Rare Earth Elements in Phosphate Ores and Industrial By-Products: Geochemical Behavior, Environmental Risks, and Recovery Potential
Abstract
1. Introduction
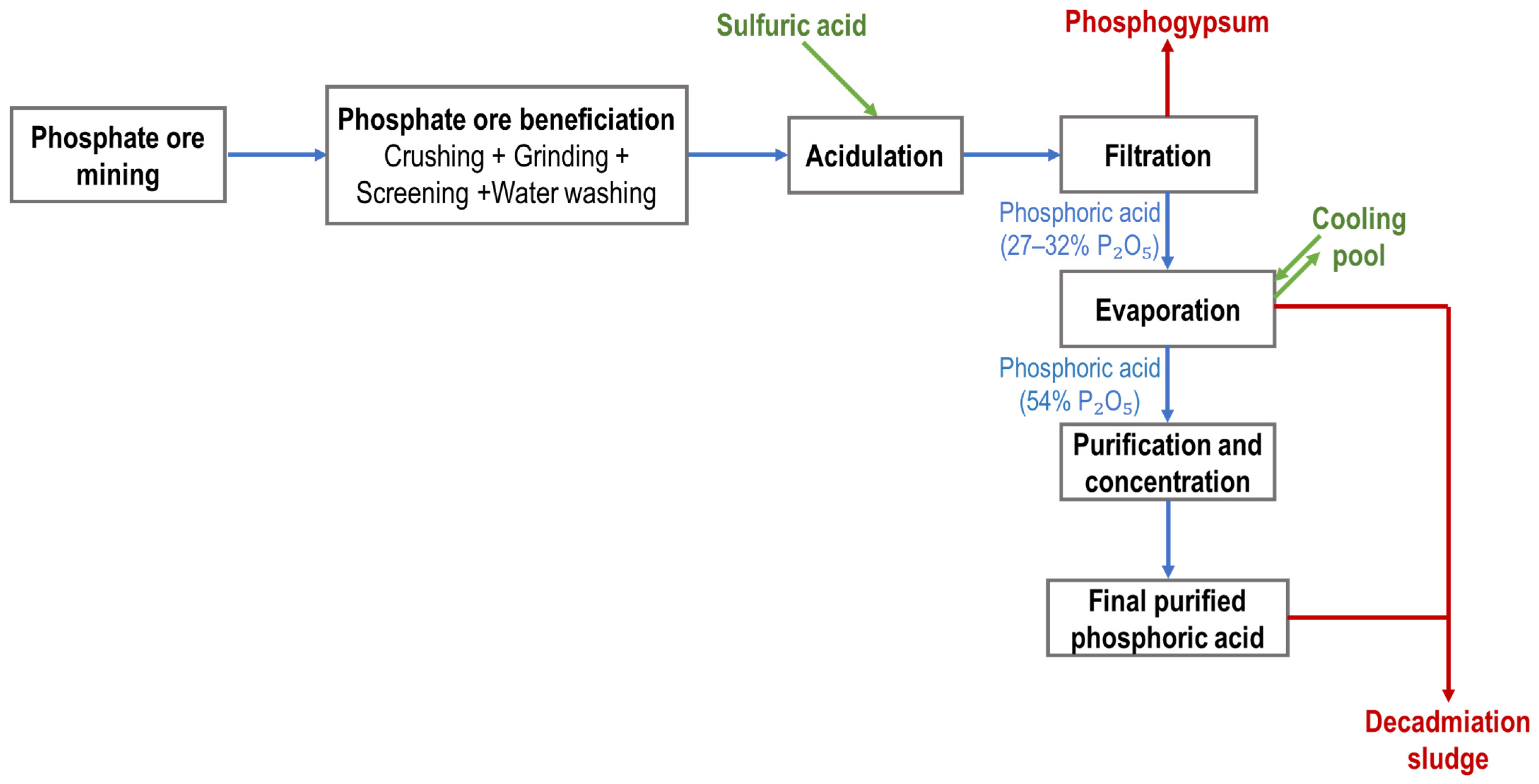
2. Global Overview of the Phosphate Industry
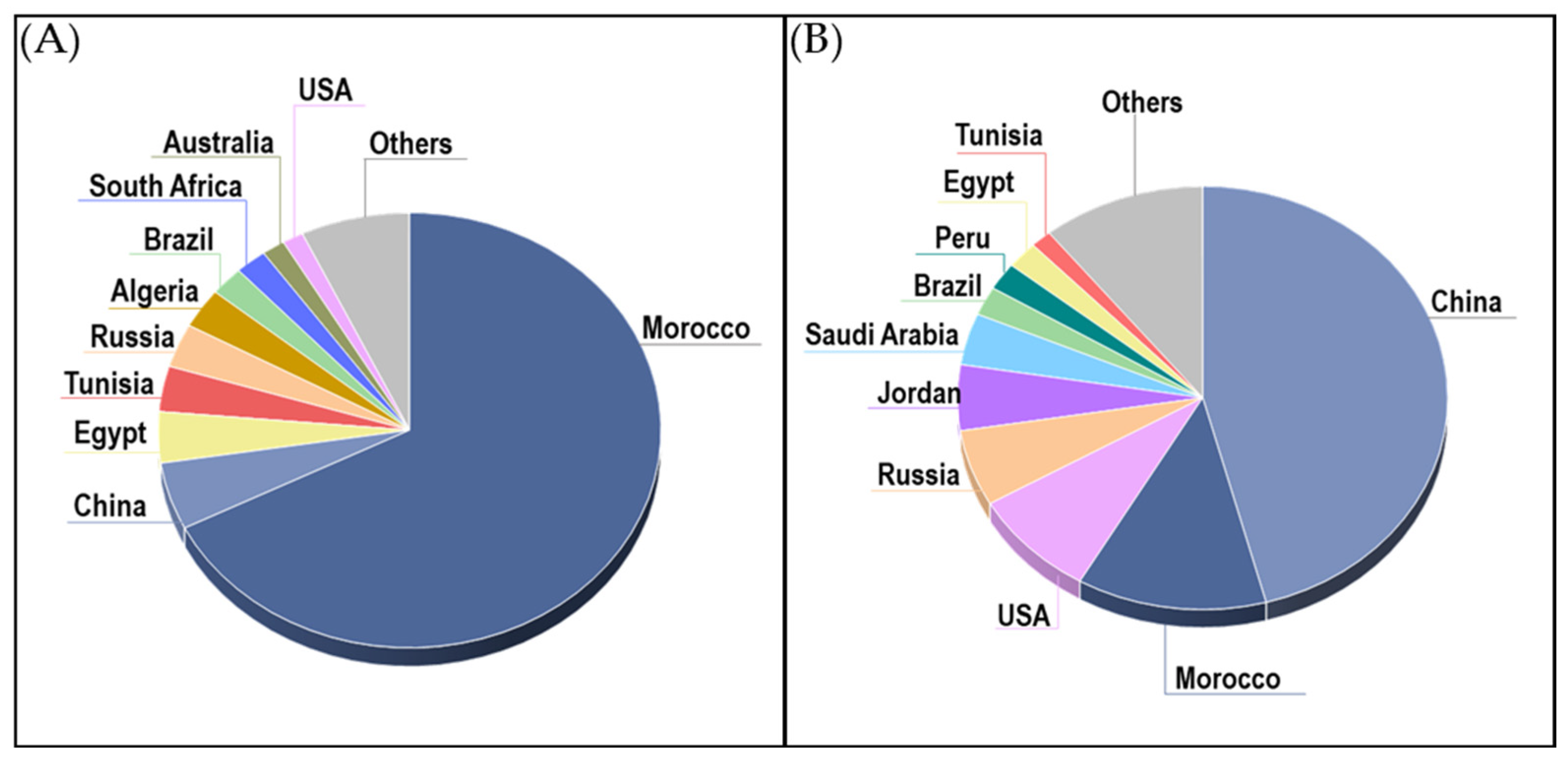
2.1. Global Production, Reserves, and Market Dynamics
2.2. Emerging Trends and Future Prospects
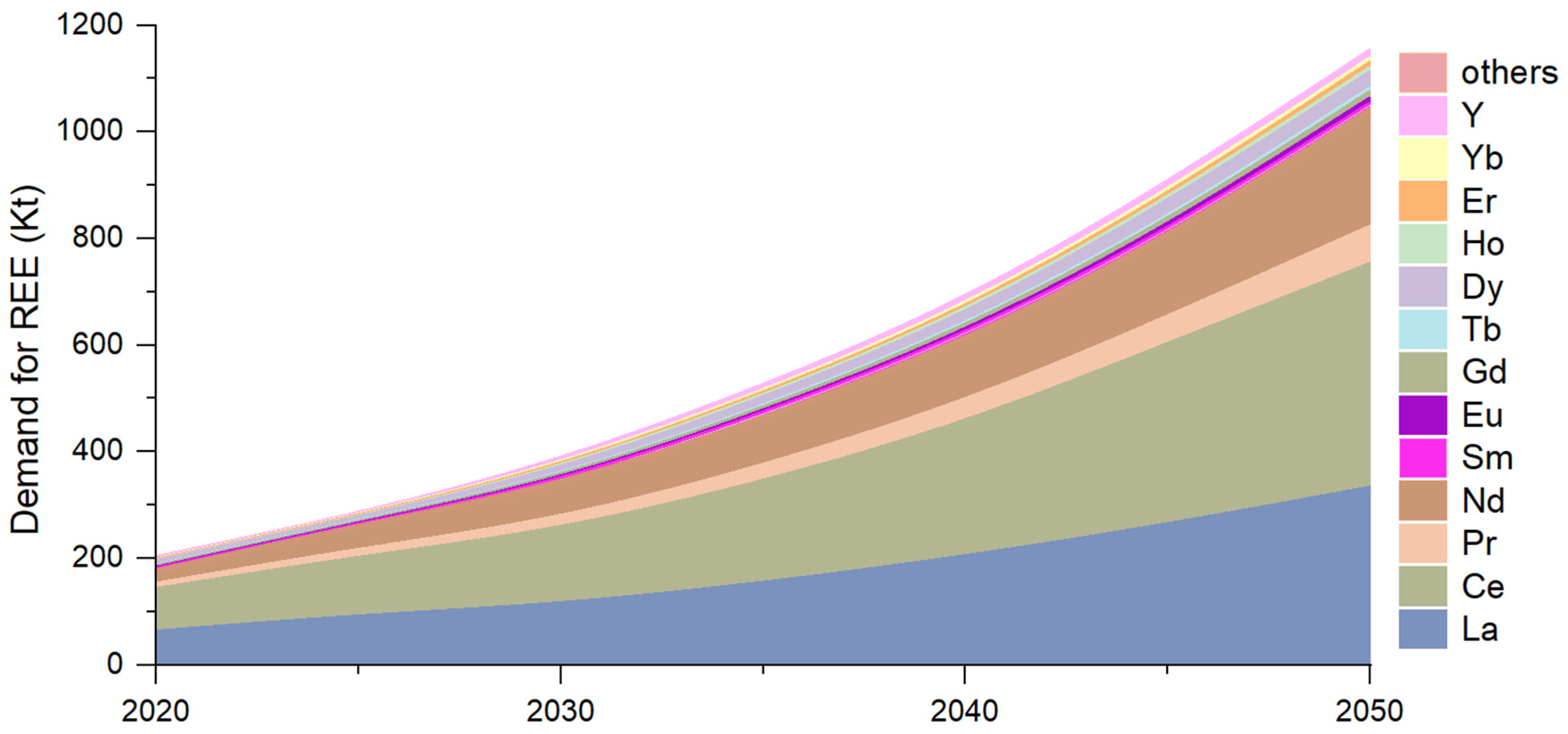
3. Economic Data and Forecasts
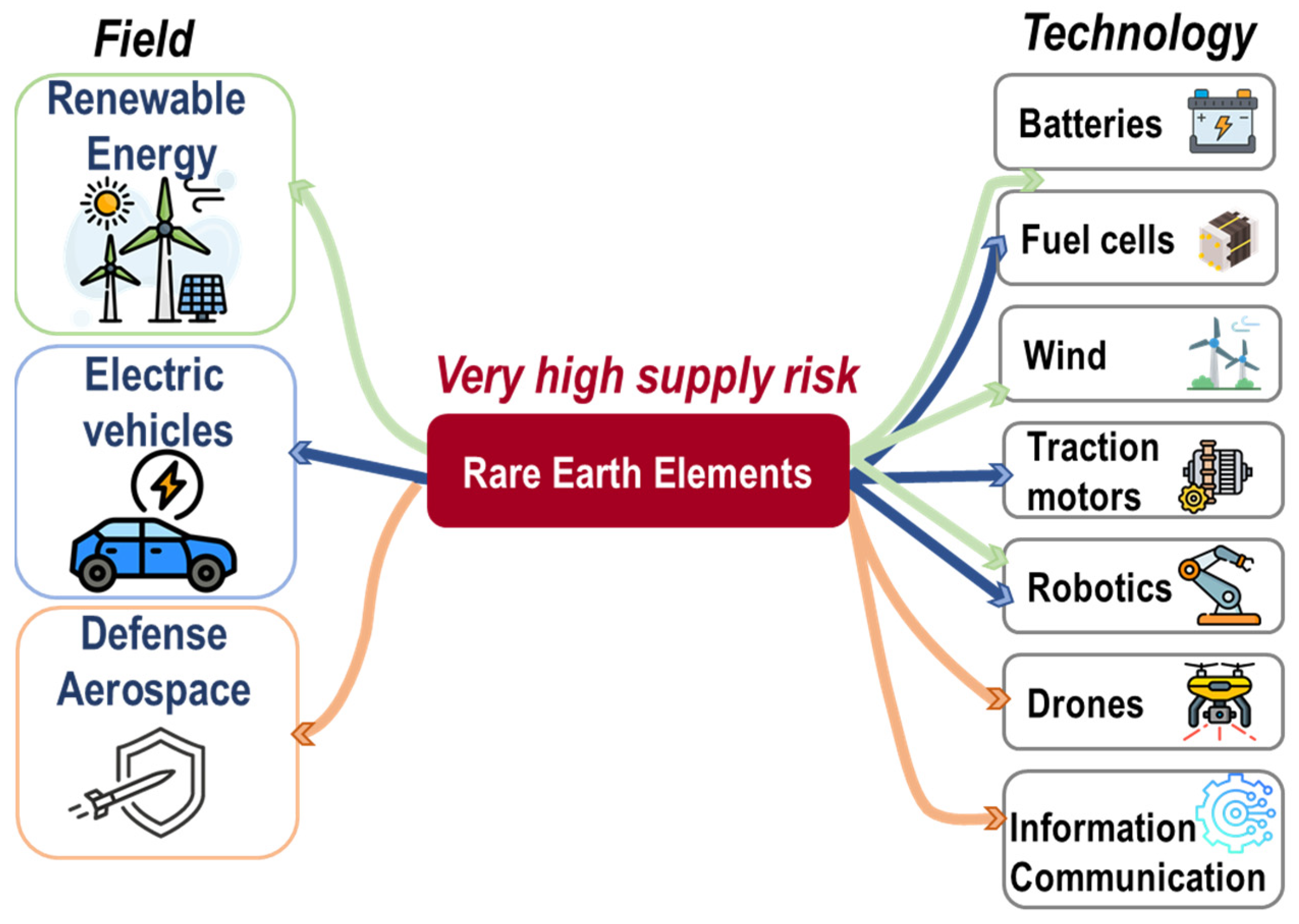
4. Context and Importance of Rare Earth Elements in Phosphates
| Phosphate Deposit | Trace Elements | Average Concentrations (mg/kg) | REE | Average Concentrations (mg/kg) | TREO (mg/kg) | TREO/(U + Th) | References |
|---|---|---|---|---|---|---|---|
| Gafsa Basin, Tunisia | Cd; Cr; Pb; Sr; Zn; U | 24.25; 175.5; 4.52; 1349.5; 196; 59.7 | La; Ce; Nd; Sm; Eu; Gd; Tb; Yb | 51.26; 79.31; 50.14; 9.65; 3.34; 10.7; 6.1; 5.52 | 256.7 | -- | Smida et al. (2021) [108] |
| Gafsa Basin, Tunisia | N.A. | N.A. | Sc; Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 3.55; 123.5; 84; 123.5; 18.35; 79; 14.85; 3.39; 14.1; 2.02; 13.1; 2.84; 8.34; 1.15; 7.58; 1.19 | 600.2 | -- | El Zrelli et al. (2021) [32] |
| Gafsa Basin, Tunisia | Ba; Br; Cd; Cr; Cu; Mo; Ni; Sc; Sr; U; Y; Zn; Zr | 39; 12.41; 34; 238; 9.32; 5.96; 17.9; 3.68; 1788; 30; 99; 228; 41 | La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 74; 108.16; 15.38; 62.58; 13; 2.78; 11.73; 1.8; 10.2; 2.31; 6.76; 0.95; 6.31; 1 | 376.6 | -- | Garnit et al. (2017) [109] |
| Gafsa Basin, Tunisia | Cd; Zn; Cr; Cu; Ni; Sr; Sc; Co; Rb; Sb; Cs; Ba; Hf; Ta; Th; U; As; Mo; Zr | 43.68; 337.91; 226.12; 18.05; 25.21; 1501.35; 3.21; 1.69; 7.3; 0.5; 0.48; 57.45; 0.65; 0.24; 7.02; 23.88; 6.07; 14.92; 79.5 | La; Ce; Nd; Sm; Eu; Gd; Tb; Dy; Yb | 63.49; 101.92; 57.94; 10.7; 3; 12.86; 1.53; 10.43; 5.72 | 318.2 | 10.30 | Galfati et al. (2010) [110] |
| Algeria | V; Cr; Li; Co; Ni; Cu; Zn; As; Rb; Sr; Zr; Nb; Mo; Cd; Cs; Ba; Hf; Ta; W; Tl; Pb; Th; U | 100.52; 208.89; 5.87; 1.14; 28.56; 14.72; 209.82; 7.18; 4.64; 1834.58; 19.15; 1.81; 2.90; 28.06; 2.32; 57.61; 0.32; 0.16; 0.54; 1.38; 2.14; 5.20; 55.74 | Sc; Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Tb; Dy; Ho; Er; Tm; Yb; Lu; ∑REE | 3.95; 190.35; 92.05; 78.95; 15.91; 68.81; 12.52; 3.28; 15.49; 2.18; 14.93; 3.38; 10.37; 1.42; 8.12; 1.54; 328.95 | 630.3 | 10.34 | Kechiched et al. (2020) [111] |
| Morocco | Minor elements (MEs) | ΣMEs = 3538 mg/kg | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm Yb; Lu | 143; 24.06; 13; 3.63; 17.52; 3.68; 1; 5.14; 0.84; 5.67; 1.3; 4.31; 0.62; 4.2; 0.79 | 282.2 | -- | Amine et al. (2019) [112] |
| South-West China | Cr; Zr; Co; Ni; Th; U; V; As; Sb; Mn; Sr | 24; 32; 6; 106; 4; 28; 38.6; 63; 11.4; 599.33; 488.33 | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 242.6; 160.6; 121; 30.4; 132; 24.5; 6.4; 29.7; 4; 24.3; 5; 13.13; 1.56; 7.6; 1 | 970.6 | 30.33 | Zhang et al. (2021) [113] |
| South China | N.A. | N.A. | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 165.76; 198.77; 407.19; 53.26; 221.32; 39.72; 9.59; 36.32; 5.04; 24.5; 4.29; 11.46; 1.19; 5.88; 0.75 | 1426.2 | -- | Xin et al. (2015) [114] |
| South China | N.A. | N.A. | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm Yb; Lu | 93.23; 33.11; 47.94; 10.48; 49.04; 11.20; 2.81; 12.05; 1.96; 11.43; 2.32; 6.20; 0.72; 3.7; 0.48 | 346.8 | -- | Xin et al. (2016) [115] |
| South China | N.A. | N.A. | La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu; Y | Total reserves (tons) 164536; 253849; 3355; 115949; 32901; 2975; 25758; 3641; 2003; 4291; 12599; 1803; 9442; 1431; 147387 | -- | -- | Emsbo et al. (2015) [30] |
| USA | N.A. | N.A. | Sc; Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 5.82; 110.33; 64.00; 105.14; 6.49; 74.12; 0.00; 8.05; 14.36; 1.73; 12.16; 0.00; 8.50; 3.03; 7.42; 1.19 | 503.4 | -- | Liang et al. (2017) [85] |
| Arkansas, USA | N.A. | N.A. | La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu | 207.9; 566.37; 124.11; 790.62; 477.25; 314.96; 582.71; 70; 278.53; 82.77; 82.18; 8.7; 42; 5.91 | 4255 | -- | Murthy et al. (2004) [116] |
| Mountain Pass, USA | N.A. | N.A. | La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu; Y | REE total ore concentration (tons) 743495; 1042279; 88027; 247033; 18913; 2350; 4701; 351; 765; 90; 136; 46; 46; 27; 2642 | -- | -- | Long et al. (2012) [117] |
| Love Hollow, USA | N.A. | N.A. | La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu; Y | REE concentrations (tons/100km2) 2009; 443543; 4074; 182148; 369; 8370; 4176; 5494; 32981; 6477; 1715; 1934; 10328; 1215; 242496 | -- | -- | Emsbo et al. (2015) [30] |
| Egypt | U; Th | 127.26; 22.942 | Sc; Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu; ∑REE | 3.71; 46.74; 34.81; 51.75; 6.59; 27.57; 5.12; 1.35; 6.09; 1.02; 5.978; 1.29; 4.21; 0.6; 4.16; 0.76; 151.3 | 239 | 1.59 | Shahin et al. (2020) [118] |
| Jordan | Nb; Rb; Sr; Th; U; V; Zr; Mo; Cu; Pb; Zn; Ni; As; Cd; Sb; Cr; Ba; Co; Ga; Hf | 0.63; 2.32; 1466.27; 0.68; 58.42; 160.11; 16.40; 8.64; 35.86; 1.82; 217.38; 50.80; 9.38; 19.48; 1.25; 170.90; 590.79; 1.58; 1.09; 0.30 | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Ho; Er; Tm; Yb; Lu | 37.4; 13.4; 8.1; 1.91; 8.2; 1.67; 0.44; 2.36; 0.66; 2.12; 0.32; 2.06; 0.36 | 96.5 | 1.63 | Abed et al. (2016) [119] |
| Jordan | Ba; Cd; Cr; Cs; Cu; Ga; Hf; Mo; Nb; Ni; Rb; Sb; Sc; Se; Sr; Th; Tl; U; V; Zn; Zr | 66.89; 19.18; 190.53; 0.07; 10.24; 0.70; 1.18; 3.68; 0.73; 50.21; 1.39; 0.39; 1.22; 0.81; 340.91; 0.61; 0.07; 24.48; 64.08; 113.84; 50.92 | Y; La; Ce; Pr; Nd; Sm; Eu; Gd; Tb; Dy; Ho; Er; Tm; Yb; Lu; ∑ REE | 13.91; 5.59; 6.33; 0.99; 4.03; 0.81; 0.21; 0.98; 0.15; 0.96; 0.24; 0.73; 0.11; 0.70; 0.12; 21.95 | 43.6 | 1.74 | Amireh et al. (2019) [120] |
| Brazil | Ba; Zr; Th | 6480; 2450; 103 | La; Ce; Nd; Sm; Eu; Tb; Yb; Lu | 2220; 5310; 1490; 256; 68; 13.0; 14.8; 0.65 | 11272.5 | -- | De Oliveira et al. (2007) [121] |
| Brazil | N.A. | N.A. | Sc; Y; La; Ce; Nd; Pr; Sm; Sc; Eu; Dy; Gd; Er; Yb; Ho; Tb; Lu | 2.95; 41.95; 32.6; 44.3; 36.85; 8.05; 6.25; 1.9; 7.15; 8.15; 3.1; 2.65; 1.15; 1.35; 0.45 | 235.6 | -- | Silva et al. (2019) [122] |
5. Rare Earth Elements in Phosphate Deposits and Phosphogypsum: Historical Progress, Global Initiatives, and Challenges
6. Geochemistry of Rare Earth Elements in Phosphates, Global Distribution, Traceability, and Environmental Impact
6.1. Geochemistry
6.2. Global Distribution
6.3. Traceability and Environmental Impact
7. Environmental and Sustainability Challenges in the Phosphate Industry
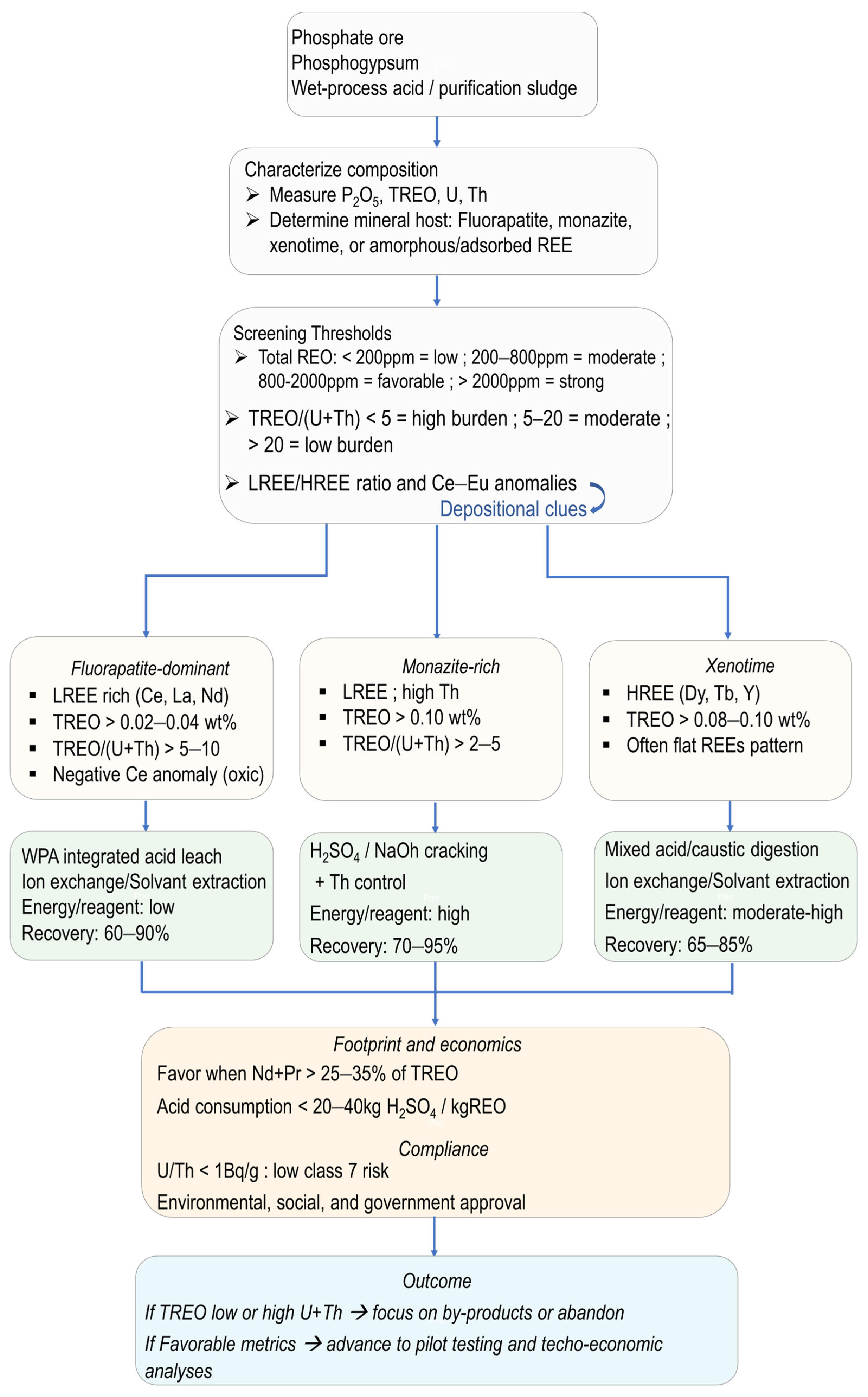
8. Opportunities and Strategies for REE Recovery from Phosphate Ores and Industrial By-Products
9. Executive Summary
10. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey. Mineral Commodity Summaries 2025; Version 1.2; U.S. Geological Survey: Reston, VA, USA, 2025; p. 212. [Google Scholar]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Spears, B.M.; Brownlie, W.J.; Cordell, D.; Hermann, L.; Mogollón, J.M. Concerns about Global Phosphorus Demand for Lithium-Iron-Phosphate Batteries in the Light Electric Vehicle Sector. Commun. Mater. 2022, 3, 14. [Google Scholar] [CrossRef]
- Cisse, L.; Mrabet, T. World Phosphate Production: Overview and Prospects. Phosphorus Res. Bull. 2004, 15, 21–25. [Google Scholar] [CrossRef]
- Tahir, M.; Khalid, U.; Ijaz, M.; Shah, G.M.; Naeem, M.A.; Shahid, M.; Mahmood, K.; Ahmad, N.; Kareem, F. Combined Application of Bio-Organic Phosphate and Phosphorus Solubilizing Bacteria (Bacillus Strain MWT 14) Improve the Performance of Bread Wheat with Low Fertilizer Input under an Arid Climate. Braz. J. Microbiol. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Alexandratos, N. World Agriculture Towards 2030/2050: The 2012 Revision; Food and Agriculture Organization of the UN: Rome, Italy, 2012. [Google Scholar]
- Scholz, R.W.; Wellmer, F.-W. Approaching a Dynamic View on the Availability of Mineral Resources: What We May Learn from the Case of Phosphorus? Glob. Environ. Change 2013, 23, 11–27. [Google Scholar] [CrossRef]
- Elser, J.; Bennett, E. A Broken Biogeochemical Cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Weiner, M.L.; Salminen, W.F.; Larson, P.R.; Barter, R.A.; Kranetz, J.L.; Simon, G.S. Toxicological Review of Inorganic Phosphates. Food Chem. Toxicol. 2001, 39, 759–786. [Google Scholar] [CrossRef]
- Gallala, W.; Herchi, F.; Ali, I.B.; Abbassi, L.; Gaied, M.E.; Montacer, M. Beneficiation of Phosphate Solid Coarse Waste from Redayef (Gafsa Mining Basin) by Grinding and Flotation Techniques. Procedia Eng. 2016, 138, 85–94. [Google Scholar] [CrossRef]
- Becker, P. Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process, 2nd ed.; Revised and Expanded; Marcel Dekker, Inc.: New York, NY, USA, 1989; Volume 6, ISBN 978-0-8247-7976-4. [Google Scholar]
- Awwad, N.S.; El-Nadi, Y.A.; Hamed, M.M. Successive Processes for Purification and Extraction of Phosphoric Acid Produced by Wet Process. Chem. Eng. Process. Process Intensif. 2013, 74, 69–74. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G.J. Extraction of Lanthanides from the Phosphoric Acid Production Process to Gain a Purified Gypsum and a Valuable Lanthanide By-Product. Hydrometallurgy 2000, 58, 51–60. [Google Scholar] [CrossRef]
- Calderón-Morales, B.R.S.; García-Martínez, A.; Pineda, P.; García-Tenório, R. Valorization of Phosphogypsum in Cement-Based Materials: Limits and Potential in Eco-Efficient Construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental Impact and Management of Phosphogypsum (Review). J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of Phosphogypsum in Soil Stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The Application of Phosphogypsum in Agriculture and the Radiological Impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a Construction Material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Bouargane, B.; Laaboubi, K.; Biyoune, M.G.; Bakiz, B.; Atbir, A. Effective and Innovative Procedures to Use Phosphogypsum Waste in Different Application Domains: Review of the Environmental, Economic Challenges and Life Cycle Assessment. J. Mater. Cycles Waste Manag. 2023, 25, 1288–1308. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of Waste Phosphogypsum to Prepare Hydroxyapatite Nanoparticles and Its Application towards Removal of Fluoride from Aqueous Solution. J. Hazard. Mater. 2012, 241–242, 418–426. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental Impacts of Phosphogypsum. Sci. Total Environ. 1994, 149, 1–38. [Google Scholar] [CrossRef]
- Zairi, M.; Rouis, M.J. Impacts Environnementaux Du Stockage Du Phosphogypse à Sfax (Tunisie). Bull.-Lab. Des. Ponts Et Chaussées 1999, 219, 29–40. [Google Scholar]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M. Changes in Mobility of Toxic Elements during the Production of Phosphoric Acid in the Fertilizer Industry of Huelva (SW Spain) and Environmental Impact of Phosphogypsum Wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef]
- Ajam, L.; Ben Ouezdou, M.; Felfoul, H.S.; Mensi, R.E. Characterization of the Tunisian Phosphogypsum and Its Valorization in Clay Bricks. Constr. Build. Mater. 2009, 23, 3240–3247. [Google Scholar] [CrossRef]
- Ajmal, P.Y.; Bhangare, R.C.; Tiwari, M.; Sahu, S.K.; Pandit, G.G. External Gamma Radiation Levels and Natural Radioactivity in Soil around a Phosphate Fertilizer Plant at Mumbai. J. Radioanal. Nucl. Chem. 2014, 300, 23–27. [Google Scholar] [CrossRef]
- El Afifi, E.M.; Hilal, M.A.; Attallah, M.F.; EL-Reefy, S.A. Characterization of Phosphogypsum Wastes Associated with Phosphoric Acid and Fertilizers Production. J. Environ. Radioact. 2009, 100, 407–412. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.B.; Klar, J.K.; Castet, S.; Grégoire, M.; Courjault-Radé, P.; Fabre, S. Spatial Distribution Patterns, Eco-Environmental Risk Assessment, and Human Health Impacts of Uranium and Thorium in Beach Sediments in the Central Gulf of Gabes (Southern Mediterranean Sea). Sustainability 2025, 17, 1283. [Google Scholar] [CrossRef]
- Gulbrandsen, R.A. Chemical Composition of Phosphorites of the Phosphoria Formation. Geochim. Et Cosmochim. Acta 1966, 30, 769–778. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; Du Bray, E.A.; Koenig, A.E. Rare Earth Elements in Sedimentary Phosphate Deposits: Solution to the Global REE Crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; De Carvalho, T.S.; Chaves, L.C.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in Raw Materials and Products of the Phosphate Fertilizer Industry in South America: Content, Signature, and Crystalline Phases. J. Geochem. Explor. 2016, 168, 177–186. [Google Scholar] [CrossRef]
- El Zrelli, R.; Baliteau, J.Y.; Yacoubi, L.; Castet, S.; Grégoire, M.; Fabre, S.; Sarazin, V.; Daconceicao, L.; Courjault-Radé, P.; Rabaoui, L. Rare Earth Elements Characterization Associated to the Phosphate Fertilizer Plants of Gabes (Tunisia, Central Mediterranean Sea): Geochemical Properties and Behavior, Related Economic Losses, and Potential Hazards. Sci. Total Environ. 2021, 791, 148268. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C. European Commission: Directorate-General for Internal Market, I., Entrepreneurship and SMEs. In Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Lu, S.-B.; Warmadewanthi; Liu, J.-C. Recovery of Rare Earth Elements from Phosphogypsum Using Subcritical Water Extraction. Chem. Eng. Process.—Process Intensif. 2023, 190, 109433. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Macías, F.; Pérez López, R.; Nieto, J.M. Mobility of Rare Earth Elements, Yttrium and Scandium from a Phosphogypsum Stack: Environmental and Economic Implications. Sci. Total Environ. 2018, 618, 847–857. [Google Scholar] [CrossRef]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving Toward a Circular Economy for Phosphorus: The Role of Phosphate Rock Mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef]
- Rout, S.; Abhilash; Meshram, P.; Zhang, P. A Comprehensive Review on Occurrence and Processing of Phosphate Rock Based Resources- Focus on REEs. Miner. Process. Extr. Metall. Rev. 2024, 45, 368–388. [Google Scholar] [CrossRef]
- Zhang, P. Comprehensive Recovery and Sustainable Development of Phosphate Resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Bobba, S.; Carrara, S.; Huisman, J.; Mathieux, F.; Pavel, C.; Europäische Kommission. Critical Raw Materials for Strategic Technologies and Sectors in the EU: A Foresight Study; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-15337-5. [Google Scholar]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Natural Earth Free Vector and Raster Map Data 2023. Available online: https://www.naturalearthdata.com/downloads/10m-cultural-vectors/10m-admin-0-countries/ (accessed on 7 November 2025).
- Mason, G.T.; Arndt, R.E. U.S. Geological Survey Mineral Resources Data System (MRDS) 2023; U.S. Geological Survey: Reston, VA, USA, 2023. [Google Scholar]
- Ulrich, A.E.; Frossard, E. On the History of a Reoccurring Concept: Phosphorus Scarcity. Sci. Total Environ. 2014, 490, 694–707. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Tracking Phosphorus Security: Indicators of Phosphorus Vulnerability in the Global Food System. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The Future Distribution and Production of Global Phosphate Rock Reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Grand View Research. Phosphate-Rock-Market. Available online: https://www.grandviewresearch.com/press-release/global-phosphate-rock-market (accessed on 7 November 2025).
- Khabarov, N.; Obersteiner, M. Global Phosphorus Fertilizer Market and National Policies: A Case Study Revisiting the 2008 Price Peak. Front. Nutr. 2017, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Vaccari, D.A.; Powers, S.M.; Liu, X. Demand-Driven Model for Global Phosphate Rock Suggests Paths for Phosphorus Sustainability. Environ. Sci. Technol. 2019, 53, 10417–10425. [Google Scholar] [CrossRef]
- Technavio. Phosphate Rock Market Analysis—Size and Forecast 2025–2029. Available online: https://www.technavio.com/report/phosphate-rock-market-analysis (accessed on 7 November 2025).
- Issaoui, R.; Rösch, C.; Woidasky, J.; Schmidt, M.; Viere, T. Cradle-to-Gate Life Cycle Assessment of Beneficiated Phosphate Rock Production in Tunisia. NachhaltigkeitsManagementForum 2021, 29, 107–118. [Google Scholar] [CrossRef]
- Jalali, J.; Gaudin, P.; Capiaux, H.; Ammar, E.; Lebeau, T. Fate and Transport of Metal Trace Elements from Phosphogypsum Piles in Tunisia and Their Impact on Soil Bacteria and Wild Plants. Ecotoxicol. Environ. Saf. 2019, 174, 12–25. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Abda, H.; Daghbouj, N.; Pérez-López, R.; Castet, S.; Aigouy, T.; Bejaoui, N.; Courjault-Radé, P. Characterization of the Role of Phosphogypsum Foam in the Transport of Metals and Radionuclides in the Southern Mediterranean Sea. J. Hazard. Mater. 2019, 363, 258–267. [Google Scholar] [CrossRef]
- Ben Amor, R.; Fathallah, S.; Gueddari, M. Impact of Phosphogypsum Waste on the Geochemistry of the Coastal Water of Ghannouche-Gabes (SE of Tunisia). In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 19–24 April 2009; p. 7999. [Google Scholar]
- El Kateb, A.; Stalder, C.; Rüggeberg, A.; Neururer, C.; Spangenberg, J.E.; Spezzaferri, S. Impact of Industrial Phosphate Waste Discharge on the Marine Environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, B.; Gzam, M.; Souid, F.; Telahigue, F.; Chahlaoui, A.; Ouarrak, K.; Kharroubi, A. Assessment of Heavy Metal Contamination in Gulf of Gabès Coastland (Southeastern Tunisia): Impact of Chemical Industries and Drift Currents. Arab. J. Geosci. 2020, 13, 1180. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of Phosphate Rock and Phosphogypsum from Gabes Phosphate Fertilizer Factories (SE Tunisia): High Mining Potential and Implications for Environmental Protection. Environ. Sci. Pollut. Res. 2018, 25, 14690–14702. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Ben Alaya, M.; Daghbouj, N.; Castet, S.; Besson, P.; Michel, S.; Bejaoui, N.; Courjault-Radé, P. Seawater Quality Assessment and Identification of Pollution Sources along the Central Coastal Area of Gabes Gulf (SE Tunisia): Evidence of Industrial Impact and Implications for Marine Environment Protection. Mar. Pollut. Bull. 2018, 127, 445–452. [Google Scholar] [CrossRef]
- Ben Amor, R.; Gueddari, M. Major Ion Geochemistry of Ghannouch–Gabes Coastline (at Southeast Tunisia, Mediterranean Sea): Study of the Impact of Phosphogypsum Discharges by Geochemical Modeling and Statistical Analysis. Environ. Earth Sci. 2016, 75, 851. [Google Scholar] [CrossRef]
- Melki, S.; Gueddari, M. Impact Assessment of Phosphogypsum Leachate on Groundwater of Sfax-Agareb (Southeast of Tunisia): Using Geochemical and Isotopic Investigation. J. Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Klinger, J.M. A Historical Geography of Rare Earth Elements: From Discovery to the Atomic Age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Meng, D.; Wang, M.; Feng, Z.; Xia, C.; Zhao, Y.; Huang, X. Behavior of Phase Transformation of Baotou Mixed Rare Earth Concentrate during Oxidation Roasting. J. Rare Earths 2022, 40, 981–987. [Google Scholar] [CrossRef]
- Tuncay, G.; Yuksekdag, A.; Mutlu, B.K.; Koyuncu, I. A Review of Greener Approaches for Rare Earth Elements Recovery from Mineral Wastes. Environ. Pollut. 2024, 357, 124379. [Google Scholar] [CrossRef]
- Alonso, E.; Wallington, T.; Sherman, A.; Everson, M.; Field, F.; Roth, R.; Kirchain, R. An Assessment of the Rare Earth Element Content of Conventional and Electric Vehicles. SAE Int. J. Mater. Manuf. 2012, 5, 473–477. [Google Scholar] [CrossRef]
- Alonso, E.; Pineault, D.G.; Gambogi, J.; Nassar, N.T. Mapping First to Final Uses for Rare Earth Elements, Globally and in the United States. J. Ind. Ecol. 2023, 27, 312–322. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of Critical Metals in the Future Markets of Clean Energy Technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global Rare Earth Elements Projects: New Developments and Supply Chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the Recovery of Rare Earth Elements (REE) from Phosphogypsum Waste—Case Study of the WIZÓW Chemical Plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Kurkinen, S.; Virolainen, S.; Sainio, T. Recovery of Rare Earth Elements from Phosphogypsum Waste in Resin-in-Leach Process by Eluting with Biodegradable Complexing Agents. Hydrometallurgy 2021, 201, 105569. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. The Potential for Mining Trace Elements from Phosphate Rock. J. Clean. Prod. 2015, 91, 337–346. [Google Scholar] [CrossRef]
- Lütke, S.F.; Oliveira, M.L.S.; Waechter, S.R.; Silva, L.F.O.; Cadaval, T.R.S.; Duarte, F.A.; Dotto, G.L. Leaching of Rare Earth Elements from Phosphogypsum. Chemosphere 2022, 301, 134661. [Google Scholar] [CrossRef]
- Gupta, A.; Williams, E.; Gaustad, G. Forecasting Revenue from Primary and Secondary Sources of Rare Earth Elements. Resour. Conserv. Recycl. 2024, 207, 107612. [Google Scholar] [CrossRef]
- Elshkaki, A. Sustainability of Emerging Energy and Transportation Technologies Is Impacted by the Coexistence of Minerals in Nature. Commun. Earth Environ. 2021, 2, 186. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Blais, J.-F.; Royer-Lavallée, A.; Coudert, L.; Neculita, C.M. Review of Recent Work on the Recovery of Rare Earth Elements from Secondary Sources; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Dushyantha, N.P.; Ratnayake, N.P.; Premasiri, H.M.R.; Ilankoon, I.M.S.K.; Hemalal, P.V.A.; Jayawardena, C.L.; Chandrajith, R.; Rohitha, L.P.S.; Abeysinghe, A.M.K.B.; Dissanayake, D.M.D.O.K.; et al. Leaching of Rare Earth Elements (REEs) from Lake Sediments around Eppawala Phosphate Deposit, Sri Lanka: A Secondary Source for REEs. Hydrometallurgy 2021, 205, 105751. [Google Scholar] [CrossRef]
- Khelifi, F.; Batool, S.; Kechiched, R.; Padoan, E.; Ncibi, K.; Hamed, Y. Abundance, Distribution, and Ecological/Environmental Risks of Critical Rare Earth Elements (REE) in Phosphate Ore, Soil, Tailings, and Sediments: Application of Spectroscopic Fingerprinting. J. Soils Sediments 2024, 24, 2099–2118. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwon, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global Demand for Rare Earth Resources and Strategies for Green Mining. Environ. Res. 2016, 150, 182–190. [Google Scholar] [CrossRef]
- Garnit, H.; Bouhlel, S.; Barca, D.; Chtara, C. Application of LA-ICP-MS to Sedimentary Phosphatic Particles from Tunisian Phosphorite Deposits: Insights from Trace Elements and REE into Paleo-Depositional Environments. Geochemistry 2012, 72, 127–139. [Google Scholar] [CrossRef]
- Christmann, P. A Forward Look into Rare Earth Supply and Demand: A Role for Sedimentary Phosphate Deposits? Procedia Eng. 2014, 83, 19–26. [Google Scholar] [CrossRef]
- Altschuler, Z.S.; Berman, S.; Cuttitta, F. Rare Earths in Phosphorites—Geochemistry and Potential Recovery; U.S. Geological Survey: Reston, VA, USA, 1967. [Google Scholar]
- Baturin, G.N.; Bliskovskii, V.Z.; Mineev, D.A. Rare Earth Elements in Phosphorites from the Ocean Floor. In Proceedings of the Doklady Akademii Nauk SSSR; Academy of Sciences of the USSR: Moscow, Russia, 1972; Volume 207, pp. 954–957. [Google Scholar]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-Earth Leaching from Florida Phosphate Rock in Wet-Process Phosphoric Acid Production. Miner. Metall. Process. 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Ptáček, P. Mining and Beneficiation of Phosphate Ore. In Apatites and Their Synthetic Analogues—Synthesis, Structure, Properties and Applications; Ptáček, P., Ed.; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2266-1. [Google Scholar]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of Biological Recovery of Rare-Earth Elements from Industrial and Electronic Wastes: A Review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Rakovan, J.; Reeder, R.J. Intracrystalline Rare Earth Element Distributions in Apatite: Surface Structural Influences on Incorporation during Growth. Geochim. Et Cosmochim. Acta 1996, 60, 4435–4445. [Google Scholar] [CrossRef]
- Get’man, E.I.; Loboda, S.N.; Ignatov, A.V.; Prisedsky, V.V.; Abdul Jabar, M.A.B.; Ardanova, L.I. Isomorphous Substitution of Rare-Earth Elements in Lacunary Apatite Pb8Na2(PO4)6. Inorg. Chem. 2016, 55, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jackson, W.D.; Christiansen, G. International Strategic Minerals Inventory Summary Report—Rare-Earth Oxides; Circular; U.S. Geological Survey: Reston, VA, USA, 1993. [Google Scholar]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Engi, M. Petrochronology Based on REE-Minerals: Monazite, Allanite, Xenotime, Apatite. Rev. Mineral. Geochem. 2017, 83, 365–418. [Google Scholar] [CrossRef]
- Chen, W.; Honghui, H.; Bai, T.; Jiang, S. Geochemistry of Monazite within Carbonatite Related REE Deposits. Resources 2017, 6, 51. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. Crystal Chemistry of the Monazite and Xenotime Structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Rudolph, M.; Leistner, T.; Gutzmer, J.; Peuker, U.A. A Review of Rare Earth Minerals Flotation: Monazite and Xenotime. Int. J. Min. Sci. Technol. 2015, 25, 877–883. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Z.; He, D.; Deng, J.; Zhao, H.; Li, H. Simultaneous Leaching of Rare Earth Elements and Phosphorus from a Chinese Phosphate Ore Using H3PO4. Green Process. Synth. 2021, 10, 258–267. [Google Scholar] [CrossRef]
- Jang, G.G.; Thompson, J.A.; Meyer, P.A.; Zhang, P.; Shen, Z.; Tsouris, C. Technoeconomic Assessment of Phosphoric Acid and Rare Earth Element Recovery from Phosphoric Acid Sludge. Sustainability 2024, 16, 6984. [Google Scholar] [CrossRef]
- Salem, M.; Souissi, R.; Souissi, F.; Abbes, N.; Moutte, J. Phosphoric Acid Purification Sludge: Potential in Heavy Metals and Rare Earth Elements. Waste Manag. 2019, 83, 46–56. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of Rare Earth Elements from Phosphate Rock by Hydrometallurgical Processes—A Critical Review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Diwa, R.R.; Tabora, E.U.; Haneklaus, N.H.; Ramirez, J.D. Rare Earths Leaching from Philippine Phosphogypsum Using Taguchi Method, Regression, and Artificial Neural Network Analysis. J. Mater. Cycles Waste Manag. 2023, 25, 3316–3330. [Google Scholar] [CrossRef]
- Maina, L.; Kiegiel, K.; Samczyński, Z.; Haneklaus, N.; Zakrzewska-Kołtuniewicz, G. Sulfuric Acid Leaching Recovery of Rare Earth Elements from Wizów’s Phosphogypsum in Poland. Sustainability 2024, 16, 9059. [Google Scholar] [CrossRef]
- Battsengel, A.; Batnasan, A.; Narankhuu, A.; Haga, K.; Watanabe, Y.; Shibayama, A. Recovery of Light and Heavy Rare Earth Elements from Apatite Ore Using Sulphuric Acid Leaching, Solvent Extraction and Precipitation. Hydrometallurgy 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Yu, S.; Ao, X.; Liang, L.; Mao, X.; Guo, Y. Recovery of Rare Earth Elements from Sedimentary Rare Earth Ore via Sulfuric Acid Roasting and Water Leaching. J. Rare Earths 2024, 43, 805–814. [Google Scholar] [CrossRef]
- Cheremisina, O. Recovery of Rare Earth Metals from Phosphogypsum—Apatite Ore Sulfuric Acid Leaching Product. In Proceedings of the 19th SGEM International Multidisciplinary Scientific GeoConference EXPO, Sofia, Bulgaria, 20 June 2019. [Google Scholar]
- Wang, L.; Long, Z.; Huang, X.; Yu, Y.; Cui, D.; Zhang, G. Recovery of Rare Earths from Wet-Process Phosphoric Acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Tanaka, M.; Hoshino, M.; Kon, Y.; Watanabe, Y. Selective Recovery of Heavy Rare Earth Elements from Apatite with an Adsorbent Bearing Immobilized Tridentate Amido Ligands. Sep. Purif. Technol. 2016, 159, 157–160. [Google Scholar] [CrossRef]
- Smida, O.; Souissi, R.; Salem, M.; Souissi, F. Geochemical Assessment and Mobility of Undesired Elements in the Sludge of the Phosphate Industry of Gafsa-Metlaoui Basin, (Southern Tunisia). Appl. Sci. 2021, 11, 1075. [Google Scholar] [CrossRef]
- Garnit, H.; Bouhlel, S.; Jarvis, I. Geochemistry and Depositional Environments of Paleocene–Eocene Phosphorites: Metlaoui Group, Tunisia. J. Afr. Earth Sci. 2017, 134, 704–736. [Google Scholar] [CrossRef]
- Galfati, I.; Sassi, A.B.; Zaïer, A.; Bouchardon, J.L.; Bilal, E.; Joron, J.L.; Sassi, S. Geochemistry and Mineralogy of Paleocene-Eocene Oum El Khecheb Phosphorites (Gafsa-Metlaoui Basin) Tunisia. Geochem. J. 2010, 44, 189–210. [Google Scholar] [CrossRef]
- Kechiched, R.; Laouar, R.; Bruguier, O.; Kocsis, L.; Salmi-Laouar, S.; Bosch, D.; Ameur-Zaimeche, O.; Foufou, A.; Larit, H. Comprehensive REE + Y and Sensitive Redox Trace Elements of Algerian Phosphorites (Tébessa, Eastern Algeria): A Geochemical Study and Depositional Environments Tracking. J. Geochem. Explor. 2020, 208, 106396. [Google Scholar] [CrossRef]
- Amine, M.; Asafar, F.; Bilali, L.; Nadifiyine, M. Hydrochloric Acid Leaching Study of Rare Earth Elements from Moroccan Phosphate. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Niu, H.; Xing, J.; Yan, S.; Li, A.; Weng, Q.; Zhao, X. Enrichment of Rare Earth Elements in the Early Cambrian Zhijin Phosphorite Deposit, SW China: Evidence from Francolite Micro-Petrography and Geochemistry. Ore Geol. Rev. 2021, 138, 104342. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, S.-Y.; Yang, J.-H.; Wu, H.-P.; Pi, D.-H. Rare Earth Element and Sr–Nd Isotope Geochemistry of Phosphatic Rocks in Neoproterozoic Ediacaran Doushantuo Formation in Zhangcunping Section from Western Hubei Province, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 440, 712–724. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, S.; Yang, J.; Wu, H.; Pi, D. Rare Earth Element Geochemistry of Phosphatic Rocks in Neoproterozoic Ediacaran Doushantuo Formation in Hushan Section from the Yangtze Gorges Area, South China. J. Earth Sci. 2016, 27, 204–210. [Google Scholar] [CrossRef]
- Murthy, R.; Kidder, D.; Mapes, R.; Hannigan, R. Rare-Earth Element Chemistry of Mississippian–Age Phosphate Nodules in the Fayetteville Shale of Oklahoma and Arkansas. Environ. Geosci. 2004, 11, 99–111. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective. In Non-Renewable Resource Issues; Sinding-Larsen, R., Wellmer, F.-W., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 131–155. ISBN 978-90-481-8678-5. [Google Scholar]
- Shahin, M.; Elmongy, S.; Saad, E.; Shazly, A.; Ezzat, A. Evaluation of Rare Earth Elements in Black Sand and Phosphate Ores, EGYPT. Egypt. J. Chem. 2020, 63, 4185–4193. [Google Scholar] [CrossRef]
- Abed, A.M.; Jaber, O.; Alkuisi, M.; Sadaqah, R. Rare Earth Elements and Uranium Geochemistry in the Al-Kora Phosphorite Province, Late Cretaceous, Northwestern Jordan. Arab. J. Geosci. 2016, 9, 187. [Google Scholar] [CrossRef]
- Amireh, B.S.; Amaireh, M.N.; Taha, S.A.; Abed, A.M. Petrogenesis, Provenance, and Rare Earth Element Geochemistry, Southeast Desert Phosphorite, Jordan. J. Afr. Earth Sci. 2019, 150, 701–721. [Google Scholar] [CrossRef]
- De Oliveira, S.M.B.; Da Silva, P.S.C.; Mazzilli, B.P.; Favaro, D.I.T.; Saueia, C.H. Rare Earth Elements as Tracers of Sediment Contamination by Phosphogypsum in the Santos Estuary, Southern Brazil. Appl. Geochem. 2007, 22, 837–850. [Google Scholar] [CrossRef]
- Silva, F.B.V.; Nascimento, C.W.A.; Alvarez, A.M.; Araújo, P.R.M. Inputs of Rare Earth Elements in Brazilian Agricultural Soils via P-Containing Fertilizers and Soil Correctives. J. Environ. Manag. 2019, 232, 90–96. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. REE Extraction from Phosphoric Acid, Phosphoric Acid Sludge, and Phosphogypsum. Miner. Process. Extr. Metall. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Yang, X.; Makkonen, H.T.; Pakkanen, L. Rare Earth Occurrences in Streams of Processing a Phosphate Ore. Minerals 2019, 9, 262. [Google Scholar] [CrossRef]
- Amaral, J.C.B.S.; Sá, M.L.C.G.; Morais, C.A. Recovery of Uranium, Thorium and Rare Earth from Industrial Residues. Hydrometallurgy 2018, 181, 148–155. [Google Scholar] [CrossRef]
- Khawassek, Y.M.; Eliwa, A.A.; Gawad, E.A.; Abdo, S.M. Recovery of Rare Earth Elements from El-Sela Effluent Solutions. J. Radiat. Res. Appl. Sci. 2015, 8, 583–589. [Google Scholar] [CrossRef]
- Hamza, M.F.; El-Aassy, I.E.; Guibal, E. Integrated Treatment of Tailing Material for the Selective Recovery of Uranium, Rare Earth Elements and Heavy Metals. Miner. Eng. 2019, 133, 138–148. [Google Scholar] [CrossRef]
- Samsonov, M.D.; Trofimov, T.I.; Kulyako, Y.M.; Vinokurov, S.E.; Malikov, D.A.; Batorshin, G.S.; Myasoedov, B.F. Recovery of Rare Earth Elements, Uranium, and Thorium from Monazite Concentrate by Supercritical Fluid Extraction. Radiochemistry 2015, 57, 343–347. [Google Scholar] [CrossRef]
- Graupner, T.; Mühlbach, C.; Schwarz-Schampera, U.; Henjes-Kunst, F.; Melcher, F.; Terblanche, H. Mineralogy of High-Field-Strength Elements (Y, Nb, REE) in the World-Class Vergenoeg Fluorite Deposit, South Africa. Ore Geol. Rev. 2015, 64, 583–601. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Rare-Earth Elements in Marine Sediments and Geochemical Standards. Chem. Geol. 1990, 88, 333–347. [Google Scholar] [CrossRef]
- Menendez, A.; James, R.H.; Roberts, S.; Peel, K.; Connelly, D. Controls on the Distribution of Rare Earth Elements in Deep-Sea Sediments in the North Atlantic Ocean. Ore Geol. Rev. 2017, 87, 100–113. [Google Scholar] [CrossRef]
- Khadijeh, R.E.S.; Elias, S.B.; Wood, A.K.; Reza, A.M. Rare Earth Elements Distribution in Marine Sediments of Malaysia Coasts. J. Rare Earths 2009, 27, 1066–1071. [Google Scholar] [CrossRef]
- Toyoda, K.; Nakamura, Y.; Masuda, A. Rare Earth Elements of Pacific Pelagic Sediments. Geochim. Cosmochim. Acta 1990, 54, 1093–1103. [Google Scholar] [CrossRef]
- Milinovic, J.; Rodrigues, F.J.L.; Barriga, F.J.A.S.; Murton, B.J. Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites. Minerals 2021, 11, 142. [Google Scholar] [CrossRef]
- Ouyang, A.; Xiong, W.; Li, X.; Chen, D.; Zhang, L.; Jiang, P. Occurrence and Screening- Flotation Separation for the Beneficiation of Rare Earth Elements and Yttrium (REY) in Core Sediments from the Pacific Ocean. Mar. Geol. 2023, 462, 107097. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, L.; Wang, L.; Huang, X.; Zhang, Y.; Feng, Z.; Cui, D. Simultaneous Recovery of Rare Earth Elements and Phosphorus from Phosphate Rock by Phosphoric Acid Leaching and Selective Precipitation: Towards Green Process. J. Rare Earths 2019, 37, 652–658. [Google Scholar] [CrossRef]
- Hammache, Z.; Berbar, Y.; Bensaadi, S.; Trari, M.; Amara, M. Recovery of Light Rare Earth Elements by Leaching and Extraction from Phosphate Mining Waste (Fluorapatite and Carbonate-Fluorapatite). J. Afr. Earth Sci. 2020, 171, 103937. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals 2018, 8, 416. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Radiation Protection and Management of NORM Residues in the Phosphate Industry; Safety reports series; International Atomic Energy Agency: Vienna, Austria, 2013; ISBN 978-92-0-135810-3. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Pérez-López, R.; Macías, F.; Chapron, S.; Nieto, J.M.; Pellet-Rostaing, S. Exploration of Fertilizer Industry Wastes as Potential Source of Critical Raw Materials. J. Clean. Prod. 2017, 143, 497–505. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J.; Stauffer, P.H.; Hendley, J.W., II. Rare Earth Elements—Critical Resources for High Technology; Fact Sheet; U.S. Geological Survey: Reston, VA, USA, 2002. [Google Scholar]
- Park, S.; Tracy, C.L.; Ewing, R.C. Reimagining US Rare Earth Production: Domestic Failures and the Decline of US Rare Earth Production Dominance–Lessons Learned and Recommendations. Resour. Policy 2023, 85, 104022. [Google Scholar] [CrossRef]
- Hellman, P.L.; Duncan, R.K. Evaluating Rare Earth Element Deposits. ASEG Ext. Abstr. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Lynas Rare Earths. Residue and Tailings Management. Available online: https://lynasrareearths.com/sustainability/residue-tailings-management/ (accessed on 7 November 2025).
- Chen, L.; Zhou, S.; Wu, S.; Wang, C.; Li, B.; Li, Y.; Wang, J. Combining Emission Inventory and Isotope Ratio Analyses for Quantitative Source Apportionment of Heavy Metals in Agricultural Soil. Chemosphere 2018, 204, 140–147. [Google Scholar] [CrossRef]
- Wang, Z.; Hill, R.; Williams, G.; Dwyer, G.S.; Hu, J.; Schnug, E.; Bol, R.; Sun, Y.; Coleman, D.S.; Liu, X.-M.; et al. Lead Isotopes and Rare Earth Elements Geochemistry of Global Phosphate Rocks: Insights into Depositional Conditions and Environmental Tracing. Chem. Geol. 2023, 639, 121715. [Google Scholar] [CrossRef]
- Shields, G. Stratigraphic Trends in Cerium Anomaly in Authigenic Marine Carbonates and Phosphates: Diagenetic Alteration or Seawater Signals? Mineral. Mag. 1998, 62A, 1387–1388. [Google Scholar] [CrossRef]
- Shields, G.; Stille, P. Diagenetic Constraints on the Use of Cerium Anomalies as Palaeoseawater Redox Proxies: An Isotopic and REE Study of Cambrian Phosphorites. Chem. Geol. 2001, 175, 29–48. [Google Scholar] [CrossRef]
- Wu, Y. Review of Comprehensive Utilization of Phosphate Rock-Rare Earth Recovery. J. Wuhan Inst. Chem. Ind. 1983, 1, 1–13. [Google Scholar]
- Singh, D.K.; Kain, V. Phosphatic Resources: A Valuable Wealth of Rare Earths. In Critical and Rare Earth Elements; CRC Press: Boca Raton, FL, USA, 2019; pp. 331–342. [Google Scholar] [CrossRef]
- Goldstein, I.J. Preparation of the Precipitate and of Rare Earth Oxides from Kola Apatites. Rev. Chem. 1965, 16, 359–360. [Google Scholar]
- Habashi, F. The Recovery of the Lanthanides from Phosphate Rock. J. Chem. Technol. Biotechnol. Chem. Technol. 1985, 35, 5–14. [Google Scholar] [CrossRef]
- Rollat, A. Recovery of Rare Earths from Wet-Process Phosphoric Acid, the Solvay Experience. Procedia Eng. 2016, 138, 273–280. [Google Scholar] [CrossRef]
- Mashkovtsev, M.; Botalov, M.; Smyshlyaev, D.; Pajarre, R.; Kangas, P.; Rychkov, V.; Koukkari, P. Pilot-Scale Recovery of Rare Earths and Scandium from Phosphogypsum and Uranium Leachates. E3S Web Conf. 2016, 8, 01026. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Production of a Rare Earths Concentrate after Phosphogypsum Treatment with Dietary NaCl and Na2CO3 Solutions. Miner. Eng. 2019, 132, 169–174. [Google Scholar] [CrossRef]
- Masmoudi-Soussi, A.; Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M. Rare Earths Recovery by Fractional Precipitation from a Sulfuric Leach Liquor Obtained after Phosphogypsum Processing. Hydrometallurgy 2020, 191, 105253. [Google Scholar] [CrossRef]
- Khater, A.E.M.; Galmed, M.A.; Nasr, M.M.; El-Taher, A. Uranium and Rare Earth Elements in Hazm El-Jalamid Phosphate, Saudi Arabia: Concentrations and Geochemical Patterns Comparison. Environ. Earth Sci. 2016, 75, 1261. [Google Scholar] [CrossRef]
- Ji, B.; Li, Q.; Huang, Q.; Zhang, W. Enhanced Leaching Recovery of Rare Earth Elements from a Phosphatic Waste Clay through Calcination Pretreatment. J. Clean. Prod. 2021, 319, 128654. [Google Scholar] [CrossRef]
- Togizov, K.; Issayeva, L.; Muratkhanov, D.; Kurmangazhina, M.; Swęd, M.; Duczmal-Czernikiewicz, A. Rare Earth Elements in the Shok-Karagay Ore Fields (Syrymbet Ore District, Northern Kazakhstan) and Visualisation of the Deposits Using the Geography Information System. Minerals 2023, 13, 1458. [Google Scholar] [CrossRef]
- Xie, G.; Guan, Q.; Zhou, F.; Yu, W.; Yin, Z.; Tang, H.; Zhang, Z.; Chi, R. A Critical Review of the Enhanced Recovery of Rare Earth Elements from Phosphogypsum. Molecules 2023, 28, 6284. [Google Scholar] [CrossRef]
- Podbiera-Matysik, K.; Gorazda, K.; Wzorek, Z. Potencial Management of Waste Phosphogypsum with Particular Focus on Recovery of Rare Earth Metals. Pol. J. Chem. Technol. 2015, 17, 55–61. [Google Scholar] [CrossRef]
- Qing, J.; Zhao, D.; Zeng, L.; Zhang, G.; Zhou, L.; Du, J.; Li, Q.; Cao, Z.; Wu, S. Comprehensive Recovery of Rare Earth Elements and Gypsum from Phosphogypsum: A Wastewater Free Process Combining Gravity Separation and Hydrometallurgy. J. Rare Earths 2025, 43, 362–370. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare Earths Concentration from Phosphogypsum Waste by Two-Step Leaching Method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Yang, J.; Dong, S.; Ma, L.; Dai, Q.; Zheng, D.; Huang, B.; Sun, M.; Hu, B.; Du, W.; Xie, L.; et al. Review on High-Value Utilization of Phosphogypsum: Utilization of Calcium and Oxygen Resources Present in Phosphogypusm. Sep. Purif. Technol. 2024, 344, 127246. [Google Scholar] [CrossRef]
- Yahorava, V.; Bazhko, V.; Freeman, M. Viability of Phosphogypsum as a Secondary Resource of Rare Earth Elements. In Proceedings of the XXVIII International Mineral Processing Congress Proceedings, Quebec City, QC, Canada, 11–15 September 2016; pp. 11–15. [Google Scholar]
- Soltani, F.; Abdollahy, M.; Petersen, J.; Ram, R.; Becker, M.; Javad Koleini, S.M.; Moradkhani, D. Leaching and Recovery of Phosphate and Rare Earth Elements from an Iron-Rich Fluorapatite Concentrate: Part I: Direct Baking of the Concentrate. Hydrometallurgy 2018, 177, 66–78. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Zhang, J.; Chao, J. Occurrence of Yttrium in the Zhijin Phosphorus Deposit in Guizhou Province, China. Open Geosci. 2022, 14, 776–784. [Google Scholar] [CrossRef]
- Sadaqah, R.M.; Abed, A.M.; Grimm, K.A.; Pufahl, P.K. The Geochemistry of Rare Earth Elements (REE), Yttrium (Y) and Scandium (Sc) in Some Upper Cretaceous Jordanian Phosphorites. J. Afr. Earth Sci. 2005, 32, 104635. [Google Scholar]
- Kogarko, L.N. Chemical Composition and Petrogenetic Implications of Apatite in the Khibiny Apatite–Nepheline Deposits (Kola Peninsula). Minerals 2018, 8, 532. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of Rare Earth Elements from Phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Tzifas, I.; Godelitsas, A.; Magganas, A.; Androulakaki, E.; Eleftheriou, G.; Mertzimekis, T.J.; Perraki, M. Uranium-Bearing Phosphatized Limestones of NW Greece. J. Geochem. Explor. 2014, 143, 62–73. [Google Scholar] [CrossRef]
- Buccione, R.; Kechiched, R.; Mongelli, G.; Sinisi, R. REEs in the North Africa P-Bearing Deposits, Paleoenvironments, and Economic Perspectives: A Review. Minerals 2021, 11, 214. [Google Scholar] [CrossRef]
- Bouabdallah, M.; Elgharbi, S.; Horchani-Naifer, K.; Barca, D.; Fattah, N.; Férid, M. Chemical, Mineralogical and Rare Earth Elements Distribution Study of Phosphorites from Sra Ouertane Deposit (Tunisia). J. Afr. Earth Sci. 2019, 157, 103505. [Google Scholar] [CrossRef]
- Preston, J.S.; Cole, P.M.; Craig, W.M.; Feather, A.M. The Recovery of Rare Earth Oxides from a Phosphoric Acid By-Product. Part 1: Leaching of Rare Earth Values and Recovery of a Mixed Rare Earth Oxide by Solvent Extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar] [CrossRef]
- Merroune, A.; Ait Brahim, J.; Achiou, B.; Kada, C.; Mazouz, H.; Beniazza, R. Closed-Loop Purification Process of Industrial Phosphoric Acid: Selective Recovery of Heavy Metals and Rare Earth Elements via Solvent Extraction. Desalination 2024, 580, 117515. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of Rare Earth Elements (REEs) and Impurities from Phosphogypsum: A Preliminary Insight for Further Recovery of Critical Raw Materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- El-Didamony, H.; Ali, M.M.; Awwad, N.S.; Fawzy, M.M.; Attallah, M.F. Treatment of Phosphogypsum Waste Using Suitable Organic Extractants. J. Radioanal. Nucl. Chem. 2012, 291, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Wamser, C.A.; Bruen, C.P. Recovery of Fluorine, Uranium and Rare Earth Metal Values from Phosphoric Acid By-Product Brine Raffinate. U.S. Patent 3,937,783, 10 February 1976. [Google Scholar]
- Bunus, F.T. Uranium and Rare Earth Recovery from Phosphate Fertilizer Industry by Solvent Extraction. Miner. Process. Extr. Metall. Rev. 2000, 21, 381–478. [Google Scholar] [CrossRef]
- Bunuş, F.; Dumitrescu, R. Simultaneous Extraction of Rare Earth Elements and Uranium from Phosphoric Acid. Hydrometallurgy 1992, 28, 331–338. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, H.; Jin, Z.; DePaoli, D.; Miller, J.; Lin, C.; Crossman, R. Rare Earths in Phosphate: Characterization and Extraction. In Rare Earth Elements: Sustainable Recovery, Processing, and Purification; Karamalidis, A.K., Eggert, R., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 99–165. ISBN 978-1-119-51503-6. [Google Scholar]
- He, N.; Zhang, Z.; Meng, X.; Davaasambuu, S.; Zhao, H. Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia Cepacia and Aspergillus Niger. Minerals 2024, 14, 1055. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards Zero-Waste Valorisation of Rare-Earth-Containing Industrial Process Residues: A Critical Review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Bech, J.; Suarez, M.; Reverter, F.; Tume, P.; Sánchez, P.; Bech, J.; Lansac, A. Selenium and Other Trace Elements in Phosphate Rock of Bayovar–Sechura (Peru). J. Geochem. Explor. 2010, 107, 136–145. [Google Scholar] [CrossRef]
- Nourhen Omri. Analyses Inorganiques, Isotopiques et de Spéciation Solide Avancées Pour Le Suivi Géochimique Des Éléments Traces et Des Terres Rares: Du Bassin Phosphatier de Gafsa à Leur Enregistrement Dans Les Sédiments Du Golfe de Gabès (Tunisie). Doctoral Dissertation, Université de Pau et des Pays de l’Adour, Pau, France, 2025.
- Wang, L.; Huang, X.; Yu, Y.; Zhao, L.; Wang, C.; Feng, Z.; Cui, D.; Long, Z. Towards Cleaner Production of Rare Earth Elements from Bastnaesite in China. J. Clean. Prod. 2017, 165, 231–242. [Google Scholar] [CrossRef]
- Pradip; Fuerstenau, D.W. Design and Development of Novel Flotation Reagents for the Beneficiation of Mountain Pass Rare-Earth Ore. Min. Metall. Explor. 2013, 30, 1–9. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsieh, C.-H.; Miller, J.D. Characterization of Rare-Earth Resources at Mountain Pass, CA Using High-Resolution X-Ray Microtomography (HRXMT). Min. Metall. Explor. 2013, 30, 10–17. [Google Scholar] [CrossRef]
- Li, S.; Malik, M.; Azimi, G. Extraction of Rare Earth Elements from Phosphogypsum Using Mineral Acids: Process Development and Mechanistic Investigation. Ind. Eng. Chem. Res. 2022, 61, 102–114. [Google Scholar] [CrossRef]
- Bolivar, J. Radioecological Study of an Estuarine System Located in the South of Spain. Water Res. 2000, 34, 2941–2950. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of Uncontrolled Fertilization and Heavy Metal Toxicity Associated with Arsenic(As), Lead(Pb) and Cadmium (Cd), and Possible Remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Villalobos, M.; Vioque, I.; Mantero, J.; Manjón, G. Radiological, Chemical and Morphological Characterizations of Phosphate Rock and Phosphogypsum from Phosphoric Acid Factories in SW Spain. J. Hazard. Mater. 2010, 181, 193–203. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Van Beek, P.; Castet, S.; Souhaut, M.; Grégoire, M.; Courjault-Radé, P. Natural Radioactivity and Radiation Hazard Assessment of Industrial Wastes from the Coastal Phosphate Treatment Plants of Gabes (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2019, 146, 454–461. [Google Scholar] [CrossRef]
- El Zrelli, R.; Courjault-Radé, P.; Rabaoui, L.; Castet, S.; Michel, S.; Bejaoui, N. Heavy Metal Contamination and Ecological Risk Assessment in the Surface Sediments of the Coastal Area Surrounding the Industrial Complex of Gabes City, Gulf of Gabes, SE Tunisia. Mar. Pollut. Bull. 2015, 101, 922–929. [Google Scholar] [CrossRef] [PubMed]
- El Zrelli, R.; Courjault-Radé, P.; Rabaoui, L.; Daghbouj, N.; Mansour, L.; Balti, R.; Castet, S.; Attia, F.; Michel, S.; Bejaoui, N. Biomonitoring of Coastal Pollution in the Gulf of Gabes (SE, Tunisia): Use of Posidonia Oceanica Seagrass as a Bioindicator and Its Mat as an Archive of Coastal Metallic Contamination. Environ. Sci. Pollut. Res. 2017, 24, 22214–22225. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential Uses and Problems—A Review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Markiewicz, J.; Wolf, W.M. Heavy Metal Uptake by Herbs. IV. Influence of Soil pH on the Content of Heavy Metals in Valeriana officinalis L. Water Air Soil. Pollut. 2015, 226, 106. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The Impact of Soil pH on Heavy Metals Uptake and Photosynthesis Efficiency in Melissa Officinalis, Taraxacum Officinalis, Ocimum Basilicum. Molecules 2022, 27, 4671. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Essam, M.; Boukhriss, A.; Kamar, M.; Midani, M. Properties, Purification, and Applications of Phosphogypsum: A Comprehensive Review Towards Circular Economy. Mater. Circ. Econ. 2024, 6, 9. [Google Scholar] [CrossRef]
- Jacomino, V.M.F.; Oliveira, K.A.P.D.; Taddei, M.H.T.; Siqueira, M.C.; Carneiro, M.E.D.P.; Nascimento, M.R.L.; Silva, D.F.D.; Mello, J.W.V.D. Radionuclides and Heavy Metal Contents in Phosphogypsum Samples in Comparison to Cerrado Soils. Rev. Bras. Ciênc. Solo 2009, 33, 1481–1488. [Google Scholar] [CrossRef]
- Saueia, C.H.R.; Mazzilli, B.P. Distribution of Natural Radionuclides in the Production and Use of Phosphate Fertilizers in Brazil. J. Environ. Radioact. 2006, 89, 229–239. [Google Scholar] [CrossRef]
- Guerrero Márquez, J.L. Evaluation of the Impact Produced by the Huelva Phosphogypsum Stacks on Their Estuarine Environment. Ph.D. Thesis, Universidad de Huelva, Huelva, Spain, 2021; p. 207. [Google Scholar]
- Hamed, Y.; Gentilucci, M.; Mokadem, N.; Khalil, R.; Ayadi, Y.; Hadji, R.; Elaloui, E. Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis. Hydrology 2024, 11, 84. [Google Scholar] [CrossRef]
- Ben Garali, A.; Salah, S.; Henchiri, M.; Srarfi, F. Assessment of Heavy Metals Contamination/Pollution of Phosphogypsum Waste of the Mdhilla Region (Gafsa, Southern Tunisia). Environ. Monit. Assess. 2024, 196, 1204. [Google Scholar] [CrossRef]
- Hassoune, H.; Lachehab, A.; Hajjaji, K.E.; Mertah, O.; Kherbeche, A. Dynamic of Heavy Metals and Environmental Impact of Waste Phosphogypsum. In Fate and Transport of Subsurface Pollutants; Gupta, P.K., Bharagava, R.N., Eds.; Springer: Singapore, 2021; pp. 57–77. ISBN 978-981-15-6564-9. [Google Scholar]
- Santos, A.J.G.; Silva, P.S.C.; Mazzilli, B.P.; Fávaro, D.I.T. Radiological Characterisation of Disposed Phosphogypsum in Brazil: Evaluation of the Occupational Exposure and Environmental Impact. Radiat. Prot. Dosim. 2006, 121, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ettoumi, M.; Jouini, M.; Neculita, C.M.; Khalil, A.; Bouhlel, S.; Taha, Y.; Benzaazoua, M. Challenges of Phosphate By-Product Management: A Case-Study of the Tunisian Mining Basins. Mine Water Environ. 2025, 44. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, D.; Chen, Y.; Wu, K.; Tu, M.; Wang, P.; Zhang, Z.; Zhou, L. Characteristic Pollutant Purification Analysis of Modified Phosphogypsum Comprehensive Utilization. Environ. Sci. Pollut. Res. 2022, 29, 67456–67465. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, A.; Duan, H.; Zhang, H.; Yang, J. Status and Development Trends of Phosphogypsum Utilization in China. Circ. Econ. 2024, 3, 100116. [Google Scholar] [CrossRef]
- Chouaybi, I.; Azifa, A.; Moujahid, E.M.; Bettach, M. Waste to Wealth: Synthesis of Hydrocalumite from Moroccan Phosphogypsum and Aluminum Wastes. Waste Manag. 2023, 171, 26–31. [Google Scholar] [CrossRef]
- Noli, F.; Sidirelli, M.; Tsamos, P. The Impact of Phosphate Fertilizer Factory on the Chemical and Radiological Pollution of the Surrounding Marine Area (Seawater and Sediments) in Northwestern Greece. Reg. Stud. Mar. Sci. 2024, 73, 103458. [Google Scholar] [CrossRef]
- Gargouri, D.; Annabi-Trabelsi, N.; Karam, Q.; Ali, M.; Ayadi, H. Assessment of Metallic Pollution in the Waters, Suspended Particulate Matter, and Surface Sediments of the Central Coastal Area of the Gulf of Gabès, Mediterranean Sea; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Kobbi-Rebai, R.; Annabi-Trabelsi, N.; Khemakhem, H.; Ayadi, H.; Aleya, L. Impacts of Restoration of an Uncontrolled Phosphogypsum Dumpsite on the Seasonal Distribution of Abiotic Variables, Phytoplankton, Copepods, and Ciliates in a Man-Made Solar Saltern. Environ. Monit. Assess. 2013, 185, 2139–2155. [Google Scholar] [CrossRef]
- Vaccari, D.A.; Strigul, N. Extrapolating Phosphorus Production to Estimate Resource Reserves. Chemosphere 2011, 84, 792–797. [Google Scholar] [CrossRef]
- Koppelaar, R.; Weikard, H.P. Assessing Phosphate Rock Depletion and Phosphorus Recycling Options. Glob. Environ. Change 2013, 23, 1454–1466. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; Crissien, T.J.; Santosh, M.; Bolivar, J.; Shao, L.; Dotto, G.L.; Gasparotto, J.; Schindler, M. A Review on the Environmental Impact of Phosphogypsum and Potential Health Impacts through the Release of Nanoparticles. Chemosphere 2022, 286, 131513. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Wu, F.; Li, D.; Yang, J.; Chen, J.; Liang, J.; Lou, X.; Chen, H. Phosphorus Recovery and Resource Utilization from Phosphogypsum Leachate via Membrane-Triggered Adsorption and Struvite Crystallization Approach. Chem. Eng. J. 2023, 471, 144310. [Google Scholar] [CrossRef]
- Bandara, A.M.T.S.; Senanayake, G. Leachability of Rare-Earth, Calcium and Minor Metal Ions from Natural Fluorapatite in Perchloric, Hydrochloric, Nitric and Phosphoric Acid Solutions: Effect of Proton Activity and Anion Participation. Hydrometallurgy 2015, 153, 179–189. [Google Scholar] [CrossRef]
- Lee, H.; Coulon, F.; Beriro, D.J.; Wagland, S.T. Recovering Metal(Loids) and Rare Earth Elements from Closed Landfill Sites without Excavation: Leachate Recirculation Opportunities and Challenges. Chemosphere 2022, 292, 133418. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of Contaminants in Phosphogypsum of the Fertilizer Industry of Huelva (SW Spain): From Phosphate Rock Ore to the Environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Yang, X.; Salvador, D.; Makkonen, H.T.; Pakkanen, L. Phosphogypsum Processing for Rare Earths Recovery—A Review. Nat. Resour. 2019, 10, 325–336. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Su, X.; Du, H.; Lu, Y.; Zhang, Q. Rare Earth Extraction from Phosphogypsum by Aspergillus Niger Culture Broth. Molecules 2024, 29, 1266. [Google Scholar] [CrossRef]
- Naz, R.; Khan, M.S.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y.; et al. Assessment of Phytoremediation Potential of Native Plant Species Naturally Growing in a Heavy Metal-Polluted Industrial Soils. Braz. J. Biol. 2024, 84, e264473. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Faz, A.; Zornoza, R.; Martinez-Martinez, S.; Acosta, J.A. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(Loid)s and Rare Earth Elements. Plants 2023, 12, 1219. [Google Scholar] [CrossRef]
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the Transfer of Trace Metals to Spontaneous Plants on Abandoned Pyrrhotite Mine: Potential Application for Phytostabilization of Phosphate Wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Gonçalves, F.; Römbke, J.; Pereira, R. Phosphogypsum as a Soil Fertilizer: Ecotoxicity of Amended Soil and Elutriates to Bacteria, Invertebrates, Algae and Plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bumanis, G.; Vaičiukynienė, D.; Tambovceva, T.; Puzule, L.; Sinka, M.; Nizevičienė, D.; Fornés, I.; Bajare, D. Circular Economy in Practice: A Literature Review and Case Study of Phosphogypsum Use in Cement. Recycling 2024, 9, 63. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.-L. Exploring the Potential Reuse of Phosphogypsum: A Waste or a Resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef]
- Fornés, I.V.; Vaiciukyniene, D.; Nizeviciene, D.; Bajare, D.; Borg, R.P.; Bistrickaite, R. By-Product Phosphogypsum Valorisation Possibilities in the Context of Circular Economy of Building Materials. In Creating a Roadmap Towards Circularity in the Built Environment; Bragança, L., Cvetkovska, M., Askar, R., Ungureanu, V., Eds.; Springer Tracts in Civil Engineering; Springer Nature: Cham, Switzerland, 2024; pp. 107–118. ISBN 978-3-031-45979-5. [Google Scholar]
- Peiravi, M.; Dehghani, F.; Ackah, L.; Baharlouei, A.; Godbold, J.; Liu, J.; Mohanty, M.; Ghosh, T. A Review of Rare-Earth Elements Extraction with Emphasis on Non-Conventional Sources: Coal and Coal Byproducts, Iron Ore Tailings, Apatite, and Phosphate Byproducts. Min. Metall. Explor. 2021, 38, 1–26. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Murali, G.; Azab, M. Recent Research in Utilization of Phosphogypsum as Building Materials: Review. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- da Silva, E.F.; Mlayah, A.; Gomes, C.; Noronha, F.; Charef, A.; Sequeira, C.; Esteves, V.; Marques, A.R.F. Heavy Elements in the Phosphorite from Kalaat Khasba Mine (North-Western Tunisia): Potential Implications on the Environment and Human Health. J. Hazard. Mater. 2010, 182, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.B. Radioactive Material, Fluorine and Rare-Earth Element Quantification in Phosphogypsum Using Nuclear Analytical Techniques. Master’s Thesis, Universidade Nova de Lisboa, Caparica, Portugal, 2024. Available online: http://hdl.handle.net/10362/178030 (accessed on 7 November 2025).
- Haschke, M.; Friedrich, B.; Stopic, S.; Panias, D.; Schneider, P.; Dittrich, C. Extraction of Critical Technology Elements and Radionuclides from Phosphogypsum Tailings. In Proceedings of the OPMR2016—Opportunities in Processing of Metal Resources in South East Europe (OPMR 2016), Budapest, Hungary, 28–30 November 2016. [Google Scholar]
- Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare Earth Element Recovery from Acidic Extracts of Florida Phosphate Mining Materials Using Chelating Polymer 1-Octadecene, Polymer with 2,5-Furandione, Sodium Salt. Minerals 2019, 9, 477. [Google Scholar] [CrossRef]



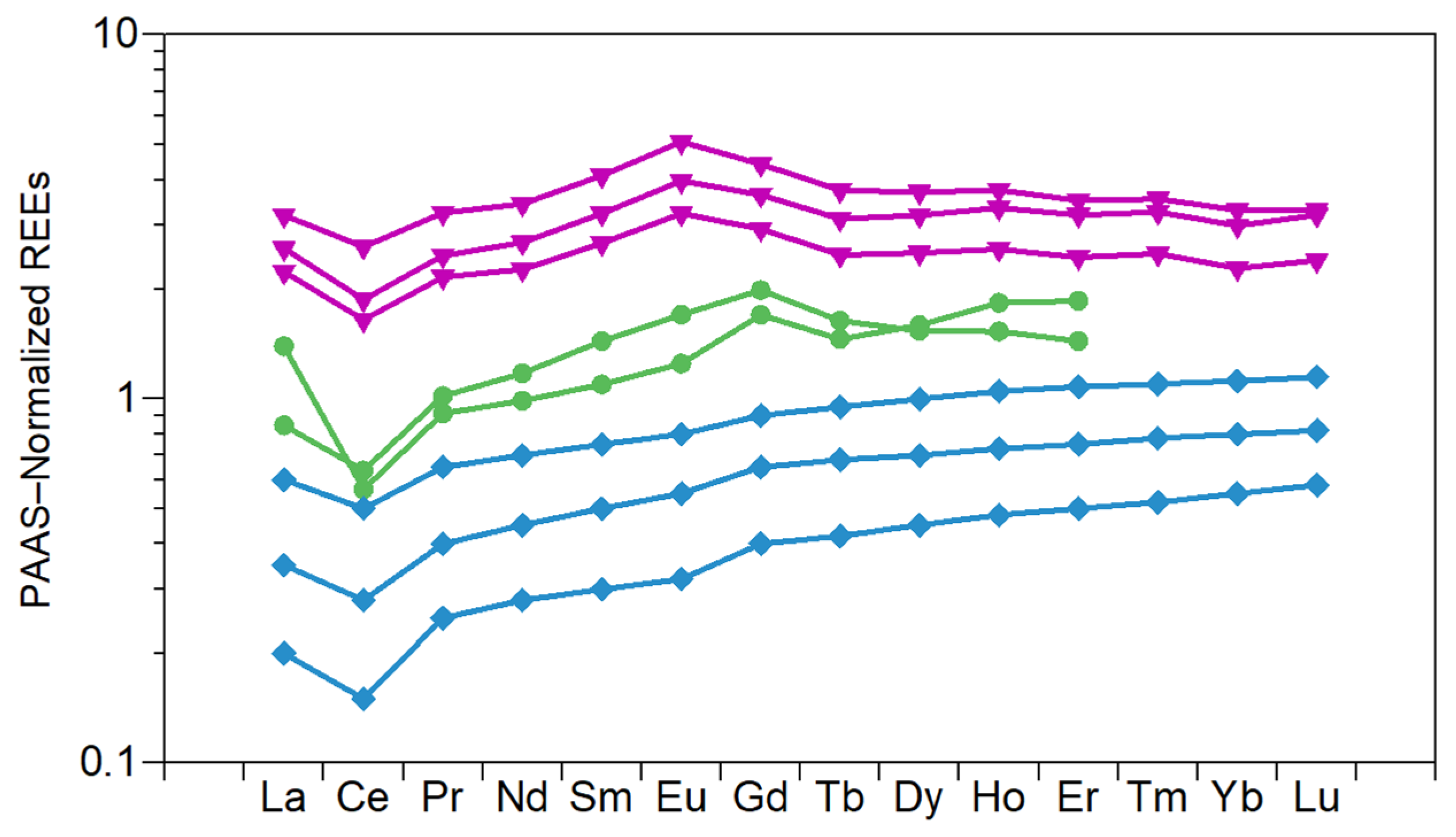
| REE Deposit | TREO (wt%) | U (mg·kg−1) | Th (mg·kg−1) | TREO/(U + Th) | Reference |
|---|---|---|---|---|---|
| Mountain Pass (USA) | 9.3 | 17 | 175.8 | 414.1 | Haxel et al. (2002) [142] Park et al.(2023) [143] |
| Mount Weld (Australia) | 8.8 | 25.4 | 659.1 | 125.6 | Hellman & Duncan (2018) [144] Lynas Rare Earths (2025) [145] |
| Dominant REE Host Mineral | Typical REE Pattern | Extraction Approach | Considerations |
|---|---|---|---|
| Fluorapatite | LREE-enriched; high LREE/HREE; often negative Ce anomaly [83,93,95] | Sulfuric acid leaching (wet-process), recovery from phosphoric acid, phosphogypsum, or purification sludge [100,101,102,103,104,105,106] | Recovery integrated into fertilizer production; REE co-leach with P2O5; possible selectivity |
| Monazite | LREE-rich, may show subdued anomalies; high Th/U [93,95,96] | Hot concentrated acid digestion | Requires radioelement management; high REE grade but higher processing cost |
| Xenotime | HREE-enriched; flatter REE profile; possible positive Eu anomaly [93,95,96] | Mixed-acid digestion | Strategic for HREE; more aggressive reagents needed |
| Adsorbed/ amorphous phases | Low total REE; variable patterns [99,184,203,241] | Bioleaching, ion exchange, or weak acid leaching [99,184,191,241] | Environmentally friendlier; slower kinetics; suitable for PG or process waters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omri, N.; Souissi, R.; Souissi, F.; Gleyzes, C.; Zaaboub, N.; Abderrazak, H.; Donard, O.F.X.; Rddad, L. Rare Earth Elements in Phosphate Ores and Industrial By-Products: Geochemical Behavior, Environmental Risks, and Recovery Potential. Minerals 2025, 15, 1232. https://doi.org/10.3390/min15121232
Omri N, Souissi R, Souissi F, Gleyzes C, Zaaboub N, Abderrazak H, Donard OFX, Rddad L. Rare Earth Elements in Phosphate Ores and Industrial By-Products: Geochemical Behavior, Environmental Risks, and Recovery Potential. Minerals. 2025; 15(12):1232. https://doi.org/10.3390/min15121232
Chicago/Turabian StyleOmri, Nourhen, Radhia Souissi, Fouad Souissi, Christine Gleyzes, Noureddine Zaaboub, Houyem Abderrazak, Olivier F. X. Donard, and Larbi Rddad. 2025. "Rare Earth Elements in Phosphate Ores and Industrial By-Products: Geochemical Behavior, Environmental Risks, and Recovery Potential" Minerals 15, no. 12: 1232. https://doi.org/10.3390/min15121232
APA StyleOmri, N., Souissi, R., Souissi, F., Gleyzes, C., Zaaboub, N., Abderrazak, H., Donard, O. F. X., & Rddad, L. (2025). Rare Earth Elements in Phosphate Ores and Industrial By-Products: Geochemical Behavior, Environmental Risks, and Recovery Potential. Minerals, 15(12), 1232. https://doi.org/10.3390/min15121232






