Mineralogical and Geochemical Characteristics of the Fe-Ti Mineralized Mafic-Ultramafic Intrusions at Wajilitag, Tarim Basin, China: With Special Emphasis on the Role of Apatite
Abstract
1. Introduction
2. Geological Setting
3. Petrology
3.1. Petrographic Characteristics
3.2. Magmatic Evolution of Mafic–Ultramafic Intrusion in the Wajilitag Area
3.3. Apatite as a Useful Petrogenetic Indicator
4. Samples and Methods
5. Results
5.1. Major Elements
5.2. Trace Elements
6. Discussion
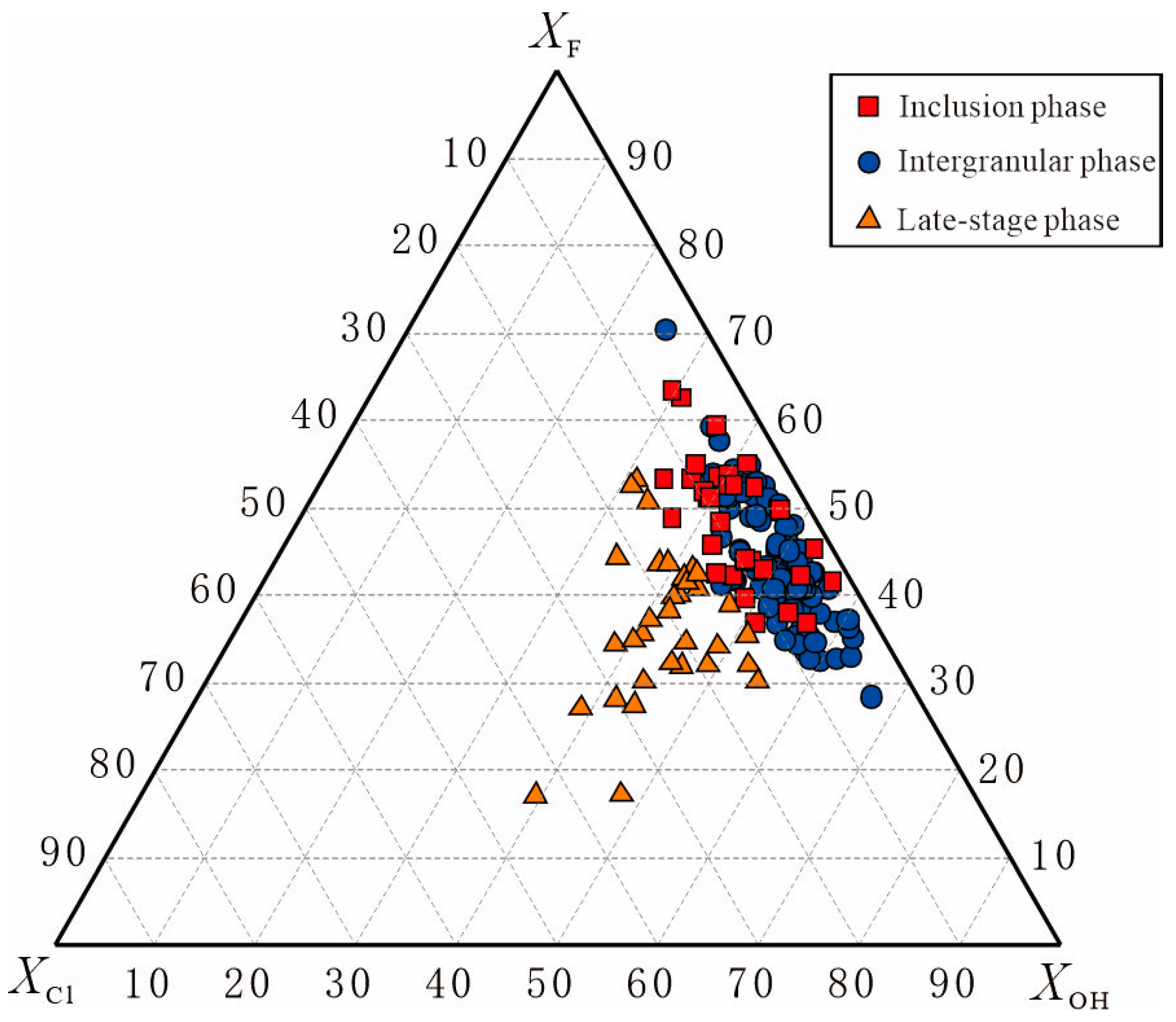
6.1. Estimating Melt Volatile Compositions from Apatite: Insights into Magmatic Sources and Ore-Forming Environments
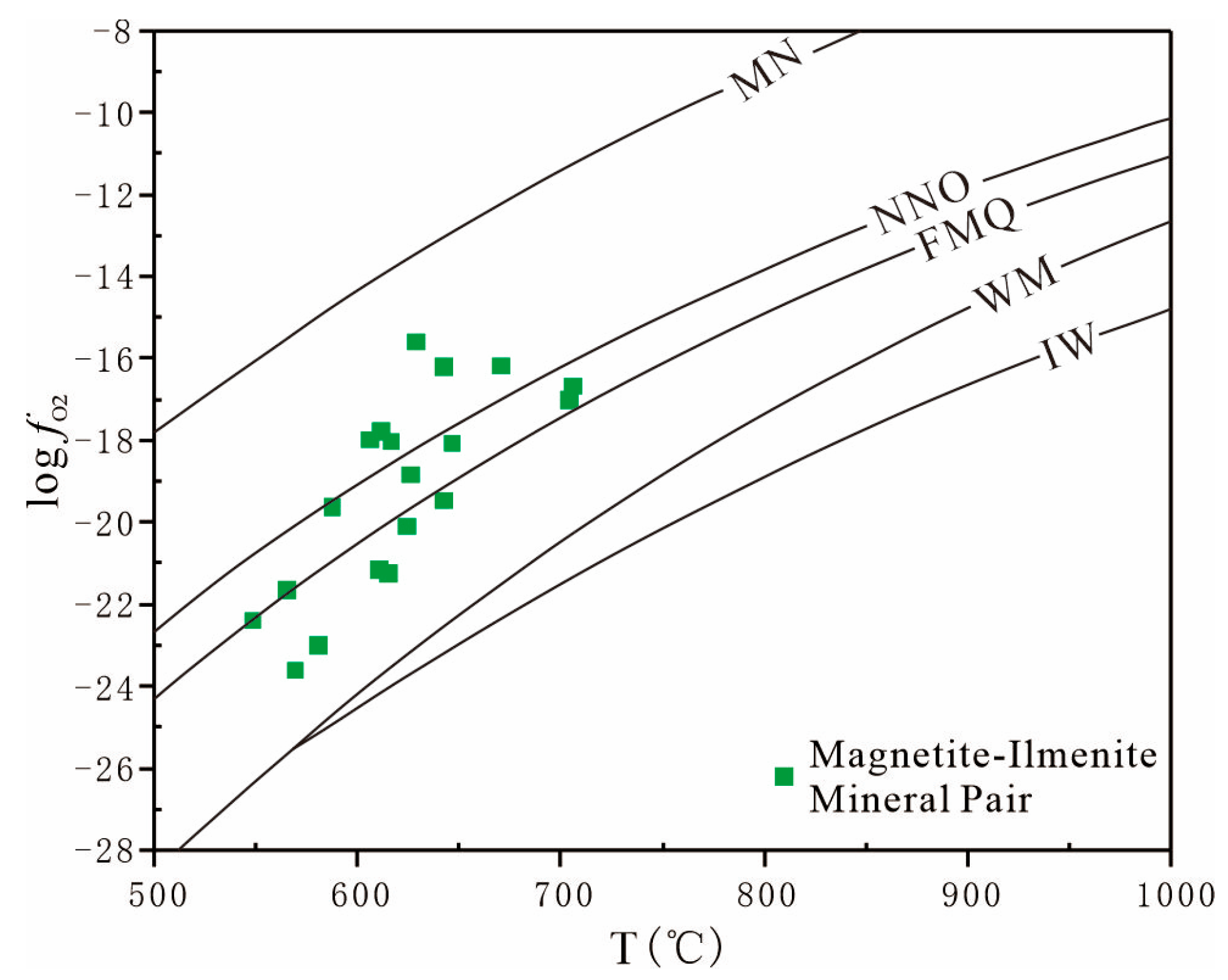
6.2. Apatite Reveals Magma Oxygen Fugacity
6.3. Apatite Geochemistry of Mafic–Ultramafic Intrusion in the Wajilitag Area: Constraints on Magma Source and Evolution
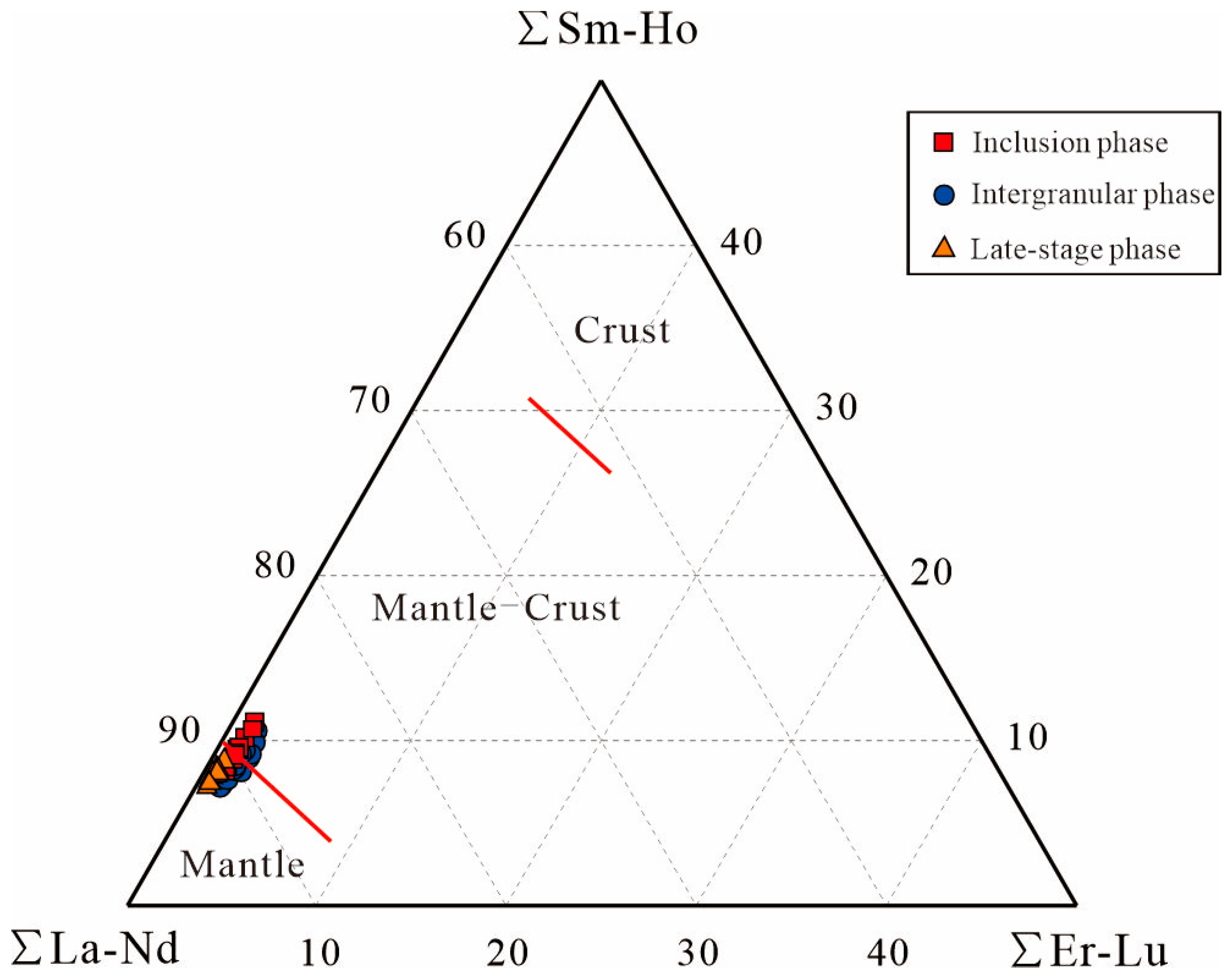
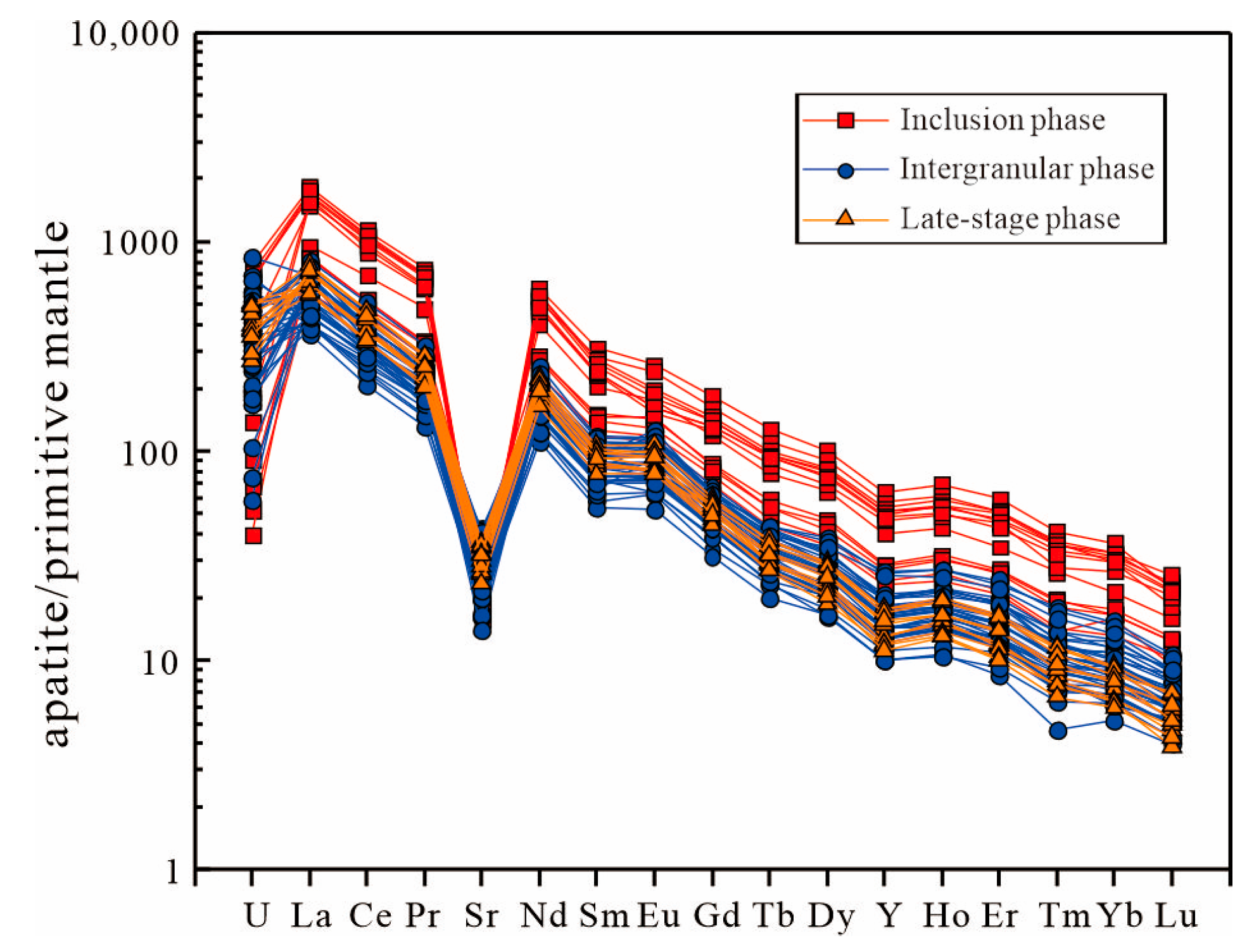
6.3.1. Tracing the Mantle Source: Evidence from Apatite Trace Elements
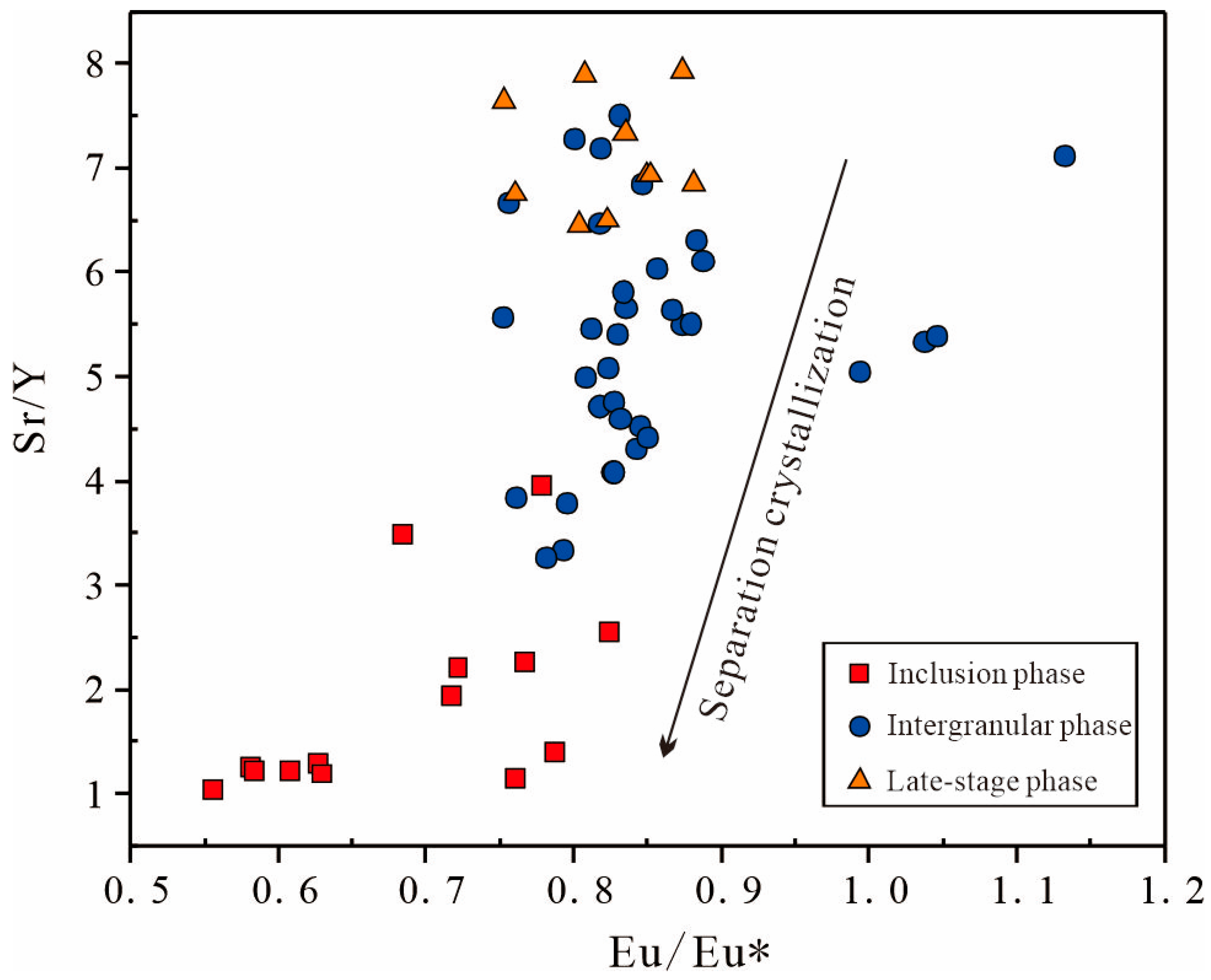
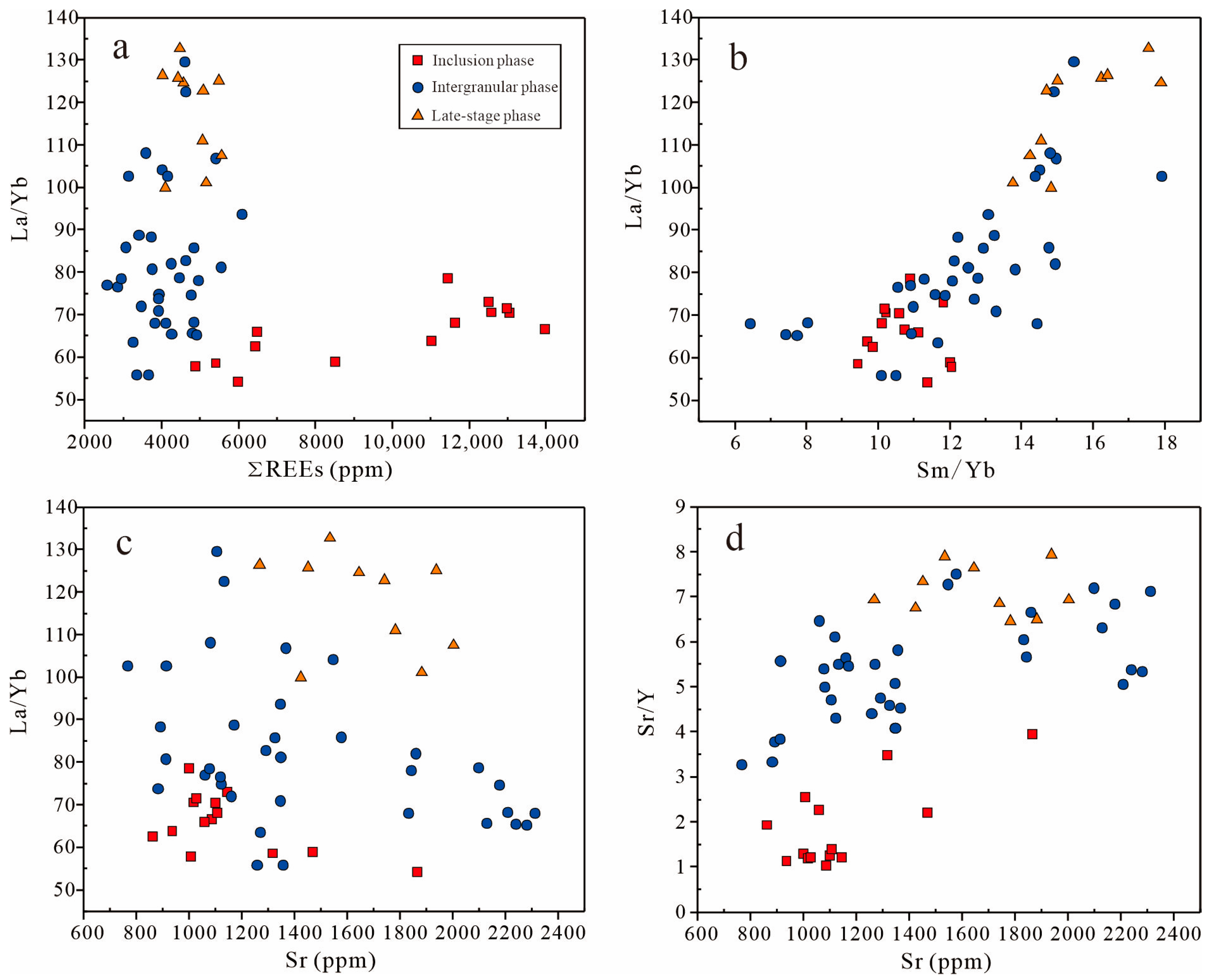
6.3.2. Apatite Reveals Crystallization History and Magmatic Evolution
6.4. Implications for Genesis of Fe-Ti Oxides
- (1)
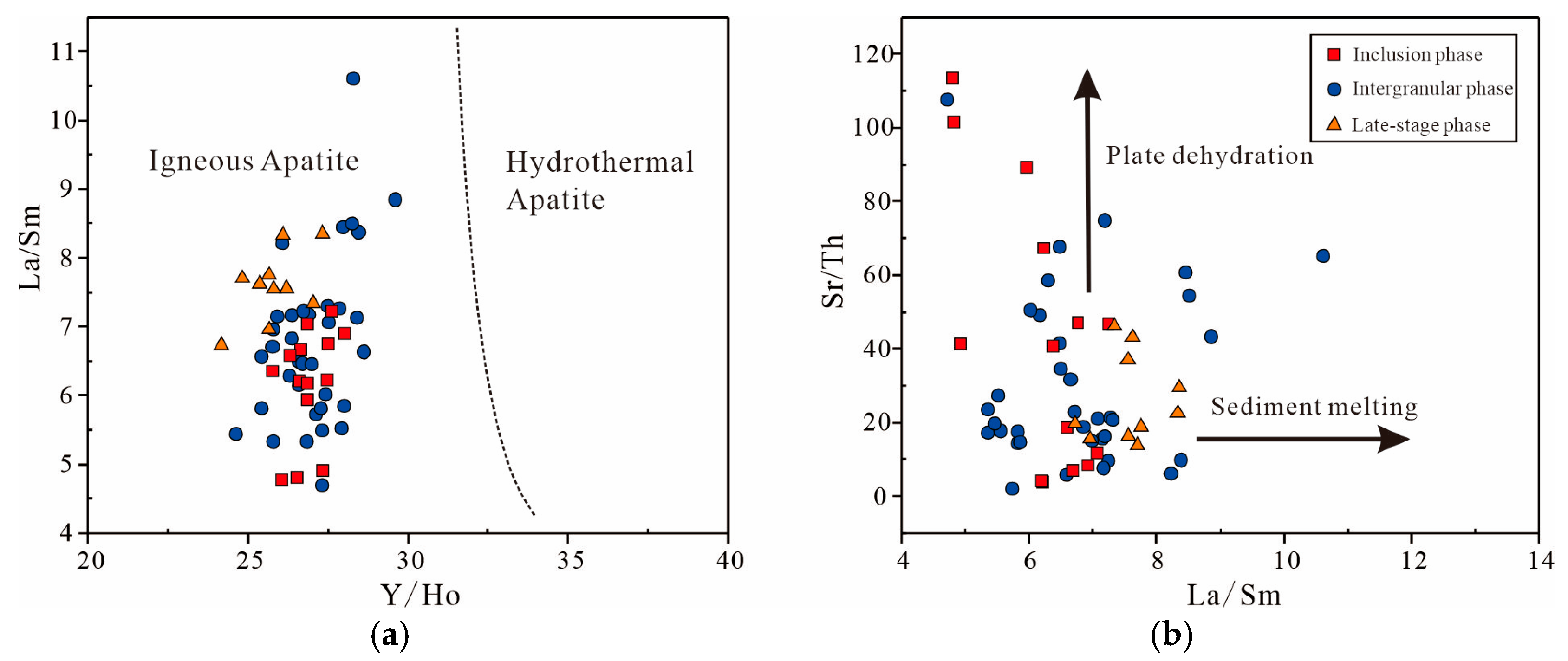
- In the pre-subduction stage, the subducting slab underwent deep dehydration, releasing fluids enriched in large ion lithophile elements (LILE), light rare earth elements (LREE), F, Cl, and H2O, with elevated oxygen fugacity (fO2). These fluids migrated upward, extensively metasomatizing the overlying lithospheric mantle. During this process, F, because of its compatibility, became incorporated into metasomatic minerals such as muscovite and amphibole. Cl predominantly remained in the fluid phase, while sulfur (S) was oxidized to sulfate (SO42−) and subsequently migrated out of the system with the fluid. This resulted in the formation of a metasomatized lithospheric mantle region characterized by elevated fO2, abundant volatiles (F/Cl/H2O), and sulfur-poor metasomatic rocks, establishing the material and geochemical foundation for mineralization.
- (2)
- In the triggering phase, ascending mantle plumes from the deep mantle interacted with the metasomatized lithospheric mantle. The primary role of these plumes was to supply substantial heat flux, rather than provide mineralizing materials. This thermal effect has been shown to induce thermal erosion and lithospheric thinning, significantly lowering solidus temperatures and triggering high-grade partial melting in the enriched mantle source region. Without the thermal input from the mantle plume, this enriched mantle would have undergone only minor melting and would not have been capable of generating substantial amounts of magma.
- (3)
- In the melting and evolution stage, the altered, enriched mantle underwent extensive partial melting, producing large volumes of sulfur-undersaturated parent magmas. These magmas inherited the source region’s high F/Cl ratio, high fO2, and sulfur-poor characteristics. Following magmatic emplacement, the magma experienced significant crystallization and differentiation within the chamber. Volatiles such as F and Cl acted as fluxing agents, reducing viscosity and solidus temperatures while prolonging the duration of differentiation. The initial crystallization of mafic silicate minerals (e.g., olivine, clinopyroxene) resulted in the exceptional enrichment of incompatible elements such as Fe, Ti, V, and P in the residual melt.
- (4)
- During the mineralization process, ferrous ions (Fe2+) were oxidized to ferric ions (Fe3+) under conditions of elevated oxygen fugacity. Simultaneously, vanadium (V) was incorporated into the oxide lattice as a solid solution. Late-stage melts reached a state of supersaturation with respect to Fe-Ti oxide, which induced extensive crystallization, leading to the formation of ore bodies with layered or quasi-layered morphology. Late-stage exsolution or assimilation of chlorine-rich fluids from host rocks further activated and concentrated ore-forming elements (e.g., Fe, Ti, and V as chlorine complexes). This process is key to explaining the complex ore body structure and the presence of locally high-chlorine apatite.
7. Conclusions
- (1)
- A negative correlation between fluorine (F) and chlorine (Cl) in apatite is evident. Inclusion-phase and intergranular-phase apatites are F-rich and Cl-poor, while late-stage apatite is the opposite. All apatites are magmatic in origin. The elevated F/Cl ratio suggests that the parent magma originated from an F-rich, subducted metamorphic mantle source, with late-stage Cl enrichment likely linked to high volatile content or fluid dissolution in magma.
- (2)
- Estimates of magmatic volatilization and oxygen fugacity, based on apatite composition, indicate similar F, Cl, and sulfur contents across the three apatite types, with low sulfur content and high oxygen fugacity.
- (3)
- Apatite exhibits strong LREE-HREE fractionation, significant REEs’ enrichment, a weak negative Euanomaly, and distinct trace-element patterns (enriched in Th, U; depleted in Rb, Ba, Sr, Nb, Zr, Hf). This geochemistry indicates a predominantly mantle-derived magma with minor crustal contamination, further supported by Sr/Th and La/Sm ratios that reflect fluid input from subduction dehydration.
- (4)
- We hypothesize that ancient subduction fluids pre-altered the lithospheric mantle, creating a source region rich in volatiles (F, Cl, H2O), high in oxygen fugacity, and sulfur-poor. This preconditioning provided the material basis and oxidizing environment for mineralization. As mantle plumes ascended, they induced large-scale partial melting, with magma undergoing fractional crystallization and ultimately forming Fe-Ti oxide deposits.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Element | Crystal | Spectral Line | Counting Time (s) | Standard Reference Material | Detection Limit (ppm) | |
|---|---|---|---|---|---|---|
| Peak | Background | |||||
| Na | TAP | Kα | 10 | 5 | Jadeite | 71–87 |
| Mg | TAP | Kα | 10 | 5 | Forsterite | 65–74 |
| Al | TAP | Kα | 10 | 5 | Jadeite | 77–81 |
| F | TAP | Kα | 10 | 5 | Topaz | 1098–1174 |
| K | PETJ | Kα | 10 | 5 | K-feldspar | 48–53 |
| Ca | PETJ | Kα | 10 | 5 | Wollastonite | 64–77 |
| Si | PETJ | Kα | 10 | 5 | Jadeite | 83–96 |
| Fe | LIF | Kα | 10 | 5 | Hematite | 87–99 |
| Ti | LIF | Kα | 10 | 5 | Rutile | 236–244 |
| P | PETJ | Kα | 10 | 5 | Apatite | 84–93 |
| Cr | LIFH | Kα | 10 | 5 | Cr2O3 | 112–137 |
| Mn | LIFH | Kα | 10 | 5 | MnO | 104–113 |
| Ni | LIF | Kα | 10 | 5 | NiO | 136–154 |
| V | LIFH | Kα | 10 | 5 | V2O5 | 104–192 |
| Cl | PETH | Kα | 10 | 5 | NaCl | 222–289 |
| ICP-MS Instrument Parameters | |
| Instrument Model | Bruker M90 |
| Sampling Depth (mm) | 6 |
| Carrier Gas (Ar) Flow Rate (L/min) | 1.08 |
| Signal Acquisition Mode | Time Resolution |
| Laser Ablation System Parameters | |
| Instrument Model | RESOlution S-155 |
| Carrier Gas & Flow Rate (L/min) | He: 0.6 |
| Energy Density (J/cm2) | 6 |
| Laser Spot Size (μm) | 30/38 |
| Repetition Rate (Hz) | 5 |
| Sample | F | Cl | MgO | Al2O3 | Na2O | P2O5 | CaO | SiO2 | K2O | MnO | FeO | TiO | SO3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion Phase | ||||||||||||||
| ZK4506 | ||||||||||||||
| b1-2-1 | 1.98 | 0.29 | 0.06 | 0.03 | 0.04 | 41.65 | 54.77 | 0.57 | 0.02 | 0.00 | 0.25 | 0.04 | 0.07 | 99.77 |
| b1-3-1 | 2.08 | 0.25 | 0.05 | 0.00 | 0.09 | 41.58 | 54.57 | 0.40 | 0.01 | 0.08 | 0.21 | 0.00 | 0.03 | 99.34 |
| b1-3-2 | 2.25 | 0.31 | 0.01 | 0.00 | 0.13 | 41.60 | 54.24 | 0.39 | 0.00 | 0.02 | 0.19 | 0.00 | - | 99.13 |
| b3-3-1 | 1.50 | 0.78 | 0.04 | 0.04 | 0.04 | 41.57 | 55.13 | 0.18 | 0.00 | 0.03 | 0.23 | 0.02 | 0.01 | 99.58 |
| b3-4-1 | 1.60 | 0.32 | 0.03 | 0.01 | 0.04 | 41.40 | 56.50 | 0.29 | 0.00 | 0.08 | 0.10 | 0.00 | 0.01 | 100.39 |
| b9-3-1 | 2.02 | 0.87 | 0.04 | 0.00 | 0.12 | 41.74 | 53.48 | 0.33 | 0.00 | 0.00 | 0.52 | 0.00 | 0.04 | 99.15 |
| b9-3-2 | 1.85 | 0.97 | 0.03 | 0.00 | 0.10 | 41.39 | 53.65 | 0.37 | 0.00 | 0.04 | 0.70 | 0.00 | 0.04 | 99.12 |
| b9-3-3 | 1.61 | 0.89 | 0.05 | 0.00 | 0.06 | 41.58 | 53.91 | 0.45 | 0.02 | 0.09 | 0.74 | 0.00 | - | 99.40 |
| b13-4-1 | 1.73 | 0.81 | 0.00 | 0.01 | 0.03 | 41.54 | 54.83 | 0.17 | 0.01 | 0.06 | 0.17 | 0.00 | - | 99.37 |
| b18-1-1 | 1.40 | 0.46 | 0.32 | 0.00 | 0.03 | 41.85 | 54.47 | 0.55 | 0.01 | 0.18 | 0.45 | 0.03 | - | 99.76 |
| b18-1-2 | 2.02 | 0.49 | 0.05 | 0.02 | 0.07 | 41.42 | 54.48 | 0.36 | 0.02 | 0.08 | 0.39 | 0.04 | 0.05 | 99.49 |
| b18-2-1 | 2.37 | 0.43 | 0.03 | 0.00 | 0.06 | 41.38 | 54.99 | 0.32 | 0.03 | 0.07 | 0.08 | 0.00 | 0.02 | 99.77 |
| b18-2-2 | 2.03 | 0.41 | 0.05 | 0.00 | 0.08 | 41.68 | 54.43 | 0.36 | 0.05 | 0.06 | 0.13 | 0.01 | 0.03 | 99.32 |
| b18-3-1 | 2.40 | 0.47 | 0.02 | 0.01 | 0.02 | 41.65 | 54.76 | 0.41 | 0.02 | 0.02 | 0.11 | 0.00 | 0.02 | 99.89 |
| b18-3-2 | 1.99 | 0.45 | 0.00 | 0.00 | 0.05 | 41.46 | 55.03 | 0.63 | 0.00 | 0.11 | 0.23 | 0.00 | 0.07 | 100.03 |
| b19-1-1 | 1.60 | 0.77 | 0.02 | 0.00 | 0.16 | 41.31 | 55.60 | 0.19 | 0.00 | 0.05 | 0.08 | 0.00 | 0.03 | 99.82 |
| b19-2-1 | 2.02 | 0.69 | 0.02 | 0.00 | 0.13 | 41.60 | 54.33 | 0.33 | 0.00 | 0.04 | 0.14 | 0.00 | 0.04 | 99.33 |
| b19-2-2 | 1.88 | 0.21 | 0.01 | 0.00 | 0.13 | 41.70 | 54.67 | 0.42 | 0.04 | 0.00 | 0.18 | 0.00 | - | 99.22 |
| b19-2-3 | 1.94 | 0.65 | 0.01 | 0.02 | 0.10 | 41.55 | 54.79 | 0.31 | 0.02 | 0.00 | 0.14 | 0.01 | 0.01 | 99.54 |
| b19-3-1 | 2.08 | 0.59 | 0.02 | 0.00 | 0.15 | 41.66 | 54.70 | 0.23 | 0.01 | 0.00 | 0.13 | 0.00 | 0.03 | 99.60 |
| b19-4-1 | 1.40 | 0.81 | 0.05 | 0.00 | 0.26 | 41.41 | 54.93 | 0.22 | 0.01 | 0.03 | 0.07 | 0.00 | 0.02 | 99.19 |
| b19-4-2 | 1.99 | 0.42 | 0.00 | 0.00 | 0.11 | 41.47 | 54.84 | 0.25 | 0.00 | 0.04 | 0.12 | 0.00 | - | 99.24 |
| b20-1-1 | 1.57 | 0.13 | 0.09 | 0.02 | 0.04 | 42.16 | 55.77 | 0.26 | 0.02 | 0.08 | 0.34 | 0.08 | 0.01 | 100.56 |
| b20-1-2 | 1.71 | 0.13 | 0.03 | 0.00 | 0.03 | 41.60 | 55.13 | 0.25 | 0.01 | 0.04 | 0.26 | 0.02 | 0.03 | 99.24 |
| b22-1-1 | 1.96 | 0.65 | 0.04 | 0.02 | 0.08 | 41.53 | 54.55 | 0.31 | 0.00 | 0.08 | 0.22 | 0.00 | 0.02 | 99.46 |
| b22-1-2 | 1.82 | 0.66 | 0.04 | 0.00 | 0.06 | 41.33 | 55.18 | 0.43 | 0.01 | 0.02 | 0.26 | 0.00 | 0.13 | 99.84 |
| b22-1-3 | 1.94 | 0.53 | 0.02 | 0.01 | 0.10 | 41.60 | 54.74 | 0.19 | 0.00 | 0.03 | 0.19 | 0.02 | 0.11 | 99.48 |
| b22-2-1 | 1.66 | 0.59 | 0.05 | 0.01 | 0.05 | 41.56 | 55.51 | 0.36 | 0.00 | 0.09 | 0.15 | 0.00 | 0.04 | 100.06 |
| b22-2-2 | 1.62 | 0.55 | 0.05 | 0.02 | 0.07 | 41.73 | 54.69 | 0.20 | 0.00 | 0.11 | 0.21 | 0.00 | 0.02 | 99.27 |
| b22-2-3 | 1.67 | 0.64 | 0.02 | 0.03 | 0.04 | 41.31 | 55.16 | 0.50 | 0.00 | 0.02 | 0.16 | 0.00 | 0.14 | 99.70 |
| b22-3-1 | 1.66 | 0.64 | 0.03 | 0.00 | 0.06 | 41.42 | 55.42 | 0.26 | 0.00 | 0.06 | 0.12 | 0.00 | 0.09 | 99.76 |
| b22-3-2 | 1.93 | 0.64 | 0.05 | 0.00 | 0.05 | 41.42 | 54.99 | 0.32 | 0.01 | 0.04 | 0.08 | 0.00 | 0.06 | 99.58 |
| b22-3-3 | 1.44 | 0.54 | 0.08 | 0.00 | 0.07 | 41.51 | 53.35 | 0.24 | 0.00 | 0.07 | 0.10 | 0.00 | 0.08 | 97.48 |
| intergranular phase | ||||||||||||||
| ZK3306 | ||||||||||||||
| b3-1-01 | 1.76 | 0.71 | 0.09 | 0.01 | 0.03 | 41.63 | 55.22 | 0.22 | 0.01 | 0.11 | 0.31 | 0.02 | 100.11 | |
| b3-1-02 | 1.59 | 0.79 | 0.11 | 0.02 | 0.14 | 41.62 | 55.53 | 0.38 | 0.03 | 0.07 | 0.32 | 0.01 | 100.60 | |
| b5-1-01 | 1.74 | 0.34 | 0.05 | 0.00 | 0.06 | 41.47 | 55.31 | 0.14 | 0.00 | 0.04 | 0.42 | 0.04 | 99.60 | |
| b5-1-02 | 1.46 | 0.39 | 0.10 | 0.01 | 0.05 | 41.70 | 55.10 | 0.17 | 0.00 | 0.06 | 0.12 | 0.00 | 99.14 | |
| b10-1-01 | 1.47 | 0.60 | 0.00 | 0.00 | 0.01 | 41.79 | 54.97 | 0.13 | 0.01 | 0.00 | 0.31 | 0.00 | 99.28 | |
| b10-1-02 | 1.64 | 0.52 | 0.10 | 0.02 | 0.03 | 41.45 | 54.77 | 0.22 | 0.00 | 0.08 | 0.27 | 0.00 | 99.11 | |
| b10-1-03 | 1.34 | 0.59 | 0.25 | 0.04 | 0.08 | 41.38 | 55.30 | 0.30 | 0.04 | 0.00 | 0.48 | 0.05 | 99.86 | |
| b11-1-01 | 1.31 | 0.48 | 0.10 | 0.00 | 0.08 | 41.57 | 55.52 | 0.18 | 0.01 | 0.00 | 0.28 | 0.03 | 0.08 | 99.66 |
| b11-2-01 | 1.73 | 0.31 | 0.08 | 0.01 | 0.06 | 41.31 | 55.42 | 0.15 | 0.01 | 0.00 | 0.32 | 0.00 | 0.06 | 99.45 |
| b11-3-01 | 1.72 | 0.37 | 0.16 | 0.03 | 0.10 | 42.09 | 54.40 | 0.28 | 0.01 | 0.04 | 0.41 | 0.03 | 0.10 | 99.75 |
| b11-4-01 | 1.54 | 0.39 | 0.10 | 0.01 | 0.02 | 41.63 | 55.00 | 0.25 | 0.00 | 0.01 | 0.42 | 0.01 | 99.38 | |
| b14-1-01 | 1.33 | 0.50 | 0.04 | 0.00 | 0.02 | 41.43 | 55.00 | 0.26 | 0.00 | 0.00 | 0.33 | 0.00 | 98.91 | |
| b14-1-02 | 2.66 | 0.28 | 0.11 | 0.00 | 0.01 | 41.72 | 55.55 | 0.25 | 0.00 | 0.06 | 0.22 | 0.03 | 100.89 | |
| b14-2-01 | 1.59 | 0.47 | 0.12 | 0.03 | 0.04 | 41.55 | 54.90 | 0.21 | 0.02 | 0.06 | 0.39 | 0.02 | 99.38 | |
| b14-3-01 | 1.23 | 0.53 | 0.10 | 0.03 | 0.03 | 41.42 | 55.42 | 0.38 | 0.01 | 0.03 | 0.25 | 0.00 | 99.43 | |
| b24-1-01 | 1.30 | 0.61 | 0.06 | 0.02 | 0.03 | 41.49 | 55.88 | 0.23 | 0.00 | 0.00 | 0.39 | 0.00 | 100.01 | |
| b24-2-01 | 2.24 | 0.34 | 0.73 | 0.11 | 0.07 | 41.43 | 52.84 | 0.91 | 0.02 | 0.02 | 1.54 | 0.14 | 100.38 | |
| b24-3-01 | 1.70 | 0.64 | 0.12 | 0.03 | 0.12 | 41.55 | 55.15 | 0.11 | 0.01 | 0.00 | 0.36 | 0.00 | 99.79 | |
| b34-1-01 | 1.83 | 0.38 | 0.48 | 0.07 | 0.30 | 41.81 | 54.26 | 0.20 | 0.07 | 0.05 | 0.35 | 0.02 | 99.81 | |
| b34-1-02 | 1.52 | 0.43 | 0.25 | 0.04 | 0.17 | 41.77 | 54.80 | 0.18 | 0.03 | 0.04 | 0.25 | 0.02 | 99.50 | |
| b34-2-01 | 1.85 | 0.43 | 0.29 | 0.06 | 0.10 | 41.50 | 53.16 | 0.33 | 0.04 | 0.00 | 0.39 | 0.00 | 98.13 | |
| b34-2-02 | 2.05 | 0.37 | 0.30 | 0.05 | 0.43 | 41.44 | 53.64 | 0.19 | 0.10 | 0.02 | 0.34 | 0.02 | 98.94 | |
| b40-2-01 | 1.70 | 0.38 | 0.10 | 0.00 | 0.02 | 42.06 | 55.08 | 0.12 | 0.01 | 0.00 | 0.16 | 0.00 | 99.63 | |
| b40-2-02 | 1.54 | 0.39 | 0.07 | 0.00 | 0.03 | 41.73 | 54.99 | 0.16 | 0.00 | 0.05 | 0.18 | 0.00 | 99.14 | |
| b40-3-01 | 1.24 | 0.40 | 0.19 | 0.10 | 0.11 | 41.70 | 54.56 | 0.36 | 0.02 | 0.00 | 0.28 | 0.08 | 99.04 | |
| b42-1-01 | 1.96 | 0.39 | 0.06 | 0.00 | 0.05 | 41.76 | 55.01 | 0.12 | 0.01 | 0.00 | 0.46 | 0.05 | 99.88 | |
| b42-1-02 | 1.43 | 0.34 | 0.89 | 0.13 | 0.00 | 41.78 | 52.54 | 1.51 | 0.00 | 0.02 | 0.51 | 0.02 | 99.17 | |
| b42-1-03 | 1.73 | 0.37 | 0.04 | 0.01 | 0.00 | 41.38 | 55.34 | 0.22 | 0.00 | 0.00 | 0.23 | 0.06 | 99.38 | |
| b42-2-01 | 1.51 | 0.33 | 0.03 | 0.00 | 0.03 | 41.49 | 55.31 | 0.12 | 0.00 | 0.00 | 0.23 | 0.06 | 99.11 | |
| b42-2-03 | 1.54 | 0.33 | 0.06 | 0.00 | 0.05 | 41.47 | 55.14 | 0.19 | 0.00 | 0.01 | 0.30 | 0.00 | 99.09 | |
| b42-3-01 | 2.18 | 0.35 | 0.04 | 0.00 | 0.10 | 42.03 | 55.48 | 0.14 | 0.01 | 0.03 | 0.31 | 0.00 | 100.67 | |
| b46-1-01 | 1.07 | 0.31 | 0.20 | 0.11 | 0.06 | 41.74 | 54.96 | 0.91 | 0.01 | 0.05 | 0.29 | 0.10 | 99.81 | |
| b46-2-01 | 1.90 | 0.19 | 0.05 | 0.00 | 0.09 | 41.62 | 54.58 | 0.29 | 0.00 | 0.08 | 0.37 | 0.02 | 99.21 | |
| b46-2-02 | 1.33 | 0.21 | 0.06 | 0.00 | 0.02 | 41.37 | 55.35 | 0.28 | 0.02 | 0.00 | 0.29 | 0.03 | 98.94 | |
| b46-2-03 | 1.37 | 0.20 | 0.03 | 0.10 | 0.08 | 41.52 | 55.57 | 0.54 | 0.00 | 0.05 | 0.07 | 0.00 | 99.51 | |
| b46-3-01 | 1.66 | 0.29 | 0.14 | 0.31 | 0.06 | 41.43 | 54.15 | 0.71 | 0.00 | 0.03 | 1.15 | 0.02 | 99.95 | |
| b46-3-02 | 1.56 | 0.34 | 0.04 | 0.03 | 0.13 | 41.56 | 55.03 | 0.19 | 0.03 | 0.00 | 0.10 | 0.03 | 99.05 | |
| b48-1-01 | 1.56 | 0.90 | 0.11 | 0.00 | 0.05 | 41.47 | 55.15 | 0.20 | 0.02 | 0.08 | 0.26 | 0.02 | 99.81 | |
| b48-2-01 | 1.45 | 0.51 | 0.30 | 0.01 | 0.07 | 41.52 | 54.39 | 0.54 | 0.01 | 0.08 | 0.29 | 0.02 | 99.19 | |
| b48-2-04 | 1.54 | 0.36 | 0.06 | 0.02 | 0.09 | 41.43 | 55.83 | 0.28 | 0.01 | 0.00 | 0.22 | 0.00 | 99.83 | |
| b48-3-01 | 1.54 | 0.63 | 0.10 | 0.00 | 0.05 | 41.66 | 54.98 | 0.21 | 0.00 | 0.01 | 0.23 | 0.00 | 99.43 | |
| b48-3-02 | 1.62 | 0.59 | 0.03 | 0.00 | 0.04 | 41.47 | 55.06 | 0.13 | 0.00 | 0.00 | 0.12 | 0.02 | 99.07 | |
| b54-1-02 | 1.39 | 0.67 | 0.08 | 0.03 | 0.09 | 41.43 | 55.05 | 0.26 | 0.00 | 0.12 | 0.31 | 0.04 | 99.45 | |
| b54-2-01 | 1.58 | 0.78 | 0.11 | 0.03 | 0.05 | 41.39 | 54.99 | 0.13 | 0.00 | 0.09 | 0.09 | 0.00 | 99.24 | |
| b54-2-02 | 1.32 | 0.67 | 0.09 | 0.00 | 0.07 | 41.76 | 54.87 | 0.07 | 0.01 | 0.04 | 0.15 | 0.02 | 99.07 | |
| b54-3-01 | 1.41 | 0.17 | 0.10 | 0.01 | 0.09 | 41.57 | 55.40 | 0.26 | 0.02 | 0.01 | 0.29 | 0.00 | 99.34 | |
| b54-3-02 | 1.70 | 0.30 | 0.07 | 0.01 | 0.13 | 41.63 | 55.05 | 0.09 | 0.01 | 0.06 | 0.29 | 0.05 | 99.40 | |
| b54-4-01 | 1.46 | 0.65 | 0.12 | 0.00 | 0.00 | 41.33 | 55.51 | 0.24 | 0.01 | 0.07 | 0.29 | 0.00 | 99.68 | |
| ZK4506 | ||||||||||||||
| b3-1-1 | 1.89 | 0.54 | 0.03 | 0.01 | 0.01 | 41.69 | 54.77 | 0.19 | 0.02 | 0.00 | 0.14 | 0.00 | 0.02 | 99.30 |
| b3-1-2 | 1.93 | 0.51 | 0.02 | 0.01 | 0.08 | 41.59 | 55.32 | 0.25 | 0.00 | 0.00 | 0.08 | 0.02 | 0.06 | 99.86 |
| b3-1-3 | 2.03 | 0.52 | 0.06 | 0.00 | 0.10 | 41.79 | 54.69 | 0.09 | 0.00 | 0.05 | 0.04 | 0.06 | 0.07 | 99.50 |
| b3-1-4 | 2.01 | 0.42 | 0.03 | 0.01 | 0.08 | 41.76 | 55.47 | 0.16 | 0.00 | 0.02 | 0.12 | 0.06 | 0.01 | 100.14 |
| b5-1-1 | 1.56 | 0.44 | 0.06 | 0.00 | 0.03 | 41.61 | 54.98 | 0.25 | 0.01 | 0.01 | 0.29 | 0.00 | 0.02 | 99.23 |
| b5-1-2 | 1.98 | 0.38 | 0.04 | 0.01 | 0.07 | 41.67 | 55.08 | 0.12 | 0.00 | 0.04 | 0.92 | 0.19 | - | 100.50 |
| b5-2-1 | 1.94 | 0.33 | 0.01 | 0.00 | 0.01 | 41.40 | 55.19 | 0.17 | 0.00 | 0.02 | 0.17 | 0.00 | - | 99.25 |
| b5-3-1 | 1.75 | 0.28 | 0.05 | 0.01 | 0.00 | 41.58 | 55.62 | 0.26 | 0.00 | 0.00 | 0.27 | 0.04 | 0.04 | 99.89 |
| b5-3-2 | 1.99 | 0.22 | 0.03 | 0.00 | 0.01 | 41.77 | 54.85 | 0.08 | 0.02 | 0.00 | 0.19 | 0.01 | 0.02 | 99.17 |
| b5-3-3 | 1.82 | 0.17 | 0.01 | 0.00 | 0.04 | 41.36 | 55.67 | 0.25 | 0.00 | 0.03 | 0.17 | 0.03 | - | 99.54 |
| b5-4-1 | 1.88 | 0.22 | 0.06 | 0.00 | 0.06 | 41.61 | 56.48 | 0.23 | 0.00 | 0.02 | 0.14 | 0.01 | 0.03 | 100.73 |
| b5-4-2 | 1.71 | 0.23 | 0.04 | 0.00 | 0.02 | 41.55 | 54.98 | 0.21 | 0.02 | 0.02 | 0.16 | 0.03 | 0.004 | 98.98 |
| b5-4-3 | 1.61 | 0.22 | 0.05 | 0.00 | 0.00 | 41.79 | 55.43 | 0.17 | 0.00 | 0.05 | 0.14 | 0.04 | - | 99.50 |
| b5-4-4 | 1.80 | 0.23 | 0.00 | 0.00 | 0.01 | 41.31 | 55.38 | 0.21 | 0.00 | 0.08 | 0.17 | 0.10 | - | 99.30 |
| b6-1-1 | 2.00 | 0.25 | 0.05 | 0.02 | 0.07 | 41.42 | 55.33 | 0.24 | 0.00 | 0.00 | 0.19 | 0.00 | 0.05 | 99.62 |
| b6-1-2 | 1.88 | 0.28 | 0.00 | 0.00 | 0.00 | 41.58 | 55.64 | 0.26 | 0.00 | 0.08 | 0.16 | 0.00 | 0.01 | 99.90 |
| b6-1-3 | 1.25 | 0.30 | 0.03 | 0.02 | 0.01 | 41.72 | 55.57 | 0.24 | 0.00 | 0.06 | 0.39 | 0.07 | - | 99.66 |
| b6-2-1 | 1.85 | 0.39 | 0.05 | 0.01 | 0.00 | 41.51 | 56.09 | 0.26 | 0.00 | 0.04 | 0.19 | 0.01 | 0.004 | 100.40 |
| b6-3-1 | 1.70 | 0.64 | 0.04 | 0.00 | 0.02 | 41.40 | 54.78 | 0.19 | 0.02 | 0.07 | 0.06 | 0.00 | 0.01 | 98.92 |
| b6-4-1 | 1.54 | 0.18 | 0.04 | 0.01 | 0.04 | 41.74 | 55.75 | 0.20 | 0.02 | 0.07 | 0.14 | 0.00 | 0.002 | 99.74 |
| b7-3-1 | 1.54 | 0.55 | 0.12 | 0.02 | 0.02 | 41.43 | 54.98 | 0.47 | 0.01 | 0.01 | 0.38 | 0.06 | - | 99.61 |
| b10-1-1 | 1.61 | 0.45 | 0.11 | 0.02 | 0.06 | 41.76 | 55.66 | 0.23 | 0.00 | 0.05 | 0.19 | 0.00 | 0.13 | 100.27 |
| b10-1-2 | 1.55 | 0.44 | 0.10 | 0.00 | 0.03 | 41.71 | 54.79 | 0.31 | 0.00 | 0.08 | 0.26 | 0.00 | 0.04 | 99.30 |
| b10-2-1 | 1.26 | 0.58 | 0.08 | 0.03 | 0.04 | 41.59 | 54.84 | 0.31 | 0.00 | 0.13 | 0.19 | 0.02 | 0.02 | 99.09 |
| b10-2-2 | 1.37 | 0.52 | 0.07 | 0.03 | 0.05 | 41.59 | 55.13 | 0.20 | 0.00 | 0.00 | 0.14 | 0.02 | 0.02 | 99.12 |
| b10-2-3 | 1.95 | 0.53 | 0.06 | 0.02 | 0.01 | 41.78 | 54.65 | 0.17 | 0.01 | 0.00 | 0.11 | 0.00 | 0.06 | 99.35 |
| b12-3-1 | 1.60 | 0.47 | 0.11 | 0.00 | 0.08 | 41.42 | 55.75 | 0.19 | 0.00 | 0.10 | 0.28 | 0.00 | 0.04 | 100.04 |
| b12-3-2 | 1.24 | 0.58 | 0.12 | 0.00 | 0.06 | 41.39 | 55.43 | 0.18 | 0.02 | 0.05 | 0.26 | 0.03 | 0.01 | 99.37 |
| b25-2-1 | 1.93 | 0.24 | 0.08 | 0.00 | 0.06 | 41.72 | 55.17 | 0.15 | 0.00 | 0.01 | 0.12 | 0.00 | 0.04 | 99.53 |
| b25-2-2 | 1.61 | 0.23 | 0.06 | 0.01 | 0.04 | 41.77 | 55.55 | 0.18 | 0.01 | 0.07 | 0.18 | 0.00 | 0.02 | 99.72 |
| b25-4-1 | 2.07 | 0.24 | 0.03 | 0.00 | 0.09 | 41.76 | 54.85 | 0.25 | 0.03 | 0.00 | 0.06 | 0.00 | 0.02 | 99.39 |
| b25-4-2 | 1.40 | 0.27 | 0.05 | 0.00 | 0.06 | 41.64 | 54.85 | 0.19 | 0.00 | 0.03 | 0.16 | 0.00 | 0.002 | 98.65 |
| b25-4-3 | 1.67 | 0.25 | 0.05 | 0.00 | 0.06 | 41.77 | 55.16 | 0.21 | 0.01 | 0.00 | 0.14 | 0.00 | 0.03 | 99.33 |
| b25-4-4 | 1.53 | 0.51 | 0.04 | 0.01 | 0.04 | 41.64 | 55.13 | 0.28 | 0.00 | 0.00 | 0.16 | 0.00 | - | 99.34 |
| late-stage phase | ||||||||||||||
| ZK4506 | ||||||||||||||
| b3-2-1 | 1.65 | 1.16 | 0.07 | 0.00 | 0.01 | 41.75 | 54.07 | 0.39 | 0.00 | 0.09 | 0.24 | 0.03 | 0.04 | 99.51 |
| b7-1-1 | 1.56 | 1.12 | 0.02 | 0.00 | 0.09 | 41.41 | 54.54 | 0.20 | 0.00 | 0.07 | 0.25 | 0.00 | 0.05 | 99.31 |
| b7-1-2 | 1.98 | 1.12 | 0.02 | 0.01 | 0.12 | 41.46 | 54.98 | 0.26 | 0.00 | 0.12 | 0.14 | 0.00 | 0.00 | 100.21 |
| b7-1-3 | 1.50 | 1.26 | 0.04 | 0.00 | 0.10 | 41.45 | 54.24 | 0.25 | 0.00 | 0.00 | 0.23 | 0.02 | 0.03 | 99.12 |
| b7-2-1 | 1.29 | 1.16 | 0.04 | 0.00 | 0.06 | 41.61 | 54.53 | 0.22 | 0.01 | 0.00 | 0.20 | 0.00 | 0.03 | 99.15 |
| b9-1-1 | 1.63 | 1.02 | 0.03 | 0.00 | 0.09 | 41.70 | 54.49 | 0.54 | 0.03 | 0.00 | 0.10 | 0.02 | 0.04 | 99.68 |
| b9-1-2 | 1.91 | 1.06 | 0.03 | 0.02 | 0.10 | 41.44 | 54.30 | 0.55 | 0.02 | 0.03 | 0.14 | 0.03 | 0.08 | 99.72 |
| b9-2-1 | 1.51 | 1.22 | 0.04 | 0.01 | 0.15 | 41.77 | 55.03 | 0.58 | 0.00 | 0.00 | 0.12 | 0.00 | 0.12 | 100.66 |
| b9-2-2 | 1.65 | 1.23 | 0.17 | 0.17 | 0.16 | 41.33 | 53.42 | 1.02 | 0.32 | 0.10 | 0.65 | 0.13 | 0.16 | 100.50 |
| b10-3-1 | 1.47 | 0.91 | 0.10 | 0.01 | 0.09 | 41.55 | 54.77 | 0.14 | 0.00 | 0.05 | 0.19 | 0.01 | 0.06 | 99.35 |
| b10-3-2 | 1.60 | 1.01 | 0.07 | 0.00 | 0.07 | 41.66 | 54.55 | 0.09 | 0.00 | 0.08 | 0.22 | 0.00 | - | 99.35 |
| b12-1-1 | 1.02 | 2.33 | 0.01 | 0.02 | 0.11 | 41.35 | 55.07 | 0.16 | 0.00 | 0.00 | 0.07 | 0.00 | 0.05 | 100.18 |
| b12-1-2 | 1.06 | 2.05 | 0.03 | 0.00 | 0.08 | 41.52 | 54.49 | 0.16 | 0.00 | 0.00 | 0.08 | 0.00 | 0.02 | 99.50 |
| b12-1-3 | 0.65 | 2.39 | 0.05 | 0.00 | 0.07 | 41.32 | 55.64 | 0.13 | 0.01 | 0.00 | 0.05 | 0.00 | - | 100.30 |
| b12-2-1 | 1.14 | 1.80 | 0.03 | 0.00 | 0.09 | 41.73 | 54.51 | 0.12 | 0.01 | 0.00 | 0.18 | 0.00 | 0.02 | 99.62 |
| b12-2-2 | 1.30 | 1.84 | 0.06 | 0.00 | 0.06 | 41.59 | 54.09 | 0.25 | 0.02 | 0.00 | 0.15 | 0.03 | 0.04 | 99.38 |
| b12-2-3 | 1.04 | 1.95 | 0.09 | 0.00 | 0.08 | 41.66 | 53.96 | 0.22 | 0.02 | 0.05 | 0.21 | 0.02 | - | 99.29 |
| b13-1-1 | 1.34 | 0.91 | 0.03 | 0.03 | 0.08 | 41.90 | 55.00 | 0.21 | 0.01 | 0.07 | 0.15 | 0.00 | 0.09 | 99.81 |
| b13-2-1 | 2.00 | 1.06 | 0.02 | 0.04 | 0.09 | 41.65 | 53.55 | 0.48 | 0.01 | 0.00 | 0.24 | 0.02 | 0.05 | 99.21 |
| b13-3-1 | 1.21 | 1.02 | 0.03 | 0.02 | 0.09 | 41.76 | 54.15 | 0.72 | 0.04 | 0.11 | 0.19 | 0.00 | 0.09 | 99.43 |
| b16-1-1 | 1.21 | 1.30 | 0.02 | 0.00 | 0.01 | 41.61 | 54.69 | 0.24 | 0.00 | 0.10 | 0.26 | 0.00 | - | 99.43 |
| b16-2-1 | 0.65 | 2.96 | 0.04 | 0.00 | 0.07 | 41.62 | 53.47 | 0.24 | 0.01 | 0.04 | 0.17 | 0.00 | - | 99.27 |
| b16-3-1 | 1.20 | 1.47 | 0.06 | 0.00 | 0.06 | 41.76 | 54.46 | 0.12 | 0.00 | 0.00 | 0.19 | 0.02 | - | 99.34 |
| b16-3-2 | 1.34 | 1.62 | 0.03 | 0.00 | 0.06 | 41.62 | 54.31 | 0.12 | 0.00 | 0.11 | 0.23 | 0.00 | 0.01 | 99.45 |
| b16-3-3 | 1.31 | 1.71 | 0.05 | 0.00 | 0.04 | 41.45 | 54.05 | 0.16 | 0.00 | 0.08 | 0.19 | 0.06 | 0.04 | 99.14 |
| b20-2-1 | 1.54 | 1.06 | 0.03 | 0.00 | 0.09 | 41.66 | 54.70 | 0.46 | 0.06 | 0.11 | 0.38 | 0.01 | - | 100.11 |
| b20-2-2 | 1.14 | 1.02 | 0.15 | 0.08 | 0.12 | 41.56 | 53.34 | 0.95 | 0.30 | 0.11 | 0.72 | 0.13 | 0.002 | 99.61 |
| b20-3-1 | 1.58 | 1.12 | 0.01 | 0.02 | 0.10 | 41.50 | 53.97 | 0.42 | 0.06 | 0.05 | 0.25 | 0.04 | 0.002 | 99.13 |
| b25-1-1 | 1.22 | 1.53 | 0.02 | 0.00 | 0.07 | 41.76 | 55.00 | 0.32 | 0.01 | 0.02 | 0.06 | 0.00 | 0.06 | 100.07 |
| b25-1-2 | 1.67 | 1.49 | 0.03 | 0.01 | 0.06 | 41.77 | 54.38 | 0.22 | 0.02 | 0.01 | 0.10 | 0.00 | - | 99.75 |
| b25-1-3 | 1.40 | 1.51 | 0.04 | 0.00 | 0.04 | 41.75 | 54.53 | 0.25 | 0.00 | 0.04 | 0.06 | 0.00 | 0.03 | 99.65 |
| b25-3-1 | 1.44 | 1.35 | 0.04 | 0.01 | 0.06 | 41.57 | 54.46 | 0.19 | 0.00 | 0.00 | 0.14 | 0.01 | - | 99.26 |
| b25-3-2 | 1.31 | 1.36 | 0.01 | 0.00 | 0.05 | 41.44 | 55.25 | 0.17 | 0.00 | 0.11 | 0.11 | 0.01 | - | 99.81 |
| Sample | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | K2O | V2O3 | Cr2O3 | ZnO | NiO | CuO | CoO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZK4506 | |||||||||||||||
| b1-Mt-1 | 0.02 | 1.11 | 0.28 | 92.29 | 0.05 | 0.13 | 0.00 | 0.00 | 0.25 | 0.12 | 0.06 | 0.00 | 0.05 | 0.17 | 94.51 |
| b1-Ilm-1 | 0.00 | 47.81 | 0.03 | 50.40 | 1.26 | 0.27 | 0.00 | 0.01 | 0.47 | 0.00 | 0.03 | 0.00 | 0.00 | 0.07 | 100.34 |
| b1-Mt-2 | 0.00 | 0.58 | 0.14 | 92.94 | 0.04 | 0.01 | 0.00 | 0.00 | 0.24 | 0.05 | 0.00 | 0.00 | 0.00 | 0.17 | 94.17 |
| b1-Ilm-2 | 0.05 | 46.11 | 0.03 | 51.24 | 1.47 | 0.21 | 0.00 | 0.00 | 0.68 | 0.00 | 0.12 | 0.00 | 0.00 | 0.06 | 99.98 |
| b5-Mt-1 | 0.02 | 1.59 | 0.48 | 92.16 | 0.10 | 0.48 | 0.00 | 0.02 | 0.41 | 0.01 | 0.00 | 0.00 | 0.00 | 0.15 | 95.40 |
| b5-Ilm-1 | 0.03 | 52.68 | 0.00 | 41.24 | 0.81 | 4.58 | 0.11 | 0.01 | 0.62 | 0.06 | 0.02 | 0.00 | 0.00 | 0.09 | 100.23 |
| b5-Mt-2 | 0.01 | 2.30 | 6.29 | 75.57 | 0.16 | 2.94 | 0.02 | 0.00 | 0.47 | 0.08 | 0.10 | 0.03 | 0.06 | 0.22 | 88.23 |
| b5-Ilm-2 | 0.00 | 51.27 | 0.01 | 42.91 | 0.62 | 4.06 | 0.01 | 0.01 | 0.44 | 0.00 | 0.03 | 0.02 | 0.00 | 0.09 | 99.47 |
| b6-Mt-1 | 0.01 | 5.65 | 1.58 | 82.21 | 0.10 | 1.03 | 0.00 | 0.00 | 0.44 | 0.20 | 0.05 | 0.00 | 0.08 | 0.22 | 91.57 |
| b6-Ilm-1 | 0.00 | 49.95 | 0.00 | 41.27 | 0.59 | 3.60 | 0.00 | 0.00 | 0.61 | 0.00 | 0.04 | 0.00 | 0.00 | 0.11 | 96.16 |
| b6-Mt-2 | 0.00 | 7.19 | 1.42 | 80.90 | 0.19 | 0.75 | 0.01 | 0.00 | 0.43 | 0.12 | 0.05 | 0.02 | 0.02 | 0.23 | 91.33 |
| b6-Ilm-2 | 0.02 | 50.82 | 0.02 | 41.49 | 0.54 | 3.63 | 0.00 | 0.00 | 0.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 97.16 |
| b9-Mt-1 | 0.00 | 1.01 | 0.24 | 91.63 | 0.00 | 0.05 | 0.06 | 0.00 | 0.38 | 0.08 | 0.10 | 0.00 | 0.14 | 0.19 | 93.87 |
| b9-Ilm-1 | 0.01 | 48.17 | 0.02 | 47.19 | 0.99 | 0.93 | 0.12 | 0.01 | 0.55 | 0.06 | 0.11 | 0.00 | 0.00 | 0.13 | 98.28 |
| b9-Mt-2 | 0.00 | 0.96 | 0.34 | 91.62 | 0.04 | 0.12 | 0.00 | 0.00 | 0.30 | 0.04 | 0.00 | 0.00 | 0.00 | 0.16 | 93.58 |
| b9-Ilm-2 | 0.00 | 48.42 | 0.03 | 46.32 | 1.68 | 1.03 | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 98.12 |
| b10-Mt-1 | 0.00 | 1.60 | 2.00 | 87.47 | 0.04 | 0.84 | 0.00 | 0.01 | 0.43 | 0.21 | 0.13 | 0.04 | 0.04 | 0.16 | 92.98 |
| b10-Ilm-1 | 0.02 | 51.29 | 0.03 | 44.00 | 0.95 | 2.46 | 0.00 | 0.00 | 0.54 | 0.08 | 0.15 | 0.00 | 0.00 | 0.10 | 99.62 |
| b10-Mt-2 | 0.00 | 1.23 | 0.43 | 91.78 | 0.03 | 0.15 | 0.00 | 0.00 | 0.48 | 0.18 | 0.00 | 0.00 | 0.00 | 0.16 | 94.46 |
| b10-Ilm-2 | 0.00 | 50.74 | 0.03 | 46.37 | 0.82 | 1.76 | 0.00 | 0.00 | 0.70 | 0.03 | 0.00 | 0.00 | 0.10 | 0.06 | 100.61 |
| b22-Mt-1 | 0.02 | 1.34 | 0.54 | 92.06 | 0.10 | 0.15 | 0.00 | 0.01 | 0.54 | 0.00 | 0.01 | 0.00 | 0.00 | 0.17 | 94.93 |
| b22-Ilm-1 | 0.00 | 49.40 | 0.02 | 46.15 | 1.63 | 1.47 | 0.02 | 0.01 | 0.54 | 0.00 | 0.05 | 0.00 | 0.00 | 0.12 | 99.42 |
| b22-Mt-2 | 0.00 | 2.24 | 0.66 | 90.00 | 0.25 | 0.27 | 0.00 | 0.00 | 0.42 | 0.00 | 0.11 | 0.00 | 0.00 | 0.17 | 94.13 |
| b22-Ilm-2 | 0.02 | 48.03 | 0.02 | 47.07 | 1.62 | 1.35 | 0.00 | 0.02 | 0.62 | 0.00 | 0.03 | 0.00 | 0.00 | 0.10 | 98.86 |
| b25-Mt-1 | 0.07 | 3.25 | 0.43 | 90.22 | 0.22 | 0.23 | 0.00 | 0.00 | 0.50 | 0.14 | 0.06 | 0.00 | 0.00 | 0.19 | 95.30 |
| b25-Ilm-1 | 0.03 | 49.35 | 0.04 | 46.19 | 1.32 | 1.20 | 0.00 | 0.00 | 0.41 | 0.04 | 0.00 | 0.00 | 0.04 | 0.11 | 98.73 |
| b25-Mt-2 | 0.00 | 7.09 | 1.97 | 81.37 | 0.27 | 0.96 | 0.00 | 0.00 | 0.46 | 0.08 | 0.03 | 0.00 | 0.00 | 0.18 | 92.42 |
| b25-Ilm-2 | 0.02 | 49.74 | 0.02 | 46.37 | 1.41 | 1.59 | 0.00 | 0.00 | 0.54 | 0.02 | 0.12 | 0.02 | 0.00 | 0.12 | 99.97 |
| ZK3306 | |||||||||||||||
| b34-Mt-1 | 0.03 | 10.45 | 1.93 | 75.99 | 0.30 | 1.83 | 0.00 | 0.00 | 0.43 | 0.29 | 0.00 | 0.00 | 0.06 | 0.19 | 91.50 |
| b34-Ilm-1 | 0.03 | 50.95 | 0.04 | 39.47 | 0.46 | 4.38 | 0.00 | 0.00 | 0.41 | 0.03 | 0.14 | 0.00 | 0.01 | 0.08 | 96.01 |
| b34-Mt-2 | 0.01 | 10.56 | 1.97 | 73.85 | 0.37 | 2.09 | 0.04 | 0.00 | 0.34 | 0.31 | 0.01 | 0.00 | 0.00 | 0.08 | 89.63 |
| b34-Ilm-2 | 0.00 | 50.33 | 0.04 | 37.94 | 0.43 | 5.28 | 0.03 | 0.01 | 0.43 | 0.00 | 0.07 | 0.00 | 0.00 | 0.03 | 94.60 |
| b40-Mt-1 | 0.01 | 10.08 | 1.75 | 78.37 | 0.25 | 1.85 | 0.00 | 0.01 | 0.52 | 0.32 | 0.00 | 0.08 | 0.00 | 0.17 | 93.42 |
| b40-Ilm-1 | 0.00 | 52.44 | 0.02 | 38.97 | 0.49 | 4.92 | 0.00 | 0.00 | 0.63 | 0.00 | 0.06 | 0.00 | 0.00 | 0.02 | 97.54 |
| b40-Mt-2 | 0.00 | 10.37 | 2.08 | 77.16 | 0.24 | 1.89 | 0.00 | 0.01 | 0.48 | 0.34 | 0.11 | 0.05 | 0.00 | 0.14 | 92.86 |
| b40-Ilm-2 | 0.00 | 52.37 | 0.01 | 39.33 | 0.46 | 5.28 | 0.00 | 0.00 | 0.59 | 0.07 | 0.14 | 0.00 | 0.00 | 0.10 | 98.34 |
| b48-Mt-1 | 0.02 | 8.59 | 2.11 | 76.21 | 0.34 | 1.30 | 0.00 | 0.00 | 0.36 | 0.41 | 0.03 | 0.00 | 0.00 | 0.17 | 89.53 |
| b48-Ilm-1 | 0.01 | 47.62 | 0.01 | 40.68 | 0.46 | 3.70 | 0.03 | 0.00 | 0.47 | 0.04 | 0.00 | 0.00 | 0.00 | 0.01 | 93.03 |
| b48-Mt-2 | 0.03 | 6.89 | 1.40 | 79.78 | 0.21 | 0.79 | 0.00 | 0.01 | 0.45 | 0.51 | 0.10 | 0.06 | 0.00 | 0.17 | 90.41 |
| b48-Ilm-2 | 0.02 | 48.62 | 0.00 | 41.27 | 0.51 | 3.08 | 0.00 | 0.04 | 0.46 | 0.03 | 0.00 | 0.00 | 0.00 | 0.08 | 94.11 |
| Inclusion Phase | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZK4506 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample | b1-2-1 | b9-3-1 | b9-3-2 | b19-1-1 | b19-2-1 | b19-2-2 | b19-2-3 | b19-3-1 | b19-4-1 | b22-2-1 | b22-2-2 | b22-3-2 | b22-3-3 | b22-3-4 | |||||||||||||||||||||||||||||||||||||
| Rb | 0.63 | 1.33 | 0.94 | 0.55 | 0.74 | 0.61 | 0.86 | 0.67 | 0.74 | 0.31 | 0.30 | 0.63 | 0.90 | 0.73 | |||||||||||||||||||||||||||||||||||||
| Ba | 1.05 | 1.21 | 3.02 | 4.46 | 2.63 | 10.43 | 3.85 | 3.52 | 5.53 | 1.04 | 1.98 | 11.99 | 125.70 | 10.81 | |||||||||||||||||||||||||||||||||||||
| Th | 35.34 | 267.30 | 261.43 | 21.33 | 118.94 | 154.62 | 87.55 | 49.99 | 23.43 | 9.89 | 16.43 | 11.79 | 19.52 | 21.10 | |||||||||||||||||||||||||||||||||||||
| U | - | 42.72 | 36.26 | 9.42 | 35.46 | 38.41 | 35.18 | 21.47 | 7.45 | 2.81 | 4.98 | 2.15 | 3.94 | 3.71 | |||||||||||||||||||||||||||||||||||||
| Nb | 0.13 | 0.24 | - | 0.10 | 0.08 | 0.08 | - | - | 0.09 | 0.02 | 0.09 | 0.06 | 0.09 | - | |||||||||||||||||||||||||||||||||||||
| La | 1595.40 | 3063.52 | 2762.62 | 2678.24 | 2814.58 | 2921.97 | 2946.87 | 2488.59 | 2579.64 | 958.34 | 1149.06 | 1385.72 | 1180.69 | 1405.66 | |||||||||||||||||||||||||||||||||||||
| Ce | 3672.17 | 6010.18 | 5405.43 | 4965.86 | 5521.65 | 5692.80 | 5667.50 | 4709.69 | 5149.95 | 2025.67 | 2562.05 | 2787.28 | 2341.14 | 2802.60 | |||||||||||||||||||||||||||||||||||||
| Pb | 1.55 | 11.39 | 8.99 | 13.57 | 4.49 | 5.64 | 3.88 | 2.75 | 4.40 | 3.40 | 3.11 | 3.40 | 6.27 | 3.62 | |||||||||||||||||||||||||||||||||||||
| Pr | 439.16 | 681.46 | 610.33 | 553.90 | 624.64 | 645.51 | 626.05 | 542.56 | 563.41 | 235.27 | 290.92 | 307.79 | 256.73 | 299.01 | |||||||||||||||||||||||||||||||||||||
| Sr | 1466.44 | 1083.70 | 1143.52 | 998.66 | 1015.48 | 1097.86 | 1027.47 | 934.29 | 1106.42 | 1004.43 | 1864.66 | 1055.64 | 1316.90 | 859.78 | |||||||||||||||||||||||||||||||||||||
| Nd | 1828.32 | 2716.78 | 2391.50 | 2184.25 | 2439.13 | 2533.72 | 2523.89 | 2152.31 | 2237.52 | 1047.07 | 1268.27 | 1296.09 | 1058.66 | 1248.02 | |||||||||||||||||||||||||||||||||||||
| Sm | 325.09 | 493.35 | 446.01 | 370.46 | 407.04 | 437.69 | 417.98 | 377.68 | 381.56 | 199.24 | 240.10 | 233.03 | 189.59 | 221.02 | |||||||||||||||||||||||||||||||||||||
| Zr | 11.10 | 15.82 | 9.21 | 3.19 | 1.62 | - | - | - | 9.94 | 9.98 | 13.37 | 13.35 | 10.61 | 13.74 | |||||||||||||||||||||||||||||||||||||
| Hf | 0.00 | 0.08 | - | - | - | - | - | - | 0.05 | - | 0.12 | - | - | - | |||||||||||||||||||||||||||||||||||||
| Eu | 69.32 | 101.09 | 94.21 | 58.89 | 70.78 | 77.70 | 74.61 | 63.28 | 63.82 | 46.08 | 55.72 | 56.66 | 43.53 | 50.39 | |||||||||||||||||||||||||||||||||||||
| Gd | 266.07 | 414.39 | 362.11 | 284.50 | 312.52 | 328.74 | 314.60 | 294.15 | 293.21 | 172.39 | 195.70 | 189.91 | 154.32 | 182.86 | |||||||||||||||||||||||||||||||||||||
| Tb | 29.73 | 48.54 | 42.08 | 32.78 | 36.16 | 38.38 | 36.26 | 35.49 | 35.71 | 18.08 | 22.30 | 20.50 | 16.48 | 20.65 | |||||||||||||||||||||||||||||||||||||
| Dy | 154.04 | 240.82 | 216.31 | 165.67 | 189.44 | 198.66 | 194.82 | 182.04 | 178.91 | 91.63 | 113.01 | 107.92 | 85.90 | 99.36 | |||||||||||||||||||||||||||||||||||||
| Y | 661.71 | 1045.70 | 932.12 | 768.78 | 845.99 | 874.00 | 839.31 | 811.40 | 787.08 | 392.21 | 470.63 | 464.69 | 376.66 | 442.06 | |||||||||||||||||||||||||||||||||||||
| Ho | 24.23 | 39.32 | 34.72 | 27.85 | 30.20 | 32.84 | 31.24 | 30.85 | 28.63 | 14.80 | 18.08 | 17.31 | 13.71 | 17.15 | |||||||||||||||||||||||||||||||||||||
| Er | 52.35 | 89.36 | 78.16 | 67.93 | 71.25 | 77.36 | 75.39 | 69.73 | 64.98 | 32.83 | 41.06 | 38.84 | 31.10 | 39.90 | |||||||||||||||||||||||||||||||||||||
| Tm | 5.95 | 9.30 | 8.17 | 6.25 | 7.95 | 8.33 | 8.05 | 7.51 | 7.24 | 3.19 | 4.38 | 4.02 | 3.08 | 4.32 | |||||||||||||||||||||||||||||||||||||
| Yb | 27.06 | 46.00 | 37.77 | 34.06 | 39.85 | 41.42 | 41.14 | 38.98 | 37.83 | 16.55 | 21.14 | 20.97 | 20.13 | 22.43 | |||||||||||||||||||||||||||||||||||||
| Lu | 3.24 | 4.53 | 3.73 | 4.14 | 4.85 | 4.63 | 5.22 | 4.45 | 4.35 | 2.08 | 2.54 | 2.52 | 1.93 | 2.63 | |||||||||||||||||||||||||||||||||||||
| Intergranular Phase | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ZK4506 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample | b3-1-3 | b5-1-1 | b5-2-1 | b5-3-1 | b5-4-1 | b6-1-1 | b6-2-1 | b6-3-1 | b6-4-1 | b10-1-1 | b10-2-1 | b12-3-1 | b12-3-2 | b25-2-1 | b25-4-1 | ||||||||||||||||||||||||||||||||||||
| Rb | 0.51 | 1.14 | 0.56 | 0.24 | 0.21 | - | 296.41 | - | - | 0.24 | 0.36 | 0.17 | 0.33 | 0.33 | 0.32 | ||||||||||||||||||||||||||||||||||||
| Ba | 3.95 | 5.54 | 1.35 | 0.50 | 5.64 | - | 1682.84 | 6.43 | 4.35 | 8.18 | 3.98 | 4.79 | 3.05 | 1.43 | 5.93 | ||||||||||||||||||||||||||||||||||||
| Th | 86.78 | 417.37 | 76.98 | 88.91 | 17.02 | 93.73 | 180.16 | 110.63 | 67.71 | 17.97 | 38.85 | 68.03 | 41.69 | 44.30 | 37.12 | ||||||||||||||||||||||||||||||||||||
| U | 20.48 | 31.45 | 30.55 | 37.86 | 5.70 | 28.35 | 46.40 | 26.65 | 20.84 | 3.17 | 13.32 | 22.54 | 13.95 | 10.52 | 9.09 | ||||||||||||||||||||||||||||||||||||
| Nb | 0.06 | 0.44 | - | - | - | - | 12.35 | - | - | 0.10 | - | - | 0.08 | - | - | ||||||||||||||||||||||||||||||||||||
| La | 1263.26 | 670.20 | 797.88 | 669.52 | 801.87 | 950.76 | 1157.64 | 1128.88 | 877.56 | 1346.84 | 1203.61 | 1044.98 | 1094.55 | 1077.77 | 1028.37 | ||||||||||||||||||||||||||||||||||||
| Ce | 2401.12 | 1335.26 | 1641.17 | 1279.03 | 1694.54 | 1746.72 | 2038.54 | 2007.75 | 1823.87 | 2749.28 | 2470.41 | 2024.57 | 2124.22 | 2197.85 | 2092.01 | ||||||||||||||||||||||||||||||||||||
| Pb | 3.10 | 11.88 | 2.88 | 2.76 | 1.49 | 10.16 | 28.83 | 33.25 | 249.04 | 30.03 | 59.25 | 39.17 | 31.90 | 2.89 | 2.57 | ||||||||||||||||||||||||||||||||||||
| Pr | 257.32 | 159.90 | 198.66 | 146.52 | 200.78 | 188.09 | 213.24 | 222.83 | 211.43 | 289.86 | 261.62 | 215.22 | 221.85 | 235.75 | 230.68 | ||||||||||||||||||||||||||||||||||||
| Sr | 1365.74 | 911.70 | 1344.92 | 1576.06 | 1832.47 | 1545.63 | 1129.81 | 1104.40 | 1858.16 | 1344.80 | 1346.96 | 1288.36 | 1324.59 | 1840.68 | 2176.67 | ||||||||||||||||||||||||||||||||||||
| Nd | 989.61 | 658.65 | 836.58 | 623.10 | 910.12 | 749.90 | 821.37 | 858.07 | 872.59 | 1151.25 | 1066.81 | 897.84 | 931.81 | 952.76 | 927.58 | ||||||||||||||||||||||||||||||||||||
| Sm | 176.87 | 117.01 | 149.34 | 115.12 | 170.10 | 132.38 | 140.81 | 134.68 | 159.59 | 187.85 | 185.34 | 152.82 | 164.95 | 166.49 | 163.53 | ||||||||||||||||||||||||||||||||||||
| Zr | 12.83 | 6.87 | 9.18 | 6.56 | 12.81 | 8.46 | 22.73 | 8.48 | 9.34 | 17.27 | 17.00 | 14.89 | 15.30 | 8.57 | 6.97 | ||||||||||||||||||||||||||||||||||||
| Hf | 0.00 | 0.43 | - | - | 0.11 | - | 0.18 | - | - | 0.12 | 0.11 | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Eu | 42.50 | 25.13 | 37.28 | 28.71 | 43.35 | 29.98 | 35.08 | 32.18 | 35.87 | 45.55 | 44.37 | 37.08 | 38.87 | 40.75 | 40.57 | ||||||||||||||||||||||||||||||||||||
| Gd | 134.15 | 89.37 | 128.32 | 97.13 | 140.82 | 99.03 | 107.17 | 107.77 | 132.31 | 151.23 | 144.98 | 122.94 | 124.10 | 133.57 | 131.39 | ||||||||||||||||||||||||||||||||||||
| Tb | 14.34 | 8.93 | 12.97 | 10.54 | 15.20 | 10.55 | 11.06 | 11.39 | 13.59 | 16.15 | 16.07 | 13.39 | 13.33 | 15.06 | 14.45 | ||||||||||||||||||||||||||||||||||||
| Dy | 69.84 | 38.35 | 60.05 | 49.70 | 69.74 | 51.67 | 54.22 | 54.09 | 65.27 | 79.29 | 78.94 | 64.12 | 69.68 | 74.24 | 70.74 | ||||||||||||||||||||||||||||||||||||
| Y | 301.68 | 163.33 | 263.98 | 209.92 | 302.95 | 212.40 | 205.52 | 233.86 | 278.58 | 328.56 | 329.35 | 270.40 | 287.88 | 324.59 | 317.85 | ||||||||||||||||||||||||||||||||||||
| Ho | 10.63 | 6.03 | 9.84 | 8.26 | 11.10 | 7.90 | 7.89 | 8.22 | 10.20 | 12.46 | 12.40 | 10.26 | 10.07 | 12.17 | 12.10 | ||||||||||||||||||||||||||||||||||||
| Er | 24.15 | 12.71 | 21.16 | 16.95 | 24.31 | 17.17 | 19.60 | 19.03 | 22.26 | 29.80 | 28.19 | 22.67 | 24.12 | 29.01 | 27.38 | ||||||||||||||||||||||||||||||||||||
| Tm | 2.77 | 1.04 | 2.08 | 1.83 | 2.35 | 1.85 | 1.72 | 1.58 | 2.28 | 3.07 | 2.85 | 2.29 | 2.65 | 2.53 | 2.57 | ||||||||||||||||||||||||||||||||||||
| Yb | 11.82 | 6.53 | 11.25 | 7.79 | 11.79 | 9.13 | 9.45 | 8.71 | 10.69 | 14.38 | 14.82 | 12.61 | 12.77 | 13.79 | 13.78 | ||||||||||||||||||||||||||||||||||||
| Lu | 1.52 | 0.82 | 1.28 | 1.06 | 1.44 | 1.21 | 1.09 | 1.34 | 1.16 | 1.91 | 1.70 | 1.42 | 1.73 | 1.63 | 1.63 | ||||||||||||||||||||||||||||||||||||
| Intergranular Phase | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ZK4506 | ZK3306 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample | b25-4-2 | b25-4-3 | b10-1-1 | b11-1-1 | b11-3-1 | b11-4-1 | b24-2-1 | b40-1-1 | b40-1-2 | b40-3-1 | b42-2-1 | b42-3-1 | b46-1-1 | b46-3-1 | b46-3-2 | ||||||||||||||||||||||||||||||||||||
| Rb | 0.31 | 1.11 | 0.24 | 0.25 | - | 0.36 | 0.42 | 0.17 | 0.47 | 0.19 | 0.25 | 2.56 | 0.84 | 2.42 | 0.69 | ||||||||||||||||||||||||||||||||||||
| Ba | 5.12 | 9.08 | 5.73 | 2.48 | 2.16 | 3.05 | 1.24 | 2.58 | 11.97 | 2.11 | 1.48 | 3.94 | 4.33 | 8.78 | 8.71 | ||||||||||||||||||||||||||||||||||||
| Th | 42.66 | 42.06 | 16.54 | 75.91 | 63.47 | 52.94 | 191.46 | 52.21 | 50.07 | 70.72 | 50.91 | 51.62 | 35.45 | 37.42 | 40.53 | ||||||||||||||||||||||||||||||||||||
| U | 10.84 | 10.21 | 4.06 | 15.19 | 11.25 | 9.79 | 36.16 | 19.14 | 25.06 | 17.60 | 13.98 | 15.04 | 21.36 | 25.54 | 24.22 | ||||||||||||||||||||||||||||||||||||
| Nb | - | - | 0.02 | - | - | 0.01 | - | - | 1.30 | - | 0.02 | 0.06 | 0.12 | 0.24 | 0.25 | ||||||||||||||||||||||||||||||||||||
| La | 952.32 | 1029.62 | 867.02 | 664.95 | 646.99 | 724.93 | 743.16 | 669.02 | 607.75 | 681.89 | 746.81 | 841.43 | 1094.16 | 1275.09 | 1263.02 | ||||||||||||||||||||||||||||||||||||
| Ce | 1945.88 | 2077.00 | 1691.86 | 1469.34 | 1408.71 | 1569.80 | 1523.55 | 1214.00 | 1091.44 | 1255.02 | 1491.08 | 1547.55 | 1625.83 | 2084.31 | 2061.48 | ||||||||||||||||||||||||||||||||||||
| Pb | 4.42 | 5.99 | 7.29 | 15.21 | 6.91 | 7.99 | 17.83 | 10.22 | 8.58 | 12.76 | 6.57 | 20.10 | 3.81 | 4.16 | 4.55 | ||||||||||||||||||||||||||||||||||||
| Pr | 214.69 | 232.62 | 183.19 | 165.88 | 160.70 | 180.28 | 172.27 | 133.35 | 120.16 | 134.45 | 161.46 | 166.88 | 151.99 | 205.09 | 200.54 | ||||||||||||||||||||||||||||||||||||
| Sr | 2096.09 | 2129.59 | 1120.35 | 1356.03 | 1268.46 | 1257.35 | 1159.78 | 1118.75 | 1057.62 | 1076.32 | 1169.78 | 1078.10 | 2312.03 | 2278.94 | 2206.64 | ||||||||||||||||||||||||||||||||||||
| Nd | 891.76 | 954.24 | 776.21 | 690.47 | 674.31 | 755.53 | 689.78 | 550.36 | 504.69 | 563.25 | 671.23 | 679.47 | 559.04 | 805.57 | 790.31 | ||||||||||||||||||||||||||||||||||||
| Sm | 154.70 | 171.09 | 134.24 | 120.11 | 118.67 | 135.84 | 113.20 | 92.02 | 85.94 | 97.85 | 111.32 | 115.11 | 103.11 | 150.88 | 148.54 | ||||||||||||||||||||||||||||||||||||
| Zr | 6.17 | 7.61 | 10.48 | 8.22 | 9.14 | 9.73 | 9.06 | 6.94 | 13.07 | 4.51 | 3.75 | 8.55 | 7.04 | 11.64 | 9.66 | ||||||||||||||||||||||||||||||||||||
| Hf | - | - | 0.06 | - | - | - | - | 0.06 | 0.23 | - | - | 0.13 | - | - | - | ||||||||||||||||||||||||||||||||||||
| Eu | 37.35 | 43.77 | 34.24 | 29.77 | 31.62 | 35.03 | 29.40 | 24.26 | 20.81 | 25.06 | 27.38 | 28.02 | 36.88 | 49.14 | 46.70 | ||||||||||||||||||||||||||||||||||||
| Gd | 126.04 | 134.48 | 115.10 | 99.50 | 101.99 | 117.19 | 95.16 | 76.03 | 70.59 | 87.23 | 95.66 | 97.72 | 96.23 | 139.10 | 139.15 | ||||||||||||||||||||||||||||||||||||
| Tb | 14.23 | 15.63 | 12.50 | 11.05 | 10.73 | 13.01 | 10.11 | 8.70 | 7.51 | 9.09 | 9.82 | 10.05 | 11.67 | 16.47 | 16.46 | ||||||||||||||||||||||||||||||||||||
| Dy | 69.18 | 75.98 | 61.76 | 54.45 | 55.10 | 62.76 | 47.83 | 42.11 | 39.49 | 48.25 | 49.62 | 52.19 | 66.34 | 91.31 | 88.78 | ||||||||||||||||||||||||||||||||||||
| Y | 291.02 | 337.42 | 260.17 | 232.89 | 230.36 | 284.38 | 205.41 | 182.92 | 163.44 | 198.78 | 213.74 | 215.78 | 324.69 | 427.14 | 436.52 | ||||||||||||||||||||||||||||||||||||
| Ho | 10.96 | 12.31 | 9.65 | 8.34 | 9.36 | 11.04 | 8.09 | 6.57 | 5.94 | 7.71 | 8.30 | 7.85 | 11.49 | 15.27 | 15.46 | ||||||||||||||||||||||||||||||||||||
| Er | 24.87 | 28.57 | 22.31 | 19.37 | 19.51 | 25.03 | 18.05 | 16.28 | 13.90 | 18.11 | 17.64 | 18.48 | 28.03 | 34.86 | 36.47 | ||||||||||||||||||||||||||||||||||||
| Tm | 2.33 | 2.83 | 2.39 | 2.09 | 2.09 | 2.73 | 2.12 | 1.62 | 1.42 | 1.73 | 1.71 | 1.84 | 3.06 | 4.01 | 3.86 | ||||||||||||||||||||||||||||||||||||
| Yb | 12.10 | 15.67 | 11.58 | 11.92 | 10.18 | 12.97 | 10.32 | 8.72 | 7.89 | 8.68 | 8.42 | 7.78 | 16.07 | 19.51 | 18.50 | ||||||||||||||||||||||||||||||||||||
| Lu | 1.49 | 1.81 | 1.38 | 1.37 | 1.25 | 1.59 | 1.27 | 1.02 | 0.87 | 1.01 | 1.02 | 1.09 | 1.79 | 2.17 | 2.09 | ||||||||||||||||||||||||||||||||||||
| Intergranular Phase | Late-Stage Phase | ||||||||||||||||||||||||||||||||||||||||||||||||||
| ZK3306 | ZK4506 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample | b46-3-3 | b48-2-1 | b48-2-2 | b48-3-1 | b54-2-2 | b12-1-1 | b12-1-2 | b12-2-1 | b12-2-2 | b12-2-3 | b25-1-1 | b25-1-2 | b25-1-3 | b25-3-1 | b25-3-2 | ||||||||||||||||||||||||||||||||||||
| Rb | 0.31 | 0.19 | 0.22 | 0.30 | 0.14 | 0.14 | 0.22 | 0.14 | 0.24 | 0.15 | 0.34 | 0.25 | - | 0.21 | 0.24 | ||||||||||||||||||||||||||||||||||||
| Ba | 3.86 | 3.56 | 25.21 | 3.15 | 9.79 | 7.36 | 10.59 | 1.78 | 6.17 | 3.49 | 2.23 | 1.32 | 2.79 | 2.08 | 2.25 | ||||||||||||||||||||||||||||||||||||
| Th | 51.61 | 60.53 | 60.78 | 98.52 | 91.78 | 105.70 | 72.49 | 77.10 | 94.08 | 91.64 | 54.09 | 40.84 | 85.94 | 41.45 | 59.12 | ||||||||||||||||||||||||||||||||||||
| U | 18.92 | 16.81 | 20.24 | 19.78 | 26.72 | 27.81 | 20.97 | 21.81 | 24.73 | 27.22 | 20.49 | 19.15 | 26.54 | 14.72 | 16.02 | ||||||||||||||||||||||||||||||||||||
| Nb | 0.13 | 0.04 | - | - | - | - | - | - | 0.03 | 0.04 | 0.05 | 0.05 | - | - | 0.04 | ||||||||||||||||||||||||||||||||||||
| La | 1127.19 | 824.33 | 797.21 | 954.86 | 879.70 | 1043.78 | 936.84 | 1034.56 | 1068.35 | 952.58 | 1285.87 | 1183.96 | 1301.31 | 1193.19 | 1227.08 | ||||||||||||||||||||||||||||||||||||
| Ce | 1815.77 | 1695.08 | 1620.34 | 1812.62 | 1643.87 | 2032.36 | 1771.27 | 1974.50 | 2003.96 | 1808.73 | 2509.57 | 2318.76 | 2499.91 | 2257.72 | 2323.88 | ||||||||||||||||||||||||||||||||||||
| Pb | 4.22 | 13.85 | 12.13 | 20.44 | 14.87 | 51.68 | 43.07 | 105.32 | 88.33 | 58.72 | 2.41 | 1.86 | 3.88 | 1.52 | 1.84 | ||||||||||||||||||||||||||||||||||||
| Pr | 175.57 | 186.35 | 179.69 | 191.00 | 165.78 | 215.57 | 193.44 | 205.84 | 208.08 | 185.51 | 261.95 | 241.17 | 259.71 | 233.51 | 229.55 | ||||||||||||||||||||||||||||||||||||
| Sr | 2238.79 | 881.15 | 909.01 | 764.34 | 888.42 | 1646.18 | 1424.86 | 1450.65 | 1535.56 | 1267.72 | 2002.57 | 1883.72 | 1937.21 | 1782.84 | 1742.17 | ||||||||||||||||||||||||||||||||||||
| Nd | 672.97 | 777.03 | 754.32 | 781.53 | 676.93 | 877.39 | 810.50 | 842.40 | 838.81 | 743.29 | 1029.06 | 963.89 | 996.98 | 932.12 | 889.11 | ||||||||||||||||||||||||||||||||||||
| Sm | 127.38 | 141.54 | 136.22 | 133.62 | 121.60 | 149.98 | 139.15 | 133.58 | 141.33 | 123.70 | 170.33 | 161.22 | 156.20 | 156.56 | 146.87 | ||||||||||||||||||||||||||||||||||||
| Zr | 5.75 | 7.55 | 7.38 | 8.81 | 5.04 | 3.65 | 2.99 | 2.49 | 2.60 | 3.56 | 3.44 | 3.34 | 1.73 | 4.70 | 3.29 | ||||||||||||||||||||||||||||||||||||
| Hf | 0.04 | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Eu | 43.19 | 34.17 | 31.33 | 32.15 | 29.16 | 33.13 | 31.56 | 32.56 | 31.64 | 30.78 | 42.22 | 38.81 | 38.93 | 36.57 | 36.95 | ||||||||||||||||||||||||||||||||||||
| Gd | 125.28 | 122.81 | 116.41 | 118.68 | 103.25 | 120.64 | 115.95 | 106.46 | 101.73 | 99.33 | 134.60 | 129.01 | 118.60 | 123.61 | 111.87 | ||||||||||||||||||||||||||||||||||||
| Tb | 14.90 | 12.36 | 11.92 | 12.54 | 11.01 | 11.12 | 11.01 | 11.15 | 10.22 | 10.27 | 14.24 | 14.42 | 12.37 | 13.56 | 12.19 | ||||||||||||||||||||||||||||||||||||
| Dy | 82.60 | 61.88 | 53.29 | 59.12 | 54.03 | 56.28 | 53.99 | 51.73 | 44.61 | 48.60 | 70.70 | 68.85 | 61.63 | 66.97 | 59.84 | ||||||||||||||||||||||||||||||||||||
| Y | 415.53 | 263.63 | 236.20 | 233.53 | 234.30 | 215.52 | 211.15 | 197.53 | 194.34 | 183.03 | 288.88 | 289.84 | 244.29 | 276.31 | 254.43 | ||||||||||||||||||||||||||||||||||||
| Ho | 14.04 | 9.67 | 8.44 | 9.02 | 8.77 | 8.40 | 8.74 | 7.70 | 7.42 | 7.37 | 11.20 | 10.72 | 9.36 | 10.89 | 9.31 | ||||||||||||||||||||||||||||||||||||
| Er | 33.26 | 20.89 | 17.78 | 20.66 | 19.10 | 18.13 | 17.62 | 17.17 | 15.54 | 15.18 | 25.02 | 23.74 | 21.27 | 24.42 | 20.79 | ||||||||||||||||||||||||||||||||||||
| Tm | 3.59 | 2.15 | 1.93 | 2.03 | 2.00 | 1.79 | 1.89 | 1.70 | 1.72 | 1.50 | 2.61 | 2.57 | 2.03 | 2.39 | 2.15 | ||||||||||||||||||||||||||||||||||||
| Yb | 17.20 | 11.16 | 9.87 | 9.29 | 9.95 | 8.38 | 9.38 | 8.23 | 8.05 | 7.53 | 11.97 | 11.72 | 10.40 | 10.75 | 9.99 | ||||||||||||||||||||||||||||||||||||
| Lu | 1.86 | 1.48 | 1.25 | 1.24 | 1.20 | 0.94 | 1.11 | 1.00 | 0.79 | 0.88 | 1.40 | 1.20 | 1.06 | 1.43 | 1.25 | ||||||||||||||||||||||||||||||||||||
| Element | U | La | Ce | Pr | Sr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | 2.6 | 2.44 | 2.99 | 3.32 | 2.59 | 3.38 | 3.59 | 2.34 | 3.78 | 3.53 | 3.25 | 3.61 | 3.46 | 3.14 | 3.04 | 2.58 | 2.77 |
References
- BoudreauI, A.E.; Mathez, E.A.; McCallum, I.S. Halogen geochemistry of the Stillwater and Bushveld Complexes: Evidence for transport of the platinum-group elements by Cl-rich fluids. J. Petrol. 1986, 27, 967–986. [Google Scholar] [CrossRef]
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite trace element compositions: A robust new tool for mineral exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Tan, H.M.R.; Huang, X.W.; Qi, L.; Gao, J.F.; Meng, Y.M.; Xie, H. Research progres on chemical composition ofapatite: Application in petrogenesis, ore genesis and mineral exploration. Acta Petrol. Sin. 2022, 38, 3067–3084, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xu, S.H. Apatite Geochemical Constraints on Petrogenesisand Metallogenesis of Mafic-Ultramaficintrusions in the Emeishan Large Igneous Province. Master’s Thesis, Lanzhou University, Lanzhou, China, 2022. (In Chinese with English Abstract). [Google Scholar]
- Shen, J.F.; Li, S.R.; Huang, S.F.; Qing, M.; Zhang, H.F.; Xu, B. The Decennary New Advances on the Genetic Mineralogy and Prospecting Mineralogy (2010–2020). Bull. Mineral. Petrol. Geochem. 2021, 40, 0610–0623+0777, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.; Klügel, A. S-rich apatite-hosted glass inclusions in xenoliths from La Palma: Constra, ints on the volatile partitioning in evolved alkaline magmas. Contrib. Mineral. Petrol. 2011, 162, 463–478. [Google Scholar] [CrossRef]
- Chelle-Michou, C.; Chiaradia, M. Amphibole and apatite insights into the evolution and mass balance of Cl and S in magmas associated with porphyry copper deposits. Contrib. Mineral. Petrol. 2017, 172, 105. [Google Scholar] [CrossRef]
- Stonadge, G.; Miles, A.; Smith, D.; Large, S.; Knott, T. The volatile record of volcanic apatite and its implications for the formation of porphyry copper deposits. Geology 2023, 51, 1158–1162. [Google Scholar] [CrossRef]
- Toplis, M.; Carroll, M. Differentiation of ferro-basaltic magmas under conditions open and closed to oxygen: Implications for the Skaergaard intrusion and other natural systems. J. Petrol. 1996, 37, 837–858. [Google Scholar] [CrossRef]
- Tollari, N.; Barnes, S.J.; Cox, R.A.; Nabil, H. Trace element concentrations in apatites from the Sept-Îles Intrusive Suite, Canada—Implications for the genesis of nelsonites. Chem. Geol. 2008, 252, 180–190. [Google Scholar] [CrossRef]
- VanTongeren, J.; Mathez, E. Large-scale liquid immiscibility at the top of the Bushveld Complex, South Africa. Geology 2012, 40, 491–494. [Google Scholar] [CrossRef]
- Cawthorn, R. Rare earth element abundances in apatite in the Bushveld Complex—A consequence of the trapped liquid shift effect. Geology 2013, 41, 603–606. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Santosh, M.; Cheng, Z.; He, H.; Kang, J. Perovskite and baddeleyite from kimberlitic intrusions in the Tarim large igneous province signal the onset of an end-Carboniferous mantle plume. Earth Planet. Sci. Lett. 2013, 361, 238–248. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zou, H.B.; Wang, H.Y.; Li, H.K.; Ye, H.M. Multiple phases of the Neoproterozoic igneous activity in Quruqtagh of the northeastern Tarim Block, NW China: Interaction between plate subduction and mantle plume? Precambrian Res. 2012, 222, 488–502. [Google Scholar] [CrossRef]
- Guo, Z.J.; Yin, A.; Robinson, A.; Jia, C.Z. Geochronology and geochemistry of deep-drill-core samples from the basement of the central Tarim basin. J. Asian Earth Sci. 2005, 25, 45–56. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.H.; Chen, G.; An, P.; Yang, C.D.; Ren, C.H. Magmatic evolution and mineralization of Xiaohaizi complex in Bachu County, Xinjiang: Constraints from geochronology and geochemistry. Miner. Depos. 2024, 43, 1054–1080. [Google Scholar] [CrossRef]
- Xu, Y.G.; Wei, X.; Luo, Z.Y.; Liu, H.Q.; Cao, J. The Early Permian Tarim Large Igneous Province: Main characteristics and a plume incubation model. Lithos 2014, 204, 20–35. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Xie, Q.; Cheng, Z.; Jin, S. Olivine from aillikites in the Tarim large igneous province as a window into mantle metasomatism and multi-stage magma evolution. Am. Mineral. 2021, 106, 1064–1076. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Giuliani, A.; Cheng, Z.; Liu, B.; Kong, W. Geochemical and O–C–Sr–Nd isotopic constraints on the petrogenetic link between aillikites and carbonatites in the Tarim large igneous province. J. Petrol. 2021, 62, egab017. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, Z.; Cheng, Z.; Liu, B.; Santosh, M.; Wei, B.; Ke, S.; Xu, L.; Zhang, X. Mantle source of tephritic porphyry in the Tarim Large Igneous Province constrained from Mg, Zn, Sr, and Nd isotope systematics: Implications for deep carbon cycling. GSA Bull. 2022, 134, 487–500. [Google Scholar] [CrossRef]
- Yu, S.Y.; Chen, L.M.; Lan, J.B.; He, Y.S.; Chen, Q.; Song, X.Y. Controls of mantle source and condition of melt extraction on generation of the picritic lavas from the Emeishan large igneous province, SW China. J. Asian Earth Sci. 2020, 203, 104534. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Reichow, M.K.; Tian, W.; Kong, W.; Liu, B. Tracing decarbonated eclogite in the mantle sources of Tarim continental flood basalts using Zn isotopes. Bulletin 2023, 135, 1768–1782. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.Y.; Xing, C.M.; Xu, Y.G. Origin of the early Permian Wajilitag igneous complex and associated Fe–Ti oxide mineralization in the Tarim large igneous province, NW China. J. Asian Earth Sci. 2014, 84, 51–68. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Song, B.; Li, Y.; Yang, S.; Yu, X. Temporal evolution of the Permian large igneous province in Tarim Basin in northwestern China. J. Asian Earth Sci. 2011, 42, 917–927. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Hou, T.; Santosh, M.; Zhang, D.; Ke, S. Petrogenesis of nephelinites from the Tarim Large Igneous Province, NW China: Implications for mantle source characteristics and plume–lithosphere interaction. Lithos 2015, 220–223, 15. [Google Scholar] [CrossRef]
- Wei, X.; Xu, Y.G.; He, B.; Zhang, L.; Shi, X.F. Zircon U-Pb age and Hf-O isotope insights into genesis of Permian Tarim felsic rocks, NW China: Implications for crustal melting in response to a mantle plume. Gondwana Res. 2019, 76, 290–302. [Google Scholar] [CrossRef]
- Yang, S.Y.; Jiang, S.Y.; Mao, Q.; Chen, Z.Y.; Rao, C.; Li, X.L.; Li, W.C.; Yang, W.Q.; He, P.L.; Li, X. Electron Probe Microanalysis in Geosciences: Analytical Procedures and Recent Advances. At. Spectrosc. 2022, 43, 186–200. [Google Scholar] [CrossRef]
- Vukadinovic, D.; Edgar, A.D. Phase relations in the phlogopite-apatite system at 20 kbar; implications for the role of fluorine in mantle melting. Contrib. Mineral. Petrol. 1993, 114, 247–254. [Google Scholar] [CrossRef]
- Sun, S.S.; Mcdonough, W.F. Chemical and isotopic systematics of ocean basalts: Implications for mantle composition and processes, in Magmatism in the Ocean Basins. Geol. Soc. Lond. Spec. Publ. 1989, 423, 13–345. [Google Scholar]
- Piccoli, P.M.; Candela, P.A. Apatite in Igneous Systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Xing, C.M.; Wang, C.Y. Cathodoluminescence images and trace element compositions of fluorapatite from the Hongge layered intrusion in SW China: A record of prolonged crystallization and overprinted fluid metasomatism. Am. Mineral. 2017, 102, 1390–1401. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhou, M.F.; Su, S.G.; Chen, X.G. Contrasting Geochemistry of Apatite from Peridotites and Sulfide Ores of the Jinchuan Ni-Cu Sulfide Deposit, NW China. Econ. Geol. 2021, 116, 1073–1092. [Google Scholar] [CrossRef]
- BoudreauI, A.E.; McCallum, I.S. Investigations of the Stillwater Complex: Part V. Apatites as indicators of evolving fluid composition. Contrib. Mineral. Petrol. 1989, 102, 138–153. [Google Scholar] [CrossRef]
- Webster, J.D.; Tappen, C.M.; Mandeville, C.W. Partitioning behavior of chlorine and fluorine in the system apatite–melt–fluid. II: Felsic silicate systems at 200 MPa. Geochim. Et Cosmochim. Acta 2009, 73, 559–581. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, H.Y.; Zhao, Y.; Ling, M.X.; Sun, W.D. Investigation of the geochemical characteristics of apatite trace elements from the Yulong porphyr copper belt, Eastern Tibet. Geochimica 2018, 47, 14–32, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zheng, T.Z.S.; Liu, Z.X.; Duan, G.; Su, Y.P.; Xiao, B.; Lai, X.D.; Zhou, L. Volatile-rich and oxidized ore-forming magmas of the Zijinshan porphyry Cu deposit, China: A mineral composition perspective. Ore Geol. Rev. 2024, 165, 105898. [Google Scholar] [CrossRef]
- Jin, S.K. Geologic Characteristics and Prtrogenesis of TheWajilitag Mafic-Ultramafic Intrusion from the Permiantarimlarge igneous Province, NW China. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2019. (In Chinese with English Abstract). [Google Scholar]
- Zhu, S.Z.; Huang, X.L.; Yang, F.; He, P.L. Petrology and geochemistry of early Permian mafic–ultramafic rocks in the Wajilitag area of the southwestern Tarim Large Igneous Province: Insights into Fe-rich magma of mantle plume activity. Lithos 2021, 398-399, 106355. [Google Scholar] [CrossRef]
- Tang, Q.; Li, L.; Zhang, Y.; Li, Z.; Song, H.; Liu, W.; Zhao, C. Major element geochemistry of apatite from Fe-Ti-V oxide-bearing malic-ultramafic intrusions in the Emeishan large igneous province and their petrogenesis and mineralization. Acta Petrol. Sin. 2024, 40, 2169–2185, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, J.H. Geochemical Characteristics and Significance of Apatite from the Baima Intrusion in the Emeishan Large Igneous Province, SW China. Master’s Thesis, Lanzhou University, Lanzhou, China, 2023. (In Chinese with English Abstract). [Google Scholar]
- Zhang, X.Q.; Song, X.Y.; Chen, L.M.; Xie, W.; Yu, S.Y.; Zheng, W.Q.; Deng, Y.F.; Zhang, J.F.; Gui, S.G. Fractional crystallization and the formation of thick Fe-Ti-V oxide layers in the Baima layered intrusion, SW China. Ore Geol. Rev. J. Compr. Stud. Ore Genes. Ore Explor. 2012, 49, 96–108. [Google Scholar] [CrossRef]
- Mccubbin, F.M.; Jones, R.H. Extraterrestrial Apatite: Planetary Geochemistry to Astrobiology. Elements 2015, 11, 183–188. [Google Scholar] [CrossRef]
- Li, H.; Hermann, J. Chlorine and fluorine partitioning between apatite and sediment melt at 2.5 GPa, 800 °C: A new experimentally derived thermodynamic model. Am. Mineral. 2017, 102, 580–594. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F. Sulfur partitioning between apatite and melt and effect of sulfur on apatite solubility at oxidizing conditions. Contrib. Mineral. Petrol. 2004, 147, 201–212. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.O. Sulfur partition coefficient between apatite and rhyolite: The role of bulk S content. Contrib. Mineral. Petrol. 2005, 150, 643–651. [Google Scholar] [CrossRef]
- Peng, G.; Luhr, J.F.; Mcgee, J.J. Factors controlling sulfur concentrations in volcanic apatite. Am. Mineral. 1997, 82, 1210–1224. [Google Scholar] [CrossRef]
- Konecke, B.A.; Fiege, A.; Simon, A.C.; Linsler, S.; Holtz, F. An experimental calibration of a sulfur-in-apatite oxybarometer for mafic systems. Geochim. Et Cosmochim. Acta 2019, 265, 242–258. [Google Scholar] [CrossRef]
- Sadove, G.; Brian, K.; Fiege, A.; Simon, A. Structurally bound S2-, S1-, S4+ S6+ in terrestrial apatite: The redox evolution of hydrothermal fluids at the Phillips mine, New York, USA. Ore Geol. Rev. J. Compr. Stud. Ore Genes. Ore Explor. 2019, 107, 1084–1096. [Google Scholar] [CrossRef]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas? Geochim. Et Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef]
- Marks, M.A.; Scharrer, M.; Ladenburger, S.; Markl, G. Comment on “Apatite: A new redox proxy for silicic magmas?”[Geochimica et Cosmochimica Acta 132 (2014) 101-119]. Geochim. Et Cosmochim. Acta 2016, 183, 267–270. [Google Scholar] [CrossRef]
- Wang, H.; Cai, K.; Sun, M. Apatite as a magma redox indicator and its application in metallogenic research. Lithos Int. J. Mineral. Petrol. Geochem. 2022, 422–423, 106749. [Google Scholar]
- Cao, M.; Li, G.; Qin, K.; Yusupovha, S.E.; Liu, Y. Major and Trace Element Characteristics of Apatites in Granitoids from Central Kazakhstan: Implications for Petrogenesis and Mineralization. Resour. Geol. 2012, 62, 63–83. [Google Scholar] [CrossRef]
- Bromiley, G.D. Do concentrations of Mn, Eu and Ce in apatite reliably record oxygen fugacity in magmas? Lithos 2020, 384–385, 105900. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Apatite in granitoids related to polymetallic mineral deposits in southeastern Hunan Province, Shi-Hang zone, China: Implications for petrogenesis and metallogenesis. Ore Geol. Rev. 2015, 69, 14. [Google Scholar] [CrossRef]
- Li, Z.M.; Linghu, M.M.; Sun, J.F.; Sun, Z.H.; Zhou, B.Q.; Yang, J.H. Geochemical characterization and application of apatite in magmatic rocks. Acta Petrol. Sin. 2025, 41, 321–338, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- LI, J.; Ma, Z.B.; Yang, Y.; Huang, J.H.; Zhang, R.Z.; Han, J.W. Reviews on apatite and its application in the field of ore deposit geology. Geol. China 2025, 52, 574–596, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Huang, Y.; Wang, G. REE content and distribution in apatite and its geological tracing significance. Chin. Rare Earths 2004, 25, 41–45. [Google Scholar]
- Kendrick, M.A.; Scambelluri, M.; Honda, M.; Phillips, D. High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction. Nat. Geoence 2011, 4, 807–812. [Google Scholar] [CrossRef]
- Xu, L.L.; Bi, X.W.; Zhang, X.C.; Huang, M.L.; Liu, G. Mantle contribution to the generation of the giant Jinduicheng porphyry Mo deposit, Central China: New insights from combined in-situ element and isotope compositions of zircon and apatite. Chem. Geol. 2023, 616, 121238. [Google Scholar] [CrossRef]
- Chu, M.F.; Wang, K.L.; Griffin, W.L.; Chung, S.L.; O'Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite Composition: Tracing Petrogenetic Processes in Transhimalayan Granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar] [CrossRef]
- Watson, E.B.; Green, T.H. Apatite/liquid partition coefficients for the rare earth elements and strontium. Earth Planet. Sci. Lett. 1981, 56, 405–421. [Google Scholar] [CrossRef]
- Bédard, J.H. Parental magmas of the Nain Plutonic Suite anorthosites and mafic cumulates: A trace element modelling approach. Contrib. Mineral. Petrol. 2001, 141, 747–771. [Google Scholar] [CrossRef]
- Prowatke, S.; Klemme, S. Trace element partitioning between apatite and silicate melts. Geochim. Et Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Ji, D.; Dygert, N. Trace element partitioning between apatite and silicate melts: Effects of major element composition, temperature, and oxygen fugacity, and implications for the volatile element budget of the lunar magma ocean. Geochim. Et Cosmochim. Acta 2024, 369, 141–159. [Google Scholar] [CrossRef]
- Zhang, B.Q. Genesis of the Wajilitag Layered Intrusion in ThePermian Tarim Large Igneous Province. Master’s Thesis, Chang’an University, Xi’an, China, 2024. (In Chinese with English Abstract). [Google Scholar]
- Eugster, H.P.; Wones, D.R. Stability relations of the ferruginous biotite, annite. J. Petrol. 1962, 3, 82–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhi, X.; Chen, L.; Saunders, A.D.; Reichow, M.K. Re–Os isotopic compositions of picrites from the Emeishan flood basalt province, China. Earth Planet. Sci. Lett. 2008, 276, 30–39. [Google Scholar] [CrossRef]
- Chen, L.M.; Teng, F.Z.; Song, X.Y.; Luan, Y.; Yu, S.Y.; Kang, J. Origins and implications of magnesium isotopic heterogeneity in Fe-Ti oxides in layered mafic intrusions. Geochim. Et Cosmochim. Acta J. Geochem. Soc. Meteorit. Soc. 2021, 308, 273–290. [Google Scholar] [CrossRef]
- Chen, L.-M.; Teng, F.-Z.; Song, X.-Y.; Hu, R.-Z.; Yu, S.-Y. Magnesium isotopic evidence for chemical disequilibrium among cumulus minerals in layered mafic intrusion. Earth Planet. Sci. Lett. Lett. J. Devoted Dev. Time Earth Planet. Syst. 2018, 487, 74–83. [Google Scholar] [CrossRef]
- Chen, L.M.; Song, X.Y.; Zhu, X.K.; Zhang, X.Q.; Yu, S.Y.; Yi, J.N. Iron isotope fractionation during crystallization and sub-solidus re-equilibration: Constraints from the Baima mafic layered intrusion, SW China. Chem. Geol. 2014, 380, 97–109. [Google Scholar] [CrossRef]
- Zhou, M.F.; Chen, W.T.; Wang, C.Y.; Prevec, A.S.; Liu, P.P.; Howarth, G.H. Two stages of immiscible liquid separation in the formation of Panzhihua-type Fe-Ti-V oxide deposits, SW China. Geosci. Front. 2013, 4, 481–502. [Google Scholar] [CrossRef]
- Zhou, M.F.; Arndt, N.T.; Malpas, J.; Wang, C.Y.; Kennedy, A.K. Two magma series and associated ore deposit types in the Permian Emeishan large igneous province, SW China. Lithos 2008, 103, 352–368. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.Y.; Huang, F.; Zhang, Z. Iron isotope systematics of the Panzhihua mafic layered intrusion associated with giant Fe-Ti oxide deposit in the Emeishan large igneous province, SW China. J. Geophys. Res. Solid Earth 2019, 124, 358–375. [Google Scholar] [CrossRef]
- Wang, K.; Xing, C.M.; Ren, Z.; Wang, Y. Liquid immiscibility in the Panzhihua mafie layered intrusion: Evidence from melt inclusions in apatite. Acta Petrol. Sin. 2013, 29, 3503–3518, (In Chinese with English Abstract). [Google Scholar]
- Cheng, Z.; Zhang, Z.; Xie, Q.; Hou, T.; Ke, S. Subducted slab-plume interaction traced by magnesium isotopes in the northern margin of the Tarim Large Igneous Province. Earth Planet. Sci. Lett. 2018, 489, 100–110. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Kong, Z.; Chen, M.; Yin, J.; Maimaiti, M.; Liu, D. Mineralogical and Geochemical Characteristics of the Fe-Ti Mineralized Mafic-Ultramafic Intrusions at Wajilitag, Tarim Basin, China: With Special Emphasis on the Role of Apatite. Minerals 2025, 15, 1208. https://doi.org/10.3390/min15111208
Wang W, Kong Z, Chen M, Yin J, Maimaiti M, Liu D. Mineralogical and Geochemical Characteristics of the Fe-Ti Mineralized Mafic-Ultramafic Intrusions at Wajilitag, Tarim Basin, China: With Special Emphasis on the Role of Apatite. Minerals. 2025; 15(11):1208. https://doi.org/10.3390/min15111208
Chicago/Turabian StyleWang, Weicheng, Zhigang Kong, Maohong Chen, Jinmao Yin, Maihemuti Maimaiti, and Donghui Liu. 2025. "Mineralogical and Geochemical Characteristics of the Fe-Ti Mineralized Mafic-Ultramafic Intrusions at Wajilitag, Tarim Basin, China: With Special Emphasis on the Role of Apatite" Minerals 15, no. 11: 1208. https://doi.org/10.3390/min15111208
APA StyleWang, W., Kong, Z., Chen, M., Yin, J., Maimaiti, M., & Liu, D. (2025). Mineralogical and Geochemical Characteristics of the Fe-Ti Mineralized Mafic-Ultramafic Intrusions at Wajilitag, Tarim Basin, China: With Special Emphasis on the Role of Apatite. Minerals, 15(11), 1208. https://doi.org/10.3390/min15111208






