The Influence of Sodium Butyl Xanthate and Ammonium Dibutyl Dithiophosphate on the Flotation Behavior of Chalcopyrite and Bornite
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Flotation Collectors
2.2. Flotation Tests
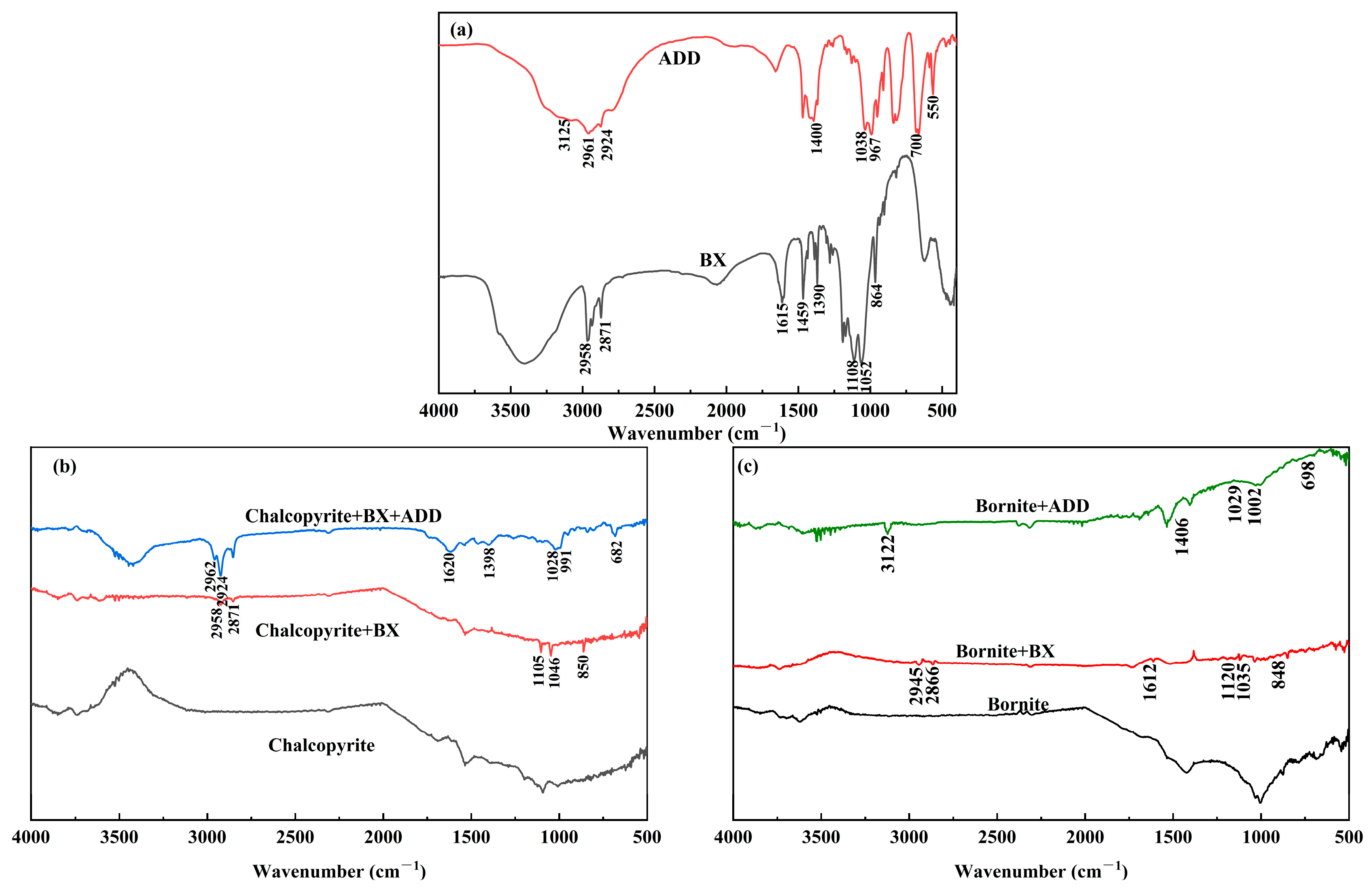
2.3. Fourier Transform Infrared Spectroscopy Measurements
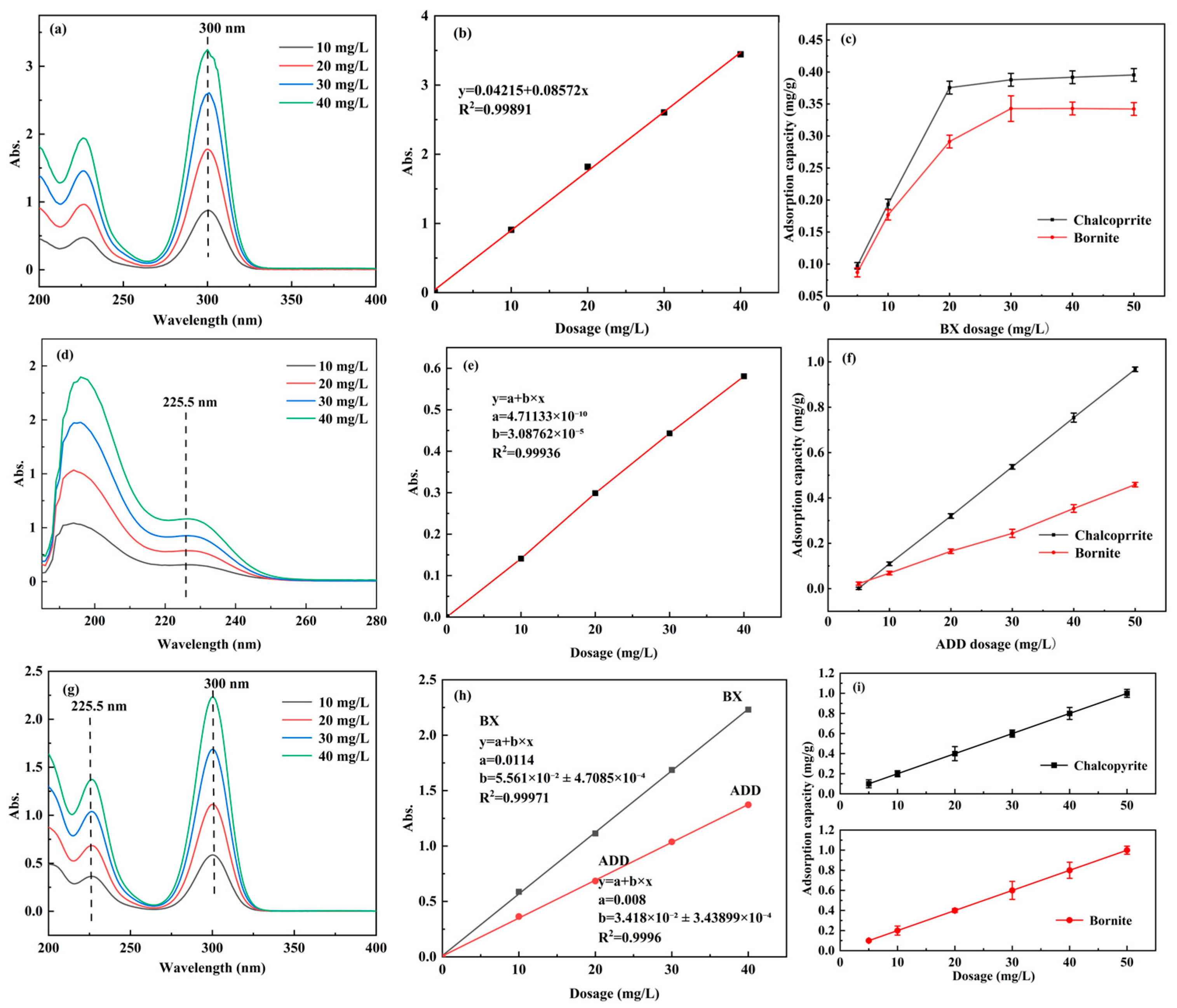
2.4. Adsorption Measurements
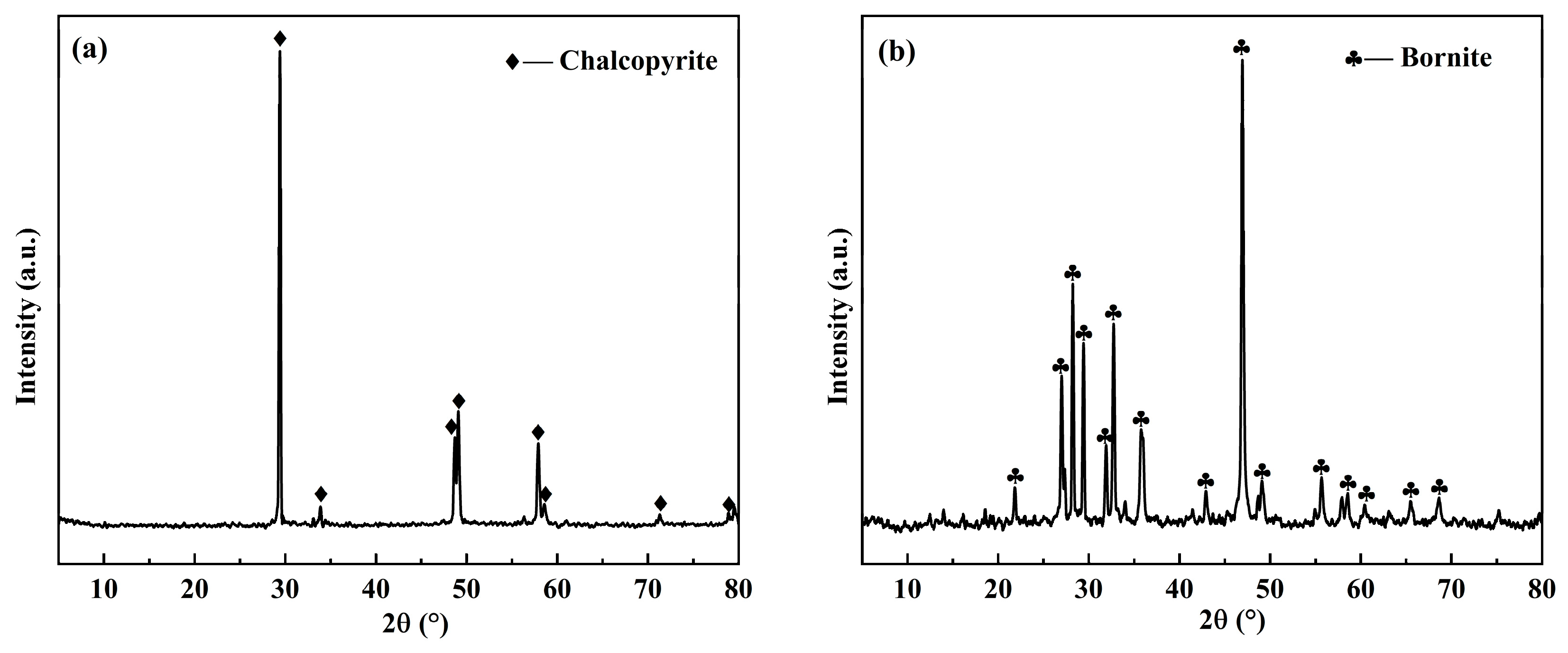
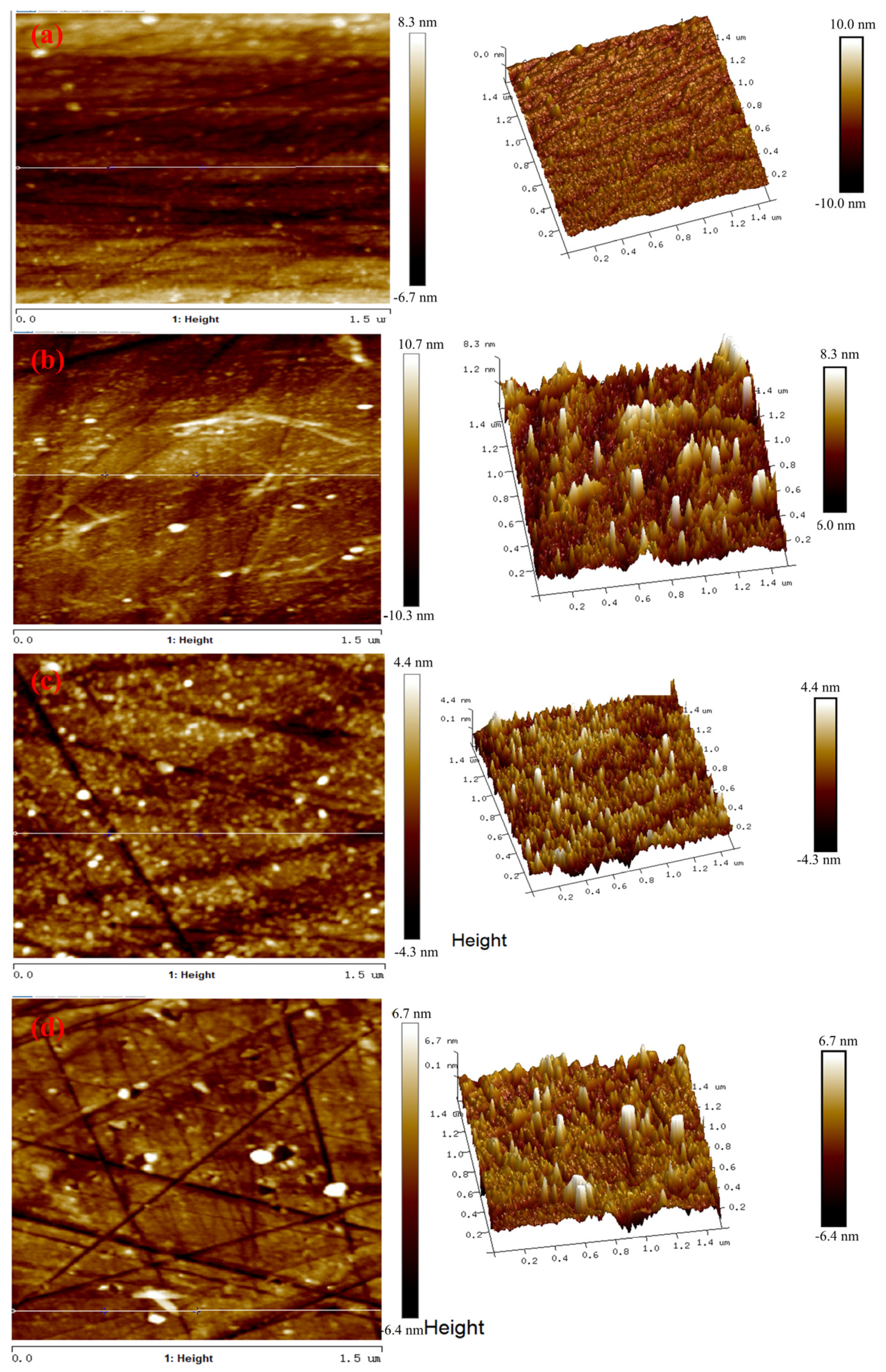
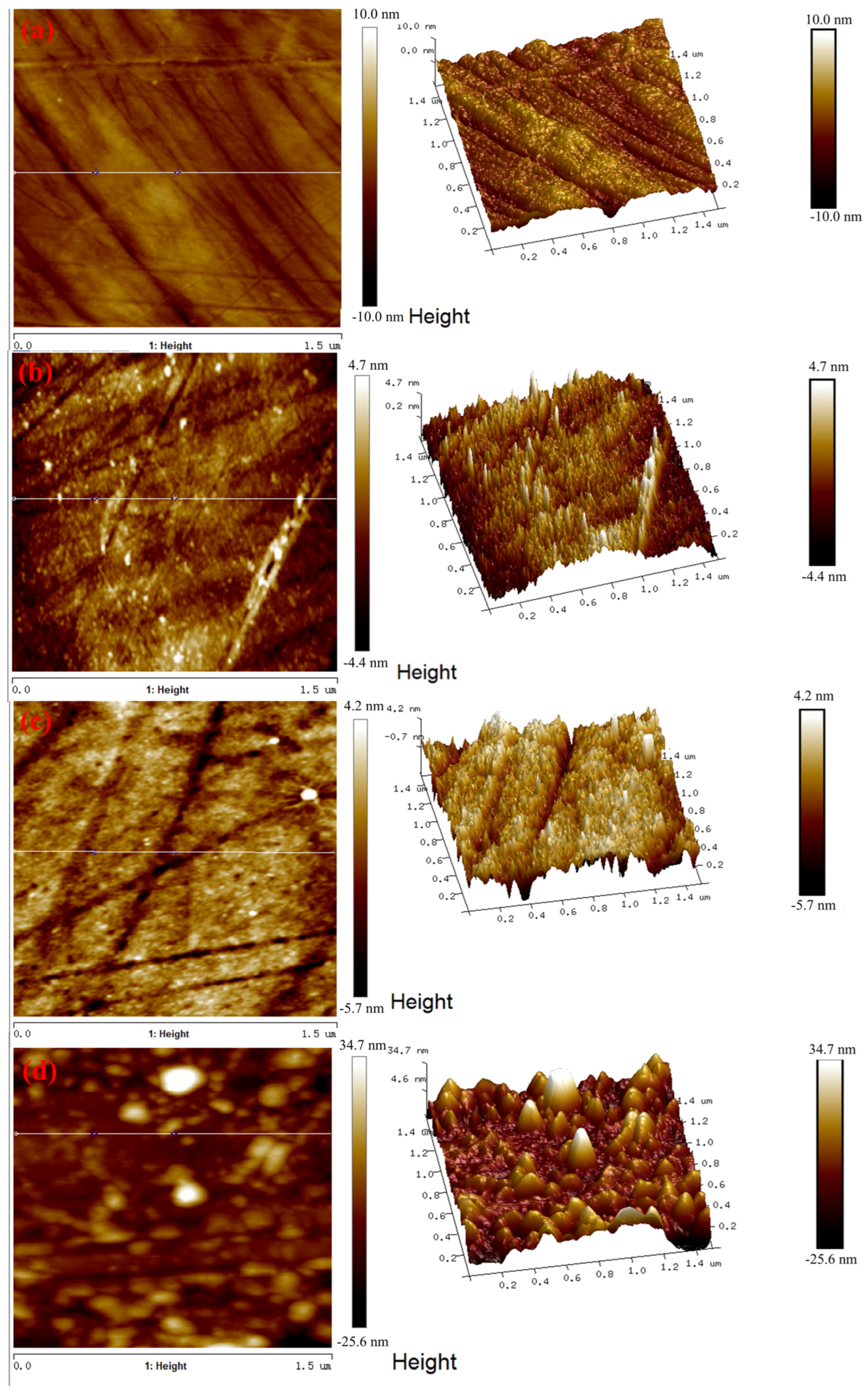
2.5. Surface Image Analysis
3. Results and Discussion
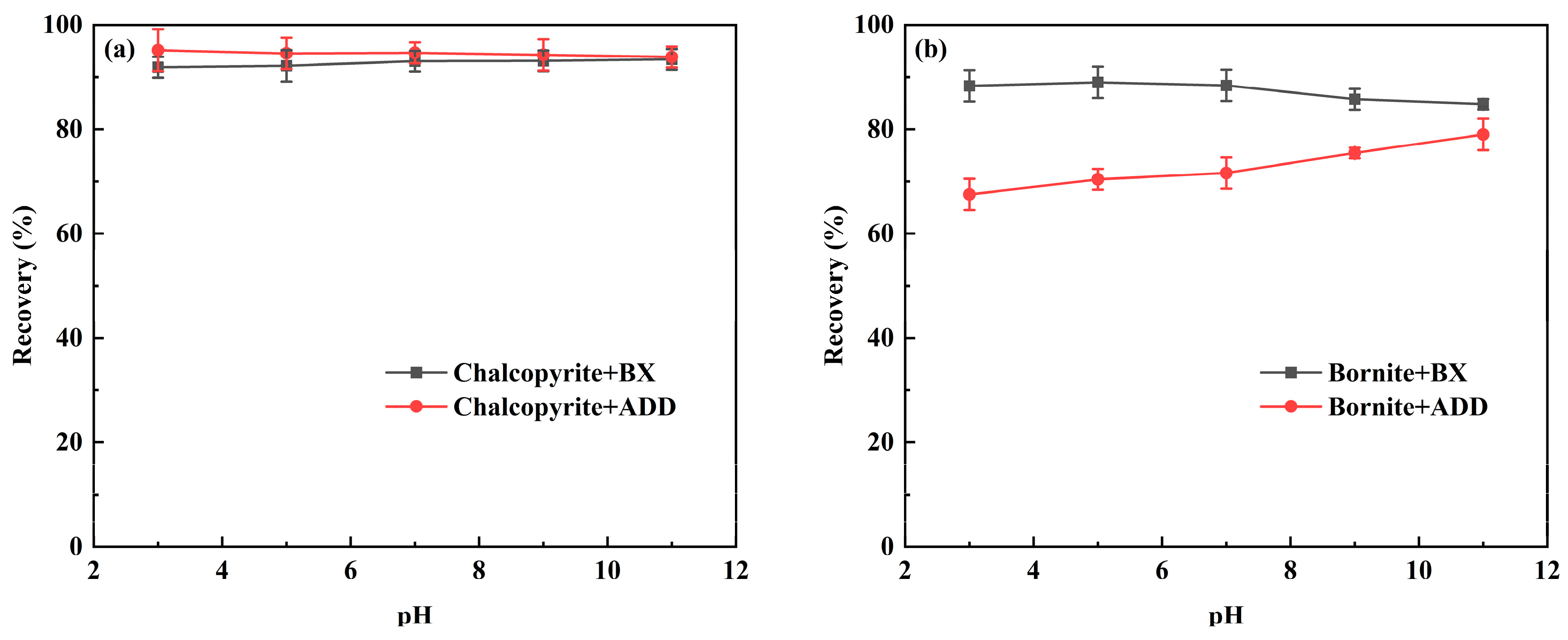
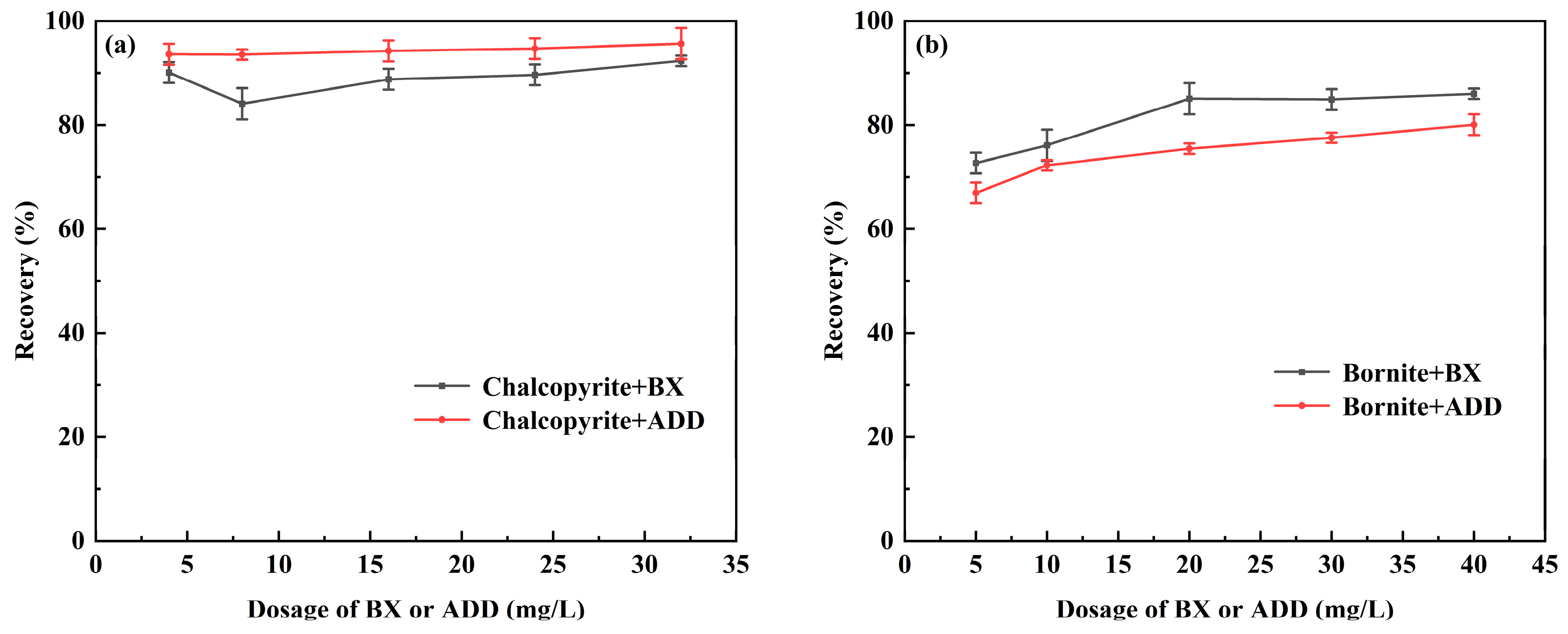
3.1. Flotation Experiments
3.2. Fourier-Transform Infrared Spectroscopy Measurements
3.3. Adsorption Capacity Measurement
3.4. AFM Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, Z.; Feng, Q.; Wen, S.; Wang, H. Flotation separation of chalcopyrite from galena based on selective adsorption of pyrogallic acid: Experiments and MD simulations. Appl. Surf. Sci. 2024, 667, 160398. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.-q.; Sun, W.; Wang, L.; Dong, Y.-h.; Wang, C.-t. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of sodium silicate and sodium sulfite. Trans. Nonferrous Met. Soc. China 2020, 30, 1091–1101. [Google Scholar] [CrossRef]
- Sun, D.; Li, M.; Zhang, M.; Cui, R.; Yang, Z.; Yu, L.; Wang, D.; Yao, W. Utilization and Mechanisms of Tannic Acid as a Depressant for Chalcopyrite and Pyrite Separation. ACS Omega 2023, 8, 30474–30482. [Google Scholar] [CrossRef]
- Sun, L.; Jiao, W.; Cao, Y.; Wang, Q.; Sun, W.; Zhao, K.; Song, Z.; Xie, J.; Lu, B. Sustainable Flotation of Complex Copper-Nickel Sulfide Ores: A Review of Integrated Process Strategies. Miner. Process. Extr. Metall. Rev. 2025, 1–16. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, G.; Liu, Q.; Zhong, H.; Zhang, M. Separation of pyrite from chalcopyrite and molybdenite by using selective collector of N-isopropoxypropyl-N′-ethoxycarbonyl thiourea in high salinity water. Miner. Eng. 2017, 100, 93–98. [Google Scholar] [CrossRef]
- Feng, K.; Wu, D.; Cao, J.; Li, J.; Bai, S. Surface oxidation modification mechanism of sodium persulfate on arsenopyrite and its impact on the flotation separation of bornite and arsenopyrite. J. Hazard. Mater. 2025, 492, 138243. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Cao, J.; Wu, D.; Li, J.; Zuo, Q.; Bai, S. Selective passivation of arsenopyrite surface using a novel eco-friendly oxidant, sodium percarbonate, for Cu–As flotation separation. Chem. Eng. J. 2025, 515, 163664. [Google Scholar] [CrossRef]
- Barde, A.; Lewis, D.J. Fabrication of High Quality Bornite and Chalcopyrite Thin Films by Aerosol-Assisted Chemical Vapor Deposition. J. Phys. Chem. C 2023, 127, 13969–13977. [Google Scholar] [CrossRef]
- Sharma, R.K. Geology of the Copper Mineralization in Proterozoic Ajabgarh Meta-Sediments of, Dokan-Dariba Belt, Sikar District Rajasthan Northwestern India. Open J. Geol. 2023, 13, 384–430. [Google Scholar] [CrossRef]
- Moimane, T.; Huai, Y.; Peng, Y. The critical degree of bornite surface oxidation in flotation. Miner. Eng. 2020, 155, 106445. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Investigation of Copper Recovery from a New Copper Ore Deposit (Nussir) in Northern Norway: Dithiophosphates and Xanthate-Dithiophosphate Blend as Collectors. Minerals 2019, 9, 146. [Google Scholar] [CrossRef]
- Hwang, M.; Mu, Y.; Cao, L.; Peng, Y. The influence of NaCl on xanthate adsorption on chalcopyrite surface and chalcopyrite flotation. Miner. Eng. 2024, 218, 109026. [Google Scholar] [CrossRef]
- Sun, W.; Dai, S.; Zhang, H.; Chen, Y.; Yu, X.; Li, P.; Liu, W. Selective flotation of chalcopyrite from galena using a novel collector benzoic diethylcarbamothioic thioanhydride: An experimental and theoretical investigation. J. Mol. Liq. 2022, 365, 120027. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, L.; Yan, H.; Zhang, H.; Cui, L.; Liu, X. The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation. Minerals 2022, 12, 209. [Google Scholar] [CrossRef]
- Nadezhda, V.V.; Ekaterina, A.V.; Alexander, E.B.; Igor, B.R. Xanthates and Dithiocarbamates: Synthesis, Characterization and Application in Flotation Processes. Mini-Rev. Org. Chem. 2025, 22, 44–53. [Google Scholar]
- Zhang, J.; Zhang, W. AFM Image Analysis of the Adsorption of Xanthate and Dialkyl Dithiophosphate on Chalcocite. Minerals 2022, 12, 1018. [Google Scholar] [CrossRef]
- Chen, J.; Lan, L.; Chen, Y. Computational simulation of adsorption and thermodynamic study of xanthate, dithiophosphate and dithiocarbamate on galena and pyrite surfaces. Miner. Eng. 2013, 46–47, 136–143. [Google Scholar] [CrossRef]
- Andreev, G.N.; Barzev, A. Raman spectroscopic study of some chalcopyrite–xanthate flotation products. J. Mol. Struct. 2003, 661–662, 325–332. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hamilton, I.C.; Woods, R. Investigation of the surface oxidation of bornite by linear potential sweep voltammetry and X-ray photoelectron spectroscopy. J. Appl. Electrochem. 1984, 14, 63–74. [Google Scholar] [CrossRef]
- Hepel, T.; Pomianowski, A. Diagrams of electrochemical equilibria of the system copper-potassium ethylxanthate-water at 25°C. Int. J. Miner. Process. 1977, 4, 345–361. [Google Scholar] [CrossRef]
- Mustafa, S.; Hamid, A.; Naeem, A. Xanthate adsorption studies on chalcopyrite ore. Int. J. Miner. Process. 2004, 74, 317–325. [Google Scholar] [CrossRef]
- Roos, J.R.; Celis, J.P.; Sudarsono, A.S. Electrochemical control of metallic copper and chalcopyrite-xanthate flotation. Int. J. Miner. Process. 1990, 28, 231–245. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Li, Y.; Chen, J. Co-adsorption mechanism of selective Aerophine 3418A/DTP collectors and their industrial application for chalcopyrite flotation. Miner. Eng. 2025, 228, 109304. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Comparison of single and mixed reagent systems for flotation of copper sulphides from Nussir ore. Miner. Eng. 2019, 142, 105930. [Google Scholar] [CrossRef]
- Grano, S.R.; Cnossen, H.; Skinner, W.; Prestidge, C.A.; Ralston, J. Surface modifications in the chalcopyrite-sulphite ion system, II. Dithiophosphate collector adsorption study. Int. J. Miner. Process. 1997, 50, 27–45. [Google Scholar] [CrossRef]
- Monte, M.B.M.; Lins, F.F.; Oliveira, J.F. Adsorption of the thiol compound on gold and pyrite and its influence on their selective flotation. In Proceedings of the XXI International Mineral Processing Congress, Rome, Italy, 23–27 July 2000. [Google Scholar]
- Ackerman, P.K.; Harris, G.H.; Klimpel, R.R.; Aplan, F.F. Evaluation of flotation collectors for copper sulfides and pyrite, I. Common sulfhydryl collectors. Int. J. Miner. Process. 1987, 21, 105–127. [Google Scholar] [CrossRef]
- Clarke, P.; Fornasiero, D.; Ralston, J.; Smart, R.S.C. A study of the removal of oxidation products from sulfide mineral surfaces. Miner. Eng. 1995, 8, 1347–1357. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Sun, W.; Hu, Y.; Cao, J.; Gao, Z. Selective flotation of chalcopyrite from pyrite using diphosphonic acid as collector. Miner. Eng. 2019, 140, 105890. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Sun, W.; Chen, D.; Chen, J.; Wang, R.; Han, M.; Zhang, C. The effects of hydroxyl on selective separation of chalcopyrite from pyrite: A mechanism study. Appl. Surf. Sci. 2023, 608, 154963. [Google Scholar] [CrossRef]
- Sun, Q.-y.; Yin, W.-z.; Cao, S.-h.; Yang, B.; Sun, H.-r.; Tang, Y.; Wang, D.-h.; Yao, J. Adsorption kinetics and thermodynamics of sodium butyl xanthate onto bornite in flotation. J. Cent. South Univ. 2020, 26, 2998–3007. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, G.; Ma, L.; Liu, J. 5-Heptyl-1,3,4-oxadiazole-2-thione: Synthesis and flotation mechanism to chalcopyrite. J. Ind. Eng. Chem. 2018, 61, 331–339. [Google Scholar] [CrossRef]
- Sepehrnia, A.; Gharabaghi, M.; Abdollahi, H.; Katal, R. Investigating the Synergy Effect of Using Combined Collector and Nanocollector on Recovery of Copper Sulfide. J. Sustain. Metall. 2025, 11, 505–516. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H.-J. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interface Sci. 2018, 256, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Liu, S.; Wen, S.; Han, G.; Feng, Q. Unveiling the depression role of quercetin in selective flotation separation of chalcopyrite from pyrite at low alkalinity. J. Environ. Chem. Eng. 2024, 12, 114618. [Google Scholar] [CrossRef]
- Kelebek, S.; Smith, G.W.; Finch, J.A.; Yörük, S. Critical Surface Tension of Wetting and Flotation Separation of Hydrophobic Solids. Sep. Sci. Technol. 1987, 22, 1527–1546. [Google Scholar] [CrossRef]
- Huangfu, Z.; Sun, W.; Zhang, H.; Chen, C.; Zhang, C. Hydrophilic Modification of Macroscopically Hydrophobic Mineral Talc and Its Specific Application in Flotation. Langmuir 2024, 40, 25988–25996. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Zhang, J. An Investigation of the Adsorption of Xanthate on Bornite in Aqueous Solutions Using an Atomic Force Microscope. Minerals 2021, 11, 906. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of H2O2 and potassium amyl xanthate on separation of enargite and tennantite from chalcopyrite and bornite using flotation. Miner. Eng. 2020, 152, 106371. [Google Scholar] [CrossRef]

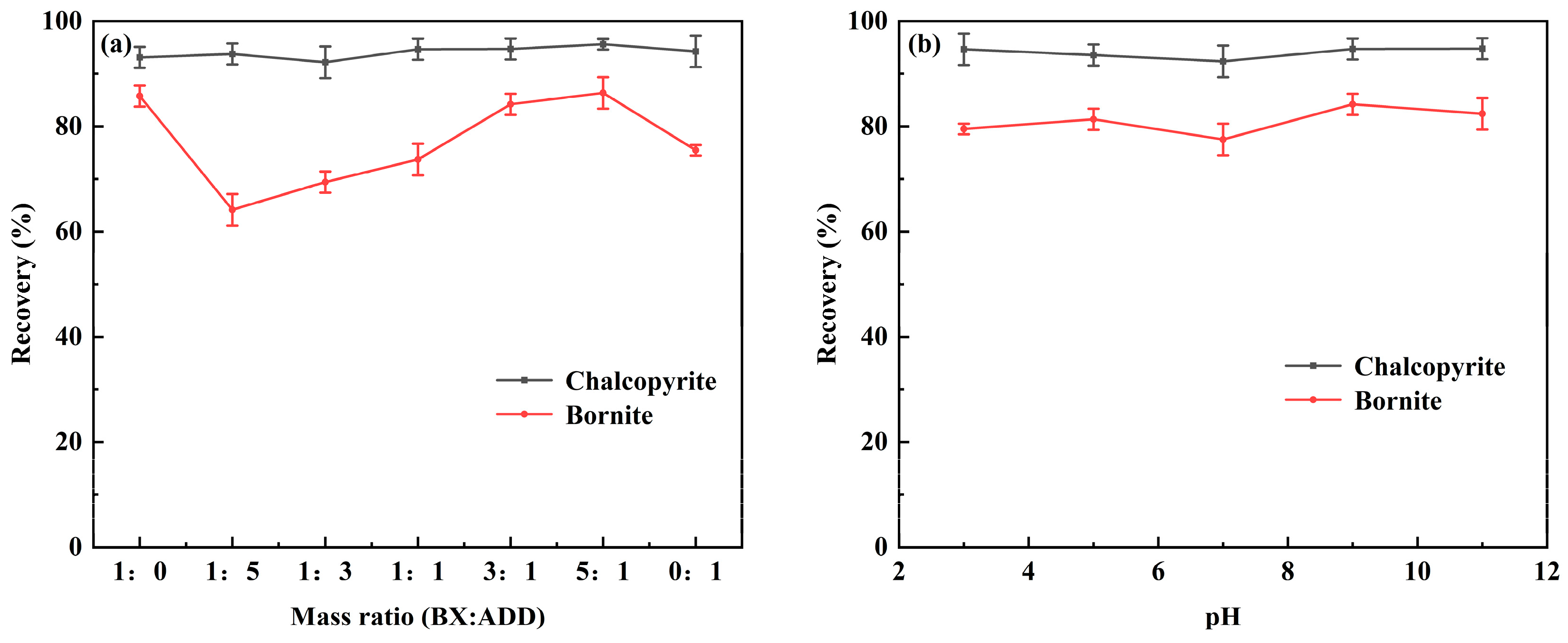
| Mineral/Collector System | BX | ADD | BX/ADD (3:1) |
|---|---|---|---|
| Chalcopyrite | 93.1% | 94.25% | 94.7% |
| Bornite | 85.75% | 75.45% | 84.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Li, L.; Xu, Y.; Wang, Z.; Huang, Y.; Wang, Y. The Influence of Sodium Butyl Xanthate and Ammonium Dibutyl Dithiophosphate on the Flotation Behavior of Chalcopyrite and Bornite. Minerals 2025, 15, 1148. https://doi.org/10.3390/min15111148
Jiang H, Li L, Xu Y, Wang Z, Huang Y, Wang Y. The Influence of Sodium Butyl Xanthate and Ammonium Dibutyl Dithiophosphate on the Flotation Behavior of Chalcopyrite and Bornite. Minerals. 2025; 15(11):1148. https://doi.org/10.3390/min15111148
Chicago/Turabian StyleJiang, Hao, Le Li, Yanling Xu, Zhijie Wang, Yan Huang, and Yanhong Wang. 2025. "The Influence of Sodium Butyl Xanthate and Ammonium Dibutyl Dithiophosphate on the Flotation Behavior of Chalcopyrite and Bornite" Minerals 15, no. 11: 1148. https://doi.org/10.3390/min15111148
APA StyleJiang, H., Li, L., Xu, Y., Wang, Z., Huang, Y., & Wang, Y. (2025). The Influence of Sodium Butyl Xanthate and Ammonium Dibutyl Dithiophosphate on the Flotation Behavior of Chalcopyrite and Bornite. Minerals, 15(11), 1148. https://doi.org/10.3390/min15111148







