Experimental Investigation of CO2–Mineral Interactions in Tight Clastic Rock Reservoirs: Implications for Geological Carbon Sequestration
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Mineral Identification
3.1.1. Feldspar Identification
3.1.2. Clay Mineral Identification
3.2. Feldspar Reactions with CO2
3.3. Clay Mineral Reactions with CO2
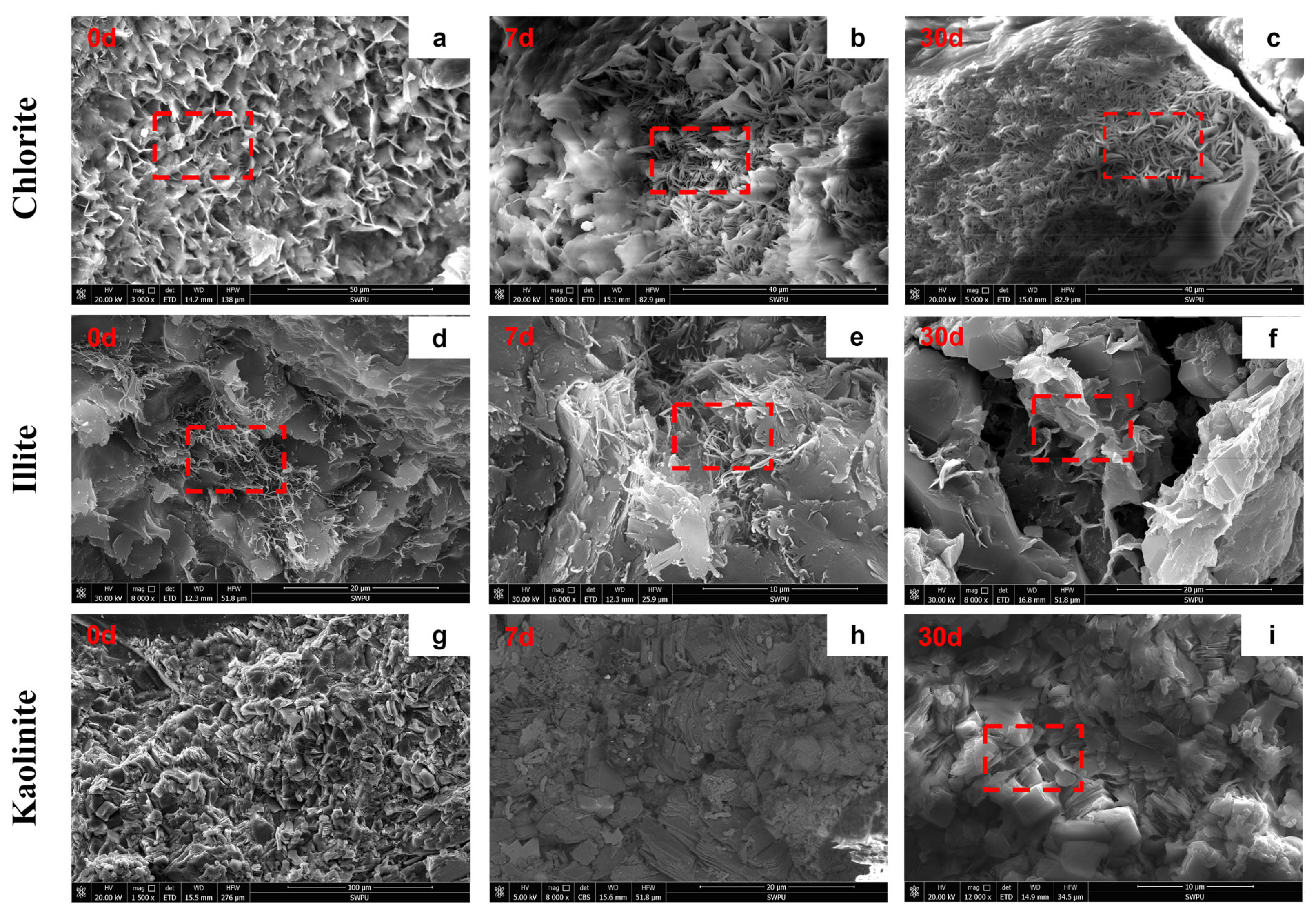
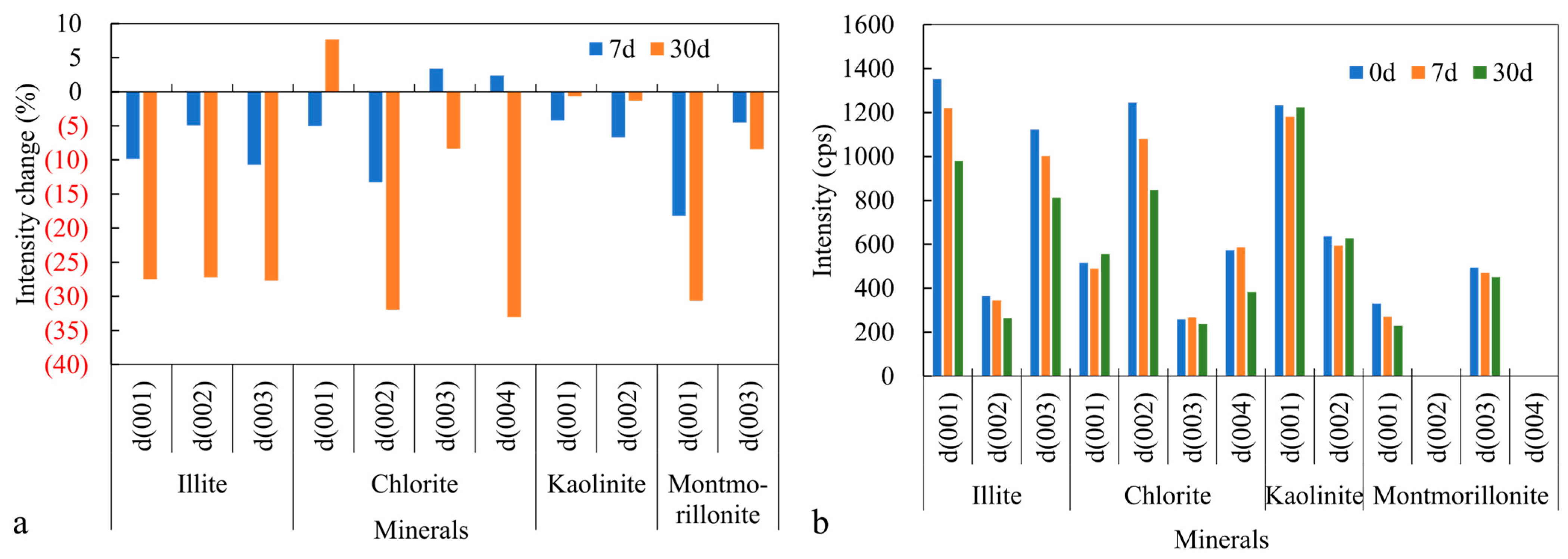
3.3.1. Changes in Crystal Structure of Clay Minerals
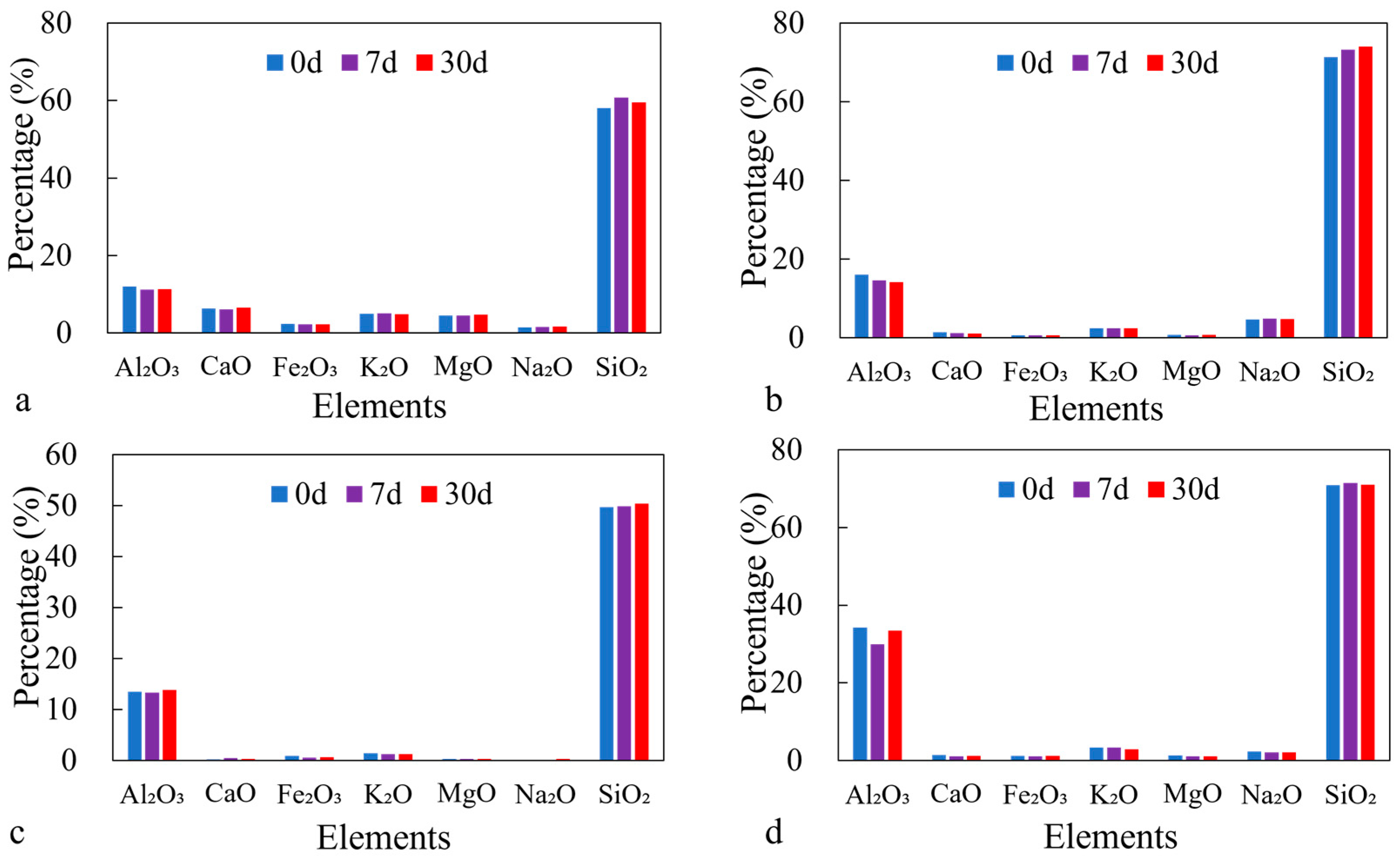
3.3.2. Mineral Morphology and Elemental Composition Changes
3.3.3. Element Release and Ion Concentration Changes
4. Discussion
4.1. Analysis of the Reaction Mechanism Between Feldspar and CO2
4.2. Analysis of the Reaction Mechanism Between Clay Minerals and CO2
- (1)
- Reaction Mechanism of Chlorite with CO2
- (2)
- Reaction Mechanism of Illite with CO2
- (3)
- Reaction Mechanism of Kaolinite with CO2
- (4)
- Reaction Mechanism of Montmorillonite with CO2
- (5)
- Comparison of Reaction Intensities among Common Clay Minerals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2019, 21, 241–258. [Google Scholar] [CrossRef]
- Cai, B.; Pang, L.; Cao, L.; Li, Q.; Liu, G.; Zhong, P.; Zhang, X.; Yang, Y.; Fan, C.; Li, Q.; et al. Two-year implementation assessment (2016–2018) of China’s technical guideline on environmental risk assessment for carbon dioxide capture, utilization and storage (on trial). Environ. Eng. 2019, 37, 1–7. [Google Scholar]
- Guo, P.; Sun, L.; Sun, L.; Li, S.; Peng, P.; Yue, L. Influences of injection gas on physical behavior of crude. J. Southwest Pet. Inst. 2000, 22, 57–60. [Google Scholar]
- Wang, T.; Yao, Y.; Li, X.; Li, H.; Shi, J.; Yang, Z. Factors affecting and analysis of CO2 flooding effectiveness. China Pet. Chem. Ind. 2008, 24, 30–33. [Google Scholar]
- Wang, M.; He, J.; Liu, S.; Zeng, C.; Jia, S.; Nie, Z.; Wang, S.; Wang, W.; Zhang, C. Effect of Sedimentary Facies Characteristics on Deep Shale Gas Desserts: A Case from the Longmaxi Formation, South Sichuan Basin, China. Minerals 2023, 13, 476. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Wanniarachchi, W.A.M.; Yang, S.Q.; Lashin, A.; Al Arifi, N. An experimental investigation of coupled chemico-mineralogical and mechanical changes in varyingly-cemented sandstones upon CO2 injection in deep saline aquifer environments. Energy 2017, 133, 404–414. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Cheng, Y.; Yu, X.; Duan, X.; Kang, Z.; Xiong, Y. Fractal insights into permeability control by pore structure in tight sandstone reservoirs, Heshui area, Ordos Basin. Open Geosci. 2025, 17, 20250791. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y.; Enechukwu, C. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2–water–rock interactions and their effects. J. CO2 Util. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Hitchon, B. Aquifer Disposal of Carbon Dioxide: Hydrodynamic and Mineral Trapping-Proof of Concept; Geoscience Publishing Ltd.: London, UK, 1996. [Google Scholar]
- Gunter, W.; Wong, S.; Gentzis, T. Field-testing CO2 sequestration and enhanced coalbed methane recovery in Alberta, Canada-Historical perspective and future plans. Abstr. Pap. Am. Chem. Soc. 2000, 220, U396. [Google Scholar]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT—A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.Z.; Turcu, R.V.F.; Rosso, K.M.; Ilton, E.S.; Wang, C.; Sears, J.A.; Engelhard, M.H.; Felmy, A.R.; Hoyt, D.W. The role of H2O in the carbonation of forsterite in supercritical CO2. Int. J. Greenh. Gas Control. 2011, 5, 1081–1092. [Google Scholar] [CrossRef]
- Loring, J.S.; Thompson, C.J.; Zhang, C.; Wang, Z.; Schaef, H.T.; Rosso, K.M. In Situ Infrared Spectroscopic Study of Brucite Carbonation in Dry to Water-Saturated Supercritical Carbon Dioxide. J. Phys. Chem. A 2012, 116, 4768–4777. [Google Scholar] [CrossRef]
- Shi, L.; Hu, H.; Zhang, Y.; Gao, Y.; Zhang, J.; Zhang, H.; Wang, L. Interaction of tight glutenite mineral with supercritical CO2 and formation water. Oilfield Chem. 2019, 36, 640–645. [Google Scholar]
- Luo, Y.; Xiao, M.; Cheng, J.; Yu, Y.; Xu, F.; Zhao, R.; Ahmat, K. Experimental study on the interaction of Sc-CO2 with feldspar and clay minerals: Implication for carbon sequestration in sandstone reservoirs. Gas Sci. Eng. 2025, 139, 205639. [Google Scholar] [CrossRef]
- Wang, M.; Tang, H.; Zhao, F.; Liu, S.; Yang, Y.; Zhang, L.; Liao, J.; Lu, H. Controlling factor analysis and prediction of the quality of tight sandstone reservoirs: A case study of the He8 Member in the eastern Sulige Gas Field, Ordos Basin, China. J. Nat. Gas Sci. Eng. 2017, 46, 680–698. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, T.; Sun, H.; Zhang, X.; Zhao, D. Dissolution process and mechanism of montmorillonite in oxalic acid and sulfuric acid media at various pH levels. Appl. Clay Sci. 2024, 261, 107573. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y.; et al. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Yanzhong, W.; Nianmin, Z.; Xu, C.; Yingchang, C.; Guanghui, Y.; Gluyas, J.G.; Miruo, L. Geologic CO2 storage in arkosic sandstones with CaCl2-rich formation water. Chem. Geol. 2020, 558, 119867. [Google Scholar] [CrossRef]
- Gianni, E.; Tyrologou, P.; Behnous, D.; Pál Farkas, M.; Fernández-Canteli Álvarez, P.; García Crespo, J.; Chacartegui Ramirez, R.; Koukouzas, N.; Carneiro, J. CO2 sequestration potential in Depleted Hydrocarbon fields? A geochemical approach [version 2; peer review: 2 approved]. Open Res. Eur. 2025, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Strand, S.; Puntervold, T.; Mamonov, A.; Piñerez Torrijos, I.D.; Khan, M.A.I. Geochemical effects of carbonated water on reservoir and caprock minerals for carbon capture and storage. Gas Sci. Eng. 2024, 124, 205246. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Li, R.; Gao, L. Effects of pH on the solubility of the feldspar and the development of secondary porosity. Bull. Mineral. Petrol. Geochem. 2001, 20, 103–107. [Google Scholar]
- Chi, E.; Lan, B.; Xiao, Y. Impact of temperature and CO2 in solution on feldspar solubility. J. Water Resour. Water Eng. 2014, 25, 230–232. [Google Scholar]
- Jiao, C.; Xiao, J.; Pi, J.; Sun, X. Application of PHREEQC in simulation of groundwater chemical formation in geothermal field of Tangshi. Miner. Eng. Res. 2018, 33, 49–53. [Google Scholar]
- Watson, M.N.; Zwingmann, N.; Lemon, N.M. The Ladbroke Grove–Katnook carbon dioxide natural laboratory: A recent CO2 accumulation in a lithic sandstone reservoir. Energy 2004, 29, 1457–1466. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control. 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Ryzhenko, B.N. Genesis of dawsonite mineralization: Thermodynamic analysis and alternatives. Geochem. Int. 2006, 44, 835–840. [Google Scholar] [CrossRef]
- Tang, H.; Meng, Y.; Li, G.; Yang, X.; Yan, R. Study on stability of feldspar in acidization system. Nat. Gas Ind. 2004, 24, 116–118. [Google Scholar]
- Zhao, T.; Yan, Z.; Zhang, J.; Wang, Y.; Jiang, L. Analysis of the effects of temperature and CO2 on feldspar solubility based on PHREEQC simulation. Light Ind. Sci. Technol. 2016, 32, 84–86. [Google Scholar]
- Fischer, S.; Liebscher, A.; Wandrey, M. CO2–brine–rock interaction—First results of long-term exposure experiments at in situ P–T conditions of the Ketzin CO2 reservoir. Geochemistry 2010, 70, 155–164. [Google Scholar] [CrossRef]
- Wandrey, M.; Fischer, S.; Zemke, K.; Liebscher, A.; Scherf, A.-K.; Vieth-Hillebrand, A.; Zettlitzer, M.; Würdemann, H. Monitoring petrophysical, mineralogical, geochemical and microbiological effects of CO2 exposure—Results of long-term experiments under in situ conditions. Energy Procedia 2011, 4, 3644–3650. [Google Scholar] [CrossRef][Green Version]
- Pearce, J.K.; Dawson, G.K.W.; Golab, A.; Knuefing, L.; Sommacal, S.; Rudolph, V.; Golding, S.D. A combined geochemical and μCT study on the CO2 reactivity of Surat Basin reservoir and cap-rock cores: Porosity changes, mineral dissolution and fines migration. Int. J. Greenh. Gas Control. 2019, 80, 10–24. [Google Scholar] [CrossRef]
- Cai, D. Study on Interaction Between CO2 and Water/Rock Minerals in Tight Glutenite Reservoirs. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2018. [Google Scholar]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental investigation of CO2–brine–rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Tang, H.; Tang, H.; He, J.; Zhao, F.; Zhang, L.; Liao, J.; Wang, Q.; Yuan, X. Damage mechanism of water-based fracturing fluid to tight sandstone gas reservoirs: Improvement of The Evaluation Measurement for Properties of Water-based Fracturing Fluid: SY/T 5107-2016. Nat. Gas Ind. 2020, 40, 55–63. [Google Scholar] [CrossRef]
- Xing, X.; Tang, H.; Zhao, F.; Li, G.; Xie, X. Reaction experiment of illite with mud acid and fluorboric. J. Southwest Pet. Univ. 2007, 29, 29–31. [Google Scholar]
- Ni, X.; Yu, Y.; Wang, Y.; Gao, S. Dissolution kinetics of Si/Al elements of illites in carbonic aicd solutions. Nat. Gas Ind. 2014, 34, 20–26. [Google Scholar]
- Guo, H.; Wang, Y.; Ni, X.; Wang, J.; Han, W. Digestion kinetics analysis of silicon and aluminum during kaolinite-water-CO2 interaction. J. China Univ. Min. Technol. 2016, 45, 591–596. [Google Scholar]
- Yuan, B.; Wood, D.A. Formation Damage During Improved Oil Recovery: Fundamentals and Applications; Gulf Professional Publishing: Houston, TX, USA, 2018. [Google Scholar]
- Wu, H.; Liu, P.; Gao, S.; He, G.; Wang, W. Characterization of interfacial reactions at kaolinite/water. Geochimica 2005, 410–416. [Google Scholar]
- Liu, N. Study on Fluids Transportation and Water-Rock Interactions of CO2 Geological Storage in Sandstone Reservoirs in Continental Sedimentary Basins. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2018. [Google Scholar]
- Jeon, P.R.; Kim, D.-W.; Lee, C.-H. Dissolution and reaction in a CO2-brine-clay mineral particle system under geological CO2 sequestration from subcritical to supercritical conditions. Chem. Eng. J. 2018, 347, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Li, X. Effect of fluoboric acid and mud acid on clay swelling property. Oil Drill. Prod. Technol. 1995, 17, 56–60. [Google Scholar]
- Wang, D.; Guo, J.; Wang, F.; Zhang, H. Acidification effects on the composition and structure of montmorillonite. Acta Miner. Sin. 1998, 18, 189–193. [Google Scholar]
- Stone, T.; Boon, J.; Bird, G.W. Modelling Silica Transport in Large-Scale Laboratory Experiments. J. Can. Pet. Technol. 1986, 25, PETSOC-86-01-06. [Google Scholar] [CrossRef]

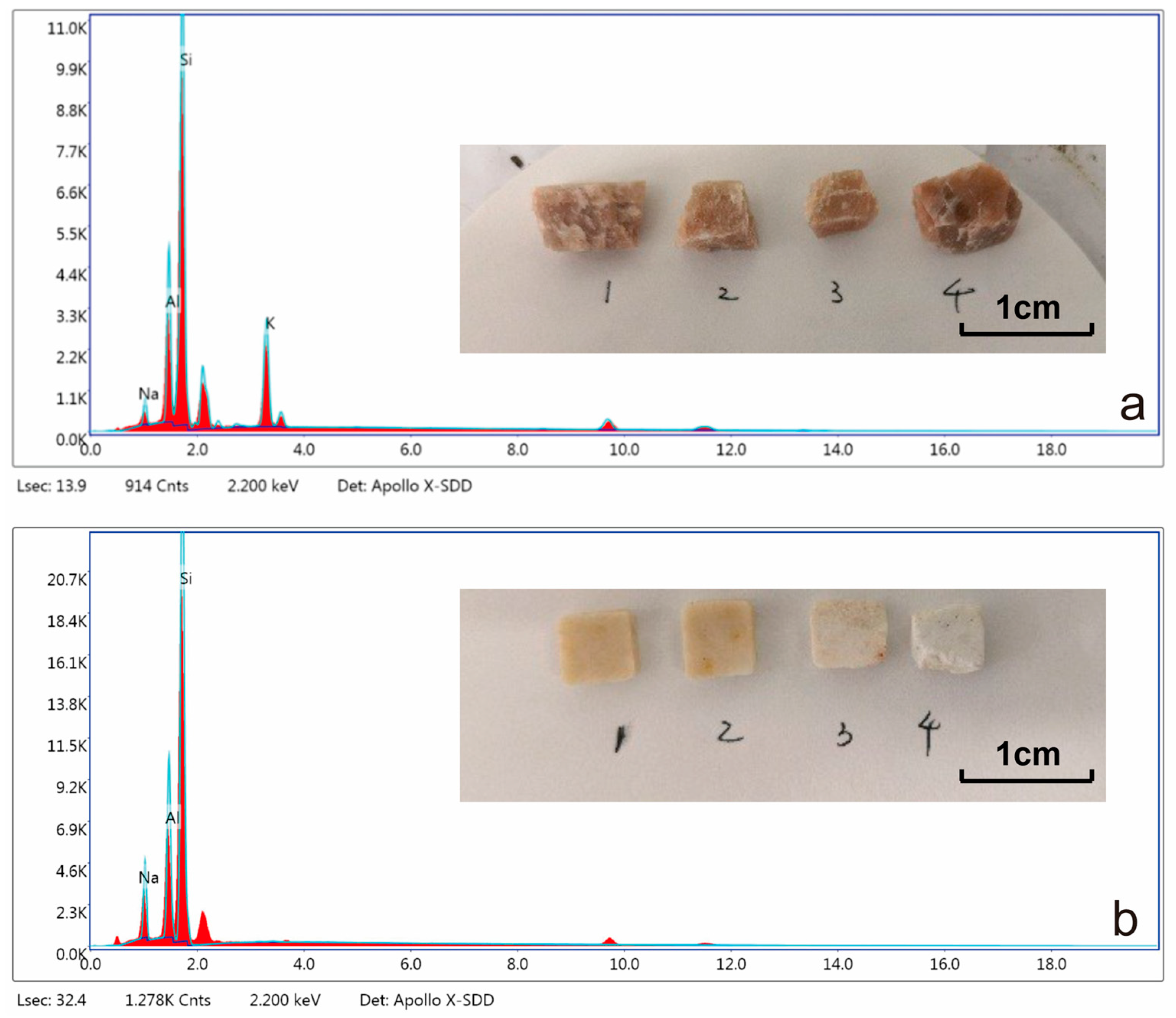
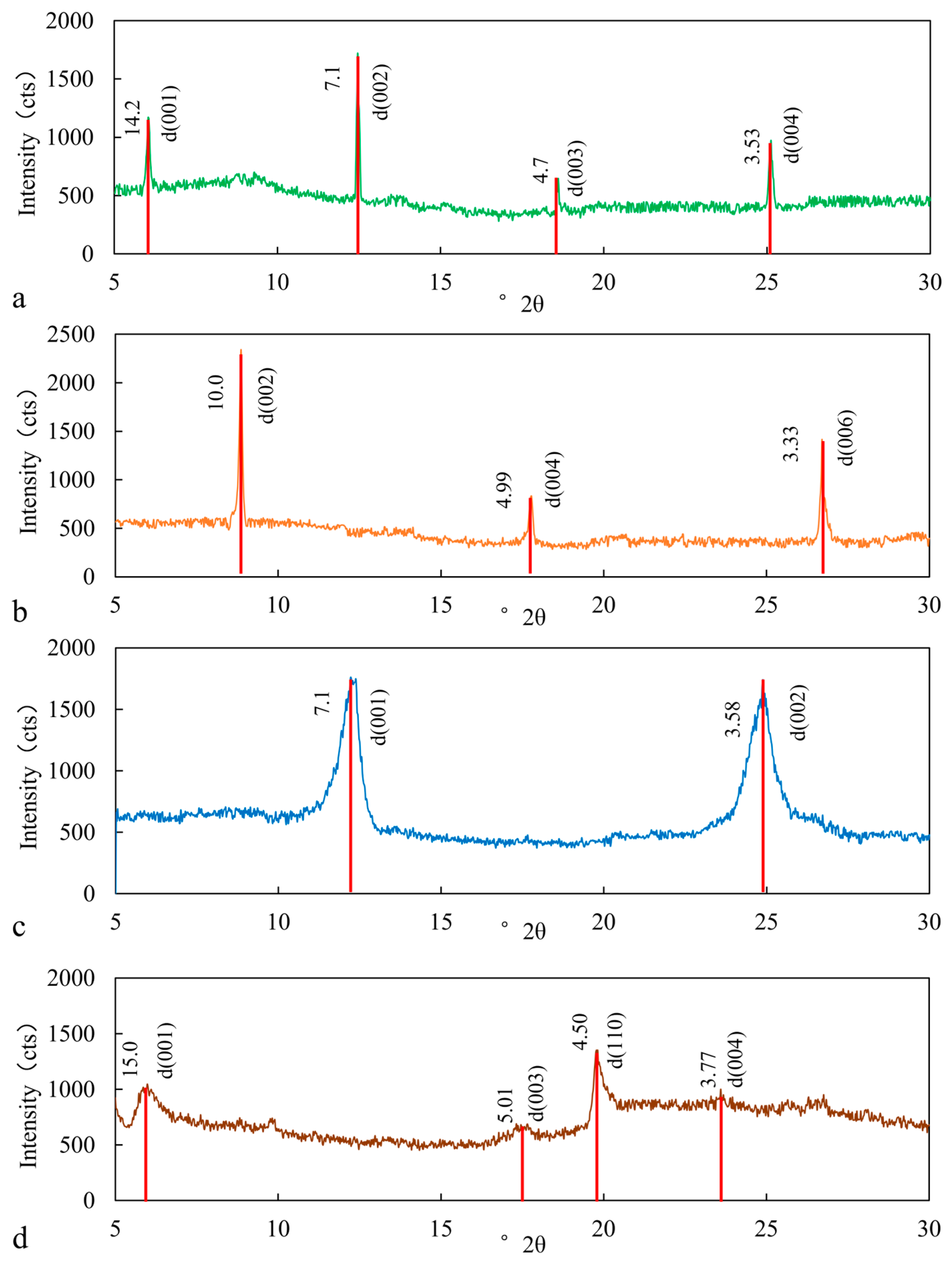
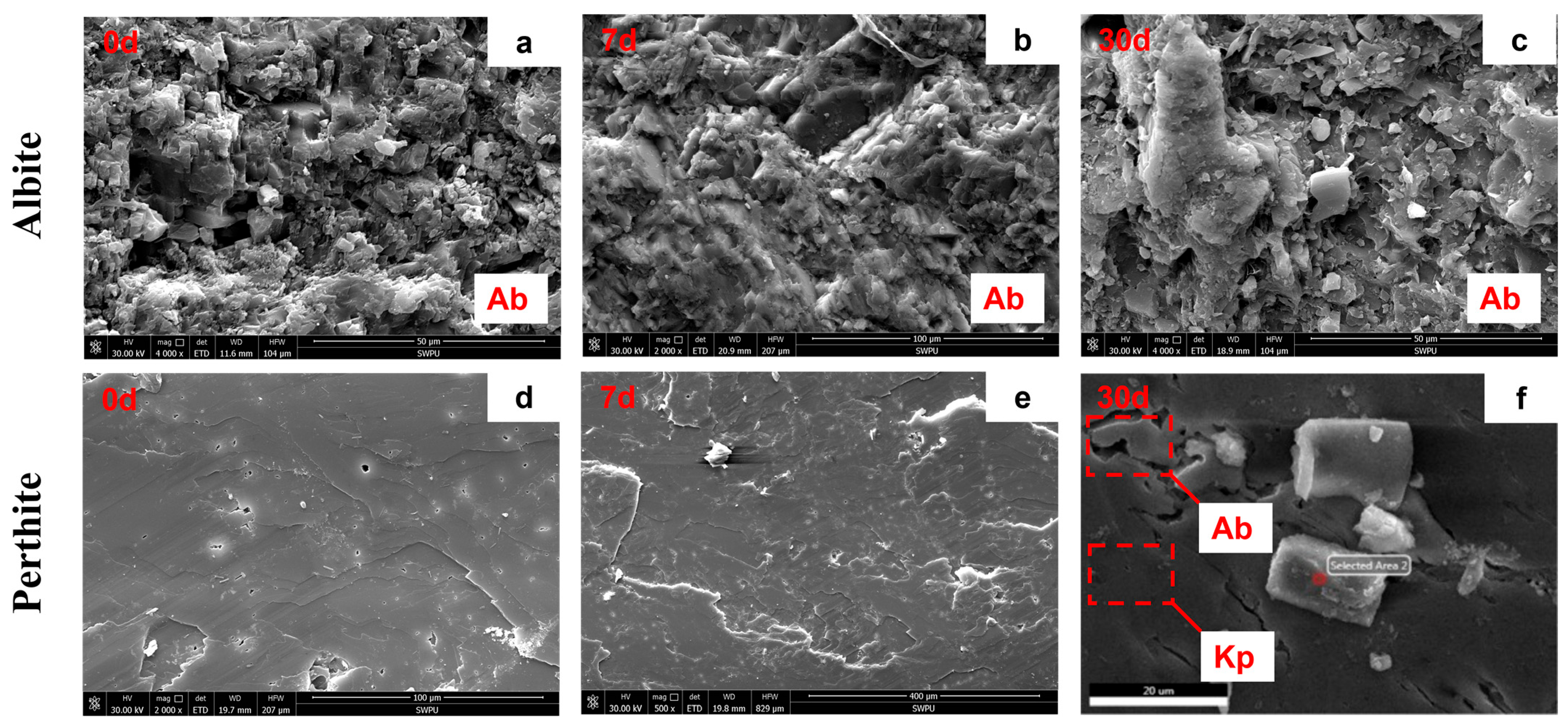
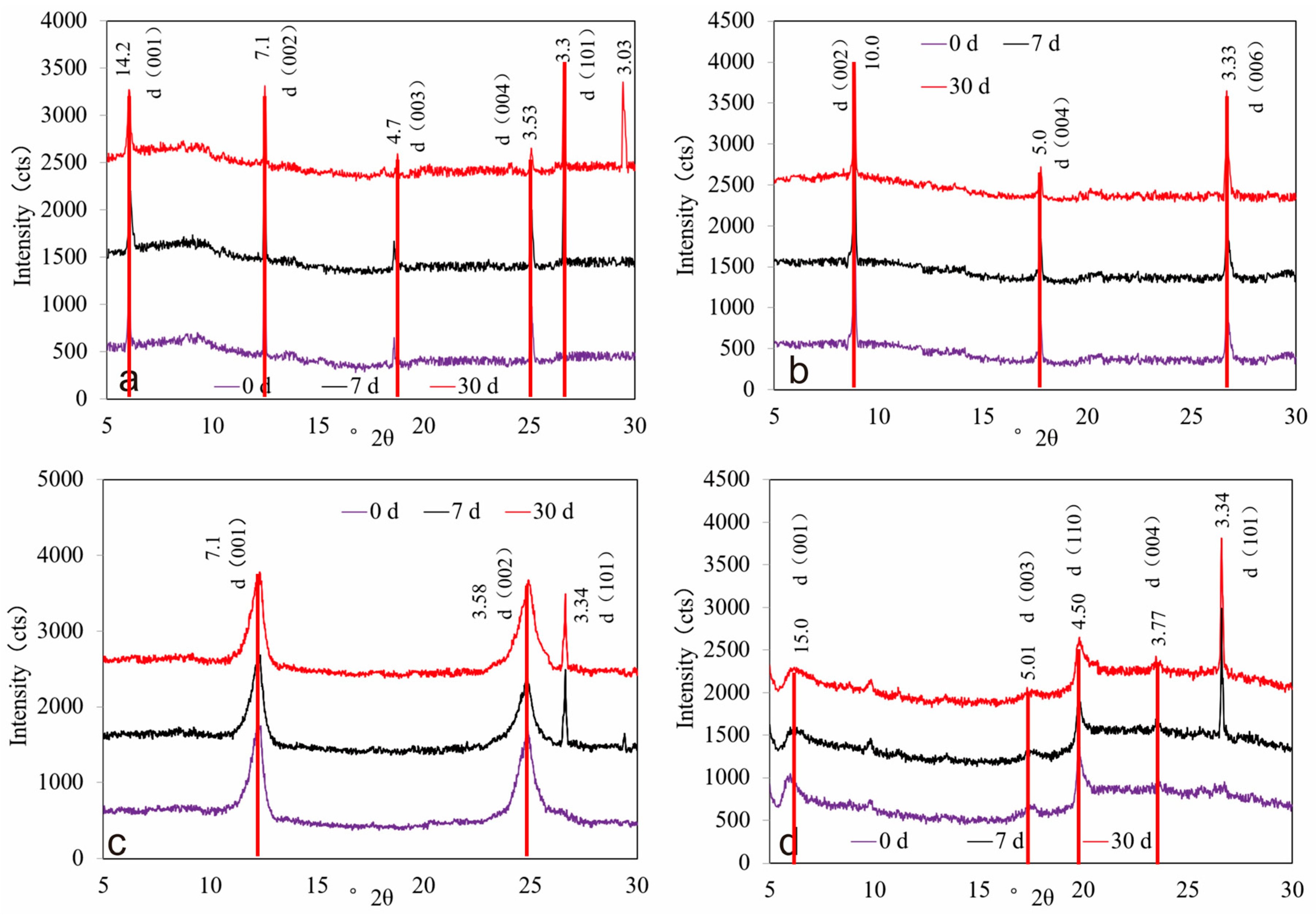

| Elements | Sodium Feldspar | Potassium Feldspar | ||
|---|---|---|---|---|
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |
| Na | 5.01 | 7.06 | 11.80 | 13.94 |
| Al | 15.29 | 18.36 | 17.99 | 18.12 |
| Si | 51.00 | 58.84 | 70.22 | 67.94 |
| K | 16.59 | 13.75 | ||
| Minerals | Al2O3/% | CaO/% | Fe2O3/% | K2O/% | MgO/% | Na2O/% | SiO2/% |
|---|---|---|---|---|---|---|---|
| Potassium feldspar | 18.32 | 0.09 | 0.81 | 11.35 | <0.01 | 3.47 | 65.38 |
| Sodium feldspar | 19.35 | 0.47 | 0.76 | 0.07 | 0.02 | 16.94 | 67.84 |
| Kaolinite | 13.46 | 0.20 | 0.89 | 1.39 | 0.30 | 0.10 | 49.70 |
| Illite | 17.04 | 1.35 | 0.64 | 2.40 | 0.73 | 4.70 | 70.36 |
| Montmorillonite | 34.26 | 1.36 | 1.13 | 3.36 | 1.24 | 2.29 | 70.87 |
| Chlorite | 12.03 | 5.52 | 3.40 | 4.95 | 4.57 | 1.4 | 58.06 |
| Reaction Time (d) | Mineral | Elements (%) | |||
|---|---|---|---|---|---|
| K | Na | Al | Si | ||
| 0 | Perthite | 14.36 | 7.35 | 20.44 | 57.85 |
| Albite | / | 21.73 | 20.77 | 57.51 | |
| 7 | Perthite | 13.75 | 7.06 | 20.36 | 58.84 |
| Albite | / | 21.07 | 20.94 | 57.99 | |
| 30 | Perthite | 13.81 | 6.90 | 20.75 | 58.94 |
| Albite | / | 20.93 | 21.04 | 58.16 | |
| Properties | Clay Minerals | |||
|---|---|---|---|---|
| Kaolinite | Illite | Montmorillonite | Chlorite | |
| Cation exchange capacity mg/100 g (%) | 3.00–15.00 | 10.00–40.00 | 80.00–150.00 | 10.00–40.00 |
| Specific surface (m2/cm3) | 8.80 | 39.60 | 34.90 | 14.00 |
| Layer spacing (nm) | 0.72 | 1.00 | 0.96–4.00 | 1.40 |
| Main force | Hydrogen bond | Ionic bond | Van Der Waals force | hydrogen bond, Ionic bond, Van Der Waals force |
| Charge distribution | Edge | Si-O tetrahedron | Al-O octahedral | Magnesite octahedron |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, L.; Liu, S.; Wang, M.; Tang, H.; Peng, D.; Yu, X.; Duan, X. Experimental Investigation of CO2–Mineral Interactions in Tight Clastic Rock Reservoirs: Implications for Geological Carbon Sequestration. Minerals 2025, 15, 1142. https://doi.org/10.3390/min15111142
Wang Z, Zhang L, Liu S, Wang M, Tang H, Peng D, Yu X, Duan X. Experimental Investigation of CO2–Mineral Interactions in Tight Clastic Rock Reservoirs: Implications for Geological Carbon Sequestration. Minerals. 2025; 15(11):1142. https://doi.org/10.3390/min15111142
Chicago/Turabian StyleWang, Ziyi, Liehui Zhang, Shu Liu, Meng Wang, Hongming Tang, Dongyu Peng, Xinan Yu, and Xingming Duan. 2025. "Experimental Investigation of CO2–Mineral Interactions in Tight Clastic Rock Reservoirs: Implications for Geological Carbon Sequestration" Minerals 15, no. 11: 1142. https://doi.org/10.3390/min15111142
APA StyleWang, Z., Zhang, L., Liu, S., Wang, M., Tang, H., Peng, D., Yu, X., & Duan, X. (2025). Experimental Investigation of CO2–Mineral Interactions in Tight Clastic Rock Reservoirs: Implications for Geological Carbon Sequestration. Minerals, 15(11), 1142. https://doi.org/10.3390/min15111142





