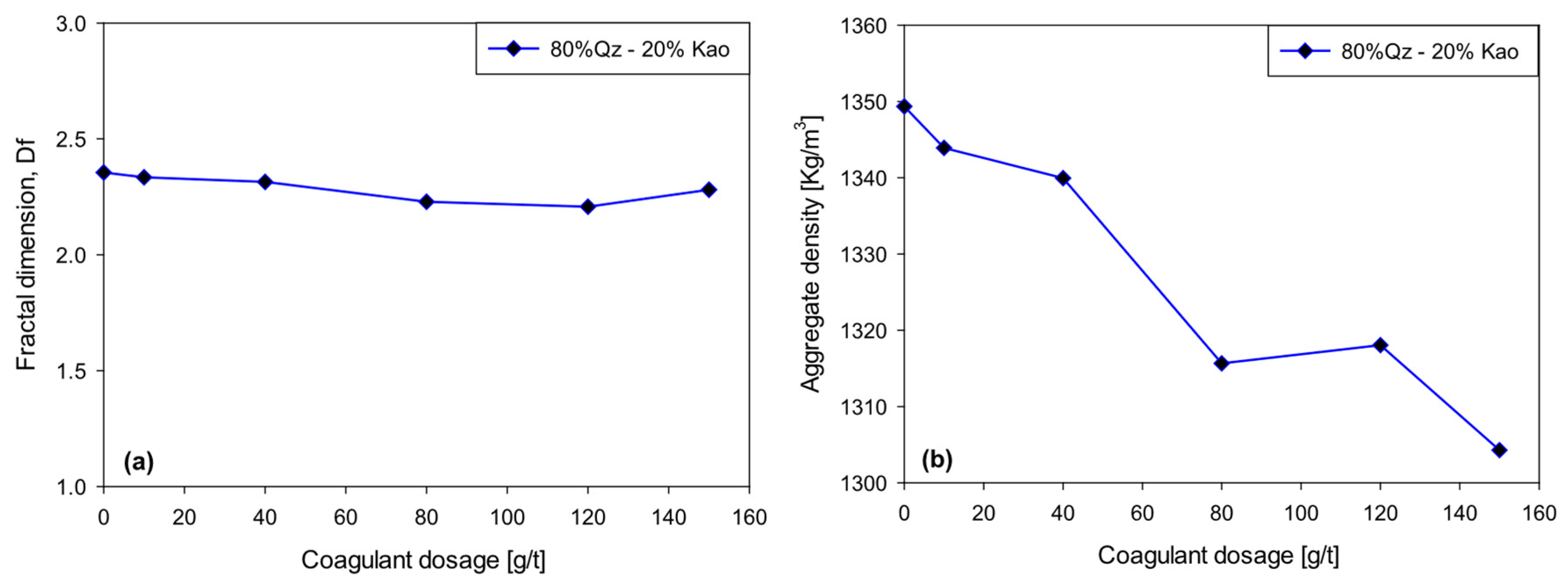
Figure 7 shows the evolution of the fractal dimension and bulk density of the aggregates formed in Qz–Kao (80:20) suspensions flocculated with 20 g/t of SNF 604
®, as a function of the Magnafloc 1727
® coagulant dosage.
The fractal dimension decreases slightly from 2.35 to 2.20 as the coagulant dosage increases from 0 to 120 g/t. Similarly, the bulk density of the aggregates decreases from 1349 kg/m3 to 1304 kg/m3 over the same dosage range. However, these changes are relatively small in magnitude, indicating that the internal structure of the flocs remains stable overall.
This slight decrease in (Df) and aggregate density is not interpreted as a significant mechanical weakening of the flocs, but rather as a natural consequence of the growth in aggregate size. Theoretically, there is an inverse relationship between floc size and its internal density. As flocs grow, their structure becomes slightly more open, resulting in a marginal decrease in fractal dimension. This phenomenon is consistent with reaction-limited diffusive aggregation (DLA) models, where the growth of macroflocs generates more branched geometries without necessarily compromising mechanical stability.
From an operational perspective, the formation of larger but slightly less dense flocs can be favorable, provided the mechanical integrity of the aggregate is maintained. In this case, the results suggest that the coagulant dosage range evaluated (up to 120 g/t) maximizes floc size and improves sedimentological efficiency without generating fragile or unstable structures. This is a key aspect for optimizing the performance of thickeners in mining operations, where high sedimentation rates and low suspended solids content in the supernatant are simultaneously sought. Finally, joint monitoring of fractal dimension, density, and aggregate size using in situ techniques such as FBRM can be a valuable tool for dynamically adjusting reagent dosage and agitation conditions, optimizing water recovery and tailings consolidation under realistic plant conditions.
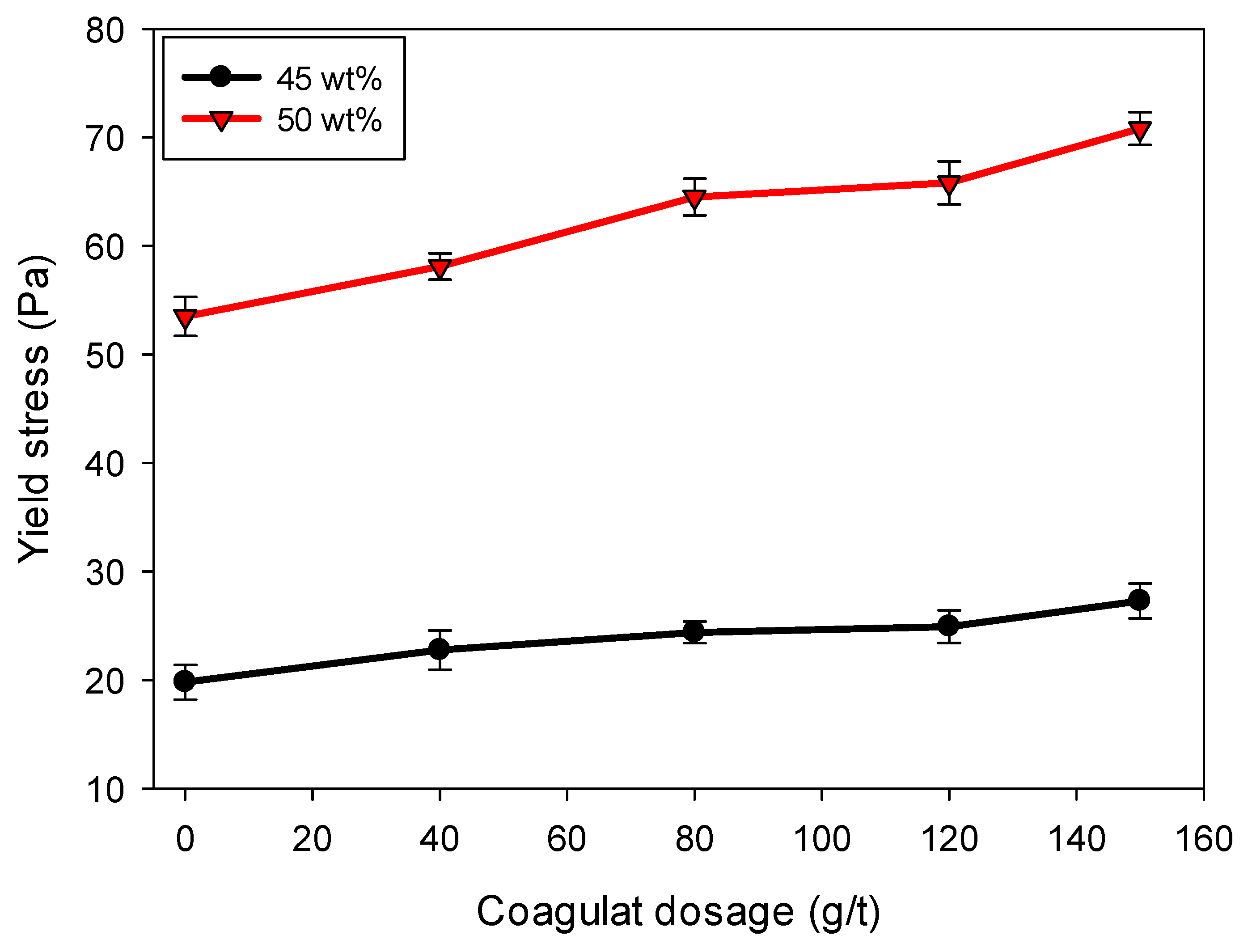
3.5. Yield Stress of Quartz-Kaolin Sludge
Figure 8 shows the evolution of yield stress as a function of coagulant dosage in quartz/kaolinite suspensions flocculated with high molecular weight anionic polyacrylamide (SNF 604), for two solids concentrations: 45 and 50 wt%. In both cases, yield stress increases with increasing coagulant dosage (0–150 g/t), although the magnitude of this effect varies considerably depending on the solids content. At 50 wt%, yield stress increases markedly from approximately 55 Pa to over 70 Pa with increasing dosage, demonstrating a substantial strengthening of the structural network. In contrast, the 45 wt% suspension shows a more moderate increase, from ~22 Pa to ~27 Pa.
This trend suggests that adding a coagulant before the flocculant improves interparticle interactions by partially neutralizing surface charges and promoting the formation of bridges or doublets between particles, which facilitates the development of a more cohesive and rigid floc network after flocculation. The impact is more pronounced in the suspension with higher solids concentrations due to the smaller interparticle distance, which favors percolation and the formation of continuous flocculated structures capable of withstanding stresses.
From a structural perspective, reducing coagulant-induced electrostatic repulsion decreases the separation between the clay laminae and quartz particles, forming denser and stronger interflocculant bonds after flocculant addition. These connections limit the capacity for internal rearrangement under stress, resulting in greater resistance to deformation and increased yield strength. This behavior is characteristic of transitioning from a weakly structured network to a viscoplastic material with internal stiffness.
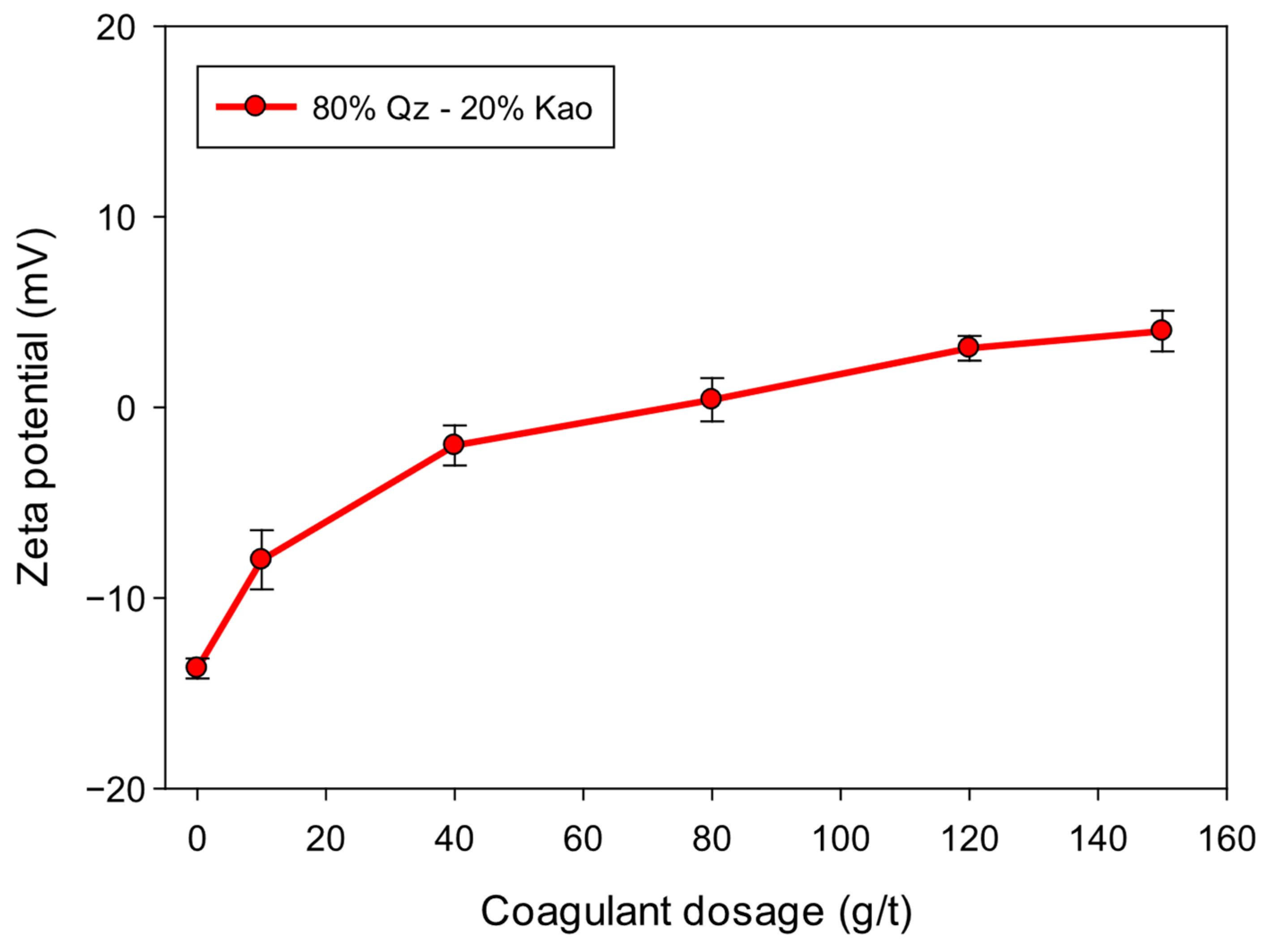
3.6. Zeta Potential
Figure 9 shows the evolution of the zeta potential of Qz–Kao (80:20) suspensions flocculated with 20 g/t of SNF 604
® as a function of the Magnafloc 1727
® coagulant dosage under high alkalinity conditions (pH 11).
In the absence of a coagulant, the system exhibits a zeta potential of −13.2 mV, characteristic of strongly negative particles due to quartz and kaolinite under alkaline conditions. As the coagulant dosage increases, the zeta potential progressively shifts toward less negative values, reaching electrostatic neutrality (≈0 mV) around 40 g/t. The zeta potential reverses at higher dosages, reaching +4.0 mV at 150 g/t of coagulant.
This behavior reflects the classic coagulation mechanism by neutralization and surface charge inversion. Adding a cationic coagulant promotes the adsorption of positively charged groups onto initially negatively charged particles, reducing the electrostatic repulsion forces that stabilize the suspension. The charge reversal observed at high doses indicates surface saturation and the formation of an excess cationic polymer shell.
From a colloidal aggregation perspective, neutralization near 0 mV corresponds to the point of greatest suspension instability, where particles are most likely to aggregate due to van der Waals forces. The formation of dense, stable flocs observed in the previous sections coincides with this near-neutral zeta potential range.
Operationally, zeta potential control can monitor and dynamically adjust coagulant dosage in thickening plants. Achieving and maintaining near-neutral zeta potentials can simultaneously maximize settling rate and clear water recovery, minimize excessive reagent consumption, and reduce the risk of particle redispersion.
4. Conclusions
The sequential application of a cationic coagulant (Magnafloc 1727®) followed by an anionic flocculant (SNF 604®) simultaneously optimized the sedimentation and clarification of synthetic tailings suspensions in alkaline industrial water, achieving a 46% reduction in turbidity and an increase in sedimentation velocity of up to 11%.
The shift in zeta potential from −13.2 mV to +4.0 mV demonstrated effective neutralization of surface charges. This condition favored the formation of larger flocs with lower apparent density, with more open but stable structures, as indicated by the decrease in fractal dimension from 2.35 to 2.20.
Rheological characterization showed a substantial increase in yield stress in suspensions at 50 wt% with increasing coagulant dosage, which was interpreted as evidence of forming a continuous flocculated network, capable of resisting applied stresses and limiting internal particle mobility.
Reducing the ultrafine particle content through precoagulation improved the efficiency of the flocculant’s polymeric bridging mechanism by reducing competition for active sites and allowing for greater structural consolidation of the macroflocs.