Characterization of Mineralogical Species in a Copper Concentrate After Acid Pretreatment
Abstract
1. Introduction
2. Materials and Methods
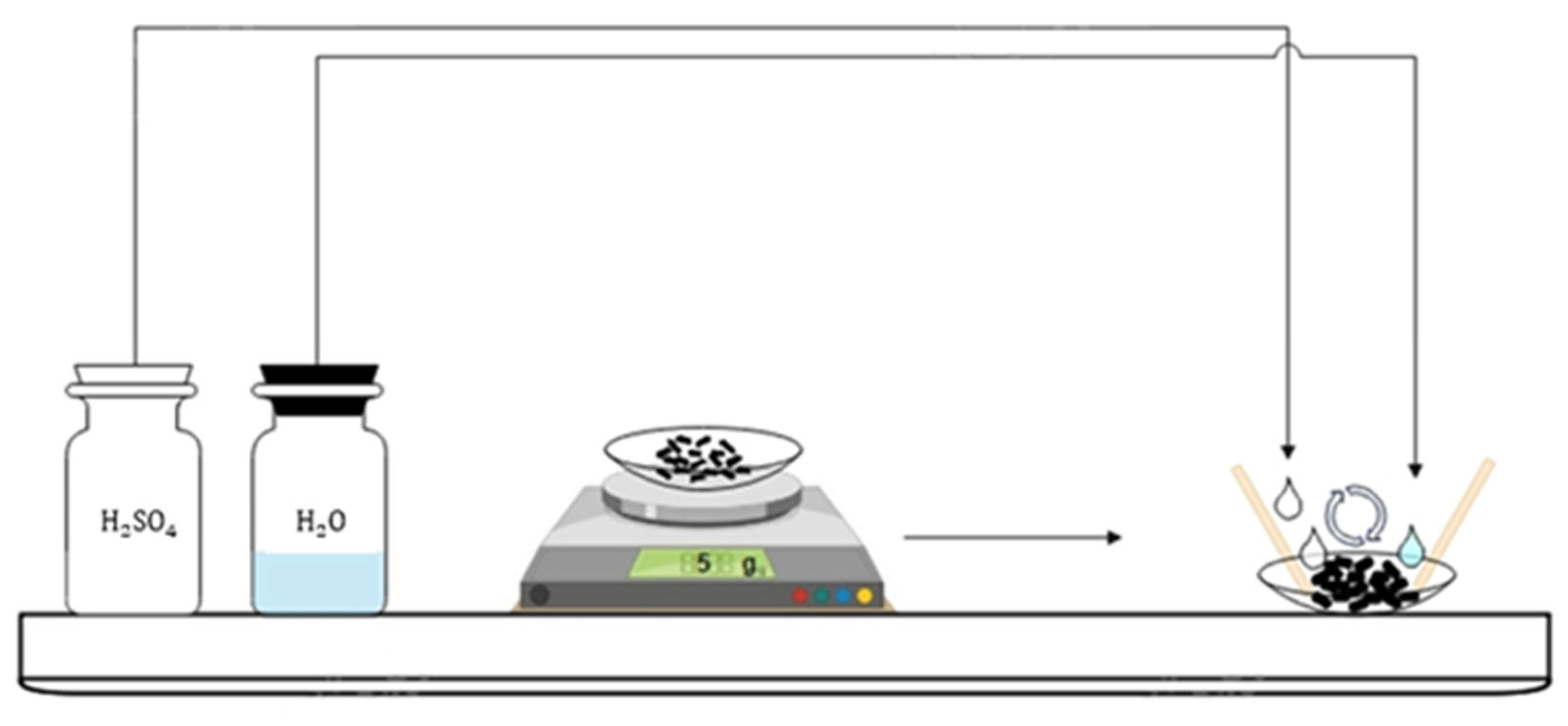
2.1. Pretreated Sample of Copper Concentrate
2.2. Mineralogical Characterization
3. Results and Discussion
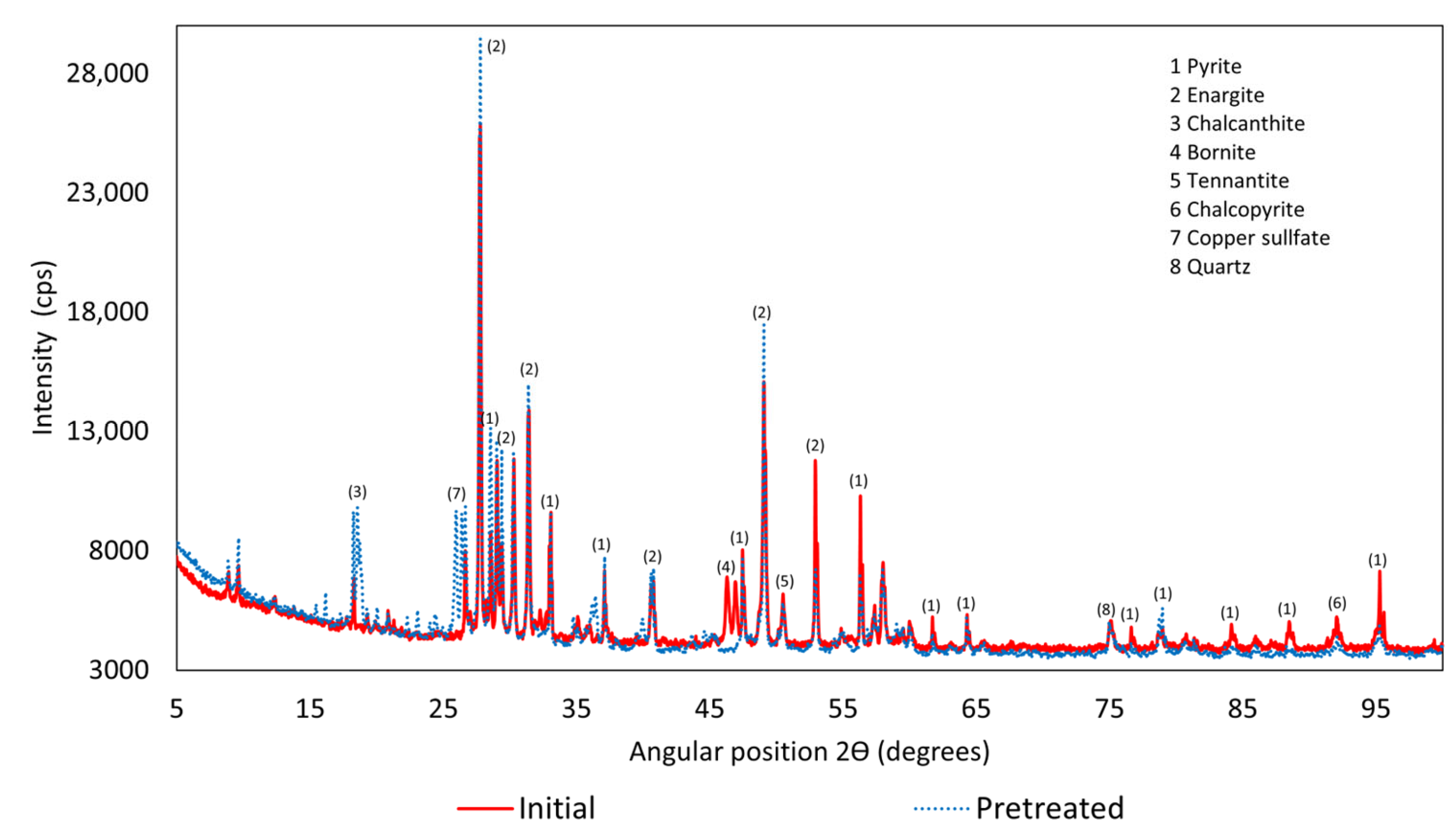
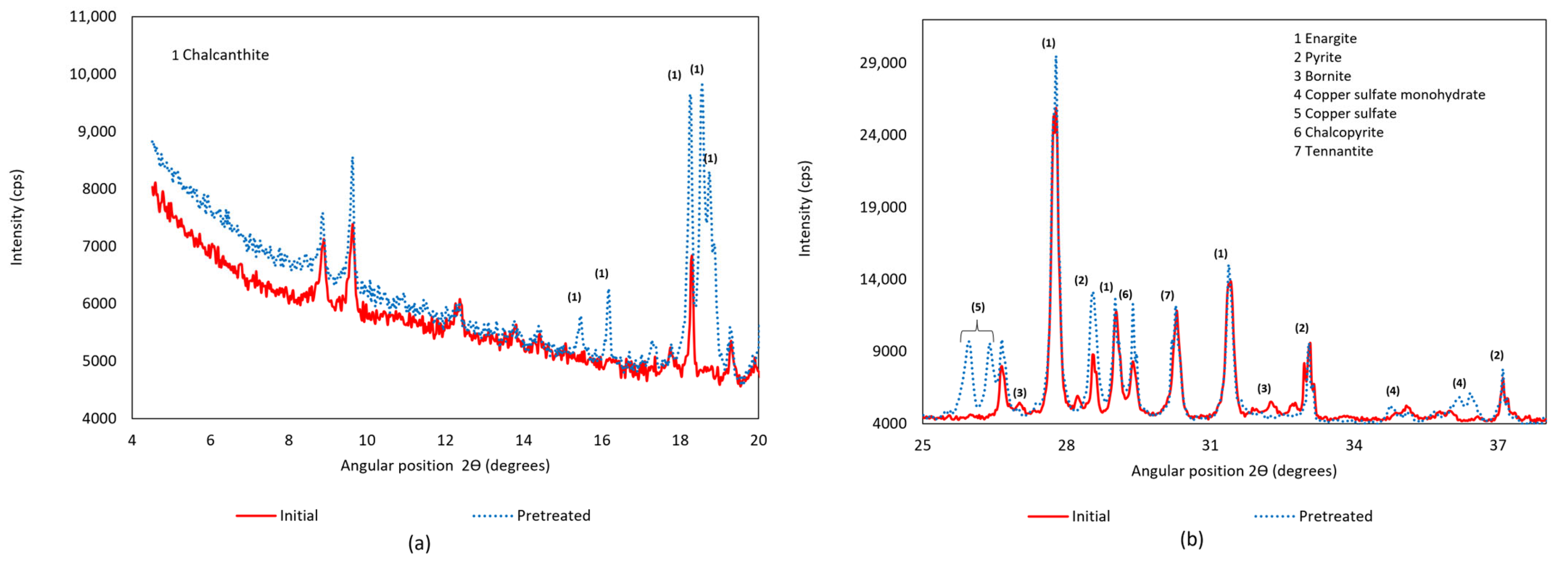
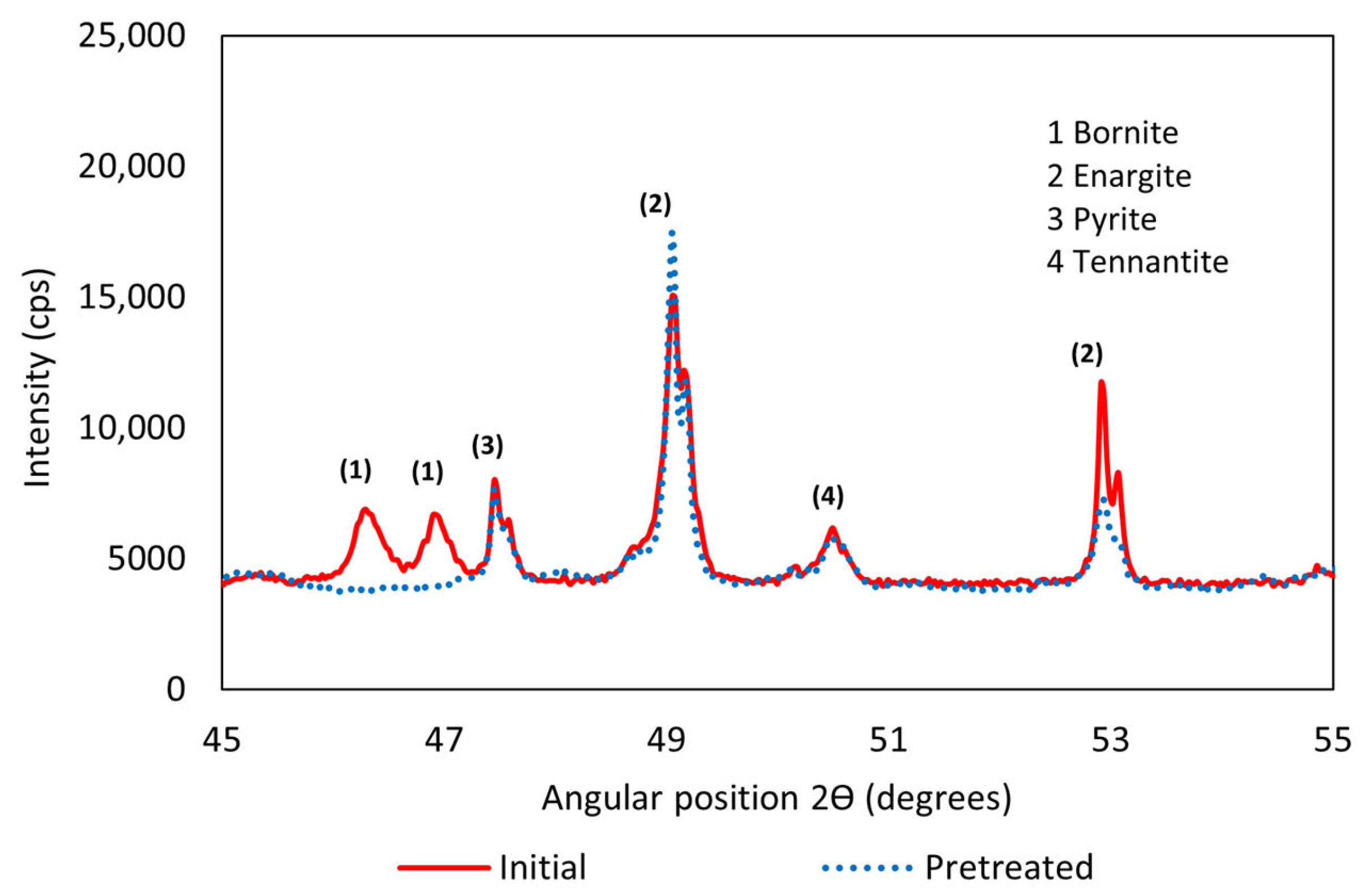
3.1. X-Ray Diffraction (XRD)
3.2. Field Emission Scanning Electron Microscopy (FESEM)
3.3. X-Ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
References
- Safarzadeh, M.S.; Miller, J.D. International Journal of Mineral Processing Acid bake—Leach process for the treatment of arsenopyrite, tennantite, and tetrahedrite. Int. J. Miner. Process. 2013, 124, 128–131. [Google Scholar] [CrossRef]
- COCHILCO RPI. Proyección de la Producción de Cobre en Chile 2023–2034; Comisión Chilena del Cobre (Cochilco): Santiago, Chile, 2024. [Google Scholar]
- COCHILCO RPI. Proyección de la Producción de Cobre en Chile 2022–2033; Comisión Chilena del Cobre (Cochilco): Santiago, Chile, 2022. [Google Scholar]
- Mandal, B.K.; Suzuki, T. Arsenic round the world: A review. Talanta 2002, 57, 201–235. [Google Scholar] [CrossRef]
- Taylor, P.R.; Putra, T.A.R. Pyrometallurgical Processing Technologies for Treating High Arsenic Copper Concentrates; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–198. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Wang, G.; Yang, H. Copper leaching from complex chalcopyrite-rich ores: Utilizing mechanical activation and wastewater-based sulfuric acid system. Sep. Purif. Technol. 2025, 354, 128631. [Google Scholar] [CrossRef]
- Vargas, T.; Rojas, F.; Bahamondez, C.; Castro, R.; Ihle, C.F.; Caraballo, M. Physical and chemical transformations of gangue materials during leaching of copper sulphides, and their influence on copper leaching kinetics. J. S. Afr. Inst. Min. Met. 2017, 117, 727–730. [Google Scholar] [CrossRef][Green Version]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. the effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, E.; Jamett, N.E.; Ghorbani, Y. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals 2017, 8, 1. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl–H2SO4–O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E.; Hernández, M. Pretreatment to leaching for a primary copper sulphide ore in chloride media. Metals 2021, 11, 1260. [Google Scholar] [CrossRef]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. Hydrometallurgy Acid bake-leach process for the treatment of enargite concentrates. Hydrometallurgy 2012, 119–120, 30–39. [Google Scholar] [CrossRef]
- Quezada, V.; Villagrán, G.; Calisaya-Azpilcueta, D.; Marín, N. Effect of Pretreatment on a Copper Concentrate with High Arsenic. Minerals 2024, 14, 419. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth-Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Quezada, V.; Zepeda, S.; Benavente, O.; Hernández, M.; Melo, E. Effect of curing time and ferric chloride on a copper concentrate with a high arsenic content. Minerals 2024, 14, 1063. [Google Scholar] [CrossRef]
- Young, C.L.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution. Chem. Eng. J. 2013, 228, 731–740. [Google Scholar] [CrossRef]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Electron Spectrosc. Relat. Phenom. 2007, 156–158, 310–314. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray photoelectron spectroscopic study of copper minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Waltham, MA, USA, 1992. [Google Scholar]
- Zhao, H.; Huang, X.; Hu, M.; Zhang, C.; Zhang, Y.; Wang, J.; Qin, W.; Qiu, G. Insights into the surface transformation and electrochemical dissolution process of bornite in bioleaching. Minerals 2018, 8, 173. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Borda, L.; Bia, G.; Borgnino, L.; Chiaramonte, N.; García, M.G. Understanding arsenic-ulexite interactions in evaporite environments: Evidence from XRPD, micro-XRF, micro-FT-IR, and XPS studies. J. Hazard. Mater. 2024, 473, 134547. [Google Scholar] [CrossRef] [PubMed]

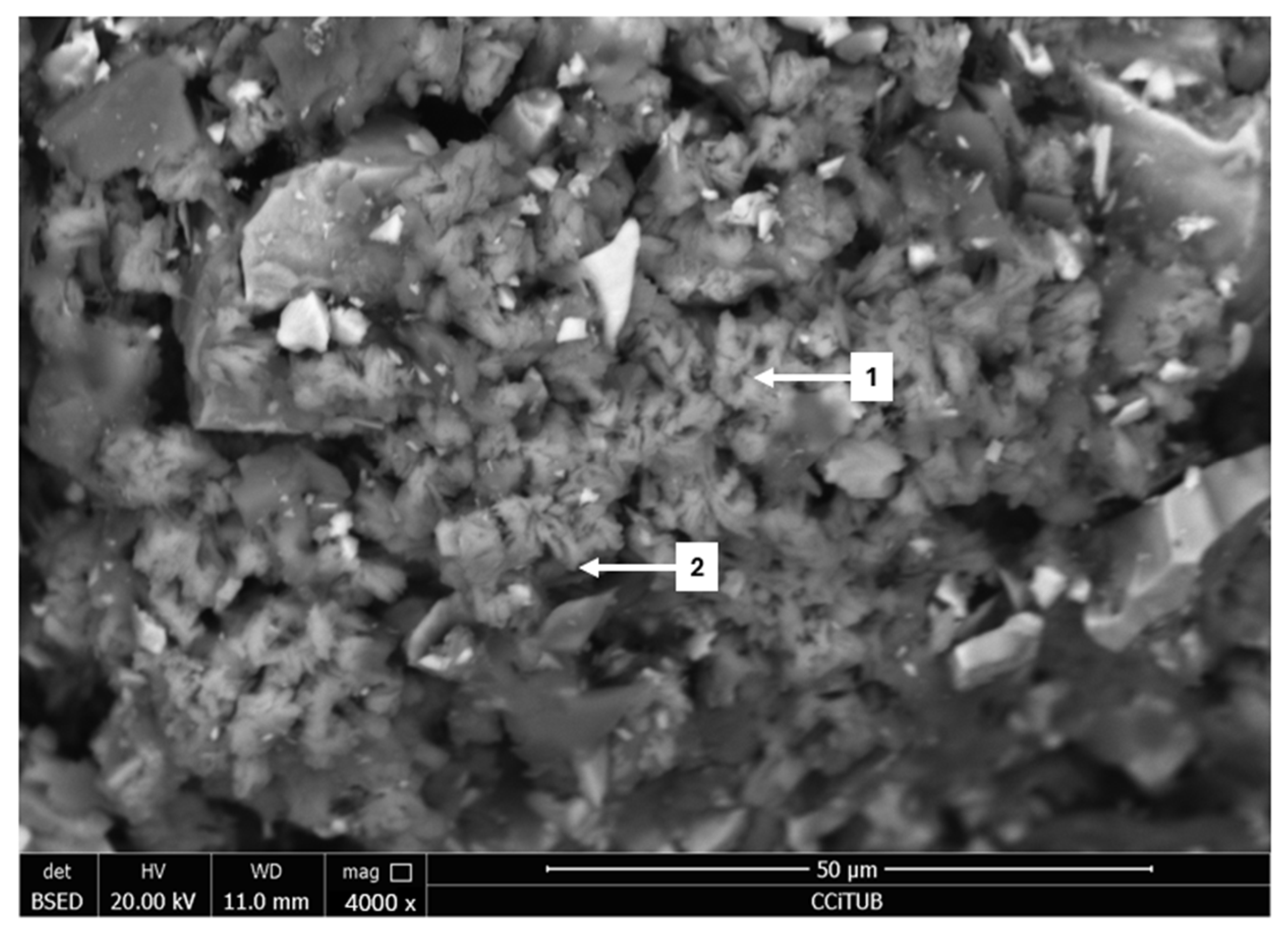

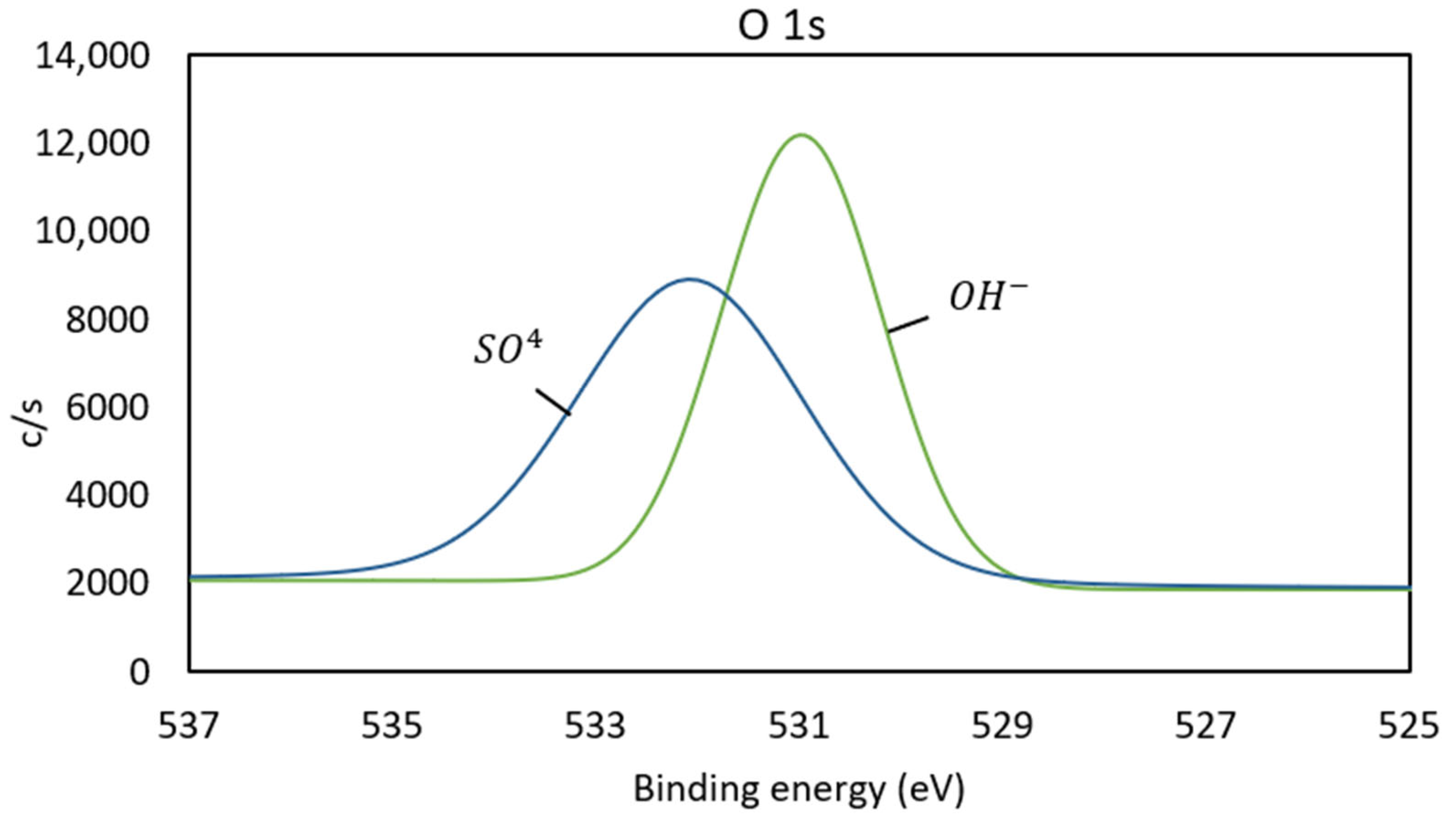
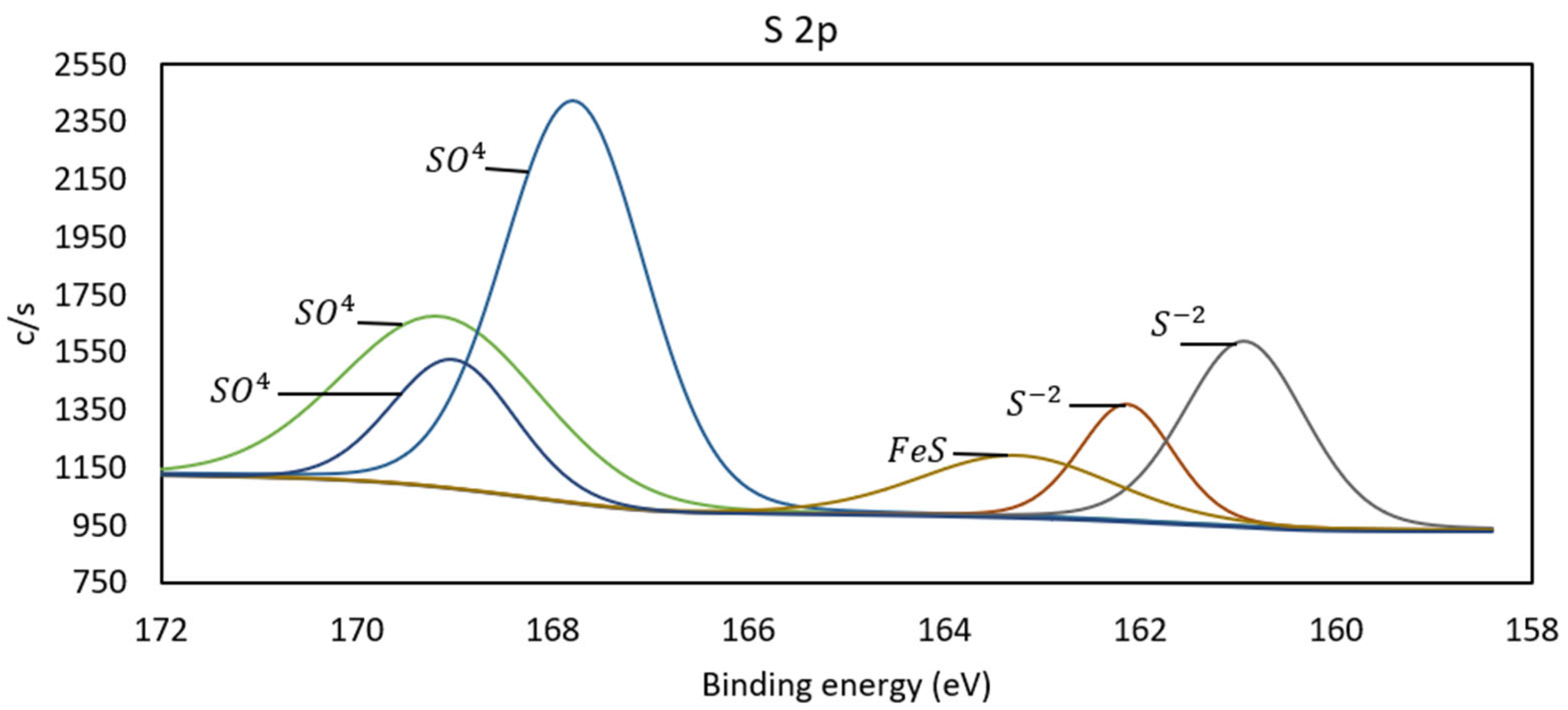

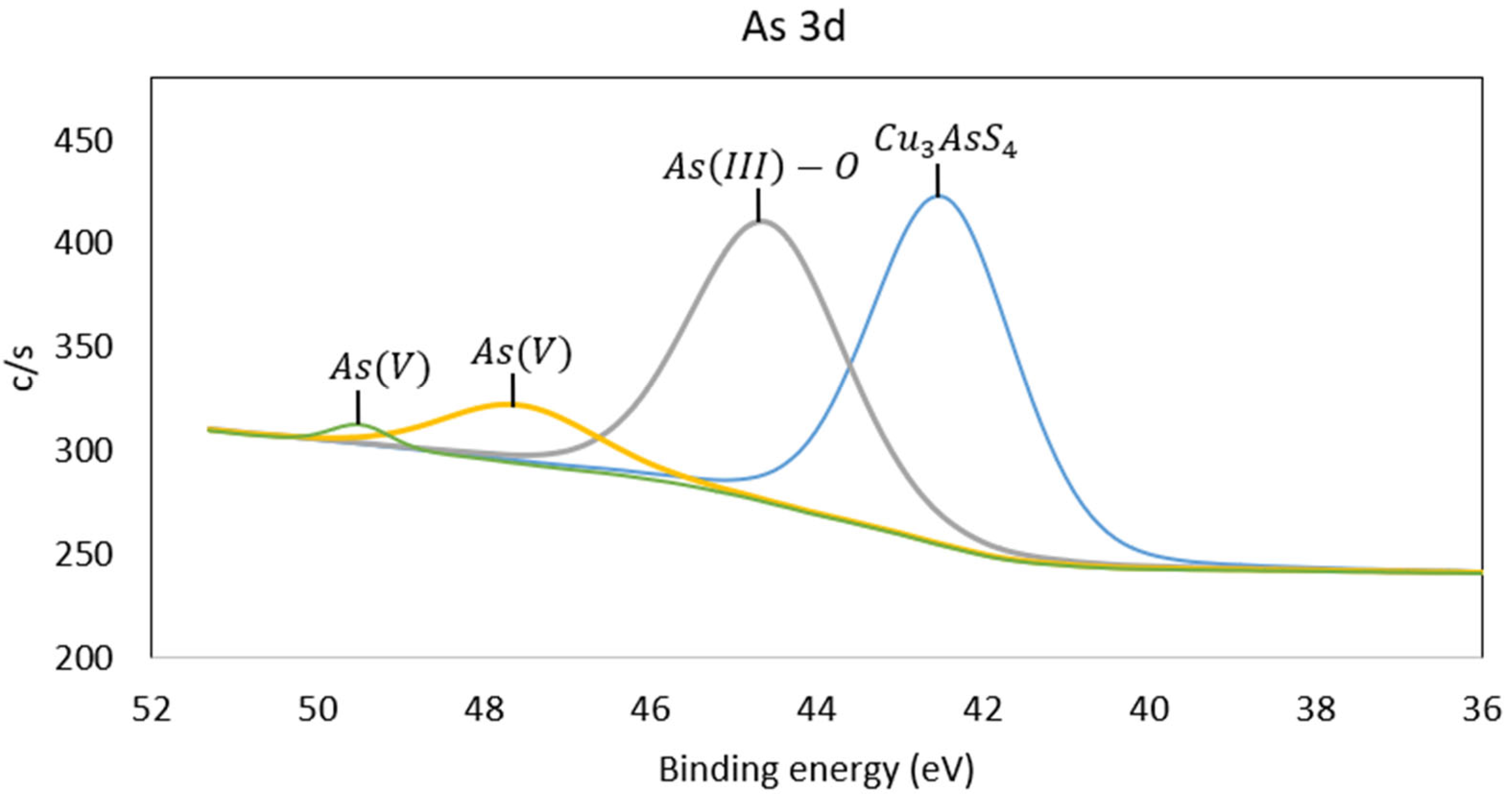
| N° | Mineral Species | O | Al | Si | S | K | Fe | Cu | As | Sb | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Oxidized Enargite | 5.62 | - | - | 31.09 | - | - | 46.13 | 16.66 | 0.50 | 100 |
| 2 | Oxidized Enargite | 9.91 | - | - | 32.20 | - | - | 41.93 | 14.16 | 1.80 | 100 |
| 3 | Copper Sulfate | 47.30 | - | - | 19.62 | - | - | 32.25 | 0.82 | - | 100 |
| 4 | Enargite | - | 0.32 | 0.42 | 36.17 | - | 0.67 | 45.94 | 16.48 | - | 100 |
| 5 | Enargite | - | 0.46 | 0.64 | 35.98 | - | 0.69 | 46.08 | 16.15 | - | 100 |
| 6 | Copper Sulfate | 18.81 | 0.19 | 0.44 | 27.30 | - | 7.64 | 45.62 | - | 100 | |
| 7 | Oxidized Enargite | 8.65 | 0.77 | 1.04 | 29.38 | - | 0.92 | 44.23 | 15.01 | - | 100 |
| N° | Mineral Species | O | Al | Si | S | K | Ca | Fe | Cu | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Copper Sulfate | 46.00 | 0.60 | 0.62 | 19.31 | - | 0.21 | 2.21 | 31.05 | 100 |
| 2 | Copper Sulfate | 40.20 | 1.33 | 1.49 | 20.95 | 0.24 | 0.55 | 2.64 | 32.60 | 100 |
| N° | Mineral | O | Al | Si | S | K | Fe | Cu | Zn | As | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pyrite | 4.06 | 0.70 | 1.09 | 35.56 | 0.13 | 52.64 | 4.44 | 0.78 | 0.60 | 100 |
| 2 | Covellite | 2.59 | 0.71 | 1.41 | 27.17 | - | 1.13 | 66.99 | - | - | 100 |
| 3 | Covellite | 5.35 | - | 1.59 | 30.96 | 0.16 | 1.74 | 58.74 | - | 1.46 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, G.V.; Quezada, V.; Rius-Ayra, O.; Biserova-Tahchieva, A.; Llorca-Isern, N. Characterization of Mineralogical Species in a Copper Concentrate After Acid Pretreatment. Minerals 2025, 15, 520. https://doi.org/10.3390/min15050520
Santana GV, Quezada V, Rius-Ayra O, Biserova-Tahchieva A, Llorca-Isern N. Characterization of Mineralogical Species in a Copper Concentrate After Acid Pretreatment. Minerals. 2025; 15(5):520. https://doi.org/10.3390/min15050520
Chicago/Turabian StyleSantana, Geraldine Villagrán, Víctor Quezada, Oriol Rius-Ayra, Alisiya Biserova-Tahchieva, and Nuria Llorca-Isern. 2025. "Characterization of Mineralogical Species in a Copper Concentrate After Acid Pretreatment" Minerals 15, no. 5: 520. https://doi.org/10.3390/min15050520
APA StyleSantana, G. V., Quezada, V., Rius-Ayra, O., Biserova-Tahchieva, A., & Llorca-Isern, N. (2025). Characterization of Mineralogical Species in a Copper Concentrate After Acid Pretreatment. Minerals, 15(5), 520. https://doi.org/10.3390/min15050520








