4.1. Petrography and Geochemistry of Metavolcanic Rocks
The sulfide mineralization in the Xylagani area is hosted in metamorphosed mafic lava and pyroclastics of Jurassic age, which consist of chlorite-, sericite-, tremolite-, actinolite-, and epidote-greenschists (
Figure 3a–c). They belong to the Makri unit, which represents the upper crustal section of the Evros ophiolite in the CRB at Thrace.
These rocks are foliated on a macro-to-microscopic scale and are sometimes extensively altered, especially when they are associated with mineralization. They often show pillow structures (
Figure 3a), while primary textures, such as porphyritic, subophitic, or amygdaloidal textures (
Figure 3b,c), are well preserved. Based on textural and mineralogical features, the metavolcanic rocks can be subdivided into pyroxene-phyric lava, aphyric-oligophyric lava, and albite-rich lava.
The secondary minerals observed in metavolcanic rocks, such as quartz, chlorite, sericite, prehnite, pumpellyite, epidote, zoisite, titanite, actinolite, and calcite, are characteristic of very low-to-low-grade metamorphism, from prehnite–pumpellyite to greenschist facies. Frequently, volcanic rocks are rich in vesicles filled with quartz, prehnite, pumpellyite, chlorite, and calcite, forming characteristic amygdaloidal textures (
Figure 3d–f). Silicification is the main alteration feature resulting from the interaction of hydrothermal solutions with lava during the ore-forming process. In addition to silicification, the devitrification and chloritization of volcanic glass also occur. Sporadically, massive milky quartz veins were observed, crosscutting the metavolcanic rocks and their foliation. These veins range in width from 0.5 to 5 cm and are believed to have formed during the orogenic metamorphism that accompanied the Alpine Orogeny.
Major and trace elements were determined for six representative samples of the metavolcanic rocks that are related with the mineralization, and the results are presented in
Table 1. Concentrations of SiO
2 (48.60–59.00 wt%), Na
2O+K
2O (1.81–5.23 wt%), CaO (4.09–8.86 wt%), MgO (6.46–13.89 wt%), and Fe
2O
3 (6.51–9.45 wt%) show a broad distribution, while TiO
2 (0.28–0.53 wt%), Zr (17–20 ppm) and Y (9.7–13.8 ppm) are low.
The metavolcanic rocks of Xylagani are characterized by relatively high contents of Cu (15–1090 ppm), Zn (40–4145 ppm), Cr (29–240 ppm), V (177–272 ppm), and Ni (37–105 ppm). The abundance of rare earth elements (REEs) (
Table 1) and chondrite-normalized patterns of the metavolcanic rocks (
Figure 4) have also been used to interpret their eruptive setting. The REE concentrations are low (2 to 11 times chondritic values), with their low total content (ΣREE) ranging from 10.38 to 39.34 ppm.
4.2. Types of Mineralization
Field observations show that the mineralization at Xylagani, which is concentrated mainly around Mylorema Creek near the Nea Petra village (
Figure 2), occurs in the form of silicified bodies hosted in the metavolcanic rocks. Over 20 ore outcrops occur in the area, and local residents report that this mineralization was exploited by a French–Italian company between 1900 and 1910. The intense mining activity in the area is witnessed by 23 inactive underground galleries and surface workings, along with stockpiles of mined material (
Figure 5a,b). These works were probably exploiting pyrite and chalcopyrite ore. The galleries, which have a length of up to 120 m, are flooded, and entry to most of them is rather difficult. Remnants of mining installations or ore concentration facilities have not been found in the region, indicating that the ore was likely transported out of the mined area.
The main ore bodies are up to 100 m long and more than 10 m thick and cover an area of ~2 km2. They form lenses or layers, which are conformable with the foliation of the associated metavolcanic rocks at different stratigraphic levels and exhibit a stratabound or stratiform structure. The mineralization is disseminated at the edge and is massive compared to the core of the ore bodies. Disseminated mineralization also occurs in the host rocks close to the ore bodies. Stockworks of stringer-type ore were not observed in the area.
Based on textural features, five types of mineralization are recognized: (1) Thin layers, consisting mainly of pyrite, aligned parallel to the bedding and occasionally folded (
Figure 5c) with thicknesses of 0.5 to 3 mm. (2) All the studied silicified ore bodies contain disseminated sulfides (<10–20%), mainly pyrite. Significant disseminated sulfide enrichment also occurs in altered metavolcanics. This is the most widespread type of mineralization. (3) Semi-massive mineralization occurs in the central parts of lenses and contains about 50 to 80% sulfides, mainly pyrite and chalcopyrite (
Figure 5d). (4) Massive mineralization is characterized by a very high sulfide content (80%) and is subdivided into two types: massive pyrite and massive chalcopyrite–pyrite mineralization. Massive pyrite occurs as lenses, up to 30 cm long and 10 cm thick, forming stratabound mineralization (
Figure 5e) or as layers, extending up to 10 m in length and 5 cm in thickness. Massive chalcopyrite–pyrite mineralization occurs as layers (
Figure 5f) with a length of up to 10 m and a thickness of up to 10 cm, forming stratiform mineralization. (5) Milky quartz veins have a low sulfide content, crosscutting metavolcanic rocks and their foliation.
Chemical analyses from the different mineralization types in the Xylagani area (
Table 2;
Supplementary Table S1) showed that the studied ore bodies demonstrate a Cu concentration ranging from 132 ppm to 19.00 wt%. In contrast, Zn and Pb are lower, with contents reaching 4290 ppm and 5190 ppm, respectively. The contents of As, Co, and Ni in the mineralized bodies are relatively low, up to 248 ppm, 333 ppm, and 13 ppm, respectively. The ore bodies contain values of Au between 0.1 and 6.4 ppm, with an average of 3.5 ppm (
n = 28). The highest average Au concentration (5 ppm) is found in semi-massive mineralization. In addition, the concentration of Ag reaches 54 ppm, with the highest content in massive chalcopyrite–pyrite mineralization.
4.3. Ore Mineralogy and Alteration Minerals
The main ore minerals in the mineralization at Xylagani are pyrite and chalcopyrite, with sphalerite as the minor phase. Pyrrhotite, galena, tennantite-(Zn), and gold occur in traces (
Figure 6). Secondary iron and copper minerals, such as goethite, malachite, and covellite, are rare. Quartz, sericite, and chlorite are the most common alteration minerals. The ore mineral assemblages, characterizing the different mineralization types, are presented in
Table 3, and the microprobe analyses of the ore minerals are represented in
Table 4 and
Supplementary Table S2.
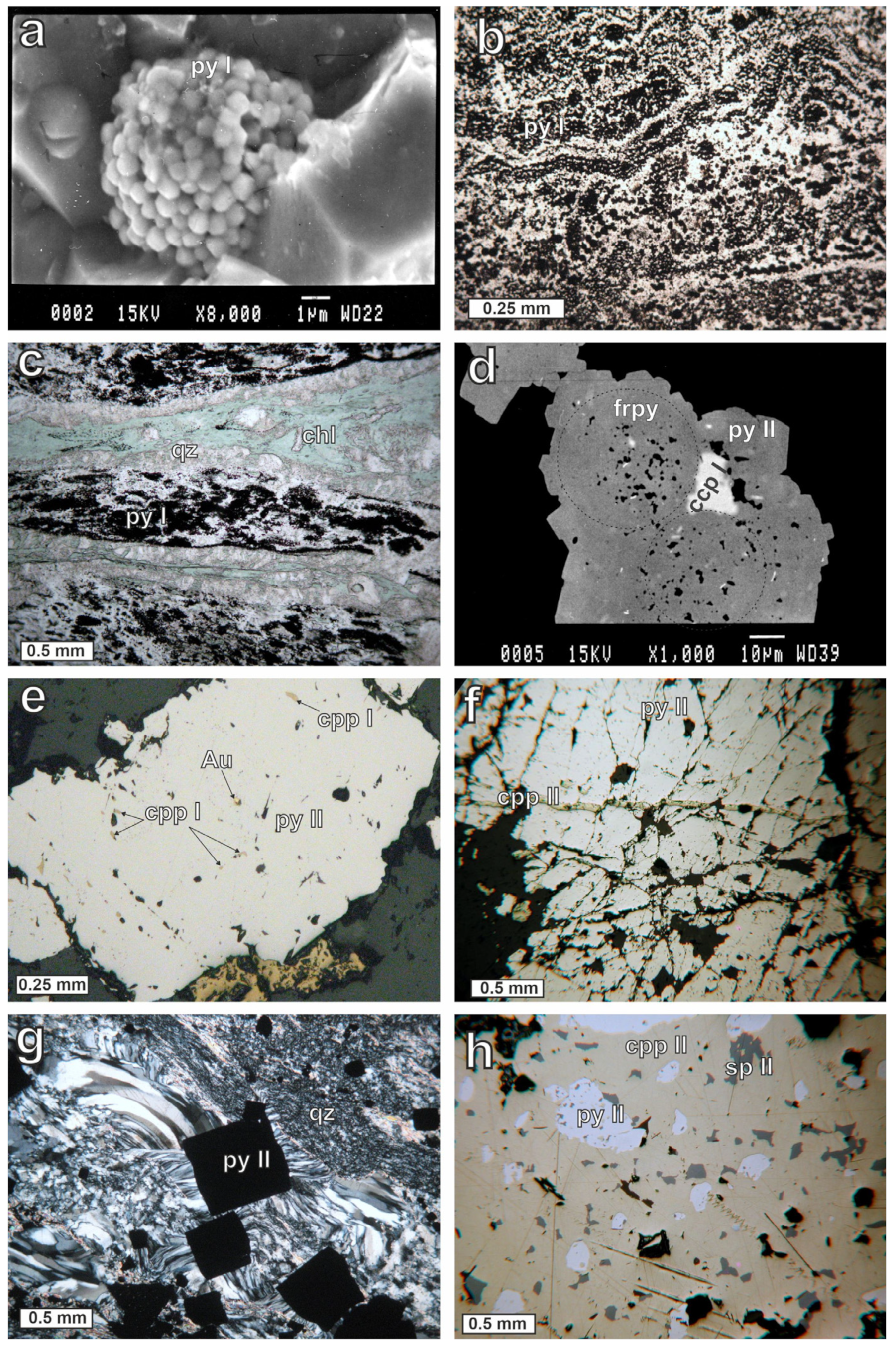
Pyrite is the predominant sulfide of all ore types during mineralization and occurs in three generations (py I, py II, py III). Framboidal and fine-grained pyrite was found in the thin layers and in the disseminated and semi-massive ore types and represents the early generation (py I); it does not contain inclusions of other sulfides. The framboids (
Figure 7a) are found either isolated or in clusters within fine-grained quartz. Thin layers consisting of framboidal pyrite, parallel to the host rock bedding with a thickness of about 100 μm, have also been observed in a matrix of hydrothermal quartz and chlorite (
Figure 7b,c). The framboids are spherical and, more rarely, oval-shaped, ranging from 3 to 70 μm across. In some cases, they display a concentric internal arrangement of microcrystals. Microprobe analyses of py I (
Table 4;
Supplementary Table S2) showed As contents ranging from 0.07 to 0.91 wt% (0.33 wt% average), corresponding to a chemical formula of Fe
1.00(S,As)
2.00.
Following the deposition of framboidal and fine-grained pyrite, the second generation of pyrite (py II) displays coarse-grained euhedral and subhedral textures with various sizes of up to 5 mm. Frequently, a growth of py II in the form of radiating, subhedral pyrite and thin pyritic zones occurs around framboids (
Figure 7d). This second pyrite generation also forms veinlets crosscutting the framboidal layers. Within some pyrite grains, well-preserved outlines of framboidal pyrite (
Figure 7d) suggest that coarse pyrite (py II) postdates the formation of py I. The second pyrite generation is characterized by inclusions of other sulfides, such as chalcopyrite and sphalerite, and less abundant pyrrhotite, tennantite-(Zn), and gold (
Figure 7e). These inclusions range between 5 and 200 μm across and are rounded, oval-shaped, or irregular. The cataclastic deformation of pyrite is common, with chalcopyrite and quartz filling the cracks and fractures of pyrite (
Figure 7f). Quartz fibers are usually developed in pressure fringes around euhedral pyrite (py II) (
Figure 7g).
The second generation of pyrite (py II) shows lower As contents than py I and up to 0.37 wt% (0.09 wt% on average). Gold reaches 0.17 wt% (0.06 wt% on average). The average chemical formula of py II corresponds to Fe
1.00S
2.00. The Co and Ni concentrations of pure pyrite (py II) from the massive pyrite ore type (
Table 5), ranged from 59 to 70 ppm and from 16 to 19 ppm, respectively. The Co/Ni ratios range from 3.11 to 3.94. Arsenic varies from 291 to 299 ppm, Cu varies from 262 to 338 ppm, Pb varies from 51 to 53 ppm, and Au varies from 1.7 to 1.9 ppm. The third generation of pyrite (py III) contains even less As than the older generations, up to 0.16 wt% (0.06 wt% on average). Gold is also lower, reaching 0.06 wt%. The average chemical formula of py III is Fe
1.00S
2.00.
The most abundant sulfide after pyrite is chalcopyrite, which occurs mainly as disseminated anhedral grains ranging between 5 μm and 1 mm across. It may be massive and locally very abundant, mainly in the semi-massive and the massive chalcopyrite–pyrite ore types. It is intergrown mainly with pyrite and sphalerite and less frequently with pyrrhotite and galena. Chalcopyrite occurs in three generations. The first early generation (ccp I) appears as interstitial fillings among pyrite framboids and as inclusions within pyrite (
Figure 7d,e).
Figure 7d demonstrates a good example of early-formed chalcopyrite that was crystallized after framboidal pyrite (py I) and before coarse subhedral pyrite (py II). The abundance of chalcopyrite blebs (<1 μm) in sphalerite indicates “chalcopyrite disease”, and they belong to the first chalcopyrite generation (ccp I). Often, these blebs are orientated along dislocations in sphalerite.
The second generation of chalcopyrite (ccp II), which is more widespread in mineralization, appears as disseminated grains, void fillings in quartz, veinlets in pyrite, and massive layers parallel to the foliation of the host rocks. The chalcopyrite veinlets crosscutting pyrite grains (
Figure 7f) indicate that part of chalcopyrite was formed later than the main pyrite deposition (py II). The third generation of chalcopyrite (ccp III) occurs in the milky quartz veins and is intergrown with pyrite, sphalerite, galena, and tennantite-(Zn). Chalcopyrite is quite homogeneous in composition (
Table 4,
Supplementary Table S2). In ccp II, Co and Ni concentrations are low (up to 0.05 wt%), whereas As and Au reach 0.16 and 0.13 wt%, respectively. Variable Se values were detected in chalcopyrite at up to 0.16 wt%. In ccp III, Ni reaches up to 0.03 wt%, As reaches up to 0.11 wt%, Au reaches up to 0.02 wt%, and Se reaches up to 0.41 wt%. Chalcopyrite has stoichiometric average chemical formulae: Cu
1.00Fe
1.00S
2.00 for ccp II and Cu
1.00Fe
0.99S
2.01 for ccp III.
Pyrrhotite occurs exclusively as discrete minute inclusions (5 to 200 μm) in pyrite, and in some cases, it is intergrown with chalcopyrite. Microprobe analyses (
Table 4,
Supplementary Table S2) revealed that the composition of pyrrhotite varies within a narrow range from 60.46 to 61.05 wt% Fe and is homogeneous within individual grains. The calculated chemical formula is Fe
0.90S
1.00.
Sphalerite is found in anhedral grains up to 1 mm and occurs in three generations (sp I, sp II, and sp III). Sphalerite I contains fine blebs of chalcopyrite (chalcopyrite disease). Sphalerite II is intergrown with pyrite and chalcopyrite (
Figure 7h), while the third generation of sphalerite (sp III) occurs in milky quartz veins, and is intergrown with pyrite, chalcopyrite, galena, and tennantite-(Zn). Sphalerite (
Table 4,
Supplementary Table S2) of all generations has a similar chemical composition and is characterized by low contents in Cd (up to 0.25 wt%) and Mn (up to 0.09 wt%) and variable Fe concentrations (0.16–3.88 wt%; 0 to 6.45 mole% FeS). The average chemical composition of the analyzed sphalerite is (Zn, Fe)
1.00S
1.00.
Galena has been identified in traces in massive chalcopyrite–pyrite mineralization (gn I) and in milky quartz veins (gn II). It occurs as rounded or elongated inclusions within chalcopyrite and pyrite. Silver contents reach 0.19 wt%, and the highest concentrations (0.17 wt% on average) are confined to the massive chalcopyrite–pyrite ore type. With respect to Se contents, two chemically distinct types of galena exist (
Table 4,
Supplementary Table S2): one with a higher Se concentration (1.10 to 1.68 wt%) in massive chalcopyrite–pyrite mineralization (gn I), and the other with lower Se contents (up to 0.42 wt%) hosted in quartz veins (gn II). These two variations in galena correspond to the chemical formulae Pb
0.99(S,Se)
1.01 and Pb
1.00(S,Se)
1.00, respectively.
Tennantite-(Zn), the only identified sulfosalt phase at Xylagani, is rare and occurs in disseminated and semi-massive mineralization (tn I) and in quartz veins (tn II). It is associated with pyrite, sphalerite, galena, and chalcopyrite, and the size of the grains range between 10 and 300 μm. Microprobe analyses (
Table 4,
Supplementary Table S2) revealed that Ag is low, reaching 0.35 wt% in tn I and 0.77 wt% in tn II. The average chemical formula is (Cu,Ag,Cd)
9.91(Fe,Zn)
2.01(As,Sb)
4.09(S,Se)
13.00 for tn I, and (Cu, Ag,Cd)
9.72(Fe,Zn)
2.16(As,Sb)
4.11(S,Se)
13.01 for tn II. Both tennantite types are characterized as tennantite-(Zn) due to the elevated Zn content, which is 6.13 wt% (1.41 apfu) for tn I and 7.16 wt% (1.65 apfu) for tn II.
Native gold occurs in pyrite from the semi-massive ore type and forms elongated, angular, or rounded grains of up to 20 μm across (
Figure 7e). Data obtained from microprobe analyses (
Table 6) show that Ag contents range between 1.94 and 5.94 wt% and for Cu from 0.13 to 0.26 wt%, while Bi rises in one case up to 0.24 wt%. The average chemical formula is (Au,Ag,Cu,Bi)
1.00.
Mineralization is associated with extensive hydrothermal alterations, e.g., silicification and chloritization, which often envelopes the mineralized lenses and replace volcanic rocks. Alteration intensity increases from the margins toward the center of the mineralized zones, progressing from weak to pervasive, with complete replacement of the host rocks by quartz, chlorite, sericite, and sulfides.
Hydrothermal quartz, the main host of the ore minerals, shows a variety of textures. Most commonly, it is polycrystalline with adulatory extinction and suturing of the contacts between crystals, implying extended recrystallization. In many cases, brittle deformation has produced a cataclastic texture. One of the most common textures is the development of quartz fibers around euhedral pyrite crystals, forming pressure fringes (
Figure 7g). Hydrothermal white mica (sericite) and chlorite were deposited with quartz (
Figure 7c), and all were interpreted as syngenetic with sulfide mineralization.
Thirty microanalyses of hydrothermal chlorite from the mineralization at Xylagani (
Supplementary Table S3) are plotted on the classification diagram of [
23] and fall in the compositional field of ripidolite and pycnochlore (
Figure 8). The contents of the calculated Al
IV range between 1.926 and 2.714 atoms per formula unit (apfu), and the Fe/(Fe + Mg) ratio varies from 0.322 to 0.420 (
Figure 8).
Chlorite composition is related to formation conditions, e.g., temperature, pressure, redox conditions, and fluid and bulk-rock composition [
24]. One of the most significant factors is temperature, and various methods have been proposed to determine it based on the chemical composition of chlorite. The most common methods use the empirical geothermometer that relates the temperature of the formation to the tetrahedral aluminum (Al
IV) content of chlorite [
25,
26] or the semi-empirical geothermometer that incorporates the influence of the Fe/(Fe + Mg) ratio in addition to the Al
IV content [
27]. The application of both geothermometers at the hydrothermal chlorites of the mineralization at Xylagani yields similar temperatures to chlorite formation. The empirical thermometer of Cathelineau and Nieva [
25] yielded temperatures between 222 and 301 °C, and the semi-empirical thermometer of Zang and Fyfe [
27] yielded temperatures from 222 to 306 °C.