Abstract
This study investigates the adsorption behavior of natural sepiolite for the removal of cadmium (Cd2+), copper (Cu2+), and nickel (Ni2+) ions from aqueous solutions under batch conditions. The sepiolite was extensively characterized prior to adsorption experiments. Mineralogical analysis confirmed the presence of crystalline sepiolite, while DTG-TGA revealed thermal stability with distinct weight loss linked to surface and structural water. BET analysis indicated a high surface area of 194 m2/g and a mesoporous structure favorable for adsorption. Batch experiments evaluated the effects of contact time, pH, adsorbent dosage, and initial metal concentration. Adsorption was highly pH-dependent, with maximum removal near-neutral pH values. Higher adsorbent dosages reduced in a lower adsorption capacity per unit mass, primarily because the fixed amount of solute was distributed over a larger number of available sites, leading to unsaturation of the adsorbent surface and possible particle agglomeration. Isotherm modeling revealed that the Langmuir model provided the best fit, indicating monolayer adsorption with maximum adsorption capacities of 15.95 mg/g for Cd(II), 37.31 mg/g for Cu(II), and 17.83 mg/g for Ni(II). Langmuir constants indicated favorable interactions. Kinetics showed rapid adsorption within the first hour, reaching equilibrium at 240 min through surface adsorption and intraparticle diffusion. Cu(II) exhibited the fastest uptake, while Ni(II) adsorbed more slowly, suggesting differences in diffusion rates among the metal ions. Desorption using 0.1 N HCl achieved over 80% efficiency for all metals, confirming sepiolite reusability. Overall, raw sepiolite is an effective, low-cost adsorbent for removing potentially toxic elements from water.
1. Introduction
The persistent contamination of water bodies by potentially toxic elements such as cadmium (Cd2+), copper (Cu2+), and nickel (Ni2+) represents a critical environmental and public health challenge. These metals are non-biodegradable, toxic even at low concentrations, and capable of bioaccumulating in aquatic ecosystems and food chains [1]. Cadmium is highly toxic and non-essential to biological systems. Chronic exposure has been linked to kidney dysfunction, skeletal damage, and carcinogenic effects. Its concentration in unpolluted natural waters is generally <1 µg/L, but values exceeding 10 µg/L have been reported in contaminated sources [2]. Copper, while essential in trace amounts, can be toxic at higher concentrations, particularly to aquatic organisms. Natural background levels typically range from 1–10 µg/L, but industrial effluents can raise concentrations to several hundred µg/L [3]. Nickel, another transition metal, is known to induce dermatological and respiratory disorders upon prolonged exposure; it is usually found at levels below 10 µg/L in natural waters but can reach higher values near industrial discharges [4]. According to the World Health Organization (WHO), the recommended guideline limits for drinking water are 3 µg/L for Cd, 2 mg/L for Cu, and 0.02 mg/L for Ni.
Conventional water treatment methods, including chemical precipitation, membrane filtration, and ion exchange, often involve high operational costs and generate secondary waste [3]. In contrast, adsorption has gained prominence due to its simplicity, cost-effectiveness, and high efficiency [5], especially when low-cost natural materials are employed as adsorbents [6,7,8,9,10,11]. Clay minerals, abundant, chemically stable, and rich in surface functional groups, have gained attention as cost-effective, efficient, and environmentally friendly alternatives for heavy metal removal from solution through ion exchange, surface complexation, and electrostatic interactions [12,13].
Sepiolite is a naturally occurring hydrated magnesium silicate clay with the idealized formula Mg8Si12O30(OH)4·8H2O. It is a fibrous trioctahedral clay [14] characterized by a unique crystalline structure that imparts its distinctive morphology. The mineral’s structure, first described by Brauner and Preisinger [15], consists of continuous sheets of tetrahedral silica, in which an apical oxygen is periodically inverted. This inversion occurs every three tetrahedral chains along the [001] crystallographic direction, resulting in a ribbon-like architecture. These tetrahedral sheets are bonded to octahedral sheets, which are continuous and elongated in the [001] direction but discontinuous in the [010] direction. The periodic inversion of apical oxygen atoms in the tetrahedral sheets causes discontinuities in the octahedral sheets, leading to the formation of structural channels that run parallel to the c-axis of the crystal aligned with the direction of the fiber itself [16]. This unique arrangement creates a porous framework providing a large surface area, high sorption capacity, and the presence of both external silanol (Si-OH) groups and intra-crystalline channels that facilitate diffusion and binding of contaminants [6]. In recent years, a distinction has been made between “channels,” referring to surface-accessible voids with an open face, and “tunnels,” which describe the internal, enclosed voids within the crystal structure. The tunnels typically exhibit cross-sectional dimensions of approximately 4 × 11 Å2 [17]. Compared to other clay minerals such as bentonite, kaolinite, or montmorillonite, sepiolite exhibits unique textural and surface chemical properties that make it especially effective for adsorbing toxic metals from aqueous systems [18,19,20,21,22].
Over the last decades, numerous studies have demonstrated the effectiveness of natural and modified sepiolite in removing toxic metals. For instance, Padilla-Ortega et al. [22] found natural sepiolite capable of removing over 90% of Pb2+ and Cd2+ from aqueous solutions under optimized conditions. Hojati and Khademi [23] investigated the adsorption of Cd2+ using Iranian sepiolite and reported a maximum uptake of 19.2 mg/g. Similarly, sepiolite used in metallurgical wastewater [24] showed strong performance, achieving capacities of 85.5 mg/g for Pb2+, 62.4 mg/g for Cd2+, 13.05 mg/g for Cu2+, and 15.7 mg/g for Zn, with optimal performance at pH 6 and equilibrium achieved in 45 min. Moreover, Doğan et al. [20] reported significant uptake of Cu2+ and Zn2+ by acid-activated and organo-modified sepiolite, highlighting the role of surface area enhancement and functional group availability. Adsorption mechanisms proposed in such studies typically involve complexation with silanol groups, electrostatic attraction to negatively charged surfaces, and cation exchange with Mg2+ present in the sepiolite lattice.
Comparative studies with other clay minerals also show sepiolite to be highly competitive. For example, Jiang et al. [25] observed that natural kaolinite exhibited a selectivity order of Cu > Cd > Ni, closely paralleling trends seen with sepiolite, suggesting that both ionic radius and hydration energy play key roles in selectivity. A recent study by El-Rayyes et al. [26] further confirmed the applicability of natural clay minerals, including sepiolite, for reusable treatment systems; their study showed that metal ions such as arsenic could be efficiently desorbed using mild acidic solutions without significant loss in capacity, underscoring the material’s regenerative potential.
Other investigations have expanded the range of contaminants studied. Sabah and Ouki [27] demonstrated that raw sepiolite also effectively adsorbs organic pollutants such as pyrene (a polycyclic aromatic hydrocarbon), indicating its broader applicability beyond metal ions. More recently, Li et al. [28] showed that raw sepiolite could remove up to 94.8% of graphene oxide (GO) from water under acidic conditions (pH 3), following pseudo-second-order kinetics and fitting Langmuir and Temkin isotherm models. Overall, these studies confirm that raw sepiolite performs effectively in the removal of both inorganic and organic pollutants from aqueous systems, especially when optimized for pH, contact time, and dosage.
Although sepiolite has been extensively studied as an adsorbent, its adsorption performance strongly depends on the specific physicochemical and mineralogical characteristics of each natural deposit. Consequently, systematic evaluation of its adsorption–desorption behavior towards Cd(II), Cu(II), and Ni(II) under identical conditions remains limited. Moreover, few studies have correlated these intrinsic material properties with adsorption and desorption efficiency. In view of the growing demand for low-cost, high-efficiency adsorbents and the favorable properties of sepiolite, the current provides new insights into the adsorption and regeneration behavior of natural sepiolite under batch conditions. This work includes a detailed evaluation of adsorption kinetics and isotherm models, as well as regeneration performance, with the goal of better understanding the mechanisms governing heavy metal uptake and assessing the potential of sepiolite for practical water treatment applications.
2. Materials and Methods
2.1. Characterization of Raw Sepiolite
The sepiolite used in this study was a natural, raw clay sample sourced from Spain. The sample was air dried, ground, and sieved to obtain the <63 μm size fraction to ensure homogeneity and facilitate subsequent analysis. Mineralogical composition was determined by X-ray powder diffraction (XRD) using a Philips PW1710 diffractometer (Philips Analytical, Eindhoven, The Netherlands) with Ni-filtered CuKa radiation, operating at 35 kV and 25 mA, in the range of 3–63° 2θ at a scan speed of 1.2°/min. Samples were analyzed in both randomly oriented, oriented, and ethylene glycolated mounts. The bulk chemical composition of the sepiolite was determined at ACME Analytical Laboratories (Vancouver, BC, Canada) using a standard whole rock analysis package. The analytical procedure involved lithium metaborate/tetraborate fusion followed by ICP-ES (inductively coupled plasma–emission spectrometry) and ICP-MS (mass spectrometry) for high-precision quantification. Thermal behavior was analyzed through simultaneous differential thermal analysis (DTA) and thermogravimetric analysis (TG) using a TA Instruments SD 2960 analyzer (TA Instruments, Inc., New Castle, DE, USA). Approximately 15 ± 2 mg of powdered sample was placed in an alumina crucible, with a second, empty crucible used as a reference. All measurements were conducted under a nitrogen atmosphere at a constant heating rate of 10 °C/min, covering a temperature range of 25–1000 °C.
Surface area measurements were performed using nitrogen adsorption at 77 K with an Autosorb system (Quantachrome Instruments, Boynton Beach, FL, USA). The specific surface area (SBET) was calculated from the initial portion of the nitrogen adsorption isotherm, specifically within the relative pressure range P/P0 < 0.5. High-purity nitrogen gas (99.99%) was employed throughout the analysis to ensure accuracy and consistency. The cation exchange capacity (CEC) of the sepiolite, expressed in mmol/100 g, was determined following the procedure of Alexiades and Jackson [29] briefly described in Bourliva et al. [10].
2.2. Batch Adsorption Experiments
Adsorption experiments were carried out by introducing a pre-weighed amount of sepiolite into 100 mL of aqueous metal ion solution (Cd2+, Cu2+, and Ni2+) and agitating the mixture on a vertical rotary shaker at a speed of 100 rpm for predetermined time intervals to ensure homogeneous mixing and effective contact between the adsorbent and the solution. Based on the kinetic study, an equilibrium contact time of 240 min was established and used for all subsequent equilibrium experiments, including the pH, adsorbent dose, and isotherm analyses. Upon completion of the contact time, the suspensions were centrifuged at 3000 rpm for 15 min to separate the solid and liquid phases. The concentrations of Cd2+, Cu2+, and Ni2+ before and after adsorption were measured using flame atomic absorption spectrometry (FAAS) with a Perkin Elmer 503 instrument (PerkinElmer, Inc., Shelton, CT, USA). All measurements were performed in duplicate, and the average values are reported with an estimated relative error of approximately 5%.
Control experiments were conducted in parallel: one without the adsorbent and another without the metal ion, to ensure the reliability of the adsorption results. The effect of various parameters such as solution pH, adsorbent dosage, initial Cd2+, Cu2+, Ni2+ concentration, and contact time was systematically investigated. To adjust the pH to the desired level, either 0.01 M NaOH or 0.01 M HNO3 was added dropwise, with the pH monitored both prior to and following the adsorption process. Experimental conditions were selected to closely mimic the chemical environment of typical industrial wastewater. The experimental parameters maintained across the various sets of experiments are summarized in Table S1.
The amount, qe, of the metal ions adsorbed per unit mass of sepiolite and the extent of removal (%) were calculated using the following equations:
where Co is the initial metal concentration in the solution, Ce is the metal concentration in the solution at equilibrium (mg/L), m is the sepiolite mass (g), and V is the solution volume (L).
2.3. Regeneration Tests
The potential of sepiolite to be reused was examined via recycling the Cd(II)-, Cu(II)-, and Ni(II)-adsorbent load by using different eluting agents. After the adsorption process, distilled water, and 0.1 M HCl were used as eluting agents to wash the Cd(II)-, Cu(II)-, and Ni(II)-adsorbent load and the metal concentration desorbed was evaluated by FAAS. The desorption percentage (%D) was computed using the equation:
where D represent the desorption percentage (%), AD denote metal amount desorbed, and AA the metal amount adsorbed.
2.4. Adsorption Isotherms and Kinetic Models
The equilibrium data were analyzed using three widely applied isotherm models: Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) models.
The Langmuir isotherm assumes monolayer adsorption onto a surface with finite identical sites [30] and is expressed as:
where qe (mg/g) is the amount of metal adsorbed at equilibrium, Ce (mg/L) is the equilibrium concentration, Qm (mg/g) is the maximum monolayer adsorption capacity, and b (L/mg) is the Langmuir constant related to adsorption energy. To assess the favorability of the adsorption process, the dimensionless separation factor (RL) was calculated as:
where Co is the initial metal ion concentration (mg/L). An RL value between 0 and 1 indicates favorable adsorption, RL = 1 suggests linear adsorption, RL > 1 implies unfavorable adsorption, and RL = 0 reflects irreversible adsorption.
The Freundlich isotherm [31] describes heterogeneous surface adsorption and is expresses by equation:
where Kf (mg1−n Ln g−1) and n (dimensionless) are empirical constants indicative of adsorption capacity and intensity, respectively.
The Dubinin-Radushkevich (D-R) isotherm provides insight into the adsorption mechanism and energy and is expressed as [32]:
where qm (mg/g) is the theoretical D-R monolayer saturation capacity, B (mol2/kJ2) is related to the mean free energy of adsorption, and ε is the Polanyi potential calculated as follows:
where R is the gas constant (8.314 J/mol·K) and T is the temperature (K). The constant B gives the mean adsorption energy, E, of sorption per molecule of the adsorbate when it is transferred to the surface of the solid from infinity in the solution and can be computed using the relationship:
The removal mechanism occurs basically via physical interaction when E < 8 kJ/mol, and via ion-exchange if 8 < E < 16 kJ/mol [33,34].
To evaluate the adsorption kinetics, three models were applied to time-dependent data: the pseudo-first-order (PFO), pseudo-second-order (PSO), and Weber–Morris intraparticle diffusion (IPD) models. The pseudo-first-order model [35,36] is represented as:
where qt (mg/g) is the adsorbed amount at time t (min), qe is the equilibrium uptake, and k1 (1/min) is the rate constant.
The pseudo-second-order model assumes chemisorption as the rate-limiting step [37,38] and is described as follows:
where k2 (g/mg·min) is the rate constant of second-order adsorption.
The intraparticle diffusion model proposed by Weber and Morris [39] was also applied to assess diffusion mechanisms:
where kid (mg/g·min0.5) is the intraparticle diffusion rate constant and C (mg/g) indicates boundary layer thickness. When intra-particle diffusion plays a significant role in controlling the kinetics of the adsorption processes, the plots of qt vs. t0.5 yield straight lines passing through the origin and the slope gives the rate constant kid. When pore diffusion limits the adsorption process, the relationship between the initial solute concentration and the rate of adsorption is not linear [40]. Multi-linearity in the intraparticle plots was interpreted to reflect multiple diffusion stages, including external surface adsorption and internal pore diffusion. Adsorption isotherm and kinetic models’ parameters were obtained by linear regression, and correlation coefficients (R2) were used to assess the best fit.
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. Mineralogical Characterization of Raw Sepiolite
XRD study verified that the predominant mineral constituent of the raw adsorbent material was a well crystallized sepiolite, as identified by the characteristic diffraction peak at d110 = 12.25 Å shown in Figure 1a. The average crystallite size of sepiolite was approximately 9.24 nm, indicating a nanocrystalline structure consistent with its fibrous morphology.
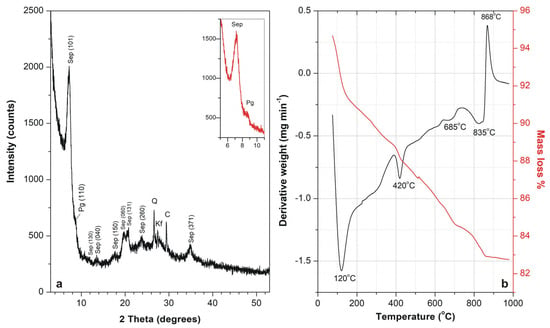
Figure 1.
X-ray diffraction pattern (a) and Differential Thermal Analysis (DTA) and Thermogravimetric analysis (TG) curves (b) of sepiolite. Sep: Sepiolite, Pg: Palygorskite, Q: Quartz, Kf: K-Feldspars, C: Calcite.
The basal spacing of the (110) reflection was slightly higher than typical literature values at 12.01 Å and 11.96 Å reported by Post et al. [41] and Sánchez del Rio et al. [42], respectively. This peak shifting is typical due to the presence of palygorskite-type polysomes [43,44]. The coexistence of palygorskite-type domains within the sepiolite matrix was detected by the presence of a distinct shoulder at ~10.4 Å adjacent to the main (110) reflection in the XRD pattern (Figure 1a). This is indicative of polysomatic intergrowths, a phenomenon commonly observed in natural deposits, where alternating structural modules of sepiolite and palygorskite lead to characteristic broadening or asymmetry in the basal reflection [45]. Moreover, upon ethylene glycol treatment, the XRD pattern showed distinct reflections at 12.35 Å and ~10.3 Å, verifying the coexistence of sepiolite and palygorskite. Quartz (d101 = 3.34 Å), calcite (d104 = 3.03 Å), and K-feldspars (d002 = 3.24 Å) were also identified in small amounts.
3.1.2. Chemical Composition of Raw Sepiolite
The chemical composition of the natural clay sample was determined and the results are summarized in Table 1. The dominant oxides were SiO2 (51.37%) and MgO (20.32%), consistent with the typical composition of sepiolite. A SiO2/MgO weight ratio of approximately 2.53 was calculated, which aligns closely with the theoretical values of 2.23, calculated from the chemical formula of sepiolite [46]. When converted to molar proportions, the sample yields a Si/Mg molar ratio of 1.56, very close to the theoretical value of 1.5 (Si:Mg = 6:4), further confirming the predominance of sepiolite as the main mineral phase. The relatively higher Al2O3 (7.34%) content in our sample than in typical sepiolite, is attributed to the presence of a minor aluminous clay phase, such as palygorskite as verified by XRD patterns. Trace quantities of CaO (1.21%) reinforces the presence of carbonate impurities, such as calcite, which is frequently associated with natural sepiolite beds.

Table 1.
Physicochemical data of sepiolite used in this study.
3.1.3. DTG-TA Analysis of Raw Sepiolite
Thermal behavior of the sepiolite sample was examined and the results are shown in Figure 1b. The DTA curve displays distinct endothermic and exothermic events. The first, a pronounced endothermic peak near 110 °C, corresponds to the loss of hygroscopic and zeolitic water located in the outer surfaces and trapped in the channels of the sepiolite structure. The second sharp endothermic effect, observed around 400 °C, is attributed to the removal of half the coordinated (bound) water associated with structural Mg2+ ions. The other half of Mg-coordinated water remains in the collapsed channels up to ~670 °C when it removes [47]. In the high temperature zones, there is a very pronounced endothermic effect at 800–825 °C due to the dehydroxylation of sepiolite, marking the loss of octahedral OH and probably the loss of OH of some edge SiOH [48]. This effect is accompanied by an additional sharp, strong exothermic one at near 865 °C corresponding to crystallization of enstatite (MgSiO3) or other reorganization of the silicate framework upon heating. The TG curve (red line) reveals a progressive mass loss of approximately 12% which is attributed to the release of structurally and physically bound water molecules.
3.1.4. Specific Surface Area and CEC of Raw Sepiolite
The textural properties of the natural sepiolite were examined using nitrogen adsorption–desorption analysis at 77 K (Figure S1). The isotherm displayed type IV behavior with a distinct hysteresis loop, indicating the presence of pores formed by parallel plates. The sepiolite sample exhibited a specific surface area SBET = 194.8 m2/g (Table 1), which is in line with the textural characteristics of sepiolite reported in previous studies [19,49]. For instance, Suárez et al. [45] reported surface areas ranging from 122 to 376 m2/g for raw Turkish sepiolites, while Sheikhhosseini et al. [50] found values of 102 m2/g for natural sepiolite used in heavy metal removal. Also, the obtained SBET value was within the range of values reported in the literature for sepiolite samples from the deposits of Spain which are typically between 189 m2/g [51] and 340 m2/g [52]. According to the BJH (Barrett–Joyner–Halenda) method, the total pore volume was 0.39 cm3/g, with a BET average pore diameter of 8.12 nm (Table 1). These values reflect the tubular mesostructure of sepiolite, which consists of parallel channels formed by the inversion of silica ribbons, facilitating a high degree of porosity and accessibility for adsorbates. The CEC value of the sepiolite was 58.7 mmol/100 g (Table 1). Several authors have determined the CEC of sepiolite and reported values within the range from 9.5 mmol/100 g to 60 mmol/100 g [6,19].
3.2. Adsorption Studies
3.2.1. Effect of pH
The solution pH plays a pivotal role in determining the efficiency of heavy metal adsorption by influencing both the ionization state of the adsorbate and the surface charge of the adsorbent. The adsorption behavior of Cd(II), Cu(II), and Ni(II) onto sepiolite was examined over a pH range of 3 to 8, and the results are depicted in Figure 2.
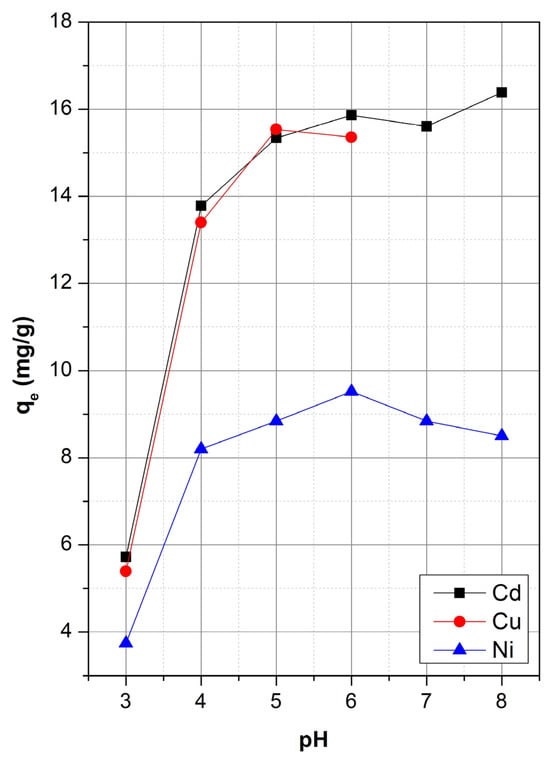
Figure 2.
Effect of pH on the adsorption of Cd2+, Cu2+, and Ni2+ onto sepiolite.
A consistent trend was observed for all three metal ions with adsorption capacity increasing with increasing pH, particularly between pH 3 and pH 6. At low pH (pH = 3), adsorption capacities were relatively low across all metals, with Cd(II), Cu(II), and Ni(II) showing qe values of 5.7 mg/g, 5.4 mg/g, and 3.7 mg/g, respectively. This reduced uptake is likely ascribed to the elevated contents of H+ ions in the solution competing with metal cations for active adsorption sites on the sepiolite surface [53]. As the pH increased to 6, metal uptake improved markedly, indicating reduced competition from protons and increased availability of negatively charged functional groups (e.g., Si–O−) on the sepiolite surface. The surface charge of sepiolite plays a crucial role in governing its interaction with metal cations. The point of zero charge (pHPZC) of natural sepiolite has been reported in the range of 6.5–7.2 [20,43]. Below this pH, the sepiolite surface carries a net positive charge, resulting in electrostatic repulsion with cationic metal species. As the solution pH approaches and exceeds the pHPZC deprotonation of surface hydroxyl groups (≡Si–OH) leads to the formation of negatively charged ≡Si–O− sites, which promote the adsorption of divalent metal ions through electrostatic attraction and surface complexation. At pH = 6 adsorption capacity for Cd(II) and Cu(II) were observed to be 15.8 mg/g and 15.3 mg/g, respectively. For Ni(II), adsorption capacity also peaked at pH 6 (9.5 mg g−1), though its overall uptake was consistently lower than that of Cd(II) and Cu(II) across the pH range.
For Cd(II) and Ni(II), which remained in soluble form in pH values beyond 6, allowing these to be studied over a broader pH range, adsorption capacities began to level off (plateau) or slightly decline. Specifically, for Cd(II), a small increase to 16.38% was observed at pH 8, suggesting minor enhancement due to more negatively charged sites, while Ni(II) showed no significant further improvement but some reduction in Ni(II) uptake. The decline or plateau at higher pH values may be attributed to the onset of metal hydroxide precipitation (e.g., Cd(OH)2, Ni(OH)2), which competes with adsorption and complicates accurate assessment of surface interactions. The speciation behavior of Cd(II), Cu(II), and Ni(II) as a function of pH is illustrated in the speciation diagrams given in Figure S2. These metals predominantly exist as free divalent cations (M2+) under acidic to near-neutral conditions (pH < 6), facilitating electrostatic attraction to the negatively charged sepiolite surface. As the pH increases, hydrolysis reactions become significant, leading to the gradual formation of soluble metal-hydroxide complexes (Figure S2).
These results confirm that the adsorption of potentially toxic elements onto sepiolite is highly pH-dependent which is consistent with prior literature [22,26]. Optimal adsorption of divalent metal ions on clay-based materials typically occurs in slightly acidic to neutral conditions [10,54]. At this pH range, the surface of sepiolite becomes increasingly negative, enhancing electrostatic attraction with positively charged metal ions, while still avoiding precipitation artifacts.
3.2.2. Effect of Adsorbent Dosage
The influence of adsorbent dosage on the adsorption capacity (qe, mg/g) of sepiolite for Cd(II), Cu(II), and Ni(II) ions was investigated across a range of adsorbent concentrations (1–10 g/L). The results, illustrated in Figure 3, demonstrate a decrease in the adsorption capacity per unit mass of sepiolite (qe, mg/g) as the adsorbent dosage increased. At lower adsorbent dosages (1 g/L), metal uptake per gram of sepiolite was significantly higher, with maximum qe values of approximately 13.4 mg/g for Cd, 17.4 mg/g for Cu, and 9.9 mg/g for Ni. However, as the adsorbent concentration increased, the adsorption capacity decreased sharply. The decline in qe with increasing dose can be attributed to the “adsorbent overdose effect,” where the relative availability of active sites surpasses the number of metal ions present in the solution [32]. Additionally, agglomeration of adsorbent particles at higher dosages may lead to a reduction in effective surface area and active site accessibility, further lowering adsorption efficiency [55].
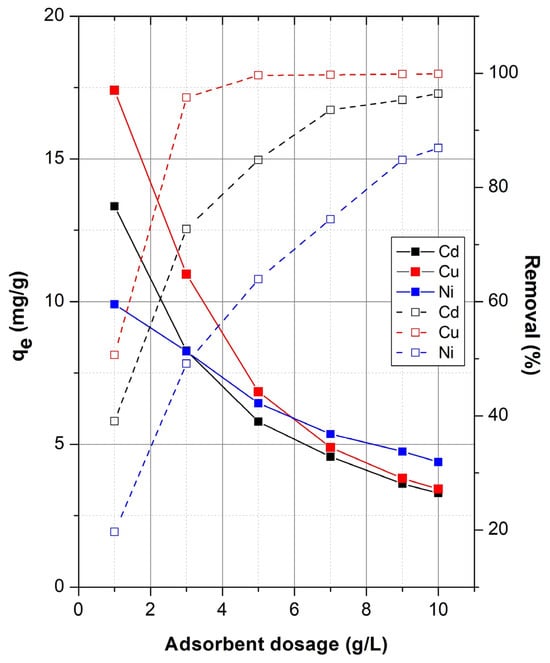
Figure 3.
Effect of adsorbent dosage on the adsorption of Cd2+, Cu2+, and Ni2+ onto sepiolite.
This is further reinforced by the fact that the clear inverse relationship between adsorbent dose and adsorption capacity per unit mass follows a power-law trend with high correlation coefficients (R2 > 0.93) in compliance to the relationship qe = m (dose)−n [56]. The negative n values (Figure 3) suggest that the adsorbed metal species may reduce the number of available active sites for adsorption by obstructing access to the interior pores or by causing particles to agglomerate. Among the metals studied, Cu2+ showed the highest initial adsorption capacity, while Ni2+ exhibited the least sensitivity to increasing dosage, as reflected by the shallower slope of its regression curve. The higher decay rate observed for Cd2+ (n = −0.719) suggests that its adsorption is more strongly impacted by dosage increases, potentially due to stronger competition for available sites at lower metal concentrations.
On the other hand, a steep increase in removal efficiency was observed for all metal ions at lower adsorbent doses, followed by a plateau at higher concentrations, indicating near-saturation of adsorption sites as shown in Figure 3. This effect can be explained by the increase in available surface area as the concentration of sepiolite rises, which consequently provides a greater number of active binding sites within the same solution volume, thereby enhancing the total removal of metal ions [57]. Cu(II) showed the highest affinity for sepiolite, achieving >95% removal at just 3 g/L. Cd(II) removal efficiency continued to increase until about 7 g/L, beyond which the change was minimal. In contrast, Ni(II) removal exhibited a slower progression, with maximum removal (~87%) achieved at the highest tested dose (10 g/L). These results confirm that Cu(II) exhibits stronger and more efficient interaction with sepiolite, followed by Cd(II) and Ni(II), likely due to differences in hydration energy, ionic radius, and binding affinity. The observed trends are consistent with previously reported findings [9,10,26].
3.2.3. Adsorption Isotherms
The equilibrium adsorption behavior of Cd(II), Cu(II), and Ni(II) ions onto sepiolite was evaluated by plotting the equilibrium uptake (qe, mg/g) against the equilibrium concentration (Ce, mg/L) and the resulting adsorption isotherms are given in Figure 4.
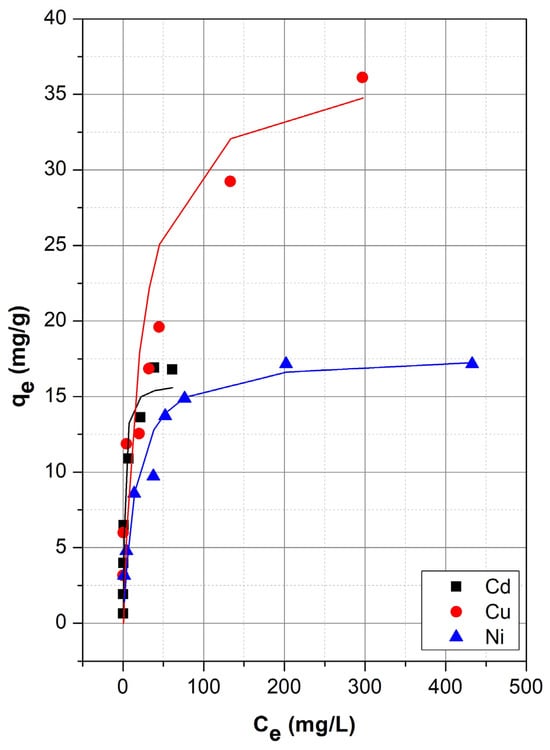
Figure 4.
Adsorption isotherms for Cd2+, Cu2+, and Ni2+ ions.
As seen, adsorption isotherms revealed characteristic features of Type I (Langmuir-type) isotherms behavior, where the adsorption capacity increases rapidly at lower concentrations and gradually levels off as the available adsorption sites become saturated [58]. This behavior suggests monolayer coverage of metal ions on the surface of the sepiolite and supports the assumption of finite and energetically equivalent adsorption sites.
Among the three metals, Cu(II) exhibited the highest adsorption capacity, with qe values reaching approximately 36 mg/g at elevated Ce values. The steep initial slope of the isotherm, followed by a gradual plateau, indicates a strong interaction between Cu(II) ions and the sepiolite surface, along with a progressive saturation of the available sites. In contrast, Cd(II) showed a similar but more moderate adsorption pattern, attaining a maximum qe of around 17 mg/g. The narrower range of Ce values over which adsorption occurred implies that Cd(II) ions may have a higher affinity for sepiolite at lower concentrations but quickly reach equilibrium as sites are occupied. On the other hand, Ni(II) displayed the lowest adsorption uptake, with qe values peaking around 17 mg/g even at considerably high equilibrium concentrations, suggesting a relatively weaker interaction with the sepiolite surface. The more gradual increase in qe and broader Ce range observed for Ni(II) could be attributed to slower adsorption kinetics, lower affinity for the adsorbent, or competitive interactions with other cations for limited surface sites. Overall, the isotherm profiles (Figure 4) demonstrate that sepiolite has a heterogeneous surface capable of adsorbing multiple heavy metal ions with varying degrees of affinity. The relative adsorption capacities observed for the tested metals follow the order Cu(II) > Ni(II) ~ Cd(II) with the sepiolite adsorption capacity towards Cu(II) being more than 2 times higher than that towards Cd(II) and Ni(II).
To further interpret the adsorption mechanism and evaluate the nature of the isotherms, the experimental data were fitted to three common isotherm models: Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) and the results are presented in Figure S3, while the obtained parameters are summarized in Table 2. These models help elucidate the adsorption mechanism, surface characteristics, and capacity of the sepiolite adsorbent.

Table 2.
Adsorption isotherms parameter values for the adsorption of Cd2+, Cu2+, and Ni2+ ions on sepiolite.
The Langmuir model is based on the assumption of monolayer adsorption onto a homogeneous surface with identical and energetically equivalent sites. It also assumes no interactions between adsorbed molecules [30]. Linearized Langmuir plots (Ce/qe vs. Ce) for each metal are shown in Figure S3a. The straight lines and high correlation coefficients (R2 > 0.960) confirm that the adsorption data fit well with the Langmuir model. Among the three metal ions, Ni(II) exhibited the best fit to Langmuir model, with a correlation coefficient (R2) of 0.997, while Cd(II) and Cu(II) also fit well, with R2 values of 0.992 and 0.964, respectively. The calculated maximum adsorption capacities (Qₘ) followed the trend Cu(II) > Ni(II) > Cd(II), with values of 37.31 mg/g, 17.83 mg/g, and 15.95 mg/g, respectively, in line with previous studies [9,22,25]. The Langmuir constants further support the favorable nature of the process. The dimensionless separation factor, RL, was found to be below 1 for all ions, indicating favorable adsorption conditions across the studied concentration range. Despite the high Qm values for Cu(II), a lower b value suggests that the strength of binding per site is weaker compared to Cd(II) and Ni(II).
The Freundlich model describes adsorption on heterogeneous surfaces and is particularly suitable for systems where multilayer adsorption may occur [31]. Linearized Freundlich plots (logqe vs. logCe) are given in Figure S3b. The model fits were slightly lower than those of the Langmuir model but still acceptable, with R2 values ranging from 0.889 for Cd(II) to 0.931 for Ni(II) supporting the hypothesis that the adsorption occurs on a heterogeneous surface. The Freundlich constants provided insight into adsorption intensity and capacity. The adsorption intensity parameter (n) was greater than 1 for all ions with 1/n values well below 1, confirming favorable adsorption. Among the three metals, Cu(II) showed the highest Freundlich constant (Kf = 5.37), followed by Cd(II) (Kf = 4.40) and Ni(II) (Kf = 3.29), suggesting also that Cu(II) adsorbs more effectively even under low concentration conditions. The higher values of n and Kf for Cu(II) indicate strong and efficient adsorption on sepiolite’s active sites, while the relatively lower values for Ni(II) point to weaker interactions. The trend is consistent with the Langmuir maximum adsorption capacities (Qₘ), reinforcing the conclusion that sepiolite demonstrates superior adsorption performance toward Cu(II). The favorable fits of the data to the Freundlich model also suggest that the adsorption mechanism may involve multilayer formation and interactions with a range of active sites differing in energy and accessibility. Such behavior is typical for naturally occurring mineral adsorbents like sepiolite, which possess a complex porous structure and surface chemistry.
The Dubinin-Radushkevich model provides insight into the nature of adsorption processes, especially for porous materials like sepiolite. Unlike Langmuir, it does not assume surface homogeneity or a constant adsorption potential. This model also estimates the adsorption energy, which can be used to distinguish between physical and chemical adsorption mechanisms. Linearized D-R plots (ln qe vs. ε2) are presented in Figure S3c. The model fits were lower than those of the Langmuir model but still high, with R2 values ranging from 0.889 for Cu(II) to 0.977 for Ni(II). The D-R model yielded higher maximum adsorption capacities (Qm) compared to the Langmuir model (Table 2) consistent with previously reported findings [10,59]. Cd(II) showed the highest capacity at 51.82 mg/g, followed by Ni(II) at 35.64 mg/g, and Cu(II) at 24.57 mg/g. These elevated values, particularly for Cd(II), suggest that micropore filling significantly contributes to the overall uptake, especially at lower concentrations [60].
The mean adsorption energies E, derived from the model, were above 8 kJ/mol for all three metals. This indicates that the adsorption process is primarily chemical in nature-rather than physical [61,62,63]. The relatively low energy values also support the reversibility of the adsorption process, which is favorable for potential regeneration and reuse of the adsorbent [64].
The comparative analysis of the isotherm models shows that the Langmuir model provided the best overall fit for all metal ions, implying that monolayer coverage dominates the adsorption process on the sepiolite surface. The higher Qm and Kf values for Cu(II) in both Langmuir and Freundlich models affirm the superior binding affinity of sepiolite for this metal. For Cd(II), although showing lower Langmuir capacity, exhibited the highest D-R capacity, suggesting that it can penetrate more deeply into the porous structure of sepiolite. On the other hand, Ni(II), while adsorbed favorably, showed comparatively weaker interactions with the adsorbent, as reflected in its lower Freundlich constants and a slower approach to equilibrium.
The Langmuir maximum adsorption capacities Qm (Table 2) indicated a selectivity sequence of Cu(II) > Ni(II) > C(II) which is consistent with prior findings on sepiolite and various clay minerals. For example, Padilla-Ortega et al. [22] reported exchange capacities of 13.8 mg/g for Cu(II) and 7.6 mg/g for Ni(II) on sepiolite. In addition, Jiang et al. [25] demonstrated that kaolinite exhibits a strong affinity for Cu(II), with lower affinity for Cd(II) and Ni(II), following the order: Cu(II) > Cd(II) > Ni(II), while our previous work on bentonite [9] revealed a similar selectivity sequence, even under competitive multi-metal conditions. Despite differences in experimental conditions, this persistent preference of sepiolite and related phyllosilicates to favor Cu(II) adsorption is governed by its higher electronegativity Cu (1.9) > Ni (1.8) > Cd (1.7) and first hydrolysis constant (Kh) values, Cu (10–8.0) > Ni (10–9.9) > Cd (10–10.1), which promote stronger electrostatic interactions and inner-sphere complexation with clay minerals’ surface sites.
Compared to literature data (Table 3), the sepiolite used in this study shows enhanced or competitive adsorption performance. For instance, Padilla-Ortega et al. [22] reported capacities of 13.8 and 7.6 mg/g for Cu(II) and Ni(II), respectively, while Huang et al. [65] documented 27.8 mg/g for Cd(II). On the other hand, Garcia-Sanchez et al. [66] reported notably lower adsorption capacities for sepiolite calculated as 8.3 mg/g for Cd(II) and 6.9 mg/g for Cu(II). Modified sepiolites may exhibit higher uptake, such as Cu(II) capacities up to 118.8 mg/g reported by Doğan et al. [67], but these materials require chemical treatments that may not be economically or environmentally favorable. Our results demonstrate that even untreated natural sepiolite possesses excellent adsorption characteristics, especially for Cu(II), likely due to its fibrous morphology, high surface area, and abundance of exchangeable sites.

Table 3.
Reported results on the adsorption capacity (in mg/g) of sepiolite for Cd2+, Cu2+, and Ni2+ ions.
3.2.4. Effect of Contact Time and Adsorption Kinetics
Understanding the kinetics of heavy metal adsorption is essential for evaluating the rate-limiting steps and potential application of sepiolite in wastewater treatment. The adsorption of Cd(II), Cu(II), and Ni(II) was monitored over a contact time range from 0 to 360 min, and the results are summarized in Figure 5.
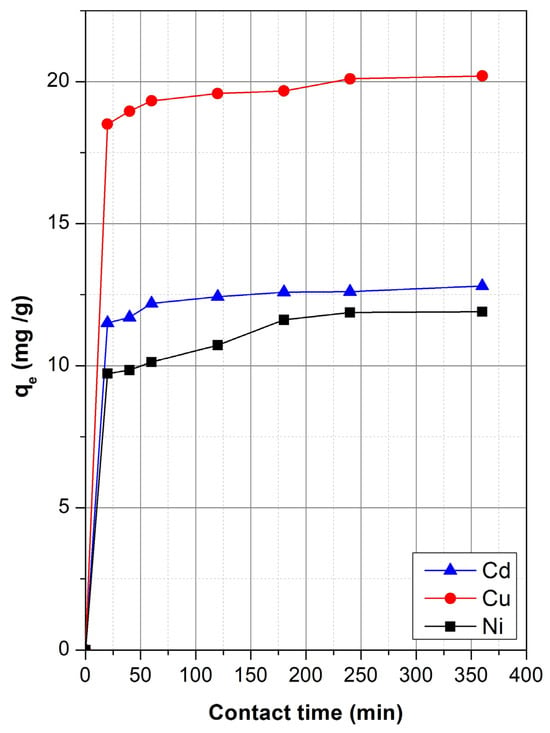
Figure 5.
Effect of contact time on the adsorption of Cd2+, Cu2+, and Ni2+ onto sepiolite.
The kinetics data indicate a two-stage adsorption mechanism: an initial fast phase dominated by surface adsorption on external sites, followed by a slower phase attributed to intraparticle diffusion or pore filling. Specifically, at the initial stages of contact (within the first 20 min), a rapid increase in metal uptake was observed for all ions, indicative of abundant available active sites on the sepiolite surface. Cu(II) exhibited the highest initial uptake, increasing to 18.5 mg/g at 20 min and gradually reaching 20.2 mg/g by 240 min. Cd(II) and Ni(II) showed slower adsorption profiles, with final uptakes of 12.8 mg/g and 11.9 mg/g, respectively. The adsorption process for Cu(II) was characterized by both a higher rate and greater overall capacity, consistent with previous isotherm findings. Notably, Ni(II) exhibited a steady but slower increase over time, while Cd(II) followed a similar trend, although the plateau began earlier, suggesting that equilibrium was nearly achieved within 180 min. The rapid saturation recorded for Cu(II), may be related to its stronger electrostatic interaction and higher affinity with sepiolite, while the relatively slower approach to equilibrium for Cd(II) and Ni(II) could be due to their weaker interactions and larger hydrated radii. In general, the equilibrium was nearly achieved within 180–240 min for all three metals. This indicates that sepiolite exhibits reasonably fast adsorption kinetics, making it a suitable candidate for practical applications, particularly in systems where longer contact times are feasible.
To further elucidate the adsorption kinetics of Cd2+, Cu2+ and Ni2+ onto sepiolite, the linearized forms of Lagergren pseudo-first order (PFO), the pseudo-second order (PSO) and the Weber–Morris intraparticle diffusion (IPD) models were applied by plotting log (qe-qt) vs. t, t/qt vs. t, and qt vs. t0.5, respectively, and are summarized in Figure S4, while the obtained parameters are presented in Table 4.

Table 4.
Comparison of the pseudo-first order, pseudo-second order and intraparticle diffusion kinetic models for Cd2+, Cu2+, and Ni2+ ions onto sepiolite.
The regression coefficients (R2) and slopes of the fitted lines show that the PFO model fits moderately well (Figure S4a), particularly for Cu(II) (R2 = 0.860) and Ni(II) (R2 = 0.881), while Cd(II) exhibits a slightly lower correlation (R2 = 0.834). The relatively good linearity suggests that the PFO model provides a fair approximation of the kinetic behavior, particularly during the early stages of adsorption. However, the fact that the calculated qe values (qe,calc) derived from the model deviate from the experimental values (qe,exp, Table 4) implies that the PFO model may not fully capture the complexity of the adsorption mechanism. This is particularly the case for systems where chemisorption or intraparticle diffusion plays a significant role-evident from previous intraparticle diffusion analyses [26,62].
On the other hand, the linearity of the plots of t/qt vs. t and the high correlation coefficients (R2 > 0.995 for all metals) indicate excellent agreement with the PSO model (Figure S4b). Moreover, the closeness of the experimental (qe,exp) and calculated (qe,calc) qe values (Table 4) indicate that the PSO model best fits the adsorption kinetics of Cu(II), Cd(II), and Ni(II), supporting a chemisorption-controlled mechanism involving valence forces through electron sharing or exchange between the metal ions and functional groups on the sepiolite surface [10,65,71].
Finally, to further examine the diffusion mechanisms, the Weber–Morris IDP model was applied and the plots of qt vs. t0.5 (Figure S4c) revealed that for Cu(II) and Cd(II), the curves were multilinear, indicating that the adsorption process occurred in multiple stages: an initial sharper region corresponding to rapid external surface adsorption or film diffusion, followed by a second, more gradual linear portion associated with slower diffusion within the pores. However, the lower correlation coefficients (R2 = 0.670–0.873) obtained for the second stage are primarily due to the system approaching equilibrium, where qt changes slightly with t0.5. This reflects that most active sites are already occupied and the diffusion rate significantly decreases as equilibrium is reached, rather than indicating simultaneous surface and intraparticle diffusion mechanisms. This interpretation is consistent with typical behavior observed in similar adsorption systems [62,71,72,73]. For Ni(II), however, the plot was linear without distinct breaks, suggesting that intraparticle diffusion was the primary mechanism without significant external mass transfer resistance. The multilinear behavior observed for Cu(II) and Cd(II) is characteristic of boundary layer effects in the early stages, followed by intraparticle diffusion dominating in the later stages. Notably, the non-zero intercepts (C) confirm that intraparticle diffusion is not the sole rate-controlling mechanism, but occurs in conjunction with external mass transfer [62]. Compared to other metals, the higher intercept values for Cu(II) (Table 4) indicate a greater contribution from the boundary layer effect, which may be attributed to its stronger initial affinity to sepiolite surfaces. This aligns with the overall higher adsorption capacity observed for Cu(II), and supports the greater affinity for sepiolite among the metals tested.
3.3. Regeneration Study
The regeneration potential of sepiolite was examined via recycling the Cd(II)-, Cu(II)-, and Ni(II)-loaded adsorbent by using two different eluting agents, distilled water and 0.1 N HCl and the results are summarized in Figure 6.
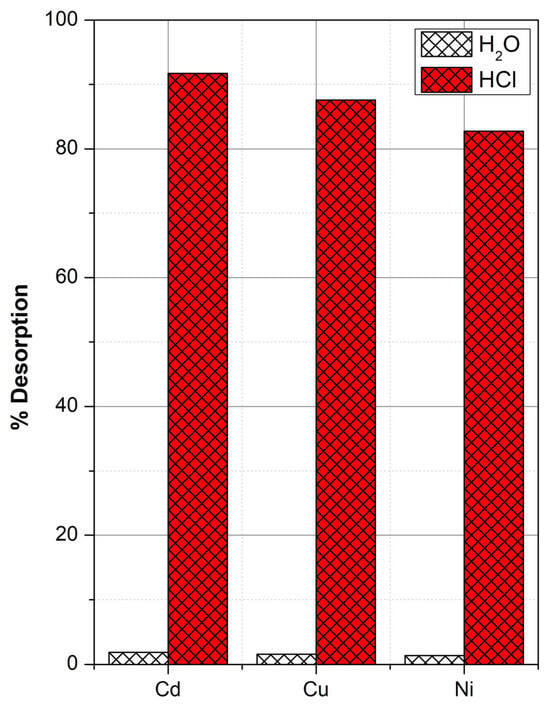
Figure 6.
Desorption study of Cd(II)-, Cu(II)-, and Ni(II)-loaded sepiolite.
As shown, desorption with water was minimal across all metals, with values ranging from 1.5% to 2.2%, indicating that the metal ions are strongly retained on the sepiolite surface, likely due to chemisorption mechanisms. Conversely, HCl proved to be a better eluting agent, achieving desorption efficiencies of approximately 92% for Cd(II), 88% for Cu(II), and 83% for Ni(II). These results suggest that sepiolite can be efficiently regenerated under mild acidic conditions. These findings are consistent with previous studies. For instance, El-Rayyes et al. [26] also reported HCl as the best eluting agent for As(V) desorption by natural clay, while Padilla-Ortega et al. [22] reported successful desorption of toxic metals from natural sepiolite using diluted acid maintaining adsorption performance over cycles. In the present study, only a single adsorption–desorption cycle was performed to establish the basic regeneration potential of sepiolite. Future investigations will focus on multi-cycle adsorption–desorption experiments (3–5 cycles) to evaluate the long-term reusability, structural stability, and performance consistency of the material.
4. Conclusions
The present study comprehensively evaluated the physicochemical properties and adsorption performance of natural sepiolite for the removal of Cd(II), Cu(II), and Ni(II) ions from aqueous solutions under batch conditions. Extensive characterization confirmed that the studied sepiolite is a highly crystalline fibrous magnesium silicate with minor palygorskite, quartz, and calcite impurities. X-ray diffraction revealed a well-defined lattice structure, while DTG–TGA analysis identified distinct dehydration and dehydroxylation stages, demonstrating high thermal stability. BET analysis showed a high specific surface area (SBET = 194.8 m2/g), mesoporous texture, and a total pore volume of 0.39 cm3/g, properties that are highly favorable for adsorption applications.
Adsorption efficiency was strongly pH-dependent, with optimal uptake for all metals occurring near pH 6. At low pH, competition between H+ ions and metal cations reduced removal efficiency, while near-neutral conditions promoted electrostatic attraction between negatively charged surface sites and divalent metal ions. Variation in adsorbent dosage revealed that while higher doses improved the overall removal percentages, the adsorption capacity per unit mass decreased due to particle aggregation and site overlap.
Equilibrium studies indicated that the Langmuir isotherm provided the best fit for all tested metals, suggesting monolayer adsorption on homogeneous active sites. The maximum adsorption capacities (Qm) obtained from the Langmuir model were 15.95 mg/g for Cd(II), 37.31 mg/g for Cu(II), and 17.83 mg/g for Ni(II). The selectivity trend followed Cu(II) > Ni(II) > Cd(II), consistent with differences in ionic radius, hydration energy, and electronegativity. Freundlich modeling supported the presence of surface heterogeneity and possible multilayer adsorption, while Dubinin-Radushkevich analysis indicated that the adsorption process was predominantly chemical in nature, with mean adsorption energies above 8 kJ/mol (8.45–21.32 kJ/mol) confirming the involvement of valence interactions and ion-exchange mechanisms.
Kinetic analysis showed that adsorption proceeded rapidly during the initial contact period, followed by a slower phase attributed to intraparticle diffusion. The pseudo-second-order kinetic model provided the best fit to experimental data for all three metals, suggesting that chemisorption involving valence forces and electron sharing plays a dominant role, even though physical adsorption is also significant. Intraparticle diffusion plots further indicated that both surface adsorption and pore diffusion contribute to the overall rate-limiting steps, with Cu(II) exhibiting a more pronounced boundary layer effect.
Regeneration experiments demonstrated that mild acid treatment (0.1 N HCl) could desorb over 80% of the adsorbed metals, with efficiencies of approximately 92% for Cd(II), 88% for Cu(II), and 83% for Ni(II). These results confirm the strong regeneration potential of natural sepiolite under mild acidic conditions, indicating its suitability for reuse after proper treatment. Only a single adsorption–desorption cycle was performed in this study to establish the basic regeneration potential of sepiolite; however, future work will focus on multi-cycle adsorption–desorption testing to assess the long-term reusability and stability of sepiolite for practical wastewater applications.
In conclusion, the combination of high surface area, mesoporous structure, and favorable adsorption capacity positions natural sepiolite as an effective, low-cost, and environmentally benign material for the remediation of heavy metal-contaminated water. Its ability to be regenerated efficiently further enhances its practical applicability. Given its competitive performance compared to other natural and modified adsorbents, sepiolite can be considered a promising candidate for scalable wastewater treatment processes targeting multi-metal contamination. Future work will focus on surface modification of sepiolite to evaluate how such treatments influence surface area, functional groups, and metal ion selectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15111110/s1, Table S1. Experimental conditions for the removal of Cd(II), Cu(II), and Ni(II) ions by adsorption on sepiolite. Figure S1. Linear plot of N2 adsorption onto sepiolite. Figure S2. Speciation diagrams of (a) Cd(II), (b) Cu(II), and (c) Ni(II) as a function of pH, generated using Visual MINTEQ (v3.1) at 25 °C for a total metal concentration of 1 × 10−4 M. Figure S3. Linear plots of (a) Langmuir (Ce/qe vs. Ce), (b) Freundlich (logqe vs. logCe), and (c) Dubinin-Radushkevich (ln qe vs. ε2) adsorption isotherm models for Cd(II), Cu(II), and Ni(II) adsorption onto sepiolite. Figure S4. Linear plots of (a) Lagergren pseudo-first order (log (qe − qt) vs. t), (b) pseudo-second order (t/qt vs. t), and Weber-Morris intraparticle diffusion (qt vs. t^0.5) kinetic models for the adsorption of Cd(II), Cu(II), and Ni(II) onto sepiolite.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The author wishes to express sincere gratitude to Emeritus Prof. C. Sikalidis for kindly providing the sepiolite sample used in this study. Special thanks are also extended to Dr. Maria Betsiou for conducting the chemical analysis, and to Dr. S. Konopisi for the thermal analysis. Their contributions and expertise were instrumental in the successful completion of this research. Finally, three anonymous reviewers are highly acknowledged for their insightful comments and valuable suggestions, which greatly improved this work.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef] [PubMed]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol./Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Tang, J.; Wang, B.; Huang, F. Advance of the treatment of heavy metal wastewater by adsorption. Chem. Ind. Eng. Prog. 2013, 11, 2749–2756. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Aziz, K.H.H. Removal of toxic heavy metals from aquatic systems using low-cost and sustainable biochar: A review. Desalination Water Treat. 2024, 320, 100757. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono- and multi-metal systems. Environ. Earth Sci. 2015, 73, 5435–5444. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Lead removal from aqueous solutions by natural Greek bentonites. Clay Miner. 2013, 48, 771–787. [Google Scholar] [CrossRef]
- Fathy, A.T.; Moneim, M.A.; Ahmed, E.A.; El-Ayaat, A.; Dardir, F.M. Effective removal of heavy metal ions (Pb, Cu, and Cd) from contaminated water by limestone mine wastes. Sci. Rep. 2025, 15, 1680. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Newman, A.C.D.; Brown, G. The chemical constitution of clays. In The Chemistry of Clays and Clay Minerals; Newman, A.C.D., Ed.; Mineralogical Society of London: London, UK, 1987; pp. 1–128. [Google Scholar]
- Brauner, K.; Presinger, A. Struktur und Entstehung des Sepioliths. Tschermak’s Mineral. Petrogr. Mitteilungen 1956, 6, 120–140. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Doi, H.; Kamigaito, O. Pore distribution and adsorption selectivity of sepiolite. Clay Miner. 1990, 25, 99–105. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Handbook of Clay Science; Bergaya, F., Theng, B.H.K., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar]
- Bahabadi, F.N.; Farpoor, M.H.; Mahrizi, M.H. Removal of Cd, Cu and Zn ions from aqueous solutions using natural and Fe modified sepiolite, zeolite and palygorskite clay minerals. Water Sci. Technol. 2013, 75, 340–349. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Lugli, C.; Poppi, L. Kinetics of heavy-metal removal and recovery in sepiolite. Appl. Clay Sci. 2000, 16, 45–57. [Google Scholar] [CrossRef]
- Doğan, M.; Turhan, Y.; Alkan, M.; Namli, H.; Turan, P.; Demirbaş, Ö. Functionalized sepiolite for heavy metal ions adsorption. Desalination 2008, 230, 248–268. [Google Scholar] [CrossRef]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Mendoza-Barron, J.; Guerrero-Coronado, R.M.; Jacobo-Azuara, A.; Aragon-Piña, A. Adsorption of heavy metals ions from aqueous solution onto sepiolite. Adsorpt. Sci. Technol. 2011, 29, 569–584. [Google Scholar] [CrossRef]
- Hojati, S.; Khademi, H. Cadmium sorption from aqueous solutions onot Iranian sepiolite: Kinetics and isotherms. J. Cent. South Univ. 2013, 20, 3627–3632. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Xue, X.X. Study on adsorption of heavy metalion in metallurgical wastewater by sepiolite. Adv. Mater. Res. 2011, 726–731, 2585–2588. [Google Scholar]
- Jiang, M.-Q.; Jin, X.-Y.; Lu, X.-Q.; Chen, Z.-L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- El-Rayyes, A.; Hefawy, M.; Refat, M.S.; Ogunbamowo, O.E.; Babatimehin, A.M.; Ngueagni, P.T.; Ofudje, E.A.; Alsuhaibani, A.M. Kinetics, equilibrium and thermodynamics studies on natural and heat treated clays for the removal of arsenate ions from aqueous solution. Sci. Rep. 2025, 15, 15526. [Google Scholar] [CrossRef]
- Sabah, E.; Ouki, S. Adsorption of pyrene from aqueous solutions onto sepiolite. Clays Clay Miner. 2017, 65, 14–26. [Google Scholar] [CrossRef]
- Li, N.; Yan, X.; Dai, W.; Lv, B.; Wang, W. Adsorption properties and mechanism of sepiolite to grapheme oxide in aqueous solution. Arab. J. Chem. 2023, 16, 104595. [Google Scholar] [CrossRef]
- Alexiades, C.A.; Jackson, M.L. Quantitative clay mineralogical analysis of soils and sediments. Clays Clay Miner. 1966, 14, 35–52. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber die Adsorption in Loesungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Veli, S.; Alyuz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.L.; Chen, C.L.; Wang, X.K. Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: Effect of pH, ionic strength, foreign ions and temperature. Appl. Clay Sci. 2008, 41, 37–46. [Google Scholar] [CrossRef]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Batch lead(II) removal from aqueous solution by peat: Equilibrium and kinetics. Trans. Inst. Chem. Eng. 1999, 77, 165–173. [Google Scholar]
- Ho, Y.S.; Ng, J.C.; McKay, G. Removal of lead(II) from effluents by sorption on peat using second-order kinetics. Sep. Sci. Technol. 2001, 36, 241–261. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption of carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–63. [Google Scholar] [CrossRef]
- Knocke, W.R.; Hemphill, L.H. Mercury (II) sorption by waste rubber. Water Res. 1981, 15, 275–282. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L.; Heaney, P.J. Synchroton powder X-ray diffraction study of the structure and dehydration behavior of sepiolite. Am. Mineral. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Sánchez del Rio, M.; García-Romero, E.; Suárez, M.; da Silva, I.; Fuentes Moreno, L.; Martín-Criado, G. Variability in sepiolite: Diffraction studies. Am. Mineral. 2011, 96, 1443–1454. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Variability of the surface properties of sepiolite. Appl. Clay Sci. 2012, 67–68, 72–82. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Advances in the crystal-chemistry of sepiolite and palygorskite. In Developments in Clay Science; Galan, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 33–65. [Google Scholar]
- Suárez, M.; García-Rivas, J.; García-Romero, E.; Jara, N. Mineralogical characterization and surface properties of sepiolite from Polatli (Turkey). Appl. Clay Sci. 2016, 131, 124–130. [Google Scholar] [CrossRef]
- Karakaya, N.; Celik Karakaya, M.; Temel, A.; Küpeli, S.; Tunoğlu, C. Mineralogical and chemical characterization of sepiolite occurrences at Karapinar (Konya basin, Turkey). Clays Clay Miner. 2004, 52, 495–509. [Google Scholar] [CrossRef]
- Perraki, T.; Orfanoudaki, A. Study of raw and thermally treated sepiolite from the Mantoudi area, Euboea, Greece: X-ray diffraction, TG/DTG/DTA and FTIR investigations. J. Therm. Anal. Calorim. 2008, 91, 589–593. [Google Scholar] [CrossRef]
- Pėrez-Rodríguez, J.L.; Galán, E. Determination of impurity in sepiolite by thermal analysis. J. Therm. Anal. 1994, 42, 131–141. [Google Scholar] [CrossRef]
- Lazarevic, S.; Jankovic-Častvan, I.; Jovanovic, D.; Milonjic, S.; Janackovic, D.; Petrovic, R. Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl. Clay Sci. 2007, 37, 47–57. [Google Scholar] [CrossRef]
- Sheikhhosseini, A.; Shirvani, M.; Shariatmadari, H. Competitive sorption of nickel, cadmium, zinc and copper on palygorskite and sepiolite silicate clay minerals. Geoderma 2013, 192, 249–253. [Google Scholar] [CrossRef]
- Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Camazano, M. Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: Influence of clay type and pesticide hydrophobicity. Appl. Clay Sci. 2006, 31, 216–228. [Google Scholar] [CrossRef]
- Casal, B.; Merino, J.; Serratosa, J.M.; Ruiz-Hitzky, E. Sepiolite-based materials for the photo- and thermal-stabilization of pesticides. Appl. Clay Sci. 2001, 18, 245–254. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef]
- Kumrić, K.R.; Đuckić, A.B.; Trtić-Petrović, T.M.; Vukelć, N.S.; Stojanović, Z.; Grbović Novakovi, J.D.; Matović, L.L. Simultaneous removal of divalent heavy metals from aqueous solutions using raw and mechanochemically treated interstratified montmorillonite/kaolinite clay. Ind. Eng. Chem. Res. 2013, 52, 7930–7939. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Kannan, N.; Rengasamy, G. Comparison of cadmium adsorption on various activated carbons. Water Air Soil Pollut. 2005, 163, 185–201. [Google Scholar] [CrossRef]
- Sen, T.K.; Gomez, D. Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination 2011, 267, 286–294. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm: I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Onursal, N. Application of a new adsorption kinetic model for the removal of Zn(II) ions present in aqueous solutions with Malatya clay. J. King Saud Univ.-Sci. 2025, 37, 4232024. [Google Scholar] [CrossRef]
- Dubinin, M.; Astakhov, V. Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents–Communication 1. Bull. Acad. Sci. USSR Div. Chem. Sci. 1971, 20, 3–7. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Gokirmak Sogut, E.; Alacabey, I. Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kul, A.R.; Koyuncu, H. Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. J. Hazard. Mater. 2010, 179, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Shreadah, M.A.; Heiba, H.F.; Ahmed, A.M. Validity of Egyptian Na-montmorillonite for adsorption of Pb2+, Cd2+ and Ni2+ under acidic conditions: Characterization, isotherm, kinetics, thermodynamics and application study. Asia-Pac. J. Chem. Eng. 2017, 12, 292–306. [Google Scholar] [CrossRef]
- Girish, C.R. Determination of thermodynamic parameters in adsorption studies: A review. Chem. Pap. 2025, 79, 5687–5706. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.; Chen, L.; Sun, Y. The sorption of Cd(II) and U(VI) on sepiolite: A combined experimental and modeling studies. J. Mol. Liq. 2015, 209, 706–712. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Alastuey, A.; Querol, X. Heavy metal adsorption by different minerals: Application to the remediation of polluted soils. Sci. Total Environ. 1999, 242, 179–188. [Google Scholar] [CrossRef]
- Doğan, M.; Türkyilmaz, A.; Alkan, M.; Demirbaş, Ö. Adsorption of copper (II) ions onto sepiolite and electrokinetic properties. Desalination 2009, 238, 257–270. [Google Scholar] [CrossRef]
- Ansanay-Alex, S.; Lomenech, C.; Hurel, C.; Marmier, N. Adsorption of nickel and arsenic from aqueous solution on natural sepiolite. Int. J. Nanotechnol. 2012, 9, 204–215. [Google Scholar] [CrossRef]
- Samieifard, R.; Landi, A.; Pourreza, N. Adsorption of Cd, Co, and Zn from multi-ionic solutions onto Iranian sepiolite isotherms. Cent. Asian J. Environ. Sci. Technol. 2021, 3, 102–118. [Google Scholar]
- Turhan, Y.; Turan, P.; Doğan, M.; Alkan, M.; Namli, H.; Demirbaş, Ö. Characterization and adsorption properties of chemically modified sepiolite. Ind. Eng. Chem. Res. 2008, 47, 1883–1895. [Google Scholar] [CrossRef]
- Sun, W.J.; Tang, Q.T.; Lu, T.H.; Fan, R.D.; Sun, G.G.; Tan, Y.Z. Adsorption performance of bentonite and clay for Zn(II) in landfill leachate. Geoenviron. Disasters 2024, 11, 4. [Google Scholar] [CrossRef]
- Ciosek, A.L.; Luk, G.K. Kinetic modeling of the removal of multiple heavy metallic ions from mine waste by natural zeolite sorption. Water 2017, 9, 482. [Google Scholar] [CrossRef]
- Lach, J.; Okoniewska, E. Equilibrium, kinetic, and diffusion mechanism of lead (II) and cadmium (II) adsorption onto commercial activated carbons. Molecules 2024, 29, 2418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).