Application of SDS-Coated Polystyrene Nanoparticles as Advanced Collectors for Selective Coal Flotation: A Combined Experimental and Theoretical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral Sample
2.2. Synthesis of Polystyrene Nanoparticles
2.3. Nanoparticle Characterization
2.4. Coal Flotation Experiments
2.5. Total Organic Carbon Quantification
2.6. Zeta Potential Study
2.7. Study of Physicochemical Variables on Coal Flotation and Optimization
2.8. Contact Angle Measurements
2.9. Computational Details
3. Results and Discussion
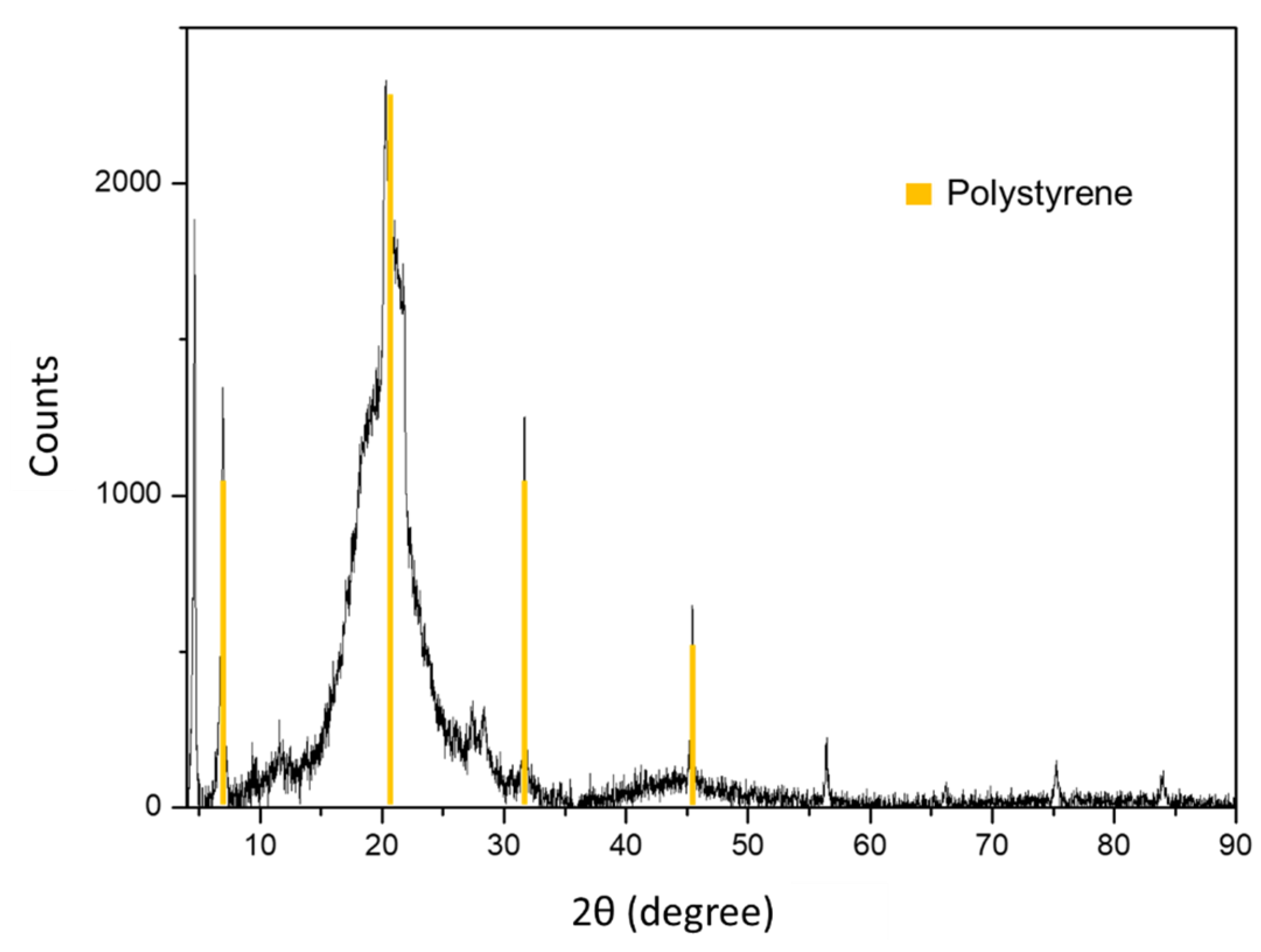
3.1. Polystyrene Nanoparticle Characterization
3.2. Coal Flotation Experiments
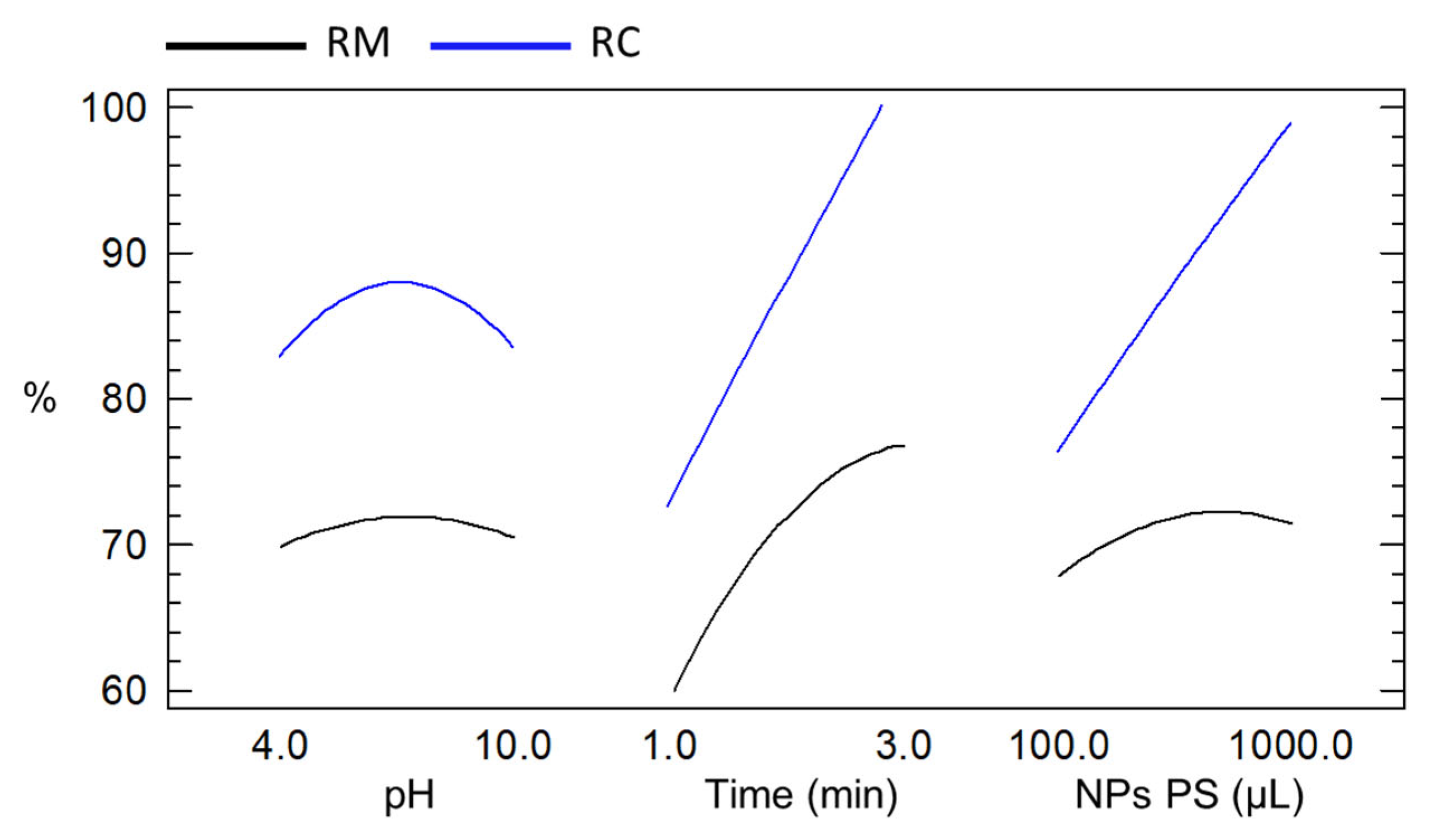
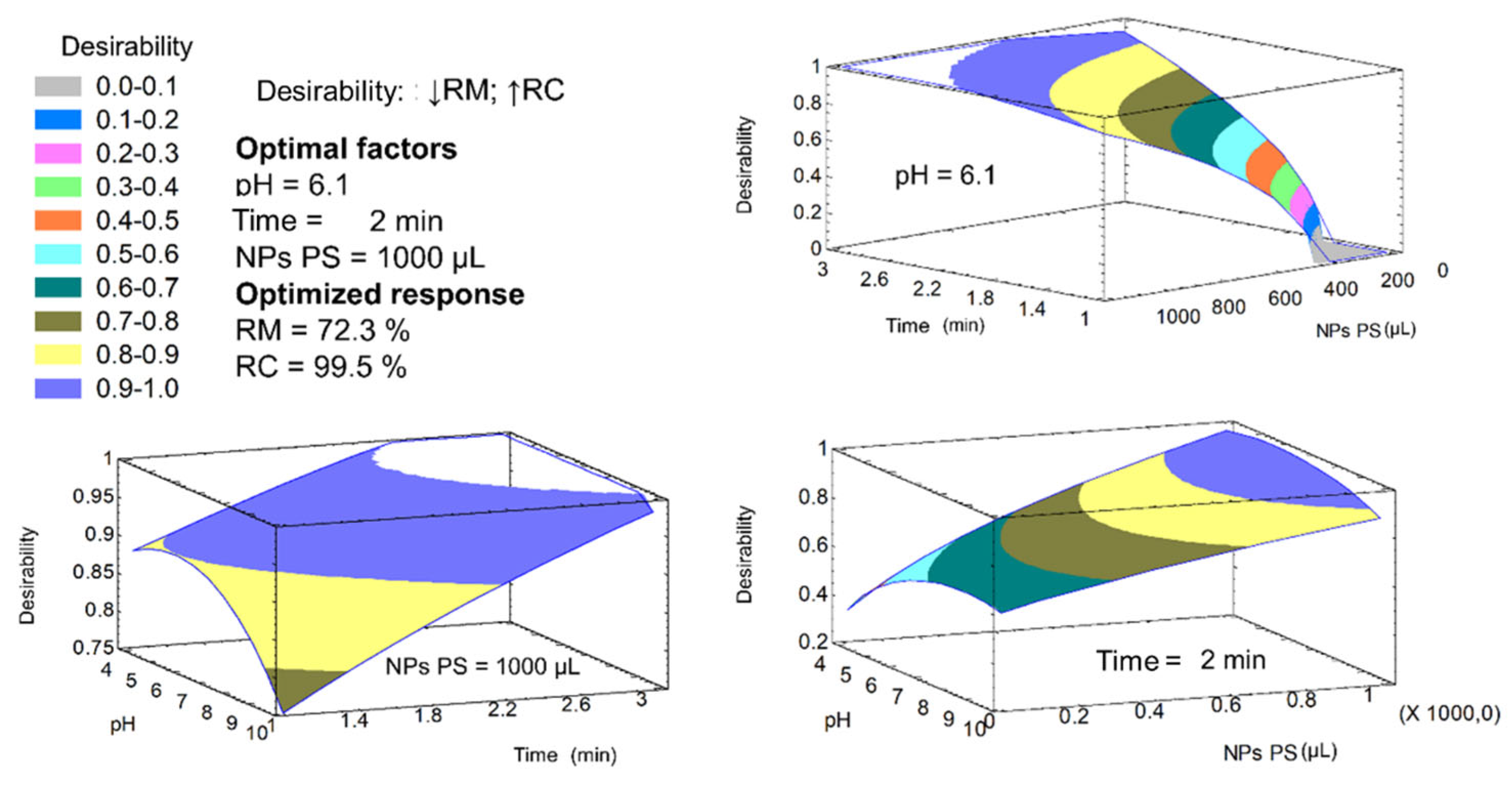
3.3. Effect of Physicochemical Variables on Coal Flotation and Optimal Conditions

0.941667·pH·Time − 0.00272222·pH·NPsPS − 3.77917·Time2 − 0.00583333·Time·NPsPS −
0.0000110082·NPsPS2
0.291667·pH·Time − 0.0027963·pH·NPsPS − 0.325·Time2 − 0.017·Time·NPsPS − 0.00000135802·NPs PS2
3.4. Study of the CM–Polystyrene Nanoparticles Interaction
3.4.1. X-Ray Photoelectron Spectroscopy (XPS)
3.4.2. Contact Angle
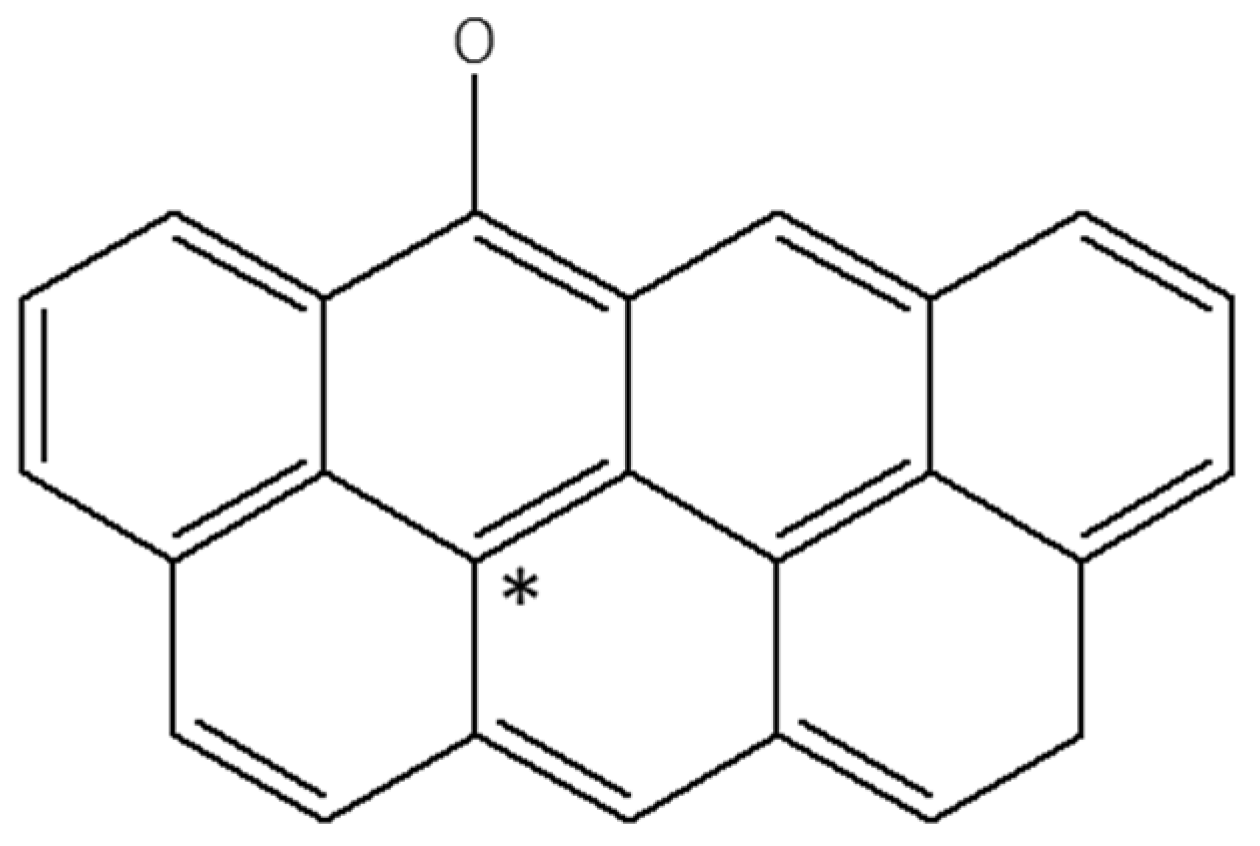
3.4.3. DFT Analysis
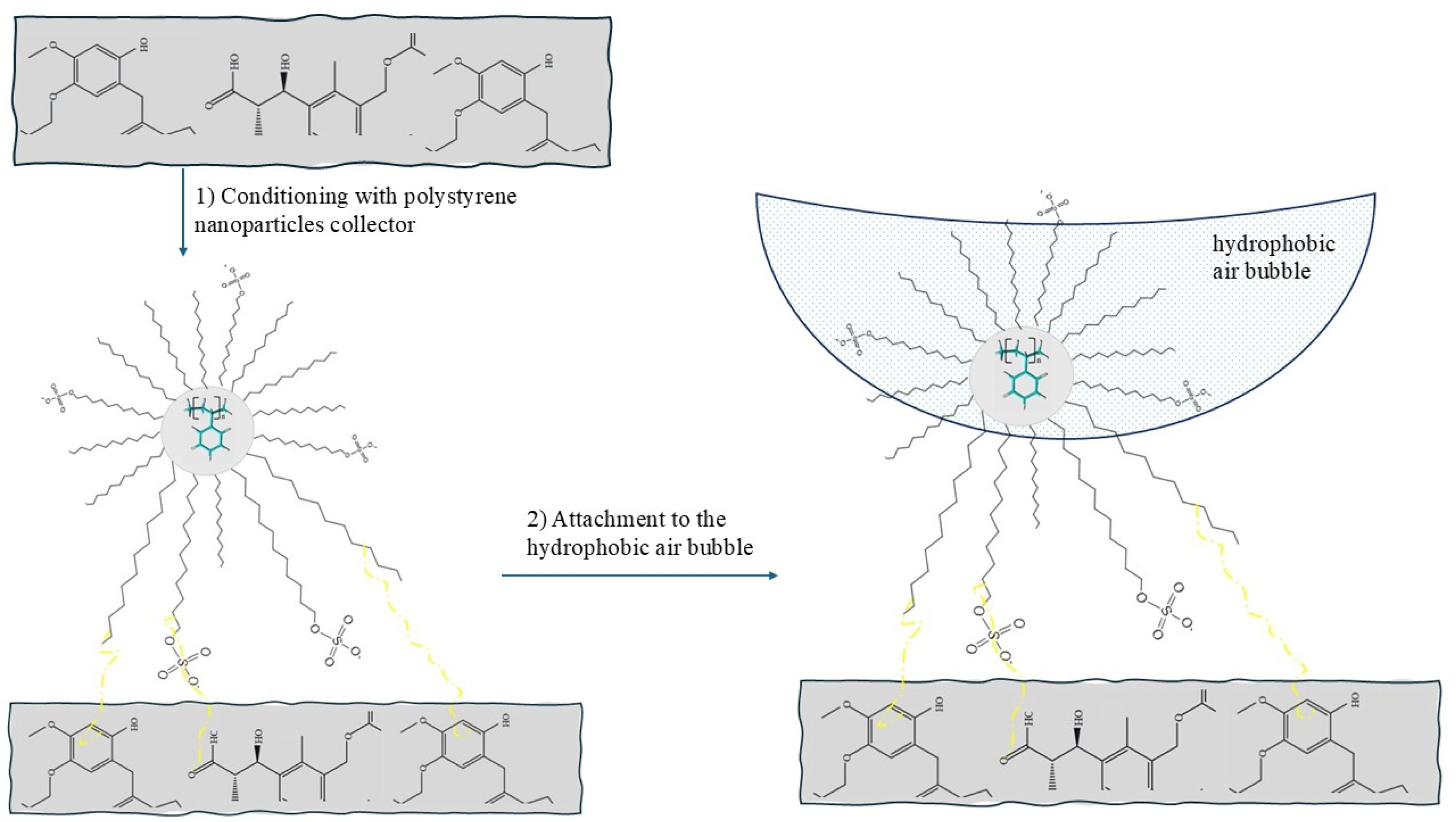
3.4.4. Mechanism of Interaction—Polystyrene Nanoparticles–Oxidized Coal Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagheri, B.; Mehrabani, J.V.; Farrokhpay, S. Recovery of sphalerite from a high zinc grade tailing. J. Hazard. Mater. 2020, 381, 120946. [Google Scholar] [CrossRef] [PubMed]
- Bakalarz, A.; Duchnowska, M.; Pawlos, W. Influence of hydrodynamics on preflotation process in a flotation machine. Miner. Metall. Process. 2018, 35, 19–23. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Z.; Chen, L.; Tao, X.; Tang, L.; He, H. Wetting thermodynamics of low-rank coal and attachment in flotation. Fuel 2017, 207, 214–225. [Google Scholar] [CrossRef]
- Jena, M.S.; Biswal, S.K.; Rudramuniyappa, M.V. Study on flotation characteristics of oxidized Indian high ash sub-bituminous coal. Int. J. Miner. Process. 2008, 87, 42–50. [Google Scholar] [CrossRef]
- Li, S.; Gao, L.; Wang, J.; Rong, G.; Cao, Y. Enhancement of floatability of low-rank coal using oxidized paraffin soap. RSC Adv. 2020, 10, 15098–15106. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.; Xia, Y.; Guo, F.; Ding, S.; Tan, J.; Che, T.; Meng, F.; Gui, X. Synergistic adsorption mechanism of anionic and cationic surfactant mixtures on low-rank coal flotation. ACS Omega 2020, 5, 20630–20637. [Google Scholar] [CrossRef]
- An, M.; Liao, Y.; Gui, X.; Zhao, Y.; He, Y.; Liu, Z.; Lai, Q. An investigation of coal flotation using nanoparticles as a collector. Int. J. Coal Prep. Util. 2017, 40, 679–690. [Google Scholar] [CrossRef]
- Liao, L.; An, M.; Hao, X.; Song, X.; Yang, Z.; Ren, H.; Liu, Z. Enhanced floatability of low-rank coal using surface functionalized polystyrene nanoparticles as collectors. J. Clean. Prod. 2021, 284, 124763. [Google Scholar] [CrossRef]
- Vilasó, J.E.; Ávila, D.M.; Reyes, I.A.; Blanco, A.; Gutiérrez, E.J. Coal flotation in a low-rank carbonaceous mineral using 3-phenyl-1-propanol as a collector reagent. Fuel 2021, 304, 121363. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z. A novel method for preparing monodispersed polystyrene nanoparticles. Front. Chem. China 2007, 2, 17–20. [Google Scholar] [CrossRef]
- Blythe, T.; Bloor, D. Measurement of Electric Properties, of Electrical Properties of Polymers; Cambridge University Press: Cambridge UK, 2005; pp. 154–185. [Google Scholar]
- Xia, W.; Li, Y.; Nguyen, A.V. Improving coal flotation using the mixture of candle soot and hydrocarbon oil as a novel flotation collector. J. Clean. Prod. 2018, 195, 1183–1189. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Oney, O.; Samanli, S.; Celik, H.; Tayyar, N. Optimization of operating parameters for flotation of fine coal using a Box-Behnken Design. Int. J. Coal Prep. Util. 2015, 35, 233–246. [Google Scholar] [CrossRef]
- Oney, O. Optimization of operating parameters of graphite flotation circuit using Box-Behnken design. Indian J. Chem. Technol. 2018, 25, 170–178. [Google Scholar]
- Ye, G.; Ma, L.; Li, L.; Liu, J.; Yuan, J.; Huang, G. Application of Box–Behnken design and response surface methodology for modeling and optimization of batch flotation of coal. Int. J. Coal Prep. Util. 2017, 40, 131–145. [Google Scholar] [CrossRef]
- Espinal, J.F.; Montoya, A.; Mondragon, F.; Truong, T.N. A DFT study of the interaction of carbon monoxide with carbonaceous materials. J. Phys. Chem. B 2004, 108, 1003–1008. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yu, D.G.; Zhu, L.M.; Branford-White, C.J.; Yang, J.H.; Wang, X.; Li, Y.; Qian, W. Solid dispersions in the form of electrospun core-sheath nanofibers. Int. J. Nanomed. 2011, 6, 3271–3280. [Google Scholar] [CrossRef]
- Wankasi, D.; Dikio, E.D. Comparative study of polystyrene and polymethylmethacrylate wastes as adsorbents for sorption of Pb2+ from aqueous solution. Chem. Asian J. 2014, 26, 8295–8302. [Google Scholar] [CrossRef]
- Al Najjar, T.; Allam, N.K.; El Sawy, E.N. Anionic/nonionic surfactants for controlled synthesis of highly concentrated sub-50 nm polystyrene spheres. Nanoscale Adv. 2021, 3, 5626–5635. [Google Scholar] [CrossRef]
- Schobert, H. Structural features of low-rank coals. Resour. Conserv. Recycl. 1990, 3, 111–123. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low-rank coal by surfactants. A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Sarikaya, M.; Ozbayoglu, G. Flotation characteristics of oxidized coal. Fuel 1995, 74, 291–294. [Google Scholar] [CrossRef]
- Qu, J.; Tao, X.; He, H.; Zhang, X.; Xu, N.; Zhang, B. Synergistic effect of surfactants and a collector on the flotation of a low-rank Coal. Int. J. Coal Prep. Util. 2015, 35, 14–24. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Li, X.; Liang, Y.; Wang, Y.; Zhang, H. Effect of mixed cationic/anionic surfactants on the low-rank coal wettability by an experimental and molecular dynamics simulation. Fuel 2021, 289, 119886. [Google Scholar] [CrossRef]
- Wołowicz, A.; Staszak, K. Study of surface properties of aqueous solutions of sodium dodecyl sulfate in the presence of hydrochloric acid and heavy metal ions. J. Mol. Liq. 2020, 299, 112170. [Google Scholar] [CrossRef]
- Harvey, P.A.; Nguyen, A.V.; Evans, G.M. Influence of electrical double-layer interaction on coal flotation. J. Colloid Interface Sci. 2002, 250, 337–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Albijanic, B.; Tadesse, B.; Wang, Y.; Yang, J.; Zhu, X. Desulphurization of coals of different ranks in the presence of slimes by reverse flotation. Energy Rep. 2019, 5, 1316–1323. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Geng, P.; Li, J. Carrier Flotation of Low-Rank Coal with Polystyrene. Minerals 2020, 10, 452. [Google Scholar] [CrossRef]
- Rahman, A.; Brown, C.W. Effect of pH on the critical micelle concentration of sodium dodecyl sulphate. J. Appl. Pol. Sci. 1983, 28, 1331–1334. [Google Scholar] [CrossRef]
- Brown, W.; Zhao, J. Adsorption of sodium dodecyl sulfate on polystyrene latex particles using dynamic light scattering and zeta potential measurements. Macromolecules 1993, 26, 2711–2715. [Google Scholar] [CrossRef]
- Bahrami, A.; Kazemi, F.; Ghorbani, Y. Effect of different reagent regimes on the kinetic model and recovery in gilsonite flotation. J. Mater. Res. Technol. 2019, 8, 4498–4509. [Google Scholar] [CrossRef]
- Welsby, S.D.D.; Vianna, S.M.S.M.; Franzidis, J.-P. Assigning physical significance to floatability components. Int. J. Miner. Process. 2010, 97, 59–67. [Google Scholar] [CrossRef]
- Kozyreva, E.N.; Nepeina, E.S. Specific surface and porosity of coal. Coke Chem. 2019, 62, 498–501. [Google Scholar] [CrossRef]
- Linge, H.G. The surface area of coal particles. Fuel 1989, 68, 111–113. [Google Scholar] [CrossRef]
- Shi, M.; Xin, Y.; Chen, X.; Zou, K.; Jing, W.; Sun, J.; Chen, Y.; Liu, Y. Coal-derived porous activated carbon with ultrahigh specific surface area and excellent electrochemical performance for supercapacitors. J. Alloys Compd. 2021, 859, 157856. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Li, B.; Xie, Z.; Guo, J.; Liu, S. Experimental and molecular dynamics simulation study on the enhancement of low-rank coal flotation by mixed collector. Fuel 2020, 266, 117046. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, Y.; Hao, Y.; Cao, Y.; Xing, Y.; Gui, X. Enhancing flotation performance of low-rank coal using environment-friendly vegetable oil. Minerals 2023, 13, 717. [Google Scholar] [CrossRef]
- Zarrouk, T.; Ibragimova, R.; Bartók, A.P.; Caro, M.A. Experiment-driven atomistic materials modeling: A case study combining X--Ray Photoelectron Spectroscopy and Machine Learning Potentials to infer the structure of oxygen-rich amorphous carbon. J. Am. Chem. Soc. 2024, 146, 14645–14659. [Google Scholar] [CrossRef]
- Goodson, M.L.; Lagle, R.; Guggilla, P. X-Ray Photoelectron Spectroscopy of polystyrene composite films. J. Adv. Mater. Sci. Eng. 2022, 2, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J. Investigation of polystyrene-based microspheres from different copolymers and their structural color coatings on wood surface. Coatings 2021, 11, 14. [Google Scholar] [CrossRef]
- Langreth, D.C.; Dion, M.; Rydberg, H.; Schröder, E.; Hyldgaard, P.; Lundqvist, B.I. Van der Waals density functional theory with applications. Int. J. Quantum Chem. 2005, 101, 599–610. [Google Scholar] [CrossRef]
- Klimeš, J.; Michaelides, A. Perspective: Advances and challenges in treating van der Waals dispersion forces in density functional theory. J. Chem. Phys. 2012, 137, 120901. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, B.D.; Hong, S. Van der Waals density functional theory study for bulk solids with BCC, FCC, and diamond structures. Curr. Appl. Phys. 2015, 15, 885–891. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, W. Empirical correction to density functional theory for van der Waals interactions. J. Chem. Phys. 2002, 116, 515–524. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Yoon, R.H.; Flinn, D.H.; Rabinovich, Y.I. Hydrophobic interactions between dissimilar surfaces. J. Colloid Interface Sci. 1997, 185, 363–370. [Google Scholar] [CrossRef]





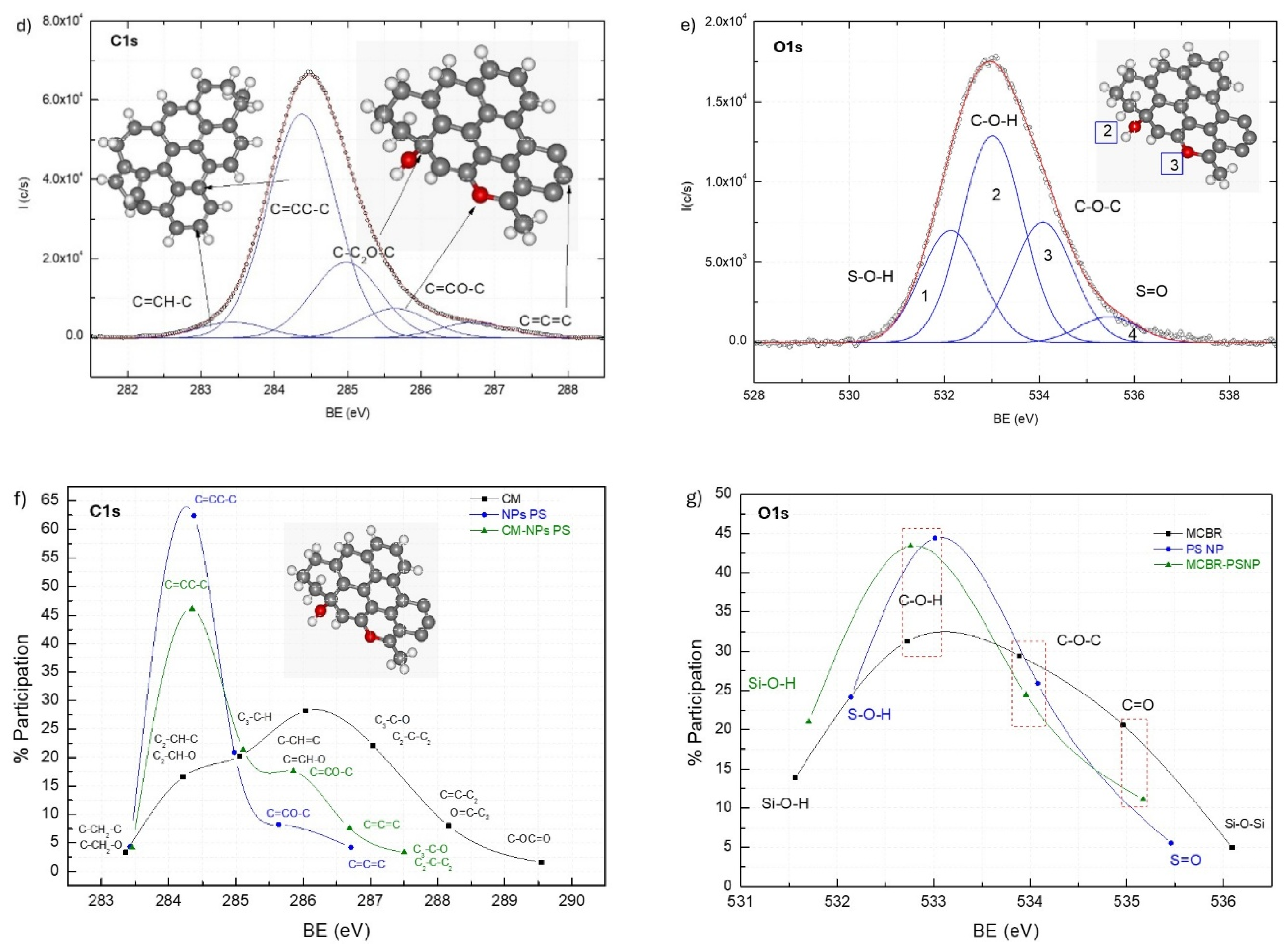
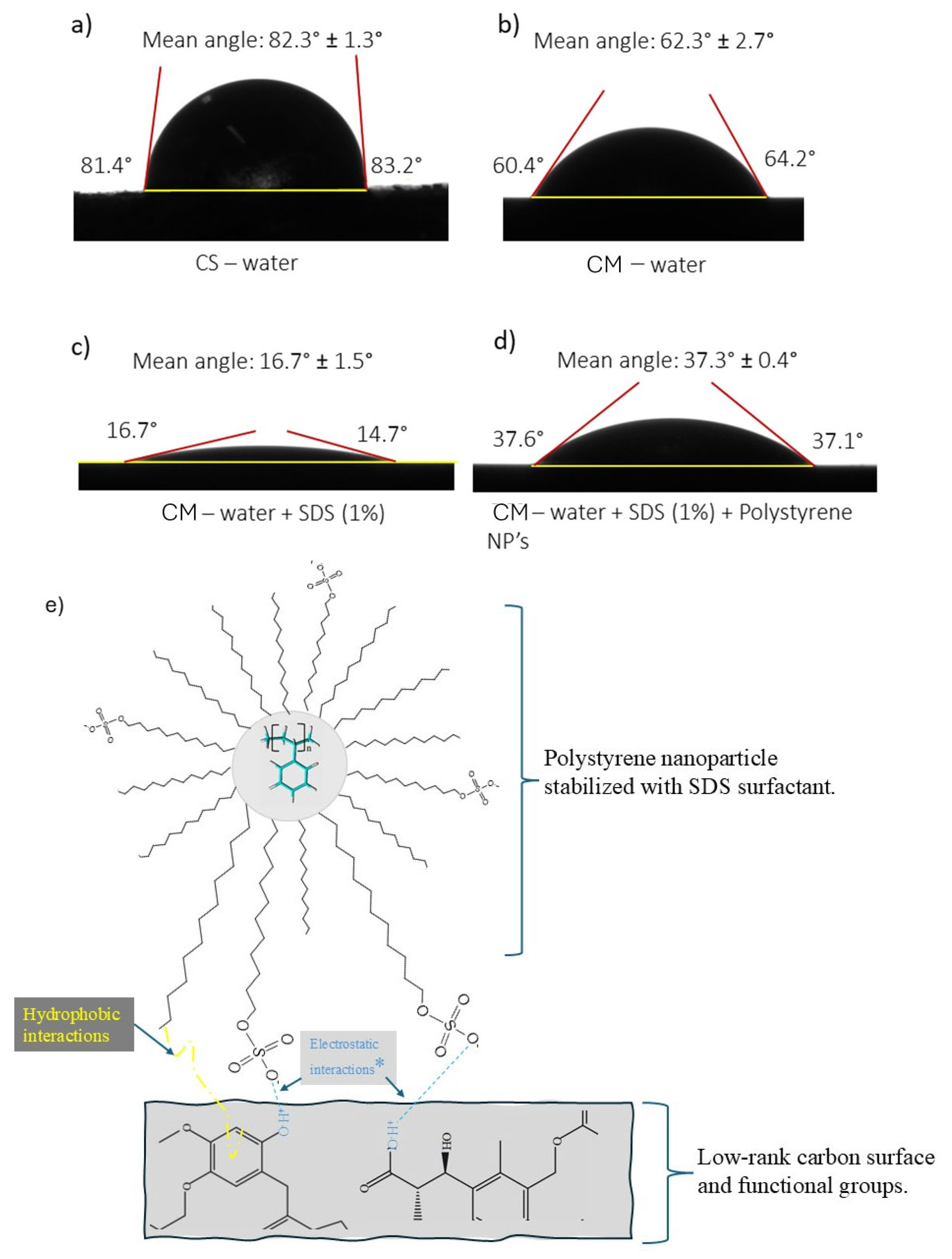
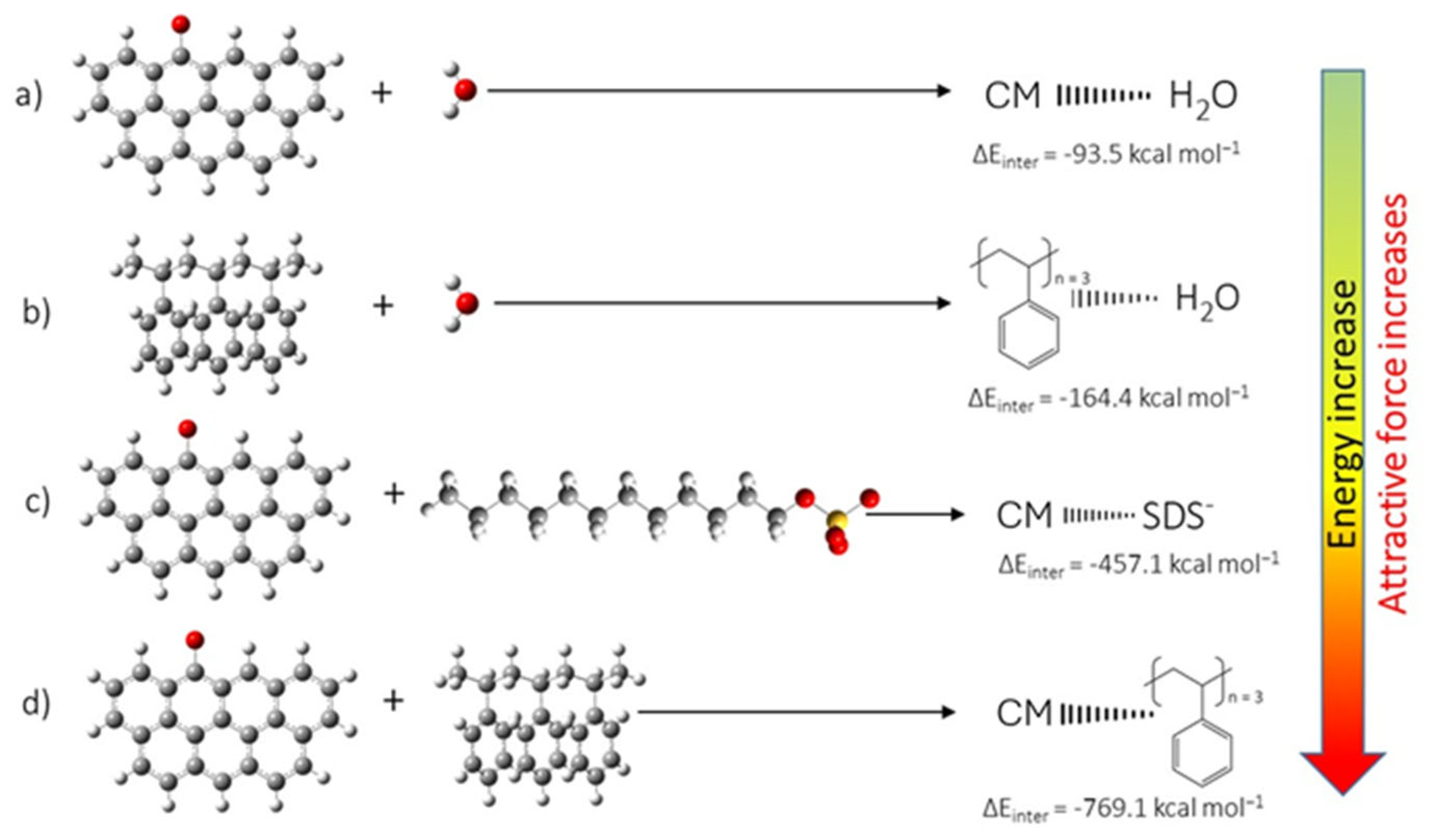
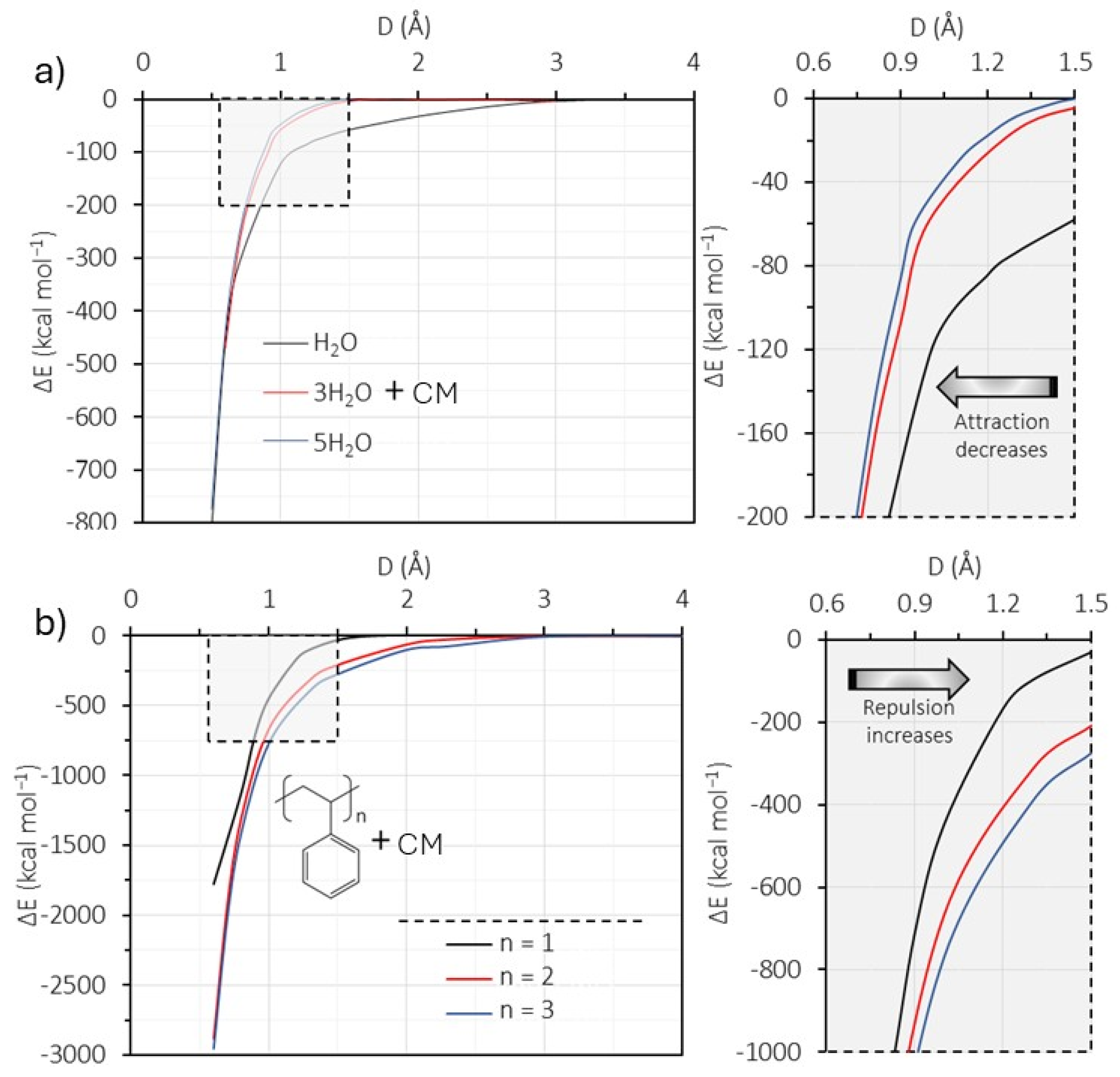
| Physicochemical Properties | Value ± SD |
|---|---|
| pH | 8.1 ± 0.1 |
| Carbonates (%) | 20 ± 0.4 |
| TOC (%) | 2.0 ± 0.03 |
| Acidic groups (mEq/g) | 0.28 ± 0.1 |
| Basic groups (mEq/g) | 2.1 ± 0.6 |
| Materials | σ (S/cm) |
|---|---|
| CM | 1.46 × 10−6 |
| CM-NPs PS | 2.7 × 10−7 |
| CM-SDS (1%) | 3.57 × 10−6 |
| Experiment | pH | t (Min) | NPs PS (µL) | RM (%) | RC (%) |
|---|---|---|---|---|---|
| 1 | 7 | 3 | 1000 | 74.2 | 100 |
| 2 | 7 | 2 | 550 | 75.1 | 89.2 |
| 3 | 4 | 3 | 550 | 80.8 | 100 |
| 4 | 10 | 2 | 1000 | 65.3 | 95.6 |
| 5 | 10 | 2 | 100 | 73.7 | 73.3 |
| 6 | 7 | 2 | 550 | 72.0 | 88.5 |
| 7 | 4 | 2 | 100 | 63.3 | 62.6 |
| 8 | 10 | 3 | 550 | 73.4 | 100 |
| 9 | 7 | 1 | 100 | 52.3 | 59.2 |
| 10 | 4 | 2 | 1000 | 69.6 | 100.0 |
| 11 | 7 | 2 | 550 | 70.0 | 89.8 |
| 12 | 4 | 1 | 550 | 53.8 | 67.4 |
| 13 | 7 | 2 | 550 | 69.8 | 89.0 |
| 14 | 7 | 3 | 100 | 71.2 | 100 |
| 15 | 10 | 1 | 550 | 57.7 | 63.9 |
| 16 | 7 | 2 | 550 | 69.3 | 89.9 |
| 17 | 7 | 1 | 1000 | 65.8 | 89.8 |
| 18 | 7 | 2 | 550 | 75.1 | 80.7 |
| Source | Sum of Squares | DF | Mean Square | F Stat | p-Value |
|---|---|---|---|---|---|
| A: pH | 0.845 | 1 | 0.845 | 0.12 | 0.7433 |
| B: Time | 612.5 | 1 | 612.5 | 86.83 | 0.0002 |
| C: NPs PS | 25.92 | 1 | 25.92 | 3.67 | 0.1134 |
| AA | 12.3037 | 1 | 12.3037 | 1.74 | 0.2438 |
| AB | 31.9225 | 1 | 31.9225 | 4.53 | 0.0867 |
| AC | 54.0225 | 1 | 54.0225 | 7.66 | 0.0395 |
| BB | 62.3219 | 1 | 62.3219 | 8.84 | 0.0311 |
| BC | 27.5625 | 1 | 27.5625 | 3.91 | 0.1050 |
| CC | 21.6837 | 1 | 21.6837 | 3.07 | 0.1399 |
| Lack of fit | 84.41 | 3 | 28.1367 | 3.99 | 0.0853 |
| Error | 35.2683 | 5 | 7.05367 | ||
| Total | 987.0 | 17 |
| Source | Sum of Squares | DF | Mean Square | F Stat | p-Value |
|---|---|---|---|---|---|
| A: pH | 0.98 | 1 | 0.98 | 0.08 | 0.7910 |
| B: Time | 1791.01 | 1 | 1791.01 | 142.84 | 0.0001 |
| C: NPs PS | 1019.26 | 1 | 1019.26 | 81.29 | 0.0003 |
| AA | 96.3927 | 1 | 96.3927 | 7.69 | 0.0392 |
| AB | 3.0625 | 1 | 3.0625 | 0.24 | 0.6421 |
| AC | 57.0025 | 1 | 57.0025 | 4.55 | 0.0862 |
| BB | 0.460909 | 1 | 0.460909 | 0.04 | 0.8555 |
| BC | 234.09 | 1 | 234.09 | 18.67 | 0.0076 |
| CC | 0.33 | 1 | 0.33 | 0.03 | 0.8775 |
| Lack of fit | 157.017 | 3 | 52.3392 | 4.17 | 0.0790 |
| Error | 62.695 | 5 | 12.539 | ||
| Total | 3426.69 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Márquez, D.M.; Blanco-Flores, A.; González Torres, M.; Toledo Jaldin, H.P. Application of SDS-Coated Polystyrene Nanoparticles as Advanced Collectors for Selective Coal Flotation: A Combined Experimental and Theoretical Study. Minerals 2025, 15, 594. https://doi.org/10.3390/min15060594
Ávila-Márquez DM, Blanco-Flores A, González Torres M, Toledo Jaldin HP. Application of SDS-Coated Polystyrene Nanoparticles as Advanced Collectors for Selective Coal Flotation: A Combined Experimental and Theoretical Study. Minerals. 2025; 15(6):594. https://doi.org/10.3390/min15060594
Chicago/Turabian StyleÁvila-Márquez, Delia Monserrat, Alien Blanco-Flores, Maribel González Torres, and Helen Paola Toledo Jaldin. 2025. "Application of SDS-Coated Polystyrene Nanoparticles as Advanced Collectors for Selective Coal Flotation: A Combined Experimental and Theoretical Study" Minerals 15, no. 6: 594. https://doi.org/10.3390/min15060594
APA StyleÁvila-Márquez, D. M., Blanco-Flores, A., González Torres, M., & Toledo Jaldin, H. P. (2025). Application of SDS-Coated Polystyrene Nanoparticles as Advanced Collectors for Selective Coal Flotation: A Combined Experimental and Theoretical Study. Minerals, 15(6), 594. https://doi.org/10.3390/min15060594







