Recycling Galvanic Sludge to Produce Geopolymer Modified with Algae
Abstract
1. Introduction
- Dissolution of aluminosilicate materials in a concentrated alkaline solution (KOH or NaOH);
- Diffusion of dissolved silicon and aluminum from the particle surface into the intermolecular space;
- Polycondensation of oligomers and formation of an aluminosilicate gel phase;
- Hardening of the gel phase following polymerization and stabilization of the semicrystalline structures.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Geopolymers Preparation
2.2.2. Sorption Experiments
3. Results
3.1. Physicochemical Characteristics of the Geopolymer
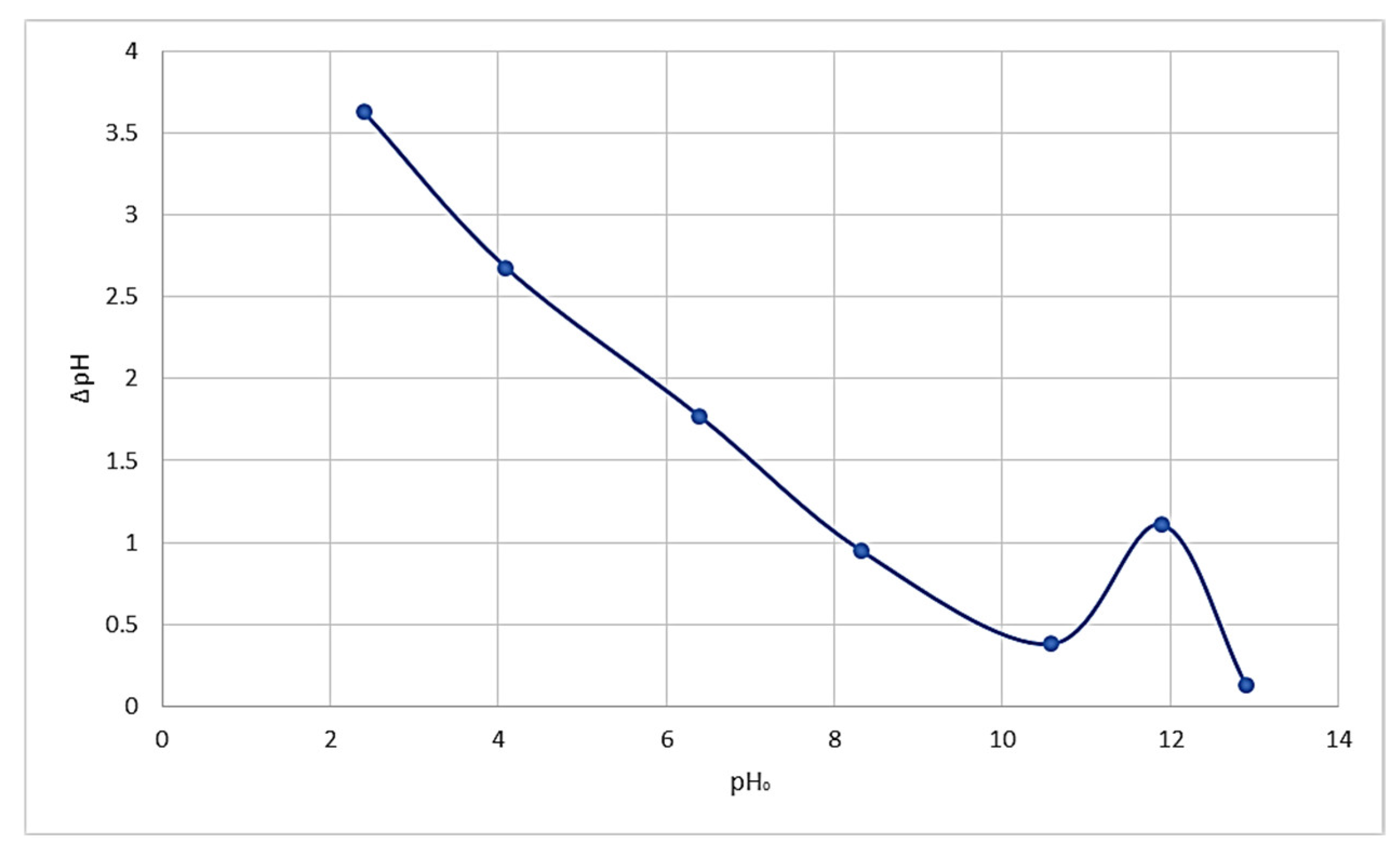
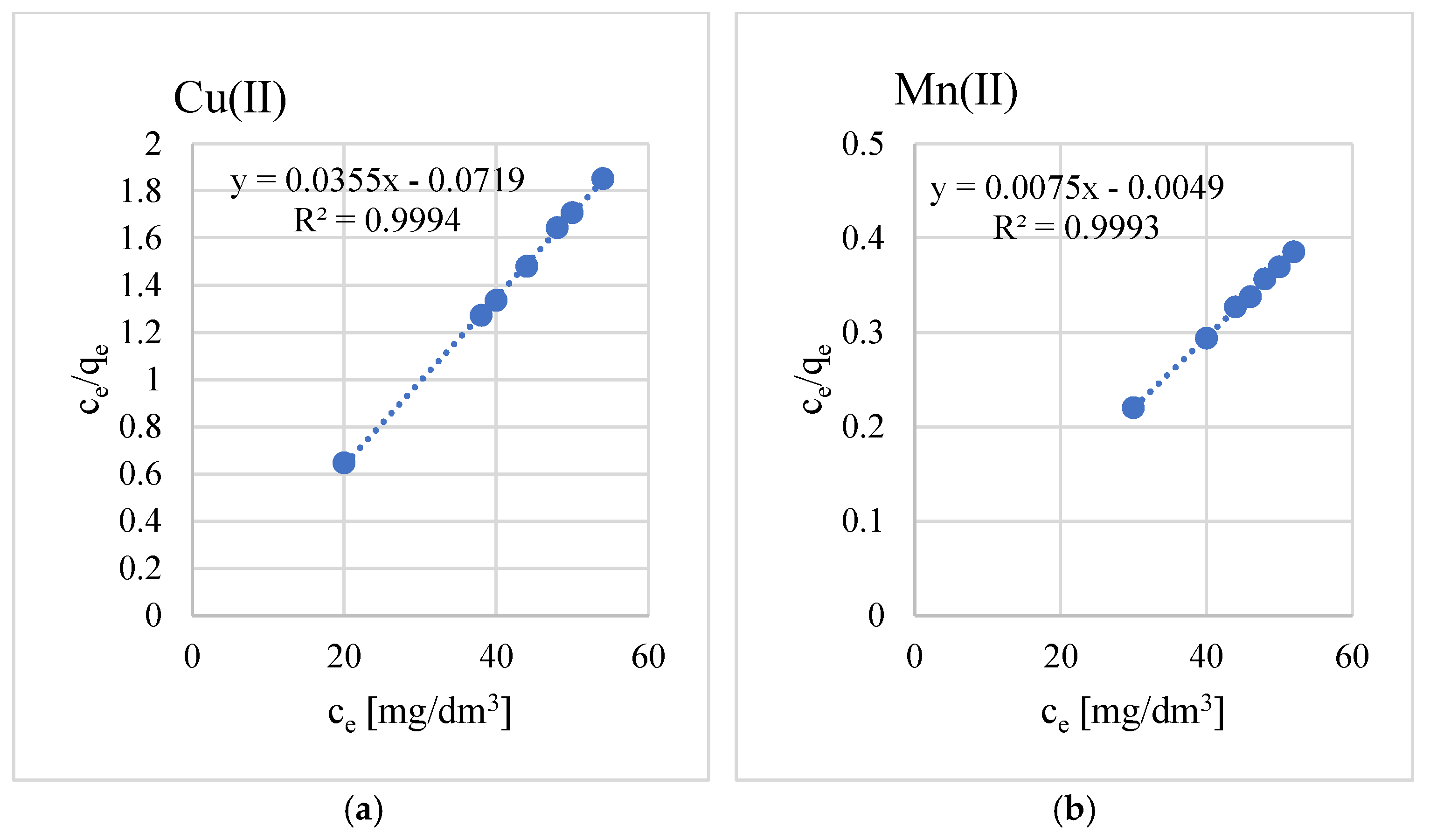
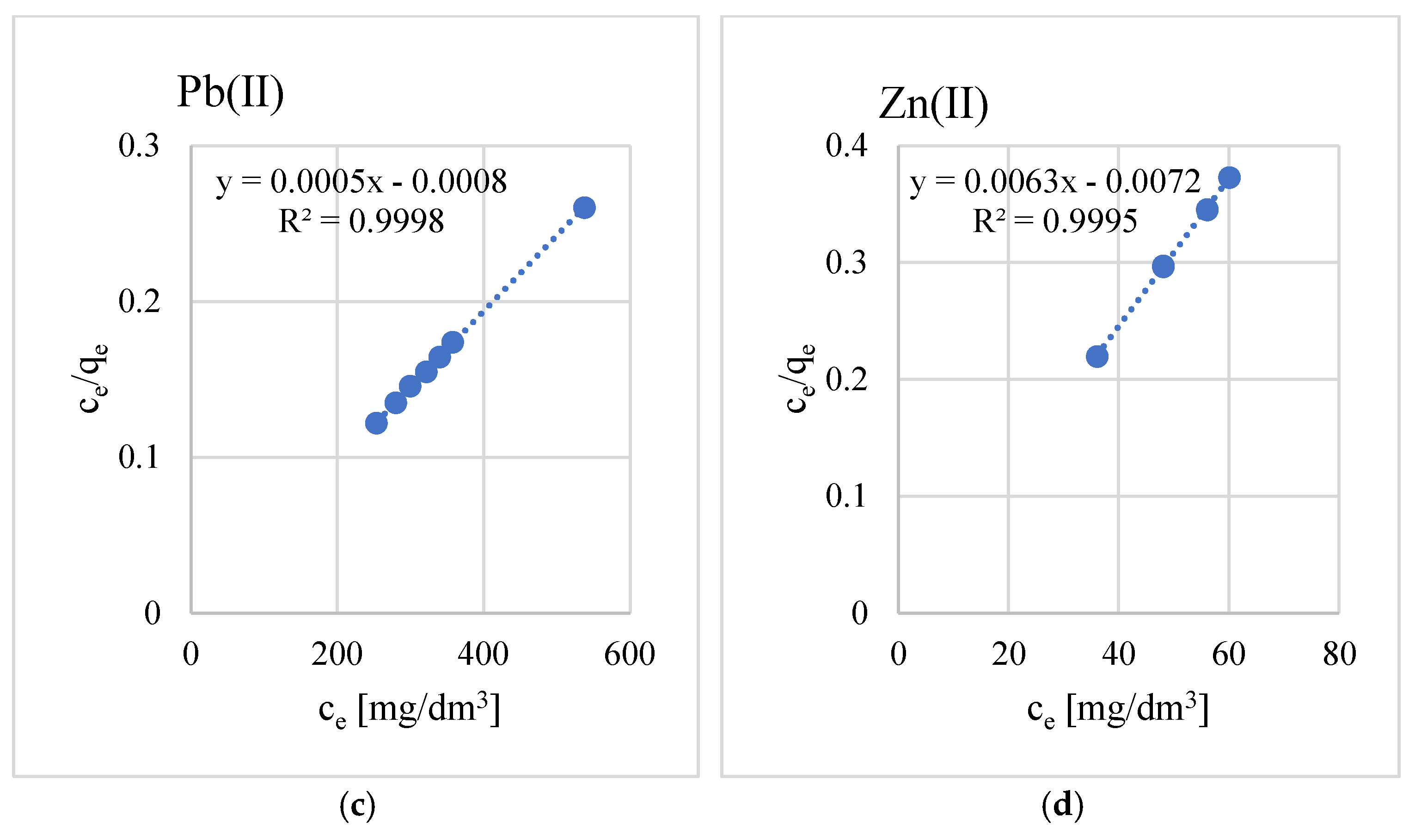
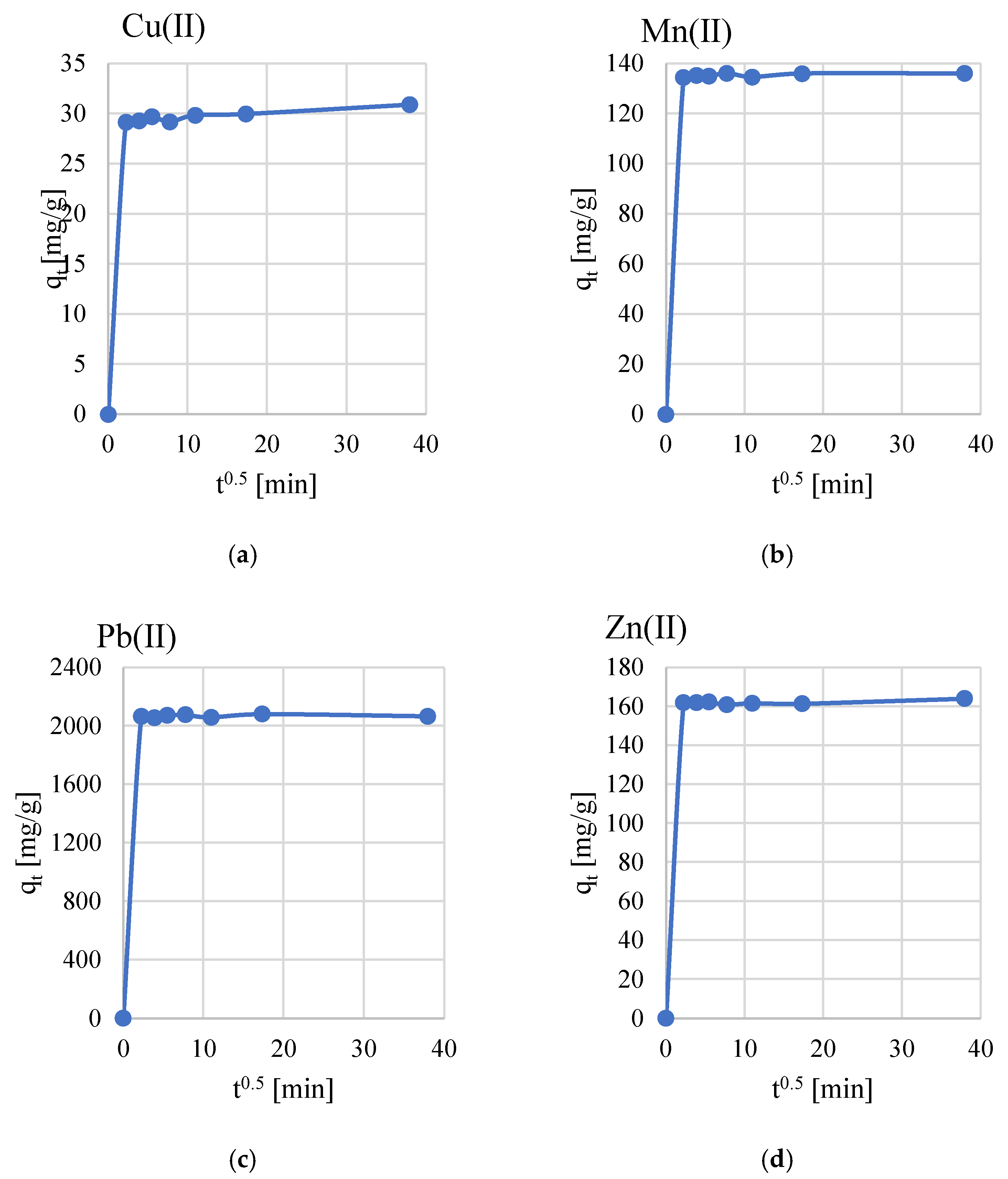
3.2. Sorption Properties
3.3. Spectroscopic Analyses
4. Discussion
5. Conclusions
- The C-(N)-A-S-H gel is predominant in the structure of the obtained geopolymer material with the addition of algae. Its presence is an essential factor that influences the mechanism, properties, and durability of the geopolymer material obtained.
- The adsorbent used shows the high adsorption efficiency of Cu(II), Mn(II), Pb(II), and Zn(II) ions in the studied model systems, respectively, 96.9—Cu(II), 98.9—Mn(II), 99.7—Pb(II), and 99.5%—Zn(II).
- The adsorption process is well described by the Langmuir isotherm. The equilibrium parameters indicate that the geopolymer used has a good adsorption capacity and that the process occurs by chemisorption.
- The adsorption process efficiency and equilibrium concentration of metal ions were found to increase with time. The adsorption efficiency of the studied metal ions on geopolymers can be written in the following series: Pb(II) < Zn(II)< Mn(II) < Cu(II).
- The maximum sorption capacities for the studied metal ions are equal to, respectively, Cu(II)—29, Mn(II)—135, Pb(II)—161, and Zn(II)—2060 mg/g.
- The adsorption of the investigated Cu(II), Mn(II), Pb(II), and Zn(II) ions on geopolymers obtained from galvanic sewage sludge and algae follows the pseudo-second-order model.
- The analysis of the FTIR spectra of the geopolymer after adsorption of the studied metal ions indicates the involvement of functional groups, i.e., O-H and C-O in the adsorption process, which confirms that the adsorption process of Cu(II), Mn(II), Pb(II), and Zn(II) ions occurs mainly by a chemical sorption mechanism.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of refuse derived fuel through torrefaction and carbonization: Evaluation of RDF char fuel properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Bessi, C.; Lombardi, L.; Meoni, R.; Canovai, A.; Corti, A. Solid recovered fuel: An experiment on classification and potential applications. Waste Manag. 2016, 47, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Maged, A.; Eloffy, M.G.; Zahran, M.; Kharbish, S.; Elwakeel, K.Z.; Bhatnagar, A. Geopolymers as sustainable eco-friendly materials: Classification, synthesis routes, and applications in wastewater treatment. Sep. Purif. Technol. 2023, 324, 124631. [Google Scholar] [CrossRef]
- Maged, A.; El-Fattah, H.A.; Kamel, R.M. A comprehensive review on sustainable clay-based geopolymers for wastewater treatment: Circular economy and future outlook. Environ. Monit. Assess. 2023, 195, 693. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.; Sadique, M.; Ming, L.Y.; Mohd Salleh, M.A.A.; Mohd Arif Zainol, M.R.R.; Ghazali, C.N.R. Diverse material based geopolymer towards heavy metals removal: A review. J. Mater. Res. Technol. 2023, 22, 126–156. [Google Scholar] [CrossRef]
- Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and functionalization strategies for the development of sustainable materials in construction industry and cultural heritage applications: A review. Materials 2020, 15, 1725. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Hossain, S.S.; Akhtar, F. Recent progress of geopolymers for carbon dioxide capture, storage and conversion. J. CO2 Util. 2023, 78, 102631. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Nergis, D.D.B.; Rahim, S.Z.A.; Omar, M.F.; Sandu, A.V.; Syafwandi, P.V. Potential of soil stabilization using Ground Granulated Blast Furnace Slag (GGBFS) and fly ash via geopolymerization method: A review. Materials 2022, 15, 375. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Sitarz-Palczak, E.; Kalembkiewicz, J.; Galas, D. Comparative study on the characteristics of coal fly ash and biomass ash geopolymers. Arch. Environ. Prot. 2019, 45, 126–135. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.I.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-basen geopolymer. Appl. Clay Sci. 2012, 5, 90–96. [Google Scholar] [CrossRef]
- Felaous, K.; Aziz, A.; Achab, M. Physico-mechanical and durability properties of new eco-material based on blast furnace slag activated by Moroccan diatomite gel. Environ. Sci. Pollut. Res. 2023, 30, 3549–3561. [Google Scholar] [CrossRef]
- Sitarz-Palczak, E. Utilization of galvanic sewage sludge to produce alkali-activated materials. J. Ecol. Eng. 2024, 25, 305–313. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, H.; He, T.; Gao, S. Review on the effect collision between hazardous metal ions and geopolymer as adsorbents or in situ stabilization/solidification. Appl. Clay Sci. 2024, 249, 107258. [Google Scholar] [CrossRef]
- Jin, H.Z.; Qiu, C.X.; Li, Y.S.; Liu, B.; Liu, J.Y.; Chen, Q.; Lu, X.F.; Li, C.X.; Wang, Q.K. Structural and functional design of geopolymer adsorbents: A review. Tungsten 2024, 6, 48–76. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, J.; Wang, R.; Zhang, W.; Ye, J. Adsorption properties and mechanisms of geopolymers and their composites in different water environments: A comprehensive review. J. Water Process. Eng. 2024, 62, 105393. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhao, D.; Zhong, G.; Sun, Y.; Hu, X.; Sun, J.; Li, X.; Zhu, W.; Li, M. Research and application progress of geopolymers in adsorption: A review. Nanomaterials 2022, 12, 3002. [Google Scholar] [CrossRef]
- Saafi, M.; Andrew, K.; Tang, P.L.; McGhon, D.; Taylor, S.; Rahman, M.; Yang, S.; Zhou, X. Multifunctional properties of carbon nanotube/fly ash geopolymeric nanocomposites. Constr. Buil. Mater. 2013, 49, 46–55. [Google Scholar] [CrossRef]
- Shamsol, A.S.; Apandi, N.M.; Zailani, W.W.A.; Izwan, K.N.K.; Zakaria, M.; Zulkarnain, N.N. Graphene oxide as carbon-based materials: A review of geopolymer with addition of graphene oxide towards sustainable construction materials. Constr. Buil. Mater. 2024, 411, 134410. [Google Scholar] [CrossRef]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully reacted high strength geopolymer made with diatomite as a fumed silica alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Alzeer, M.I.M.; MacKenzie, K.J.D. Fiber composites of inorganic polymers (geopolymers) reinforced with natural fibers. Compos. Mater. Ser. 2021, 5, 117–147. [Google Scholar] [CrossRef]
- Amor, A.B.; Arenas, M.; Martín, J.; Ouakouak, A.; Santos, J.L.; Aparicio, J.; Alonso, E.; Hamdi, N. Alginate/geopolymer hybrid beads as an innovative adsorbent applied to the removal of 5-fluorouracil from contaminated environmental water. Chemosphere 2023, 335, 139092. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Alaskar, A.; Sarmad, M.; Nassar, M.R.U.D.; Zaid, O.; Althoey, F.; Arbili, M.M. Systematic review on geopolymer composites modified with nanomaterials and thin films: Enhancing performance and sustainability in construction. Constr. Build. Mater. 2023, 409, 133888. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muňoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Świerk, K.; Bielicka, A.; Bojanowska, I.; Maćkiewicz, Z. Investigation of heavy metals leaching from industrial wastewater sludge. Pol. J. Environ. Stud. 2007, 16, 447–451. [Google Scholar]
- Galas, D.; Kalembkiewicz, J.; Sitarz-Palczak, E. Physicochemistry, morphology and leachability of selected metals from post-galvanized sewage sludge from screw factory in Łańcut, SE Poland. Contemp. Trends Geosci. 2016, 5, 83–91. [Google Scholar] [CrossRef]
- Sitarz-Palczak, E.; Galas, D.; Kalembkiewicz, J. Study of the adsorption of Cu(II), Mn(II), Pb(II), and Zn(II) ions on geopolymers obtained from ashes from biomass combustion. In Geopolymer: Synthesis, Properties and Applications; Glenn, J.E., Ed.; NOVA SCIENCE Publishers: New York, NY, USA, 2022; pp. 39–85. [Google Scholar]
- Grabowska, B.; Kaczmarska, K.; Bobrowski, A.; Kurleto, Ż.; Mrówka, N.; Żymankowska-Kumon, S. The cation Exchange Capacity (CEC) of attapulgite—The aluminosilicate of Palygorskites Group. Arch. Foundry Eng. 2015, 15, 43–46. [Google Scholar]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. IX Update. Adv. Colloid Interface Sci. 2021, 296, 102519. [Google Scholar] [CrossRef] [PubMed]
- Al-Harahsheh, M.S.; Zboon, K.A.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Xu, L.; Cui, H.; Zheng, X.; Liang, J.; Xing, X.; Yao, L.; Chen, Z.; Zhou, J. Asdorption of Cu2+ to biomass ash its modified product. Water Sci. Tech. 2017, 1, 115–125. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, Y.; Lin, C. Microstructure analysis and effects of single and mixed activators on setting time and strength of coal gangue-based geopolymers. Gels 2022, 8, 195. [Google Scholar] [CrossRef]
- Agnoli, E.; Ciapponi, R.; Levi, M.; Turri, S. Additive manufacturing of geopolymers modified with microalgal biomass biofiller from wastewater treatment plants. Materials 2019, 12, 1004. [Google Scholar] [CrossRef]
- Walkley, B.; Nicola, R.S.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Qin, L.; Qu, B.; Shi, C.; Zhang, Z. Effect of Ca/Si Ratio on the formation and characteristics of synthetic aluminosilicate hydrate gels. Mater. Rep. 2020, 34, 12057–12063. [Google Scholar] [CrossRef]
- Chenga, K.; Heidarib, Z. A new method for quantifying cation exchange capacity in clay minerals. Appl. Clay Sci. 2018, 161, 444–455. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Sun, S.; Yang, W.; Fang, L.; Ma, R.; Lin, C.; Li, H. Lead zinc slag-based geopolymer: Demonstration of heavy metal solidification mechanism from the new perspectives of electronegativity and ion potential. Environ. Pollut. 2022, 293, 118509. [Google Scholar] [CrossRef] [PubMed]
- Bumanis, G.; Novais, R.M.; Carvalheiras, J.; Bajare, D.; Labrincha, J.A. Metals removal from aqueous solutions by tailored porous waste-based granulated alkali-activated materials. App. Clay Sci. 2019, 179, 105147. [Google Scholar] [CrossRef]
- Han, L.; Wang, J.; Liu, Z.; Zhang, Y.; Jin, Y.; Li, J.; Wang, D. Synthesis of fly ash-based self-supported zeolites foam geopolymer via saturated steam treatment. J. Hazard. Mater. 2020, 393, 122468. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A green, porous and eco-friendly magnetic geopolymer adsorbent for heavy metals removal from aqueous solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Immobilization efficiency and mechanism of metal cations (Cd2+, Pb2+ and Zn2+) and anions (AsO43− and Cr2O72−) in wastes-based geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef]
- Ji, Z.; Su, L.; Pei, Y. Characterization and adsorption performance of waste-based porous open-cell geopolymer with one-pot preparation. Ceram. Int. 2021, 47, 12153–12162. [Google Scholar] [CrossRef]
- Yusuf, M.O. Bond Characterization in Cementitious Material Binders Using Fourier-Transform Infrared Spectroscopy. Appl. Sci. 2023, 13, 3353. [Google Scholar] [CrossRef]
- Sitarz-Palczak, E.; Kalembkiewicz, J. The influence of physical modification on the sorption properties of geopolymers obtained from halloysite. Pol. J. Environ. Stud. 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, A.; Palomo, A.; Fernández-Jiménez, D.E.; Macphee, C. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. Influence of alkali metal cations/type of activator on the structure of alkali-activated fly ash—ATR-FTIR studies. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 33–37. [Google Scholar] [CrossRef]
- Hervé, K.T.; Rüscher, C.H.; Kong, S.S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium water glass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.; Sufian, S.; Man, Z.; Khan, A.S.; Ullah, H.; Siyal, A.A. Short review of infra-red spectroscopic studies of geopolymers. Adv. Mater. Res. 2016, 1133, 231–235. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; El-Dessouky, M.I. Reuse of waste glass in improving properties of metakaolin-based geopolymers: Mechanical and microstructure examinations. Constr. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Cattaneo, A.S.; Mustarelli, P. Al2O3·2SiO2 powders synthesized via sol-gel as pure raw material in geopolymer preparation. J. Am. Ceram. Soc. 2017, 100, 1773–2308. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Nie, S.; Skibsted, J.; Ye, G. Structural evolution of calcium sodium aluminosilicate hydrate (C-(N-) A-S-H) gels induced by water exposure: The impact of Na leaching. Cem. Concr. Res. 2024, 178, 107432. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; Silva, E.F.; Velosa, A.; Rocha, F. The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. App. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Darmayanti, L.; Kadja, G.T.M.; Notodarmojo, S.; Damanhuri, E.; Mukti, R.R. Structural alternation within fly ash-based geopolymers governing the adsorption of Cu2+ from aqueous environment: Effect of alkali activation. J. Hazard. Mater. 2019, 377, 305–314. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Huang, P.; Zhang, Q.; Yuan, W. Efficient removal of copper from wastewater by using mechanically activated calcium carbonate. J. Environ. Manag. 2017, 203, 1–7. [Google Scholar] [CrossRef]
- Kara, I.; Tunc, D.; Sayin, F.; Akar, S.T. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal. App. Clay Sci. 2018, 161, 184–193. [Google Scholar] [CrossRef]
- Anguile, J.J.; Mbega, M.G.O.; Makani, T.; Mbadcam, J.K. Adsorption of manganese (II) ions from aqueous solution on to volcanic ash and geopolymer based volcanic ashes. Int. J. Basic Appl. Chem. Sci. 2013, 3, 7–18. [Google Scholar]
- Al-Wakeel, K.Z.; El Monem, H.A.; Khalil, M.M. Removal of divalent manganese from aqueous solution using glycine modiied chitosan resin. J. Environ. Chem. Eng. 2015, 3, 179–186. [Google Scholar] [CrossRef]
- Naghsh, M.; Shams, K. Synthesis of a kaolin-based geopolymer using a novel fusion method and its application in effective water softening. App. Clay Sci. 2017, 146, 238–245. [Google Scholar] [CrossRef]
- López, F.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-based geopolymers for targeted adsorbents to heavy metal ion separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
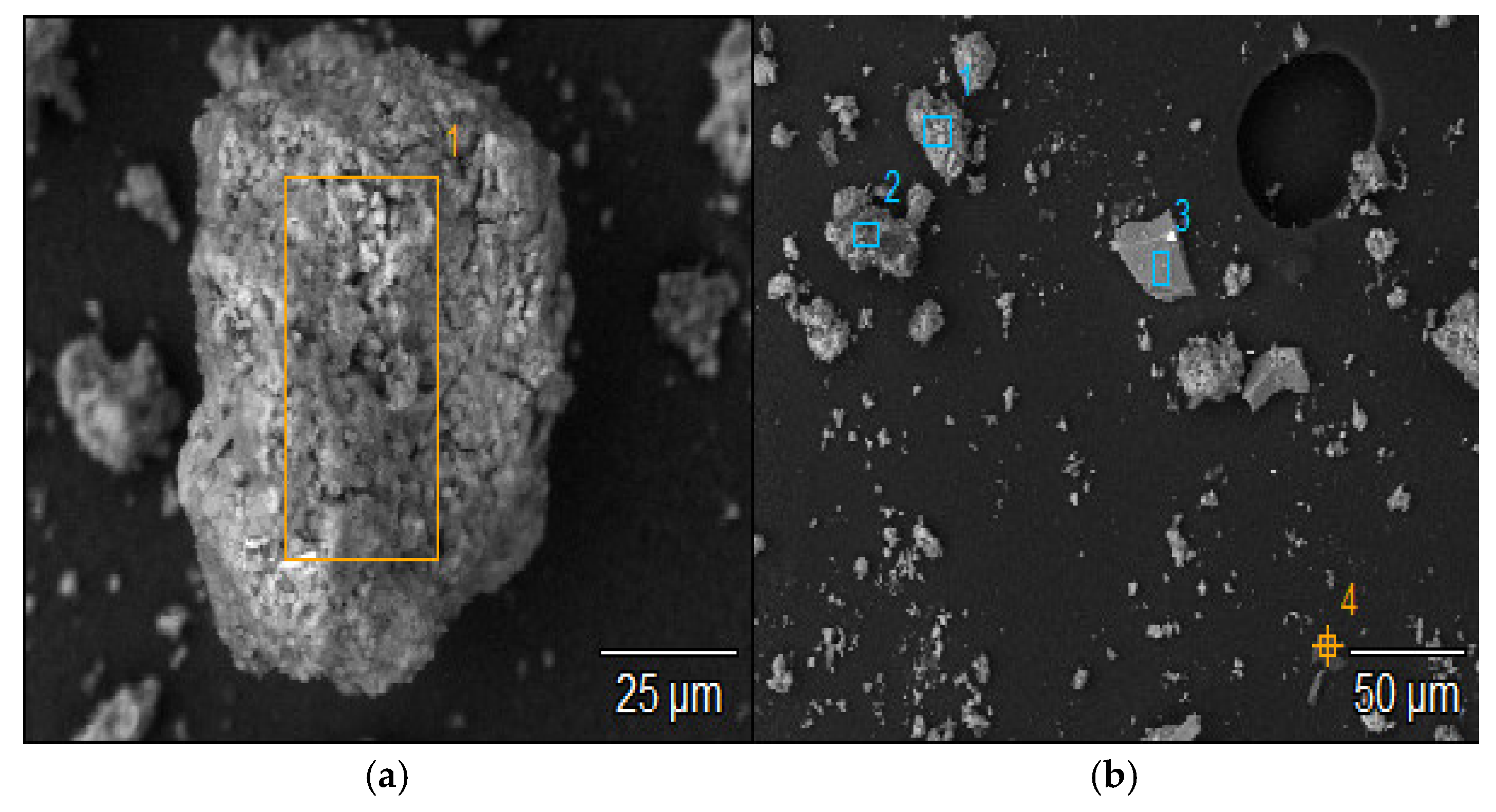

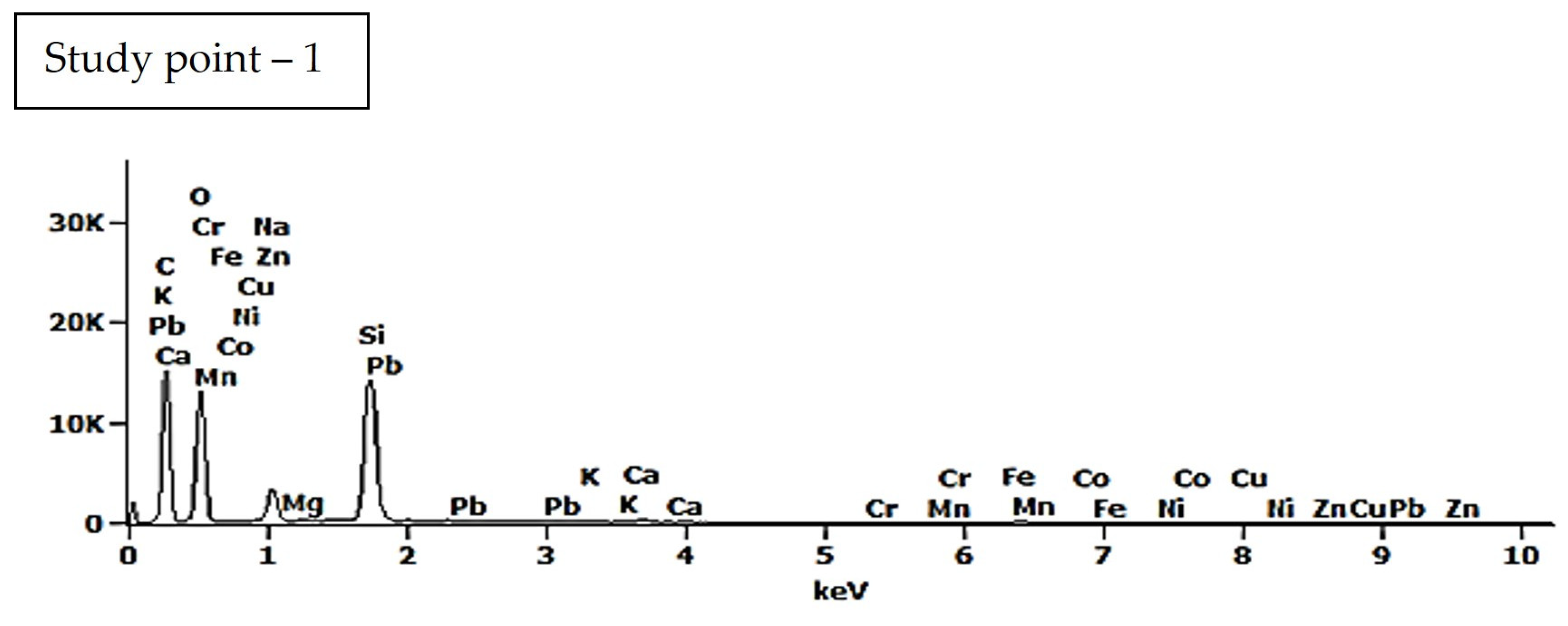
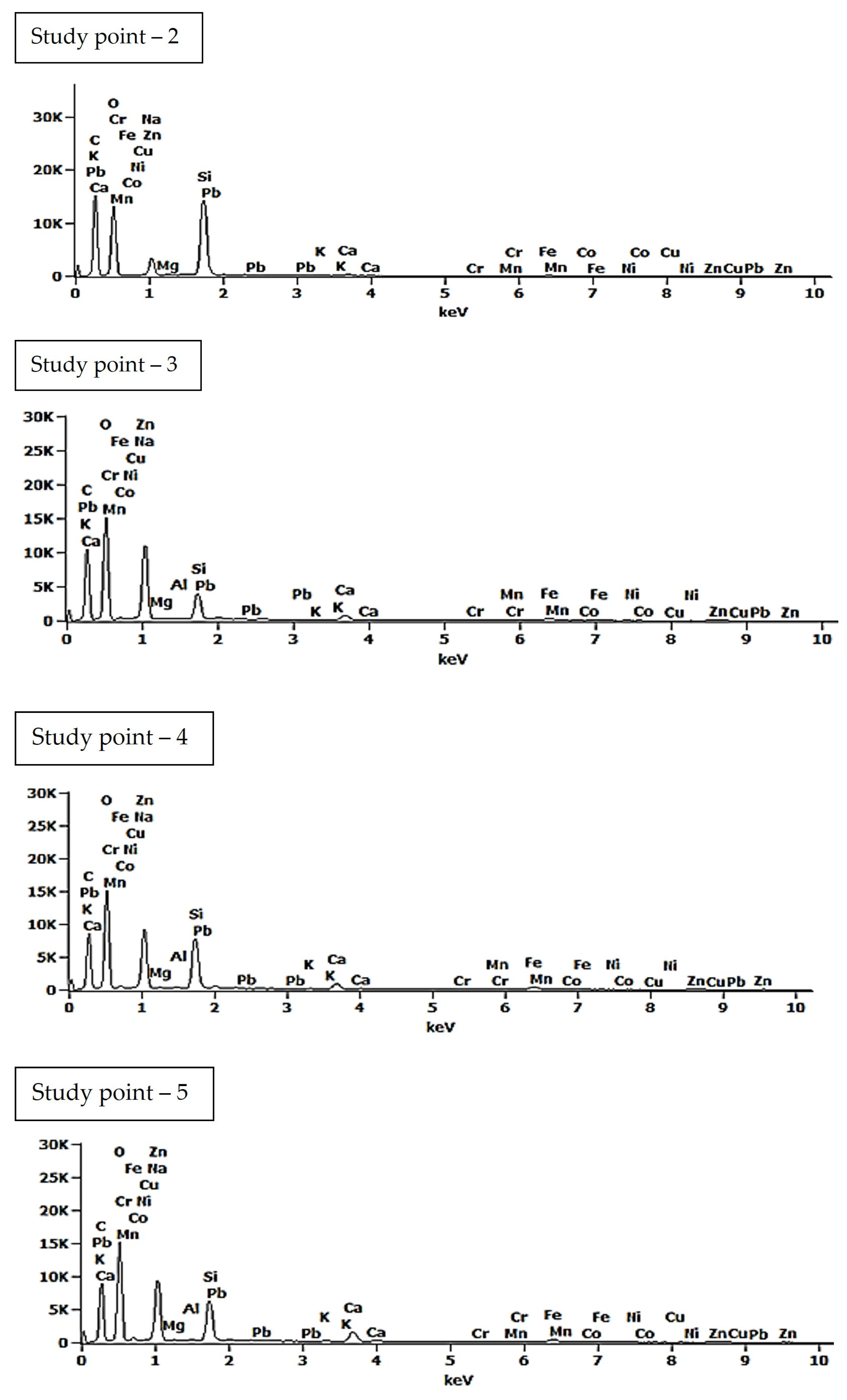
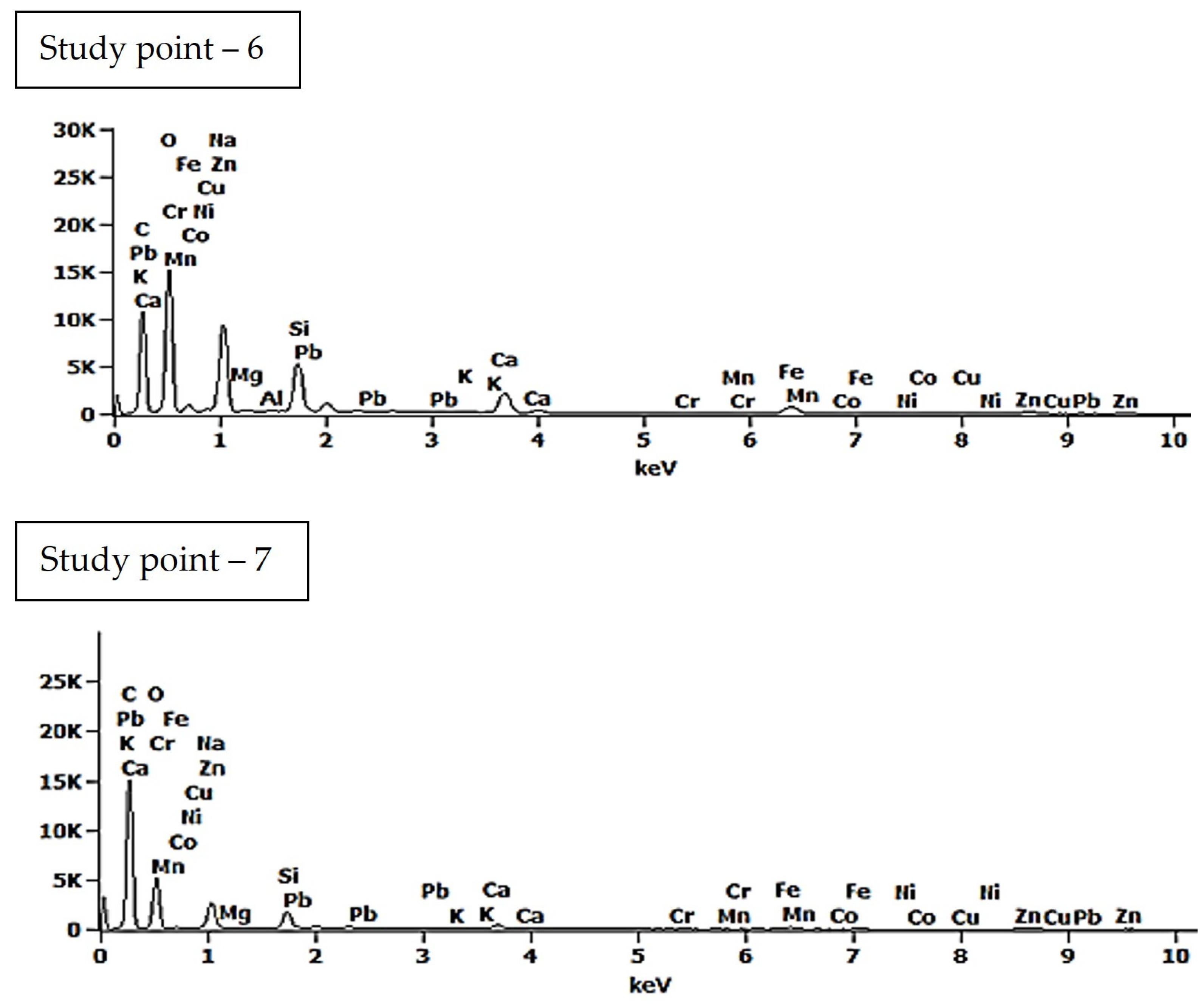

| Study Point | Ca | O | C | Na | Al | Si | Ca/Si | Al/Si | Na/Si |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 (±0.1) | 36.0 (±0.4) | 48.4 (±0.3) | 5.0 (±0.0) | 0.1 (±0.0) | 3.3 (±0.0) | 0.55 | 0.03 | 1.52 |
| 2 | 0.3 (±0.1) | 43.6 (±0.4) | 43.4 (±0.2) | 2.3 (±0.0) | 0.0 (±0.0) | 9.8 (±0.0) | 0.03 | - | 0.23 |
| 3 | 0.9 (±0.1) | 48.8 (±0.4) | 32.8 (±0.2) | 11.6 (±0.1) | 0.0 (±0.0) | 3.5 (±0.0) | 0.07 | - | 3.31 |
| 4 | 1.1 (±0.1) | 49.4 (±0.4) | 30.6 (±0.2) | 8.7 (±0.1) | 0.0 (±0.0) | 7.0 (±0.0) | 0.16 | - | 1.24 |
| 5 | 2.0 (±0.1) | 49.9 (±0.4) | 29.7 (±0.2) | 8.8 (±0.1) | 0.1 (±0.0) | 5.5 (±0.0) | 0.36 | 0.02 | 1.60 |
| 6 | 2.6 (±0.1) | 49.2 (±0.4) | 30.9 (±0.2) | 7.2 (±0.1) | 0.2 (±0.0) | 4.3 (±0.0) | 0.60 | 0.05 | 1.67 |
| 7 | 0.7 (±0.1) | 34.0 (±0.5) | 55.9 (±0.3) | 4.2 (±0.0) | 0.1 (±0.0) | 2.1 (±0.0) | 0.33 | 0.05 | 2.00 |
| Metal Ion | t [min] | ce [mg/dm3] | qe [mg/g] | A [%] | Kd [dm3/g] |
|---|---|---|---|---|---|
| Cu(II) | 5 | 54.0 | 29.15 | 91.50 | 0.54 |
| 15 | 50.0 | 29.28 | 92.13 | 0.59 | |
| 30 | 44.0 | 29.72 | 93.08 | 0.68 | |
| 60 | 48.0 | 29.20 | 92.45 | 0.61 | |
| 120 | 38.0 | 29.85 | 94.02 | 0.79 | |
| 300 | 40.0 | 29.96 | 93.71 | 0.75 | |
| 1440 | 20.0 | 30.89 | 96.85 | 1.54 | |
| Mn(II) | 5 | 48.0 | 134.47 | 98.25 | 2.80 |
| 15 | 50.0 | 135.25 | 98.18 | 2.71 | |
| 30 | 520 | 135.00 | 98.11 | 2.60 | |
| 60 | 46.0 | 136.18 | 98.33 | 2.96 | |
| 120 | 44.0 | 134.62 | 98.40 | 3.06 | |
| 300 | 40.0 | 136.00 | 98.54 | 3.40 | |
| 1440 | 30.0 | 136.12 | 98.91 | 4.54 | |
| Pb(II) | 5 | 48.0 | 161.78 | 99.27 | 3.37 |
| 15 | 56.0 | 161.90 | 99.14 | 2.89 | |
| 30 | 56.0 | 162.13 | 99.14 | 2.90 | |
| 60 | 60.0 | 160.81 | 99.08 | 2.68 | |
| 120 | 48.0 | 161.54 | 99.27 | 3.37 | |
| 300 | 48.0 | 161.30 | 99.27 | 3.36 | |
| 1440 | 36.0 | 163.89 | 99.45 | 4.55 | |
| Zn(II) | 5 | 340.0 | 2064.91 | 99.18 | 6.07 |
| 15 | 358.0 | 2054.92 | 99.14 | 5.74 | |
| 30 | 280.0 | 2071.26 | 99.32 | 7.40 | |
| 60 | 322.0 | 2075.20 | 99.22 | 6.44 | |
| 120 | 300.0 | 2056.79 | 99.28 | 6.86 | |
| 300 | 254.0 | 2079.47 | 99.39 | 8.19 | |
| 1440 | 538.0 | 2064.51 | 99.70 | 3.84 |
| Absorption Bands Position [cm−1] | Types of Vibration | References | |
|---|---|---|---|
| Before Adsorption | After Adsorption | ||
| 3427 | 3427 Pb(II) and Mn(II) 3343 Zn(II) 3392 Cu(II) | Stretching vibrations of the OH groups | [52] |
| 1650 | 1650 all metal ions | Tensile vibrations of the H-O-H | [52] |
| 1450 | 1400 Pb (II) 1430 Mn(II) 1450 Cu (II) and Zn(II) | Asymmetric and symmetric stretching vibrations of the CO group | [53] |
| 1260–1020 | 1260–1020 Pb(II), Zn(II), and Mn(II) 1085–1020 Cu(II) | Stretching vibrations of Si-O(Si) and Si-O(Al) binding | [48] |
| 870–800 | 870–800 all metal ions | Stretching vibrations of [AlO4]− groups | [54] |
| 800–500 | 800–500 all metal ions | Asymmetric vibrations of Si-O(Si) and Si-O(Al) binding and internal vibrations | [55] |
| 450–470 | 450–470 all metal ions | Bending vibrations Si-O-Si | [55] |
| Metal Ion | Type of Sorbent | qe [mg/g] | References |
|---|---|---|---|
| Cu(II) | Geopolymer with algae addition | 29.00 | In this study |
| Geopolymer based on metakaolin | 53.93 | [13] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 1:3 | 28.38 | [57] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 3:1 | 43.16 | [57] | |
| Geopolymer based on fly ash | 7.00 | [58] | |
| CaCO3 | 99.76 | [59] | |
| Mn(II) | Geopolymer with algae addition | 135.00 | In this study |
| Geopolymer based on metakaolin | 72.30 | [60] | |
| Geopolymer based on volcanic ash | 192.00 | [61] | |
| Glycine-modified chitosan | 71.40 | [62] | |
| Pb(II) | Geopolymer with algae addition | 161.00 | In this study |
| Geopolymer based on metakaolin | 100.79 | [13] | |
| Geopolymer based on fly ash | 253.80 | [13] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 1:3 | 136.51 | [57] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 3:1 | 261.22 | [57] | |
| Zn(II) | Geopolymer with algae addition | 2060.00 | In this study |
| Geopolymer based on metakaolin | 30.52 | [57] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 1:1 | 30.79 | [57] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 1:3 | 18.71 | [57] | |
| Geopolymer based on metakaolin with zeolite addition in ratio 3:1 | 35.88 | [57] |
| Metal Ion | Kinetic Equations | |||||
|---|---|---|---|---|---|---|
| Pseudo-First-Order | Pseudo-Second-Order | |||||
| k1 [1/min] | qe [mg/g] | R2 | k2 [g/mg·min] | qe [mg/g] | R2 | |
| Cu(II) | 0.0019 | 1.70 | 0.9798 | 1.74·10−4 | 30.96 | 1.00 |
| Mn(II) | 0.0005 | 1.70 | 0.2977 | 3.60·10−4 | 136.99 | 1.00 |
| Zn(II) | 0.0042 | 3.18 | 0.9413 | 7.88·10−5 | 163.93 | 1.00 |
| Pb(II) | 0.0007 | 1.45 | 0.0469 | 3.01·10−5 | 2000.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz-Palczak, E. Recycling Galvanic Sludge to Produce Geopolymer Modified with Algae. Minerals 2025, 15, 297. https://doi.org/10.3390/min15030297
Sitarz-Palczak E. Recycling Galvanic Sludge to Produce Geopolymer Modified with Algae. Minerals. 2025; 15(3):297. https://doi.org/10.3390/min15030297
Chicago/Turabian StyleSitarz-Palczak, Elżbieta. 2025. "Recycling Galvanic Sludge to Produce Geopolymer Modified with Algae" Minerals 15, no. 3: 297. https://doi.org/10.3390/min15030297
APA StyleSitarz-Palczak, E. (2025). Recycling Galvanic Sludge to Produce Geopolymer Modified with Algae. Minerals, 15(3), 297. https://doi.org/10.3390/min15030297







