Abstract
Geological Carbon Sequestration (GCS) plays a crucial role in addressing climate change, particularly in oil and gas development. Understanding the reaction of supercritical CO2 under in situ conditions and its effects on minerals is essential for advancing GCS technology. This study investigates the reaction mechanisms of feldspar (potassium and sodium feldspar) and clay minerals (chlorite, illite, montmorillonite, kaolinite) in CO2 environments. The impacts on mineral crystal structures, morphologies, and elemental compositions were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), and ion concentration measurements (ICP-OES and ICP-MS). The results show that feldspar minerals exhibit lower reaction rates, with sodium feldspar dissolving faster than potassium feldspar, due to the higher solubility of sodium ions in acidic conditions. Chlorite showed significant crystal structure damage after 30 days, while montmorillonite underwent both dissolution and precipitation, influenced by interlayer cation dissociation. Kaolinite exhibited minimal reaction, primarily showing localized dissolution. Additionally, the formation of siderite (FeCO3) was observed as Fe2+ substituted for Ca2+ in CaCO3, highlighting the role of iron-bearing carbonates in CO2 interactions. The study provides insights into the factors influencing mineral reactivity, including mineral structure, ion exchange capacity, and solubility, and suggests that chlorite, montmorillonite, and illite are more reactive under reservoir conditions, while kaolinite shows higher resistance to CO2-induced reactions. These findings offer valuable data for optimizing GCS technologies and predicting long-term sequestration outcomes.
1. Introduction
Geological Carbon Sequestration (GCS) is a key carbon reduction technology with wide applications in oil and gas industry. The application of supercritical CO2 has achieved significant progress across both conventional and unconventional oil and gas fields. In conventional reservoirs, its application is primarily based on miscible displacement theory for enhanced oil and gas recovery (CO2-EOR/EGR). In unconventional reservoirs, it is utilized based on competitive adsorption theory for enhanced coalbed methane and shale gas recovery (CO2-ECBM/ESGR), where CO2 displaces methane adsorbed in the rock matrix. Furthermore, breakthroughs have been made in its use for fracturing and drilling technologies.
This study specifically focuses on tight sandstone and sandstone reservoirs, which are characterized by relatively low permeability. In these reservoirs, CO2 injection interacts with the mineral phases present in the rock matrix, modifying the geochemical environment [1]. When coupled with Carbon Capture and Storage (CCS), CO2-EOR is considered a promising method for reducing the carbon footprint of hydrocarbon production while enhancing recovery. This approach, often termed Carbon Capture, Utilization, and Storage (CCUS)-EOR, can store net CO2 underground [2]. CO2 not only carries and releases remaining oil from reservoirs but can also ‘stay’ in the reservoir after oil and gas extraction. Compared to water flooding, CO2 has advantages such as reducing viscosity, lowering interfacial tension, and acidizing to clear blockages, making it especially suitable for low-permeability reservoirs. Compared to chemical flooding, CO2 is more cost-effective, more easily obtained, less toxic, and less damaging to reservoirs. Furthermore, compared to other gas flooding methods (e.g., nitrogen or hydrocarbon gases), CO2 has higher viscosity and lower volume coefficient, making it easier to inject and more efficient, while helping to alleviate ecological environmental pressure [3,4,5]. The effectiveness of CO2-EOR varies with reservoir conditions—for example, miscible flooding at pressures above the minimum miscibility pressure (MMP) and in lighter oils typically yields higher gains, whereas sub-MMP pressures, heavier oils, and low-permeability/heterogeneous formations mainly see smaller improvements due to oil swelling, viscosity reduction, and gas channeling [4]. Previous studies have extensively investigated the mechanisms of dissolution and precipitation during CO2 reactions and their dominance under different reservoir conditions, but experimental results for similar reservoirs still show differences, and no consistent understanding of the controlling factors for dissolution and precipitation has yet emerged [6,7,8].
Current research focuses on general evaluations of oil recovery and reaction effects, with little in-depth investigation of microscopic characteristics such as mineral composition and clay mineral microstructures. After CO2 injection, CO2–brine–rock interactions generate a suite of geochemical reactions that modify porosity and permeability through dissolution, precipitation, and secondary mineral formation [6]. The observed variability reflects mineral-dependent heterogeneity in reaction pathways and rates. For instance, dissolution of feldspars liberates K+/Na+ that couple with CO2-derived dissolved inorganic carbon species to precipitate carbonates, thereby altering the reservoir geochemical milieu [9,10,11]. On the other hand, clay minerals such as illite, chlorite, and montmorillonite exhibit complex reaction behaviors during CO2 injection, influencing the pH and major ion concentrations of fluids, thereby further affecting the stability of minerals and pore structures [12,13,14]. Due to the complex composition of reservoir cores, directly reacting cores with CO2 makes it difficult to summarize reaction mechanisms, necessitating a detailed study of individual factors to reveal specific reaction pathways and interactions of different minerals during CO2 injection.
2. Materials and Methods
This study was conducted at the National Key Laboratory of Oil and Gas Reservoir Geology and Development Engineering at Southwest Petroleum University. It aims to systematically investigate the reaction mechanisms of feldspar and clay minerals (illite, chlorite, montmorillonite) with CO2, and their impact on the reservoir pore structure. The mineral samples used in the experiments include potassium feldspar, sodium feldspar, kaolinite, illite, chlorite, and montmorillonite. While quartz is a common component of most reservoirs, particularly those with feldspars and clay minerals, it was not included in the experimental samples due to its generally low reactivity under CO2 injection conditions. The primary focus of this study is on the reactions of feldspars and clay minerals, as these minerals are more reactive and play a significant role in altering the geochemical environment of the reservoir. These mineral samples were obtained from laboratory specimens and from the Lixing Kaning and Tarim regions of Xinjiang, with a purity greater than 80%. Prior to the experiments, the mineral samples were mechanically crushed and sieved to a 200-mesh (75 µm) size. This step was performed to achieve a uniform particle size and ensure consistent exposure of the minerals to CO2 during the reactions, allowing for more controlled and reproducible experimental conditions. Bulk powders for non-clay analyses were oven-dried at 105 °C for 12 h; however, clay XRD mounts were not oven-dried and were prepared as oriented mounts, analyzed in air-dried and ethylene-glycol-solvated conditions (≤40 °C for drying prior to glycolation).
Before the reaction experiments, the mineral samples were analyzed using a -Smartlab 9KW X-ray diffractometer (XRD, RIGAKU, Tokyo, Japan), under the conditions of Cu-Kα radiation (with an energy of 8.04 keV), a working voltage of 35 kV, and a current of 30 mA (Figure 1a). The scanning range was 2θ = 5–30°, with a scanning time of 5 min, a scanning speed of 2°/min, and a step size of 0.02°. The quartz d(101) peak was used as a reference for calibration. At the same time, a Magix PW-2424 X-ray fluorescence spectrometer (XRF, PANalytical, Almelo, The Netherlands) was used to determine the major elemental composition of the samples, using an Rh-target X-ray tube and a power setting of 4 kW (Figure 1b). The measurement time was 30 s, and the detection precision was up to 0.1%. The micro-morphology of the mineral surfaces was observed using an Quanta 650 FEG field emission scanning electron microscope (FE-SEM, FEI, Brno, the Czech Republic) at an accelerating voltage of 10–20 kV and a working distance of 10 mm, using secondary electron mode (SE) to record cleavage characteristics, fractures, and surface roughness of the samples (Figure 1c).
Figure 1.
Experiment instruments. (a) XRD, (b) XRF, (c) SEM-EDS, (d) ICP-OES, (e) high-temperature and high-pressure reactor.
Static reaction experiments were conducted in a CWYF-1 high-temperature and high-pressure reactor- (Haian County Petroleum Scientific Research Instruments, Haian, China) (Figure 1e). The experimental conditions were set to simulate in situ reservoir conditions at a depth of approximately 2600 m, where the temperature is around 65 °C. This temperature was selected to match typical conditions at this depth in deep reservoirs. The pressure was set at 10 MPa, which is lower than the actual reservoir pressure of 31 MPa, to create more controlled conditions for studying reaction mechanisms without the complications of high pressure. The solid–liquid ratio was set at 1:3 (by mass) to ensure sufficient reactants while maintaining practical and reproducible conditions. The reaction times were 7 days and 30 days. Prior to the experiments, mineral powders were homogenized with deionized water and loaded into the reactor. To minimize sedimentation without disturbing system equilibrium, the reactor’s top-mounted sealed stirring rod (mechanical feedthrough) was used to manually agitate the suspension at 7-day intervals, without opening or venting the reactor. After the reaction, the samples were removed from the reactor, and the upper turbid liquid was allowed to stand for 24 h to separate the clear liquid from the rock powder. The upper clear liquid was analyzed for major elements (such as Si, Al, K, Na, etc.) using a PE Optima 5300 DV inductively coupled plasma optical emission spectrometer (ICP-OES, Perkin Elmer, Waltham, USA). The RF power was set to 1300 W, the cooling airflow to 10 L/min, the sample flow rate to 1 mL/min, and the detection limit was 0.01 mg/L (Figure 1d). Trace elements were analyzed using an Agilent-7900 inductively coupled plasma mass spectrometer (ICP-MS, Agilent, Tokyo, Japan) with the RF power set to 1550 W, the nebulizer flow rate to 0.1 L/min, and a detection limit of 0.1 µg/L. The remaining rock powder was rinsed and soaked in deionized water 2–3 times to remove soluble salts and then dried for further use.
After the reaction, the mineral samples were analyzed using X-ray diffraction (XRD) for mineral phase identification, with a scanning range of 5–60° and a scanning speed of 2°/min. The Rietveld method was used for quantitative phase analysis, using the quartz d(101) peak as the reference. Additionally, the surface morphology and elemental distribution changes of the rock powder after the reaction were observed using—Quanta 650 FEG field emission scanning electron microscope (FE-SEM, FEI, Brno, the Czech Republic) combined with energy dispersive spectroscopy (EDS). The magnification range was set between 500×–5000×. The X-ray fluorescence spectrometer (XRF) was used to measure the changes in the major elemental content of the solid-phase samples to monitor the migration and transformation of elements during mineral reactions.
3. Results
3.1. Mineral Identification
3.1.1. Feldspar Identification
Potassium feldspar (KAlSi3O8) belongs to the monoclinic crystal system, with the chemical formula KAlSi3O8. It typically appears pinkish-red, with two complete cleavage directions. Its density ranges from 2.54 to 2.57 g/cm3, and its specific gravity is between 2.56 and 2.59, with a hardness of 6. Energy-dispersive spectroscopy (EDS) analysis shows that the potassium feldspar used in this study contains small amounts of sodium, categorizing it as strip feldspar. The theoretical Si:Al ratio in potassium feldspar is 3:1, and the total content of potassium (K) and sodium (Na) is comparable to that of aluminum (Al). To compare the dissolution rates of alkali feldspar and plagioclase feldspar, we selected potassium feldspar as the K-feldspar host (matrix) and sodium feldspar as the exsolved albite lamellae for the CO2 reaction experiments.
Potassium feldspar (KAlSi3O8) belongs to the monoclinic crystal system, with the chemical formula KAlSi3O8. It typically appears pinkish-red, with two complete cleavage directions. Its density ranges from 2.54 to 2.57 g/cm3, and its specific gravity is between 2.56 and 2.59, with a hardness of 6. Energy-dispersive spectroscopy (EDS) analysis shows that the potassium feldspar used in this study contains small amounts of sodium, consistent with a perthitic K-feldspar. The theoretical Si:Al ratio in potassium feldspar is 3:1, and the total content of potassium (K) and sodium (Na) is comparable to that of aluminum (Al). To compare reaction rates between alkali and plagioclase feldspars within a single specimen, we used a perthitic K-feldspar in which a K-feldspar host (matrix) contains exsolved albite lamellae; the CO2 reaction experiments thus compared the host K-feldspar with the albite lamellae under identical conditions.
Sodium feldspar (NaAlSi3O8) belongs to the triclinic crystal system, with the chemical formula NaAlSi3O8. It typically appears white to grayish-white, with two complete cleavage directions and a hardness of 6–6.5. Its density ranges from 2.61 to 2.64 g/cm3 and is a common acidic plagioclase mineral. Sodium feldspar is rich in silicon and poor in aluminum, with a theoretical Si:Al ratio of 3:1. EDS analysis shows that the sodium feldspar used in this study is relatively pure (Figure 2, Table 1).
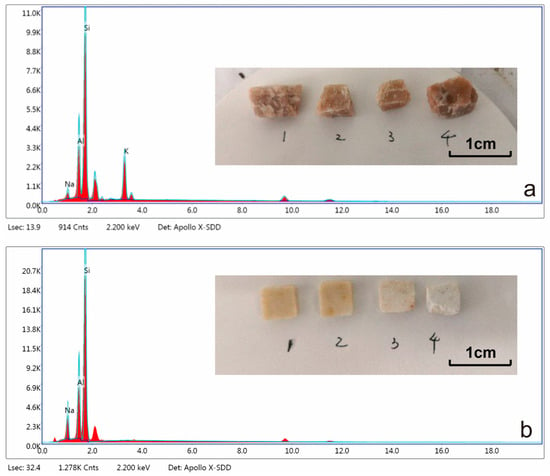
Figure 2.
EDS analysis of (a) sodium feldspar and (b) potassium feldspar.

Table 1.
EDS analysis of feldspar.
3.1.2. Clay Mineral Identification
The clay minerals used in this study include chlorite, illite, kaolinite, and montmorillonite. Their specific identification is as follows (Figure 3, Table 2):
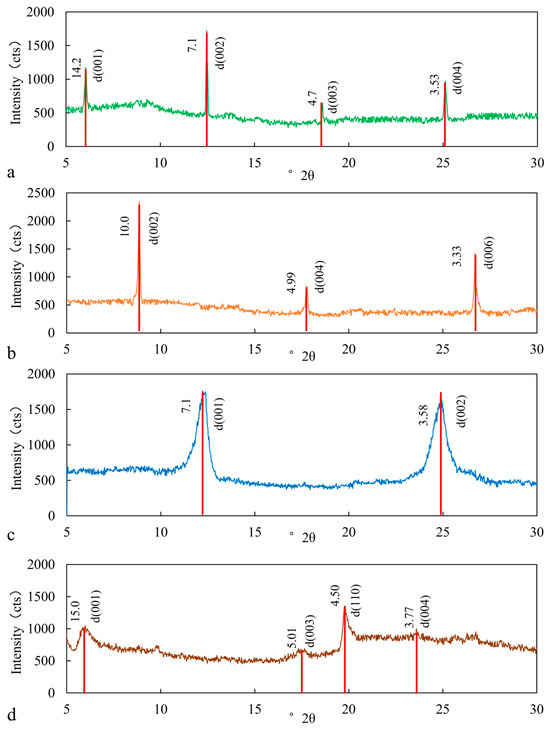
Figure 3.
XRD curve of (a) chlorite, (b) illite, (c) kaolinite, (d) montmorillonite.

Table 2.
XRF analysis of different minerals.
Chlorite is a 2:1-type layered aluminum silicate mineral, with a structure similar to 2:1 montmorillonite but with an additional octahedral (alumina) sheet in the chlorite structural unit, which acts as an interlayer cation. Chlorite is typically monoclinic, with a specific gravity of 2.6–3.3 and a hardness of 2–3. Its molecular formula is (Mg, Fe)(AlSi3O10)(OH)8 [15]. Based on the Fe2+/Fe3+ and Fe2+/Mg2+ ratios, chlorite can be classified as iron-rich, magnesium-rich, or intermediate types (Figure 3a). The chlorite used in this study is magnesium-rich.
Illite is a typical 2:1 (TOT)-type layered aluminosilicate mineral. Its fundamental structural unit consists of an aluminum-hydroxide octahedral sheet sandwiched between two silicon–oxygen tetrahedral sheets. The adjacent TOT layers are primarily held together by strong ionic bonds formed between the interlayer potassium ions (K+) and the tetrahedral sheets [16]. Additionally, hydrogen bonds, originating from the hydroxyl groups in the octahedral sheet, contribute to the stability of the layered structure. Illite is monoclinic, with a molecular formula of KAl2(Si,Al)4O102·nH2O, and it typically appears as a white, clayey material with a hardness of 1–2 and a specific gravity of 2.6–2.9 (Figure 3b).
Kaolinite is a 1:1-type (TO) dioctahedral layered silicate mineral. It is typically earthy or blocky, with a hardness of 2–2.5, a molecular formula of Al4(Si4O10)(OH)8, and belongs to the triclinic system, with a density ranging from 2.60 to 2.63 g/cm3 and a specific gravity of 2.16–2.68 (Figure 3c).
Montmorillonite belongs to the smectite group of minerals, typically occurring as blocky or earthy forms. Its molecular formula is (Na, Ca)0.33(Al, Mg)2(Si4O10)(OH)2·nH2O. Its structure consists of a central aluminum–oxygen octahedral layer flanked by silicon–oxygen tetrahedral layers, forming a 3-layer structure (TOT) [17]. The layers contain water molecules and exchangeable cations, giving montmorillonite a high cation-exchange capacity and water absorption ability. It is monoclinic in structure (Figure 3d).
3.2. Feldspar Reactions with CO2
In the first 7 and 30 days after CO2 pressurization, both sodium feldspar and perthitic K-feldspar exhibited reaction, but the early-stage surface alteration of standalone albite (actual sodium feldspar) appeared modest and localized. According to SEM-EDS, the relative Na content in albite decreased from 21.73% to 20.93%, while Al and Si changed only slightly, indicating a mild early-stage response. Over the full reaction duration, however, solution chemistry (ICP-OES/ICP-MS) shows that sodium feldspar is overall more reactive than potassium feldspar [16]. In contrast, the perthitic K-feldspar shows more complex behavior. Electron-microscopy observations indicate preferential dissolution in Na-rich microdomains (albite exsolution lamellae), whereas K-rich regions (K-feldspar host) dissolve more slowly. Specifically, the relative Na content decreased from 7.35% to 6.90%, while K decreased from 14.36% to 13.81% [15]. The before-and-after differences—especially the contrast between Na and K—indicate preferential leaching of Na-rich domains within the perthite (Figure 4; Table 3).
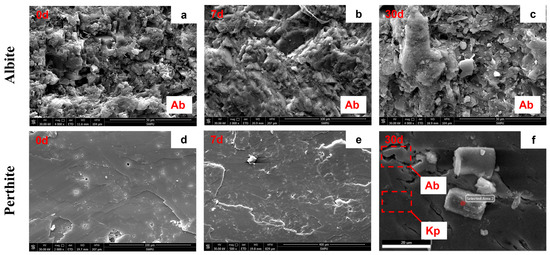
Figure 4.
SEM analysis of albite: (a) before reaction, (b) reaction for 7 d, (c) reaction for 30 d, has localized dissolution; perthite: (d) before reaction, (e) reaction for 7 d, (f) reaction for 30 d, sodium part (Ab) has higher dissolution than potassium part (Kp).

Table 3.
EDS analysis results of different feldspars before and after reaction.
Regarding the changes in ion concentrations in the reaction solution, both sodium feldspar and potassium feldspar exhibited a significant increase in the concentrations of Na+, Al3+, and Si4+. Specifically, for sodium feldspar, over 0 to 30 days, the Na+ concentration increased from 9302 mg/L to 14,630 mg/L, the Al3+ concentration increased from 0 to 1.52 mg/L, and the Si4+ concentration increased from 0 to 11.13 mg/L. In comparison, the changes in the ion concentrations for potassium feldspar were also notable, With Na+ concentration increasing from 9302 to 11,720 mg/L, Al3+ from 0 to 0.20 mg/L, Si4+ from 0 to 11.9 mg/L, and K+ from 0 to 21.64 mg/L. While the ion concentrations in the reaction solution for both minerals increased significantly after 30 days of reaction, sodium feldspar showed a slightly higher reaction rate than potassium feldspar, indicating that the release of sodium ions was faster (Table 3).
Furthermore, the concentration changes of Ca2+ and Mg2+ ions in the reaction solutions of sodium feldspar and potassium feldspar were different. For sodium feldspar, the Ca2+ concentration decreased from 214.3 mg/L to 85.39 mg/L in the first 7 days, but then rebounded, reaching 118.69 mg/L by day 30. In contrast, the Ca2+ concentration in the potassium feldspar solution continuously decreased over the 30 days, suggesting that sodium feldspar may more readily undergo ion exchange with Ca2+. The concentration of Mg2+ in the reaction solutions for both minerals showed relatively small changes and remained fairly constant (Table 3).
In summary, sodium feldspar and potassium feldspar exhibit significant differences in their CO2 reaction behavior. Sodium feldspar has a higher dissolution rate, and the release of sodium is more rapid, whereas potassium feldspar shows a slower dissolution rate, particularly in potassium-rich areas. Changes in the ion concentrations in the reaction solutions further confirm these differences, which may be related to the crystal structure, surface properties, and reaction kinetics of CO2 with the minerals [18,19].
3.3. Clay Mineral Reactions with CO2
3.3.1. Changes in Crystal Structure of Clay Minerals
In terms of crystal-structure evolution, all four clay minerals exhibited changes in interlayer reflections to varying extents (Figure 5).
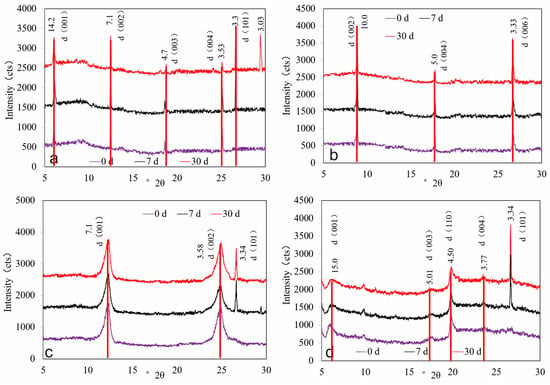
Figure 5.
Diffraction curve variation of different clay minerals: (a) chlorite, (b) illite, (c) kaolinite, (d) montmorillonite.
Chlorite. After 7 days, the d(002) peak intensity decreased by 13.25%. After 30 days, the d(002) and d(004) peaks decreased by 31.93% and 33.06%, respectively, indicating substantial structural damage. Concurrently, new reflections at 4.33 Å and 3.032 Å emerged and are indexed to CaCO3, consistent with mineral transformation during prolonged reaction (Figure 5a).
Illite. No obvious structural damage was detected after 7 days. By 30 days, the d(002), d(004), and d(006) peaks showed a gradual attenuation of 16.6–22.5%, indicating moderate damage to the illite structure after prolonged exposure, possibly due to the interaction of water within the structure with CO2, resulting in the formation of carbonic acid (H2CO3) [16,20]. The presence of carbonic acid may have contributed to the dissolution of K-feldspar and chlorite, as evidenced by changes in their XRD peaks, without the formation of new crystalline phases (Figure 5b).
Kaolinite. The crystal structure of kaolinite remained relatively stable over the first 30 days. After 7 days, the d(001) and d(002) peaks decreased by 4.20% and 6.27%, respectively; by 30 days, the decreases were 2.79% and 6.70%. These changes are minor, indicating limited structural damage and no new crystalline phases [16,21]. The quartz peak remained essentially unchanged, suggesting no formation of crystalline SiO2 (Figure 5c).
Montmorillonite. Montmorillonite underwent pronounced structural changes. After 7 days, the d(001) peak weakened by 30.61%, and the d(003) and d(004) peaks almost disappeared, indicating marked disintegration of the layered structure [17]. Additionally, a new reflection at d = 6.56 Å (2θ ≈ 13.4°, Cu Kα) appeared, consistent with a CaSO4-type sulfate phase (gypsum/bassanite family) (Figure 5d).
Overall, chlorite and montmorillonite experienced the most significant structural changes, whereas illite and kaolinite showed weaker structural responses within the same period.
3.3.2. Mineral Morphology and Elemental Composition Changes
The SEM observations (Figure 6) corroborate the XRD trends and clarify the time dependence of morphological change.
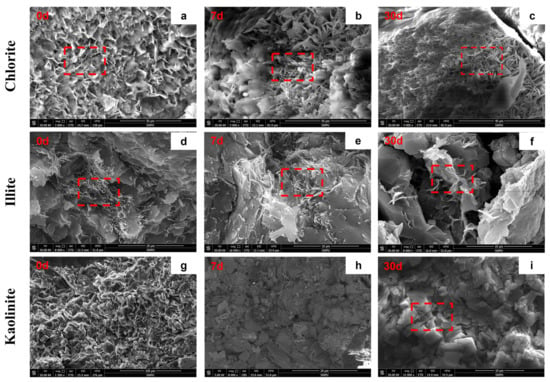
Figure 6.
SEM analysis of different clay minerals before/after reaction with CO2: (a) chlorite morphology before reaction, has petal-/needle-like outline, (b) chlorite morphology after 7 days of reaction, needle-like outline remains, (c) chlorite morphology after 30 days of reaction, needle-like outline remains; (d) illite morphology before reaction, has fibrous structure, (e) illite morphology after 7 days of reaction, fibrous structure remains, (f) illite morphology after 30 days of reaction, fibrous structure absent, (h) kaolinite morphology before reaction, (i) kaolinite morphology after 7 days of reaction, (g) kaolinite morphology after 30 days of reaction, no significant disruption.
Chlorite. At day 7, chlorite displays edge dissolution, yet the petal-/needle-like outline remains recognizable. By day 30, the surface becomes markedly fragmented, consistent with the strong XRD peak attenuation and carbonate formation reported in Section 3.3.1.
Kaolinite. Kaolinite retains its flaky aggregate at both day 7 and day 30, showing only slight edge corrosion and no significant disruption of aggregate integrity, corroborating its resistance to dissolution under the present conditions.
Illite. Illite exhibits minimal surface alteration at day 7 (fibrous habit largely preserved). By day 30, however, pronounced dissolution and fracturing render the fibrous structure nearly absent, indicating substantial consumption over time. This behavior is consistent with illite being comparatively more stable than smectite, yet not inert in CO2–brine systems.
Montmorillonite. Montmorillonite shows morphology–composition coupling, with dissolution and precipitation occurring concurrently. Release of interlayer cations exerts first-order control on texture evolution from day 7 to day 30, consistent with the structural degradation seen in Section 3.3.1.
3.3.3. Element Release and Ion Concentration Changes
During the CO2 reaction process, the ion concentration changes in chlorite showed a trend of increasing first and then decreasing, reflecting the dynamic balance between dissolution and precipitation (Figure 7). Compared with the pre-reaction solution, the concentrations of Ca2+, Fe3+, Mg2+, and Al3+ all increased at the early stage and then gradually decreased, while the Si4+ concentration increased initially and then stabilized. This indicates that, in the early stage, these ions were dissolved into the solution, and during the 7–30 day period, precipitation occurred for Al3+ and Fe3+. The decrease in ion concentrations after the initial increase is likely attributed to secondary reactions, such as precipitation of CaSO4 or other mineral phases, which consume these ions and result in their gradual reduction in the solution (Figure 8a). For example, after 30 days of reaction, the Ca2+ concentration dropped to 33 mg/L, Mg2+ decreased to 87 mg/L, Fe3+ decreased to 0.2 mg/L, and Al3+ decreased to 0.3 mg/L. Meanwhile, the Si4+ concentration increased from 0 to 46 mg/L, indicating continuous release of Si4+ and a sustained dissolution process.
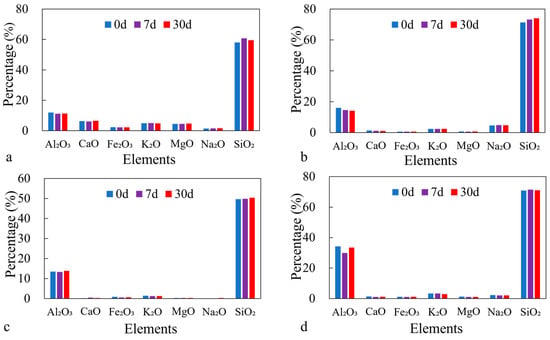
Figure 7.
XRF analysis of different clay minerals (<5 μm) before/after reaction with CO2: (a) chlorite, (b) illite, (c) kaolinite, (d) montmorillonite.
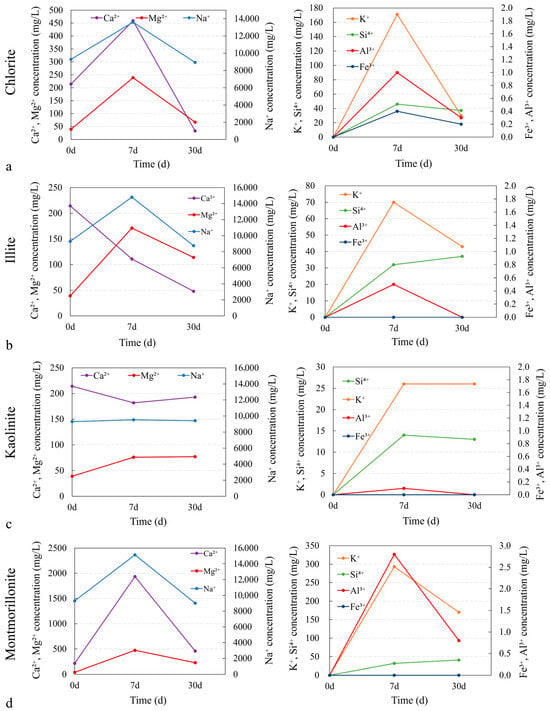
Figure 8.
Ion concentration variation of different clay minerals (<75 μm) before/after reaction with CO2: (a) chlorite, (b) illite, (c) kaolinite, (d) montmorillonite.
A similar trend was observed for illite, though the rates of ion release and the final equilibrium concentrations differed. During the reaction, the concentrations of Mg2+ and Al3+ increased initially and then decreased, whereas those of K+ and Si4+ rose in the early stage before stabilizing. For instance, after 7 days of reaction, the K+ concentration in the illite solution increased from 0 to 70 mg/L, Mg2+ from 39.13 mg/L to 170.91 mg/L, and Al3+ from 0 to 0.5 mg/L, while Si4+ increased from 0 to 37 mg/L; between 7 and 30 days, Mg2+ decreased to 114 mg/L and Al3+ dropped to 0 (Figure 8b).
Kaolinite reacted relatively slowly, with minor changes in the release of ions. After 30 days, the concentrations of Si4+, K+, and Al3+ in the kaolinite reaction solution increased at first and then leveled off; specifically, the Si4+ concentration increased from 0 to 15 mg/L and Al3+ from 0 to 0.35 mg/L (Figure 8c), with no obvious precipitation observed. This behavior is attributed to kaolinite’s higher chemical stability and its relatively closed structure.
Montmorillonite showed a distinct behavior compared to chlorite and kaolinite, particularly in terms of the rate of ion release and dissolution. In the montmorillonite system, the Ca2+ concentration increased sharply from 214.3 mg/L to 1935 mg/L within the first 7 days, followed by a decline to 459 mg/L between days 7 and 30. Similarly, the Mg2+ concentration rose from 39.1 mg/L to 473.0 mg/L in the first 7 days and then dropped to 228.0 mg/L by day 30. The Al3+ concentration increased from 0 to 2.8 mg/L in the first 7 days, then decreased to 0.8 mg/L by day 30, while the Si4+ concentration rose from 0 to 41 mg/L and then leveled off. This sharp initial increase followed by a decline in ion concentrations suggests a strong initial release of interlayer cations, which is more pronounced than in other clay minerals. The ion release behavior of montmorillonite is likely influenced by cation exchange processes, where the interlayer cations are exchanged with the surrounding aqueous phase, leading to the observed mobilization of ions. In contrast, while other clays also show a rise in ion concentrations initially, montmorillonite’s sharp early-stage release is more significant. The decreasing trend of K, Ca, and Mg concentrations in montmorillonite indicates that the interlayer cations were gradually released and mobilized during the reaction, highlighting the distinct ion release behavior of montmorillonite compared to other clays (Figure 8d).
In summary, the chemical stability and morphological changes of the minerals under CO2 reaction conditions exhibit significant differences. Chlorite and montmorillonite show similar trends in elemental changes—dissolution followed by precipitation—while kaolinite remains relatively resistant to dissolution. In contrast, illite exhibits more pronounced changes, especially with a higher dissolution rate of Mg compared to K. These findings provide important insights into the dissolution and precipitation processes in CO2 reaction systems and reveal the varied reactivity of different minerals in geochemical environments.
4. Discussion
4.1. Analysis of the Reaction Mechanism Between Feldspar and CO2
Based on the SEM-EDS analysis and the observed ion concentration changes in the reaction solutions, both potassium and sodium feldspars undergo some dissolution, although the overall reaction is relatively slow. Sodium feldspar typically dissolves faster than potassium feldspar, as Na+ ions are more weakly bonded to the feldspar structure compared to K+ ions. This makes Na+ easier to release in acidic conditions, where ion exchange accelerates the dissolution rate, resulting in a faster reaction for sodium feldspar under reservoir conditions, i.e., sodium feldspar > potassium feldspar. The presence of carbonic acid (H2CO3), produced by the interaction of illite with CO2 in the aqueous phase, may further promote the dissolution of K-feldspar and chlorite. The acidic conditions, coupled with the ion exchange processes facilitated by carbonic acid, lead to enhanced dissolution of these minerals [22,23,24].
During the feldspar dissolution process, the elements K, Na, and Al are preferentially released, while Si is released at a later stage. In the feldspar crystal lattice, the aluminum– oxygen tetrahedra (AlO4) and silicon–oxygen tetrahedra (SiO4) are connected by bridging oxygens, and the cations such as K+ and Na+ are coordinated by non-bridging oxygens. The dissolution reaction primarily occurs at the sites of both bridging and non-bridging oxygens. Notably, the (AlO4)5− tetrahedra in feldspar have a slightly larger volume than the (SiO4)4− tetrahedra, rendering the structure more unstable under acidic conditions. Consequently, aluminum is preferentially released compared to silicon [16]. This dissolution process is driven by the hydrogen ions (H+) that are generated when CO2 dissolves in water, forming carbonic acid (H2CO3), which then dissociates into H+ and HCO3− ions. The reaction of CO2 with water is as follows:
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3−
These H+ ions actively participate in the chemical reactions on the mineral surfaces, promoting the release of K, Na, Al, and Si while accompanying the dissolution of silicate minerals [14,15]. The following reactions illustrate the dissolution under acidic conditions:
For potassium feldspar (KAlSi3O8) [25,26],
KAlSi3O8 + 4H+ → K+ + Al3+ + 3SiO2 + 2H2O
For sodium feldspar (NaAlSi3O8) [27],
NaAlSi3O8 + 4H+ → Na+ + Al3+ + 3SiO2 + 2H2O
These reactions indicate that the hydrogen ions (H+) in the acidic fluid actively participate in the chemical reactions on the mineral surfaces, promoting the release of K, Na, Al, and Si while accompanying the dissolution of silicate minerals [23,24].
Furthermore, the higher dissolution rate of aluminum from the feldspar crystals results in a significant increase in the Al3+ concentration in the solution, while the release of silicon is relatively slower. The changes in the ion concentrations in the reaction solution further verify this mechanism. In the CO2 reaction system, the increase in Al3+ concentration is usually accompanied by the release of Na+ or K+, and the Si4+ concentration remains relatively lower, indicating that aluminum is preferentially released from the feldspar minerals [28].
The difference in solubility between potassium feldspar (K-feldspar) and sodium feldspar primarily stems from differences in chemical stability and ionic activities. Because potassium ions (K+) exhibit greater ionic stability compared to sodium ions (Na+), potassium feldspar generally has a lower solubility under normal conditions. Thus, when present individually, sodium feldspar tends to have a higher solubility than potassium feldspar [23]. However, when both feldspars coexist, the solubility behavior can change markedly. With increasing temperature, the solubility of sodium feldspar increases more rapidly than that of potassium feldspar [29]. This phenomenon is closely related to changes in ionic activities during the dissolution process in water and may vary under different temperature and pH conditions [22,29,30,31].
Further analysis indicates that the released sodium ions (Na+) can inhibit the dissolution of potassium feldspar. In environments with high Na+ activity, competition between Na+ and K+ may lead to reduced release of potassium ions, thereby suppressing the dissolution rate of potassium feldspar [23]. Therefore, when sodium feldspar and potassium feldspar coexist, the release of Na+ may significantly inhibit the dissolution of potassium feldspar through ion exchange.
In our experiments, the dissolution rates of sodium and potassium feldspar fragments were similar during the first 7 days, but between 7 and 30 days, sodium feldspar exhibited a significantly higher dissolution rate than potassium feldspar. No new minerals such as albite or kaolinite were detected post-reaction, and the overall dissolution rate remained low. Under the experimental condition of pH < 6, no formation of albite was observed, and the HCO3− concentration was high. Thus, both feldspars can react with acid as follows:
KAlSi3O8 + 4H2O + 4H+ → K+ + Al3+ + 3H4SiO4
NaAlSi3O8 + 4H2O + 4H+ → Na+ + Al3+ + 3H4SiO4
4.2. Analysis of the Reaction Mechanism Between Clay Minerals and CO2
- (1)
- Reaction Mechanism of Chlorite with CO2
As a typical iron-rich clay mineral, chlorite’s reaction in a CO2 solution system is primarily controlled by changes in its crystal structure and ion exchange processes. Studies have shown that the appearance of Fe3+ does not necessarily imply the dissolution of chlorite; rather, it may be due to the extraction or leaching of iron (Fe3+) by H+ [32]. During the CO2 reaction, the dissolution behaviors of Fe2+ and Fe3+ differ significantly. Due to its stronger solubility, Fe2+ is more easily replaced by H+ and enters the solution, potentially leading to the formation of siderite (FeCO3). In this context, Fe2+, as a dissolved product, can react with CO2 under low-pH conditions to form FeCO3.
The dissolution reaction related to Fe2+ can be represented as:
Fe2Si4O10(OH)2 + 4H+ → 2Fe2+ + Si4O102− + 2H2O
While the dissolution involving Fe3+ may lead to the formation of colloidal Fe(OH)3, which can further settle as a solid under lower pressure:
Fe2Si4O10(OH)2 + 6H+ → 2Fe3+ + Si4O102− + 2H2O
As the reaction progresses, Mg2+ and Al3+ in the chlorite structure are also gradually replaced by H+. According to the sequence of elemental stability (Mg2+ > Al3+ > Fe2+), the release sequence of Mg2+ and Al3+ follows this order. The dissolution of Mg2+ is particularly critical in this process and may be linked to the formation of carbonate minerals.
For example, Cai D. [33] found that after reaction, the reduction in Ca2+ and Mg2+ concentrations in dense sandstone was not only due to the precipitation of Mg2+ released from chlorite and illite (forming magnesite or sericite) but also related to the reaction of high concentrations of HCO3− in the solution. Kwak J.H. (2011) [12] suggested that the released Mg2+ might combine with HCO3− to produce magnesite (MgCO3) and amorphous hydrated magnesium carbonate (MgCO3·xH2O); under CO2 injection, magnesite predominates because the high concentration of HCO3− favors the formation of magnesite over other magnesium-based precipitates. This precipitation is particularly significant when CO2 dissolves in the fluid and increases the concentration of HCO3−, leading to magnesite formation. Once formed, magnesite is difficult to dissolve in acidic media, as its solubility is low in acidic conditions, making it more stable and less likely to re-dissolve. If this precipitation fills the pore spaces, it will affect the pore structure [8].
Rosenbauer R.J. (2005) proposed the following reaction in the presence of calcite [34]:
[Fe/Mg]5Al2Si3O10(OH)8 + 5CaCO3 + 5CO2 → 5CaFe/Mg2 + Al2Si2O5(OH)4 + SiO2 + 2H2O
Since no kaolinite was observed post-reaction and the calcite content in the sample was low—with Fe2+ and Mg2+ precipitates being detected—the reaction mechanism for chlorite in this study is proposed as:
2Mg3Fe2Al2Si3O10(OH)8 + 14H+ → 4Fe2+ + 3Mg2+ + 4Al(OH)3↓ + 6H4SiO4
- (2)
- Reaction Mechanism of Illite with CO2
The micro-morphological changes of illite are largely related to its occurrence and crystal structure. Illite often appears as hair-like coatings on particle surfaces or as flaky or fibrous fillings in pore spaces. Under high-pressure CO2 solution conditions, the crystal structure of illite is disrupted, with hair-like crystals gradually fracturing into short columnar structures or crystal fragments that mix with the materials in the pore spaces [35]. This process is closely related to the solubility and acidity of CO2, where CO2 dissolved in the solution exchanges with interlayer cations such as K+ and Mg2+ in the illite lattice, leading to dissolution and disruption of the interlayer structure.
In experiments involving rock fragments rich in illite reacting with CO2, the concentrations of Al and Si in the mineral increased slightly, whereas K+ and Mg2+ showed marked decreases, indicating the dissolution of these elements [14]. Morphological observations before and after the reaction reveal that the originally fibrous illite becomes dissolved and fragmented, with the residual illite showing significant structural damage. These changes further confirm that K+ and Mg2+ are leached out during dissolution and that the interlayer structure is severely disrupted.
Shi L. [14] reported that under conditions of 70 °C and 20 MPa, the d(001) peak of illite decreased by approximately 60%, indicating a more pronounced dissolution reaction under high temperature and pressure. This reaction depends not only on the solubility of CO2 but also on temperature, pressure, and other conditions. Different reaction environments significantly affect the dissolution rate and product formation in illite, particularly under acidic conditions where the dissolution process is notably accelerated.
Under acidic conditions, the interlayer K+ in illite is rapidly replaced by H+, causing the disintegration and dissolution of the crystal lattice. In highly acidic environments the exchange reaction for K+ is fast due to its larger ionic radius and its relatively loosely held position within the hexagonal channels of layered minerals. The dissolution of K+ leads to changes in the interlayer spacing of illite, reducing its structural stability and further promoting dissolution [36,37].
In addition, new mineral phases may form during the reaction, especially under highly acidic or high-pressure CO2 conditions. The increased CO2 solubility under high pressure leads to a higher concentration of HCO3− ions, which in turn promotes the formation of carbonates such as siderite (FeCO3) in the system. This behavior is similar to the mechanism observed in chlorite. High pressure enhances the carbonation process by increasing CO2 dissolution and shifting the equilibrium toward carbonate precipitation. In illite, especially after long reaction times, some carbonate precipitation may occur, further altering the ion distribution and mineral composition in the system. Researchers have proposed that under reservoir conditions, illite can react with CO2 according to the following equation:
Illite + H+ → 2.3Al3+ + 0.6K+ + 0.25Mg2+ + 3.5SiO2 + H2O
- (3)
- Reaction Mechanism of Kaolinite with CO2
Kaolinite is a relatively stable aluminum silicate clay mineral. Its crystal structure lacks active interlayer cations, which gives it strong chemical stability under acidic and neutral conditions. In a CO2 solution system, although kaolinite reacts only weakly with acids, under specific conditions—particularly at low pH—it may still undergo dissolution. Study by Guo H. (2016) [38] has shown that under pH conditions ranging from 4.6 to 5.7, the effect of acid on kaolinite was minimal, showing no significant dissolution. This indicates that the acidic environment does not directly compromise the structural stability of kaolinite. However, studies by Shi L. (2019) and Yuan B. (2018) have shown that when reacting with CO2 solutions, the intensity of kaolinite’s characteristic peaks decreased by approximately 26%–36%, suggesting slight acid-induced erosion [14,39]. The reaction can be represented as:
Al2Si2O5(OH)4 + 6H+ ⇌ 2Al3+ + 2SiO2 + 5H2O
This reaction indicates that under strong acidic conditions, kaolinite releases Al3+ more readily than Si. Wu H. (2005) found that when pH < 4, a protective, Si-rich and Al-poor layer may form on the surface of kaolinite, slowing down the release of Al [40]. Moreover, numerical simulations by Liu N. (2018) [41] indicate that with increasing temperature, the dissolution rate of kaolinite increases, making the dissolution process at high temperatures more significant compared to other clay minerals. However, due to the presence of hydrogen bonding in its 1:1 layered structure, CO2 cannot easily penetrate the interlayer, and the silico-aluminate framework remains largely intact. In our experiments, the reaction between kaolinite and CO2 was considered to be weak.
- (4)
- Reaction Mechanism of Montmorillonite with CO2
Montmorillonite, due to its high specific surface area and weak interlayer forces, exhibits a strong cation-exchange capacity and is highly reactive in CO2 solution systems. The interlayer forces in montmorillonite are primarily van der Waals forces, which allow H+ to easily penetrate the (001) plane and initiate reactions. Some studies have indicated that under high-pressure CO2 conditions, the adsorption of wet CO2 can lead to deformation of the montmorillonite structure. Jeon P.R. (2018) conducted kinetic studies on the dissolution reaction and structural changes in a CO2–brine–montmorillonite system at 45–65 °C and 15 MPa, finding that the (001) plane of montmorillonite underwent deformation following dissolution, as indicated by the reaction [42]:
5.92H+ + (Na0.14Ca0.06Mg0.15)(Al1.36Mg0.64)Si4.02O10(OH)2 + 4.08H2O → 0.14Na+ + 0.06Ca2+ + 0.79Mg2+ + 1.36Al3+ + 4.02H4SiO4
Moreover, the reaction mechanism of montmorillonite is complex. Its strong affinity for water leads to hydration and dispersion, and upon hydration, it exhibits a basic character that promotes CO2 dissolution. Various metal cations (such as Na+, K+, Ca2+, Mg2+) can be released from the interlayer via ion exchange, with H+ ions typically replacing them in acidic conditions. CO2 affects the reactivity of silicon and aluminum in the montmorillonite structure differently; the aluminum octahedra are more easily destroyed under acidic conditions, while the silicon tetrahedra are relatively stable. Based on previous studies, the reaction mechanism of montmorillonite in our experiment can be divided into two steps [39,42]:
First step: The interlayer aluminum ions (Al3+) in montmorillonite are replaced by H+, leading to the disintegration of the aluminum octahedra and the subsequent release of silicon from the silicon tetrahedra:
KNaCa(Al,Mg)2(Si,Al)4O10(OH)2 + 14H+ → Na+ + K+ + Ca2+ + 2Mg2+ + 2Al3+ + 2SiO2 + 8H2O
Second step: Under the acidic conditions generated by CO2 dissolution (via the formation of H2CO3 and its decomposition into H+ and HCO3−), the released metal cations (Ca2+, Mg2+, Al3+) react with HCO3− to form precipitates:
Ca2+ + Mg2+ + Al3+ + HCO3− → (Ca, Mg)CO3 + Al(OH)3↓
The reaction of montmorillonite with CO2 is of significant importance for CO2-modified reservoir systems. On one hand, CO2 reduces the montmorillonite content on rock surfaces and in pore spaces through chemical reactions, thereby increasing the pore volume and flow capacity. On the other hand, under weakly acidic conditions, the increase in H+ causes the montmorillonite surface to acquire a positive charge, reducing its cation exchange capacity (CEC). Additionally, the dehydration of H4SiO4 produces SiO2 with a very low CEC, effectively inhibiting the swelling of montmorillonite, which in turn minimizes pore throat constriction and enhances reservoir permeability and stability [42,43,44].
- (5)
- Comparison of Reaction Intensities among Common Clay Minerals
Based on the changes in the characteristic peak intensities of the diffraction curves after 30 days of CO2 injection, the decrease in peak intensities followed the order: chlorite > montmorillonite > illite > kaolinite. Combining the observations from electron microscopy, changes in the reaction solution ion compositions, comprehensive elemental analyses, and the inherent properties of the clay minerals (Table 4), it is evident that under reservoir conditions the clay minerals that undergo significant changes upon reaction with CO2 are primarily montmorillonite, chlorite, and illite, whereas kaolinite exhibits only weak reactions. In reservoir conditions, the reactivity order of the clay minerals is: montmorillonite > chlorite > illite > kaolinite (Figure 9).

Table 4.
Comparison of clay minerals properties [5].
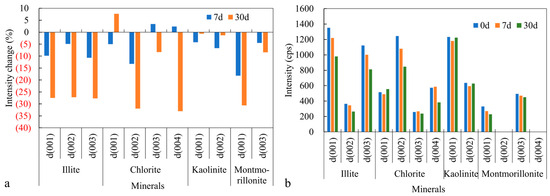
Figure 9.
Clay minerals’ variation in characteristic peak: (a) intensity change; (b) intensity.
Chlorite, which is rich in Mg and Fe, tends to release these metal ions during the reaction, which can then react with CO2 to form carbonate minerals (such as siderite, FeCO3, or dolomite, CaMg(CO3)2) or form new precipitates (e.g., Al(OH)3). These mineral transformations can significantly impact the reservoir pore structure and permeability, particularly the precipitation of siderite. The formation of siderite can block pore spaces, reduce permeability, and impair fluid flow, which may lead to failures in reservoirs, especially over prolonged periods of reaction with CO2 [25]. Montmorillonite exhibits relatively strong reactivity due mainly to its readily exchangeable interlayer cations (Na+, Ca2+), leading to structural deformation and dissolution, and the formation of precipitates such as SiO2 and Al(OH)3, which can affect hydraulic conductivity [39,42]. In contrast, illite reacts more weakly; although its interlayer cation K+ is relatively stable, its crystal surfaces still suffer partial disintegration under supercritical CO2 conditions. Kaolinite is the least reactive among them, largely due to its stable interlayer cations and compact crystal structure.
In actual CO2–rock reactions, it is important to consider the effects of the active cations (such as Na+, Ca2+, K+) released from feldspar dissolution on the overall reaction process. Due to the salting-out effect, higher ion activities can inhibit the release of other ions [45]. Additionally, during the dissolution process, the deposition of Al3+, Fe3+, and SiO2 on rock surfaces can reduce the effective reactive surface area, thereby inhibiting further reactions—especially when SiO2 precipitates in its amorphous form, which can have a more pronounced inhibitory effect [12]. Moreover, colloidal Al(OH)3 and Fe(OH)3, though not easily precipitated under high-pressure production conditions, can adsorb onto rock surfaces and hinder further acid–rock reactions. When the outlet pressure drops, these colloids may precipitate, further impacting the reaction process.
5. Conclusions
This study systematically investigated the reaction behaviors and mechanisms of feldspar and clay minerals in a CO2 environment and analyzed their potential impacts on reservoir pore structures. The main conclusions are as follows:
Feldspar Reaction Mechanism: Both sodium and potassium feldspars undergo dissolution reactions in a CO2 environment, with sodium feldspar dissolving more rapidly due to the easier detachment of Na+ from its structure. The acidic conditions in the CO2 environment accelerate this process. The dissolution of sodium feldspar releases Na+, Al, and Si, whereas the reaction in potassium feldspar is milder, indicating stronger structural stability.
Clay Mineral Reactivity: There are significant differences in the reactivity of different clay minerals. Montmorillonite and chlorite exhibit high reactivity, mainly through ion exchange and crystal structure disruption, leading to dissolution. In chlorite, the dissolution of Fe2+ and Fe3+ may lead to the formation of carbonate minerals (e.g., siderite), while montmorillonite shows pronounced interlayer cation dissolution and precipitation processes. In contrast, illite reacts more weakly, and kaolinite is the least reactive, displaying strong structural stability in a CO2 environment.
Integrated Reaction Mechanisms: The reactivity of CO2 with various minerals is closely related to the crystal structure, cation exchange capacity, and inherent solubility of the minerals. Feldspar dissolution is mainly influenced by the release of alkali cations (Na+, K+), while clay mineral reactions are governed by the interlayer cations and the stability of the crystal structure.
Implications for Geological Carbon Sequestration: This study provides essential data and theoretical support for understanding mineral reactions under CO2 injection and storage conditions. However, the reactions here focus on individual minerals, while in a real reservoir, mineral interactions in the rock matrix could lead to different behavior. The complex multivariate system in a reservoir means that results from single mineral reactions may not directly apply to the entire reservoir. This limitation should be noted, as mineral interactions are crucial for understanding the overall stability and safety of CO2 storage. Optimizing reactions between CO2 and various minerals is important, but considering mineral interactions in the reservoir is key for long-term stability and safety.
Author Contributions
Software, X.D.; Formal analysis, X.Y.; Resources, M.W.; Data curation, D.P.; Writing—original draft, Z.W.; Writing—review & editing, S.L.; Supervision, L.Z.; Project administration, H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the General Project of the Chongqing Natural Science Foundation (Grant No. CSTB2022NSCQ-MXS1642) and the Joint Fund for Innovation and Development of the Chongqing Natural Science Foundation (Grant No. CSTB2023NSCQ-LZX0078), and was also supported by the Science and Technology Research Program of the Chongqing Municipal Education Commission (Grant No. KJQN202401535).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all editors and anonymous reviewers for their helpful comments and suggestions.
Conflicts of Interest
Author Dongyu Peng was employed by the company Zhanjiang Branch of CNOOC (China). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2019, 21, 241–258. [Google Scholar] [CrossRef]
- Cai, B.; Pang, L.; Cao, L.; Li, Q.; Liu, G.; Zhong, P.; Zhang, X.; Yang, Y.; Fan, C.; Li, Q.; et al. Two-year implementation assessment (2016–2018) of China’s technical guideline on environmental risk assessment for carbon dioxide capture, utilization and storage (on trial). Environ. Eng. 2019, 37, 1–7. [Google Scholar]
- Guo, P.; Sun, L.; Sun, L.; Li, S.; Peng, P.; Yue, L. Influences of injection gas on physical behavior of crude. J. Southwest Pet. Inst. 2000, 22, 57–60. [Google Scholar]
- Wang, T.; Yao, Y.; Li, X.; Li, H.; Shi, J.; Yang, Z. Factors affecting and analysis of CO2 flooding effectiveness. China Pet. Chem. Ind. 2008, 24, 30–33. [Google Scholar]
- Wang, M.; He, J.; Liu, S.; Zeng, C.; Jia, S.; Nie, Z.; Wang, S.; Wang, W.; Zhang, C. Effect of Sedimentary Facies Characteristics on Deep Shale Gas Desserts: A Case from the Longmaxi Formation, South Sichuan Basin, China. Minerals 2023, 13, 476. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Wanniarachchi, W.A.M.; Yang, S.Q.; Lashin, A.; Al Arifi, N. An experimental investigation of coupled chemico-mineralogical and mechanical changes in varyingly-cemented sandstones upon CO2 injection in deep saline aquifer environments. Energy 2017, 133, 404–414. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Cheng, Y.; Yu, X.; Duan, X.; Kang, Z.; Xiong, Y. Fractal insights into permeability control by pore structure in tight sandstone reservoirs, Heshui area, Ordos Basin. Open Geosci. 2025, 17, 20250791. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y.; Enechukwu, C. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2–water–rock interactions and their effects. J. CO2 Util. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Hitchon, B. Aquifer Disposal of Carbon Dioxide: Hydrodynamic and Mineral Trapping-Proof of Concept; Geoscience Publishing Ltd.: London, UK, 1996. [Google Scholar]
- Gunter, W.; Wong, S.; Gentzis, T. Field-testing CO2 sequestration and enhanced coalbed methane recovery in Alberta, Canada-Historical perspective and future plans. Abstr. Pap. Am. Chem. Soc. 2000, 220, U396. [Google Scholar]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT—A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.Z.; Turcu, R.V.F.; Rosso, K.M.; Ilton, E.S.; Wang, C.; Sears, J.A.; Engelhard, M.H.; Felmy, A.R.; Hoyt, D.W. The role of H2O in the carbonation of forsterite in supercritical CO2. Int. J. Greenh. Gas Control. 2011, 5, 1081–1092. [Google Scholar] [CrossRef]
- Loring, J.S.; Thompson, C.J.; Zhang, C.; Wang, Z.; Schaef, H.T.; Rosso, K.M. In Situ Infrared Spectroscopic Study of Brucite Carbonation in Dry to Water-Saturated Supercritical Carbon Dioxide. J. Phys. Chem. A 2012, 116, 4768–4777. [Google Scholar] [CrossRef]
- Shi, L.; Hu, H.; Zhang, Y.; Gao, Y.; Zhang, J.; Zhang, H.; Wang, L. Interaction of tight glutenite mineral with supercritical CO2 and formation water. Oilfield Chem. 2019, 36, 640–645. [Google Scholar]
- Luo, Y.; Xiao, M.; Cheng, J.; Yu, Y.; Xu, F.; Zhao, R.; Ahmat, K. Experimental study on the interaction of Sc-CO2 with feldspar and clay minerals: Implication for carbon sequestration in sandstone reservoirs. Gas Sci. Eng. 2025, 139, 205639. [Google Scholar] [CrossRef]
- Wang, M.; Tang, H.; Zhao, F.; Liu, S.; Yang, Y.; Zhang, L.; Liao, J.; Lu, H. Controlling factor analysis and prediction of the quality of tight sandstone reservoirs: A case study of the He8 Member in the eastern Sulige Gas Field, Ordos Basin, China. J. Nat. Gas Sci. Eng. 2017, 46, 680–698. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, T.; Sun, H.; Zhang, X.; Zhao, D. Dissolution process and mechanism of montmorillonite in oxalic acid and sulfuric acid media at various pH levels. Appl. Clay Sci. 2024, 261, 107573. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y.; et al. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Yanzhong, W.; Nianmin, Z.; Xu, C.; Yingchang, C.; Guanghui, Y.; Gluyas, J.G.; Miruo, L. Geologic CO2 storage in arkosic sandstones with CaCl2-rich formation water. Chem. Geol. 2020, 558, 119867. [Google Scholar] [CrossRef]
- Gianni, E.; Tyrologou, P.; Behnous, D.; Pál Farkas, M.; Fernández-Canteli Álvarez, P.; García Crespo, J.; Chacartegui Ramirez, R.; Koukouzas, N.; Carneiro, J. CO2 sequestration potential in Depleted Hydrocarbon fields? A geochemical approach [version 2; peer review: 2 approved]. Open Res. Eur. 2025, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Strand, S.; Puntervold, T.; Mamonov, A.; Piñerez Torrijos, I.D.; Khan, M.A.I. Geochemical effects of carbonated water on reservoir and caprock minerals for carbon capture and storage. Gas Sci. Eng. 2024, 124, 205246. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Li, R.; Gao, L. Effects of pH on the solubility of the feldspar and the development of secondary porosity. Bull. Mineral. Petrol. Geochem. 2001, 20, 103–107. [Google Scholar]
- Chi, E.; Lan, B.; Xiao, Y. Impact of temperature and CO2 in solution on feldspar solubility. J. Water Resour. Water Eng. 2014, 25, 230–232. [Google Scholar]
- Jiao, C.; Xiao, J.; Pi, J.; Sun, X. Application of PHREEQC in simulation of groundwater chemical formation in geothermal field of Tangshi. Miner. Eng. Res. 2018, 33, 49–53. [Google Scholar]
- Watson, M.N.; Zwingmann, N.; Lemon, N.M. The Ladbroke Grove–Katnook carbon dioxide natural laboratory: A recent CO2 accumulation in a lithic sandstone reservoir. Energy 2004, 29, 1457–1466. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control. 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Ryzhenko, B.N. Genesis of dawsonite mineralization: Thermodynamic analysis and alternatives. Geochem. Int. 2006, 44, 835–840. [Google Scholar] [CrossRef]
- Tang, H.; Meng, Y.; Li, G.; Yang, X.; Yan, R. Study on stability of feldspar in acidization system. Nat. Gas Ind. 2004, 24, 116–118. [Google Scholar]
- Zhao, T.; Yan, Z.; Zhang, J.; Wang, Y.; Jiang, L. Analysis of the effects of temperature and CO2 on feldspar solubility based on PHREEQC simulation. Light Ind. Sci. Technol. 2016, 32, 84–86. [Google Scholar]
- Fischer, S.; Liebscher, A.; Wandrey, M. CO2–brine–rock interaction—First results of long-term exposure experiments at in situ P–T conditions of the Ketzin CO2 reservoir. Geochemistry 2010, 70, 155–164. [Google Scholar] [CrossRef]
- Wandrey, M.; Fischer, S.; Zemke, K.; Liebscher, A.; Scherf, A.-K.; Vieth-Hillebrand, A.; Zettlitzer, M.; Würdemann, H. Monitoring petrophysical, mineralogical, geochemical and microbiological effects of CO2 exposure—Results of long-term experiments under in situ conditions. Energy Procedia 2011, 4, 3644–3650. [Google Scholar] [CrossRef][Green Version]
- Pearce, J.K.; Dawson, G.K.W.; Golab, A.; Knuefing, L.; Sommacal, S.; Rudolph, V.; Golding, S.D. A combined geochemical and μCT study on the CO2 reactivity of Surat Basin reservoir and cap-rock cores: Porosity changes, mineral dissolution and fines migration. Int. J. Greenh. Gas Control. 2019, 80, 10–24. [Google Scholar] [CrossRef]
- Cai, D. Study on Interaction Between CO2 and Water/Rock Minerals in Tight Glutenite Reservoirs. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2018. [Google Scholar]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental investigation of CO2–brine–rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Tang, H.; Tang, H.; He, J.; Zhao, F.; Zhang, L.; Liao, J.; Wang, Q.; Yuan, X. Damage mechanism of water-based fracturing fluid to tight sandstone gas reservoirs: Improvement of The Evaluation Measurement for Properties of Water-based Fracturing Fluid: SY/T 5107-2016. Nat. Gas Ind. 2020, 40, 55–63. [Google Scholar] [CrossRef]
- Xing, X.; Tang, H.; Zhao, F.; Li, G.; Xie, X. Reaction experiment of illite with mud acid and fluorboric. J. Southwest Pet. Univ. 2007, 29, 29–31. [Google Scholar]
- Ni, X.; Yu, Y.; Wang, Y.; Gao, S. Dissolution kinetics of Si/Al elements of illites in carbonic aicd solutions. Nat. Gas Ind. 2014, 34, 20–26. [Google Scholar]
- Guo, H.; Wang, Y.; Ni, X.; Wang, J.; Han, W. Digestion kinetics analysis of silicon and aluminum during kaolinite-water-CO2 interaction. J. China Univ. Min. Technol. 2016, 45, 591–596. [Google Scholar]
- Yuan, B.; Wood, D.A. Formation Damage During Improved Oil Recovery: Fundamentals and Applications; Gulf Professional Publishing: Houston, TX, USA, 2018. [Google Scholar]
- Wu, H.; Liu, P.; Gao, S.; He, G.; Wang, W. Characterization of interfacial reactions at kaolinite/water. Geochimica 2005, 410–416. [Google Scholar]
- Liu, N. Study on Fluids Transportation and Water-Rock Interactions of CO2 Geological Storage in Sandstone Reservoirs in Continental Sedimentary Basins. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2018. [Google Scholar]
- Jeon, P.R.; Kim, D.-W.; Lee, C.-H. Dissolution and reaction in a CO2-brine-clay mineral particle system under geological CO2 sequestration from subcritical to supercritical conditions. Chem. Eng. J. 2018, 347, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Li, X. Effect of fluoboric acid and mud acid on clay swelling property. Oil Drill. Prod. Technol. 1995, 17, 56–60. [Google Scholar]
- Wang, D.; Guo, J.; Wang, F.; Zhang, H. Acidification effects on the composition and structure of montmorillonite. Acta Miner. Sin. 1998, 18, 189–193. [Google Scholar]
- Stone, T.; Boon, J.; Bird, G.W. Modelling Silica Transport in Large-Scale Laboratory Experiments. J. Can. Pet. Technol. 1986, 25, PETSOC-86-01-06. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).