Urease-Driven Microbially Induced Carbonate Precipitation (MICP) for the Circular Valorization of Reverse Osmosis Brine Waste: A Perspective Review
Abstract
1. Introduction
2. Brine Disposal Methods Are Often Environmentally Harmful
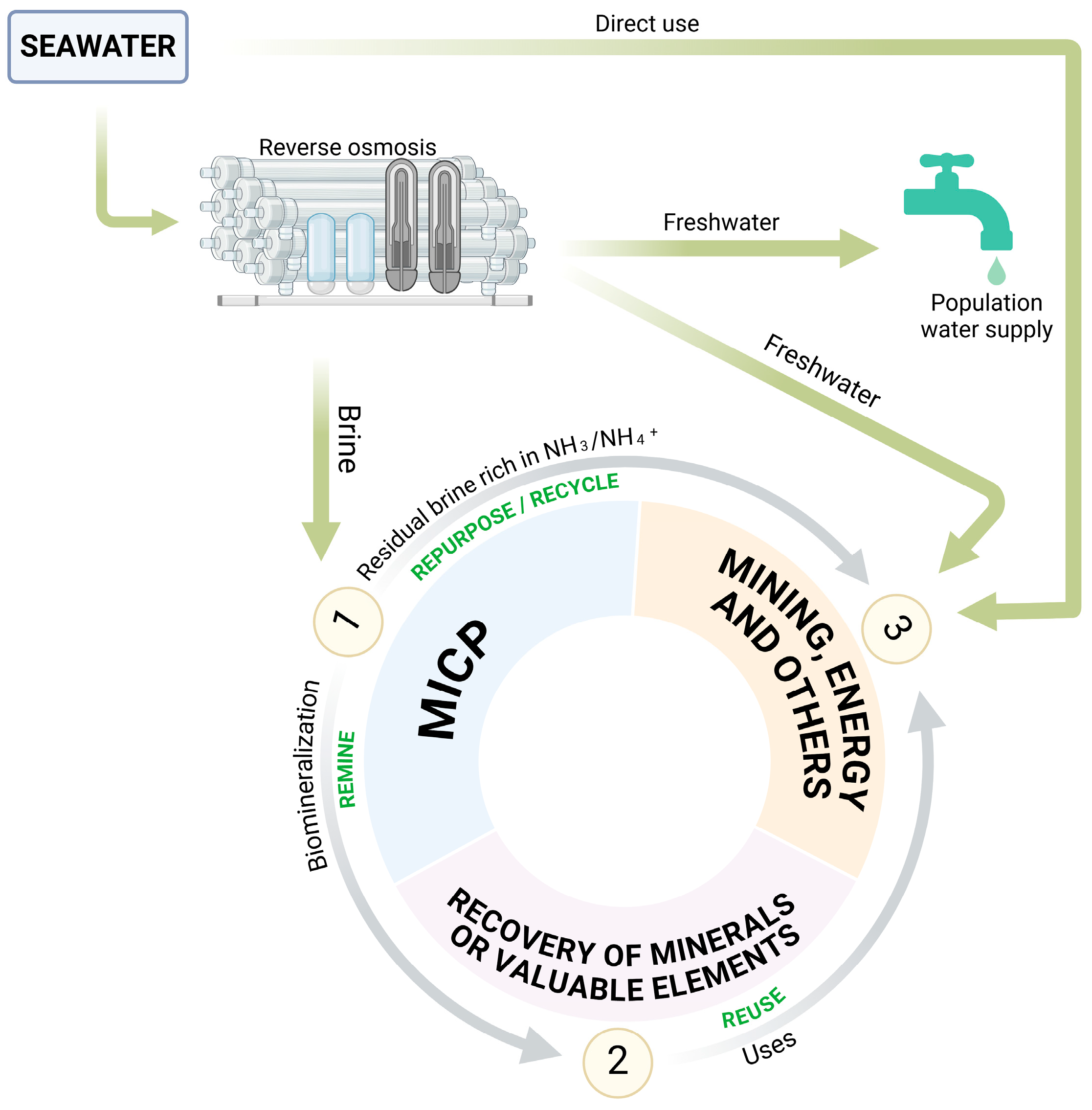
3. Resource Recovery for Brine Valorization
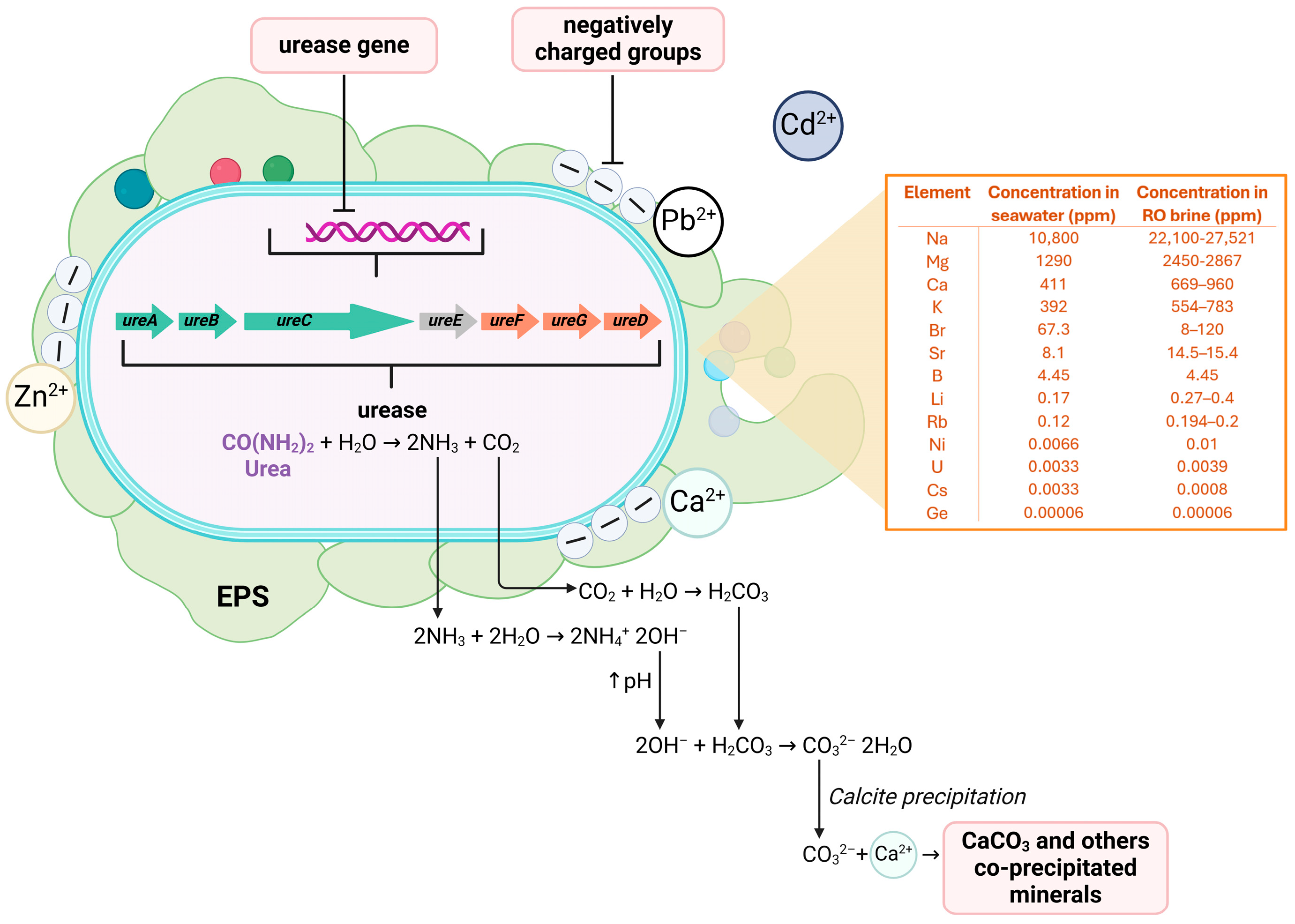
4. Biomineralization and Description of Urease-Based Microbially Induced Carbonate Precipitation (MICP)
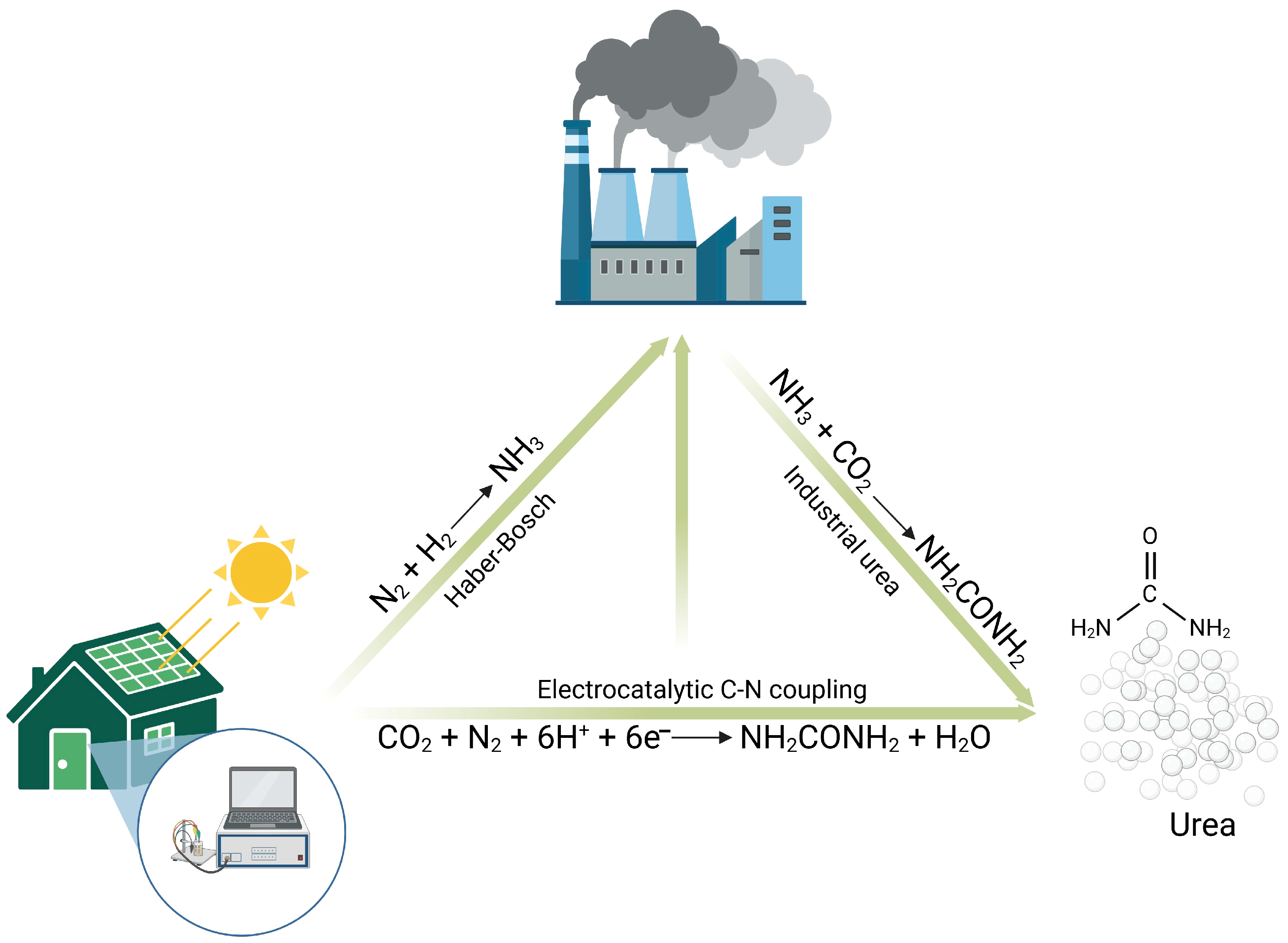
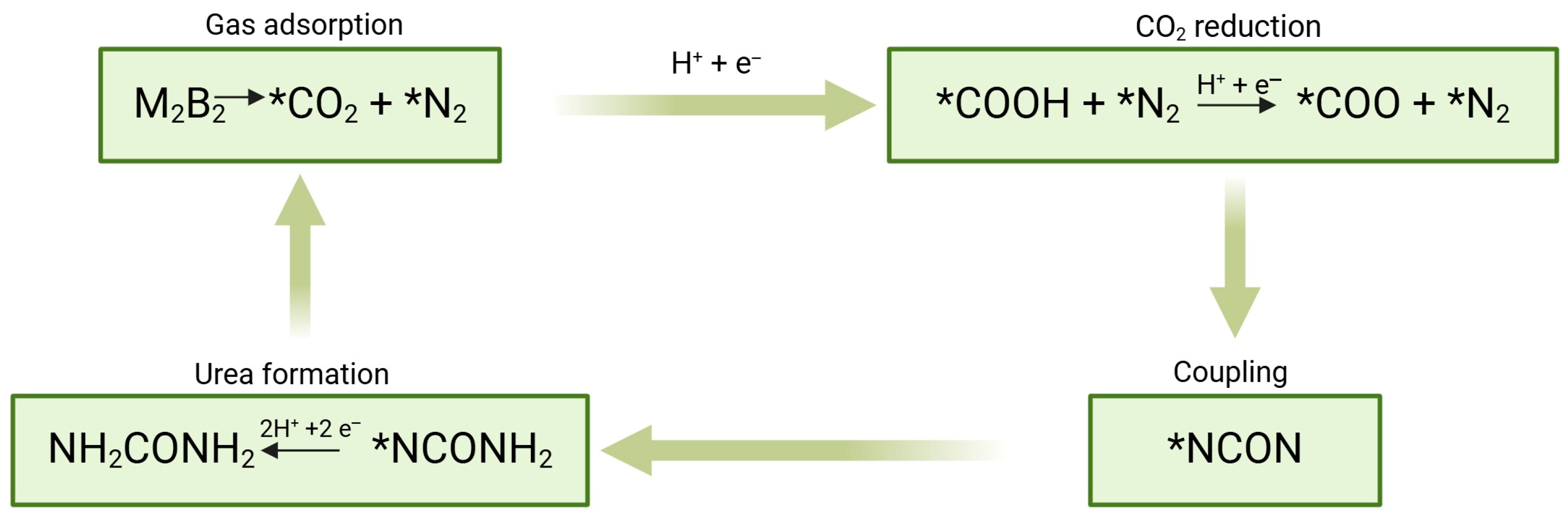
5. Perspectives on MICP as a Circular Economy Strategy for Brine Valorization
6. Future Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MICP | Microbially Induced Carbonate Precipitation |
| DT | Desalination technologies |
| RO | Reverse osmosis |
| DFT | Density functional theory |
References
- Ghernaout, D. Brine Recycling: Towards Membrane Processes as the Best Available Technology. Appl. Eng. 2019, 3, 71–84. [Google Scholar]
- United Nations. The United Nations World Water Development Report 2024: Water for Prosperity and Peace; UNESCO: Paris, France, 2024; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000388948 (accessed on 23 April 2025).
- Kurihara, M. Seawater Reverse Osmosis Desalination. Membranes 2021, 11, 10–12. [Google Scholar] [CrossRef] [PubMed]
- International Desalination Association (IDA). Desalination & Reuse Handbook 2023–2024; IDA: Topsfield, MA, USA, 2023; Available online: https://idrawater.org/ (accessed on 23 April 2025).
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy Consumption and Environmental Impact Assessment of Desalination Plants and Brine Disposal Strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Kress, N.; Gertner, Y.; Shoham-Frider, E. Seawater Quality at the Brine Discharge Site from Two Mega Size Seawater Reverse Osmosis Desalination Plants in Israel (Eastern Mediterranean). Water Res. 2020, 171, 115402. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination Brine Disposal Methods and Treatment Technologies—A Review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Zhang, Y.; Jegatheesan, V. A Review of Resource Recovery from Seawater Desalination Brine. Rev. Environ. Sci. Biotechnol. 2021, 20, 333–361. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Panagopoulos, A. Techno-Economic Assessment of Zero Liquid Discharge (ZLD) Systems for Sustainable Treatment, Minimization and Valorization of Seawater Brine. J. Environ. Manag. 2022, 306, 114488. [Google Scholar] [CrossRef]
- Panagopoulos, A. Assessing the Energy Footprint of Desalination Technologies and Minimal/Zero Liquid Discharge (MLD/ZLD) Systems for Sustainable Water Protection via Renewable Energy Integration. Energies 2025, 18, 962. [Google Scholar] [CrossRef]
- Olufisayo, O.E.; Olanrewaju, O. A Review of Renewable Energy Powered Seawater Desalination Treatment Process for Zero Waste. Water 2024, 16, 2804. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Mustafa, J.; Zafar, A.M.; Obaid, M.; Atieh, M.A.; Ghaffour, N. Waste to Wealth: A Critical Analysis of Resource Recovery from Desalination Brine. Desalination 2022, 543, 116093. [Google Scholar] [CrossRef]
- Salazar-Avalos, S.; Soliz, A.; Cáceres, L.; Conejeros, S.; Brito, I.; Galvez, E.; Galleguillos Madrid, F.M. Metal Recovery from Natural Saline Brines with an Electrochemical Ion Pumping Method Using Hexacyanoferrate Materials as Electrodes. Nanomaterials 2023, 13, 2557. [Google Scholar] [CrossRef]
- Zarzo, D. Beneficial Uses and Valorization of Reverse Osmosis Brines; Elsevier Inc.: Philadelphia, PA, USA, 2018; ISBN 9780128167120. [Google Scholar]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Ahmad, M.; Garudachari, B.; Al-Wazzan, Y.; Kumar, R.; Thomas, J.P. Mineral Extraction from Seawater Reverse Osmosis Brine of Gulf Seawater. Desalination Water Treat 2019, 144, 45–56. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine Management in Desalination Industry: From Waste to Resources Generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Abdulsalam, A.; Idris, A.; Mohamed, T.A.; Ahsan, A. An Integrated Technique Using Solar and Evaporation Ponds for Effective Brine Disposal Management. Int. J. Sustain. Energy 2017, 36, 914–925. [Google Scholar] [CrossRef]
- Tristán, C.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Recovery of salinity gradient energy in desalination plants by reverse electrodialysis. Desalination 2020, 496, 114699. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Chakraborty, A.; Purkait, M.K. Clean Energy from Salinity Gradients Using Pressure Retarded Osmosis and Reverse Electrodialysis: A Review. Sustain. Energy Technol. Assess. 2022, 49, 101687. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Microbially Induced Calcium Carbonate Precipitation: A Widespread Phenomenon in the Biological World. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a Potential Sustainable Technique to Treat or Entrap Contaminants in the Natural Environment: A Review. Environ. Sci. Ecotechnology 2021, 6, 100096. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z. Microbial-Induced Carbonate Precipitation: A Review on Influencing Factors and Applications. Adv. Civ. Eng. 2021, 2021, 9974027. [Google Scholar] [CrossRef]
- Ortega-Villamagua, E.; Gudiño-Gomezjurado, M.; Palma-Cando, A. Microbiologically Induced Carbonate Precipitation in the Restoration and Conservation of Cultural Heritage Materials. Molecules 2020, 25, 5499. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Xu, R.; Qin, H.; Liu, H.; Zhao, K. Heavy Metal Bioremediation Using Microbially Induced Carbonate Precipitation: Key Factors and Enhancement Strategies. Front. Microbiol. 2023, 14, 1116970. [Google Scholar] [CrossRef]
- Hang, L.; Yang, E.; Zhou, Y.; Song, W.; He, J. Microbially Induced Calcite Precipitation (MICP) for Stabilization of Desert Sand against the Wind-Induced Erosion: A Parametric Study. Sustainability 2022, 14, 11409. [Google Scholar] [CrossRef]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially Induced Carbonate Precipitation (MICP) for Soil Strengthening: A Comprehensive Review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, R.; Peng, J.; Dai, D. Improvement Schemes for Bacteria in MICP: A Review. Materials 2024, 17, 5420. [Google Scholar] [CrossRef]
- Liu, D.; Shao, A.; Li, H.; Jin, C.; Li, Y. A Study on the Enhancement of the Mechanical Properties of Weak Structural Planes Based on Microbiologically Induced Calcium Carbonate Precipitation. Bull. Eng. Geol. Environ. 2020, 79, 4349–4362. [Google Scholar] [CrossRef]
- Taharia, M.; Dey, D.; Das, K.; Sukul, U.; Chen, J.S.; Banerjee, P.; Dey, G.; Sharma, R.K.; Lin, P.Y.; Chen, C.Y. Microbial Induced Carbonate Precipitation for Remediation of Heavy Metals, Ions and Radioactive Elements: A Comprehensive Exploration of Prospective Applications in Water and Soil Treatment. Ecotoxicol. Environ. Saf. 2024, 271, 115990. [Google Scholar] [CrossRef]
- Kang, C.H.; Kwon, Y.J.; So, J.S. Bioremediation of Heavy Metals by Using Bacterial Mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Yang, W.; Ali, A.; Su, J.; Liu, J.; Wang, Z.; Zhang, L. Microbial Induced Calcium Precipitation Based Anaerobic Immobilized Biofilm Reactor for Fluoride, Calcium, and Nitrate Removal from Groundwater. Chemosphere 2022, 295, 133955. [Google Scholar] [CrossRef]
- Song, H.; Kumar, A.; Ding, Y.; Wang, J.; Zhang, Y. Removal of Cd2+ from Wastewater by Microorganism Induced Carbonate Precipitation (MICP): An Economic Bioremediation Approach. Sep. Purif. Technol. 2022, 297, 121540. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.W.; Mishra, S.; Zhang, W.; Zhang, Y.L.; Zhang, Q.R.; Yu, Z.G. Application of Microbial-Induced Carbonate Precipitation (MICP) Techniques to Remove Heavy Metal in the Natural Environment: A Critical Review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Nissom, P.M. Bioprecipitation of Calcium Carbonate Mediated by Ureolysis: A Review. Environ. Eng. Res. 2021, 26, 200379. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, B.; Lee, Y.S.; Park, W. Modulation of Calcium Carbonate Precipitation by Exopolysaccharide in Bacillus Sp. JH7. Appl. Microbiol. Biotechnol. 2017, 101, 6551–6561. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, L.; Ou, Y.; Yang, G.; Huang, L.; Li, F. Contribution of Selective Bacterial Extracellular Polymeric Substances to the Polymorphism and Morphologies of Formed Ca/Mg Carbonates. Int. Biodeterior. Biodegrad. 2021, 160, 105213. [Google Scholar] [CrossRef]
- Haystead, J.; Gilmour, K.; Sherry, A.; Dade-Robertson, M.; Zhang, M. Effect of (in)Organic Additives on Microbially Induced Calcium Carbonate Precipitation. J. Appl. Microbiol. 2024, 135, lxad309. [Google Scholar] [CrossRef]
- Mallick, S.; Das, S. Treatment of Low-PH Rubber Wastewater Using Ureolytic Bacteria and the Production of Calcium Carbonate Precipitate for Soil Stabilization. Chemosphere 2024, 356, 141913. [Google Scholar] [CrossRef]
- Teng, Z.; Shao, W.; Zhang, K.; Yu, F.; Huo, Y.; Li, M. Enhanced Passivation of Lead with Immobilized Phosphate Solubilizing Bacteria Beads Loaded with Biochar/Nanoscale Zero Valent Iron Composite. J. Hazard. Mater. 2020, 384, 121505. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum Conditions for Microbial Carbonate Precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Liu, Y.; Ali, A.; Su, J.F.; Li, K.; Hu, R.Z.; Wang, Z. Microbial-Induced Calcium Carbonate Precipitation: Influencing Factors, Nucleation Pathways, and Application in Waste Water Remediation. Sci. Total Environ. 2023, 860, 160439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Investigations of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP) in the Temperature Range 4–50 °C. Acta Geotech. 2023, 18, 2239–2261. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.Y. An Optimum Condition of MICP Indigenous Bacteria with Contaminated Wastes of Heavy Metal. J. Mater. Cycles Waste Manag. 2019, 21, 239–247. [Google Scholar] [CrossRef]
- Lv, C.; Tang, C.S.; Zhang, J.Z.; Pan, X.H.; Liu, H. Effects of Calcium Sources and Magnesium Ions on the Mechanical Behavior of MICP-Treated Calcareous Sand: Experimental Evidence and Precipitated Crystal Insights. Acta Geotech. 2023, 18, 2703–2717. [Google Scholar] [CrossRef]
- Danjo, T.; Kawasaki, S. Microbially Induced Sand Cementation Method Using Pararhodobacter Sp. Strain SO1, Inspired by Beachrock Formation Mechanism. Mater. Trans. 2016, 57, 428–437. [Google Scholar] [CrossRef]
- Comadran-Casas, C.; Schaschke, C.J.; Akunna, J.C.; Jorat, M.E. Cow Urine as a Source of Nutrients for Microbial-Induced Calcite Precipitation in Sandy Soil. J. Environ. Manag. 2022, 304, 114307. [Google Scholar] [CrossRef]
- Alhawari, O.; Awan, U.; Bhutta, M.K.S.; Ülkü, M. Insights from Circular Economy Literature: A Review of Extant Definitions and Unravelling Paths to Future Research. Sustainability 2021, 13, 859. [Google Scholar] [CrossRef]
- Hammes, F.; Seka, A.; De Knijf, S.; Verstraete, W. A Novel Approach to Calcium Removal from Calcium-Rich Industrial Wastewater. Water Res. 2003, 37, 699–704. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Xu, P.; Zhang, Y. Biomineralization of Hypersaline Produced Water Using Microbially Induced Calcite Precipitation. Water Res. 2021, 190, 116753. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Cui, M.; Lai, H. Experimental Investigation on Bioremediation of Heavy Metal Contaminated Solution by Sporosarcina Pasteurii under Some Complex Conditions. Water 2022, 14, 595. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, H.; Li, J.; Cheng, L.; Zhu, W. Study on Domestication of Sporosarcina Pasteurii and Cementation Effect of calcareous Sand in Seawater Environment. Rock Soil Mech. 2022, 43, 5. [Google Scholar]
- Bai, H.; Liu, D.; Zheng, W.; Ma, L.; Yang, S.; Cao, J.; Lu, X.; Wang, H.; Mehta, N. Microbially-Induced Calcium Carbonate Precipitation by a Halophilic Ureolytic Bacterium and Its Potential for Remediation of Heavy Metal-Contaminated Saline Environments. Int. Biodeterior. Biodegrad. 2021, 165, 105311. [Google Scholar] [CrossRef]
- Arias, D.; Villca, G.; Pánico, A.; Cisternas, L.A.; Jeldres, R.I.; González-Benito, G.; Rivas, M. Partial Desalination of Seawater for Mining Processes through a Fluidized Bed Bioreactor Filled with Immobilized Cells of Bacillus Subtilis LN8B. Desalination 2020, 482, 114388. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.A.; Rivas, M. Biomineralization of Calcium and Magnesium Crystals from Seawater by Halotolerant Bacteria Isolated from Atacama Salar (Chile). Desalination 2017, 405, 1–9. [Google Scholar] [CrossRef]
- Partila, A.M.; El-Bialy, H.A.A.; Gomaa, O.M. Mineral Recovery by Bioprecipitation from Desalination Brine Using Irradiated Micrococcus Luteus. Int. J. Environ. Sci. Technol. 2025, 1–14. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical Review of Abatement of Ammonia from Wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Meessen, J. Urea Synthesis. Chem. Ing. Tech. 2014, 86, 2180–2189. [Google Scholar] [CrossRef]
- Mao, N.; Ren, H.; Geng, J.; Ding, L.; Xu, K. Engineering Application of Anaerobic Ammonium Oxidation Process in Wastewater Treatment. World J. Microbiol. Biotechnol. 2017, 33, 1–11. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.Z.; Chen, D. Adsorbent Materials for Ammonium and Ammonia Removal: A Review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Aflaki, E.; Gowthaman, S.; Nakashima, K.; Kawasaki, S.; Ebadi, T. A Two-Stage Treatment Process for the Management of Produced Ammonium by-Products in Ureolytic Bio-Cementation Process. Int. J. Environ. Sci. Technol. 2022, 19, 449–462. [Google Scholar] [CrossRef]
- Williamson, A.J.; Verbruggen, F.; Chavez Rico, V.S.; Bergmans, J.; Spooren, J.; Yurramendi, L.; Laing, G.D.; Boon, N.; Hennebel, T. Selective Leaching of Copper and Zinc from Primary Ores and Secondary Mineral Residues Using Biogenic Ammonia. J. Hazard. Mater. 2021, 403, 123842. [Google Scholar] [CrossRef] [PubMed]
- Indrasis, D.; Sovik, D.; Indrajit, C.; Makarand, G. Bio-Refractory Pollutant Removal Using Microbial Electrochemical Technologies: A Short Review. J. Indian Chem. Soc. 2019, 96, 493–497. Available online: https://indianchemicalsociety.com/portal/uploads/journal/April%2013.pdf (accessed on 23 April 2025).
- Priyadarshini, M.; Ahmad, A.; Das, S.; Ghangrekar, M.M. Application of Microbial Electrochemical Technologies for the Treatment of Petrochemical Wastewater with Concomitant Valuable Recovery: A Review. Environ. Sci. Pollut. Res. 2022, 29, 61783–61802. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I. On the Importance of Identifying, Characterizing, and Predicting Fundamental Phenomena towards Microbial Electrochemistry Applications. Curr. Opin. Biotechnol. 2014, 27, 107–114. [Google Scholar] [CrossRef]
- Balaji, R.; Kannan, B.S.; Lakshmi, J.; Senthil, N.; Vasudevan, S.; Sozhan, G.; Shukla, A.K.; Ravichandran, S. An Alternative Approach to Selective Sea Water Oxidation for Hydrogen Production. Electrochem. Commun. 2009, 11, 1700–1702. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, J.; Jo, H.; Seong, A.; Lee, M.; Min, H.K.; Seo, M.G.; Choi, Y.; Kim, G. A Rigorous Electrochemical Ammonia Electrolysis Protocol with: In Operando Quantitative Analysis. J. Mater. Chem. A Mater 2021, 9, 11571–11579. [Google Scholar] [CrossRef]
- Chen, H.J.; Huang, Y.H.; Chen, C.C.; Maity, J.P.; Chen, C.Y. Microbial Induced Calcium Carbonate Precipitation (MICP) Using Pig Urine as an Alternative to Industrial Urea. Waste Biomass Valorization 2019, 10, 2887–2895. [Google Scholar] [CrossRef]
- Mei, Z.; Zhou, Y.; Lv, W.; Tong, S.; Yang, X.; Chen, L.; Zhang, N. Recent Progress in Electrocatalytic Urea Synthesis under Ambient Conditions. ACS Sustain. Chem. Eng. 2022, 10, 12477–12496. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, R.; Wang, C.; Meng, N.; Shi, Y.; Yu, Y.; Zhang, B. Direct Electrosynthesis of Urea from Carbon Dioxide and Nitric Oxide. ACS Energy Lett. 2022, 7, 284–291. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, X.; Jing, Y.; Li, Y. Electrochemical Synthesis of Urea on MBenes. Nat. Commun. 2021, 12, 4080. [Google Scholar] [CrossRef]
- Chen, C.; He, N.; Wang, S. Electrocatalytic C–N Coupling for Urea Synthesis. Small Sci. 2021, 1, 2100070. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, Y.; Jiang, J.; Liu, Y.; Sun, M.; Zhang, J.; Zhang, W.; Qin, Q. Emerging Electrocatalysts in Urea Production. Chem. A Eur. J. 2023, 29, e202301619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhu, M.; Wang, M.; He, Y.; Luo, X.; Wu, C.; Zhang, L.; Jin, Z. Review on Electrocatalytic Coreduction of Carbon Dioxide and Nitrogenous Species for Urea Synthesis. ACS Nano 2023, 17, 3209–3224. [Google Scholar] [CrossRef]
- Yuan, J.; Hu, L.; Huang, J.; Chen, Y.; Qiao, S.; Xie, H. Photo/Electrochemical Urea Synthesis via CO2 Coupling with Nitrogenous Small Molecules: Status and Challenges for the Development of Mechanism and Catalysts. Appl. Catal. B 2023, 339, 123146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, D.; Gallardo, K.; Saldana, M.; Galleguillos-Madrid, F. Urease-Driven Microbially Induced Carbonate Precipitation (MICP) for the Circular Valorization of Reverse Osmosis Brine Waste: A Perspective Review. Minerals 2025, 15, 543. https://doi.org/10.3390/min15050543
Arias D, Gallardo K, Saldana M, Galleguillos-Madrid F. Urease-Driven Microbially Induced Carbonate Precipitation (MICP) for the Circular Valorization of Reverse Osmosis Brine Waste: A Perspective Review. Minerals. 2025; 15(5):543. https://doi.org/10.3390/min15050543
Chicago/Turabian StyleArias, Dayana, Karem Gallardo, Manuel Saldana, and Felipe Galleguillos-Madrid. 2025. "Urease-Driven Microbially Induced Carbonate Precipitation (MICP) for the Circular Valorization of Reverse Osmosis Brine Waste: A Perspective Review" Minerals 15, no. 5: 543. https://doi.org/10.3390/min15050543
APA StyleArias, D., Gallardo, K., Saldana, M., & Galleguillos-Madrid, F. (2025). Urease-Driven Microbially Induced Carbonate Precipitation (MICP) for the Circular Valorization of Reverse Osmosis Brine Waste: A Perspective Review. Minerals, 15(5), 543. https://doi.org/10.3390/min15050543






