Exploring the Structure–Property Relationship in Montmorillonite–Carbon Quantum Hybrid Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
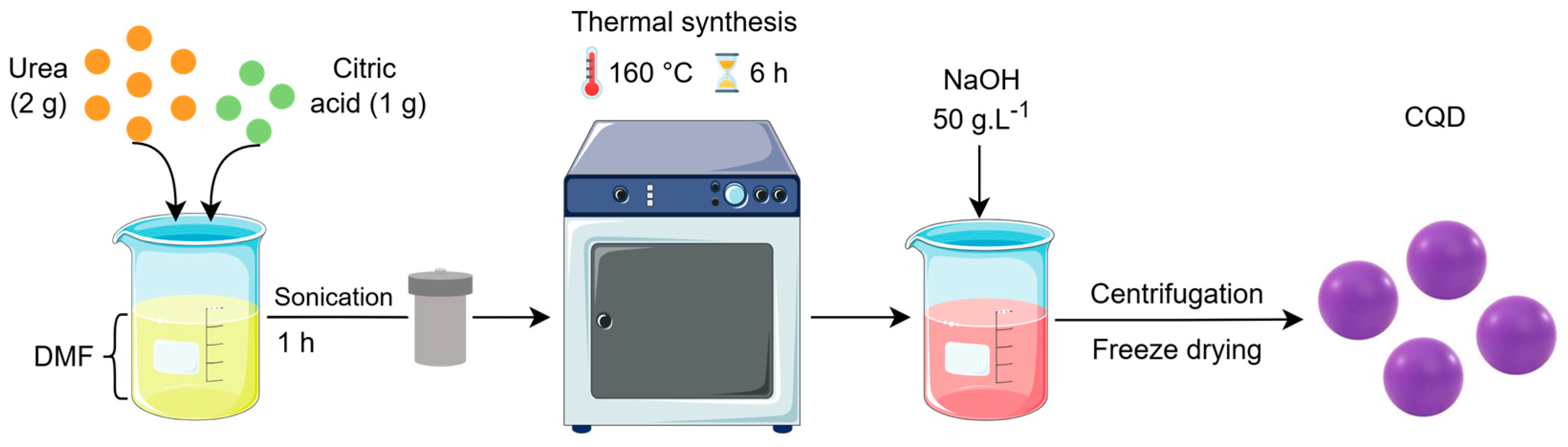
2.2. Synthesis of Carbon Quantum Dots
2.3. Preparation of Montmorillonite–Carbon Quantum Dots Hybrid Nanomaterials
2.4. Characterization
2.5. Hemolysis Test with Sheep Erythrocytes
3. Results
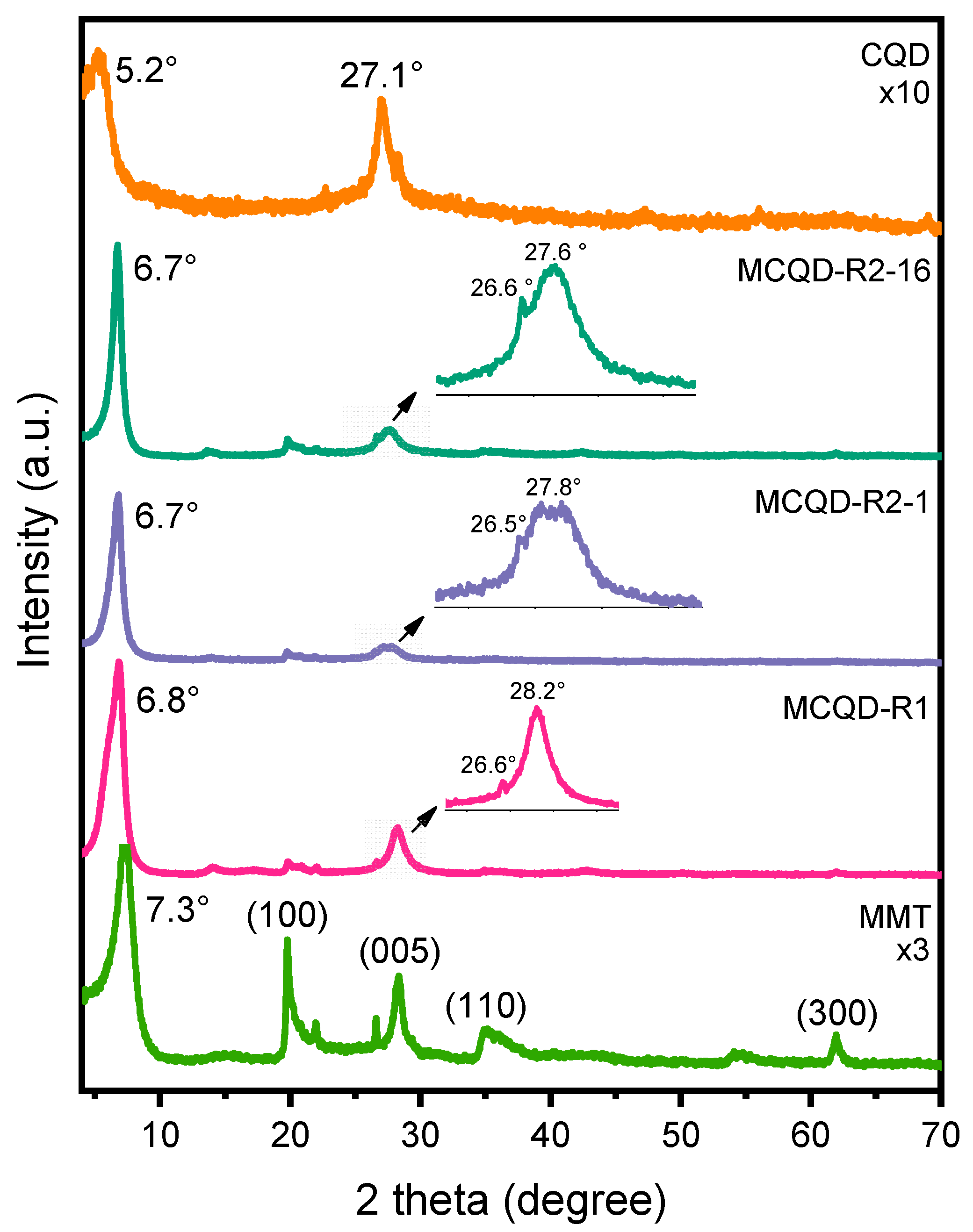
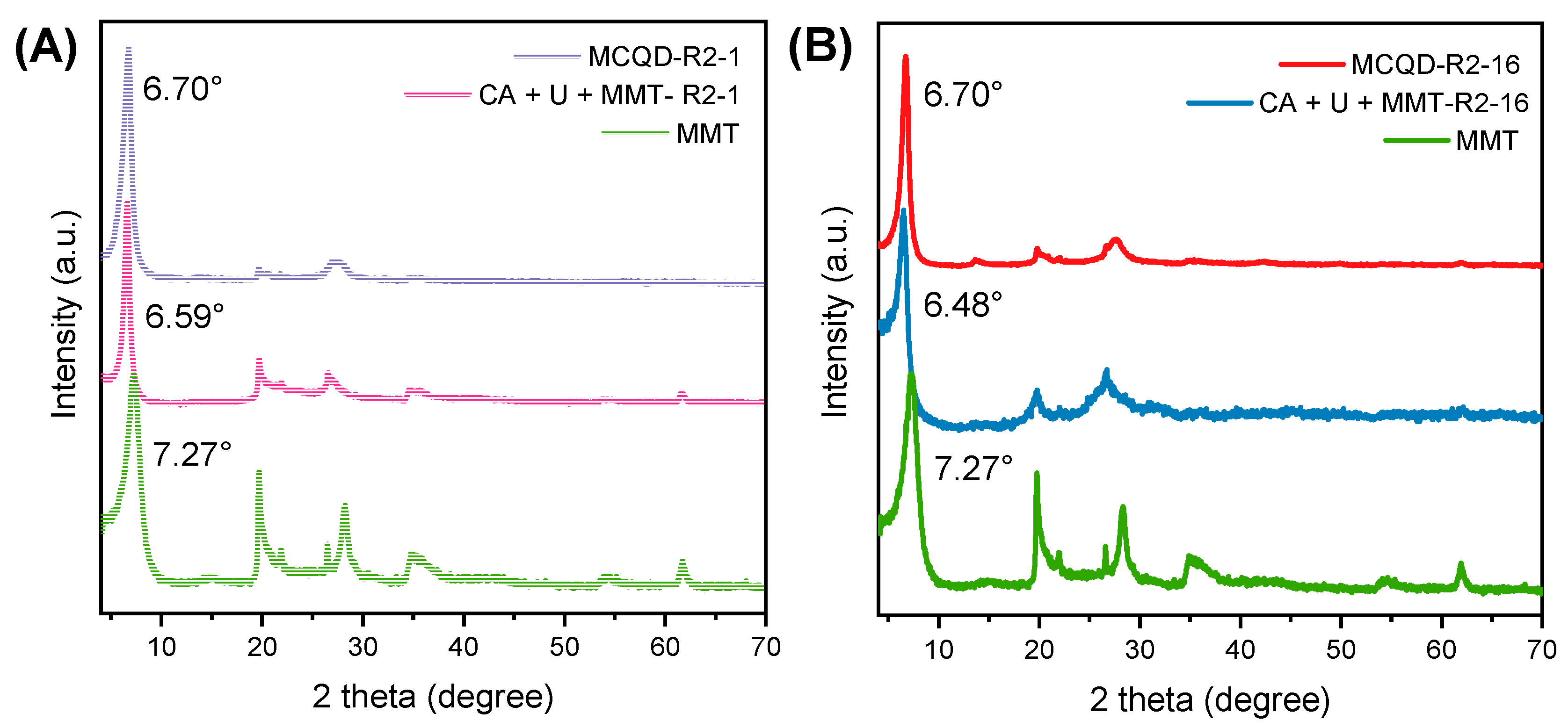
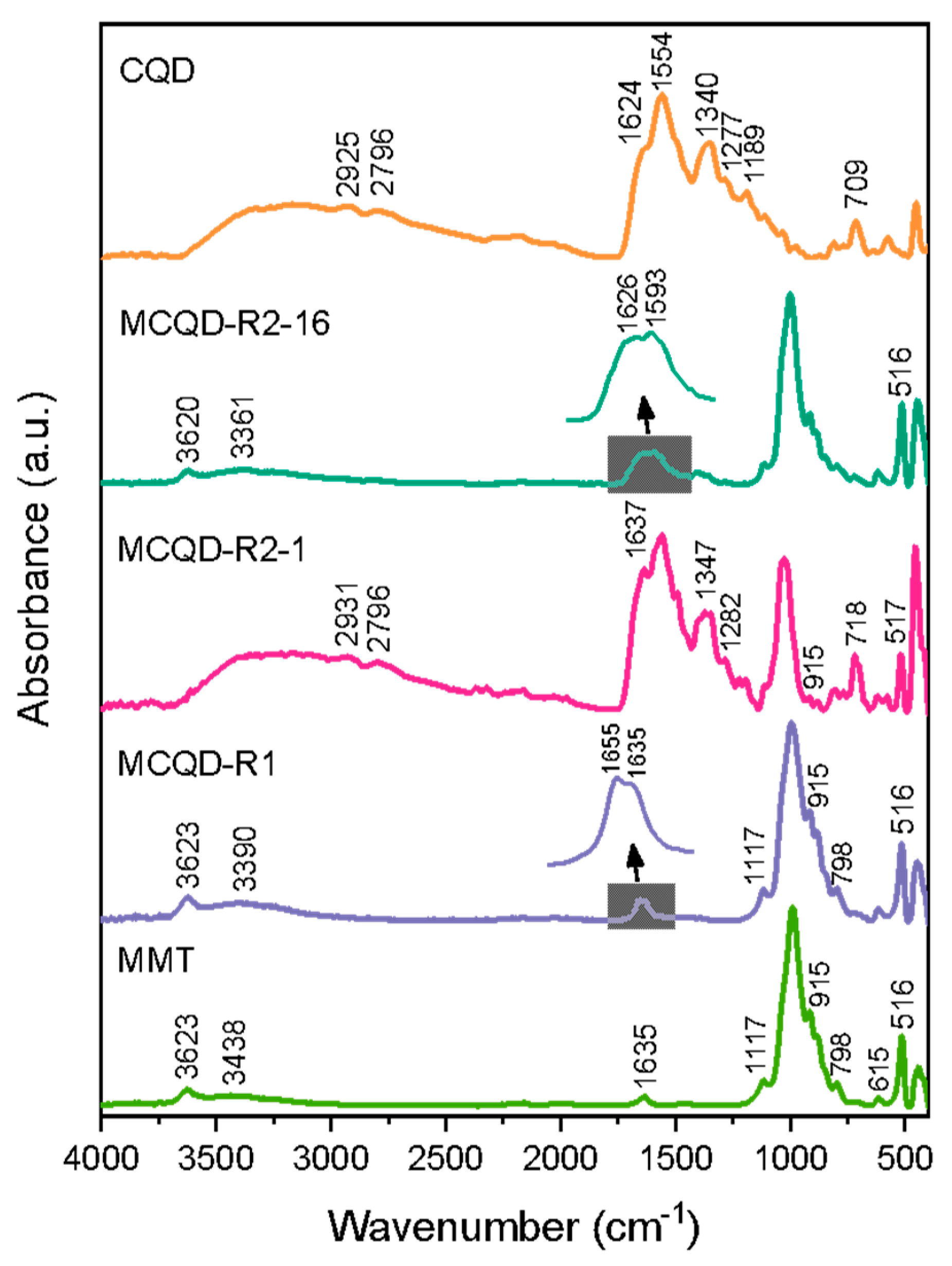
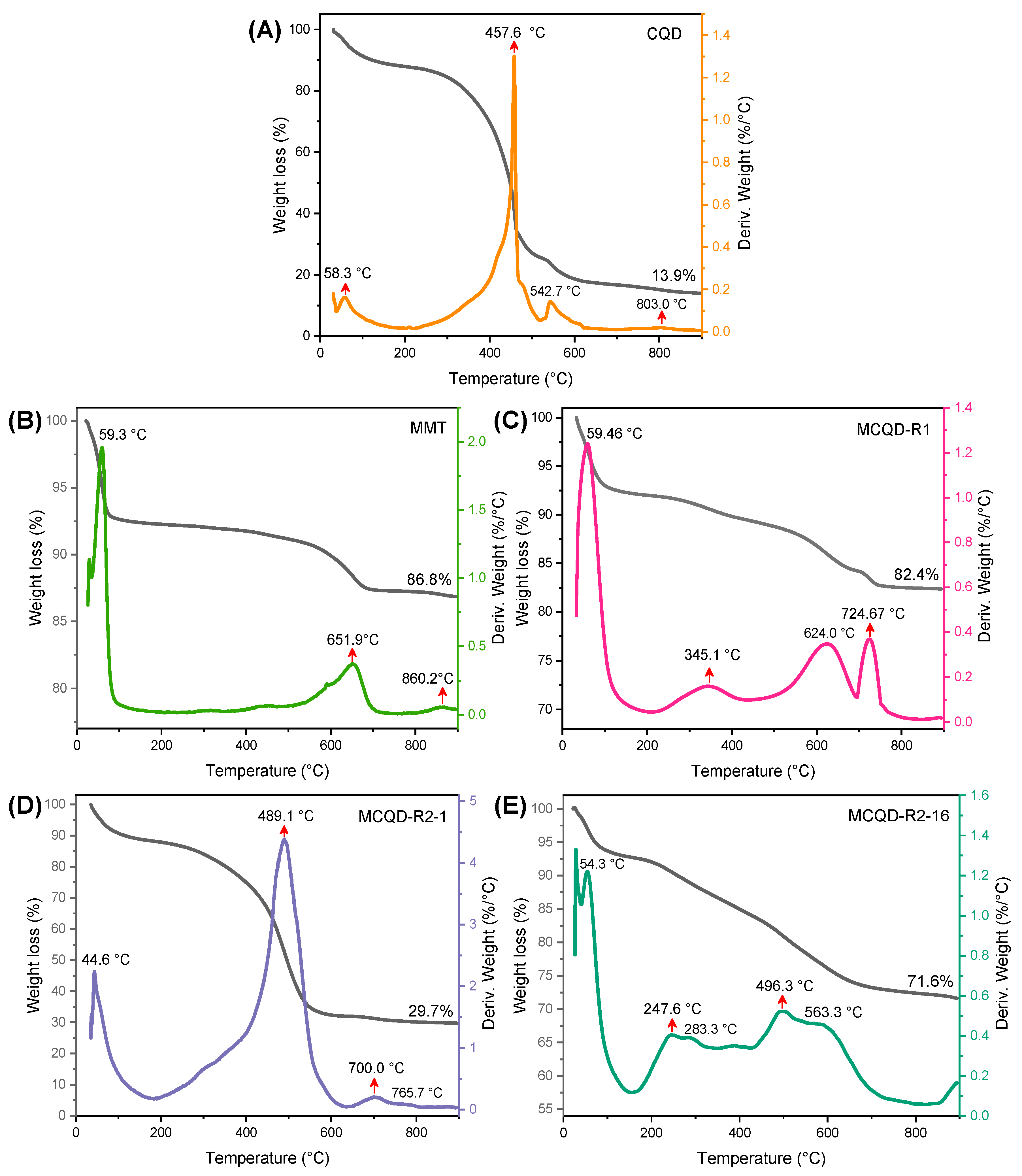
3.1. Structural Properties
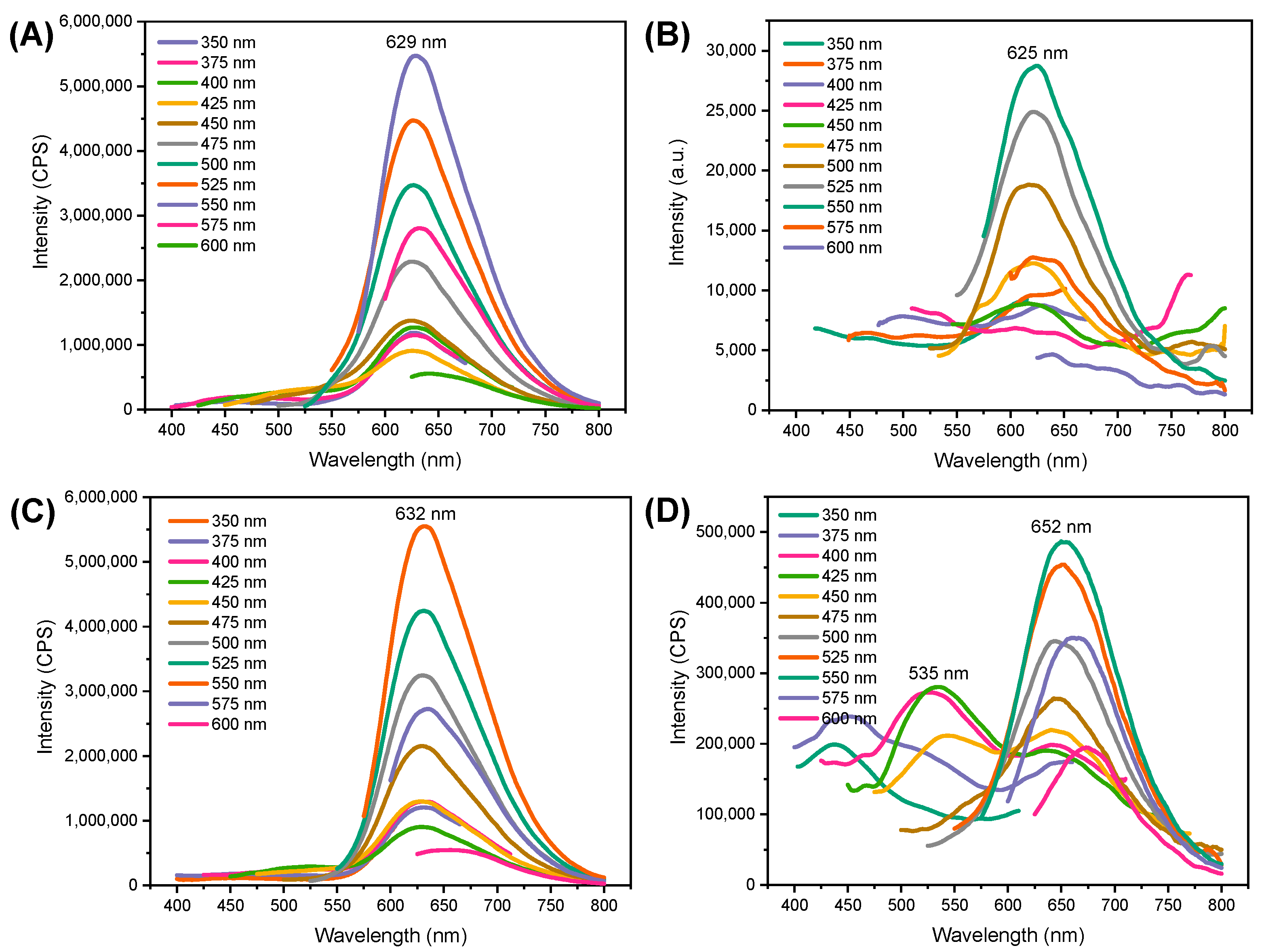
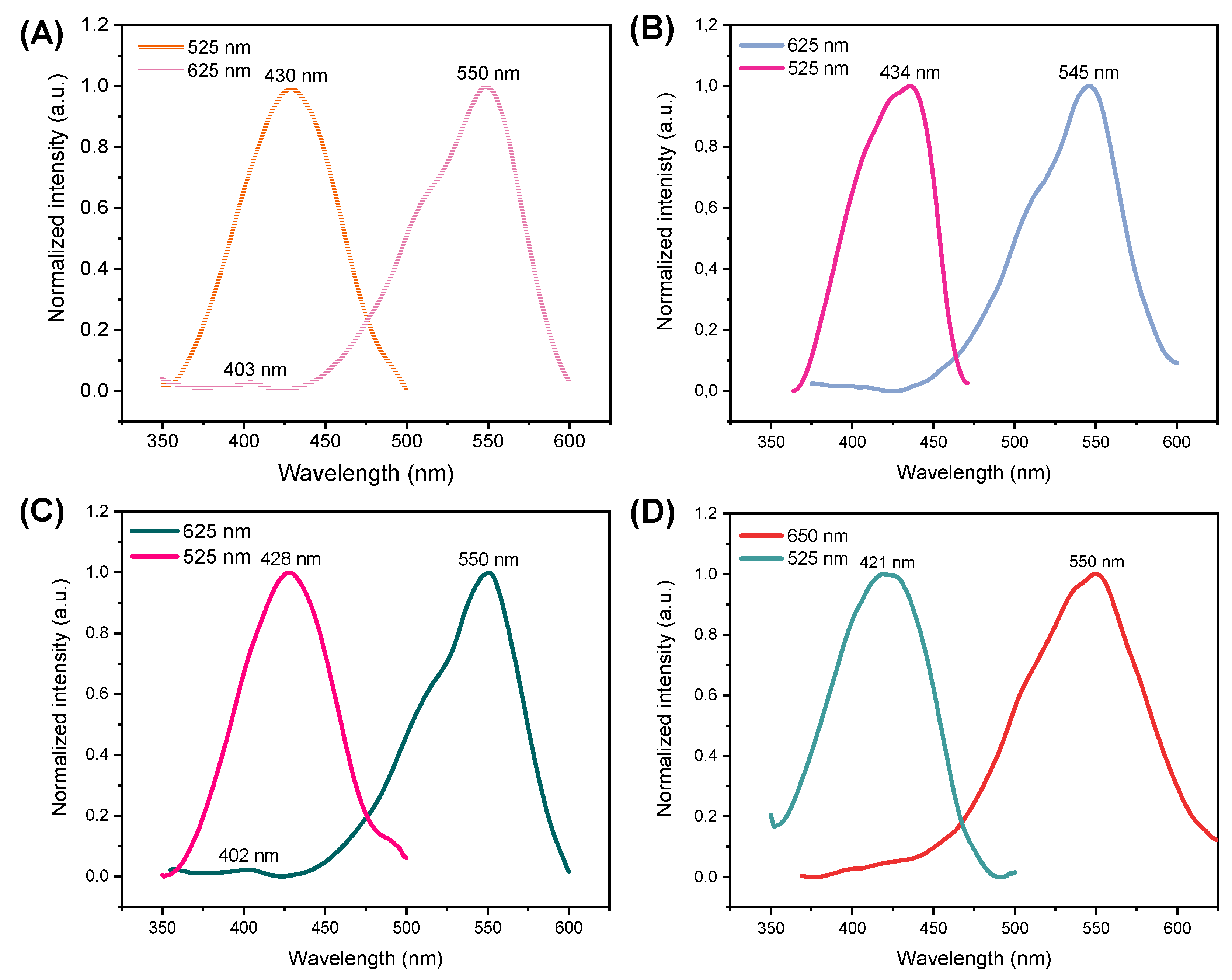
3.2. Optical Properties
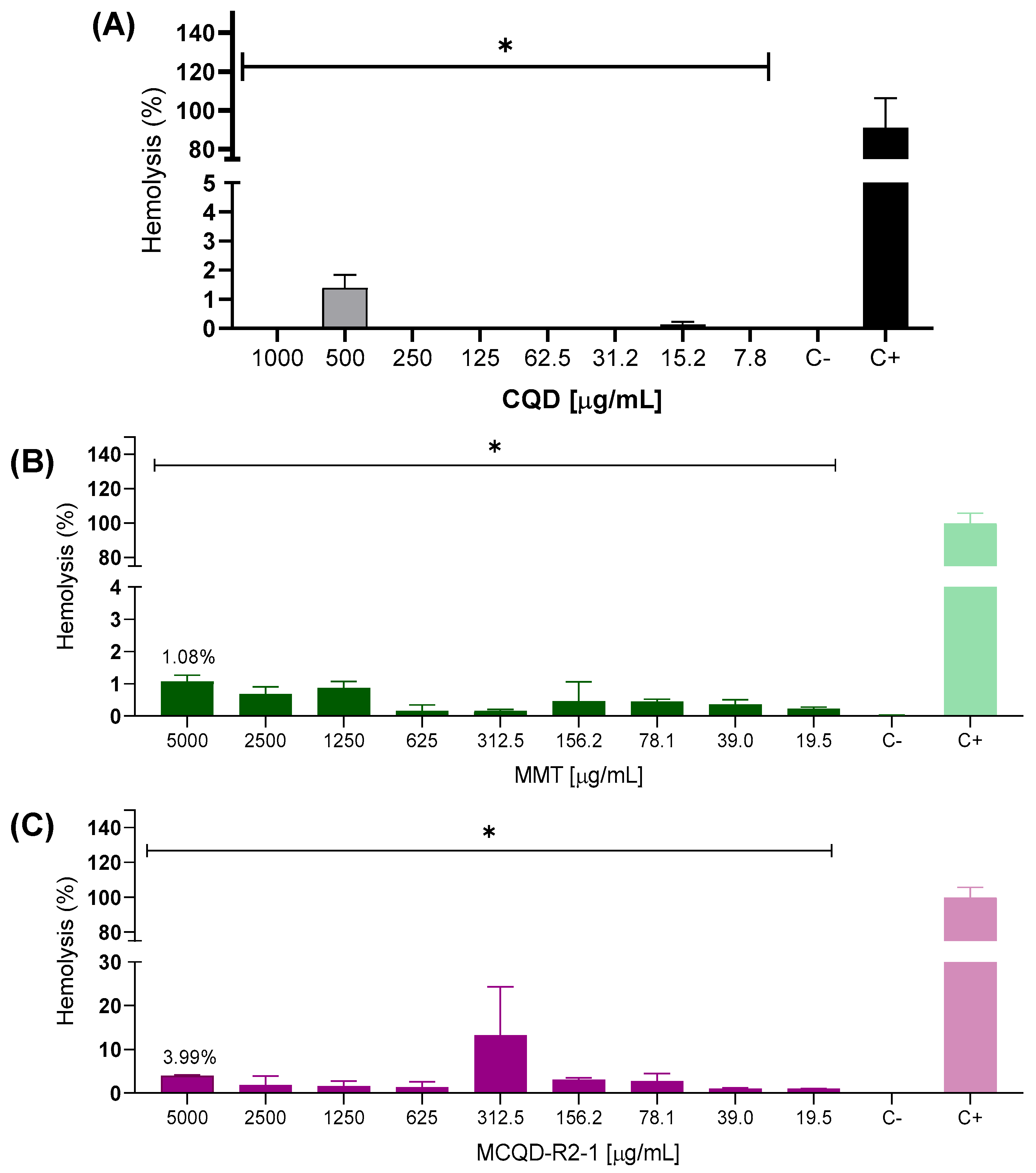
3.3. Hemolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Lu, W.; Yang, Y.; Xiang, R.; Ling, Y.; Yu, C.; Zhou, Y. Hybrid Nanomaterials for Cancer Immunotherapy. Adv. Sci. 2023, 10, 2204932. [Google Scholar] [CrossRef]
- Muhamad Azim, M.K.; Arifutzzaman, A.; Saidur, R.; Khandaker, M.U.; Bradley, D.A. Recent Progress in Emerging Hybrid Nanomaterials towards the Energy Storage and Heat Transfer Applications: A Review. J. Mol. Liq. 2022, 360, 119443. [Google Scholar] [CrossRef]
- Meroni, D.; Ardizzone, S. Preparation and Application of Hybrid Nanomaterials. Nanomaterials 2018, 8, 891. [Google Scholar] [CrossRef]
- Hayami, R.; Wada, K.; Nishikawa, I.; Sagawa, T.; Yamamoto, K.; Tsukada, S.; Gunji, T. Preparation and Properties of Organic-Inorganic Hybrid Materials Using Titanium Phosphonate Cluster. Polym. J. 2017, 49, 665–669. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Santos, J.D.J.P.; Cutrim, E.S.M.; Campos, I.R.; Campos, V.N.S.; Farias, J.R.; Guerra, R.N.M.; de Lima, R.B.; da Silva, M.C.P.; Rojas, A.; et al. Ag3PO4/Clay-Based Heterostructures as Sustainable Devices for Efficient Water Disinfection and Contaminant Removal via Cooperative Processes of Adsorption and Visible Light-Driven Degradation. J. Water Process Eng. 2025, 75, 108055. [Google Scholar] [CrossRef]
- Xie, W.; Chen, Y.; Yang, H. Layered Clay Minerals in Cancer Therapy: Recent Progress and Prospects. Small 2023, 19, e2300842. [Google Scholar] [PubMed]
- Oliveira, A.S.; Alcântara, A.C.S.; Pergher, S.B.C. Bionanocomposite Systems Based on Montmorillonite and Biopolymers for the Controlled Release of Olanzapine. Mater. Sci. Eng. C 2017, 75, 1250–1258. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Alcântara, A.C.S.; Darder, M.; Cansian, R.L.; Gómez-Hortigüela, L.; Pergher, S.B.C. Functional Carboxymethylcellulose/Zein Bionanocomposite Films Based on Neomycin Supported on Sepiolite or Montmorillonite Clays. ACS Omega 2018, 3, 13538–13550. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Souza, G.P.; Santana, S.A.A.; Pergher, S.B.C.; Alcântara, A.C.S. Bionsanocomposites Based on Cationic and Anionic Layered Clays as Controlled Release Devices of Amoxicillin. Appl. Clay Sci. 2019, 173, 35–45. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Borges, J.P.; Costa, L.C.; Silva, J.C.; Soares, P.I.P. Clay-Based Nanocomposite Hydrogels for Biomedical Applications: A Review. Nanomaterials 2022, 12, 3308. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/Orange Dual-Emissive Carbon Dots for PH Sensing and Cell Imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Li, W.; Kaminski Schierle, G.S.; Lei, B.; Liu, Y.; Kaminski, C.F. Fluorescent Nanoparticles for Super-Resolution Imaging. Chem. Rev. 2022, 122, 12495–12543. [Google Scholar] [CrossRef]
- Ghosh, T.; Nandi, S.; Bhattacharyya, S.K.; Ghosh, S.K.; Mandal, M.; Banerji, P.; Das, N.C. Nitrogen and Sulphur Doped Carbon Dot: An Excellent Biocompatible Candidate for in-Vitro Cancer Cell Imaging and Beyond. Environ. Res. 2023, 217, 114922. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Thakkar, S.V.; Ludmerczki, R.; Olla, C.; Pinna, A.; Loche, D.; Malfatti, L.; Cesare Marincola, F.; Casula, M.F. How Porosity Affects the Emission of Fluorescent Carbon Dot-Silica Porous Composites. Microporous Mesoporous Mater 2020, 305, 110302. [Google Scholar] [CrossRef]
- Jlassi, K.; Al Ejji, M.; Ahmed, A.K.; Mutahir, H.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. A Carbon Dot-Based Clay Nanocomposite for Efficient Heavy Metal Removal. Nanoscale Adv. 2023, 5, 4224–4232. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Uchida, J.; Katsurao, T.; Sakabe, H.; Ohtani, B.; Sasaki, K. Efficient Photocatalytic Degradation of Emerging Ciprofloxacin under Visible Light Irradiation Using BiOBr/Carbon Quantum Dot/Saponite Composite. Environ. Res. 2022, 212, 113635. [Google Scholar] [CrossRef]
- Massaro, M.; Barone, G.; Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Nicotra, G.; Spinella, C.; Spinelli, G.; Riela, S. Halloysite Nanotubes-Carbon Dots Hybrids Multifunctional Nanocarrier with Positive Cell Target Ability as a Potential Non-Viral Vector for Oral Gene Therapy. J. Colloid. Interface Sci. 2019, 552, 236–246. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, F.; Zhang, X.; Jing, P.; Li, D.; Yang, X.; Zhou, D.; Xu, X.; Qu, S. Synthesis of Green Emissive Carbon Dots@montmorillonite Composites and Their Application for Fabrication of Light-Emitting Diodes and Latent Fingerprints Markers. J. Colloid. Interface Sci. 2019, 554, 344–352. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, L. Rapid Synthesis of C-Dots@PGV Nanocomposites Powders for Development of Latent Fingermarks. Bull. Chem. Soc. Jpn. 2017, 90, 1217–1223. [Google Scholar] [CrossRef]
- Arjona, J.C.; Samara, M.; Ulsen, C.; Diaz, F.R.V.; Demarquette, N.R. Isoniazid adsorption and release by Cloisite and Laponite: An effect of surface charge. Mater. Chem. Phys. 2025, 344, 131097. [Google Scholar] [CrossRef]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated Sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- ISO 10993-4; Part 4: Selection of Tests for Interactions with Blood—Biological Evaluation of Medical Devices; 3rd ed. International Standard Orgnaization: Geneva, Switzerland, 2017.
- Almaaytah, A.; Tarazi, S.; Alsheyab, F.; Al-balas, Q.; Ukattash, T. Antimicrobial and Antibiofilm Activity of Mauriporin, a Multifunctional Scorpion Venom Peptide. Int. J. Pept. Res. Ther. 2014, 20, 397–408. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A Deep Investigation into the Structure of Carbon Dots. Carbon. N. Y 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Mandal, S.; Prasad, S.R.; Mandal, D.; Das, P. Bovine Serum Albumin Amplified Reactive Oxygen Species Generation from Anthrarufin-Derived Carbon Dot and Concomitant Nanoassembly for Combination Antibiotic-Photodynamic Therapy Application. ACS Appl. Mater. Interfaces 2019, 11, 33273–33284. [Google Scholar] [CrossRef]
- Bian, W.; Wang, X.; Wang, Y.; Yang, H.; Huang, J.; Cai, Z.; Choi, M.M.F. Boron and Nitrogen Co-Doped Carbon Dots as a Sensitive Fluorescent Probe for the Detection of Curcumin. Luminescence 2018, 33, 174–180. [Google Scholar] [CrossRef]
- Choppadandi, M.; Guduru, A.T.; Gondaliya, P.; Arya, N.; Kalia, K.; Kumar, H.; Kapusetti, G. Structural Features Regulated Photoluminescence Intensity and Cell Internalization of Carbon and Graphene Quantum Dots for Bioimaging. Mater. Sci. Eng. C 2021, 129, 112366. [Google Scholar] [CrossRef]
- Du, F.; Yuan, J.; Zhang, M.; Li, J.; Zhou, Z.; Li, Z.; Cao, M.; Chen, J.; Zhang, L.; Liu, X.; et al. Nitrogen-Doped Carbon Dots with Heterogeneous Multi-Layered Structures. RSC Adv. 2014, 4, 37536–37541. [Google Scholar] [CrossRef]
- Aslam, R.; Wang, Q.; Aslam, J.; Mobin, M.; Hussain, C.M.; Yan, Z. Effect of Temperature, and Immersion Time on the Inhibition Performance of Q235 Steel Using Spent Coffee Grounds Derived Carbon Quantum Dots: Electrochemical, Spectroscopic and Surface Studies. Biomass Bioenergy 2025, 200, 107961. [Google Scholar] [CrossRef]
- Corrêa, F.G.; Araujo, R.J.P.; Campos, V.N.S.; Silva, M.d.S.C.; Cutrim, E.S.M.; Rojas, A.; Teixeira, M.M.; Garcia, M.A.S.; Alcântara, A.C.S. Layered Double Hydroxides Modified with Carbon Quantum Dots as Promising Materials for Pharmaceutical Removal. Minerals 2025, 15, 899. [Google Scholar] [CrossRef]
- Farshi Azhar, F.; Olad, A. A Study on Sustained Release Formulations for Oral Delivery of 5-Fluorouracil Based on Alginate-Chitosan/Montmorillonite Nanocomposite Systems. Appl. Clay Sci. 2014, 101, 288–296. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, H.; Bai, H.; Zhao, Y.; Min, F.; Song, S. Correlation of Montmorillonite Exfoliation with Interlayer Cations in the Preparation of Two-Dimensional Nanosheets. RSC Adv. 2017, 7, 41471–41478. [Google Scholar] [CrossRef]
- Cutrim, E.S.M.; Vale, A.A.M.; Manzani, D.; Barud, H.S.; Rodríguez-Castellón, E.; Santos, A.P.S.A.; Alcântara, A.C.S. Preparation, Characterization and in Vitro Anticancer Performance of Nanoconjugate Based on Carbon Quantum Dots and 5-Fluorouracil. Mater. Sci. Eng. C 2021, 120, 111781. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, R.; Deng, W.; Xu, Y.; Zhu, J.; Tao, Q.; He, H. From Used Montmorillonite to Carbon Monolayer-Montmorillonite Nanocomposites. Appl. Clay Sci. 2014, 100, 112–117. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Shape and Aggregation Control of Nanoparticles: Not Shaken, Not Stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef]
- Kundu, A.; Lee, J.; Park, B.; Ray, C.; Sankar, K.V.; Kim, W.S.; Lee, S.H.; Cho, I.J.; Jun, S.C. Facile Approach to Synthesize Highly Fluorescent Multicolor Emissive Carbon Dots via Surface Functionalization for Cellular Imaging. J. Colloid. Interface Sci. 2018, 513, 505–514. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Thirukumaran, P.; Yoon, D.H.; Lee, Y.R.; Cheong, I.W. Simultaneous Removal of Heavy Metal Ions Using Carbon Dots-Doped Hydrogel Particles. Chemosphere 2022, 286, 131760. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-Assembly Processes in Hydrated Montmorillonite by FTIR Investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of Structural Modification in Acid Activated Montmorillonite Clay by FT-IR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 64, 273–278. [Google Scholar] [CrossRef]
- Funes, I.G.A.; Peralta, M.E.; Pettinari, G.R.; Carlos, L.; Parolo, M.E. Facile Modification of Montmorillonite by Intercalation and Grafting: The Study of the Binding Mechanisms of a Quaternary Alkylammonium Surfactant. Appl. Clay Sci. 2020, 195, 105738. [Google Scholar] [CrossRef]
- Madejová, J. FTIR Techniques in Clay Mineral Studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Matusik, J. The Effect of Surfactant-Modified Montmorillonite on the Cross-Linking Efficiency of Polysiloxanes. Materials 2021, 14, 2623. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, A.; Yang, W.; Araya, T.; Huang, Y.; Zhao, P.; Johnson, D.; Wang, J.; Ren, Z.J. Complex Formation via Hydrogen Bonding between Rhodamine B and Montmorillonite in Aqueous Solution. Sci. Rep. 2018, 8, 229. [Google Scholar] [CrossRef]
- Sheng, X.; Qin, C.; Yang, B.; Hu, X.; Liu, C.; Waigi, M.G.; Li, X.; Ling, W. Metal cation saturation on montmorillonites facilitates the adsorption of DNA via cation bridging. Chemosphere 2019, 235, 670–678. [Google Scholar] [CrossRef]
- Wang, J.; Wilson, R.S.; Aristilde, L. Electrostatic coupling and water bridging in adsorption hierarchy of biomolecules at water–clay interfaces. Proc. Natl. Acad. Sci. USA 2024, 121, e2316569121. [Google Scholar] [CrossRef]
- Leite, M.S.; Sodré, W.C.; de Lima, L.R.; Constantino, V.R.L.; Alcântara, A.C.S. Bionanocomposite Beads Based on Montmorillonite and Biopolymers as Potential Systems for Oral Release of Ciprofloxacin. Clays Clay Miner. 2021, 69, 547–560. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Wang, C.; Cao, Y.; Li, Y.; Yan, L.; Liu, M.; Lv, M.; Yang, Y.; Zhao, X.; et al. Controllable and Eco-Friendly Synthesis of P-Riched Carbon Quantum Dots and Its Application for Copper (II) Ion Sensing. Appl. Surf. Sci. 2018, 448, 589–598. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, W.; Ma, X.; Zhang, Y.; Yue, C.; Fang, W.; Hu, Y.; Wang, J.; Dang, J.; Zhao, H.; et al. Nitrogen-Doped Carbon Dots-V2O5 Nanobelts Sensing Platform for Sensitive Detection of Ascorbic Acid and Alkaline Phosphatase Activity. Anal. Chim. Acta 2019, 1089, 131–143. [Google Scholar] [CrossRef]
- Balek, V.; Beneš, M.; Málek, Z.; Matuschek, G.; Kettrup, A.; Yariv, S. Emanation Thermal Analysis Study of Na-Montmorillonite and Montmorillonite Saturated with Various Cations. J. Therm. Anal. Calorim. 2006, 83, 617–623. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; Viseras, C. Kinetic and Thermodynamic Assessment on Isoniazid/Montmorillonite Adsorption. Appl. Clay Sci. 2018, 165, 82–90. [Google Scholar] [CrossRef]
- Derkowski, A.; Kuligiewicz, A. Thermal Analysis and Thermal Reactions of Smectites: A Review of Methodology, Mechanisms, and Kinetics. Clays Clay Miner. 2022, 70, 946–972. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Zhang, H.; Zhang, X.; Zhuang, J.; Hu, C.; Liu, Y.; Lei, B.; Ma, L.; Wang, X. Enhanced Biological Photosynthetic Efficiency Using Light-Harvesting Engineering with Dual-Emissive Carbon Dots. Adv. Funct. Mater. 2018, 28, 1804004. [Google Scholar] [CrossRef]
- Deb, A.; Chowdhury, D. Unraveling the Origin of Photoluminescence in Dual Emissive Biogenic Carbon Dot. Mater. Today Commun. 2022, 31, 103777. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, B.; Ushakova, E.V.; He, B.; Xing, G.; Tang, Z.; Rogach, A.L.; Qu, S. Assignment of Core and Surface States in Multicolor-Emissive Carbon Dots. Small 2022, 19, 2204158. [Google Scholar] [CrossRef]
- De La Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for Cytotoxicity: In Vitro Methods”, 3st Ed. International Organization for Standardization: Geneva, Switzerland, 2009.


| Sample | C (%) | H (%) | N (%) | Amount of CQD (%) |
|---|---|---|---|---|
| CQD | 71.57 | 4.86 | 23.57 | 100 |
| MCQD-R1 | 4.33 | 3.35 | 1.95 | 9.63 |
| MCQD-R2-1 | 62.93 | 5.45 | 20.94 | 89.32 |
| MCQD-R2-16 | 22.10 | 3.54 | 8.36 | 34.00 |
| Samples | λem (nm) | τ1 (ns) | τ2 (ns) | τAV (ns) |
|---|---|---|---|---|
| CQD | 528 | 0.91 (56.85%) | 6.14 (43.15%) | 5.29 |
| 626 | 0.88 (94.40%) | 4.17 (5.60%) | 1.60 | |
| MCQD-R1 | 530 | 1.57 (40.17%) | 5.66 (59.82%) | 5.02 |
| MCQD-R2-1 | 560 | 1.58 (92.67%) | 5.095 (7.33%) | 2.29 |
| 590 | 2.21 (100%) | - | 2.22 | |
| MCQD-R2-16 | 551 | 1.09 (70.77%) | 5.46 (29.23%) | 4.04 |
| 600 | 0.91 (88.07%) | 4.46 (11.93%) | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutrim, E.S.M.; Figueredo, A.S.; Silva, L.A.; Fernández-Moreira, V.; Alcântara, A.C.S. Exploring the Structure–Property Relationship in Montmorillonite–Carbon Quantum Hybrid Nanomaterials. Minerals 2025, 15, 1146. https://doi.org/10.3390/min15111146
Cutrim ESM, Figueredo AS, Silva LA, Fernández-Moreira V, Alcântara ACS. Exploring the Structure–Property Relationship in Montmorillonite–Carbon Quantum Hybrid Nanomaterials. Minerals. 2025; 15(11):1146. https://doi.org/10.3390/min15111146
Chicago/Turabian StyleCutrim, Elaine S. M., Aline S. Figueredo, Lucilene A. Silva, Vanesa Fernández-Moreira, and Ana C. S. Alcântara. 2025. "Exploring the Structure–Property Relationship in Montmorillonite–Carbon Quantum Hybrid Nanomaterials" Minerals 15, no. 11: 1146. https://doi.org/10.3390/min15111146
APA StyleCutrim, E. S. M., Figueredo, A. S., Silva, L. A., Fernández-Moreira, V., & Alcântara, A. C. S. (2025). Exploring the Structure–Property Relationship in Montmorillonite–Carbon Quantum Hybrid Nanomaterials. Minerals, 15(11), 1146. https://doi.org/10.3390/min15111146









