Abstract
In Chile, copper concentrate production through mineral flotation is increasing while production through hydrometallurgical processes is decreasing due to the depletion of oxidized ores. Using the idle capacity of hydrometallurgy plants for acid pretreatment of sulfate ores before the leaching stage is an attractive alternative; however, a deeper understanding of the process and the products of such treatment is required. In this study, the mineral species formed during acid pretreatment are characterized to identify new mineralogical species. Pretreatment was conducted at 50 °C with 210 kg/t H2SO4 over 15 days on a copper concentrate mainly composed of enargite (35.93%). The characterization techniques used were X-ray diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM), and X-ray Photoelectron Spectroscopy (XPS). XRD identified copper sulfate (CuSO4) formation and the disappearance of chalcocite/digenite (Cu2S) and bornite (Cu5FeS4), indicating their transformation into sulfates. FESEM showed that enargite particles were oxidized, suggesting they did not form copper sulfates. The XPS results confirmed the presence of copper in species such as sulfides and sulfates. The results indicate that chalcocite and bornite transformed into copper sulfates, while chalcopyrite and enargite were only superficially oxidized. The combination of techniques allowed for a detailed identification of mineral transformations during pretreatment.
1. Introduction
Copper concentrate processing in Chile via flotation is increasing and will become the country’s main method for concentrate production. Despite the increase in the production of concentrates, the smelters and refineries in Chile do not have sufficient capacity to process all the copper concentrate produced domestically. Moreover, there is no expectation of a significant increase in the processing capacity of these facilities due to the lack of projects aimed at expanding refined copper production. This highlights the importance of investigating alternative treatment methods for copper concentrates [1]. At the same time, the depletion of copper oxides will reduce the contribution of hydrometallurgical processes to copper production from 24.95% (2023) to 11.1% by 2034, leaving installed hydrometallurgical plants underutilized [2].
One of the major challenges in the treatment of copper concentrates is the presence of arsenic. During pyrometallurgical processes, arsenic compounds release gases such as sulfur dioxide and arsenic trioxide [3]. Arsenic is a toxic element that must be handled carefully to comply with environmental regulations, specifically to minimize its release [1,4,5]. As such, hydrometallurgy presents itself as an alternative for treating sulfate minerals, keeping arsenic species stable in solution [6,7,8]. These sulfate minerals are refractory to conventional copper leaching due to the formation of a surface layer, primarily associated with sulfur, which inhibits the dissolution process [9,10]. Acid curing, as a pretreatment, is an alternative to promote the sulfatization of copper minerals, thereby enhancing subsequent dissolution [11,12,13].
To more fully understand the effects of acid curing, a few post-treatment studies to characterize the pretreated solid in order to identify the mineral species formed have been undertaken. A study conducted by Quezada [13] applied an acid curing pretreatment to mineral samples primarily composed of chalcopyrite (73.7%). By adding 15 kg/t of H2SO4 and 25 kg/t of NaCl over 15 days of curing, a copper extraction rate of 22.66% was achieved. The characterization of the cured mineral, using reflected light microscopy, revealed cracks on the surface of chalcopyrite particles, suggesting system reactivity and chemical crushing. X-ray diffraction identified the formation of new species such as CuSO4, Cu2Cl (OH), NaFe3(SO4)2(OH)6, and elemental sulfur (S°). The formation of natrojarosite was linked to sodium from NaCl and iron from chalcopyrite. SEM analysis showed unreacted and partially attacked chalcopyrite, with cracks associated with the transformation of chalcopyrite into chalcocite and covellite. The reaction of the pretreatment is represented by Equation (1). The acid-curing procedure differed in methodology to that suggested by [14], as Hernández used an acid-chloride-nitrate medium and proposed the formation of 2CuCl2, Fe2(SO4)3, and S, among other species.
Equation (1):
Ref. [15] performed an acid-bake-leach pretreatment on an enargite concentrate. Unlike acid curing, this acid baking was conducted at temperatures between 100 °C and 200 °C for periods ranging from 0.5 to 24 h. Subsequently, the sample was leached with water at 70 °C for 45 min, filtered, and dried at 70 °C for further analysis. They achieved a 90% copper extraction when pretreating the concentrate at 200 °C for 7 h. Additionally, they extracted 61% of the arsenic, with less than 1% of the arsenic released in gaseous form.
The pretreated solid was characterized by SEM and revealed the formation of copper sulfate crystals on the surface of enargite particles, which disappeared after leaching, leaving only unreacted enargite. It was concluded that enargite, chalcopyrite, sphalerite, and galena particles reacted to form sulfates, which enabled the high copper extraction achieved. Although several reactions were proposed, reactions (2)–(4) were considered the most likely during the acid roasting pretreatment.
Reactions (2)–(4):
In this context, a previous study conducted by Quezada et al. [16] proposed an acid-curing pretreatment for a copper concentrate with high arsenic content to facilitate subsequent dissolution. This treatment alternative would allow the unused capacity of hydrometallurgical plants to be utilized, while maintaining control over the arsenic species present in complex copper concentrates. That pretreatment study focused on evaluating the effect of four variables on acid curing: sulfuric acid concentration, sodium chloride, temperature, and curing time. The maximum copper extraction obtained using this pretreatment was 26.71%, achieved by adding 210 kg/t of sulfuric acid at a temperature of 50 °C for 15 days. It was inferred that the copper extraction corresponded to the dissolution of minerals such as chalcocite/digenite, due to their faster dissolution compared to enargite/tennantite/tetrahedrite.
Pretreatment is an attractive technique enabling the formation of more soluble species and facilitating the work of geometallurgical units. The presence of more soluble species could reduce the acid concentration at the beginning of the leaching cycle, which could result in a reduction in processing costs. This could even shorten the leaching cycle. A classic example of these benefits (among others) was achieved using the Cuprochlor process [17]. This synergy, between the identification of new mineralogical species and their effects on the process (leaching in this case), is one of the geometallurgical benefits that supports the continuous improvement of mining operations.
To further understand the effects of acid-curing pretreatment, the current study characterized the post-pretreatment copper concentrate to identify newly formed species as well as those not affected by the process [16]. The main objective of this characterization is to evaluate the formation of copper soluble species that enhance subsequent leaching efficiency. In the present study, the pretreated sample of copper concentrate used for characterization was the result of an experimental design that selected for the highest copper extraction. The following characterization techniques were applied to the pretreated sample: X-ray Diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM-EDS), and X-ray Photoelectron Spectroscopy (XPS).
2. Materials and Methods
2.1. Pretreated Sample of Copper Concentrate
The copper concentrate used for the pretreatment comes from a mining company located in the Antofagasta Region, Chile. The initial concentrate contains 35.57% CuT and is predominantly composed of tetrahedrite/tennantite/enargite (35.93%), followed by pyrite (22.35%), chalcocite/digenite (16.55%), bornite (6.01%), chalcopyrite (4.66%), and covellite (3.98%), among other species [16]. The initial chemical composition of the concentrate was determined through atomic absorption spectrometry. The initial mineralogical characterization was carried out using three techniques: Qemscan Model Zeiss EVO 50 (Zeiss, Oberkochen, Germany), a scanning electron microscope (SEM) (JEOL J-7100F, Tokyo, Japan) coupled with an energy-dispersive X-ray spectroscopy (EDS) microanalysis system (Oxford Instruments INCA, Oxfordshire, UK), and X-ray diffraction (XRD) analysis using a diffractometer (PANalytical, X’Pert PRO MPD Alpha1, Malvern, UK). X-ray diffractograms were interpreted using the X’pert HighScore Plus v.3.0e software (PANalytical, Almelo, The Netherlands) [16].
The pretreated sample was obtained from several curing tests performed in a previous study published by the authors [16]. Below is a summary of how the pretreated sample—corresponding to the test that achieved the highest copper extraction during the pretreatment—was obtained.
For the curing test, a representative 5 g sample of the copper concentrate was used. The sample was placed in a watch glass, and 210 kg/t of H2SO4 was added, followed by 0.4 mL of water to achieve the formation of stable agglomerates, by homogenizing with plastic rods, as shown in Figure 1. Then, the sample was covered with another watch glass to prevent evaporation and placed in an ELOS H110N230 oven for 15 days at 50 °C. To measure copper extraction, the samples were washed with a diluted sulfuric acid solution at a concentration of 0.5 g/L to dissolve any sulfates or soluble species formed during the pretreatment. The samples were filtered (0.2 µm), and the copper concentrations in the filtrate were determined by atomic absorption spectrometry. For the characterization presented in this study, the sample was not washed and was characterized immediately after the mentioned conditions were met.
Figure 1.
Curing test scheme.
2.2. Mineralogical Characterization
After curing, mineralogical characterization of the sample was carried out using X-ray diffraction (PANalytical X’Pert PRO MPD Alpha1, Malvern, UK) operating from 4.5° to 100° 2 θ with a step size of 0.026° and a time step of 100 s. Sample preparation included back loading of two samples into a standard cylindrical sample holder of 16 mm diameter and 2.5 mm height. Cu K (λ = 1.5418 Ǻ) radiation at 45 kV and 40 mA was used, with a Bragg –Brentano θ/2 θ geometry of 240 mm radius. Masks were used to define a beam length over the sample in the axial direction of 12 mm, Soller slits of 0.04 radians in the incident and diffracted beam, and a fixed divergence slit of 0.25°. The PIXcel detector was configured with an active length of 3.347°. A JEOL J-7100 field emission scanning electron microscope (FESEM, JEOL Ltd., Tokyo, Japan) was also used to detail morphology, and EDS microanalysis was used to determine the semiquantitative elemental composition in three different positions of each sample.
The XPS experiments were carried out in ESFOSCAN (PHI VersaProbe 4, Physical Electronics (ULVAC-PHI), Chanhassen, MN, USA) at the CCiTUB. Measurements were performed with a monochromatic-focused X-ray source (Aluminium Kalfa line of 1486.6 eV) calibrated using the 3d5/2 line of Ag with a full width at half maximum (FWHM) of 0.6 eV. The area analyzed was a circle of 100 microns in diameter, and the resolution selected for the spectra was 224 eV Pass Energy and 0.8 eV/step for the general spectra and 27 eV Pass Energy and 0.1 eV/step for high-resolution spectra of the selected elements. The analysis and fitting of the spectra were performed with Multipak software (version 8.2). A combination of a low-energy electron gun (less than 10 eV) and a low-energy argon ion gun (less than 5 eV) was used to discharge the powders when necessary. All measurements were performed in an ultra-high vacuum (UHV) chamber at a pressure between 5 × 10−10 and 5 × 10−9 Torr.
3. Results and Discussion
3.1. X-Ray Diffraction (XRD)
To identify the species formed, X-ray diffraction was performed, indicating that the species present in the pretreated sample were pyrite, enargite, chalcanthite, tennantite, chalcopyrite, copper sulfate, and quartz, as shown in Figure 2. Copper sulfate was also one of the species generated during acid curing, as reported by Quezada et al. [13]. However, natrojarosite, elemental sulfur, and copper–chloride complexes, which were also reported by the author, were not identified in this analysis. Figure 3 provides more detailed information on the species formed. To compare the species in the initial and pretreated concentrates, the initial copper concentrate was characterized using the same equipment as for the pretreated sample.
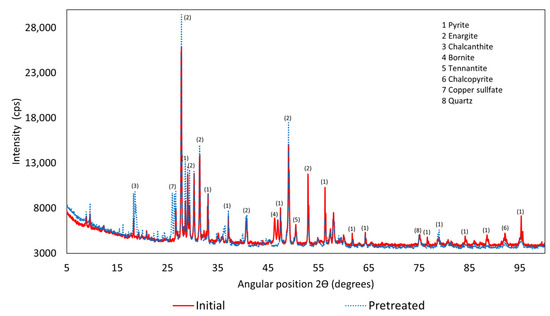
Figure 2.
X-Ray diffractometer.
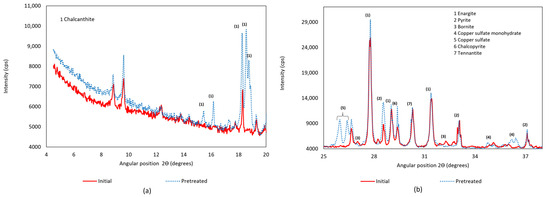
Figure 3.
Formation of copper sulfate in X-ray; (a) X-Ray diffractometer in an angular position range from 4 to 20 degrees; (b) X-Ray diffractometer in an angular position range from 25 to 38 degrees.
In Figure 3a, a comparison of the X-ray diffractogram between the initial and pretreated concentrate is presented for angular positions between 5° and 20°. Several peaks corresponding to different mineral species are observed. The formation of copper sulfate in the form of chalcanthite is shown by the peaks between 15° and 19°. The formation of copper sulfate is also identified in Figure 3b, where it is evident that copper minerals such as enargite and chalcopyrite do not show significant changes during pretreatment.
The formation of copper sulfates is associated with the transformation of bornite into new species. As shown in Figure 4, bornite disappears in the pretreated sample, giving rise to copper sulfates. In the previous Qemscan analysis, it was reported that bornite represented 6.01% of the sample, contributing 3.81% of the total copper. From this, it was inferred that 26.71% of copper extracted in the pretreatment comes from minerals such as bornite and chalcocite, which were also identified in the previous study.
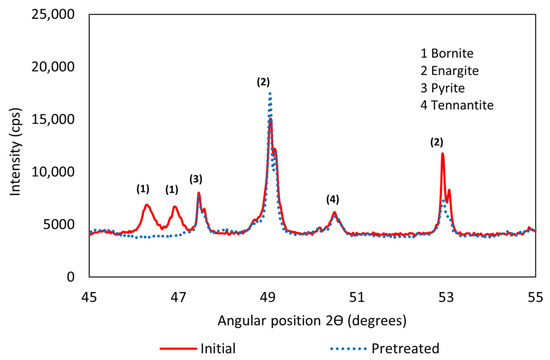
Figure 4.
X-Ray diffractometer in an angular position range from 45 to 55 degrees.
3.2. Field Emission Scanning Electron Microscopy (FESEM)
In Figure 5, the morphology of the cured sample is shown. At a scale of 100 µm, a light-toned enargite particle with darker spots is visible. These spots indicate the presence of oxygen, suggesting that the enargite was undergoing oxidation. Although a copper sulfate species is observed on the surface of the enargite particle, it is not associated with the transformation of enargite. According to Lattanzi et al. [18], the oxidation of enargite is slow; in the presence of sulfuric acid and ferric iron, the reaction involves the dissolution of copper and the formation of elemental sulfur. Therefore, more oxidative conditions are required to achieve complete oxidation, which would benefit the subsequent dissolution of copper. Additionally, they mention that a polished plate of natural enargite was exposed for 28 days in a climatic chamber at 85 °C and 80% humidity, identifying cracks with minimal amounts of a greenish species associated with antlerite through X-ray diffraction. By comparison, the higher temperature and longer time promoted the formation of these minimal amounts of sulfate, suggesting that longer periods of pretreatment could yield even better results. This behavior coincides with that reported by Quezada et al. [19], who also observed low transformation of these species, attributing it to the structural stability of the enargite and the possible formation of passivating layers on the surface of the chalcopyrite, limiting its dissolution. The elemental composition associated with this image is shown in Table 1, which presents the EDS analysis corresponding to Figure 5.
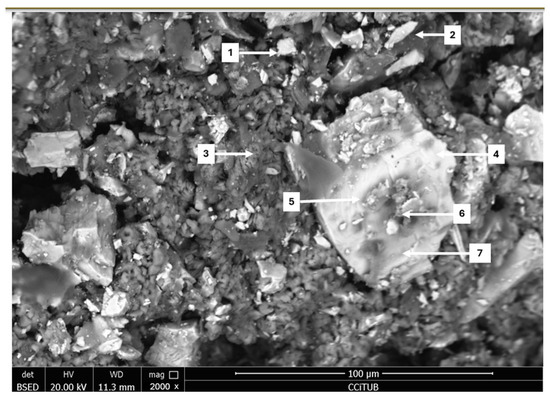
Figure 5.
Analysis through FESEM (1, 2, 7: Oxidized Enargite; 4, 5: Enargite; 3, 6: Copper Sulfate).

Table 1.
FESEM-EDS elemental composition corresponding to Figure 5.
At a scale of 50 µm, copper sulfates can be seen in much of the sample, except on the enargite particles, as shown in Figure 6. According to the X-ray diffraction results (see Section 3.1), the copper species that disappears during the pretreatment is bornite, which suggests it reacted to form copper sulfates. The elemental composition corresponding to Figure 6 is provided in Table 2.
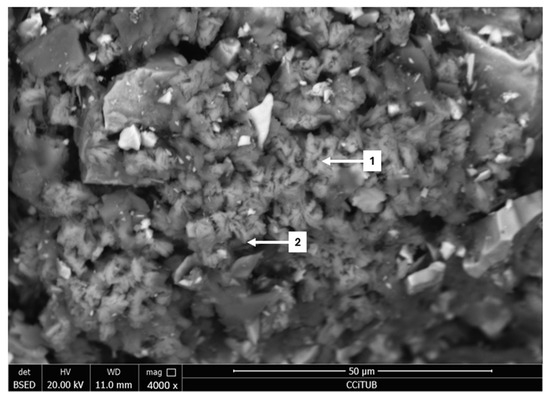
Figure 6.
Analysis through FESEM (1, 2: Copper Sulfate).

Table 2.
Elemental composition by EDS of copper sulfate phases identified in Figure 6.
In Figure 7, the morphology of sulfide minerals present in the cured sample is displayed. According to the EDS results shown in Table 3, the analyzed regions correspond to pyrite and covellite. The pyrite particle exhibits a compact and defined structure, reflecting its chemical stability under the applied curing conditions. Covellite was identified in two different zones, characterized by high copper and sulfur content, and minimal oxygen, suggesting that it remained mostly unaltered. The identification of covellite over chalcocite may be attributed to a transformation during pretreatment or to the preferential preservation of covellite. The surface reactivity of species such as digenite could coincide with what was previously identified in the study by Quezada et al [19]. The authors propose a semi-reaction associated with the first stage of the classical mechanism of chalcocite dissolution. Thus, the mechanism proposed by Cheng and Lawson in 1991 [20] could be the basis for this proposal. The identification of covellite in the present study coincides with the proposal of Quezada et al. [19] in accordance with the following reaction:

Figure 7.
Analysis through FESEM (1: Pyrite; 2, 3: Covellite).

Table 3.
Elemental composition by EDS of pyrite and covellite phases identified in Figure 7.
3.3. X-Ray Photoelectron Spectroscopy (XPS)
To identify the copper species present on the surface of the pretreated sample, a spectroscopic analysis was performed for oxygen (O), sulfur (S), copper (Cu), and arsenic (As). The results are presented in Figure 8, Figure 9, Figure 10 and Figure 11.
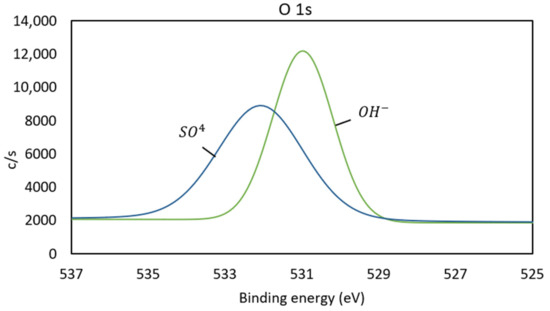
Figure 8.
Spectrum O 1s of pretreated copper sample.
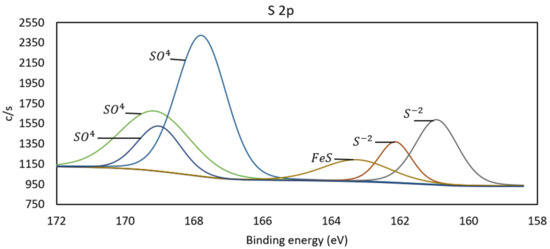
Figure 9.
Spectrum S 2p of pretreated copper sample.
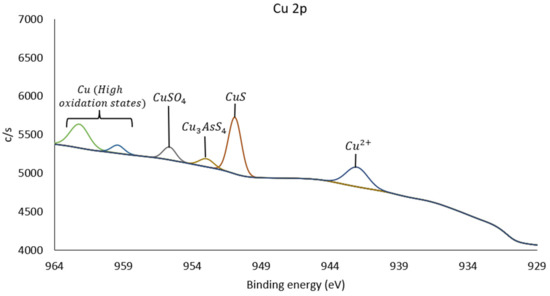
Figure 10.
Spectrum Cu 2p of pretreated copper sample.
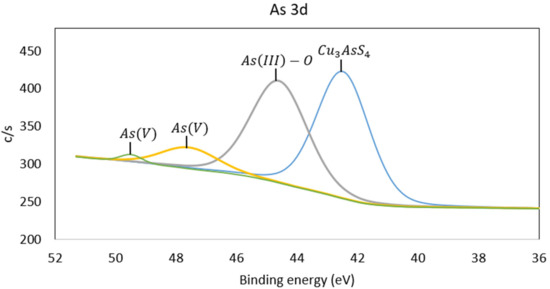
Figure 11.
As sublevel 3d spectrum of pretreated copper sample.
In Figure 8, the O 1s spectrum is shown, where peaks at 531.0 and 532.1 eV are identified. The peak at 531.0 eV is associated with the presence of hydroxides (OH) [21], which could indicate the formation of species such as antlerite. The binding energy at 532.1 eV is associated with oxygen in the form of SO42− [22], which confirms the formation of sulfates on the surface of the pretreated sample.
The S 2p spectrum is presented in Figure 9. Peaks are identified at 160.9, 162.1, 163.3, 167.8, 169.0, and 169.2 eV. Covellite (CuS) has an energy for S 2p between 161.9 and 162.9 eV, so the peaks at 160.9 and 162.1 eV are associated with sulfur, likely from a copper sulfide mineral such as CuS [23]. The sulfur at 163.3 eV is associated with Fe-S minerals, such as pyrite, which is identified in the sample through X-ray diffraction and Scanning Electron Microscopy [9]. The high-energy peaks at 167.8, 169.0, and 169.2 eV are associated with the presence of sulfur in sulfates, specifically copper sulfates in this sample [23,24]. Notably, the peak at 169.0 eV is related to antlerite, which is consistent with the O 1s spectrum at an energy of 531.0 eV [24].
In Figure 10, the spectrum for Cu 2p is presented, where peaks are identified at 942.1, 950.9, 953.0, 955.7, 959.4, and 962.2 eV. The bands found at higher energies correspond to higher oxidation states, indicating that copper in its metallic state is found at lower binding energies, specifically Cu at 932.6 eV. When oxidized, its binding energy increases to 933.7 eV (CuO) [24]. The lowest energy band found in this sample corresponds to the Cu 2p3/2 peak at 942.1 eV, indicating the presence of Cu2+ [9,25]. As oxidation increases in the sample, minerals such as covellite, which has an energy peak at 951.8 eV, can be identified. Therefore, the energy peak at 950.9 eV is associated with a copper species in the form of CuS or something similar. Enargite shows a Cu 2p1/2 peak at 952.0 eV, and tennantite at 952.6 eV, while its S 2p peak is found at 161.8 eV. Thus, the peak at 953.0 eV is associated with a species similar in composition to minerals such as enargite and tennantite [24]. The binding energy of 955.7 eV is associated with copper sulfate, while the signals observed at 959.4 and 962.2 eV correspond to copper species in even higher oxidation states. The specific identification of these species in the literature is limited due to their unusually high binding energies, which may be related to the complexity of the sample resulting from its pretreatment.
In Figure 11, the As sublevel 3d spectrum is presented. Four peaks can be observed at 42.5, 44.6, 47.6, and 49.5 eV. One of the arsenic peaks in enargite is at 42.6 eV, so the 42.5 eV peak can be related to this mineral due to the amount present in the sample [26]. The 44.6 eV peak corresponds to As(III)-O species [27]. A higher binding energy corresponds to a higher oxidation state of arsenic, such as As2O5, which has an energy peak at 46.2 eV. Therefore, the energy peaks at 47.6 and 49.5 eV are associated with arsenic as As(V) [24].
4. Conclusions
As an alternative treatment for sulfide copper ores by hydrometallurgy, acid curing offers the possibility of generating copper sulfates, which increases the solubility of the ore and consequently reduces leaching times. Three techniques were used to characterize a sample of copper concentrate pretreated with acid curing for 15 days with 210 kg/t H2SO4 at 50 °C, achieving a copper extraction of 26.71% during the pretreatment. The mineralogical characterization of the sample was performed using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and X-ray photoelectron spectroscopy (XPS).
Copper sulfate species were identified by XRD in the form of chalcanthite (CuSO4·5H2O), copper sulfate monohydrate (CuSO4⸱H2O), and copper sulfate (CuSO4). The extracted copper likely corresponds to the chemical transformation of minerals such as bornite and chalcocite, while minerals such as chalcopyrite and enargite show no significant changes in their composition.
The morphology of the cured sample was analyzed using FESEM, which allowed the identification of copper sulfate species in most of the sample, except for enargite particles, which showed signs of initial oxidation. This suggests that stronger oxidative conditions are required to achieve complete oxidation. Furthermore, the absence of chalcocite and bornite particles indicates that they reacted to form copper sulfates.
Spectroscopic analysis results confirmed the presence of copper sulfates, with energy peaks in O 1s at 531.0 eV and 532.1 eV, Cu 2p at 942.1 eV, and S 2p at 169.0 eV, as well as 169.2 eV and 167.8 eV. Copper sulfates were also identified in the form of covellite (CuS) with an S 2p peak at 162.1 eV, and minerals such as enargite and tennantite with Cu 2p peaks at 953.0 eV and As 3d peaks at 42.5 eV, along with As (III)-O species at 44.6 eV. The results of acid-curing pretreatment not only benefitted copper extraction, but also provided an alternative to keep arsenic stable in solution. The conversion of copper sulfide minerals such as bornite and chalcocite to copper sulfates was also achieved, which leads to increasing efficiency of copper recovery. At the same time, the signs of oxidation observed in the enargite particles indicate that more oxidizing conditions with longer pre-treatment times could benefit the treatment of this mineral by hydrometallurgy, further improving the feasibility of this process in the treatment of sulfide ores.
Author Contributions
Formal analysis, G.V.S.; Funding acquisition, V.Q.; Methodology, G.V.S., V.Q., A.B.-T., and N.L.-I.; Project administration, V.Q.; Software, G.V.S. and O.R.-A.; Validation, G.V.S., and V.Q.; Writing—review and editing, G.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC was funded by Agencia Nacional de Investigación y Desarrollo (ANID), Proyecto Fondecyt de Iniciación 2023, grant number 11230963.
Data Availability Statement
Date are contained within the article.
Acknowledgments
Oriol Rius-Ayra is Serra Húnter Fellow.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
References
- Safarzadeh, M.S.; Miller, J.D. International Journal of Mineral Processing Acid bake—Leach process for the treatment of arsenopyrite, tennantite, and tetrahedrite. Int. J. Miner. Process. 2013, 124, 128–131. [Google Scholar] [CrossRef]
- COCHILCO RPI. Proyección de la Producción de Cobre en Chile 2023–2034; Comisión Chilena del Cobre (Cochilco): Santiago, Chile, 2024. [Google Scholar]
- COCHILCO RPI. Proyección de la Producción de Cobre en Chile 2022–2033; Comisión Chilena del Cobre (Cochilco): Santiago, Chile, 2022. [Google Scholar]
- Mandal, B.K.; Suzuki, T. Arsenic round the world: A review. Talanta 2002, 57, 201–235. [Google Scholar] [CrossRef]
- Taylor, P.R.; Putra, T.A.R. Pyrometallurgical Processing Technologies for Treating High Arsenic Copper Concentrates; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–198. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Wang, G.; Yang, H. Copper leaching from complex chalcopyrite-rich ores: Utilizing mechanical activation and wastewater-based sulfuric acid system. Sep. Purif. Technol. 2025, 354, 128631. [Google Scholar] [CrossRef]
- Vargas, T.; Rojas, F.; Bahamondez, C.; Castro, R.; Ihle, C.F.; Caraballo, M. Physical and chemical transformations of gangue materials during leaching of copper sulphides, and their influence on copper leaching kinetics. J. S. Afr. Inst. Min. Met. 2017, 117, 727–730. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. the effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, E.; Jamett, N.E.; Ghorbani, Y. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals 2017, 8, 1. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl–H2SO4–O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Melo, E.; Hernández, M. Pretreatment to leaching for a primary copper sulphide ore in chloride media. Metals 2021, 11, 1260. [Google Scholar] [CrossRef]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. Hydrometallurgy Acid bake-leach process for the treatment of enargite concentrates. Hydrometallurgy 2012, 119–120, 30–39. [Google Scholar] [CrossRef]
- Quezada, V.; Villagrán, G.; Calisaya-Azpilcueta, D.; Marín, N. Effect of Pretreatment on a Copper Concentrate with High Arsenic. Minerals 2024, 14, 419. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth-Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Quezada, V.; Zepeda, S.; Benavente, O.; Hernández, M.; Melo, E. Effect of curing time and ferric chloride on a copper concentrate with a high arsenic content. Minerals 2024, 14, 1063. [Google Scholar] [CrossRef]
- Young, C.L.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution. Chem. Eng. J. 2013, 228, 731–740. [Google Scholar] [CrossRef]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Electron Spectrosc. Relat. Phenom. 2007, 156–158, 310–314. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray photoelectron spectroscopic study of copper minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Waltham, MA, USA, 1992. [Google Scholar]
- Zhao, H.; Huang, X.; Hu, M.; Zhang, C.; Zhang, Y.; Wang, J.; Qin, W.; Qiu, G. Insights into the surface transformation and electrochemical dissolution process of bornite in bioleaching. Minerals 2018, 8, 173. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Borda, L.; Bia, G.; Borgnino, L.; Chiaramonte, N.; García, M.G. Understanding arsenic-ulexite interactions in evaporite environments: Evidence from XRPD, micro-XRF, micro-FT-IR, and XPS studies. J. Hazard. Mater. 2024, 473, 134547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).