Characteristics of Mineralization of Refractory Gold and Its Influence on Cyanide Gold Leaching Rates: A Case Study in Pituca II, Zamora Chinchipe, Ecuador
Abstract
1. Introduction
2. Materials and Methods
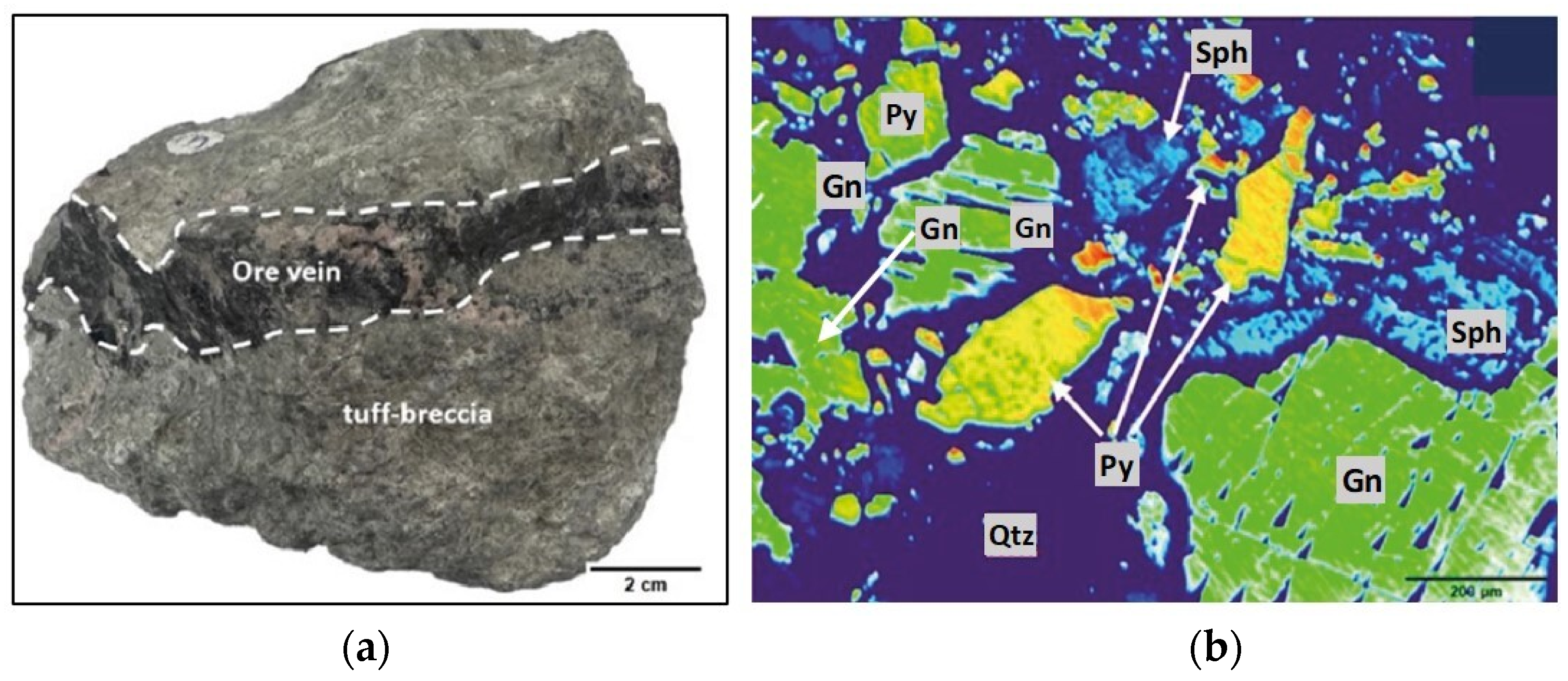
2.1. Ore Sample
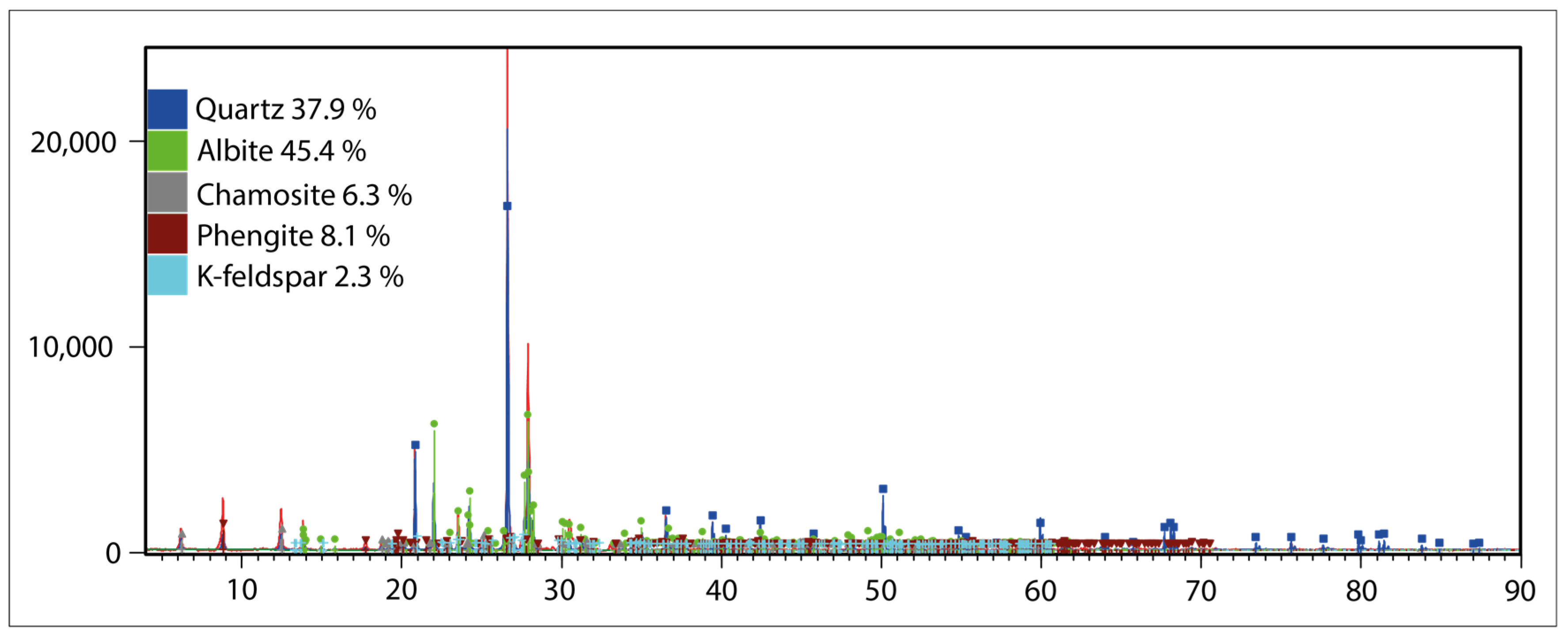
2.2. Analysis Methods
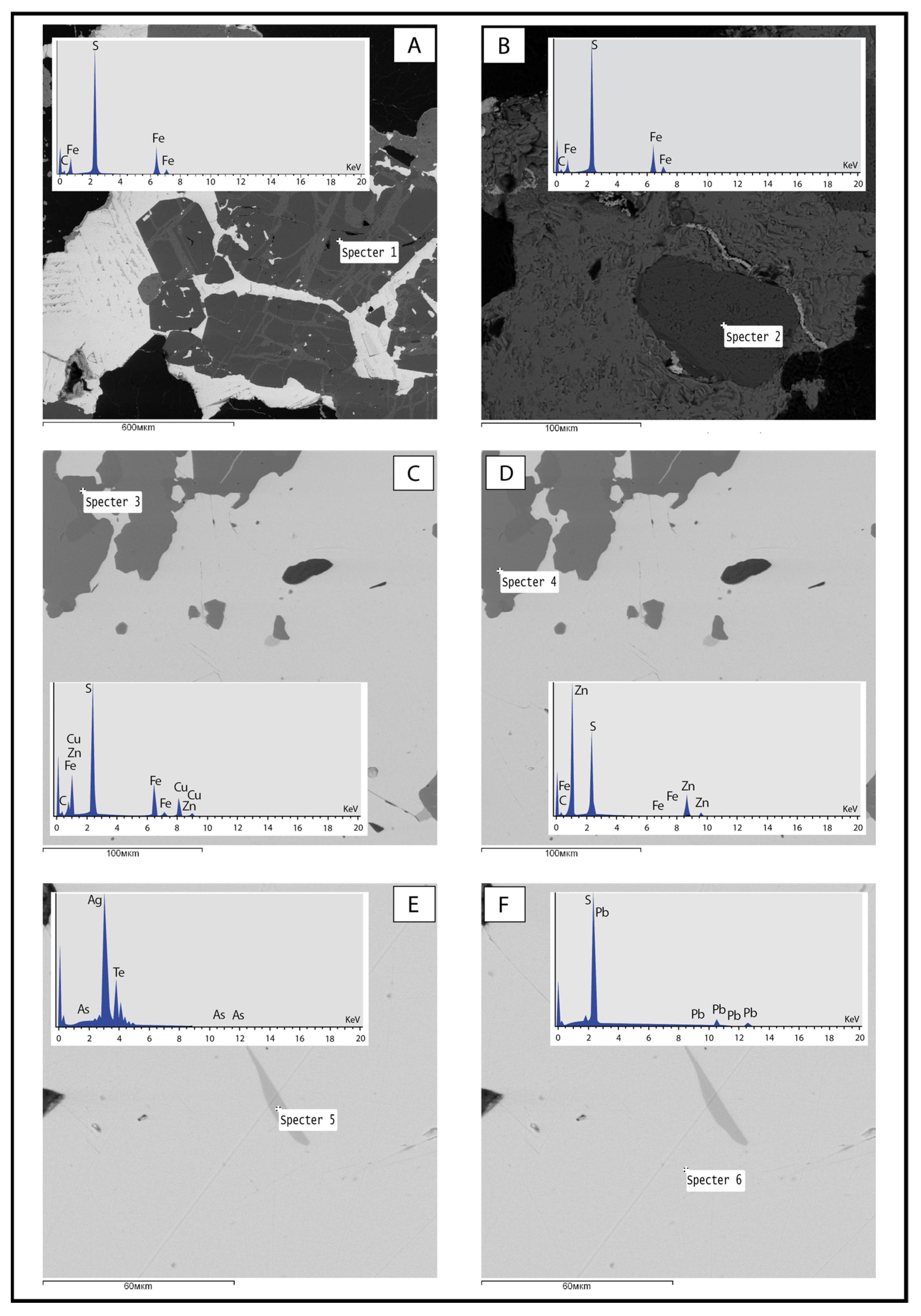
2.2.1. Electron Microprobe Analysis
2.2.2. TESCAN Integrated Mineral Analysis
2.2.3. Petrographic Analysis
2.2.4. LA-ICP-MS
2.3. Experimental Methods
2.3.1. Grinding Tests
2.3.2. Metallurgical Tests
3. Results
3.1. Mineralogical and Textural Characteristics of Ore
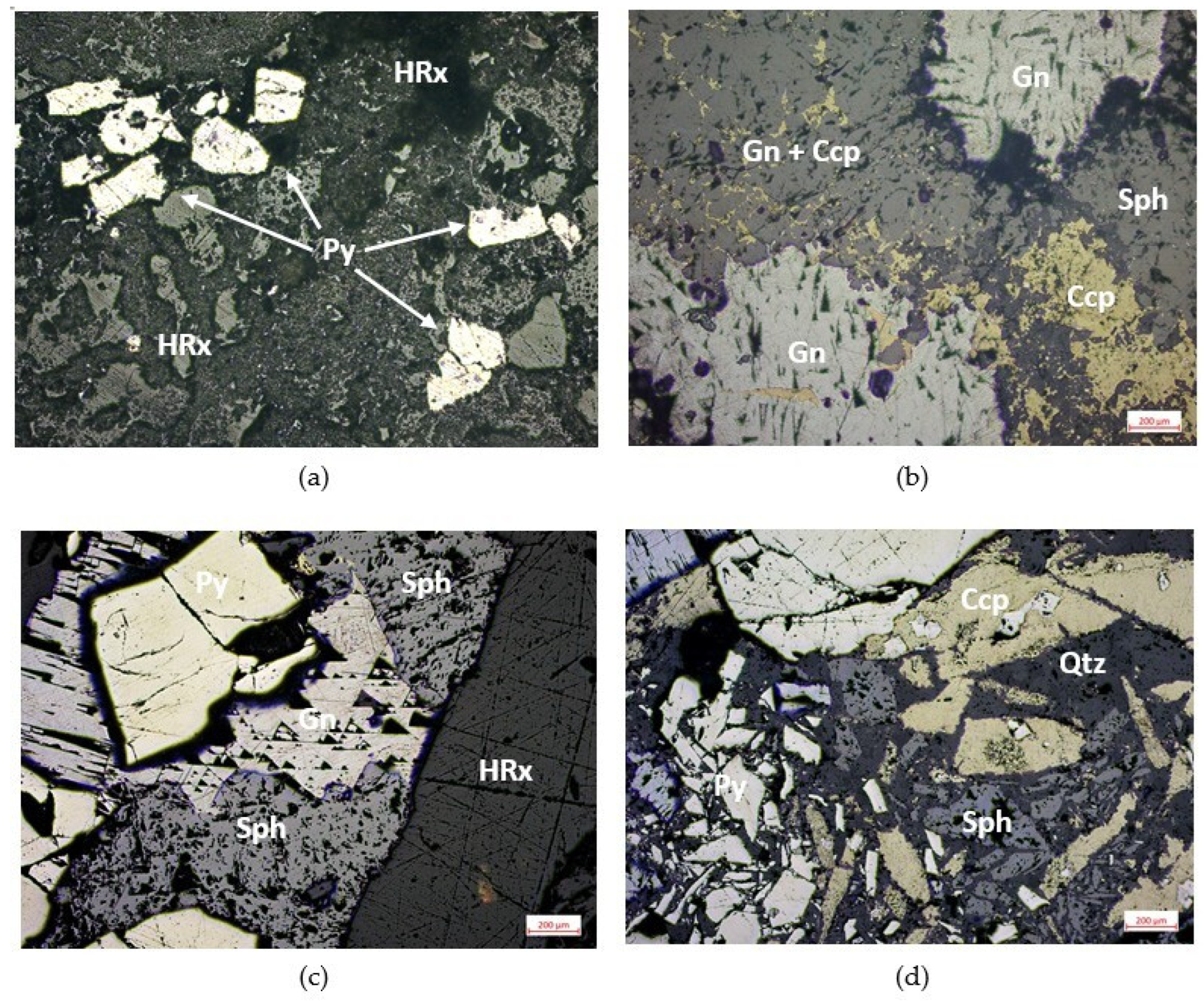
3.1.1. Pyrite
3.1.2. Chalcopyrite and Sphalerite
3.1.3. Galena
3.2. Analysis of Refractory Particles Gold Ore
3.2.1. Characterization Before Grinding
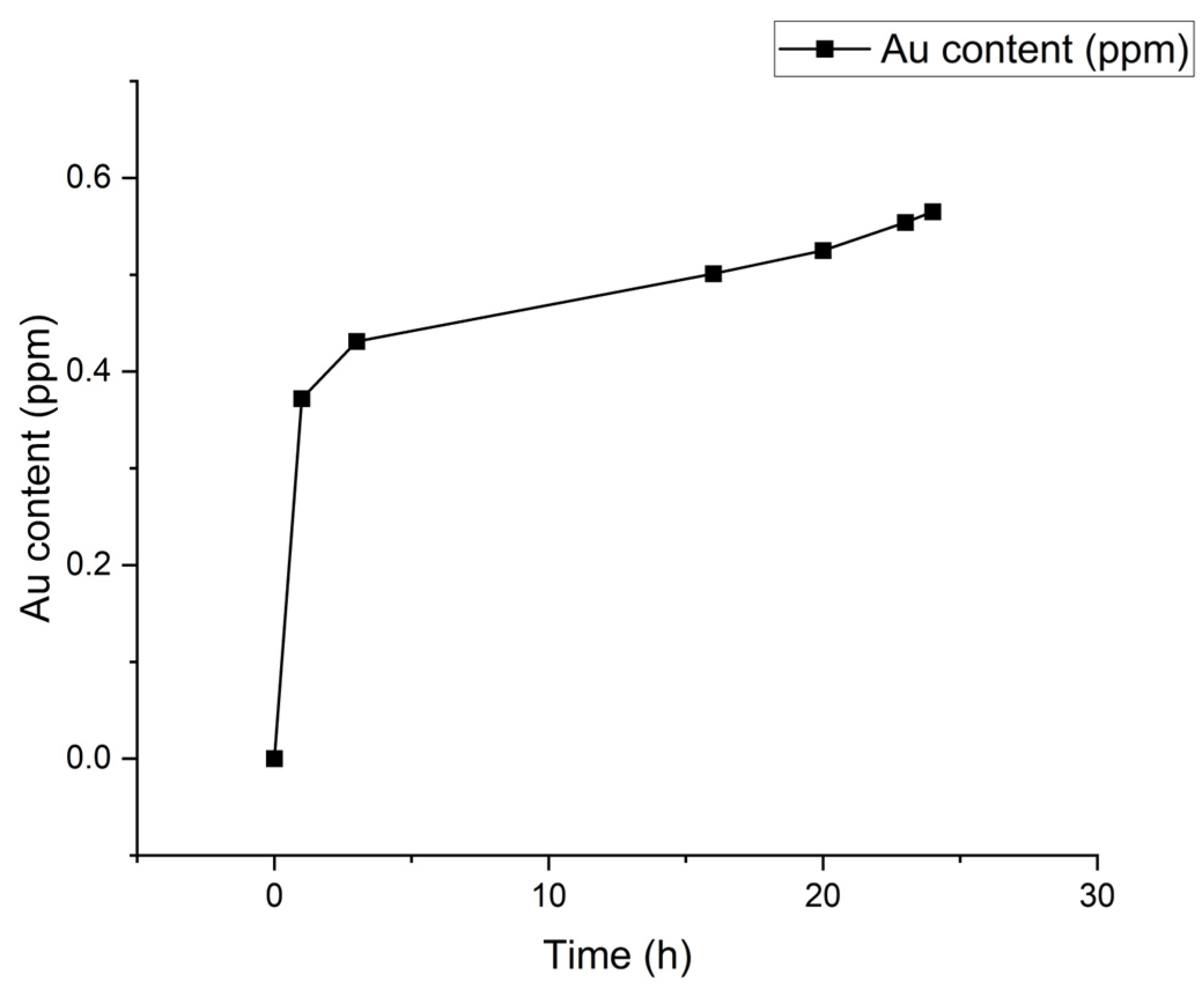
3.2.2. Cyanidation Leaching Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bazan, V.; Lara, R.; Brandalez, E. Characterization of gold refractory minerals. XIV Argen. Min. Treat. Conf. 2018, 210, 123–133. [Google Scholar]
- Yang, W.; Wang, Q.; Wang, Y.; Long, T.; Deng, S. Effects of Pyrite, Quartz and Sodium Sulfite on Roasting of a Refractory Sulfide Concentrate and Gold, Silver, Copper Leaching during Cyanidation. Hydrometallurgy 2024, 226, 106306. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Yu, D.; Zhang, L. Recovery of Gold from Sulfide Refractory Gold Ore: Oxidation Roasting Pretreatment and Gold Extraction. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Zheng, Y.-J.; Cao, P.; Li, C.-H.; Lai, S.-Z.; Wang, X. Process Mineralogy Characteristics of Acid Leaching Residue Produced in Low-Temperature Roasting-Acid Leaching Pretreatment Process of Refractory Gold Concentrates. Int. J. Miner. Metall. Mater. 2018, 25, 1132–1139. [Google Scholar] [CrossRef]
- Barbouchi, A.; Louarrat, M.; Mikali, M.; Barfoud, L.; El Alaoui-Chrifi, M.A.; Faqir, H.; Benzakour, I.; Idouhli, R.; Khadiri, M.-E.; Benzakour, J. Advancements in Improving Gold Recovery from Refractory Gold Ores/Concentrates: A Review. Can. Metall. Q. 2024, 63, 1–18. [Google Scholar] [CrossRef]
- Kargar, A.; Mohammadnejad, S. The Cyanidation of Sub Microscopic Gold: An Experimental and Molecular Modeling Study. Hydrometallurgy 2023, 222, 106177. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, S.; Zheng, Y.; Lai, S.; Liang, G.; Wang, X.; Tan, B. Identification of Elements Hindering Gold Leaching from Gold-Bearing Dust and Selection of Gold Extraction Process. Hydrometallurgy 2021, 202, 105612. [Google Scholar] [CrossRef]
- Agorhom, E.A.; Skinner, W.; Zanin, M. Influence of Gold Mineralogy on Its Flotation Recovery in a Porphyry Copper-Gold Ore. Chem. Eng. Sci. 2013, 99, 127–138. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J.; Butcher, A.R. The Use of QEMSCAN and Diagnostic Leaching in the Characterisation of Visible Gold in Complex Ores. Miner. Eng. 2005, 18, 877–886. [Google Scholar] [CrossRef]
- Nazari, A.; Ghahreman, A.; Bell, S. A Comparative Study of Gold Refractoriness by the Application of QEMSCAN and Diagnostic Leach Process. Int. J. Miner. Process. 2017, 169, 35–46. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhao, R.; Tong, L.; Chen, Q. Mineralogical Characteristics and Recovery Process Optimization Analysis of a Refractory Gold Ore with Gold Particles Mainly Encapsulated in Pyrite and Arsenopyrite. Geochemistry 2023, 83, 125945. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Tong, L.; Sand, W. Some Aspects of Industrial Heap Bioleaching Technology: From Basics to Practice. Miner. Process. Extr. Metall. Rev. 2022, 43, 510–528. [Google Scholar] [CrossRef]
- González, D. Evaluation of the Processes in the “PITUCA II” Gold Ore Recovery Plant in the Zamora Sector. Bachelor’s Thesis, Universidad Técnica Particular de Loja, Loja, Ecuador, 2016. [Google Scholar]
- Hrstka, T.; Gottlieb, P.; Skála, R.; Breiter, K.; Motl, D. Automated Mineralogy and Petrology–Applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef]
- Hastie, E.C.G.; Kontak, D.J.; Lafrance, B. Gold Remobilization: Insights from Gold Deposits in the Archean Swayze Greenstone Belt, Abitibi Subprovince, Canada. Econ. Geol. 2020, 115, 241–277. [Google Scholar] [CrossRef]
- Hastie, E.C.G.; Schindler, M.; Kontak, D.J.; Lafrance, B. Transport and Coarsening of Gold Nanoparticles in an Orogenic Deposit by Dissolution–Reprecipitation and Ostwald Ripening. Commun. Earth Environ. 2021, 2, 57. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Yang, Y.; Hu, Z. Calibration and Correction of LA-ICP-MS and LA-MC-ICP-MS Analyses for Element Contents and Isotopic Ratios. Geosci. Front. 2016, 7, 789–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, S.; Wang, D.; Liu, J.; Wang, J.; Zha, Z.; Bai, H. In Situ Trace Elements and Sulfur Isotopes of Sulfides in the Dabaiyang Te-Au Deposit, Hebei Province, China: Implications for Au Remobilization from Pyrite. Ore Geol. Rev. 2022, 140, 104626. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, G.; Zhou, F.; Cai, Y.; Pring, A. Enrichment of Precious Metals Associated with Chalcopyrite Inclusions in Sphalerite and Pyrite. Am. Mineral. 2024, 109, 150–156. [Google Scholar] [CrossRef]
- Guerrero, J. Characterization and Intensive Cyanidation Tests of Mineral Samples with Gold Content from the Ponce Enríquez Mining District—Ecuador. Undergraduate Thesis, Escuela Superior Politécnica del Litoral (ESPOL), Guayaquil, Ecuador, 2006. [Google Scholar]
- Crundwell, F. The Dissolution and Leaching of Minerals: Mechanisms, Myths and Misunderstandings. Hydrometallurgy 2013, 139, 132–148. [Google Scholar] [CrossRef]
- Huang, Z.-S.; Yang, T.-Z. Comparative Study on Refractory Gold Concentrate Kinetics and Mechanisms by Pilot Scale Batch and Continuous Bio-Oxidation. Minerals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Marsden, J.O.; House, C.I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006. [Google Scholar]



| Mineral | ||||||
|---|---|---|---|---|---|---|
| Element (W%) | Pyrite | Sphalerite | Galena | Chalcopyrite | Hessite | Gold |
| S | 53.43 | 32.87 | 13.42 | 34.87 | ||
| Fe | 46.62 | 0.48 | 30.47 | |||
| Zn | 66.96 | 0.71 | ||||
| Pb | 86.65 | |||||
| Ag | 60.09 | |||||
| Te | 40.29 | 2.01 | ||||
| Cu | 34.53 | |||||
| Au | 98.05 | |||||
| Total | 100.05 | 100.31 | 100.07 | 100.58 | 100.13 | 100.01 |
| Particle size (μm) | 20–400 | 5–300 | 50–400 | 5–300 | 20–50 | 5–40 |
| Mass ratio | Fe/S 0.87 | Zn/S 2.04 | Pb/S 6.45 | Cu/Fe/S 1:0.88:1 | Ag/Te 1.49 | - |
| Fraction | Weight (kg) | Grade (mg/kg) | Au Content (mg) |
|---|---|---|---|
| Feed | 1.5 | 0.75 | 1.125 |
| Concentrate | 0.565 | 1.438 | 0.81247 |
| Tailings | 1.5 | 0.2 | 0.3 |
| Recovery | 72.22 |
| Fraction | Weight (kg) | Grade (mg/kg) | Au Content (mg) |
|---|---|---|---|
| Feed | 1.5 | 0.75 | 1.125 |
| Concentrate | 0.692 | 1.32 | 0.91344 |
| Tailings | 1.5 | 0.13 | 0.195 |
| Recovery | 81.19 |
| Elapsed Time (h) | pH | NaCN (g) | CaO (g) |
|---|---|---|---|
| 0.00 | 11.27 | 3.00 | 1.67 |
| 1.00 | 11.05 | – | – |
| 5.00 | 10.38 | 1.26 | – |
| 8.00 | 10.16 | 0.63 | – |
| 22.00 | 9.15 | 1.74 | 1.41 |
| 24.00 | 10.16 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navas Jaramillo, S.J.; Gonzalez Zuñiga, R.E. Characteristics of Mineralization of Refractory Gold and Its Influence on Cyanide Gold Leaching Rates: A Case Study in Pituca II, Zamora Chinchipe, Ecuador. Minerals 2025, 15, 523. https://doi.org/10.3390/min15050523
Navas Jaramillo SJ, Gonzalez Zuñiga RE. Characteristics of Mineralization of Refractory Gold and Its Influence on Cyanide Gold Leaching Rates: A Case Study in Pituca II, Zamora Chinchipe, Ecuador. Minerals. 2025; 15(5):523. https://doi.org/10.3390/min15050523
Chicago/Turabian StyleNavas Jaramillo, Santiago Jose, and Renato Efren Gonzalez Zuñiga. 2025. "Characteristics of Mineralization of Refractory Gold and Its Influence on Cyanide Gold Leaching Rates: A Case Study in Pituca II, Zamora Chinchipe, Ecuador" Minerals 15, no. 5: 523. https://doi.org/10.3390/min15050523
APA StyleNavas Jaramillo, S. J., & Gonzalez Zuñiga, R. E. (2025). Characteristics of Mineralization of Refractory Gold and Its Influence on Cyanide Gold Leaching Rates: A Case Study in Pituca II, Zamora Chinchipe, Ecuador. Minerals, 15(5), 523. https://doi.org/10.3390/min15050523







