Critical Metal Potential of Tasmanian Greisen: Lithium, Rare Earth Elements, and Bismuth Distribution and Implications for Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Geochemistry
2.3. Methods
2.3.1. Comminution—Electric Pulse Fragmentation (EPF)
2.3.2. Mineralogy—Scanning Electron Microscope (SEM)-Based Automated Mineralogy
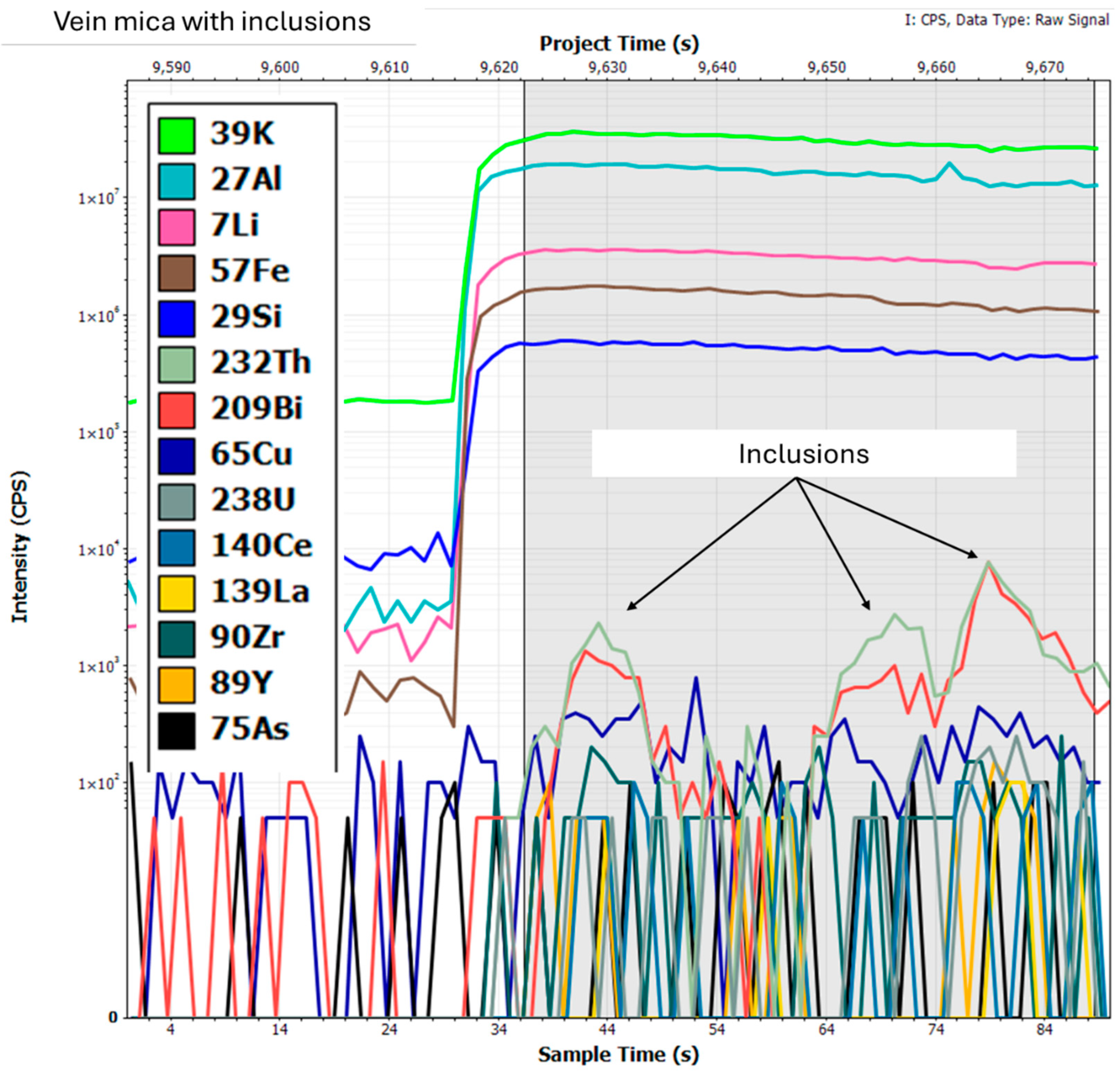
2.3.3. Element Distribution—LA ICP-MS
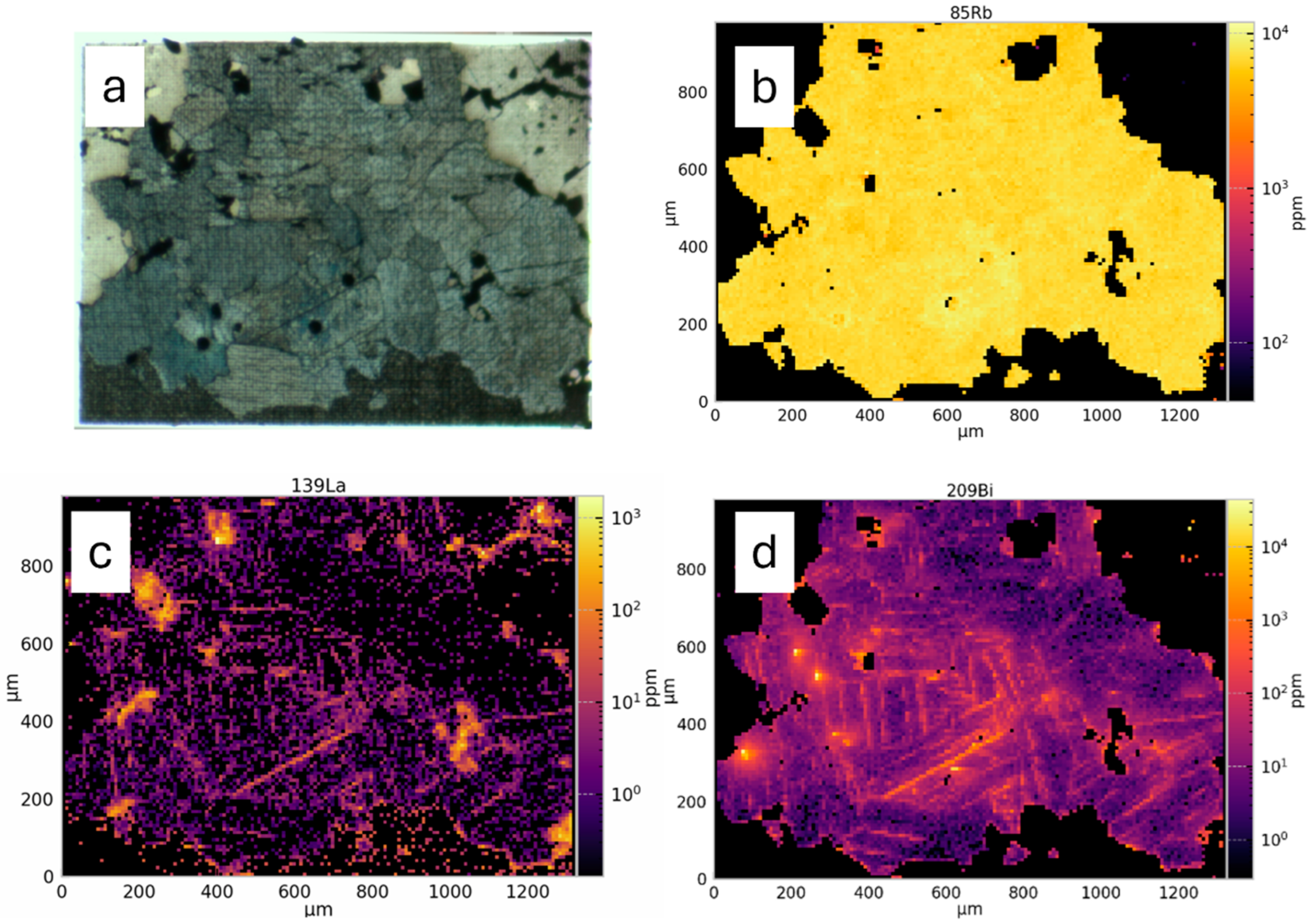
2.3.4. Element Mapping—LA ICP-TOF-MS
3. Results
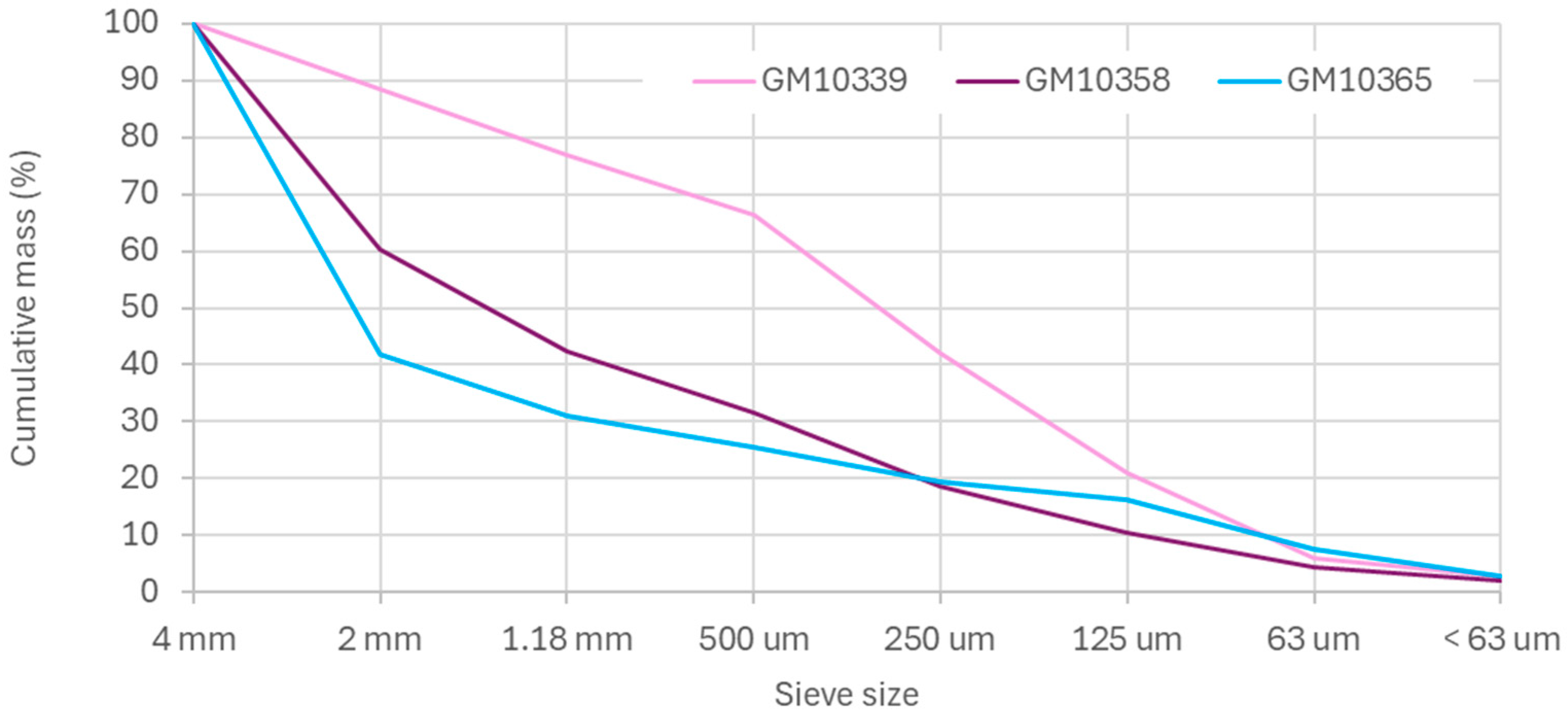
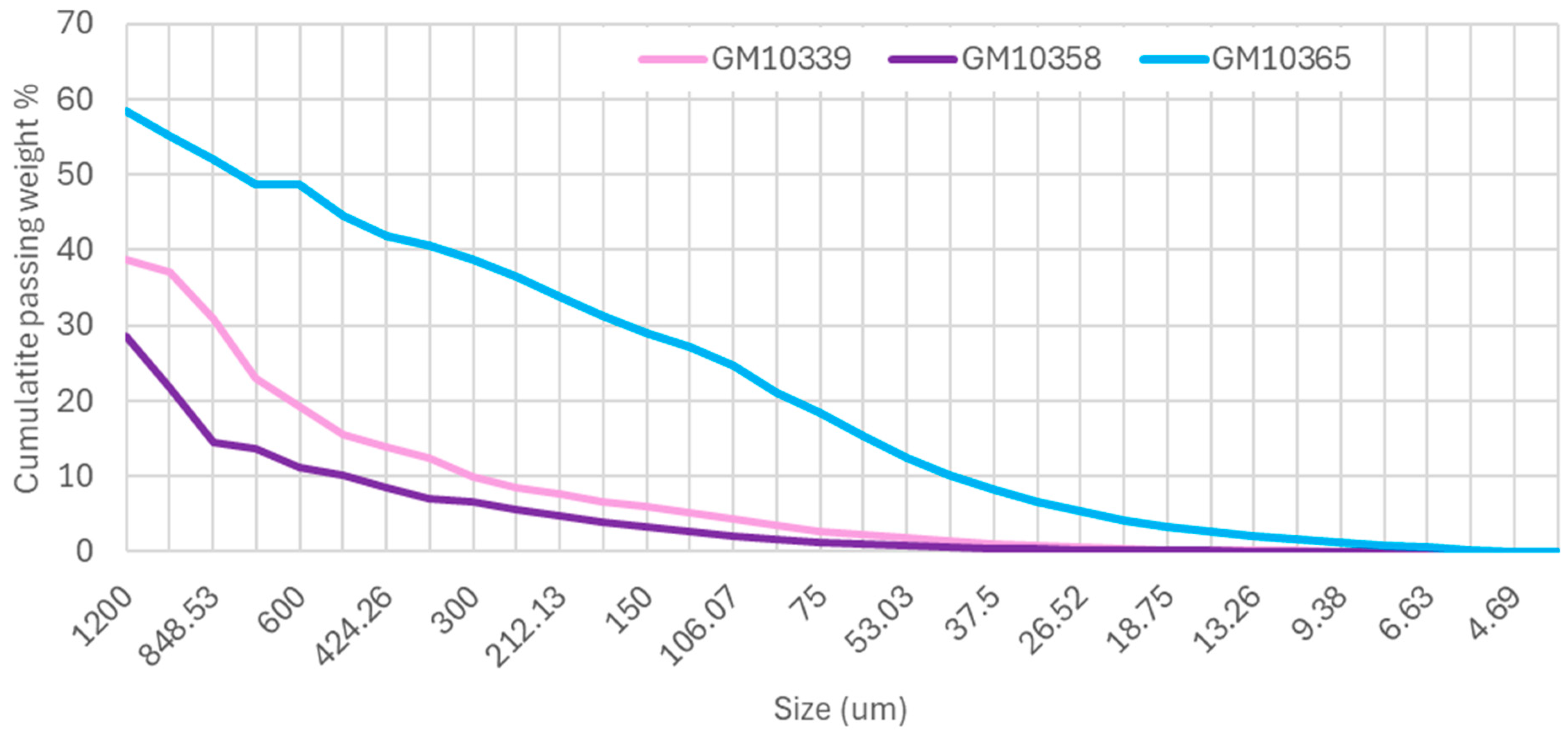
3.1. Electric Pulse Fragmentation (EPF)
3.2. Mineralogy—Abundance, Liberation, and Association
3.2.1. Abundance
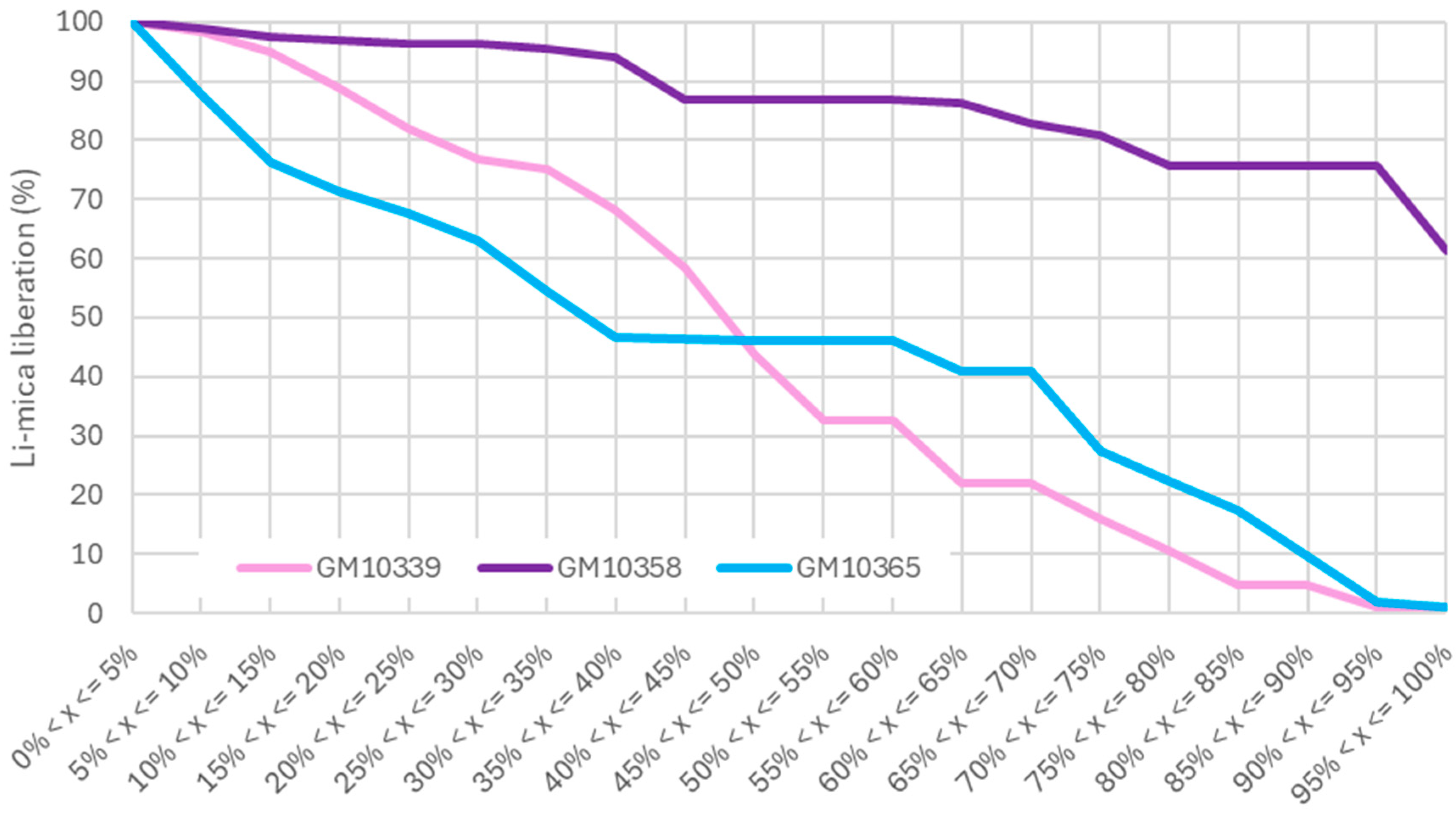
3.2.2. Liberation
3.2.3. Association
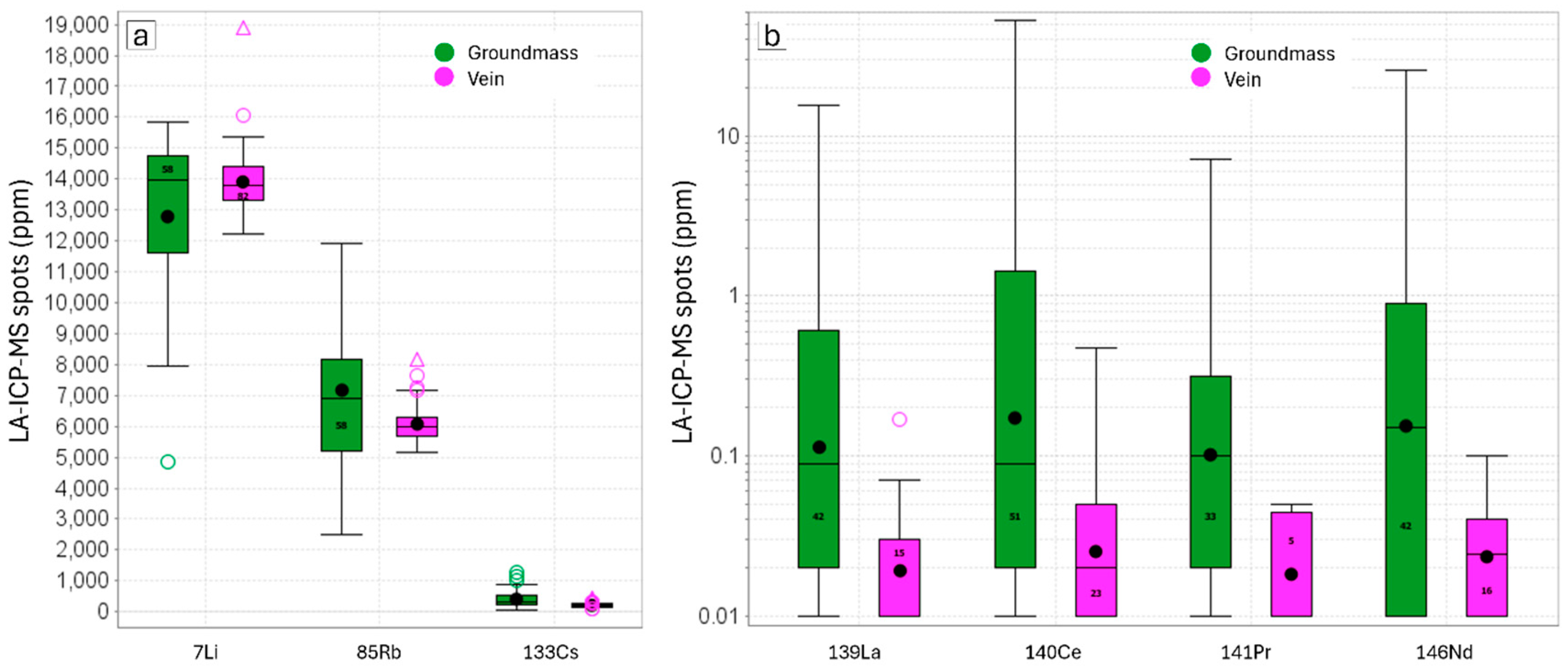
3.3. Element Distribution
3.3.1. SEM-AMICS Data
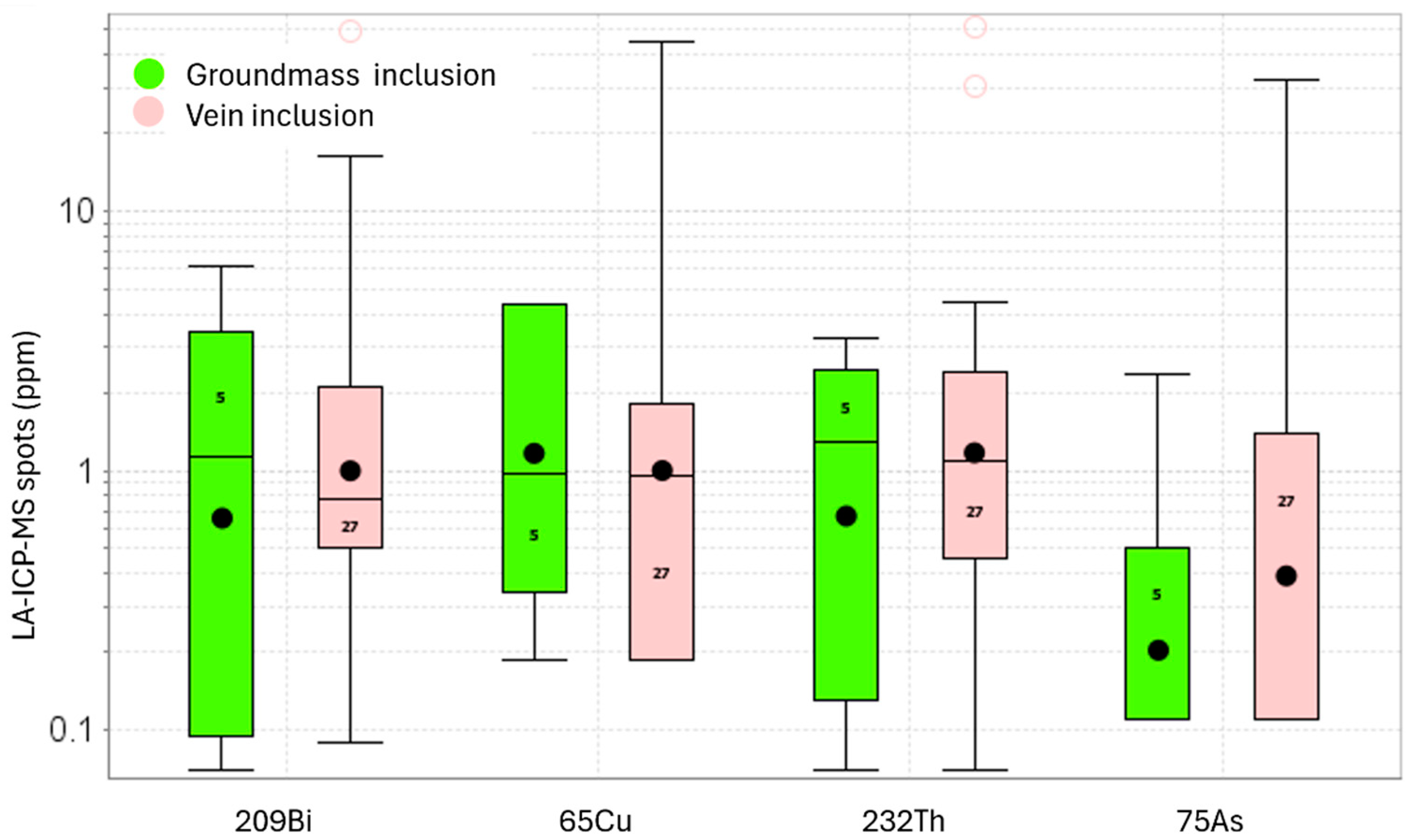
3.3.2. LA-ICP-MS Data
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pehlken, A.; Albach, S.; Vogt, T. Is there a resource constraint related to lithium ion batteries in cars? Int. J. Life Cycle Assess. 2017, 22, 40–53. [Google Scholar] [CrossRef]
- Brunelli, K.; Lee, L.; Moerenhout, T. Fact Sheet: Lithium Supply in the Energy Transition. Centre on Global Energy Policy Website. Available online: https://www.energypolicy.columbia.edu/publications/fact-sheet-lithium-supply-in-the-energy-transition/ (accessed on 3 March 2025).
- Critical Minerals at Geoscience Australia. Geoscience Australia Website. Available online: https://www.ga.gov.au/scientific-topics/minerals/critical-minerals (accessed on 2 December 2024).
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, Publications Office of the European Union: Luxembourg, 2003; ISBN 978-92-68-00414-2. [Google Scholar] [CrossRef]
- List of Critical Minerals. United States Geological Survey Website. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 2 December 2024).
- Cinovec Lithium Project Update. European Metals Holdings Limited Website. Available online: https://api.investi.com.au/api/announcements/emh/387cfa8b-b66.html (accessed on 2 December 2024).
- Zinnwald Lithium Project. Zinnwald Lithium Website. Available online: https://zinnwaldlithium.com/project/the-resource/ (accessed on 2 December 2024).
- Announcement May 2023, Testwork Realizes Continued Outstanding Lithium Recoveries. European Metals Holdings Limited Website. Available online: https://www.europeanmet.com/announcements/ (accessed on 3 March 2025).
- Process Metallurgy. British Lithium Website. Available online: https://imerysbritishlithium.com/process-metallurgy/ (accessed on 1 April 2025).
- Andres, U.; Jirestig, J.; Timoshkin, I. Liberation of minerals by high-voltage electrical pulses. Powder Technol. 1999, 104, 37–49. [Google Scholar] [CrossRef]
- Ménard, Y. Energy Savings in Comminution—Innovative Routes for Mineral Ores Embrittlement. Contribution to Deliverable 4.4—Promine Project (FP7). BRGM/RP-60127-FR. 2011. 36p. Available online: https://infoterre.brgm.fr/rapports/RP-60127-FR.pdf (accessed on 1 April 2025).
- Sperner, B.; Jonckheere, R.; Pfänder, J.A. Testing the influence of high-voltage mineral liberation on grain size, shape and yield, and on fission track and 40Ar/39Ar dating. Chem. Geol. 2014, 371, 83–95. [Google Scholar] [CrossRef]
- Rudashevsky, N.S.; Weiblen, P.W.; Stoynov, H.; Saini-Eidukat, B. Products of electric pulse desaggregation of some Keweenawan rocks. In Proceedings of the Institute on Lake Superior Geology 41st Annual Meeting, Marathon, ON, Canada, 13–18 May 1995; Volume 41, p. 61. [Google Scholar]
- Saini-Eidukat, B.; Weiblen, W. A New Method of Fossil Preparation, Using High-Voltage Electric Pulses. Curator 1996, 39, 139–144. Available online: https://www.ndsu.edu/pubweb/~sainieid/PPD/Saini-Eidukat-and-Weiblen-Liberation-of-Fossils-using-Electric-Pulses.pdf (accessed on 1 April 2025). [CrossRef]
- Andres, U. Development and prospects of mineral liberation by electrical pulses. Int. J. Miner. Process. 2010, 97, 31–38. [Google Scholar] [CrossRef]
- Wang, E.; Shi, F.; Manlapig, E. Pre-weakening of mineral ores by high voltage pulses. Miner. Eng. 2011, 24, 455–462. [Google Scholar] [CrossRef]
- Wang, E.; Shi, F.; Manlapig, E. Mineral Liberation by High Voltage Pulses and Conventional Comminution with Same Specific Energy Levels. Miner. Eng. 2012, 27–28, 28–36. [Google Scholar] [CrossRef]
- van der Wielen, K.P.; Pascoe, R.; Weh, A.; Wall, F.; Rollinson, G. The influence of equipment settings and rock properties on high voltage breakage. Miner. Eng. 2013, 46–47, 100–111. [Google Scholar] [CrossRef]
- Lyons, R.J.P. The Aberfoyle vein system, Rossarden, Tasmania. In Proceedings of the AUSIMM, Sydney, Australia, 10–15 August 1957; No. 181. pp. 75–91. Available online: https://www.ausimm.com/publications/conference-proceedings/the-ausimm-proceedings-1957/the-aberfoyle-vein-system-rossarden-tasmania/ (accessed on 2 December 2024).
- Seymour, D.B.; Green, G.R.; Calver, C.R. The Geology and Mineral Deposits of Tasmania: A Summary; Mineral Resources Tasmania Geological Survey Bulletin: Hobart, Australia, 2006. [Google Scholar]
- TinOne Resources Inc. Aberfoyle Tin Project. Available online: https://tinone.ca/ (accessed on 8 October 2024).
- Müller, A.; Herklotz, G.; Giegling, H. Chemistry of quartz related to the Zinnwald/Cínovec Sn-W-Li greisen-type deposit, Eastern Erzgebirge, Germany. J. Geochem. Explor. 2018, 190, 357–373. [Google Scholar] [CrossRef]
- High Voltage Pulse Power Machines. SELFRAG Website. Available online: https://www.selfrag.com/high-voltage-pulse-power-machines (accessed on 3 March 2024).
- AMICS Automated Mineralogy System for SEM. Bruker Website. Available online: https://www.bruker.com/en/products-and-solutions/elemental-analyzers/eds-wds-ebsd-SEM-Micro-XRF/software-amics-automated-mineralogy-system.html (accessed on 3 March 2024).
- Jochum, K.P. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- GeoReM Preferred Values. GeoRem Website. Available online: http://georem.mpch-mainz.gwdg.de/ (accessed on 24 September 2024).
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note—Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Norris, A.; Danyushevsky, L. Towards estimating the complete uncertainty budget of quantified results measured by LA-ICP-MS. In Proceedings of the Goldschmidt Conference, Boston, MA, USA, 12–17 August 2018. [Google Scholar]
- Ghosh, U.; Upadhyay, D.; Mishra, B.; Abhinay, K. In-situ trace element and Li-isotope study of zinnwaldite from the Degana tungsten deposit, India: Implications for hydrothermal tungsten mineralization. Chem. Geol. 2023, 632, 121550. [Google Scholar] [CrossRef]
- Breiter, K.; Hložková, M.; Korbelová, Z.; Galiová, M.V. Diversity of lithium mica compositions in mineralized granite-greisen system: Cinovec Li-Sn-W deposit, Erzgebirge. Ore Geol. Rev. 2019, 106, 12–27. [Google Scholar] [CrossRef]
- Technologies Leading the Next Lithium Cycle. Lepidico Website. Available online: https://lepidico.com/technology (accessed on 2 December 2024).
- Processing Technology. Cornish Lithium Website. Available online: https://cornishlithium.com/projects/lithium-in-hard-rock/processing-technology/ (accessed on 2 December 2024).
- Mende, R.; Kaiser, D.; Pavon, S.; Bertau, M. The COOL process: A holistic approach towards lithium recycling. Waste Biomass Valorization 2023, 14, 3027–3042. [Google Scholar] [CrossRef]



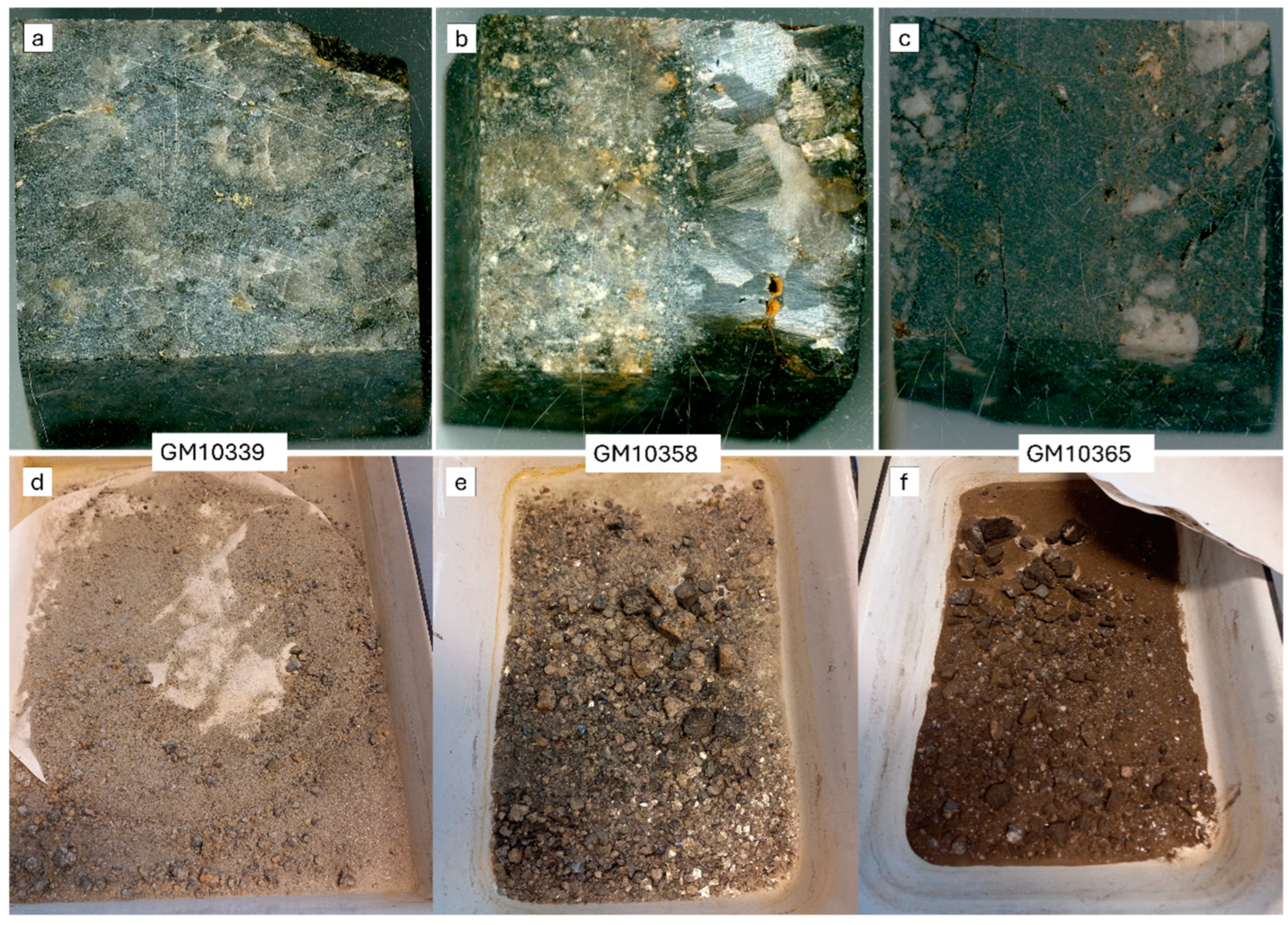


| Sample | Voltage (kV) | Electrode Gap (mm) | Pulse Rate (Hz) | Number of Pulses |
|---|---|---|---|---|
| GM10339 | 120 | 30 | 5 | 60 |
| GM10358 | 120 | 30 | 5 | 30 |
| GM10365 | 200 | 40 | 5 | 10 |
| Range | 90 to 200 | 10 to 40 | 1 to 5 | 1 to >1000 |
| Sieve Size % Mass | GM10339 | GM10358 | GM10365 |
|---|---|---|---|
| 4 mm | 11.5 | 39.7 | 58.2 |
| 2 mm | 11.7 | 17.8 | 10.8 |
| 1.18 mm | 10.4 | 10.9 | 5.5 |
| 500 μm | 24.3 | 12.8 | 6.1 |
| 250 μm | 21.2 | 8.2 | 3.2 |
| 125 μm | 14.8 | 6 | 8.8 |
| 63 μm | 3.4 | 2.3 | 4.7 |
| <63 μm | 2.7 | 2.1 | 2.7 |
| Mineral (Weight %) | GM10339 | GM10358 | GM10365 |
|---|---|---|---|
| Zinnwaldite | 27.43 | 42.28 | 12.25 |
| Quartz | 59.3 | 48.88 | 37.24 |
| Topaz | 11.53 | 7.81 | 1.68 |
| Tourmaline (schorl) | 0.01 | 0 | 44.16 |
| Muscovite-illite | 0.26 | 0.27 | 3.06 |
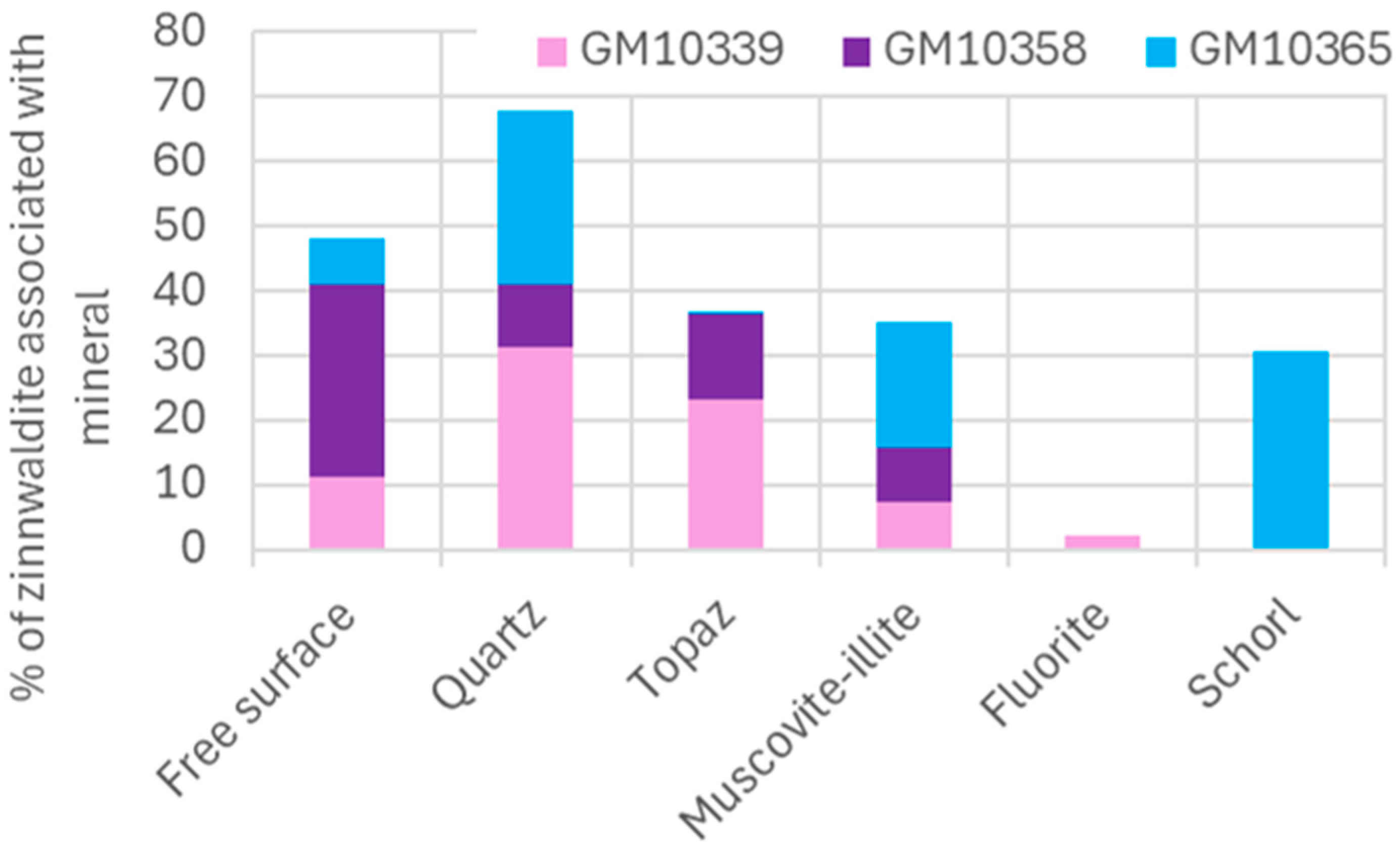
| Sample | Free Surface | Quartz | Topaz | Muscovite-Illite | Fluorite | Tourmaline (Schorl) |
|---|---|---|---|---|---|---|
| GM10339 | 11.19 | 31.37 | 23.31 | 7.40 | 2.15 | 0.08 |
| GM10358 | 29.74 | 9.58 | 13.14 | 8.47 | 0 | 0.08 |
| GM10365 | 7.06 | 26.99 | 0.45 | 19.47 | 0 | 30.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, J.; Oalmann, J.; Aâtach, M.; Pirard, E.; Fulton, R.; Feig, S. Critical Metal Potential of Tasmanian Greisen: Lithium, Rare Earth Elements, and Bismuth Distribution and Implications for Processing. Minerals 2025, 15, 462. https://doi.org/10.3390/min15050462
Hunt J, Oalmann J, Aâtach M, Pirard E, Fulton R, Feig S. Critical Metal Potential of Tasmanian Greisen: Lithium, Rare Earth Elements, and Bismuth Distribution and Implications for Processing. Minerals. 2025; 15(5):462. https://doi.org/10.3390/min15050462
Chicago/Turabian StyleHunt, Julie, Jeffrey Oalmann, Mohamed Aâtach, Eric Pirard, Russell Fulton, and Sandrin Feig. 2025. "Critical Metal Potential of Tasmanian Greisen: Lithium, Rare Earth Elements, and Bismuth Distribution and Implications for Processing" Minerals 15, no. 5: 462. https://doi.org/10.3390/min15050462
APA StyleHunt, J., Oalmann, J., Aâtach, M., Pirard, E., Fulton, R., & Feig, S. (2025). Critical Metal Potential of Tasmanian Greisen: Lithium, Rare Earth Elements, and Bismuth Distribution and Implications for Processing. Minerals, 15(5), 462. https://doi.org/10.3390/min15050462








