Effects of Fracturing Fluids on Properties of Shale Reservoir: A Case Study of the Longmaxi Formation in the Sichuan Basin
Abstract
1. Introduction
2. Materials and Samples
3. Methods
3.1. X-Ray Diffraction Analysis
3.2. Porosity and Permeability Measurement
3.3. Nuclear Magnetic Resonance Experiment
3.4. Nitrogen Adsorption-Desorption
3.5. X-Ray Computed Tomography Scanning
4. Results and Discussion
4.1. Fracturing Fluid Infiltration
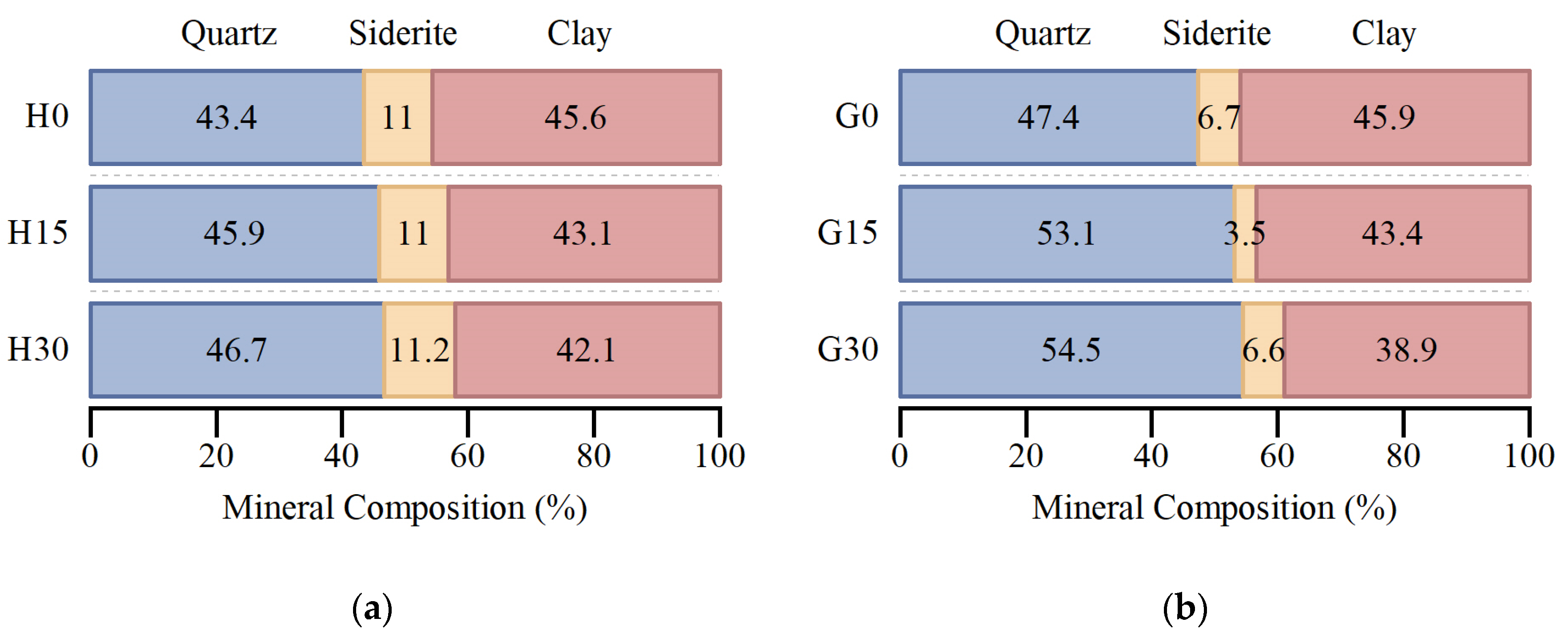
4.2. Changes in Mineral Composition
4.3. Changes in Porosity and Permeability
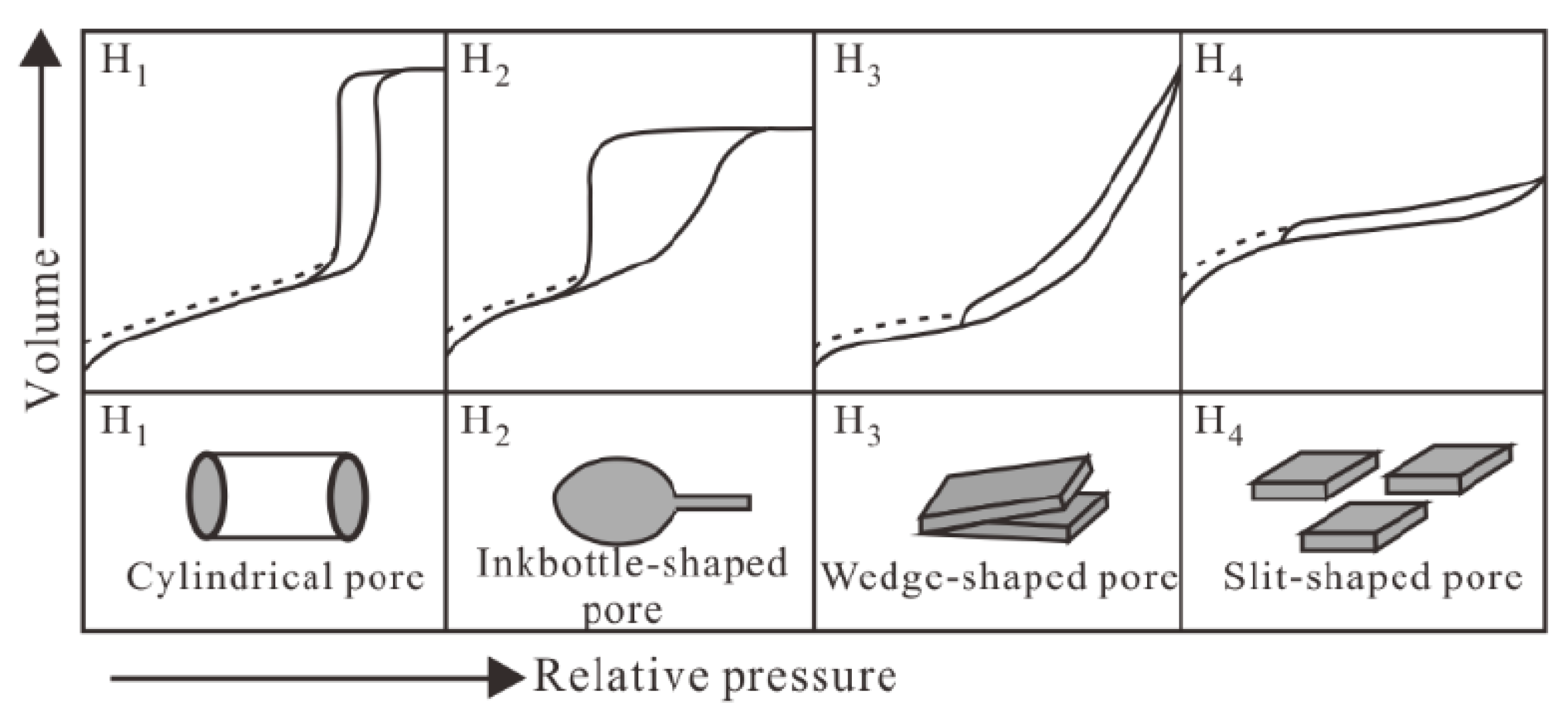
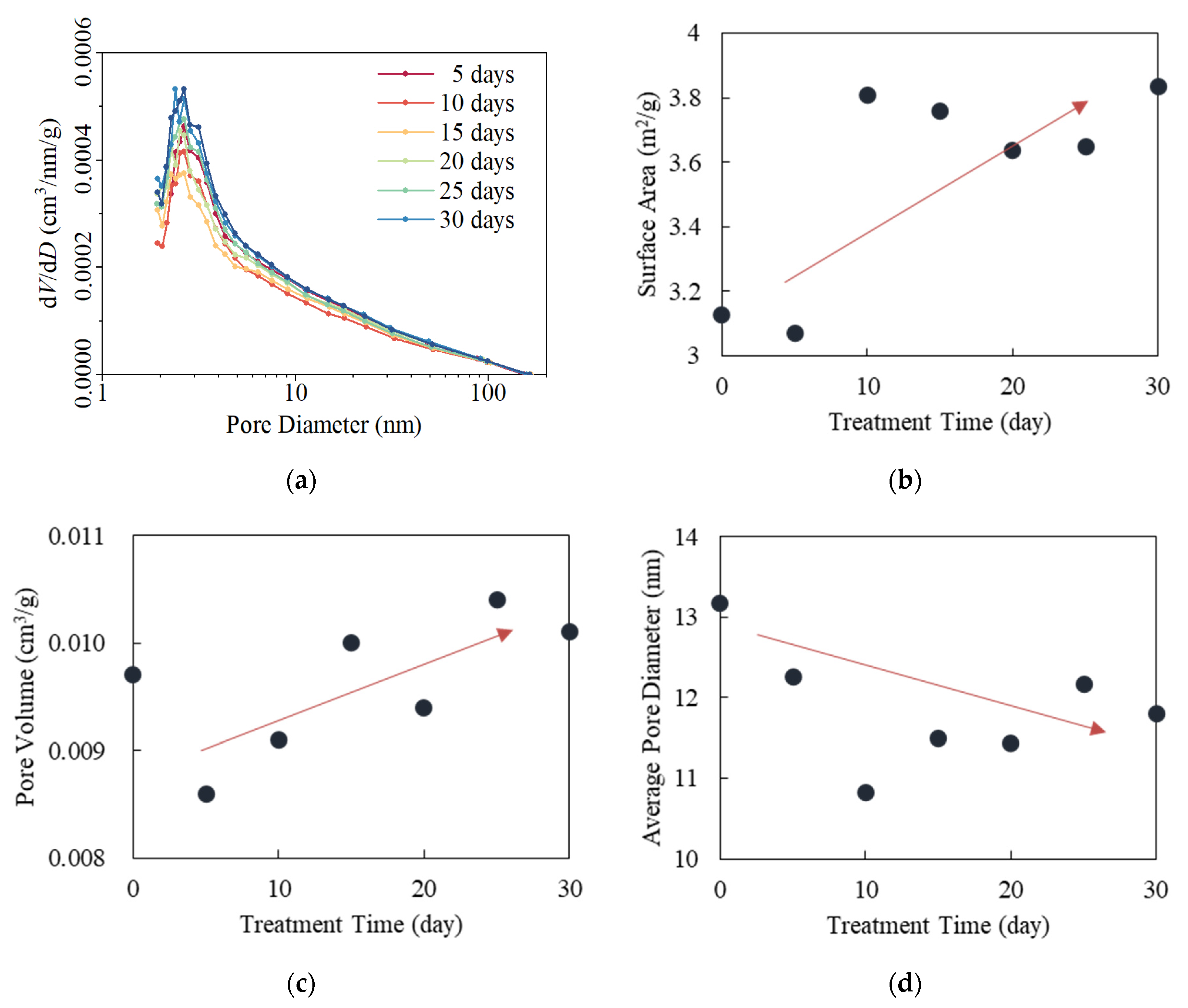
4.4. Changes in Pore Structure of Shale Mesopores
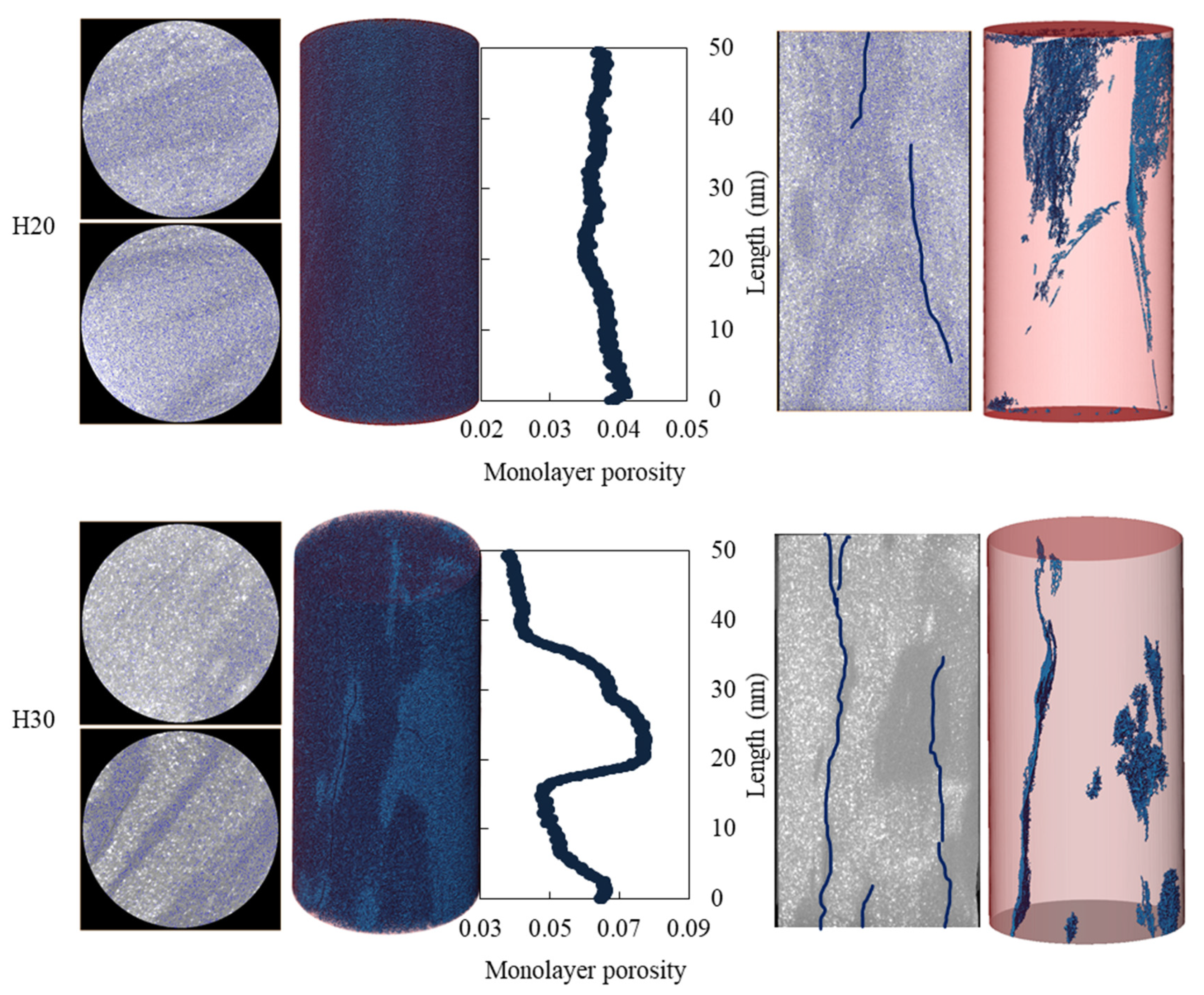
4.5. Changes in Pore Structure of Shale Macropores and Fractures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Wang, Y.; Wang, F.; Wu, J.; Usman Tahir, M.; Li, Q.; Yuan, L.; Liu, Z. Effect of thickener and reservoir parameters on the filtration property of CO2 fracturing fluid. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1705–1715. [Google Scholar]
- Li, Q.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control strategy. J Ocean. Eng. 2024, 297, 117029. [Google Scholar]
- Liu, H.; Huang, Y.; Cai, M.; Meng, S.; Tao, J. Practice and development suggestions of hydraulic fracturing technology in the Gulong shale oil reservoirs of Songliao Basin, NE China. Pet. Explor. Dev. 2023, 50, 688–698. [Google Scholar]
- Binnion, M. How the technical differences between shale gas and conventional gas projects lead to a new business model being required to be successful. Mar. Pet. Geol. 2012, 31, 3–7. [Google Scholar]
- Davies, R.J.; Almond, S.; Ward, R.S.; Jackson, R.B.; Adams, C.; Worrall, F.; Herringshaw, L.G.; Gluyas, J.G.; Whitehead, M.A. Oil and gas wells and their integrity: Implications for shale and unconventional resource exploitation. Mar. Pet. Geol. 2014, 56, 239–254. [Google Scholar]
- Mayerhofer, M.J.; Wolhart, S.L.; Rogers, J.D. Results of U.S. Department of Energy Deep Gas Well Stimulation Study. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005. [Google Scholar]
- Lin, A.; Ma, J. Stimulated-rock characteristics and behavior in multistage hydraulic-fracturing treatment. SPE J. 2015, 20, 784–789. [Google Scholar]
- Ibrahim, A.F. Optimizing cluster spacing in multistage hydraulically fractured shale gas wells: Balancing fracture interference and stress shadow impact. J. Pet. Explor. Prod. Technol. 2024, 14, 2297–2313. [Google Scholar]
- Nicot, J.-P.; Scanlon, B.R. Water Use for Shale-Gas Production in Texas, U.S. Environ. Sci. Technol. 2012, 46, 3580–3586. [Google Scholar]
- Nicot, J.-P.; Scanlon, B.R.; Reedy, R.C.; Costley, R.A. Source and fate of hydraulic fracturing water in the Barnett Shale: A historical perspective. Environ. Sci. Technol. 2014, 48, 2464–2471. [Google Scholar]
- Jiang, M.; Hendrickson, C.T.; VanBriesen, J.M. Life Cycle Water Consumption and Wastewater Generation Impacts of a Marcellus Shale Gas Well. Environ. Sci. Technol. 2014, 48, 1911–1920. [Google Scholar]
- Gao, J.; Zou, C.; Zhang, X.; Guo, W.; Yu, R.; Ni, Y.; Liu, D.; Kang, L.; Liu, Y.; Kondash, A.; et al. The water footprint of hydraulic fracturing for shale gas extraction in China. Sci. Total Environ. 2024, 907, 168135. [Google Scholar]
- Barati, R.; Liang, J.-T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Fakcharoenphol, P.; Kurtoglu, B.; Kazemi, H.; Charoenwongsa, S.; Wu, Y.-S. The effect of osmotic pressure on improve oil recovery from fractured shale formations. In Proceedings of the SPE Unconventional Resources Conference/Gas Technology Symposium, The Woodlands, TX, USA, 1–3 April 2014; SPE: St. Bellingham, WA, USA, 2014; p. D021S003R004. [Google Scholar]
- Dustin, M.K.; Bargar, J.R.; Jew, A.D.; Harrison, A.L.; Joe-Wong, C.; Thomas, D.L.; Brown, G.E., Jr.; Maher, K.J.E. Shale kerogen: Hydraulic fracturing fluid interactions and contaminant release. Energy Fuels 2018, 32, 8966–8977. [Google Scholar]
- McAdams, B.; Burrows, L.; Hakala, J.A. Abiotic Transformation Kinetics of Surfactants used in Hydraulic Fracturing Fluid. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Denver, CO, USA, 22–24 July 2019. [Google Scholar]
- Makhanov, K.; Habibi, A.; Dehghanpour, H.; Kuru, E. Liquid uptake of gas shales: A workflow to estimate water loss during shut-in periods after fracturing operations. J. Unconv. Oil Gas Resour. 2014, 7, 22–32. [Google Scholar]
- Ghanbari, E.; Dehghanpour, H. Impact of rock fabric on water imbibition and salt diffusion in gas shales. Int. J. Coal Geol. 2015, 138, 55–67. [Google Scholar]
- Li, Q.; Jew, A.D.; Kohli, A.; Maher, K.; Brown, G.E., Jr.; Bargar, J.R. Thicknesses of Chemically Altered Zones in Shale Matrices Resulting from Interactions with Hydraulic Fracturing Fluid. Energy Fuels 2019, 33, 6878–6889. [Google Scholar]
- Herz-Thyhsen, R.J.; Kaszuba, J.P.; Dewey, J.C. Mineral dissolution and precipitation induced by hydraulic fracturing of a mudstone and a tight sandstone in the Powder River Basin, Wyoming, USA. Appl. Geochem. 2020, 119, 104636. [Google Scholar]
- Renock, D.; Landis, J.D.; Sharma, M. Reductive weathering of black shale and release of barium during hydraulic fracturing. Appl. Geochem. 2016, 65, 73–86. [Google Scholar]
- Kreisserman, Y.; Emmanuel, S. Release of Particulate Iron Sulfide during Shale-Fluid Interaction. Environ. Sci. Technol. 2018, 52, 638–643. [Google Scholar] [PubMed]
- Hakala, J.A.; Phan, T.; Stuckman, M.; Edenborn, H.; Lopano, C. Role of Organic Acids in Controlling Mineral Scale Formation During Hydraulic Fracturing at the Marcellus Shale Energy and Environmental Laboratory (MSEEL) Site. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Austin, TX, USA, 24–26 July 2017. [Google Scholar]
- Hakala, J.A.; Crandall, D.; Moore, J.; Phan, T.; Sharma, S.; Lopano, C. Laboratory-Scale Studies on Chemical Reactions Between Fracturing Fluid and Shale Core From the Marcellus Shale Energy and Environmental Laboratory (MSEEL) Site. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Austin, TX, USA, 24–26 July 2017. [Google Scholar]
- Xiong, W.; Lopano, C.; Hakala, A.; Carney, B. Investigation of Barite Scaling During Reaction between Pre-Treated Hydraulic Fracturing Fluid from the Field and Marcellus Shale. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Virtual, 20–22 July 2020. [Google Scholar]
- Harrison, A.L.; Jew, A.D.; Dustin, M.K.; Thomas, D.L.; Joe-Wong, C.M.; Bargar, J.R.; Johnson, N.; Brown, G.E., Jr.; Maher, K. Element release and reaction-induced porosity alteration during shale-hydraulic fracturing fluid interactions. Appl. Geochem. 2017, 82, 47–62. [Google Scholar]
- O’Brien, N.R.; Slatt, R.M. Pore types in the Barnett and Woodford gas shales: Contribution to understanding gas storage and migration pathways in fine-grained rocks. AAPG Bull. 2011, 95, 2017–2030. [Google Scholar]
- Ross, D.J.K.; Marc Bustin, R. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar]
- Hubert, E.; King, J.; Eberle, A.P.R.; Walters, C.C.; Kliewer, C.E.; Ertas, D.; Huynh, C. Pore Architecture and Connectivity in Gas Shale. Energy Fuels 2015, 29, 1375–1390. [Google Scholar]
- Josh, M.; Esteban, L.; Delle Piane, C.; Sarout, J.; Dewhurst, D.N.; Clennell, M.B. Laboratory characterisation of shale properties. J. Pet. Sci. Eng. 2012, 88–89, 107–124. [Google Scholar]
- Kuila, U.; McCarty, D.; Derkowski, A.; Fischer, T.; Topor, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar]
- Clarkson, C.; Solano, N.; Bustin, R.; Bustin, A.; Chalmers, G.; He, L.; Melnichenko, Y.; Radlinski, A.; Blach, T. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar]
- Veselinovic, D.; Green, D.; Dick, M. Determination of Natural Fracture Porosity Using NMR. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, San Antonio, TX, USA, 1–3 August 2016. [Google Scholar]
- Song, Y.-Q.; Kausik, R. NMR application in unconventional shale reservoirs—A new porous media research frontier. Prog. Nucl. Magn. Reason. Spectrosc. 2019, 112, 17–33. [Google Scholar]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar]
- Schmitt, M.; Fernandes, C.P.; da Cunha Neto, J.A.B.; Wolf, F.G.; dos Santos, V.S.S. Characterization of pore systems in seal rocks using Nitrogen Gas Adsorption combined with Mercury Injection Capillary Pressure techniques. Mar. Pet. Geol. 2013, 39, 138–149. [Google Scholar]
- Liang, L.; Xiong, J.; Liu, X. An investigation of the fractal characteristics of the Upper Ordovician Wufeng Formation shale using nitrogen adsorption analysis. J. Nat. Gas Sci. Eng. 2015, 27 Pt 2, 402–409. [Google Scholar]
- Liu, X.; Xiong, J.; Liang, L. Investigation of pore structure and fractal characteristics of organic-rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. J. Nat. Gas Sci. Eng. 2015, 22, 62–72. [Google Scholar]
- Zou, Y.; Zhang, S.; Zhou, T.; Zhou, X.; Guo, T. Experimental Investigation into Hydraulic Fracture Network Propagation in Gas Shales Using CT Scanning Technology. Rock Mech. Rock Eng. 2016, 49, 33–45. [Google Scholar]
- Zhang, Y.; Yang, D. Simulation Study on the Heat Transfer Characteristics of Oil Shale under Different In Situ Pyrolysis Methods Based on CT Digital Rock Cores. Energies 2024, 17, 4169. [Google Scholar] [CrossRef]
- Cong, W.; Li, T.; Shi, Y.; Tang, C.A. Study on the Mechanical Properties and Failure Characteristics of Heterogenous Shale Based on CT Scanning. SPE J. 2024, 29, 7088–7107. [Google Scholar]
- Lei, W.; Wu, Z.; Song, H.; Wang, W.; Cui, H.; Tang, M. 3D Numerical Simulation Study of Brazilian Splitting in Calcite Vein-bearing Shale Based on CT Scans. KSCE J. Civ. Eng. 2024, 28, 2159–2172. [Google Scholar]
- Moore, J.; Xiong, W.; Lopano, C.; Phan, T.; Vankeuren, A.; Sharma, S.; Pilewski, J.; Jarvis, K.; Brown, S.; Crandall, D.; et al. Bench-Top Experiments Evaluating Simulated Hydraulic Fracturing Fluid Interactions With Marcellus Shale Core. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018. [Google Scholar]
- Elkady, Y.; Kovscek, A. Laboratory Visualization of Enhanced Gas Recovery in Shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020. [Google Scholar]
- Kim, T.W.; Kovscek, A. High-Temperature Imbibition for Enhanced Recovery from Diatomite. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 23–27 April 2017. [Google Scholar]
- Hascakir, B.; Ross, C.M.; Castanier, L.M.; Kovscek, A.R. Fuel Formation and Conversion During In-Situ Combustion of Crude Oil. SPE J. 2013, 18, 1217–1228. [Google Scholar]
- Sun, Z.; Ni, Y.; Wang, Y.; Wei, Z.; Wu, B.; Li, J.; Fan, W.; Wang, G.; Li, Y. Experimental investigation of the effects of different types of fracturing fluids on the pore structure characteristics of Shale Reservoir Rocks. Energy Explor. Exploit. 2020, 38, 682–702. [Google Scholar]
- Li, Y.; Feng, Y.; Liu, H.; Zhang, L.; Zhao, S. Geological characteristics and resource potential of lacustrine shale gas in the Sichuan Basin, SW China. Pet. Explor. Dev. 2013, 40, 454–460. [Google Scholar]
- Wang, Y.-M.; Wang, C.-F.; Dong, D.-Z.; Li, X.-J.; Huang, J.-L.; Zhang, C.-C.; Guan, Q.-Z. Lithofacies characterization of Longmaxi Formation of the Lower Silurian, southern Sichuan. Earth Sci. Front. 2016, 23, 119. [Google Scholar]
- Liu, G.; Tang, Y.; Liu, K.; Liu, Z.; Zhu, T.; Zou, Y.; Liu, X.; Yang, S.; Xie, A. Comparison of Pore Structure Characteristics of Shale-Oil and Tight-Oil Reservoirs in the Fengcheng Formation in Mahu Sag. Energies 2024, 17, 4027. [Google Scholar] [CrossRef]
- Tang, J.; Liu, Y.; Cheng, Q.; Jia, Y.; Lu, Y.; Xiao, Y.; Sun, X.; Liu, J. Comparative Study of Pore Structure Response in Longmaxi Marine Shale and Yanchang Continental Shale under the Combined Action of CO2 and Slickwater. Energy Fuels 2024, 38, 12718–12729. [Google Scholar]
- Wang, X.; Zhou, X. Research on quantitative analysis method of shale oil reservoir sensitivity based on mineral analysis. Geoenergy Sci. Eng. 2024, 239, 212952. [Google Scholar] [CrossRef]
- Fleury, M.; Kohler, E.; Norrant, F.; Gautier, S.; M’Hamdi, J.; Barré, L. Characterization and Quantification of Water in Smectites with Low-Field NMR. J. Phys. Chem. C 2013, 117, 4551–4560. [Google Scholar]
- Fleury, M.; Soualem, J. Quantitative analysis of diffusional pore coupling from T2-store-T2 NMR experiments. J. Colloid Interface Sci. 2009, 336, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Shi, J.; Zhao, F.; Duan, X. Characteristics of Fracturing Fluid Invasion Layer and Its Influence on Gas Production of Shale Gas Reservoirs. Energies 2023, 16, 3924. [Google Scholar] [CrossRef]
- Guo, P.; Li, X.; Li, S.; Mao, T. Combined Effect of In Situ Stress Level and Bedding Anisotropy on Hydraulic Fracture Vertical Growth in Deep Marine Shale Revealed via CT Scans and Acoustic Emission. Energies 2023, 16, 7270. [Google Scholar] [CrossRef]
- Kun, W.; Hangyu, Z.; Jie, L.; Kunjie, W.; Yin, L. Application of NMR technology in characterization of petrophysics and pore structure. Chin. J. Sci. Instrum. 2020, 41, 101–114. [Google Scholar]
- Fleury, M.; Romero-Sarmiento, M. Characterization of shales using T1–T2 NMR maps. J. Pet. Sci. Eng. 2016, 137, 55–62. [Google Scholar] [CrossRef]
- Katende, A.; O’Connell, L.G.; Rich, A.; Rutqvist, J.; Radonjic, M. A comprehensive review of proppant embedment in shale reservoirs: Experimentation, modeling and future prospects. J. Nat. Gas Sci. Eng. 2021, 95 (Suppl. C), 104143. [Google Scholar] [CrossRef]
- Dokhani, V.; Yu, M.; Bloys, B. A wellbore stability model for shale formations: Accounting for strength anisotropy and fluid induced instability. J. Nat. Gas Sci. Eng. 2016, 32, 174–184. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Han, S.; Wang, X.; Wang, L.; Yu, W.; Wang, Z. Compositional controls on pore-size distribution by nitrogen adsorption technique in the Lower Permian Shanxi Shales, Ordos Basin. J. Nat. Gas Sci. Eng. 2016, 34, 1369–1381. [Google Scholar] [CrossRef]



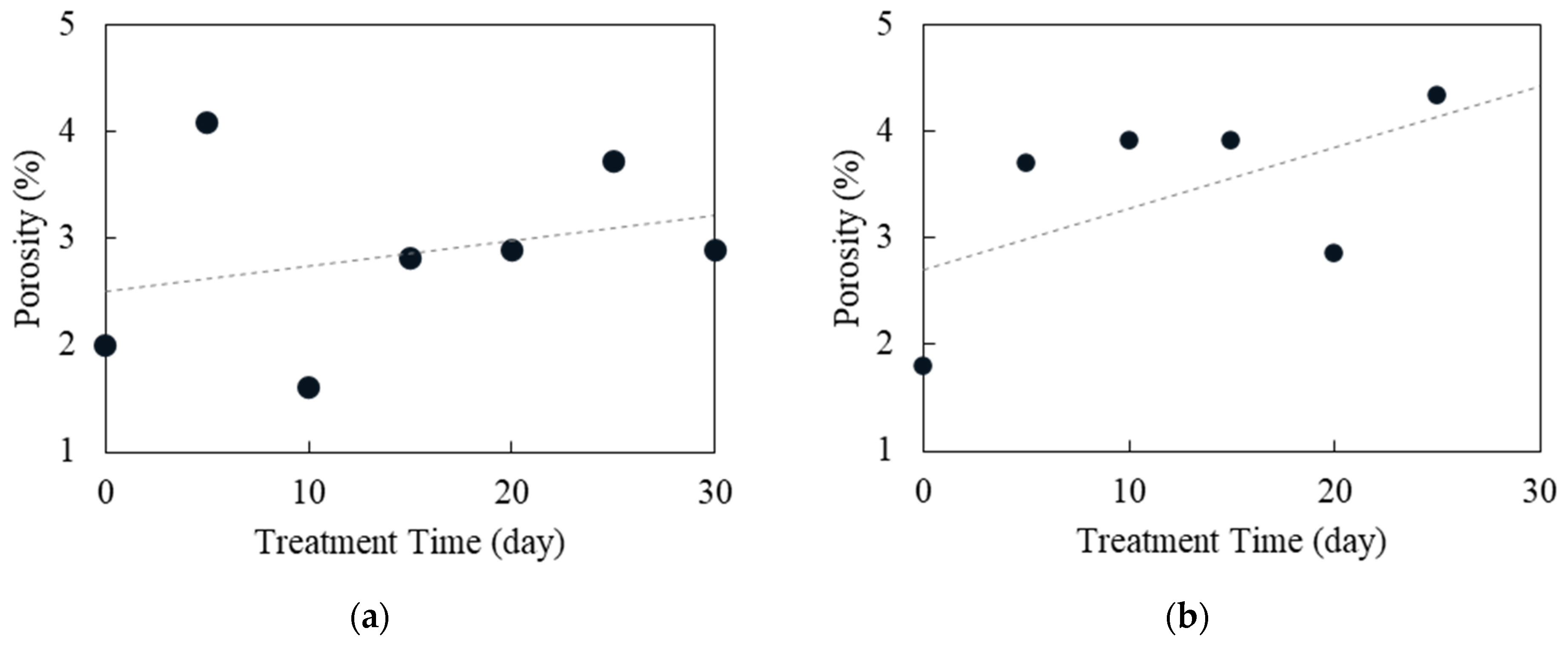



| Sample | Porosity (%) | Sample | Porosity (%) |
|---|---|---|---|
| H0 | 2.00 | G0 | 1.80 |
| H5 | 4.08 | G5 | 3.70 |
| H10 | 1.60 | G10 | 3.91 |
| H15 | 2.82 | G15 | 3.90 |
| H20 | 2.88 | G20 | 2.85 |
| H25 | 3.73 | G25 | 4.33 |
| H30 | 2.90 | G30 | -- |
| Sample | SSA (m2/g) | PV (cm3/g) | APD (nm) |
|---|---|---|---|
| G0 | 3.3375 | 0.0124 | 16.9 |
| G5 | 2.1206 | 0.0086 | 19.1 |
| G10 | 2.0022 | 0.0097 | 20.6 |
| G15 | 1.6789 | 0.0094 | 24.8 |
| G20 | 1.8014 | 0.0092 | 21.8 |
| G25 | 1.7743 | 0.0089 | 21.6 |
| G30 | 1.7287 | 0.009 | 22.9 |
| H0 | 3.128 | 0.0097 | 13.2 |
| H5 | 3.0694 | 0.0086 | 12.2 |
| H10 | 3.8088 | 0.0091 | 10.8 |
| H15 | 3.7588 | 0.01 | 11.5 |
| H20 | 3.6376 | 0.0094 | 11.4 |
| H25 | 3.6491 | 0.0104 | 12.2 |
| H30 | 3.8324 | 0.0101 | 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Li, Z.; Xu, L. Effects of Fracturing Fluids on Properties of Shale Reservoir: A Case Study of the Longmaxi Formation in the Sichuan Basin. Minerals 2025, 15, 392. https://doi.org/10.3390/min15040392
Cheng Y, Li Z, Xu L. Effects of Fracturing Fluids on Properties of Shale Reservoir: A Case Study of the Longmaxi Formation in the Sichuan Basin. Minerals. 2025; 15(4):392. https://doi.org/10.3390/min15040392
Chicago/Turabian StyleCheng, Yishan, Zhiping Li, and Longfei Xu. 2025. "Effects of Fracturing Fluids on Properties of Shale Reservoir: A Case Study of the Longmaxi Formation in the Sichuan Basin" Minerals 15, no. 4: 392. https://doi.org/10.3390/min15040392
APA StyleCheng, Y., Li, Z., & Xu, L. (2025). Effects of Fracturing Fluids on Properties of Shale Reservoir: A Case Study of the Longmaxi Formation in the Sichuan Basin. Minerals, 15(4), 392. https://doi.org/10.3390/min15040392




